Abstract
Background. Human bocavirus (HBoV) was recently discovered in children with respiratory tract disease and gastroenteritis. The causative role of HBoV in human gastroenteritis remains uncertain, and, to our knowledge, no previous case-control study has studied the relationship between HBoV and gastroenteritis.
Methods. We conducted a case-control study that examined stool samples from 397 children with diarrhea and from 115 asymptomatic control subjects. HBoV was detected using polymerase chain reaction. Real-time polymerase chain reaction was used to quantify the HBoV loads in case and control groups. Common enteric viruses were examined using enzyme-linked immunosorbent assays, polymerase chain reaction, and reverse-transcription polymerase chain reaction.
Results. At least 1 viral agent was discovered in 60.2% of cases. HBoV was detected in 14 samples, and 9 were coinfected with either rotavirus (7 of 14 samples) or human calicivirus (2 of 14). Many (8 [57.1%] of 14) of the HBoV infections occurred during September-December 2006. Most (12 [85.7%]) of the HBoV-infected children were 7–18 months of age. The percentage of children with HBoV infection did not differ significantly between case patients and control subjects (3.5% vs. 3.5%), and the statistical analysis did not support a correlation between HBoV infection and more-severe clinical symptoms. The viral load differences between the 2 groups were not statistically significant (P = .09, by log-normal Student's t test). In addition, the VP1/VP2 partial gene of HBoV from case patients and control subjects showed minimal sequence variation.
Conclusions. A single genetic lineage of HBoV was revealed in persons in China. Despite its high prevalence in stool samples, our study does not support a causative role of HBoV in gastroenteritis.
Gastroenteritis is a major cause of childhood morbidity and mortality worldwide. Between 1.5 and 2 million infants and young children die of gastroenteritis-related diseases or complications annually [1]. Pathogenic enteric bacteria are important etiologic agents of this disease, but with the improvement of molecular detection methods, a large proportion of cases of gastroenteritis is found to be associated with viral etiological agents [2, 3], such as rotaviruses, human calicivirus (HuCV), adenoviruses, and astroviruses [4]. However, the etiologic agents are still not identified in some patients with gastroenteritis, despite improvements in diagnostic technology.
In 2005, Allander et al. [5] reported the detection of a new human parvovirus in children with acute respiratory tract infections. This virus belongs to the genus Bocavirus in the subfamily parvovirinae of the family parvoviridae and is most closely related to bovine parvovirus and minute virus of canines. Therefore, it was named “human bocavirus” (HBoV). Subsequently, HBoV has been detected frequently in children with respiratory tract infections and asthma exacerbation worldwide [6–11]. Several studies confirmed that HBoV infection is indeed associated with respiratory tract symptoms [12–15]. Recently, HBoV has also been implicated in diarrhea, and its detection rates in children with gastroenteritis have a range of 0.8%–9.1%. Therefore, this newly identified virus has been suggested to be an enteric pathogen, as well as being a respiratory pathogen [16–19]. However, because most studies assessed the prevalence of HBoV in children with gastroenteritis in the absence of defined control children without enteric disease, the clinical spectrum of HBoV and the role that it plays in gastroenteritis remain to be clarified. The present case-control study investigated the prevalence of HBoV and other major viral etiologic agents in children hospitalized for gastroenteritis from July 2006 through September 2007 in Lanzhou, China, and the relationship between HBoV and gastroenteritis.
MATERIALS, PATIENTS, AND METHODS
Study participants and sample collection and processing. From July 2006 through September 2007, we conducted a casecontrol study to explore gastroenteritis-associated viral agents in hospitalized children with diarrhea and in healthy children in Lanzhou, China. Stools from 397 hospitalized children with diarrhea and from 115 asymptomatic control subjects were collected. All of the children were <5 years of age, and their parents were interviewed to determine the symptoms. Consecutive cases were collected from among children who were hospitalized for gastroenteritis in the Department of Pediatrics, The First Hospital of Lanzhou University (Lanzhou, China). Diarrhea was defined as ⩾3 loose stools in the previous 24–72 h [20]. Patients were excluded from the study if insufficient stool samples were available for a complete evaluation of viral agents, their stools had clear blood streaks, or they had another diagnosed illness, such as pneumonia. Control subjects were asymptomatic children who visited the First Hospital of Lanzhou University Pediatric Primary Care Center for a routine examination and did not have fever, diarrhea, vomiting, or a respiratory illness in the previous 3-week period [21]. All of the control subjects in this study were selected by the frequencymatching method. Control subjects received follow-up by telephone, and the children who had had the above-mentioned clinical symptoms during the week after the initial examination were excluded.
Informed consent was obtained from the parents of all of the children who provided specimens. The study protocol was approved by the hospital ethics committee. All specimens were stored at -70°C until further analysis. Viral RNA and DNA were extracted from 140 µL of 10% fecal suspension in phosphate- buffered saline with use of the QIAamp Viral RNA Mini Kit (Qiagen), which is supposed to extract viral RNA and DNA simultaneously, according to the manufacturer's instructions.
Rotavirus detection. Rotavirus antigen was detected using a commercial ELISA kit (IDEIA Rotavirus; Dako), according to the manufacturer's instructions. Positive specimens were then G and P genotyped with use of nested PCR with typespecific primers, as described elsewhere [22, 23].
Detection of common enteric viruses. Six enteric viruses were identified using multiplex RT-PCR and PCR with 3 sets of primers [24], as follows: set A, for detecting noroviruses GI and GII, sapoviruses, and astroviruses; set B, for all adenoviruses; and set C, for group B and C rotaviruses. The multiplex RT-PCR and PCR were performed in accordance with a protocol described elsewhere [24].
HBoV detection. HBoV was detected in the extracted DNA by PCR amplification of a 291-base pair fragment of the NS1 gene with forward primer HBOV-1 (5′-TATGGCCAAGGCAATCGTCCAAG-3′) and reverse primer HBOV-2 (5′-GCCGCGTGAACATGAGAAACAGA- 3′), as described elsewhere [11]. To acquire the partial VP1/VP2 gene, we used forward primer VP1/2-1 (5′-GGACCACAGTCATCAGAC-3′) and reverse primer VP1/2-2 (5′-CCACTACCATCGGGCTG-3′), targeting an 820-base pair fragment [13]. The reaction mix contained 20 pmol of each primer and 2.5 units of ExTaq DNA polymerase (Takara Bio). After 5 min at 94°C, 35 cycles of amplification (94°C for 1 min, 54°C for 1 min, and 72°C for 2 min) were performed, followed by a 10-min extension at 72°C. Positive PCR products were purified using a QIAquick PCR purification kit (Qiagen) and were sequenced by Invitrogen.
Sequence analysis and accession numbers. The nucleotide and deduced amino acid sequences of the NS1 gene and the VP1/VP2 partial gene were compared with those of HBoV strains available at the GenBank site. Phylogenetic analyses were conducted with MEGA, version 3.1 [25]. The 12 partial sequences of the VP1/VP2 gene were submitted toGenBank (accession numbers EU400116-EU400128).
Real-time PCR for HBoV. To quantify the HBoV loads, real-time PCR assays were performed. Each 25-µL reaction mixture consisted of 0.5 µL of forward primer 5′-TGC AGA CAA CGC YTA GTT GTT T-3′ and reverse primer 5′-CTG TCC CGC CCA AGA TAC A-3′ for the 88-base pair NS1 target, 0.125 µL of probe 5′-CCA GGA TTG GGT GGA ACC TGC AAA-3′ [26], and 2.5 µL of sample DNA. PCR was conducted at 50°C for 2 min and at 95°C for 10 min, followed by 50 cycles at 95°C for 15 s and at 60°C for 1 min. Serial dilutions of the pGEMT Easy vector (Promega) containing the HBoV NS1 gene were used as a quantification standard. Positive and negative controls were included. The Rotor-Gene 3000 real-time thermal cycling system (Corbett Research) was used, and the data were analyzed using Rotor-Gene software (Corbett Research), with the help of a standard curve. The minimum viral load that would allow reproducible quantification was 10 copies per reaction.
Statistical analysis. The statistical significance of rates between various groups was tested using the χ2 test and Fisher's exact test. The statistical significance of means between various groups was tested using the Student's t test and the log-normal Student's t test. Analyses were performed using SPSS, version 11.5 (SPSS).
RESULTS
Patient characteristics. The ages of children with diarrhea ranged from 15 days to 60 months (mean age ± SD, 11.18 ± 9.3 months). The majority of patients (74.8%) were 0–12 months of age. The ratio of boys to girls was 1.8:1.
Virological findings in children with diarrhea. Of the 397 specimens, 239 (60.2%) contained at least 1 viral agent. Rotaviruses were identified in 165 (41.6%) of the 397 samples: G1 was the most common G serotype (91 of 165; 55.2%), and P was the most common P genotype (112 of 165; 67.9%) [8]. We detected HuCV in 52 (13.1%) of 397 samples, astroviruses in 13 samples (3.3%), adenoviruses in 22 samples (5.5%), and HBoV in 14 samples (3.5%). Group B and C rotaviruses were not detected in this study. Detection of rotaviruses, HuCV, astroviruses, and HBoV peaked during the winter. HBoV was not detected during spring 2007. Of the adenovirus-positive samples, 77.3% (17 of 22 samples) were collected during July- September 2007, indicating a possible outbreak of adenovirus infection during that season (figure 1). The majority of patients who were positive for rotaviruses, astroviruses, and adenoviruses were 0–12 months of age, whereas HuCV and HBoV were found mostly in children aged 7–18 months (figure 2). Of the 14 HBoV-positive samples, 64.3% (9) were coinfected with either rotavirus (7) or HuCV (2). Of the patients with HBoVpositive samples, 9 were boys and 5 were girls. Of these 14 patients, 7 had fever and 11 were vomiting, and the mean duration and frequency of diarrhea were 4.6 days and 6.5 times per day, respectively (table 1).
Figure 1.
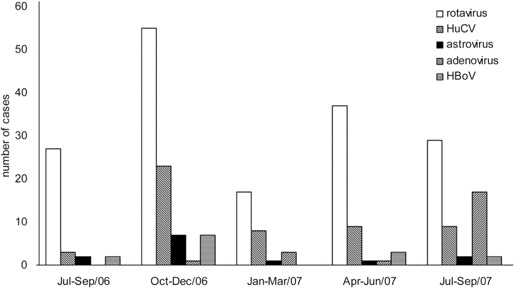
Seasonal distribution of viral gastroenteritis in pediatric patients hospitalized in the First Hospital of Lanzhou University (Lanzhou, China). HBoV, human bocavirus; HuCV, human calicivirus.
Figure 2.
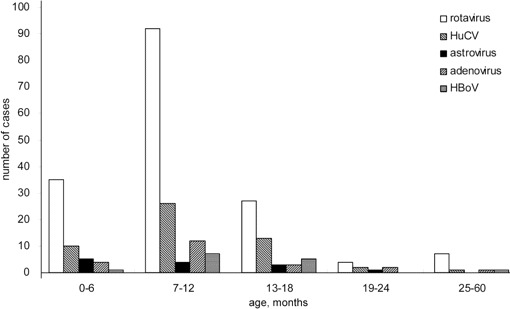
Age distribution of pediatric patients with viral gastroenteritis who were hospitalized in the First Hospital of Lanzhou University (Lanzhou, China). HBoV, human bocavirus; HuCV, human calicivirus.
Table 1.
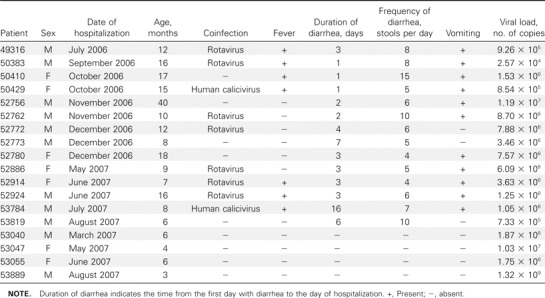
Clinical characteristics and viral loads of the 18 case and control children who were positive for human bocavirus.
Prevalence of HBoV in asymptomatic children. The ages of 115 asymptomatic control children ranged from 1 to 60 months (mean age ± SD, 14.1 ± 17.2 months). The majority of these children (78.3%) were 1–18 months of age. The ratio of boys to girls was 1.2:1. The differences in mean age (P = .08, by Student's t-test) and sex (P = .07, by χ2 test) between the case group and the control group were not statistically significant. HBoV was detected in 4 (3.5%) of 115 samples derived from the control subjects; 4 HBoV-positive cases were confirmed in subjects aged ⩽6 months. Samples were collected in March, May, June, and August. The ratio of boys to girls was 1:1.
Phylogenetic analyses of HBoV. All PCR products of the NS1 gene were confirmed by sequencing. Because a previous study demonstrated that the greatest variation in the HBoV genome was in the VP1/VP2 gene, especially at its 3′ end [13], 12 HBoV-positive specimens were randomly selected to amplify VP1/VP2 partial gene sequences (nucleotides 4370–5189 of the HBoV genome). Phylogenetic analyses indicated that all 12 VP1/VP2 genes in our study were in the same cluster as other strains from China (with >99% sequence homology) (figure 3).
Figure 3.
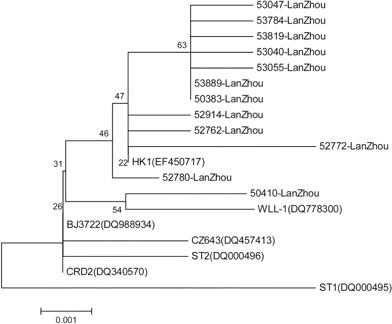
Phylogenetic tree of the VP1/VP2 gene sequences of 12 human bocavirus strains from fecal samples. Strains 53040-LanZhou, 53047-LanZhou, 53055-LanZhou, and 53889-LanZhou were from asymptomatic children; the other strains were from children with gastroenteritis. Human bocavirus strains ST1 and ST2 from Sweden are the 2 prototype strains. Strains HK1, BJ3722, WLL-1, and CZ643 are from China, and strain CRD2 is from the United States.
Quantitative analysis of HBoV DNA. We used real-time PCR to quantify the HBoV load. The mean HBoV load in the case group was 2.0 × 106 copies/mL of extract (range, 2.6 × 104–1.2 × 107 copies/mL), and the mean HBoV load in the control group was 1.5 × 107 copies/mL of extract (range, 1.8 × 106–1.3 × 109 copies/mL). There was no statitistically signficant difference between the mean values of these 2 groups (P = .09, log-normal Student's t test) (tables 1 and 2).
Table 2.

Comparison of detection rates and human bocavirus (HBoV) loads between case group and control group.
Association of HBoV with gastroenteritis. In our study, none of the HBoV-positive patients had respiratory symptoms. HBoV was not more prevalent among children with gastroenteritis than among asymptomatic children (3.5% vs. 3.5%) (table 2). HBoV was not more prevalent among children with gastroenteritis of undetectable etiology than among those in whom other viruses were detected (3.1% vs. 3.8%; P = .75, by χ2 test) (table 3). When the HBoV-positive and HBoV-negative case groups were compared, neither the rates of fever among patients nor the rates of vomiting among patients differed statistically significantly (P = .80, by χ2 test; and P = .06, by Fisher's exact test; respectively). In addition, the mean duration and frequency of diarrhea did not differ statistically significantly (P = .52 and P = .42, respectively, by Student's t test). Because the seasonal and age distributions of HBoV were similar to those of rotavirus and because there was often coinfection with HBoV and rotavirus, we compared the clinical symptoms between the group of children infected with rotavirus alone and the group of children coinfected with rotavirus and HBoV, and we did not find a statistically significant difference between these 2 groups (all P> .05, by χ2 test and Student's t test) (table 4). These results suggest that infection with HBoV did not exacerbate the clinical symptoms of gastroenteritis.
Table 3.

Comparison of detection rates between children with gastroenteritis of undetectable etiology and those in whom other viruses were detected.
Table 4.
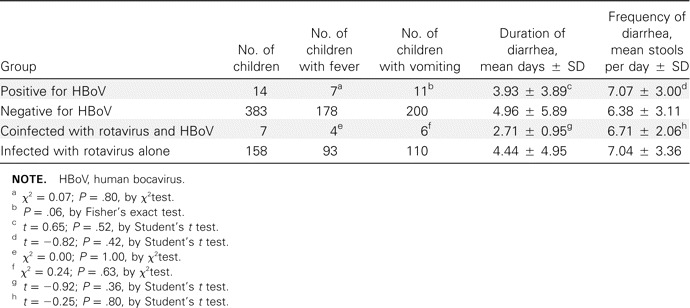
Comparison of clinical symptoms between different groups of children with gastroenteritis.
DISCUSSION
This study examined common viral etiologic agents, to delineate the clinical role played by HBoV in children hospitalized with gastroenteritis. Our results revealed that enteric viruses play an important role in pediatric diarrhea and that the most common viral agents causing severe gastroenteritis consisted of group A rotaviruses, followed by HuCV. The prevalence of adenoviruses and astroviruses was similar to that reported elsewhere [3, 27–29]. Although some studies have detected high rates of groups B and C rotaviruses in stool samples from children with diarrhea [3, 30, 31], these pathogens were not found in samples from our participants.
Previous findings [32–34] supported the association of animal bocaviruses with both respiratory symptoms and gastroenteritis, particularly in calves and puppies. Some studies raised the question about whether HBoV is a cause of human gastroenteritis [16, 35]. HBoV is thought to be swallowed during a respiratory tract infection and subsequently excreted in the feces, without further replication in the gastrointestinal tract [35]. Recently, HBoV was found to occur frequently in stool samples from children with gastroenteritis. Vicente et al. [18] found that 48 (9.1%) of 527 stool samples from Spanish patients with gastroenteritis were positive for HBoV. Lau et al. [17] detected HBoV in 2.1% of fecal samples from children with gastroenteritis in Hong Kong, and some of these patients had respiratory tract symptoms. These studies implicatedHBoV as a gastroenteritis-associated enteric virus. However, the concurrent detection of HBoV and other enteric viruses, which is unusual for known pathogens, raises concern over a causative role of HBoV in human gastroenteritis. Therefore, additional evidence is required to establish a link between HBoV and gastroenteritis.
In our study, the detection rate of HBoV (3.5%) was comparable to previously reported rates [17–19]. We also found that HBoV infections occurred more frequently during the winter months (50% of cases) in children aged <2 years. Coinfection with HBoV and another virus was common (64.3% among all HBoV-positive children with gastroenteritis). The most frequent codetection of HBoV and rotavirus further supported the idea that HBoV is an “innocent bystander” in gastroenteritis. In this regard, our results were generally consistent with previous findings [11, 18, 36], and the similar rates of HBoV in our control (3.5%) and gastroenteritis (3.5%) groups also argue against a causative role of HBoV in gastroenteritis. Furthermore, the difference in the HBoV loads, as measured in our control and gastroenteritis groups, was statistically nonsignificant.
On the basis of VP1/VP2 gene sequences, all HBoV isolates found in our study were in the same cluster as the original isolate ST2 identified by Allander et al. [5], with >99% DNA sequence homology. Moreover, the other HBoV strains identified in China—including CZ, WL, BJ, and HK—are in the same cluster. This suggests that a single genetic lineage of HBoV is circulating in humans in China and that the HBoV viruses found in both the respiratory and enteric tracts of humans in China probably belong to a single genetic lineage.
To our knowledge, this is the first case-control study to investigate the linkage between HBoV and gastroenteritis. In summary, we did not find statistically significant differences in the prevalence and HBoV loads between children with and without gastroenteritis. Concurrent detection of HBoV and other enteric pathogens, such as rotaviruses, in children with gastroenteritis was common. In addition, infection with HBoV did not significantly influence the severity of gastroenteritis. Our results do not support a causative role for HBoV in gastroenteritis, despite its frequent detection in fecal samples worldwide. To further clarify the linkage between HBoV and gastroenteritis, future priorities should include additional case-control studies that have larger cohorts of case and control samples, the establishment of serological methods to trace the viral antigens and the immune response, and the development of an animal model for HBoV infection.
Acknowledgement
Financial support. “973” National Key Basic Research Program of China (2007CB310500) and National High Technology Research and Development Program of China (2006AA02A215).
Potential conflicts of interest. All authors: no conflicts.
Footnotes
W.-x.C. and Y.J. contributed equally to this article
References
- 1.World Health Report 2005. Make every mother and child count. Geneva: World Health Organization Press; 2005. [Google Scholar]
- 2.Fodha I, Chouikha A, Peenze I, et al. Identification of viral agents causing diarrhea among children in the Eastern Center of Tunisia. J Med Virol. 2006;78:1198–203. doi: 10.1002/jmv.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen TA, Yagyu F, Okame M, et al. Diversity of viruses associated with acute gastroenteritis in children hospitalized with diarrhea in Ho Chi Minh City, Vietnam. J Med Virol. 2007;79:582–90. doi: 10.1002/jmv.20857. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelmi I, Roman E, Sanchez-Fauquier A. Viruses causing gastroenteritis. Clin Microbiol Infect. 2003;9:247–62. doi: 10.1046/j.1469-0691.2003.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102:12891–6. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung JY, Han TH, Kim SW, Kim CK, Hwang ES. Detection of viruses identified recently in children with acute wheezing. J Med Virol. 2007;79:1238–43. doi: 10.1002/jmv.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foulongne V, Olejnik Y, Perez V, Elaerts S, Rodiere M, Segondy M. Human bocavirus in French children. Emerg Infect Dis. 2006;12:1251–3. doi: 10.3201/eid1208.060213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan NM, Dove W, Abu-Zeid AF, Shamoon HE, Abd-Eldayem SA, Hart CA. Human bocavirus infection among children, Jordan. Emerg Infect Dis. 2006;12:1418–20. doi: 10.3201/eid1209.060417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma X, Endo R, Ishiguro N, et al. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol. 2006;44:1132–4. doi: 10.1128/JCM.44.3.1132-1134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu XW, Duan ZJ, Qi ZY, et al. Human bocavirus infection, People's Republic of China. Emerg Infect Dis. 2007;13:165–8. doi: 10.3201/eid1301.060824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, Mackay IM. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol. 2006;35:99–102. doi: 10.1016/j.jcv.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–10. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesebir D, Vazquez M, Weibel C, et al. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194:1276–82. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning A, Russell V, Eastick K, et al. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis. 2006;194:1283–90. doi: 10.1086/508219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fry AM, Lu X, Chittaganpitch M, et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–45. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43:283–8. doi: 10.1086/505399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau SK, Yip CC, Que TL, et al. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis. 2007;196:986–93. doi: 10.1086/521310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicente D, Cilla G, Montes M, Perez-Yarza EG, Perez-Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis. 2007;13:636–7. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JI, Chung JY, Han TH, Song MO, Hwang ES. Detection of human bocavirus in children hospitalized because of acute gastroenteritis. J Infect Dis. 2007;196:994–7. doi: 10.1086/521366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subekti D, Lesmana M, Tjaniadi P, et al. Incidence of Norwalk-like viruses, rotavirus and adenovirus infection in patients with acute gastroenteritis in Jakarta, Indonesia. FEMS Immunol Med Microbiol. 2002;33:27–33. doi: 10.1111/j.1574-695X.2002.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 21.Amar CF, East CL, Gray J, Iturriza-Gomara M, Maclure EA, Mc-Lauchlin J. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993–1996) Eur J Clin Microbiol Infect Dis. 2007;26:311–23. doi: 10.1007/s10096-007-0290-8. [DOI] [PubMed] [Google Scholar]
- 22.Gentsch JR, Glass RI, Woods P, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–73. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gouvea V, Glass RI, Woods P, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–82. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan TG, Nguyen TA, Yan H, et al. Development of a novel protocol for RT-multiplex PCR to detect diarrheal viruses among infants and children with acute gastroenteritis in eastern Russia. Clin Lab. 2005;51:429–35. [PubMed] [Google Scholar]
- 25. [Accessed 27 May 2008];Molecular Evolutionary Genetics Analysis. MEGA software. Available at: http://www.megasoftware.net/
- 26.Lu X, Chittaganpitch M, Olsen SJ, et al. Real-time PCR assays for detection of bocavirus in human specimens. J Clin Microbiol. 2006;44:3231–5. doi: 10.1128/JCM.00889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabiana A, Donia D, Gabrieli R, et al. Influence of enteric viruses on gastroenteritis in Albania: epidemiological and molecular analysis. J Med Virol. 2007;79:1844–9. doi: 10.1002/jmv.21001. [DOI] [PubMed] [Google Scholar]
- 28.Colomba C, De Grazia S, Giammanco GM, et al. Viral gastroenteritis in children hospitalised in Sicily, Italy. Eur J Clin Microbiol Infect Dis. 2006;25:570–5. doi: 10.1007/s10096-006-0188-x. [DOI] [PubMed] [Google Scholar]
- 29.Chen SY, Chang YC, Lee YS, et al. Molecular epidemiology and clinical manifestations of viral gastroenteritis in hospitalized pediatric patients in northern Taiwan. J Clin Microbiol. 2007;45:2054–7. doi: 10.1128/JCM.01519-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barman P, Ghosh S, Samajdar S, et al. RT-PCR based diagnosis revealed importance of human group B rotavirus infection in childhood diarrhoea. J Clin Virol. 2006;36:222–7. doi: 10.1016/j.jcv.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Phan TG, Nishimura S, Okame M, et al. Virus diversity and an outbreak of group C rotavirus among infants and children with diarrhea in Maizuru City, Japan during 2002–2003. J Med Virol. 2004;74:173–9. doi: 10.1002/jmv.20162. [DOI] [PubMed] [Google Scholar]
- 32.Carmichael LE, Schlafer DH, Hashimoto A. Minute virus of canines (MVC, canine parvovirus type-1): pathogenicity for pups and seroprevalence estimate. J Vet Diagn Invest. 1994;6:165–74. doi: 10.1177/104063879400600206. [DOI] [PubMed] [Google Scholar]
- 33.Durham PJ, Lax A, Johnson RH. Pathological and virological studies of experimental parvoviral enteritis in calves. Res Vet Sci. 1985;38:209–19. [PubMed] [Google Scholar]
- 34.Squires RA. An update on aspects of viral gastrointestinal diseases of dogs and cats. N Z Vet J. 2003;51:252–61. doi: 10.1080/00480169.2003.36379. [DOI] [PubMed] [Google Scholar]
- 35.Neske F, Blessing K, Tollmann F, et al. Real-time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. J Clin Microbiol. 2007;45:2116–22. doi: 10.1128/JCM.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smuts H, Hardie D. Human bocavirus in hospitalized children, South Africa. Emerg Infect Dis. 2006;12:1457–8. doi: 10.3201/eid1209.051616. [DOI] [PMC free article] [PubMed] [Google Scholar]


