Abstract
Acute respiratory distress syndrome (ARDS) and pneumonia are closely correlated in the critically ill patient. Whereas ARDS is often complicated by nosocomial pneumonia, pulmonary infection is also the most frequent single cause of ARDS. The prevalence of pneumonia during the course of ARDS seems to be particularly high, but whether persons with ARDS are more susceptible to pneumonia or simply have more risk factors remains unknown because of methodological limitations. Recent research suggests that host factors have a major bearing on the development of ARDS. To date, sepsis seems to be the principal link between pneumonia and ARDS. However, prospective observational data on this supposed sequence are not available. The individual role of specific pathogens for the development of ARDS is difficult to assess, because prospective studies are missing. Respiratory viruses have received particular attention, but this review suggests that infections with coronavirus and avian influenza virus (H5N1) are associated with a high incidence of ARDS.
Acute respiratory distress syndrome (ARDS) is currently diagnosed using 4 criteria, and its etiology can be differentiated into direct and indirect lung injury [1, 2]. Community-acquired pneumonia (CAP) is firmly diagnosed by clinical and radiographic criteria, but the diagnosis of ventilator-associated pneumonia (VAP) imposes considerable difficulties, even when adequate lower respiratory tract samples are collected (table 1). This is especially true when ARDS and pneumonia have to be differentiated in clinical practice [3]. The pathophysiology of pulmonary infiltrates in pneumonia is well defined, but the mechanisms behind the development of ARDS are still not fully understood. The hallmark of ARDS is the increased permeability of the edema, which is interpreted as being an accumulation of protein-rich edema fluid in the alveoli and is mediated by inflammation of various mechanisms [4].
Table 1.
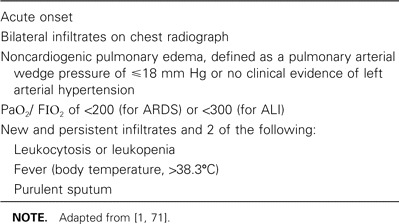
Definition of acute respiratory distress syndrome (ARDS) and acute lung injury (ALI), according to the American-European Consensus Conference and the Johanson criteria.
The diagnoses of ARDS and pneumonia both require radiographic infiltrates; severe pneumonia is frequently of acute onset and shows bilateral infiltrates on chest radiography and severe acute respiratory failure not due to cardiac failure. Thus, it is virtually impossible to differentiate acute severe bilateral pneumonia from ARDS on clinical grounds alone. Accordingly, in a recent study of the association of ARDS with pneumonia by a comparison of clinical diagnoses based on the American-European Consensus Conference Criteria [1] and histopathologic evidence for diffuse alveolar damage [5, 3], pneumonia was the most frequent mimic of ARDS. In the 43 patients who met ARDS criteria but who did not have diffuse alveolar damage, pneumonia was the most prevalent finding (32 [74%] of 43 patients) [3]. Pneumonia is also the most frequent lung condition leading to ARDS. In a series of 153 patients, Sloane et al. [6] reported pneumonia as the underlying etiology in 31% of all patients who developed ARDS, and virtually all patients with ARDS require mechanical ventilation, a major risk factor for the development of VAP [7–9].
Therefore, this review is focused on the following topics: (1) pneumonia as a cause of direct lung injury in the immunocompetent host, (2) nosocomial pneumonia as a complication of ARDS, and (3) the impact of various infectious etiologies on the induction of ARDS. This review will exclude therapeutic issues dealing with either pneumonia or ARDS, because the published information associated with these issues has been updated recently [10, 11]. We reviewed international reports identified by searches of PubMed with relevant keywords. We also searched cited references in retrieved articles, reviewed articles we have collected over many years, and used knowledge of new data presented at international scientific meetings. We gave priority to clinically relevant articles, rather than reports of randomized controlled trials, and case reports, case series reports, and retrospective studies were used for this systematic review.
Ards Complicating the Course of Pneumonia
The sequence from bacterial pneumonia to ARDS can be followed more accurately in persons with CAP [11]. Estenssoro et al. [12] observed 3050 patients admitted to intensive care units during a 15-month study period; 1193 patients (39%) were mechanically ventilated, and 235 met the criteria for ARDS (7.7% of the total number of patients, and 19.7% of the ventilated patients). The predominant etiology of ARDS was sepsis (44%), and pneumonia was the most frequent single entity (65 cases). The authors did not differentiate between CAP and nosocomial pneumonia, and they have not followed-up with patients with pneumonia who have not developed ARDS to identify risk factors. The figures given by this group were comparable with those of previous studies that used similar ARDS criteria [13–16], with pneumonia remaining the most frequent single cause of sepsis. However, to draw meaningful conclusions, we need larger, prospective cohort studies that observe patients with CAP for progression to ARDS.
To further identify the reasons why severe CAP progresses to ARDS, it is important to first discover why severe CAP progresses to sepsis. In a prospective cohort study [17], 280 patients with CAP were included, and 31 subjects (11%) were identified who met the criteria for septic shock. In a multivariate analysis incorporating age; sex; the presence of chronic pulmonary, cardiac, renal, hepatic, or neurologic disease; alcohol consumption; prior antibiotic exposure; delayed antibiotic therapy; and TNF-α genotype, the only factors that remained significant predictors of septic shock were LTα+250 genotype and increasing age. The study design was repeated with a focus on the possible role of the intracellular adhesion molecule type 1 but failed to yield a significant association between CAP and sepsis [18]. Ten (4%) of the 289 patients in the cohort had ARDS, but it was not noted whether sepsis or septic shock preceded ARDS. These data, however, did not directly address the issue of whether sepsis is the required link between ARDS and pneumonia. Thus, risk factors for both development of severe sepsis and ARDS in the course of CAP remain undefined.
Pneumonia Complicating Ards
The issue of assessing the impact of pneumonia during the natural course of ARDS is obscured by the uncertainties in diagnosing nosocomial pneumonia. All approaches to construct firm diagnostic criteria for VAP have their inherent limitations. In particular, even the most reliable measure of diagnosing VAP using quantitative cultures of bronchoscopically retrieved respiratory samples (by protected specimen brush and/or bronchoalveolar lavage) does not preclude false-negative and false-positive results in the range of 10%–30% [19, 20]. Accordingly, the incidence of pneumonia during the course of ARDS reported in various studies varies largely. The issue has been reviewed in detail by Iregui and Kollef [21].
Delclaux et al. [22] performed a prospective study of lower respiratory tract colonization and infection in 30 patients with severe ARDS by repeated quantitative culture of plugged telescoping catheter specimens every 48–72 h after the development of ARDS. Using clinical and microbiological criteria, these investigators found an incidence of VAP of 60% (4.2 episodes per 100 ventilator-days). Previous lower respiratory tract colonization with similar microorganisms preceded the development of VAP in almost all cases. In line with these data, Markowicz et al. [23] found an incidence of VAP of 37% among patients with ARDS. The incidence of early-onset pneumonia (<5 days) was 35%, and the incidence of late-onset VAP was reported to be as high as 65%.
Meduri et al. [24] found that 43% of patients with ARDS in their study had VAP using bilateral bronchoalveolar lavage. Similarly, Chastre et al. [25] obtained samples from the lower airways using bronchoalveolar lavage and protected specimen brush on critically ill patients with clinical evidence of VAP. The occurrence of VAP was significantly higher among patients with ARDS (55%) than among patients without ARDS (28%). These data suggest that ARDS may be a risk factor that predisposes ill persons to VAP, as suggested by other investigators [26]. However, the prolonged duration of mechanical ventilation for patients with ARDS may be more important in predisposing them to VAP than ARDS itself [25, 27].
We investigated a cohort of patients fulfilling ARDS criteria with diagnostic tools for nosocomial pneumonia within the first 24 h of the diagnosis. Overall, 12 (22%) of 55 patients were clinically suspected of having nosocomial pneumonia on the first day after receiving a diagnosis for ARDS. Infection could be microbiologically confirmed in 7 (58%) of them. Thus, the microbiologically confirmed pneumonia rate within 24 h of each patient's first diagnosis of ARDS was 13%. All 7 patients with microbiologically confirmed nosocomial pneumonia had been admitted to the hospital at least 6 days before receiving a diagnosis (range, 6–43 days) and had been mechanically ventilated for >48 h [28].
Helpful information regarding the differentiation between the etiologies of bilateral pulmonary infiltrates (ARDS vs. pneumonia) may come from the interpretation of triggering receptor expressed on myeloid cells [29, 30]. The investigators used soluble triggering receptor expressed on myeloid cells in samples of bronchoalveolar lavage fluid as a marker of pneumonia in patients receiving mechanical ventilation [29]. In mechanically ventilated patients with VAP, the detection of soluble triggering receptor expressed on myeloid cells type 1 was a much more accurate diagnostic tool than any clinical finding. It was the strongest independent factor, predicting pneumonia (OR, 41.5) according to a logistic regression analysis with a sensitivity of 98% and a specificity of 90%. The control group included a large number of patients with ARDS (31 [48%] of 64 patients), and the differential power of soluble triggering receptor expressed on myeloid cells should be tested under this specific hypothesis. However, because soluble triggering receptor expressed on myeloid cells has been shown to be elevated in newly admitted, critically ill patients with suspected sepsis, this will exclude a large patient group with extrapulmonary pathogenesis of ARDS caused by sepsis [31].
The Involvement of Specific Pathogens on the Development of Ards
The study of the role of specific pathogens in inducing ARDS is complex, because known risk factors for ARDS (e.g., sepsis, shock, trauma, and/or gastric aspiration) would all have to be balanced. Relevant data mainly derive from small case series and case reports. These investigations may be biased toward reporting more-severe cases, leaving milder cases unrecognized. For this reason, table 2 may represent a spectrum of more-severe illnesses caused by known bacterial, viral, and parasite infections with preceding pneumonia or pulmonary involvement.
Table 2.
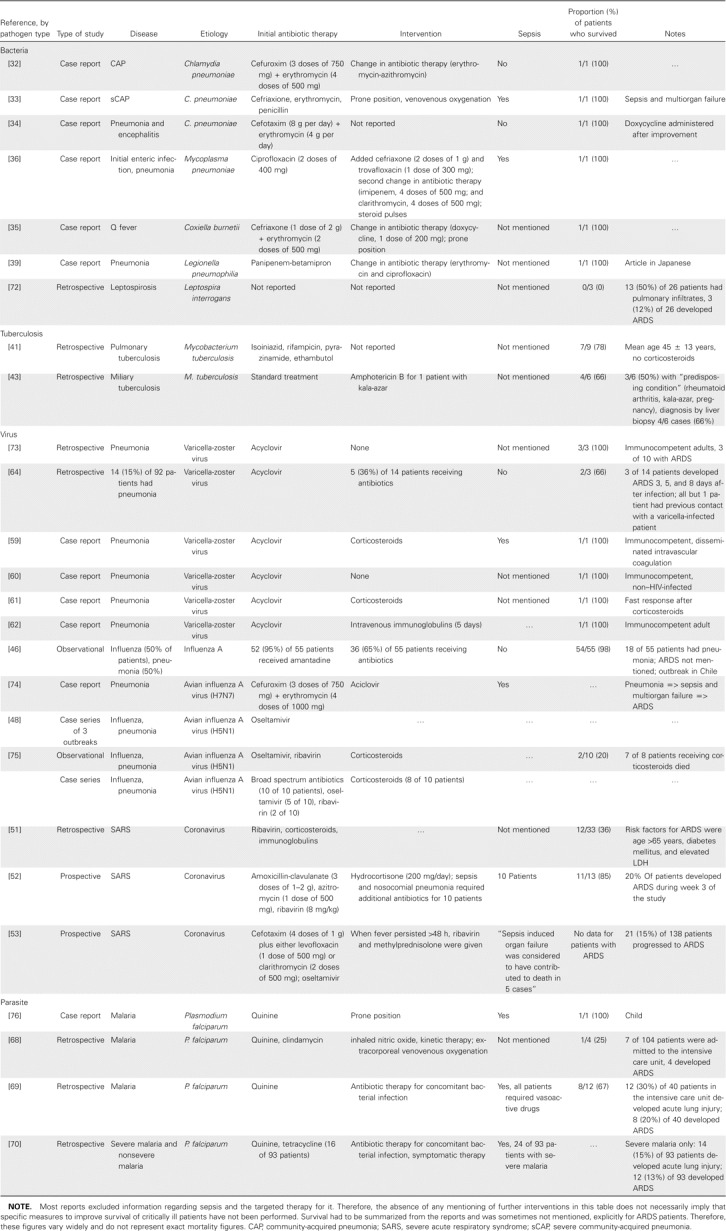
Reports of pneumonia and acute respiratory distress syndrome (ARDS), by type of pathogen.
Bacteria
The literature is obviously biased toward case reports and seems to be restricted to a group of bacteria previously referred to as “atypical” [32–36]. This is most likely because, in cases with known risk etiologies for severe pneumonias, such as Streptococcus pneumoniae and/or Pseudomonas aeruginosa infections [37], the sequence pneumonia => sepsis => ARDS is quite obvious and is not considered to be noteworthy. Markowicz et al. [23] compared 134 patients with ARDS with 744 patients without ARDS and found that nonfermenting, gram-negative bacteria caused significantly more cases of pneumonia among patients with ARDS. Mortality rates were comparable between the 2 groups, but the incidence of pneumonia increased with time on mechanical ventilation. In cases of pneumonia due to P. aeruginosa, specific cytotoxic mediators may explain the high rate of lung injury during infection [38].
The definite diagnoses of infections with Mycoplasma, Chlamydia, and Legionella species requires more effort, and infection may be undiscovered for some time, enhancing the severity and the probability of sepsis and/or ARDS. All except 1 patient [39] received an initial empiric antimicrobial treatment that cannot be considered fully ineffective against the pathogen. Routine investigation failed to identify a pathogen, and the etiology was suspected or proven later, during the course of the disease. Therefore, the available data should not be interpreted as evidence for a specific role of these pathogens in inducing lung injury.
Tuberculosis is not a common primary cause of respiratory failure requiring mechanical ventilation; therefore, it is also not instantly associated with ARDS [40]. Agarwal et al. [41] reviewed all patients (187) with ARDS and found severe pneumonia (in 65 [35%]) and sepsis (in 62 [33%]) to be the most relevant underlying illnesses. They provide information for 9 patients (5%) with ARDS and tuberculosis. All patients were mechanically ventilated, and Ziehl-Neelsen staining did not reveal acid-fast bacilli in any of the patients. Fiberoptic bronchoscopy and transbronchial lung biopsy were performed for 7 (78%) of 9 patients, and histopathological examination was used for all patients. The mortality rate (2 [22%] of 9 patients) was remarkably low, compared with those in previous reports of patients with pulmonary tuberculosis requiring mechanical ventilation (27 [66%] of 41 patients) [42] or patients with miliary tuberculosis and ARDS (2 [33%] of 6 patients) [43]. Sharma et al. [44] found a prolonged duration of illness, miliary tuberculosis, absolute lymphocytopenia, and an elevated liver enzyme level to be independent predictors for the development of ARDS. However, they reviewed a cohort of 2733 patients and reported 29 patients with ARDS (1%), confirming the low prevalence of severe lung injury in patients with tuberculosis.
Viruses
The proportion of viral etiologies in CAP has been recently investigated among 338 hospitalized patients [45]. The prevalence of viral pneumonia was 9% (31 of 338 persons), and the prevalence of mixed viral and/or bacterial pneumonia was 18% (61 of 338 persons). Influenza A was by far the most common viral etiology, and the annual prevalence showed a seasonal pattern. It seemed that persons with mixed infections were at increased risk to progress to sepsis or septic shock; however, data on ARDS were not provided in this study. Also, in their observational study, Rabagliati et al. [46] did not provide this information for their cohort of 55 hospitalized patients with influenza, but stated that 18 (33%) of 55 patients had pneumonia and that only 1 patient died. Surprisingly, no additional information from observational trials regarding the issue of Influenza A and ARDS in the immunocompetent host is available. However, it may be assumed that the progression of Influenza A infection from severe CAP to sepsis and/or septic shock to ARDS is a rare event, in contrast to the currently evaluated human cases of avian influenza virus infection.
In the clinical description of 10 cases of H5N1 infection in Vietnam, ARDS is not explicitly mentioned, but severe respiratory failure was present in 9 of 10 cases, bilateral pulmonary infiltrates “occurred,” and mortality was 80%, indicating that the criteria for ARDS may have been fulfilled in a high percentage of patients [47]. At least descriptive information regarding the prevalence of ARDS among human infections with H5N1 can be derived from the Thai pediatric case series. The pulmonary infiltration in the observed children “occasionally progressed, with subsequent deterioration to a final common pattern of acute respiratory distress syndrome” [48, p. 793], and almost all patients with ARDS died. Because of the paramount interest, virtually no confirmed cases remained unpublished; therefore, it must be assumed that the progression to ARDS is common among human avian influenza virus infections. The clinical picture of H5N1 infection has been recently reviewed, and it was reported that the average levels of plasma IFN-α among patients with avian influenza A who died were ∼3 times as high as those among healthy controls [49]. It was speculated that such responses may be responsible in part for the sepsis syndrome, ARDS, and multiorgan failure observed in many patients with H5N1 infection.
Studies of infection due to coronavirus and severe acute respiratory syndrome (SARS) have improved our understanding of viral infections and severe respiratory disease. Whereas SARS is a qualitative term that does not define the severity of lung injury, ARDS is a quantitative term [50]. Hence, coronavirus infection can provoke SARS that is severe enough to be called ARDS, but SARS is not always characterized by coronavirus infection. Consequently, Chen et al. [51] identified ARDS in only 33 (49%) of 67 patients with SARS. They described age >65 years (OR, 10.6), diabetes mellitus (OR, 13.7), and lactate dehydrogenase (OR, 8.4) as being independent predictors of ARDS. The overall mortality was 31% (21 of 67 patients), and 21 (64%) of 33 patients developed ARDS. Peiris et al. [52] found lower figures; 15 (20%) of 75 patients progressed to ARDS during the 3-week follow-up period. They also identified age and chronic hepatitis B virus infection treated with lamuvidine as being significant risk factors, but did not mention LDH. The mortality reported in this study was surprisingly low (5 [ 5%] of 75 patients); after subtracting the 2 patients who died from myocardial infarction, only 2 (15%) of 13 patients died from sepsis and ARDS [52]. Reports from several other series have suggested that a substantial number of patients develop respiratory failure and ARDS, with 17%–30% of patients requiring admission to an intensive care unit [53, 54] and a 21-day mortality of 3.6% [55].
Varicella infection (chickenpox) is a common contagious infection caused by varicella-zoster virus that has a benign outcome in children. Pneumonia is the most frequent complication of varicella infections in healthy adults [56, 57] and the leading cause of death among vaccine-preventable diseases [58]. The case reports describe young males with bilateral infiltrates with a rapid progression to ARDS [59–62]. The anti-infective treatment invariably contains acyclovir, and in some cases, the treatment contains corticosteroid and/or immunoglobulins. One review found that 6 of 15 patients had life-threatening varicella pneumonia treated with corticosteroids [63]. These patients had significantly shorter hospital and intensive care unit stays (10 and 8 days, respectively), and no patient died. A newer case series of 14 patients with severe varicella pneumonia established the diagnosis of ARDS in 3 of 14 patients, and 1 patient died. All but 1 patient in this series had previous contact with a varicella-infected patient and no varicella infection during childhood [64]. Varicella infections go along with the characteristic rash; therefore, the diagnosis and treatment are almost always established immediately, and bilateral infiltrates seem to be common.
Parasites
Parasitic infection with pulmonary involvement in immunocompetent patients may be regarded as a rare disease, depending on the geographical location [65]. It has to be considered regularly in acute eosinophilic pneumonia, and a list of pathogens can be derived from the literature [66, 67]. Malaria due to infection with Plasmodium falciparum is, however, noted remarkably often in the literature as being associated with ARDS. Losert et al. [68] reviewed 104 patients admitted to the hospital with malaria, of whom 66% had P. falciparum infections, and 7 of these were admitted to the intensive care unit. Four patients underwent intubation and mechanical ventilation and developed ARDS, and 3 patients died. In another study [69], only intensive care unit patients were considered, and 8 (20%) of 40 developed ARDS, with a mortality of 50%. Complications of malaria, such as coma, sepsis, or shock, were more prevalent among the group of patients with acute lung injury in this study and in the retrospective report by Bruneel et al. [70]. Therefore, we would like to support the hypothesis that ARDS is associated with the multiorgan failure that complicates the course of severe infection with P. falciparum, but the mechanisms seem to be independent from the causative agent [69, 70].
Conclusions
To date, sepsis seems to be the principal link between pneumonia and ARDS. However, prospective observational data on this supposed sequence are not available. The prevalence of pneumonia during the course of ARDS seems to be particularly high, but whether patients with ARDS are more susceptible to pneumonia or simply have more risk factors remains unknown because of limitations in the methodology of the diagnosis of VAP and ARDS. Recent research suggests that host factors have a major bearing on the development of ARDS. Accordingly, the individual role of specific pathogens in the development of ARDS is difficult to assess. Most recently, new respiratory viruses have received particular attention, and this review suggests that infections with coronavirus and avian influenza virus are associated with an exceptionally high incidence of lung injury and ARDS.
Acknowledgments
Financial support. Ruhr-University Bochum (grant F 397-03 to T.T.B.), German Federal Ministry of Education and Research (grant 01KI0103-105 to T.T.B. and S.E.), and the Competence Network CAPNETZ.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Bernard MS, Artigas A, Brigham KL, et al. Report on the American-European Consensus Conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Pelosi P, Caironi P, Gattinoni L. Pulmonary and extrapulmonary forms of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2001;22:259–68. doi: 10.1055/s-2001-15783. [DOI] [PubMed] [Google Scholar]
- 3.Esteban A, Fernandez-Segoviano P, Frutos-Vivar F, et al. Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med. 2004;141:440–5. doi: 10.7326/0003-4819-141-6-200409210-00009. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–27. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–66. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 6.Sloane PJ, Gee MH, Gotlieb JE. A multicenter registry of patients with acute respiratory distress syndrome. Am Rev Respir Dis. 1992;146:419–26. doi: 10.1164/ajrccm/146.2.419. [DOI] [PubMed] [Google Scholar]
- 7.Torres A, Aznar R, Gatell JM, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–8. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- 8.Celis R, Torres A, Gatell JM, Almela M, Rodriguez-Roisin R. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1988;93:318–24. doi: 10.1378/chest.93.2.318. [DOI] [PubMed] [Google Scholar]
- 9.Kollef MH. Ventilator-associated pneumonia: a multivariate analysis. JAMA. 1993;270:1965–70. [PubMed] [Google Scholar]
- 10.American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 11.Mandell LA, Bartlett JG, Dowell SF, File TM, Jr, Musher DM, Whitney C. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37:1405–33. doi: 10.1086/380488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450–6. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Valta P, Uusaro A, Nunes S, Ruokonen E, Takala J. Acute respiratory distress syndrome: frequency, clinical course, and costs of care. Crit Care Med. 1999;27:2367–74. doi: 10.1097/00003246-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Luhr O, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. Am J Respir Crit Care Med. 1999;159:1849–61. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 15.Esteban A, Anzueto A, Alia I, et al. How is mechanical ventilation employed in the intensive care unit?. An international utilization review. Am J Respir Crit Care Med. 2000;161:1450–8. doi: 10.1164/ajrccm.161.5.9902018. [DOI] [PubMed] [Google Scholar]
- 16.Roupie E, Lepage E, Wysocki M, et al. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. SRLF Collaborative Group on Mechanical Ventilation. Societe de Reanimation de Langue Francaise. Intensive Care Med. 1999;25:920–9. doi: 10.1007/s001340050983. [DOI] [PubMed] [Google Scholar]
- 17.Waterer GW, Quasney MW, Cantor RM, Wunderink RG. Septic shock and respiratory failure in community-acquired pneumonia have different TNF polymorphism associations. Am J Respir Crit Care Med. 2001;163:1599–604. doi: 10.1164/ajrccm.163.7.2011088. [DOI] [PubMed] [Google Scholar]
- 18.Quasney MW, Waterer GW, Dahmer MK, et al. Intracellular adhesion molecule Gly241Arg polymorphism has no impact on ARDS or septic shock in community-acquired pneumonia. Chest. 2002;121:85–86. doi: 10.1378/chest.121.3_suppl.85s. [DOI] [PubMed] [Google Scholar]
- 19.Torres A, Fàbregas N, Ewig S, Puig de la Bellacasa J, Bauer TT, Ramirez J. Sampling methods for ventilator-associated pneumonia: validation using different histologic and microbiologic references. Crit Care Med. 2000;28:2799–804. doi: 10.1097/00003246-200008000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Ewig S, Torres A. Approaches to suspected ventilator-associated pneumonia: relying on our own bias. Intensive Care Med. 2001;27:625–8. doi: 10.1007/s001340100881. [DOI] [PubMed] [Google Scholar]
- 21.Iregui MG, Kollef MH. Ventilator-associated pneumonia complicating the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2001;22:317–26. doi: 10.1055/s-2001-15788. [DOI] [PubMed] [Google Scholar]
- 22.Delclaux C, Roupie E, Blot F, Brochard L, Lemaire F, Brun-Buisson C. Lower respiratory tract colonization and infection during severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1092–8. doi: 10.1164/ajrccm.156.4.9701065. [DOI] [PubMed] [Google Scholar]
- 23.Markowicz P, Wolff M, Djedaini K, et al. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome: incidence, prognosis, and risk factors. Am J Respir Crit Care Med. 2000;161:1942–8. doi: 10.1164/ajrccm.161.6.9909122. [DOI] [PubMed] [Google Scholar]
- 24.Meduri GU, Reddy RC, Stanley T, El-Zeky F. Pneumonia in acute respiratory distress syndrome: a prospective evaluation of bilateral bronchoscopic sampling. Am J Respir Crit Care Med. 1998;158:870–5. doi: 10.1164/ajrccm.158.3.9706112. [DOI] [PubMed] [Google Scholar]
- 25.Chastre J, Trouillet JL, Vuagnat A, et al. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157:1165–72. doi: 10.1164/ajrccm.157.4.9708057. [DOI] [PubMed] [Google Scholar]
- 26.Meduri GU. Clinical review: a paradigm shift: the bidirectional effect of inflammation on bacterial growth. Clinical implications for patients with acute respiratory distress syndrome. Crit Care. 2002;6:24–9. doi: 10.1186/cc1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer TT, Torres A. Acute respiratory distress syndrome and nosocomial pneumonia. Thorax. 1999;54:1036–40. doi: 10.1136/thx.54.11.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer TT, Valencia M, Badia JR, et al. Respiratory microbiology patterns within the first 24 h of ARDS diagnosis: influence on outcome. Chest. 2005;128:273–9. doi: 10.1378/chest.128.1.273. [DOI] [PubMed] [Google Scholar]
- 29.Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–8. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 30.Richeldi L, Mariani M, Losi M, et al. Triggering receptor expressed on myeloid cells: role in the diagnosis of lung infections. Eur Respir J. 2004;24:247–50. doi: 10.1183/09031936.04.00014204. [DOI] [PubMed] [Google Scholar]
- 31.Gibot S, Kolopp-Sarda MN, Bene MC, et al. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004;141:9–15. doi: 10.7326/0003-4819-141-1-200407060-00009. [DOI] [PubMed] [Google Scholar]
- 32.Balis E, Boufas A, Iliopoulos I, Legakis NJ, Zerva L. Severe community-acquired pneumonia with acute hypoxemic respiratory failure due to primary infection with Chlamydia pneumoniae in a previously healthy adult. Clin Infect Dis. 2003;36:e155–7. doi: 10.1086/375063. [DOI] [PubMed] [Google Scholar]
- 33.Marik PE, Iglesias J. Severe community-acquired pneumonia, shock, and multiorgan dysfunction syndrome caused by Chlamydia pneumoniae. J Intern Med. 1997;241:441–4. doi: 10.1046/j.1365-2796.1997.119128000.x. [DOI] [PubMed] [Google Scholar]
- 34.Panagou P, Tsipra S, Bouros D. Adult respiratory distress syndrome due to Chlamydia pneumoniae in a young adult. Respir Med. 1996;90:311–3. doi: 10.1016/s0954-6111(96)90103-1. [DOI] [PubMed] [Google Scholar]
- 35.Oddo M, Jolidon RM, Peter O, Poli S, Cometta A. Q fever pneumonia complicated by acute respiratory distress syndrome. Intensive Care Med. 2001;27:615. doi: 10.1007/s001340000845. [DOI] [PubMed] [Google Scholar]
- 36.Radisic M, Torn A, Gutierrez P, Defranchi HA, Pardo P. Severe acute lung injury caused by Mycoplasma pneumoniae: potential role for steroid pulses in treatment. Clin Infect Dis. 2000;31:1507–11. doi: 10.1086/317498. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz M, Ewig S, Torres A, et al. Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160:923–9. doi: 10.1164/ajrccm.160.3.9901107. [DOI] [PubMed] [Google Scholar]
- 38.Pankhaniya RR, Tamura M, Allmond LR, et al. Pseudomonas aeruginosa causes acute lung injury via the catalytic activity of the patatin-like phospholipase domain of ExoU. Crit Care Med. 2004;32:2293–9. doi: 10.1097/01.ccm.0000145588.79063.07. [DOI] [PubMed] [Google Scholar]
- 39.Oguma A, Kojima T, Himeji D, Arinobu Y, Kikuchi I, Ueda A. A case of Legionella pneumonia complicated with acute respiratory distress syndrome treated with methylprednisolone and sivelestat sodium in combination with intravenous erythromycin and ciprofloxacin] Nihon Kokyuki Gakkai Zasshi. 2004;42:956–60. [PubMed] [Google Scholar]
- 40.Penner C, Roberts D, Kunimoto D, Manfreda J, Long R. Tuberculosis as a primary cause of respiratory failure requiring mechanical ventilation. Am J Respir Crit Care Med. 1995;151:867–72. doi: 10.1164/ajrccm/151.3_Pt_1.867. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Experience with ARDS caused by tuberculosis in a respiratory intensive care unit. Intensive Care Med. 2005;31:1284–7. doi: 10.1007/s00134-005-2721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee PL, Jerng JS, Chang YL, et al. Patient mortality of active pulmonary tuberculosis requiring mechanical ventilation. Eur Respir J. 2003;22:141–7. doi: 10.1183/09031936.03.00038703. [DOI] [PubMed] [Google Scholar]
- 43.Mohan A, Sharma SK, Pande JN. Acute respiratory distress syndrome (ARDS) in miliary tuberculosis: a twelve year experience. Indian J Chest Dis Allied Sci. 1996;38:157–62. [PubMed] [Google Scholar]
- 44.Sharma SK, Mohan A, Banga A, Saha PK, Guntupalli KK. Predictors of development of outcome in patients with acute respiratory distress syndrome due to tuberculosis. Int J Tuberc Lung Dis. 2005;10:429–35. [PubMed] [Google Scholar]
- 45.de Roux A, Marcos MA, Garcia E, et al. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125:1343–51. doi: 10.1378/chest.125.4.1343. [DOI] [PubMed] [Google Scholar]
- 46.Rabagliati R, Benitez R, Fernandez A, et al. Reconocimiento de influenza-A como etiología de síndrome febril e insuficiencia respiratoria en adultos hospitalizados durante brote en la comunidad [in Spanish] Rev Med Chil. 2004;132:317–24. [PubMed] [Google Scholar]
- 47.Hien TT, Liem NT, Dung NT, et al. Avian Influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–88. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 48.Grose C, Chokephaibulkit K. Avian influenza virus infection of children in Vietnam and Thailand. Pediatr Infect Dis J. 2004;23:793–4. doi: 10.1097/00006454-200408000-00024. [DOI] [PubMed] [Google Scholar]
- 49.The Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 50.Jih TK. Acute respiratory distress syndrome (ARDS) and severe acute respiratory syndrome (SARS): are we speaking different languages? J Chin Med Assoc. 2005;68:1–3. doi: 10.1016/S1726-4901(09)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CY, Lee CH, Liu CY, Wang JH, Wang LM, Perng RP. Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J Chin Med Assoc. 2005;68:4–10. doi: 10.1016/S1726-4901(09)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sung JJY, Wu A, Joynt GM, et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–20. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu LY, Lee CC, Green JA, et al. Severe acute respiratory syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerg Infect Dis. 2003;9:713–7. doi: 10.3201/eid0906.030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–9. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 56.Mohsen AH, McKendrick M. Varicella pneumonia in adults. Eur Respir J. 2003;21:886–91. doi: 10.1183/09031936.03.00103202. [DOI] [PubMed] [Google Scholar]
- 57.Mohsen AH, Peck RJ, Mason Z, Mattock L, McKendrick MW. Lung function tests and risk factors for pneumonia in adults with chickenpox. Thorax. 2001;56:796–9. doi: 10.1136/thorax.56.10.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention. Varicella-related deaths among adults—United States, 1997. MMWR Morb Mortal Wkly Rep. 1997;46:409–12. [PubMed] [Google Scholar]
- 59.Lee S, Ito N, Inagaki T, et al. Fulminant varicella infection complicated with acute respiratory distress syndrome, and disseminated intravascular coagulation in an immunocompetent young adult. Intern Med. 2004;43:1205–9. doi: 10.2169/internalmedicine.43.1205. [DOI] [PubMed] [Google Scholar]
- 60.Chou DW, Lee CH, Chen CW, Chang HY, Hsiue TR. Varicella pneumonia complicated by acute respiratory distress syndrome in an adult. J Formos Med Assoc. 1999;98:778–82. [PubMed] [Google Scholar]
- 61.Bautista R, Bravo-Rodriguez FA, Fuentes JF, Sancho RH. Glucocorticoids and immunoglobulins in an acute respiratory distress syndrome secondary to varicella pneumoniae. Med Clin. 2004;122:238. doi: 10.1016/s0025-7753(04)74207-4. [DOI] [PubMed] [Google Scholar]
- 62.Tokat O, Kelebek N, Turker G, Kahveci SF, Ozcan B. Intravenous immunoglobulin in adult varicella pneumonia complicated by acute respiratory distress syndrome. J Int Med Res. 2001;29:252–5. doi: 10.1177/147323000102900313. [DOI] [PubMed] [Google Scholar]
- 63.Mer M, Richards GA. Corticosteroids in life-threatening varicella pneumonia. Chest. 1998;114:426–31. doi: 10.1378/chest.114.2.426. [DOI] [PubMed] [Google Scholar]
- 64.Frangides CY, Pneumatikos I. Varicella-zoster virus pneumonia in adults: report of 14 cases and review of the literature. Eur J Intern Med. 2004;15:364–70. doi: 10.1016/j.ejim.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 65.Jindal SK, Aggarwal AN, Gupta D. Adult respiratory distress syndrome in the tropics. Clin Chest Med. 2002;23:445–55. doi: 10.1016/s0272-5231(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 66.Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA. 2004;292:2997–3005. doi: 10.1001/jama.292.24.2997. [DOI] [PubMed] [Google Scholar]
- 67.Pope-Harman AL, Davis WB, Allen ED, Christoforidis AJ, Allen JN. Acute eosinophilic pneumonia: a summary of 15 cases and review of the literature. Medicine. 1996;75:334–42. doi: 10.1097/00005792-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Losert H, Schmid K, Wilfing A, et al. Experiences with severe P. falciparum malaria in the intensive care unit. Intensive Care Med. 2000;26:195–201. doi: 10.1007/s001340050045. [DOI] [PubMed] [Google Scholar]
- 69.Gachot B, Wolff M, Nissack G, Veber B, Vachon F. Acute lung injury complicating imported Plasmodium falciparum malaria. Chest. 1995;108:746–9. doi: 10.1378/chest.108.3.746. [DOI] [PubMed] [Google Scholar]
- 70.Bruneel F, Hocqueloux L, Alberti C, et al. The clinical spectrum of severe imported falciparum malaria in the intensive care unit: report of 188 cases in adults. Am J Respir Crit Care Med. 2003;167:684–9. doi: 10.1164/rccm.200206-631OC. [DOI] [PubMed] [Google Scholar]
- 71.Johanson WG, Pierce AK, Sanford JP, Thomas GD. Nosocomial respiratory infection with Gram-negative bacilli: the significance of colonization of the respiratory tract. Ann Intern Med. 1972;77:701–6. doi: 10.7326/0003-4819-77-5-701. [DOI] [PubMed] [Google Scholar]
- 72.Martinez Garcia MA, de Diego DA, Menendez VR, Lopez Hontagas JL. Pulmonary involvement in leptospirosis. Eur J Clin Microbiol Infect Dis. 2000;19:471–4. doi: 10.1007/s100960000294. [DOI] [PubMed] [Google Scholar]
- 73.Martinez Segura JM, Gutierrez OA, Maravi PE, Jimenez U. Severe chickenpox pneumonia. Rev Clin Esp. 2003;203:591–4. doi: 10.1157/13053729. [DOI] [PubMed] [Google Scholar]
- 74.Fouchier RA, Schneeberger PM, Rozendaal FW, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004;101:1356–61. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ku AS, Chan LT. The first case of H5N1 avian influenza infection in a human with complications of adult respiratory distress syndrome and Reye's syndrome. J Paediatr Child Health. 1999;35:207–9. doi: 10.1046/j.1440-1754.1999.t01-1-00329.x. [DOI] [PubMed] [Google Scholar]
- 76.Flores JC, Imaz A, Lopez-Herce J, Serina C. Severe acute respiratory distress syndrome in a child with malaria: favorable response to prone positioning. Respir Care. 2004;49:282–5. [PubMed] [Google Scholar]


