Abstract
Background. Acute respiratory tract infections (ARTIs) are responsible for considerable morbidity in the community, but little is known about the presence of respiratory pathogens in asymptomatic individuals. We hypothesized that asymptomatic persons could have a subclinical infection and thus act as a source of transmission.
Methods. During the period of 2000–2003, all patients with ARTI who visited their sentinel general practitioner had their data reported to estimate the incidence of ARTI in Dutch general practices. A random selection of these patients (case patients) and an equal number of asymptomatic persons visiting for other complaints (control subjects) were included in a case-control study. Nose and throat swabs of participants were tested for a broad range of pathogens.
Results. The overall incidence of ARTI was 545 cases per 10,000 person-years, suggesting that, in the Dutch population, an estimated 900,000 persons annually consult their general practitioner for respiratory complaints. Rhinovirus was most common in case patients (24%), followed by influenza virus type A (11%) and coronavirus (7%). Viruses were detected in 58% of the case patients, β-hemolytic streptococci group A were detected in 11%, and mixed infections were detected in 3%. Pathogens were detected in ∼30% of control subjects, particularly in the youngest age groups.
Conclusion. This study confirms that most ARTIs are viral and supports the reserved policy of prescribing antibiotics. In both case and control subjects, rhinovirus was the most common pathogen. Of bacterial infections, only group A β-hemolytic streptococci were more common in case patients than in control subjects. Furthermore, we demonstrated that asymptomatic persons might be a neglected source of transmission.
Acute respiratory tract infections (ARTIs) are responsible for considerable morbidity in the general population. Most infections are caused by viruses, especially rhinovirus, influenza virus, and respiratory syncytial virus [1–4]. Complaints due to respiratory infections are a very common reason for consultation with a general practitioner (GP) [5]. Since 1970, the GPs from the Continuous Morbidity Registration of The Netherlands Institute of Primary Health Care have registered all patients who have consulted them about influenza-like illnesses (ILIs) [6]. Weekly incidence data show the distribution of ILIs in the population, which enables early detection of epidemics. Clinically, ILI is difficult to differentiate from other respiratory infections [7, 8]. Therefore, since the winter 1992–1993, ∼75% of the sentinel GPs have taken nose and throat swabs from a random selection of patients with ILI. These swabs are tested for respiratory viruses, Mycoplasma pneumoniae, and Chlamydophila pneumoniae. In addition to contributing to early warnings about influenza, the virological surveillance is important for early identification of new strains of influenza virus and shifts in the prevalence of other viruses.
Nevertheless, this surveillance system provided no information about the incidence of ARTI other than ILI. Extension of the system with the registration of all patients consulting with other ARTIs made it possible to estimate the incidence of both ILI and other ARTI. Besides, no data were available on the presence of respiratory pathogens in asymptomatic persons in the community. To test the hypothesis that asymptomatic persons with a subclinical infection might act as an source of transmission, the system was expanded with a case-control study between October 2000 and October 2003.
Materials and Methods
The GP network of the Continuous Morbidity Registration constitutes a representative group of ∼67 GPs in ∼45 practices. Their patient population accounts for ∼1% of the Dutch population and is representative of it with regard to age, sex, regional distribution, and degree of urbanization. Throughout the year, all practices register the number of consultations for both ILI and ARTI by age group and week (reporting study). ILI was reported for patients with an acute onset of illness (prodromal stage, ⩽3–4 days) and at least 1 of the following symptoms: cough, rhinitis, sore throat, frontal headache, retrosternal pain, or myalgia. ARTI was reported for patients with an acute respiratory illness other than ILI and with at least 1 of the following symptoms: coughing, rhinitis, or sore throat.
Almost one-half of the practices (22 practices in 2000–2001, 19 in 2001–2002, and 18 in 2002–2003) participated in the case-control study. Case patients were defined as patients who consulted their GPs because of airway complaints, who received a diagnosis of ILI or another ARTI, who consulted their GP for the first time during that episode, and who had not used antibiotics or antiviral medications in the previous 2 weeks. Control subjects were defined as patients who consulted their GPs for complaints other than respiratory complaints, who had no complaints of an ARTI in the prior 2 weeks, who did not belong to the same household as the case patient, and who had not used antibiotics or antivirals in the previous 2 weeks. Case patients and control subjects were recruited within 1 week of each other and were matched by age group (0–4, 5–14, 15–24, 25–44, 45–64, and ⩾65 years). The GP obtained 1 nose and 2 throat swab specimens from both case patients and control subjects. These swabs were accompanied by a form on which the GP registered the following data: sex, date of birth, date of onset of illness, date of sampling, symptoms, and diagnosis (ILI, common cold, acute sinusitis, otitis, pharyngitis, tonsillitis, laryngitis, tracheitis, bronchitis, or pneumonia).
Viral culture and PCR were performed at the National Institute of Public Health and the Environment (Bilthoven, The Netherlands). Both nose and throat swabs were tested for adenovirus, coronavirus, enterovirus, human metapneumovirus (hMPV), influenza virus, parainfluenza virus, rhinovirus, and respiratory syncytial virus, as well as for M. pneumoniae, C. pneumoniae, and Chlamydophila psittaci by use of viral culture and PCR [9–14]. Bacteriological cultures were performed at the Regional Public Health Laboratory (Tilburg, The Netherlands) using the throat swabs to detect all bacterial pathogens known to cause community-acquired respiratory infections. The cultures were semiquantitatively classified into 5 groups: no colonies, sporadic colonies, few colonies, several colonies, or many colonies. Standardized protocols were used for all assays.
Incidences of ILI, ARTI, and ARTI including ILI were calculated from the reporting study and were corrected for incomplete reporting years. The effects on incidence of year of study, season, participation in the case-control study, degree of urbanization, geographic region, and age of the patient were estimated univariately; independent effects were estimated by Poisson regression.
All analyses for the case-control study were performed for the complete group of participants (intention-to-treat [ITT] analysis) and for the participants who met the inclusion criteria (per-protocol [PP] analysis). Fisher's exact test was used to compare the presence of pathogens in case patients and control subjects. For effect estimates, ORs (with 95% CIs) were calculated.
Results
During the 3 study years, consultations for ILI and other ARTI were reported by 47, 45, and 43 practices per year. The overall incidence of ILI consultations was 132 consultations per 10,000 person-years, and for other ARTI consultations, it was 413 consultations per 10,000 person-years. In the season 2001–2002, the winter peak was most pronounced (figure 1). In week 9 of 2002, the highest incidence of ILI was reported (12 consultations per 10,000 person-years). For other ARTIs, there was no clear winter peak: the highest incidences were reported in week 50 of 2001, as well as weeks 10, 49, and 50 of 2002 (15 consultations per 10,000 person-years).
Figure 1.
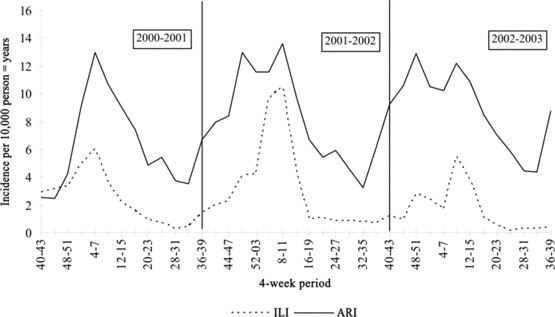
Aggregated incidence of influenza-like illnesses (ILIs) and other acute respiratory infections (ARTIs) for per 10,000 person-years for 4-week periods during October 2000 to October 2003.
The univariate and multivariate analyses of incidence yielded similar results (table 1). In the first year of study, the incidence of ARTI (including ILI) was lower than it was in the second and third year. Furthermore, the incidence was lowest in the third quarter of that year, in practices that did not participate in the case-control study, in practices with a low degree of urbanization, and in practices in the north of the country. The incidence decreased with increasing age.
Table 1.
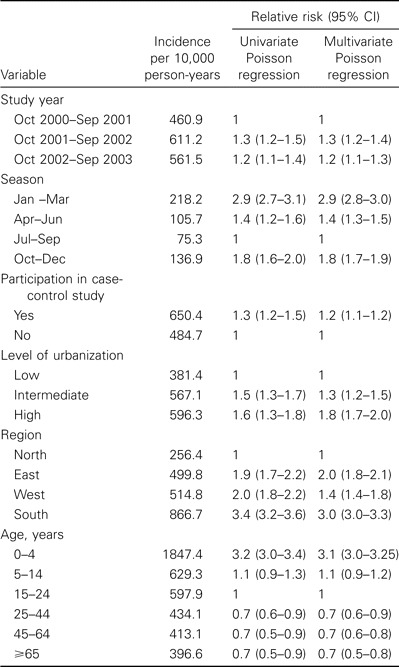
Univariate and multivariate regression analyses of the incidence of consultations for acute respiratory illnesses, including influenza-like illnesses, in the general practice network, 2000–2003.
Case-control study. The distribution of region and degree of urbanization of practices that participated in the case-control study were similar to those of the complete GP network. Nose and throat swab specimens were obtained from a total of 645 case patients and 558 control subjects, of which 541 case patients (84%) and 541 control subjects (98%) met the inclusion criteria. Characteristics of these participants are summarized in table 2. Because the ITT and PP analyses led to the same conclusions, only the data from the PP analyses are presented here, to exclude even minor respiratory complaints in control subjects.
Table 2.
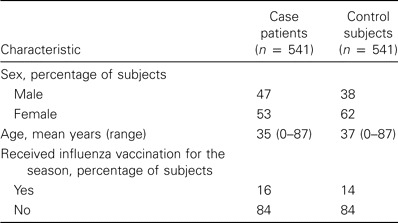
Baseline characteristics of the participants in the case-control study from consulting sentinel general practitioners, 2000–2003.
Clinical symptoms. Among case patients, there were 165 patients with ILI, 375 with another ARTI, and 1 with both ILI and ARTI (acute pharyngitis). The diagnoses most commonly reported for case patients with an ARTI other than ILI were common cold (36% of case patients), acute pharyngitis (30%), and acute tonsillitis (20%). Other diagnoses were each reported for <10% of the case patients. For 43 patients, >1 diagnosis was made. Fever was recorded for nearly 90% of the case patients with ILI (table 3). For case patients with other ARTIs, sore throat was the most common symptom (76%). Control subjects consulted their GPs for a diversity of reasons, of which joint/muscle complaints (21%) and skin disorders (14%) were most common. The complaints mentioned were either of acute nature or were related to chronic illness. Approximately 20% of control subjects consulted GPs for other reasons (e.g., to pick up prescriptions, for routine physical examinations, or to accompany relatives).
Table 3.
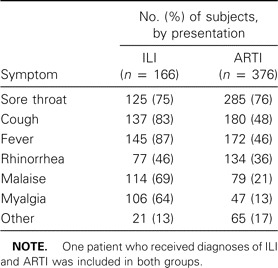
Symptoms of patients with influenza-like illnesses (ILI) or other acute respiratory infections (ARTI), consulting sentinel general practitioners, 2000–2003.
Pathogens. The distribution of swabs for case patients across the age groups was not representative for the distribution of consultations for ILI and ARTI. For patients in the age groups of <15 years and ⩾65 years, fewer nose and throat swabs were taken than representative by clinical incidences. The distribution of consultations over the age groups and the observed percentage of detected pathogens by age group were used to estimate the adjusted number of pathogens expected in case patients. To calculate the adjusted number of pathogens in control subjects, we assumed that distribution over age groups of consultations of ARTI including ILI was similar to that of other consultations.
Despite the complaints about airway conditions and the use of a large panel of diagnostic tests, no pathogen could be detected for 35% of the case patients (after correction for the distribution of consultations over the age groups). In contrast with this, pathogens were detected for 31% of the control subjects without airway complaints.
Viral pathogens, M. pneumoniaeandC. pneumoniae/C. psittaci. The adjusted numbers of case patients with ILI and with ARTI for which viral pathogens were detected were 120 (72%) and 200 (53%), respectively, regardless of whether the pathogen was detected in combination with bacterial pathogens (table 4). The adjusted number of control subjects for which a viral pathogen was detected was 156 (29%). For 59 case patients, 2 viruses were detected, and for 9 case patients, 3 viruses were detected (adjusted data). The most frequently detected pathogens were influenza virus type A for case patients with ILI (42%) and rhinovirus for those with ARTI (25%). Rhinovirus was also most common in control subjects (17%); however, it was recovered significantly less often than for case patients (adjusted OR, 1.8; 95% CI, 1.3–2.4). All other viruses, with the exception of coronavirus and enterovirus (as well as the bacteria M. pneumoniae), were also detected significantly less often in case patients. Influenza virus type B and hMPV were detected only in case patients (both those with ILI and those with ARTI), as was also the case for adenovirus and parainfluenza virus (for only those with ARTI).
Table 4.
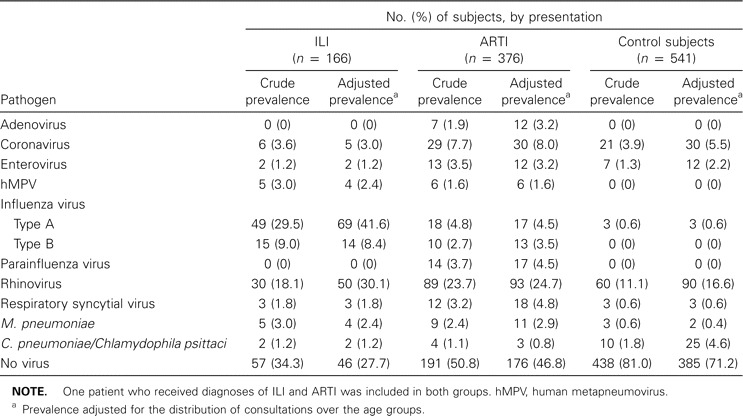
Crude and adjusted prevalence of viral pathogens, Mycobacterium pneumoniae, and Chlamydophila pneumoniae in cases and controls, consulting sentinel general practitioners, 2000–2003.
Viral pathogens were detected in 75% of the case patients aged <5 years and in 53% of those aged ⩾5 years. Among the control subjects, viruses were detected in 68% of the subjects aged 0–4 years, 40% of those aged 5–14 years, and 15% of those aged ⩾15 years. There was no association observed between the detected pathogens and diagnoses made by the GP or with reported symptoms (data not shown).
Bacterial pathogens. In addition to commensal and potential opportunistic microorganisms, bacterial pathogens were detected in 95 case patients (18%) and 43 control subjects (8%), regardless of whether viral pathogens were also present (table 5). Group A β-hemolytic streptococci were detected significantly more frequently in case patients than in control subjects (60 [11%] vs. 13 [2%]; adjusted OR, 5.1; 95% CI, 2.7–9.3). All other bacteria were detected in equal quantities in both groups. Staphylococcus aureus was the most common bacterium in control subjects. This microorganism was not detected in 0–4-year-old control subjects. A total of 246 potential opportunistic microorganisms were detected, of which Candida species predominated in both case patients and control subjects.
Table 5.
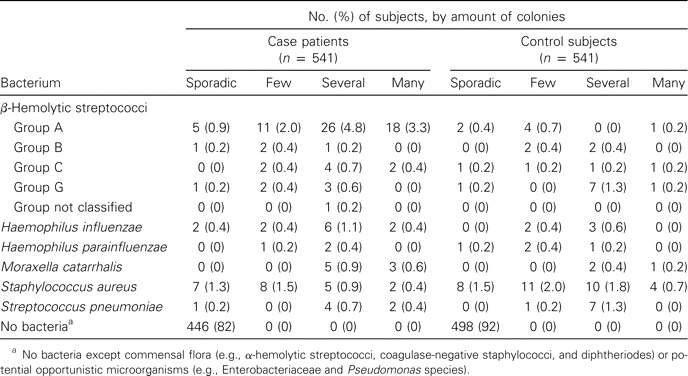
Crude prevalence of bacterial pathogens, other than Mycobacterium pneumoniae and Chlamydophila pneumoniae, in case patients and control subjects who consulted sentinel general practitioners, 2000–2003.
It was not possible to assess the presence of group A β-hemolytic streptococci from diagnoses or symptoms of patients. Fifty percent of the case patients positive for such organisms received diagnoses of acute tonsillitis. Conversely, group A β-hemolytic streptococci were detected in only 30 of the 74 patients with tonsillitis, and no bacteria (except commensal and potential opportunistic microorganisms) were detected for 29 of them. Furthermore, >90% of the case patients who tested positive for group A β-hemolytic streptococci reported having a sore throat and fever, whereas these microorganisms were actually detected in only 14% of all patients with these symptoms.
Mixed infections. A mixed infection was defined as the presence of a virus in combination with group A β-hemolytic streptococci. A. Other bacteria are ignored because they were detected with equal frequency among case patients and control subjects, making a causal relationship with the respiratory complaints not plausible. Mixed infections were detected in 21 case patients (4%) and 7 control subjects (1%). Mixed infections were more common among case patients than control subjects, and they were most pronounced in the older age groups (figure 2), although only viral infections were seen among people aged ⩾65 years.
Figure 2.
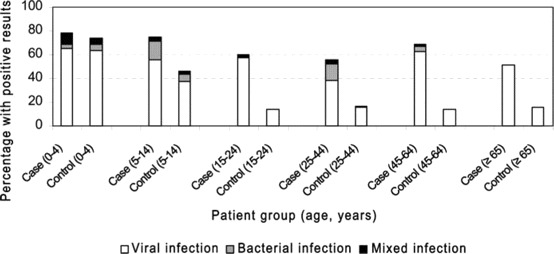
Percentage of viral, bacterial, and mixed infections in case patients and control subjects, by age group. Bacteria other than group A β-hemolytic streptococci are excluded.
Discussion
To our knowledge, this is the first study to have assessed the role of a wide range of both viruses and bacteria in a representative population of GP patients with ARTI and control subjects. A more selective study investigated the viral causes of ARTI, but this study focused on a subpopulation of community-dwelling elderly persons in The Netherlands [15]. The inclusion of control subjects made it feasible to investigate whether a causal relationship between airway complaints and detected pathogens exists. Furthermore, our study provided a unique sample from the general population, which can be used to study the relative importance of future new pathogens.
Reporting study. The incidence of ILI in general practices was estimated to be 132 cases per 10,000 person-years, and it was 413 cases per 10,000 person-years for other ARTIs, corresponding to 1 in 76 persons visiting their GPs for ILI and 1 of 24 visiting them for other ARTIs annually; for a population of 16 million individuals, this yields almost 900,000 visits annually. The incidence was highest in the first quarter of the year, reflecting the circulation of influenza virus in the winter and early spring. Comparison of incidence data with those found in other countries is practically impossible. In the European Influenza Surveillance Scheme, considerable differences in clinical morbidity rates are seen between different networks, even between neighboring countries. A number of factors can explain these differences, including circulation of influenza viruses in Europe, use of different case definitions, and differences in health care systems [16–19].
The incidence of GP consultations in our study is for a period of moderate influenza activity. During the study, the peak incidence of ILI (12 cases per 10,000 persons) was 2.5 times lower than the average peak incidence over the past 20 years [20]. Influenza activity affects the number of consultations for ILI, as well as for other respiratory illnesses [17].
Despite the policy of discouragement of GP visits for ARTI, the incidence was still considerably higher than that for other infectious diseases, such as gastroenteritis (101 visits per 10,000 person-years) [21, 22]. Of the 49 participating GPs who completed a questionnaire after 1 year of study, 80% reported discouragement of patients from visiting their practice for respiratory complaints.
The incidence of ARTI was independently associated with the degree of urbanization, the geographic region, and participation in the case-control study. The incidence was lowest in the north, as was also found in a similar study of gastroenteritis in sentinel practices in The Netherlands [21]. A higher incidence was found in areas with an intermediate or high degree of urbanization, compared with areas with a low degree of urbanization. It is possible that the risk of person-to-person transmission increases with increasing population density. A higher incidence for practices that participated in the case-control study was also seen in a study of gastroenteritis [21]. Heightened attention to respiratory infections in the participating practices may have caused information bias. However, selection bias associated with a higher participation rate for practices with higher incidences of ARTI can also account for the difference.
Age was also independently associated with incidence. The highest incidences were found in the youngest age groups. This might be attributed to the developing immune status of young children and their vulnerability to infections [23]. Furthermore, part of the increased physician's care for young children might be explained by a heightened level of parental anxiety, because interpretation of clinical signs and distinguishing between uncomplicated and significant illness can be difficult in early childhood [24]. As was also found by Monto et al. [2], the incidence decreased in the older age groups. Although respiratory viruses are known to cause substantial morbidity in elderly people, no increase in the incidence of GP consultations was seen in our study [3, 25]. The relatively low influenza activity during the study may have played a part in this [20].
Pathogens. Viruses were detected in 58% of the case patients, group A β-hemolytic streptococci were detected in 11%, and mixed infections were detected in 3%. This confirms the assumption that ARTIs are mainly viral illnesses. In agreement with other studies, we found that influenza viruses were the most common pathogens in case patients with ILI and that rhinovirus was the most common in those with ARTIs [1, 3, 26–28]. Frequencies of detected respiratory viruses vary between studies because of discrepancies in study populations and the types of performed diagnostic tests. Furthermore, the prevalence of viruses seems to vary over time and between different geographical areas [27].
In agreement with a study that involved Dutch, community-dwelling elderly persons [15], we found a relatively low diagnostic deficit (38%). The diagnostic deficit appears to be lower in the younger age groups, which might be explained by a more prolonged period of virus shedding in children [29]. Because of the development of improved detection methods, and because of identification of newly discovered pathogens, the diagnostic deficit found in our study was lower than that in earlier studies [26, 30, 31].
We detected pathogens in ∼30% of the control subjects. This percentage is considerably higher for the youngest age groups: viruses were detected in 68% and 40% of the control subjects aged 0–4 and 5–14 years, respectively. To our knowledge, only 2 other studies have investigated respiratory pathogens in persons without an ARTI [15, 32]. Johnston et al. [32] reported that 12% and 4% of asymptomatic children and adults, respectively, tested positive for rhinoviruses and enteroviruses. Graat et al. [15] reported that 4% of persons aged ⩾60 years who did not have respiratory complaints tested positive for rhinoviruses and coronaviruses. Frequencies of subclinical respiratory infections in these studies were substantial lower than were those observed in our study: 68%, 55%, and 51% for asymptomatic children (age, 0–15 years), adults, and persons aged ⩾65 years, respectively; this is probably because our study covered a wider range of pathogens. However, the percentage of subclinical infections in our study might have been overestimated, because we did not observe the control subjects for the subsequent development of symptoms. Although control subjects had no respiratory complaints before or at the moment that the nose/throat swabs were obtained, we cannot rule out the possibility that they were in the incubation period for an ARTI. From a questionnaire completed by case patients and control subjects, we know that the rates of exposure to persons with respiratory symptoms within or outside the household were 38% and 56%, respectively, for case patients and 24% and 30%, respectively, for control subjects. Graat et al. [15] included a follow-up period of 8 weeks and excluded only 8% of the control subjects because they developed respiratory symptoms. So, even if ∼10% of our control subjects would have had cases in the incubation period, we still found a substantial number of subclinical infections. For 1 of the 3 control subjects for whom influenza virus was detected, the GP reported that this child did not develop respiratory complaints in the 4 weeks after the nose/throat swab was taken.
Only group A β-hemolytic streptococci were statistically significantly more common in case patients than in control subjects. Other bacteria were reported equally often and in the same quantities in both case patients and control subjects and, therefore, seem to have no causal relationship with ARTI in the general population. This supports the current reserved policy with respect to the prescription of antibiotics. When the physician still decides to prescribe antibiotics, the use of narrow-spectrum antibiotics against group A β-hemolytic streptococci will be sufficient. However, the international trend of increasing prescription of broad-spectrum and new chemotherapeutics has also been seen in The Netherlands [33].
In conclusion, ARTI proved to be very common. We demonstrated that asymptomatic persons can harbor respiratory pathogens and, as a consequence, constitute a neglected source of infection in their environment. Furthermore, our study proved that prescription of antibiotics is usually not advisable, because the majority of cases of ARTI are caused by viruses.
Acknowledgments
We thank all participating GPs, their patients, and The Netherlands Institute of Primary Health Care, for their cooperation in the data collection; Marianne Heshusius and Wilma Kimman for aggregating the clinical and bacteriological data, respectively; Hans van der Nat, Henk Boswijk, Luise Beens, Mariam Bagheri, and Chantal Baas, for performing the virological diagnostic tests; and the technicians of the Regional Laboratory of Public Health in Tilburg, for the bacteriological diagnostic testing; Ron Altena and Ciska Röhrs, for performing cell culture and logistics; Winette van den Brandhof, for her epidemiological assistance at the beginning of the study; and Yvonne van Duynhoven, for her advice and critical reading of the manuscript.
Potential conflicts of interest. All authors: no conflicts.
Footnotes
Present affiliation: National Association of Municipal Health Services, Utrecht, The Netherlands.
References
- 1.Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–42. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monto AS. Viral respiratory infections in the community: epidemiology, agents, and interventions. Am J Med. 1995;99:24–7. doi: 10.1016/S0002-9343(99)80307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–4. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gwaltney JM. Clinical significance and pathogenesis of viral respiratory infections. Am J Med. 2002;112(Suppl 6A):13–8. doi: 10.1016/s0002-9343(01)01059-2. [DOI] [PubMed] [Google Scholar]
- 5.Butler CC, Rollnick S, Kinnersley P, Tapper-Jones L, Houston H. Communicating about expected course and re-consultation for respiratory tract infections in children: an exploratory study. Br J Gen Pract. 2004;54:536–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Bartelds AIM, Fracheboud J, van der Zee J. Utrecht, The Netherlands: Nederlands Instituut voor Onderzoek van de Gezondheidszorg (NIVEL) 1989. The Dutch sentinel practice network: relevance for public health policy. [Google Scholar]
- 7.Carrat F, Tachet A, Rouzioux C, Housset B, Valleron AJ. Evaluation of clinical case definitions of influenza: detailed investigation of patients during the 1995–1996 epidemic in France. Clin Infect Dis. 1999;28:283–90. doi: 10.1086/515117. [DOI] [PubMed] [Google Scholar]
- 8.Reina J, Ferres F, Gutierrez O, Ruiz De Gopegui E, Gonzalez-Cardenas M. Study of the clinical and epidemiological characteristics of respiratory infections caused by adenovirus in a pediatric population (1997–2003) Ann Pediatr (Barc) 2004;61:137–42. doi: 10.1016/s1695-4033(04)78371-x. [DOI] [PubMed] [Google Scholar]
- 9.Cubie HA, Inglis JM, Leslie EE, Edmunds AT, Totapally B. Detection of respiratory syncytial virus in acute bronchiolitis in infants. J Med Virol. 1992;38:283–7. doi: 10.1002/jmv.1890380410. [DOI] [PubMed] [Google Scholar]
- 10.Andeweg AC, Bestebroer TM, Huybreghs M, Kimman TG, de Jong JC. Improved detection of rhinoviruses in clinical samples by using a newly developed nested reverse transcription-PCR assay. J Clin Microbiol. 1999;37:524–30. doi: 10.1128/jcm.37.3.524-530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myint S, Johnston S, Sanderson G, Simpson H. Evaluation of nested polymerase chain methods for the detection of human coronaviruses 229E and OC43. Mol Cell Probes. 1994;8:357–64. doi: 10.1006/mcpr.1994.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorigo-Zetsma JW, Zaat SA, Wertheim-van Dillen PM, et al. Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J Clin Microbiol. 1999;37:14–7. doi: 10.1128/jcm.37.1.14-17.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouchier RA, Bestebroer TM, Herfst S, Van Der Kemp L, Rimmelzwaan GF, Osterhaus AD. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J Clin Microbiol. 2000;38:4096–101. doi: 10.1128/jcm.38.11.4096-4101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claas EC, Sprenger MJ, Kleter GE, van Beek R, Quint WG, Masurel N. Type-specific identification of influenza viruses A, B and C by the polymerase chain reaction. J Virol Methods. 1992;39:1–13. doi: 10.1016/0166-0934(92)90120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graat JM, Schouten EG, Heijnen ML, et al. A prospective, community-based study on virologic assessment among elderly people with and without symptoms of acute respiratory infection. J Clin Epidemiol. 2003;56:1218–23. doi: 10.1016/S0895-4356(03)00171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming DM, Zambon M, Bartelds AI, de Jong JC. The duration and magnitude of influenza epidemics: a study of surveillance data from sentinel general practices in England, Wales and the Netherlands. Eur J Epidemiol. 1999;15:467–73. doi: 10.1023/a:1007525402861. [DOI] [PubMed] [Google Scholar]
- 17.Fleming DM, Zambon M, Bartelds AI. Population estimates of persons presenting to general practitioners with influenza-like illness, 1987–96: a study of the demography of influenza-like illness in sentinel practice networks in England and Wales, and in The Netherlands. Epidemiol Infect. 2000;124:245–53. doi: 10.1017/s0950268899003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Influenza Surveillance Scheme . Utrecht, The Netherlands: Nederlands Instituut voor Onderzoek van de Gezondheidszorg (NIVEL) 2002. Annual report: 2001–2002 influenza season. [Google Scholar]
- 19.Paget WJ, Meerhoff TJ, Goddard NL. Mild to moderate influenza activity in Europe and the detection of novel A(H1N2) and B viruses during the winter of 2001–02. Euro Surveill. 2002;7:147–57. doi: 10.2807/esm.07.11.00373-en. [DOI] [PubMed] [Google Scholar]
- 20.Van der Plas SM, Wilbrink B, Bartelds AIM, Rimmelzwaan GF, De Jong JC, Wallinga J. Influenza-seizoen in het teken van antigene driftvariant. Infectieziekten Bulletin. 2004;3:88–90. [Google Scholar]
- 21.De Wit MA, Koopmans MP, Kortbeek LM, van Leeuwen NJ, Bartelds AI, van Duynhoven YT. Gastroenteritis in sentinel general practices, The Netherlands. Emerg Infect Dis. 2001;7:82–91. doi: 10.3201/eid0701.010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleming DM, Smith GE, Charlton JR, Charlton J, Nicoll A. Impact of infections on primary care—greater than expected. Commun Dis Public Health. 2002;5:7–12. [PubMed] [Google Scholar]
- 23.Bruijnzeels MA, Foets M, van der Wouden JC, van den Heuvel WJ, Prins A. Everyday symptoms in childhood: occurrence and general practitioner consultation rates. Br J Gen Pract. 1998;48:880–4. [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson CR, Helms PJ, Taylor MW, Baxter-Jones AD. Respiratory morbidity in primary care: a population based study, using practices from the Scottish Continuous Morbidity Recording Research Database. Health Bull (Edinb) 2000;58:489–96. [PubMed] [Google Scholar]
- 25.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 26.Tannock GA, Reid AL, Gillett SM, et al. A study of respiratory infections in a healthy adult population during the 1987 Australian winter. Fam Pract. 1993;10:378–86. doi: 10.1093/fampra/10.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wat D. The common cold: a review of the literature. Eur J Intern Med. 2004;15:79–88. doi: 10.1016/j.ejim.2004.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112(Suppl 6A):4–12. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson KG, Webster RG, Hay AJ. Textbook of influenza. 1st ed. Oxford: Blackwell Science; 2000. [Google Scholar]
- 30.Van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–73. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston SL, Sanderson G, Pattemore PK, et al. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993;31:111–7. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuyvenhoven MM, van Balen FA, Verheij TJ. Outpatient antibiotic prescriptions from 1992 to 2001 in The Netherlands. J Antimicrob Chemother. 2003;52:675–8. doi: 10.1093/jac/dkg412. [DOI] [PubMed] [Google Scholar]


