Abstract
During a 6-week period in 2003, 56 residents and 26 staff developed respiratory illness in a long-term facility; 12 residents died. Seven of 13 respiratory specimens were culture-positive for rhinovirus; 6 of the isolates were serotype 82. In elderly populations, severe illness may be associated with organisms typically considered to be “benign,” such as rhinovirus.
Rhinovirus infections may account for up to one-third of cases of “common cold.” The virus has >100 serotypes, is ubiquitous, and can cause repeated episodes of infection throughout an individual's lifetime [1]. Although infection in otherwise healthy persons is frequently self-limited, certain populations may be predisposed to severe manifestations, including bronchiolitis and pneumonia in infants and exacerbations of pre-existing airway disease in persons with chronic obstructive lung disease, asthma, and cystic fibrosis [2]. With the development of sensitive PCR techniques, there is an increasing number of reports of severe rhinovirus infection in other populations, including elderly persons [3]. We describe a large outbreak of respiratory illness in a long-term care facility (LTCF) attributed to rhinovirus and the associated high morbidity and mortality.
Methods. In June 2003, an outbreak of respiratory illness in a 99-bed LTCF was reported to the local health department and the California Department of Health Services. The LTCF performed routine surveillance for respiratory illness year-round by recording daily temperatures and assessing for symptoms. After reporting the outbreak, personnel of the LTCF initiated active surveillance for new cases of respiratory illnesses in residents and staff and, when feasible, collected specimens from ill patients. Epidemiologic and clinical data for residents were obtained by reviewing their medical and hospitalization records, and data for staff were obtained by interview with a standardized questionnaire. Nasopharyngeal swab specimens, sputum samples (from patients who could cough), and serum samples were transported on ice to the California Department of Health Services Viral and Rickettsial Laboratory.
Respiratory samples (0.2 mL) were inoculated into primary rhesus monkey kidney and human fetal diploid lung cells with gentamicin (10 µg/mL) and fungizone (1 µg/mL) in roller tubes at 33°C. Cultures were observed daily for 14 days. Hemadsorption with guinea pig RBCs was performed on rhesus monkey kidney cells between 5–7 days and 12–14 days after initiating cultures. Culture-positivity was confirmed by sequencing PCR product from the VP4-VP2 region of the genome.
Total nucleic acid was extracted using the MasterPure Complete DNA and RNA Purification Kit (Epicentre Technologies). RT-PCR was performed with reverse primers for respiratory syncytial virus, parainfluenza virus types 1–3 and influenza A and B viruses [4], parainfluenza virus type 4 [5], coronavirus 229E (reverse primer) [6], human metapneumovirus [7], and coronavirus OC43 and coronavirus 229E (forward primers) [8]. The picornavirus primers were supplied by D. Erdman (Centers for Disease Control and Prevention; Atlanta, GA) (forward primer 5′-GGCCCCTGAATG(CT)GGCTAA-3′ and reverse 5′-GAAACACGGACACCCAAAGTA-3′.) Rhinovirus primers were those described by Savolainen et al. [9]. Conventional PCR was performed with Mycoplasma pneumoniae [10] and Chlamydia pneumoniae [11] primers. Available serum samples obtained from patients were tested for detection of IgG antibodies against influenza A and B viruses, respiratory syncytial virus, parainfluenza virus types 2–4, adenovirus, M. pneumoniae, and Chlamydia species using in-house assays and for detection of IgM antibodies by means of an enzyme immunoassay against Chlamydia species [12] and M. pneumoniae (Meridian Biosciences).
Results. All 56 residents living in 2 long-term care units developed respiratory symptoms, for an attack rate of 100%. No residents in an adjacent subacute care unit became ill. The first patient developed symptoms on 15 June 2003. The outbreak lasted 6 weeks and peaked at 10 days after onset when 15 residents developed symptoms. The mean age of the infected residents was 87.6 years. Twenty-six health care workers, 22 of whom were nurses providing direct patient care, became ill in the same period (between 15 June 2003 and 3 July 2003) (figure 1).
Figure 1.
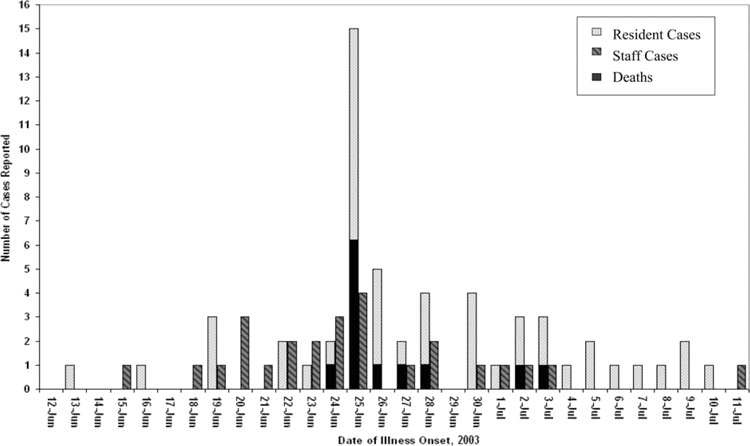
Epidemiologic curve of an outbreak of rhinovirus infection among 82 patients and staff at a long-term care facility for elderly persons (n = 82), June–July 2003.
Cases of clinical illness among residents were characterized by fever (temperature, >38.0°C), productive cough, shortness of breath, wheezing, and lethargy. Twenty-six residents (46%) had underlying medical conditions, including chronic cardiac disease [13], chronic lung disease [8], history of cerebrovascular accident [8], and dementia or Alzheimer's disease [10]. Thirty-four residents underwent chest radiography, with 15 residents (27% of all cases) showing evidence of unilobar or multilobar pneumonia. By contrast, the 26 ill health care workers reported symptoms of mild upper respiratory infection with dry cough, sore throat, and subjective, low-grade fever.
Thirty-five symptomatic residents received antibiotics orally. One resident was hospitalized. Twelve residents (21%), none of whom was a hospice patient, died, and the cause of death was directly attributed to acute infection. The mean age of deceased residents was 93.6 years, and all residents had at least 1 underlying chronic medical condition. None underwent autopsy. Fifty (89%) of the infected residents had received an influenza vaccination.
Thirteen ill residents, including 2 who subsequently died, had nasopharyngeal swab specimens collected within 7 days after the onset of symptoms. A sputum sample was collected from 1 ill resident and sent to the California Department of Health Services Viral and Rickettsial Laboratory; additional sputum specimens obtained from 3 ill residents were sent to the local public health laboratory for testing. Acute and convalescent-phase serum samples were collected from 5 ill residents, including 2 who died.
Respiratory specimens were cultured for viruses; 7 of 13 nasopharyngeal swab specimens and the only sputum sample were culture-positive for rhinovirus. One resident with rhinovirus infection had radiographically confirmed pneumonia and died. The remaining residents either had normal chest radiograph findings or did not undergo chest radiography; all had an uneventful recovery. The PCR products from the VP2-4 regions of 6 isolates were sequenced and identified as rhinovirus serogroup 82.
At the local public health laboratory, Gram staining and bacterial culture were performed on sputum samples obtained from 3 patients; 2 cultures grew nontypeable Haemophilus influenzae. Neither patient had symptoms suggestive of pneumonia, and both clinically improved. Nasopharyngeal swab specimens obtained from these same patients were cultured at the California Department of Health Services Viral and Rickettsial Laboratory and yielded rhinovirus.
Conventional PCR identified picornavirus in 7 of 13 nasopharyngeal swab specimens. Six of 7 were culture-positive for rhinovirus. No other viral pathogens were identified by PCR. All 13 nasopharyngeal swab specimens tested negative for M. pneumoniae and C. pneumoniae by PCR.
Five patients underwent the following analyses: serologic testing of acute-phase serum specimens for detection of IgM antibodies against Chlamydia species and M. pneumoniae; and serologic testing of paired serum specimens for detection of IgG antibodies against influenza A and B viruses, respiratory syncytial virus, parainfluenza virus types 2–4, adenovirus, Chlamydia species, and M. pneumoniae. All results were negative.
Discussion. This report describes an outbreak of respiratory illness attributed to rhinovirus, an organism infrequently associated with severe clinical manifestations or complications. Previous studies of respiratory illness in LTCFs have identified influenza and respiratory syncytial virus as the most common pathogens, with rhinovirus accounting for <10% of cases of infection [12]. Since this outbreak, another outbreak at a LTCF due to rhinovirus associated with 6 cases of radiologically confirmed pneumonia has been reported to the California Department of Health Services (David Schnurr, personal communication). Although other reports have described outbreaks of rhinovirus infection in LTCFs [1], none have observed the high morbidity or mortality rate that we describe.
The conclusion that rhinovirus is the causative pathogen is made cautiously. Agents isolated from the nasopharynx may represent asymptomatic colonization rather than true infection, particularly when sensitive PCR detection methods have been used. Because many of these elderly patients had a poor gag reflex and were unable to produce lower respiratory tract specimens, diagnostic testing for bacteria using Gram staining and culture was not routinely performed. H. influenzae was identified by cultures of 2 sputum samples obtained early in the outbreak. It is possible that secondary superinfection with H. influenzae or other bacterial pathogens contributed to the high morbidity and mortality associated with this outbreak.
Nevertheless, the microbiologic findings suggest that rhinovirus was the primary causative agent that precipitated the outbreak. This is supported by the lacking evidence of other viral pathogens, the temporally and geographically sharp definition of the outbreak, the observation that health care workers concurrently suffered only mild symptoms, and the discovery that a majority of the rhinovirus isolates were of the same serotype. Almost one-half of our patients had a history of underlying severe cardiopulmonary or neurologic disease; this is consistent with other reports that suggest an increased likelihood of lower respiratory tract complications from rhinovirus infection in patients with chronic underlying illness [3]. Although some studies have suggested that certain antigenically related groups are associated with higher incidence of infection, increased clinical virulence, and prolonged viral shedding, others have not found any relation between the severity of illness and the specific serotypes [14, 15]. A notable exception is rhinovirus 16, which has been associated with bronchial inflammation, airway hyperresponsivness, and asthma exacerbations [16]. Rhinovirus 82 has not been specifically associated with increased virulence in clinical or laboratory studies; further studies on the epidemiologic and clinical characteristics associated with individual serogroups are merited.
This report highlights the importance of both strict practice of respiratory hygiene and the early institution of infection-control measures (e.g., isolating and cohorting patients, daily screening for new respiratory illness in residents and staff, restricting flotation of staff between units, and closing residential units to outside visitors) after the identification of respiratory illnesses in LTCFs. Respiratory pathogens, such as rhinovirus, which may cause only mild symptoms in healthy persons, can be a source of major morbidity and mortality in elderly or immunocompromised populations. These agents may be ubiquitous in the health care worker and visitor populations who have contact with these patients. Rigorous respiratory hygiene measures have been strongly advocated in the wake of outbreaks of severe acute respiratory syndrome and influenza, their universal practice can also protect vulnerable patients from what are otherwise considered to be benign pathogens in healthy populations.
Acknowledgments
We gratefully acknowledge the contributions made by Megan Kettman and Jill Landis at the Santa Cruz County Health Services Agency, who conducted the initial epidemiologic investigation; Georgio Cosentino, David Cottam, Alex Hewitt, Natasha Huntziker, Jaime Powell, Chris Preas, Ray Sante, Lauren Wold, Elaine Yeh, and the staff at the California Department of Health Services Viral and Rickettsial Disease Laboratory, who provided laboratory and technical support; Jill Hacker, Jennifer Mark, and Will Probert, who performed the viral and Chlamydia PCR testing; Lawrence Drew, for thoughtful review of the manuscript; and Somayeh Honarmand, for assistance with formatting.
Financial support. All authors except F.N. are supported by the California Department of Health Services. F.N. is supported by the Santa Cruz County Health Services Agency.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Wald TG, Shult P, Krause P, Miller BA, Drinka P, Gravenstein S. A rhinovirus outbreak among residents of a long-term care facility. Ann Intern Med. 1995;123:588–93. doi: 10.7326/0003-4819-123-8-199510150-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol. 2004;14:17–31. doi: 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185:1338–41. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdman DD, Weinberg GA, Edwards KM, et al. GeneScan reverse transcription-PCR assay for detection of six common respiratory viruses in young children hospitalized with acute respiratory illness. J Clin Microbiol. 2003;41:4298–303. doi: 10.1128/JCM.41.9.4298-4303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguilar JC, Perez-Brena MP, Garcia ML, Cruz N, Erdman DD, Echevarria JE. Detection and identification of human parainfluenza viruses 1, 2, 3, and 4 in clinical samples of pediatric patients by multiplex reverse transcription-PCR. J Clin Microbiol. 2000;38:1191–5. doi: 10.1128/jcm.38.3.1191-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitkaranta A, Arruda E, Malmberg H, Hayden FG. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–3. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 8.Myint S, Johnston S, Sanderson G, Simpson H. Evaluation of nested polymerase chain methods for the detection of human coronaviruses 229E and OC43. Mol Cell Probes. 1994;8:357–64. doi: 10.1006/mcpr.1994.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savolainen C, Blomqvist S, Mulders N, Hovi T. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J Gen Virol. 2002;83:333–40. doi: 10.1099/0022-1317-83-2-333. [DOI] [PubMed] [Google Scholar]
- 10.Grondahl B, Puppe W, Hoppe A, Kuhne I, Weigl JA, Schmitt HJ. Rapid identification of nine microorganisms causing acute respiratory tract infections by single-tube multiplex reverse transcription-PCR: feasibility study. J Clin Microbiol. 1999;37:1–7. doi: 10.1128/jcm.37.1.1-7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman CL, Roblin PM, Gaydos CA, Quinn TC, Schachter J, Hammerschlag MR. Prevalence of asymptomatic nasopharyngeal carriage of Chlamydia pneumoniae in subjectively healthy adults: assessment by polymerase chain reaction-enzyme immunoassay and culture. Clin Infect Dis. 1995;20:1174–8. doi: 10.1093/clinids/20.5.1174. [DOI] [PubMed] [Google Scholar]
- 12.Cremer NE, Cossen CK, Shell G, Diggs J, Gallo D, Schmidt NJ. Enzyme immunoassay versus plaque neutralization and other methods for determination of immune status to measles and varicella-zoster viruses and versus complement fixation for serodiagnosis of infections with those viruses. J Clin Microbiol. 1985;21:869–74. doi: 10.1128/jcm.21.6.869-874.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falsey AR, Treanor JJ, Betts RF, Walsh EE. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J Am Geriatr Soc. 1992;40:115–9. doi: 10.1111/j.1532-5415.1992.tb01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monto AS, Bryan ER, Ohmit S. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J Infect Dis. 1987;156:43–9. doi: 10.1093/infdis/156.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Kellner G, Popow-Kraupp T, Binder C, Goedl I, Kundi M, Kunz C. Respiratory tract infections due to different rhinovirus serotypes and the influence of maternal antibodies on the clinical expression of the disease in infants. J Med Virol. 1991;35:267–72. doi: 10.1002/jmv.1890350412. [DOI] [PubMed] [Google Scholar]
- 16.Gern JE, Busse WW. Association of rhinovirus infections with asthma. Clin Micro Rev. 1999;12:9–18. doi: 10.1128/cmr.12.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]


