Abstract
Background. Induction of heat shock proteins (HSP), i.e. of the major family member HSP70, is an important cytoprotective-resistance mechanism for monocytes/ macrophages (Mphi). Patients on haemodialysis present with a high infectious morbidity and enhanced carcinoma incidence. Renal insufficiency-related alteration of microbicidal and tumoricidal functions of Mphi, major effectors of the immune system, might promote these diseases.
Methods. Freshly isolated Mphi from Sprague–Dawley rats 2 weeks after 5/6-nephrectomy and from patients on intermittent haemodialysis (IHD) were stimulated by heat shock (HS) and compared to stimulated Mphi of control rats or healthy volunteers (CTR). Expression of HSP72 (inducible HSP70) was assessed by RT-PCR, and/or flow cytometry. Apoptosis of Mphi was detected by flow cytometry (CD14/annexin V-labelling).
Results. In rat Mphi, baseline HSP72 expression was similar in both groups, but its induction was significantly impaired in renal insufficiency (214 ± 68% less HSP70-mRNA versus CTR, n = 6). In patients, HSF-1-mRNA and HSP72-mRNA/protein response to HS was significantly lower, but not affected by dialysis session itself. In parallel, apoptosis of Mphi of patients was enhanced (+83 ± 29% constitutive apoptotic Mphi versus CTR, n = 8), and HS-dependent protection from apoptosis with and without serum depletion (48 h depletion: HS, +275 ± 37% apoptotic Mphi versus CTR, n = 6; full medium: +166 ± 62% versus CTR, n = 8, P < 0.05) was inferior.
Conclusions. Impaired HSP72 stress response of Mphi in patients on haemodialysis might contribute to the observed immune dysfunction and, therefore, to the increased susceptibility to infection and malignancy. Stress impairment is not restricted to uraemia but is already present in a rat model of chronic kidney disease.
Keywords: apoptosis, haemodialysis, HSP72, human monocytes
Introduction
Patients on haemodialysis present with a high infectious morbidity, enhanced carcinoma incidence, impaired wound healing and reduced effect of vaccination—diseases that are directly related to the function of the immune system [1–4]. Immunological abnormalities of leukocytes have been described in patients on dialysis [5]. Mphi (monocytes/ macrophages) are major effectors of the immune system with widespread microbicidal and tumoricidal functions that are altered in uraemia [6–8]. Uraemic immunodeficiency is a complication of renal failure reflecting stress, e.g. exerted by as yet poorly defined uraemic toxins, imbalances of antioxidants and pro-oxidants, malnutrition or iron overload, membrane bio-incompatibility and inflammation [9–11]. Therefore, patients with end-stage renal disease (ESRD) suffer from increased oxidative stress, which can only be ineffectively treated by haemodialysis [12–14]. The response of human cells to stress, e.g. heat shock (HS), is a highly conserved adaptive cell response found in all cells in all life forms conferring resistance to different stressors, i.e. increased temperature (fever), nutritional deficiency, oxidative stress or hypoxia [15–17]. The corresponding heat shock proteins (HSPs), like the major stress-inducible family member HSP72 (70-kDa HSP, inducible HSP70, HSPA1A, HSP70-1) [18], can also be found in cells under normal physiological conditions controlling the folding of proteins. Constitutively expressed HSP70 (Hsc70) binds to newly translated immature proteins and prevents premature and improper binding and folding [19–21]. This is vital, since HSP72-deficient animals are susceptible to stress or even not viable [22]. In response to stress, housekeeping activities of HSPs, e.g. degradation of unstable and misfolded proteins, control of regulatory proteins or transport of proteins, switch towards reaction against stress to increase the cell's chance of survival. The cellular injury decreases, either by induction of cytoresistance or by enhancement of repair mechanisms [18]. Thus, HSP72 induction confers protection even against sublethal damage causing apoptosis and is, therefore, referred to as a cellular lifeguard. We and others showed HSP72-dependent injury protection of monocytes [23,24]. Of note, housekeeping functions of HSPs are critically involved, e.g. as potent immune adjuvants, into the innate and adaptive immune response in inflammation, infection and tumour defence [25–30]. In the case of infection, HSP72 induction is even crucial keeping Mphi alive and, therefore, capable for anti-hazardous actions [31–33]. Several factors and pathways have been identified as responsible triggers causing immunodeficiency in patients with ESRD [8,34,35]. But still, the underlying mechanisms determining Mphis’ dysfunction are complex and remain, to a certain extent, unknown. Thus, they are addressed in this study. Having identified a reduced stress-related HSP72 response in Mphi of rats with chronic kidney disease, we used HS, as a simple model of fever/stress, to investigate the capacity of HSP72 induction in Mphi of patients on intermittent haemodialysis (IHD).
Subjects and methods
Subjects
We enrolled 37 stable patients (20 males) on IHD with mean age of 38 (range 18–76) years chosen at random from the Dialysis Unit of the University Hospital Münster (Münster, Germany). Patients’ Mphi were compared to those of 37 apparently healthy volunteers (23 males) on no medication, who presented no complaints or active pathology with a mean age of 32 (CTR, range 22–59) years. There was no statistically significant difference in age among the groups studied. Patients had haemoglobin levels between 11.5 and 13.5 g/dl, treated with erythropoietin (epoetin alpha: 0–24 000 I.E., median 10 000 I.E./week i.v.) and intravenous iron, and standard heparin anticoagulation. Blood pressure was controlled, whereas 23 patients received a dihydropyridine calcium channel blocker and/or an angiotensin-converting enzyme inhibitor, respectively, to reach normotension. Patients received haemodialysis for 4 h on three occasions each week for more than 12 months, using polysulfone membranes (F8, FMC, Bad Homburg, Germany) and bicarbonate dialysate (32 mmol/l) containing 138 mM Na+, 3.0 mM K+, 1.5 mM Ca2+, 0.5 mM Mg2+, 111 mM Cl− and glucose 1 g/l (SK-F 313/1, FMC). Dialysate flow rate was 500 ml/min and blood flow >250 ml/min. Patients had primary arteriovenous fistulae (radiocephalic or brachiocephalic), and two needles were used for each dialysis session. The renal diagnoses were nephrosclerosis (n = 10), inactive glomerulonephritis (n = 7), interstitial nephritis (n = 5), polycystic kidney disease (n = 3), renal failure related to chronic heart failure (n = 3) and diabetic nephropathy (n = 2), and in seven patients the reason remained undefined. In both groups, the exclusion criteria were as follows: haemodialysis duration <12 months, PTH >400 pg/ml, evidence of infection, neoplasia or liver disease. Patients on antibiotic, antiinflammatory or immunosuppressive drugs were also excluded. Informed consent was obtained from all participants, and the study was approved by the ethics committee of the Münster University Hospital, Münster, Germany.
Animal model
Eight-week-old male Sprague–Dawley rats (Charles River, Sulzfeld, Germany) with free access to standard rat chow (Ssniff, Soest, Germany) and tap water were randomly assigned to surgical 5/6-nephrectomy (5/6-NX, n = 6) or control group (CTR, n = 6). Experiments were approved by a governmental committee on animal welfare and were performed in accordance with National Animal Protection guidelines.
In short, the 5/6-NX involved midline incision, removal of the right kidney and selective ligation of 2–3 branches of the left renal artery. Surgery was performed under general anaesthesia [ketamine (100 mg/kg)/xylazine (10 mg/kg)]. Body weight (BW) was measured before surgery and 2 weeks after surgery. Serum parameters, blood pressure (tail cuff plethysmography) and total kidney function were recorded after 2 weeks of disease progression. Twenty-four hours before kidney recovery, animals were housed in metabolic cages. Urine and blood samples were analysed for creatinine (enzymatic assay, Creatinine-Pap, Roche Diagnostics, Mannheim, Germany), BUN (urease-GLDH method) and protein (Bradford Blue, BioRad Laboratories, München, Germany).
Antibodies and reagents
Antibodies were obtained as described in Table 1. Saponin and D-mannitol were purchased from Merck (Darmstadt, Germany). Unless otherwise indicated, all reagents were purchased from Sigma-Aldrich (Taufkirchen, Germany).
Table 1.
Real-time PCR primers and Antibodies
| Primer | Sequence listed in the 5′ to 3′ direction |
|---|---|
| Human | |
| GAPDH sense | CAA GCT CAT TTC CTG GTA TGA C |
| GAPDH antisense | GTG TGG TGG GGG ACT GAG TGT GG |
| HSF-1 sense | CGA CAG TGG CTC AGC ACA TTC C |
| HSF-1 antisense | CAG CTC GGT GAT GTC GGA GAT G |
| HSP72 sense | CCG GCA AGG CCA ACA AGA TC |
| HSP72 antisense | CCT CCA CGG CGC TCT TCA TG |
| Rat | |
| GAPDH sense | CAA GCT CAT TTC CTG GTA TGA |
| GAPDH antisense | GTG TGG TGG GGG ACT GAG TGT |
| HSP72 sense | GCA CCA ATG AGG CGA TGG AGT G |
| HSP72 antisense | GGA CTG CAA ATA TTT GTC AGC TC |
| Antibody | |
| Annexin V | FITC-labelled, Bender Medsystems, Vienna, Austria |
| Anti-mouse FITC | FITC-labelled F(ab’)2 fragments of goat anti-mouse IgG, Dianova, Hamburg, Germany |
| Anti-rabbit HRP | DakoCytomation, Glostrup, Denmark |
| Anti-mouse HRP | DakoCytomation, Glostrup, Denmark |
| CD14 | PE-labelled, clone P9, IgG2b, Becton Dickinson, Palo Alto, USA |
| GAPDH | ab9484, abcam, Cambridge, UK |
| HSF-1 | #4356, Cell Signaling Technology, Boston, USA |
| HSP70 | Inducible HSP70, SPA-810, Stressgen, Victoria, Canada |
| Ig isotypes | PE- and FITC-conjugated murine IgG mAb of unrelated specificities, Becton Dickinson |
Histology
Portions of kidneys were snap-frozen and fixed in 4% formaldehyde in PBS. Histological changes (e.g. glomerular sclerosis, vascular walls, tubular casts and infiltration) were examined by light microscopy in paraffin-embedded tissue with periodic acid-Schiff staining.
Isolation and culture of mononuclear cells from peripheral blood
Blood samples were obtained under sterile conditions in all patients just before or before-and-after the first weekly dialysis session (long interval), respectively. In rats, trunk blood was collected after decapitation 2 weeks after 5/6-NX. Mphi were purified as published before [36]. In short, Mphi were separated from heparinized whole blood by density gradient separation (Ficoll/Hypaque; Biochrom Seromed, Berlin, Germany). Monocytes were further purified by adherence to the culture dishes, resulting in a final purity of >85%. Cells were washed and seeded in 24-well plates (Greiner, Nürtingen, Germany) at 37°C (5% CO2/95% air atmosphere) in RPMI 1640 culture medium (CM, PAA Laboratories, Pasching, Austria) supplemented with 2 mM l-glutamine, 50 μg/ml penicillin/streptomycin, 5 mM HEPES and 10 μM mercapto-ethanol. For HS, Mphi (5 × 106/ml) were seeded in preheated CM (temperature as indicated) containing 5% inactivated fetal calf serum (FCS; endotoxin content <0.01 ng/ml) and chemicals as indicated. After 40-min HS, heated CM was replaced by CM (37°C) and cells were further incubated at 37°C until measurement. For experiments with D-mannitol and urea, Mphi were incubated with CM, CM + 150 mg/dl mannitol or CM + 150 mg/dl urea. HS was performed after 60 h (40 min, 47°C), and all cells (±HS) were harvested for real-time PCR analysis after 72 h. Differing incubation times or temperatures are given in results or figure legends.
Flow cytometric detection of inducible HSP70 (HSP72)
After fixation with paraformaldehyde (4%, 15 min, 4°C) and washing, cells (5 × 106/ml) were incubated in a staining buffer (1% FCS in a 50 mM HEPES buffer, saponin 0.1%, pH 7.4) for 30 min at 4°C and labelled with mouse anti-human HSP70. After washing, the cells were incubated for 30 min with the secondary FITC-labelled goat anti-mouse-IgG1. Cytofluorometric analysis was performed with a FACScan cytometer (BD Life Science Research) for a total of 10 000 events.
Western Blot
For western blot analyses, proteins were separated by SDS-polyacrylamide (4–20%) electrophoresis, and transferred to a PVDF membrane incubated with blocking agent (Amersham, Freiburg, Germany). After incubation with the primary antibodies anti-HSF-1, or anti-HSP70 and anti-GAPDH for 90 min at room temperature, the secondary HRP antibodies goat anti-rabbit and anti-mouse (1:10 000, DakoCytomation, Glostrup, Denmark) were incubated for another 45 min. For signal development, the blots were covered with chemiluminescent substrate (SuperSignal, Pierce, Bonn, Germany) before exposure.
Flow cytometric quantification of apoptosis
Forty-eight hour serum depletion (0.2% FCS) was used to render Mphi apoptotic. Simultaneous detection of CD14 expression and annexin V-binding identifies apoptotic monocytes [37]. Mphi were deemed to be apoptotic when CD14 expression was low and annexin V-binding was high. Mphi prepared and treated as described above were double-labelled with PE-conjugated CD14 and annexin V-FITC in a staining buffer (PBS, containing 0.05% FCS and 0.05% sodium azide, pH 7.4) for 15 min on ice. PE- and FITC-conjugated murine IgG mAb of unrelated specificities were used as controls. The cells were washed after staining and flow cytometry was applied for a total of 10 000 events. Necrosis was excluded by trypan blue staining.
RT-PCR
Messanger RNA expression profiles for selected genes were validated by real-time PCR. Total RNA (10 μg) from freshly isolated monocytes was used for cDNA synthesis with the high capacity cDNA reverse transcription kit. Real-time PCR was performed using SYBR Green PCR Master Mix on an ABI PRISM 7700 sequence detection system. The specific primer pairs used for amplification are listed in Table 1 (MWG-Biotech, Ebersberg, Germany). All instruments and reagents were purchased from Applied Biosystems (Darmstadt, Germany). Relative gene expression values were evaluated with the 2−ΔΔCt method using GAPDH as a housekeeping gene [38].
Statistical analyses
Data are presented as means ± SD. Data were compared with Student's t-test or ANOVA variance analysis for multiple comparisons using GraphPad Prism 4.0 (GraphPad Software, La Jolla, CA, USA). Significance was inferred at P < 0.05.
Results
Animal model
Two weeks after 5/6-NX, subtotal renal ablation induced adaptations in remnant nephrons, whereas kidneys from controls presented with physiological histology (Figure 1A). Kidneys of 5/6-NX showed glomerular hypertrophy, fibrosis, a mild tubulo-interstitial infiltrate and thickening of the vessels’ muscular layer. Atrophy and detachment of epithelial cells, in addition to luminal proteinaceous casts, were found in proximal tubules (Figure 1B).
Fig. 1.
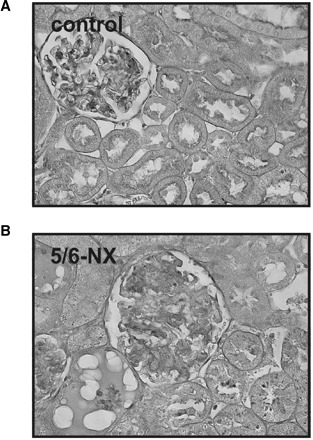
PAS staining of a representative kidney from control and from 5/6-nephrectomized rats (5/6-NX) 14 days after surgery. Whereas kidneys of control rats showed physiological histology (A), interstitial fibrosis and infiltration, glomerular sclerosis, tubular atrophy and casts were extensively present in kidneys of 5/6-NX rats (B). Original magnification ×400.
Functional data from measurements performed 2 weeks after 5/6-NX are given in Table 2. Abnormal renal histology in 5/6-NX was accompanied by elevated blood pressure and functional features of renal insufficiency. Creatinine clearance decreased and occurring polyuria was paralleled by significant proteinuria and weight loss. Retention parameters, i.e. serum creatinine and blood urea nitrogen, were increased significantly.
Table 2.
Effects of 5/6-nephrectomy on whole animal functional data (Day 14 after surgery)
| CTR | 5/6-NX | |
|---|---|---|
| Weight change post-operationem (% BW) | 43 ± 4 | −5 ± 11* |
| Urinary volume (ml/24 h) | 16 ± 3 | 39 ± 7* |
| Systolic blood pressure (mmHg) | 124 ± 4 | 167 ± 10* |
| Protein excretion (mg/mg creatinine) | 0.35 ± 0.12 | 2.64 ± 1.39* |
| Creatinine in serum (mg/dl) | 0.19 ± 0.02 | 0.34 ± 0.03* |
| Urea nitrogen in serum (mg/dl) | 16 ± 3 | 31 ± 14* |
| CrCl (ml/min/100 g BW) | 1.15 ± 0.19 | 0.56 ± 0.08* |
Mean values ± SD.
CTR: control; 5/6-NX: 5/6 nephrectomy; BW: body weight; CrCl: creatinine clearance; BUN: blood urea nitrogen. n = 5.
*Significantly different to CTR (P < 0.05).
HS induces HSP72-mRNA in rat Mphi
Twelve hours after HS, HSP72-mRNA expression of Mphi from rats with renal insufficiency (5/6-NX, n = 6) was compared to those of control rats (CTR, n = 6). Basal mRNA expression showed no difference between Mphi of CTR and 5/6-NX (Figure 2). HS significantly increased monocyte HSP72 in both CTR and 5/6-NX, but to a significantly lower extent in Mphi of 5/6-NX (214 ± 68% less than HS-CTR, n = 6).
Fig. 2.
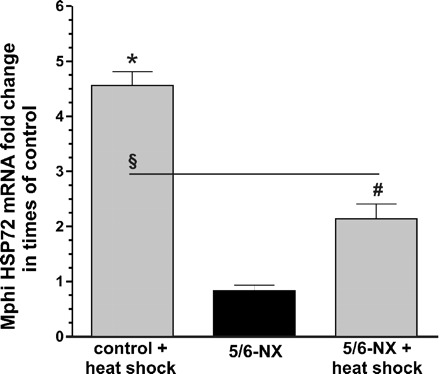
Impaired HSP70 response of Mphi from 5/6-NX. HSP72-mRNA response of freshly isolated monocytes from rats 12 h after heat shock (HS, 40 min, 47°C) with (5/6-nehrectomy, 5/6-NX, n = 6) and without (control, n = 6) renal insufficiency. mRNA expression was quantified by real-time PCR. No significant difference was found between Mphi of CTR and 5/6-NX without HS (5/6-NX HSP72-mRNA: 0.83 ± 0.26). HS significantly increased HSP72 (4.56 ± 0.62 fold) in both, Mphi of controls and of 5/6-NX rats (HSP72-mRNA: 2.13 ± 0.68 fold). Results are mean values ± SD. *Statistical difference to controls (basal, P < 0.05). #Statistical difference to basal 5/6-NX (P < 0.05). §Statistical difference to stimulated controls (P < 0.05).
Temperature- and time-dependent induction of HSP72 protein in human Mphi
To evaluate the most efficient setting for HSP72 induction (Figure 3A), Mphi isolates from six healthy subjects were exposed (40 min) to different temperatures and then allowed to recover at 37°C for 12 h. Induction was most striking after exposure to 47°C (79 ± 14% HSP72-positive cells versus 3 ± 1% basal, n = 27). To estimate to what extent induced HSP72 remains detectable after HS, samples were exposed to 47°C for 40 min and allowed to recover at 37°C during various time periods. The level of HSP72-positive Mphi increased starting 1 h after HS, with maximal values apparent after 9–12 h of recovery (Figure 3B). As shown in Figure 7 and by trypan blue staining (data not shown), HS even in the applied sublethal range did not evoke injury or apoptosis. Therefore, monocyte viability was maintained most likely by HSP induction.
Fig. 3.
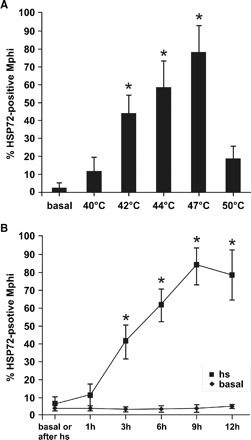
Heat shock (HS) temperature and time dependently increased HSP72 protein expression in human monocytes. (A) Twelve hours after 40 min HS at given temperatures, monocytes HSP72 protein expression was assessed by FACS-analysis. The number of HSP72-positive monocytes increased with the temperature of HS (42°C: 45 ± 10%, 44°C: 59 ± 15% versus basal: 2 ± 2%, P < 0.05). Maximum values (79 ± 14%) were observed after stimulation at 47°C. Stimulation at 50°C reduced HSP72 response, when compared to 47°C. (B) HSP72 protein expression increased significantly 3 h after stimulation (40 min at 47°C, 40 ± 9% versus 3 ± 2% basal). Six hours after HS 61 ± 9%, 9 h 82 ± 10% and 12 h 79 ± 14% HSP72-positive Mphi were detected. n = 6–27, mean values ± SD. *Statistical difference to the basal HSP72 expression (P < 0.05).
Fig. 7.
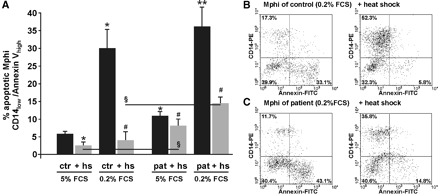
Inhibition of constitutive Mphi apoptosis by heat shock (HS, 40 min, 47°C). (A) There was slightly more basal apoptosis in Mphi of patients than in Mphi of controls (48-h cultivation in culture medium containing 5% FCS, 11± 2% versus 6 ± 1% apoptotic Mphi in CTR, n = 8, P < 0.05). HS was capable of downregulating basal apoptosis in patients to a lower extent than in controls (3 ± 2% in CTR versus 8 ± 3% in patients, n = 8, P < 0.05). Forty-eight-hour serum depletion (0.2% FCS) caused apoptosis (29 ± 5% in CTR versus 36 ± 6% in patients, n = 6, P < 0.05). HS downregulated apoptosis in both patients and controls, but controls showed a significantly reduced apoptosis rate when compared to patients (4 ± 3% in CTR versus 15 ± 2% in patients, n = 6, P < 0.05). B and C: one out of at least six-independent FACS-measurements of CD14-receptor expression and annexin V-labelling of phosphatidyl serines. Healthy cells are CD14-positive and annexin V-negative (upper left quadrant in dotplots), whereas apoptotic lose their CD14 receptor and gain annexin V-positive (lower right quadrant in dotplots). In CTR (B), HS effectively inhibited apoptosis (33.1% without HS versus 5.8% with HS). In patients on haemodialysis, inhibition of apoptosis was less effective and apoptosis rate more than twice as high as in CTR (C). Results are mean values ± SD. *Statistical difference to controls (basal) (P < 0.05). **Statistical difference to controls (0.2% FCS) (P < 0.05). #Statistical difference to matching group without HS (P < 0.05). §Statistical difference to stimulated controls (P < 0.05).
Impaired HSP72 response of Mphi of patients on haemodialysis
Having defined optimal HS-stimulation conditions for human Mphi (Figure 3), we tested our initial hypothesis, that the HS response of Mphi from patients on haemodialysis is impaired. Analogously to Mphi from rat with or without renal failure, no differences were found between basal mRNA-expression level of HSP72 from patients and controls (Figure 4A). Nevertheless, after stimulation with HS, we observed significantly increased HSP72 mRNA in Mphi of CTR and patients. In the next step, we analysed the expression of the responsible transcription factor HSF-1 and found a significantly lower expression in HS-stimulated Mphi of patients then in Mphi of CTR (Figure 4B and C).
Fig. 4.
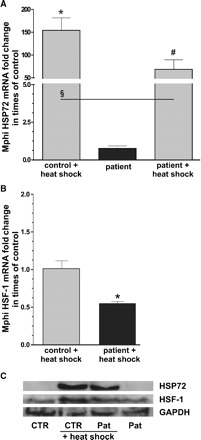
HSP72 (A) and heat shock factor 1 (HSF-1). (B) mRNA response of freshly isolated monocytes from controls and patients 12 h after heat shock. mRNA expression was quantified by real-time PCR. (A) No significant differences were found between basal mRNA expression of HSP72 [patient HSP72-mRNA 0.76 ± 0.37 fold change in times of control (fold), n = 5]. HS significantly increased HSP72 mRNA in both groups, but to a lower value in patients (CTR: 154.2 ± 54.2 versus patient: 68.5 ± 46.8, P < 0.05, n = 5). HSF-1 response to HS was 50% lower in patients, when compared to control (0.55 ± 0.05, P < 0.05, n = 5). In congruence, HSF-1 and HSP72 protein expression of patients after HS was lower than those of CTR (C, exemplary western blot, pooled Mphi, six samples per group). Results are mean values ± SD. *Statistical difference to controls (basal) (P < 0.05). #Statistical difference to basal (P < 0.05). §Statistical difference to stimulated controls (P < 0.05).
Consistent with mRNA data, the impaired HSP72-HS response of patients was evident as well, when HSP protein expression was assessed (Figures 4C, 5A and B). Again, no difference was found when basal expression was compared (Figure 5A–C).
Fig. 5.
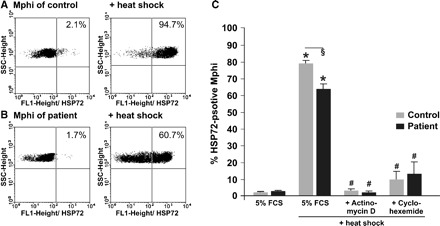
Impaired HSP72 response of Mphi of patients on haemodialysis. One exemplary experiment out of 27 independent experiments is shown in (A, B). No difference of HSP72 protein expression was observed between basal level in Mphi from controls (A, 2.1% HSP72-positive cells) and patients (B, 1.7% HSP72-positive cells). Twelve hours after heat shock (HS, 40 min, 47°C), the HSP72 protein expression is significant lower in Mphi from patients on haemodialysis (B, 60.7%) than in Mphi from controls (A, 94.7%). A summary of HSP72 protein expression measured before and after HS is presented in (C). Basal HSP72 protein expression in Mphi of CTR (2 ± 1% HSP72-positive cells, n = 27) was similar to Mphi of patients (3 ± 1%, n = 27). In contrast, HS led to a significant stronger HSP72 induction in Mphi of CTR than in Mphi of patients (79 ± 3% HSP72-positive Mphi versus 65 ± 3% in patients, n = 27, P < 0.05). To elucidate if there is a posttranscriptional difference in the HSP72 response of CTR and patients, monocytes were incubated with the transcription inhibitor actinomycin D (1 Eg/ml) ± HS or the translation inhibitor cycloheximide (15 Eg/ml) ± HS for 1 h starting at time of HS. Treatment abolished HSP72 induction usually observed 12 h after HS in both groups (actinomycin D: patients: 2 ± 1% HSP72-positive monocytes versus CTR: 3 ± 1%, n = 6, cycloheximide: patients: 13 ± 7% HSP72-positive monocytes versus CTR: 10 ± 5%, n = 3). SSCHeight: side scatter; results are mean values ± SEM. *Statistical difference to controls (basal) (P < 0.05). #Statistical difference to HS (P < 0.05). §Statistical difference to stimulated controls (P < 0.05).
To exclude additional posttranscriptional mechanisms possibly involved in the impairment of HSP70 expression of Mphi of patients on haemodialysis, Mphi were incubated either with the transcription inhibitor actinomycin D (1 μg/ml) with or without HS or with the translation inhibitor cycloheximide (15 μg/ml) with or without HS for 1 hour starting at time of HS. Treatment with inhibitors abolished HSP72 induction usually observed 12 h after HS in both groups to the same extent excluding such mechanisms.
Urea does not impair HSP72-HS response of Mphi
In the next step, we investigated if urea is the responsible trigger for the impaired HS response. To our surprise, incubation with urea significantly increased HSP72 mRNA response to HS, while the osmotic control substance mannitol did not (Figure 6).
Fig. 6.
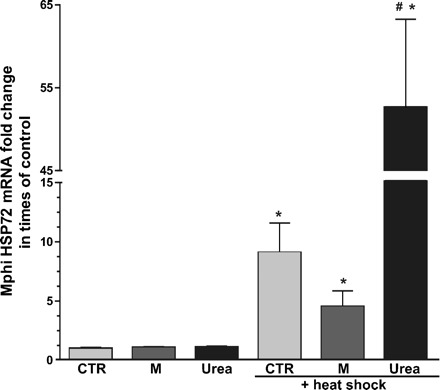
Urea increased HSP72 mRNA response of Mphi to heat shock (HS). Mphi from six healthy controls were incubated with culture medium (CM), CM + 150 mg/dl mannitol or CM + 150 mg/dl urea. HS was performed after 60 h (40 min, 47°C) and all cells (± HS) were harvested for real-time PCR analysis after 72 h. There was no significant difference in baseline HSP70 expression between CTR, mannitol [1.09 ± 0.05 fold change in times of control (fold)] and urea (1.11 ± 0.09 fold) treated Mphi. HS significantly induced HSP72 in all groups with the highest expression level measured in urea-treated Mphi (CTR 9.08 ± 2.5, mannitol 4.53 ± 1.33, urea 52.64 ± 10.63). CTR: control; M: mannitol. Results are mean values ± SD. *Statistical difference to controls (basal) (P < 0.05). #Statistical difference to stimulated controls (P < 0.05).
Impaired HS-dependent protection from apoptosis in Mphi of patients on haemodialysis
To assess the impact of the observed impaired HS response of patients’ Mphi on cellular susceptibility, apoptosis was analysed (Figure 7). First, baseline apoptosis rates were quantified. The level of constitutive apoptotic Mphi (after 48-h cultivation in CM containing 5% FCS) was rather low but doubled nearly when Mphi of patients were analysed. Nevertheless, induction of the cytoprotective, anti-apoptotic HS response by HS reduced the apoptosis rate in both, but still, the apoptosis rate of patients’ Mphi was nearly three times higher in patients than in CTR (Figure 7A). Under stress (48-h starvation due to serum depletion), the fraction of apoptotic Mphi significantly increased. HS-treated cells were effectively protected from apoptosis. Again, apoptosis rate of treated CTR was less than a third of the rate of Mphi of patients. One exemplary analysis of stress-induced apoptosis in Mphi is shown in Figure 7B and C (left). After HS, apoptosis remained twice as high in patients’ Mphi as in those of CTR (right).
Influence of haemodialysis session on HSP72-protein expression
To clarify if haemodialysis session-related stress possibly translates into HSP72 stimulation in patients’ Mphi, HSP72 expression of Mphi freshly isolated from patients before and after haemodialysis was investigated but no difference was observed (Figure 8).
Fig. 8.
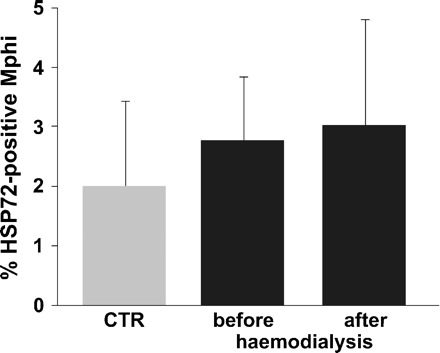
Flow cytometric detection of HSP72-specific signals of monocytes from 27 patients before and of nine patients after 4 h of haemodialysis. Haemodialysis did not change the HSP72 signal intensity significantly (2.8 ± 1.1% versus 3 ± 1.9% HSP72-positive cells, 2 ± 1.7% CTR, n = 27). Data are presented as mean ± SD.
Discussion
Uraemic stress-related dysfunction of Mphi is an important contributory factor for the increased incidence of infections and tumours, or the impaired wound healing of patients on haemodialysis [1–4,39]. We herein show that in Mphi of rats with renal insufficiency and in Mphi of patients on IHD, the stress-related HSP72 induction, an important cytoprotective, anti-apoptotic mechanism of cells, is impaired, although baseline expression remains unaffected. Reduced HSP72 expression was accompanied by significant lower mRNA expression of the responsible transcription factor HSF-1 [40] and resulted in reduced cellular resistance to pro-apoptotic stress.
Upregulation of HSP72 in human monocytes started shortly after HS (Figure 3B) and, therefore, confirmed specific detection of the inducible form of HSP70. We have chosen a high and rather long thermal stress, because lower stimulation was less effective (Figure 3A). Expression of HSP72 declined with time (peak 9–12 h, Figure 3B) but is still, as previously demonstrated, even 60 h later enhanced [23]. The rapid and massive induction of HSP acted strongly anti-apoptotic and allowed survival of monocytes, even when thermal stress with sublethal temperature or stress by serum starvation was applied (low apoptosis and necrosis rates, Figure 7). These results are consistent with previous studies mostly analysing cell lines, which confirmed the role of HSP conferring resistance to physical stress or pro-apoptotic triggers [18,21,41].
Several attempts were made, to clarify the mechanisms causing immunodeficiency in patients with ESRD. What is known so far is that the mechanism cannot be broken down to a single effector. The pathways are rather complex and not yet fully understood. Interestingly, HSP72 induction acts not only anti-apoptotic but influences Mphis’ phagocytosis capacity and resistance to intracellular infection, too [26,29,42,43]. Memoli et al. stated that the impaired immunocompetence observed in Mphi of patients on IHD results from ‘chronic over-stimulation’ caused by pro-inflammatory cytokines, complement activation and toxins, conducting a decreased resonance against further stimulation (fatigue) [35]. Nevertheless, we could neither detect dialysis session-dependent HSP72 induction (stimulation) nor find elevated baseline HSP72 in patients (Figures 4 and 8) or rats with renal insufficiency (Figure 2). Recently, and in contrast to our data, Calabrese et al. measured elevated HSP70 level in lymphocytes of patients with diabetic nephropathy [44]. As diabetes-related stress and patient's metabolism considerably differ between diabetic and non-diabetic patients and the measurements were performed on EDTA-treated lymphocytes (leading via calcium efflux to pre- and de-activation of cellular pathways), the results may vary. Further, monocytes of patients on peritoneal dialysis exhibit significant HS response after contact with peritoneal dialysis fluid meaning that they are not over-stimulated [45]. Unfortunately, this study lacks the healthy controls.
HSP70 proteins are potent immune adjuvants released e.g. upon tissue damage regulating the immune-response [26–28,30,33,46]. HSP70 also increases the expression of Toll-like receptor 4 (TLR4) [33,46]. Toll-like receptors are critical for activation of immunocompetent cells [47,48]. Ando et al. described reduced expression of TLR4 in monocytes of uraemic patients contributing to the impaired cytokine response [49]. Taken together, a reduced HSP72 response to stress might cause less effective recognition of harmful antigens and less activation of TLR4-dependent pathways.
Urea in clinically relevant concentrations (40–200 mg/dl) induces HS response in the short run (0.5–10 h) in cultured neuroblastoma cells. Maddock and Westenfelder charged protein carbamylation for being the trigger [50]. Interestingly, the HS response returned to zero by 48 h assuming some kind of cellular adaptation in the long run. This is in agreement with our data, showing the same baseline amounts of HSP72 in Mphi of healthy rats and those with increased serum BUN (Figure 2), in urea-treated Mphi (Figure 6), as well as in patients and controls (Figures 4 and 5). Consistent with Marzec et al. [45], monocytes from rats with renal insufficiency and from patients on IHD responded to HS but, in the case of HSP72, to a significantly lower extent than the ones from controls (Figures 2, 4, and 5). In contrast, skeletal muscle of chronic dialysis patients in the steady state contains increased amounts of protective HS proteins [51]. Notably, HSP72 expression in long-term urea-incubated Mphi responded to a significant higher extent to HS than controls (Figure 6). A possible explanation for urea-induced HSP72 expression in heated Mphi is that urea-mediated protein destabilization may be enhanced by increased temperature. Since disruption of the protein tertiary structure is the final common pathway of HSP induction, this mechanism could also account for urea-induced HSP72 expression at high temperature [52].
Malnutrition is frequently present in patients on IHD. Uraemia changes plasma and intracellular amino acid levels further whereas IHD alters protein turnover and amino acid transport kinetics [53]. In critically ill patients, a decreased plasma glutamine level causes an impaired monocyte function leading to substantial immunosuppression [54,55]. Glutamine deprivation increases Mphi’ susceptibility to stress and apoptosis, as well as it reduces their responsiveness to stimuli [55]. Glutamine starving reduces thermoresistance (to fever) of Mphi that is, according to Pollheimer et al., caused by impaired HSP70 response [54]. Thus, one can assume that glutamine depletion contributes to the reduced HSP72-HS response and favours apoptosis as demonstrated in patients’ Mphi (Figure 7). Nevertheless, accelerated efflux of amino acids from the muscle (protein catabolism) helps to stabilize the intracellular concentrations of amino acids during IHD [53]. Further, we performed all experiments in CM containing 2 mM L-glutamine excluding undersupply and effects were already present in the rat model of chronic kidney disease. Hence, glutamine starvation is probably not involved.
Treatment with erythropoietin induces HSP70 in organs, like heart and kidney, exhibiting anti-apoptotic, cytoprotective effects [56,57]. Recently, Ribeil et al. identified an important mechanism leading to differentiation of human erythroid precursors [58]. They showed caspase-3 activation during both terminal differentiation and (erythropoietin-starvation-induced) apoptosis. Only in the presence of erythropoietin, HSP70 protected erythroid precursors from caspase-mediated proteolysis [58]. As erythropoietin is an integral part of the pharmacological therapy in ESRD, one can speculate that in untreated ESRD patients the HSP70 response might be worse and apoptosis even elevated. In addition, the HSP72 response was already impaired in the rat model excluding medications being the main underlying trigger altering Mphi's stress response.
Our results demonstrate an impaired HSP72-stress response of Mphi from patients on IHD that is accompanied by a decreased HSF-1 response to HS. It is alarming that this impaired stress response is not necessarily bound to uraemia but is already present in chronic kidney disease as demonstrated in a rat model. Patient's Mphi show enhanced susceptibility to stress, e.g. serum starvation, as shown by increased cellular apoptosis values. Impaired HSP72 response of Mphi might explain in part, why patients on IHD are challenged by higher incidences of infections and tumours or impaired wound healing.
Acknowledgments
The authors are grateful to Kathrin Beul, Ute Neugebauer and Rita Schröter for excellent technical assistance. This study was supported by the IZKF Münster (Project D20) and the SFB 656.
Conflict of interest statement. None declared.
References
- 1.Mandayam S, Shahinian VB. Are chronic dialysis patients at increased risk for cancer? J Nephrol. 2008;21:166–174. [PubMed] [Google Scholar]
- 2.Powe NR, Jaar B, Furth SL, et al. Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int. 1999;55:1081–1090. doi: 10.1046/j.1523-1755.1999.0550031081.x. [DOI] [PubMed] [Google Scholar]
- 3.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 4.Cheung AH, Wong LM. Surgical infections in patients with chronic renal failure. Infect Dis Clin North Am. 2001;15:775–796. doi: 10.1016/s0891-5520(05)70172-0. [DOI] [PubMed] [Google Scholar]
- 5.Goldblum SE, Reed WP. Host defenses and immunologic alterations associated with chronic hemodialysis. Ann Intern Med. 1980;93:597–613. doi: 10.7326/0003-4819-93-4-597. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt S, Westhoff TH, Krauser P, et al. The uraemic toxin phenylacetic acid impairs macrophage function. Nephrol Dial Transplant. 2008;23:3485–3493. doi: 10.1093/ndt/gfn266. [DOI] [PubMed] [Google Scholar]
- 7.Athlin L, Domellof L. The phagocytic process of human peritoneal macrophages in cancer or uremia. Acta Pathol Microbiol Immunol Scand [C] 1986;94:63–68. doi: 10.1111/j.1699-0463.1986.tb02091.x. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich S, Schmidt M, Bachmann J, et al. Apoptosis of monocytes cultured from long-term hemodialysis patients. Kidney Int. 1996;49:792–799. doi: 10.1038/ki.1996.110. [DOI] [PubMed] [Google Scholar]
- 9.Galli F. Protein damage and inflammation in uraemia and dialysis patients. Nephrol Dial Transplant. 2007;22(Suppl 5):v20–v36. doi: 10.1093/ndt/gfm294. [DOI] [PubMed] [Google Scholar]
- 10.Locatelli F, Canaud B, Eckardt KU, et al. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–1280. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 11.Carracedo J, Ramirez R, Madueno JA, et al. Cell apoptosis and hemodialysis-induced inflammation. Kidney Int Suppl. 2002;80:89–93. doi: 10.1046/j.1523-1755.61.s80.17.x. [DOI] [PubMed] [Google Scholar]
- 12.Himmelfarb J. Oxidative stress in hemodialysis. Contrib Nephrol. 2008;161:132–137. doi: 10.1159/000130658. [DOI] [PubMed] [Google Scholar]
- 13.Vanholder R, Schepers E, Meert N, et al. What is uremia? Retention versus oxidation. Blood Purif. 2006;24:33–38. doi: 10.1159/000089434. [DOI] [PubMed] [Google Scholar]
- 14.Pupim LB, Himmelfarb J, McMonagle E, et al. Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int. 2004;65:2371–2379. doi: 10.1111/j.1523-1755.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RS, Singh B. Phylogenetic analysis of 70 kD heat shock protein sequences suggests a chimeric origin for the eukaryotic cell nucleus. Curr Biol. 1994;4:1104–1114. doi: 10.1016/s0960-9822(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 16.Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci USA. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 18.Aufricht C. Heat-shock protein 70: molecular supertool? Pediatr Nephrol. 2005;20:707–713. doi: 10.1007/s00467-004-1812-6. [DOI] [PubMed] [Google Scholar]
- 19.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 20.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 21.Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261–271. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- 22.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Lang D, Hubrich A, Dohle F, et al. Differential expression of heat shock protein 70 (hsp70) in human monocytes rendered apoptotic by IL-4 or serum deprivation. J Leukoc Biol. 2000;68:729–736. [PubMed] [Google Scholar]
- 24.Li CY, Lee JS, Ko YG, et al. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 25.Wieland A, Denzel M, Schmidt E, et al. Recombinant complexes of antigen with stress proteins are potent CD8 T-cell-stimulating immunogens. J Mol Med. 2008;86:1067–1079. doi: 10.1007/s00109-008-0371-x. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Town T, Gokarn V, et al. HSP70 enhances macrophage phagocytosis by interaction with lipid raft-associated TLR-7 and upregulating p38 MAPK and PI3K pathways. J Surg Res. 2006;136:58–69. doi: 10.1016/j.jss.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Anderson KM, Srivastava PK. Heat, heat shock, heat shock proteins and death: a central link in innate and adaptive immune responses. Immunol Lett. 2000;74:35–39. doi: 10.1016/s0165-2478(00)00246-7. [DOI] [PubMed] [Google Scholar]
- 28.Moseley PL. Heat shock proteins and the inflammatory response. Ann N Y Acad Sci. 1998;856:206–213. doi: 10.1111/j.1749-6632.1998.tb08327.x. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura H, Emoto M, Kimura K, et al. Hsp70 protects macrophages infected with Salmonella choleraesuis against TNF-alpha-induced cell death. Cell Stress Chaperones. 1997;2:50–59. doi: 10.1379/1466-1268(1997)002<0050:hpmiws>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van EW, Van Der ZR, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 31.Lai CC, Jou MJ, Huang SY, et al. Proteomic analysis of up-regulated proteins in human promonocyte cells expressing severe acute respiratory syndrome coronavirus 3C-like protease. Proteomics. 2007;7:1446–1460. doi: 10.1002/pmic.200600459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melcher A, Todryk S, Hardwick N, et al. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med. 1998;4:581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt E, Gehrmann M, Brunet M, et al. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- 34.Horl WH. Hemodialysis membranes: interleukins, biocompatibility, and middle molecules. J Am Soc Nephrol. 2002;13(Suppl 1):S62–S71. [PubMed] [Google Scholar]
- 35.Memoli B. Cytokine production in haemodialysis. Blood Purif. 1999;17:149–158. doi: 10.1159/000014387. [DOI] [PubMed] [Google Scholar]
- 36.Lang D, Reuter S, Buzescu T, et al. Heme-induced heme oxygenase-1 (HO-1) in human monocytes inhibits apoptosis despite caspase-3 up-regulation. Int Immunol. 2005;17:155–165. doi: 10.1093/intimm/dxh196. [DOI] [PubMed] [Google Scholar]
- 37.Heidenreich S, Schmidt M, August C, et al. Regulation of human monocyte apoptosis by the CD14 molecule. J Immunol. 1997;159:3178–3188. [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Ponce P, Cruz J, Ferreira A, et al. A prospective study on incidence of bacterial infections in Portuguese dialysis units. Nephron Clin Pract. 2007;107:c133–c138. doi: 10.1159/000110033. [DOI] [PubMed] [Google Scholar]
- 40.Trinklein ND, Chen WC, Kingston RE, et al. Transcriptional regulation and binding of heat shock factor 1 and heat shock factor 2 to 32 human heat shock genes during thermal stress and differentiation. Cell Stress Chaperones. 2004;9:21–28. doi: 10.1379/481.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 42.Degrossoli A, Colhone MC, Arrais-Silva WW, et al. Hypoxia modulates expression of the 70-kD heat shock protein and reduces Leishmania infection in macrophages. J Biomed Sci. 2004;11:847–854. doi: 10.1007/BF02254370. [DOI] [PubMed] [Google Scholar]
- 43.Kantengwa S, Polla BS. Phagocytosis of Staphylococcus aureus induces a selective stress response in human monocytes-macrophages (M phi): modulation by M phi differentiation and by iron. Infect Immun. 1993;61:1281–1287. doi: 10.1128/iai.61.4.1281-1287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calabrese V, Mancuso C, Sapienza M, et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12:299–306. doi: 10.1379/CSC-270.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzec L, Liberek T, Chmielewski M, et al. Expression of heat shock protein 72 in peritoneal leukocytes is induced by peritoneal dialysis. Perit Dial Int. 2007;27:288–295. [PubMed] [Google Scholar]
- 46.Bangen JM, Schade FU, Flohe SB. Diverse regulatory activity of human heat shock proteins 60 and 70 on endotoxin-induced inflammation. Biochem Biophys Res Commun. 2007;359:709–715. doi: 10.1016/j.bbrc.2007.05.167. [DOI] [PubMed] [Google Scholar]
- 47.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 48.Visintin A, Mazzoni A, Spitzer JH, et al. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 49.Ando M, Shibuya A, Tsuchiya K, et al. Reduced expression of Toll-like receptor 4 contributes to impaired cytokine response of monocytes in uremic patients. Kidney Int. 2006;70:358–362. doi: 10.1038/sj.ki.5001548. [DOI] [PubMed] [Google Scholar]
- 50.Maddock AL, Westenfelder C. Urea induces the heat shock response in human neuroblastoma cells. J Am Soc Nephrol. 1996;7:275–282. doi: 10.1681/ASN.V72275. [DOI] [PubMed] [Google Scholar]
- 51.Crowe AV, McArdle A, McArdle F, et al. Markers of oxidative stress in the skeletal muscle of patients on haemodialysis. Nephrol Dial Transplant. 2007;22:1177–1183. doi: 10.1093/ndt/gfl721. [DOI] [PubMed] [Google Scholar]
- 52.Beckmann RP, Lovett M, Welch WJ. Examining the function and regulation of hsp 70 in cells subjected to metabolic stress. J Cell Biol. 1992;117:1137–1150. doi: 10.1083/jcb.117.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raj DS, Welbourne T, Dominic EA, et al. Glutamine kinetics and protein turnover in end-stage renal disease. Am J Physiol Endocrinol Metab. 2005;288:E37–E46. doi: 10.1152/ajpendo.00240.2004. [DOI] [PubMed] [Google Scholar]
- 54.Pollheimer J, Zellner M, Eliasen MM, et al. Increased susceptibility of glutamine-depleted monocytes to fever-range hyperthermia: the role of 70-kDa heat shock protein. Ann Surg. 2005;241:349–355. doi: 10.1097/01.sla.0000152028.19115.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eliasen MM, Brabec M, Gerner C, et al. Reduced stress tolerance of glutamine-deprived human monocytic cells is associated with selective down-regulation of Hsp70 by decreased mRNA stability. J Mol Med. 2006;84:147–158. doi: 10.1007/s00109-005-0004-6. [DOI] [PubMed] [Google Scholar]
- 56.Xu B, Dong GH, Liu H, et al. Recombinant human erythropoietin pretreatment attenuates myocardial infarct size: a possible mechanism involves heat shock Protein 70 and attenuation of nuclear factor-kappaB. Ann Clin Lab Sci. 2005;35:161–168. [PubMed] [Google Scholar]
- 57.Yang CW, Li C, Jung JY, et al. Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J. 2003;17:1754–1755. doi: 10.1096/fj.02-1191fje. [DOI] [PubMed] [Google Scholar]
- 58.Ribeil JA, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102–105. doi: 10.1038/nature05378. [DOI] [PubMed] [Google Scholar]


