Abstract
A randomized, double-blind, placebo-controlled clinical trial was conducted to evaluate the ability of Echinacea purpurea to prevent infection with rhinovirus type 39 (RV-39). Forty-eight previously healthy adults received echinacea or placebo, 2.5 mL 3 times per day, for 7 days before and 7 days after intranasal inoculation with RV-39. Symptoms were assessed to evaluate clinical illness. Viral culture and serologic studies were performed to evaluate the presence of rhinovirus infection. A total of 92% of echinacea recipients and 95% of placebo recipients were infected. Colds developed in 58% of echinacea recipients and 82% of placebo recipients (P = .114, by Fisher's exact test). Administration of echinacea before and after exposure to rhinovirus did not decrease the rate of infection; however, because of the small sample size, statistical hypothesis testing had relatively poor power to detect statistically significant differences in the frequency and severity of illness.
Colds are the most common acute infectious illnesses in humans. Prevention of the common cold with immunization is not practical because of the antigenic diversity of the many viruses causing colds. For example, rhinoviruses, which account for ∼40% of adult colds, have >100 antigenic serotypes. Viruses of different and distinct families, such as coronavirus, parainfluenza virus, respiratory syncytial virus, influenza virus, adenovirus, and metapneumovirus, also cause colds. Products derived from Echinacea purpurea, the purple coneflower, are among the most popular herbal remedies in the United States. It is estimated that Americans spend more than $300 million/year on these products [1], which are commonly self-administered for the prevention and treatment of the common cold. The 3 most commonly used species for medicinal purposes are E. purpurea, Echinacea pallida, and Echinacea angustifola. A number of studies, which used a variety of plant parts (such as root or above-ground components) from different species of echinacea, alone or combined with other herbs or echinacea species, have been conducted to evaluate the effects on prevention and treatment of naturally occurring colds [2–21]. These studies have also used various methodologies, end points, and definitions of colds.
The experimental rhinovirus model, in which the viral etiology of each participant's cold is known, reduces some of the variability and allows for more-exact measurement of effect on infection rates, in addition to measuring clinical illness rates and severity [22, 23]. Turner et al. [2] recently reported the results of a study in which volunteers were pretreated with echinacea or placebo for 14 days before challenge with rhinovirus type 23 (RV-23), with continuation of treatment for 5 days after virus challenge. In their induced-cold study, no significant differences were observed in the rate of rhinovirus infection or illness.
Among the most commonly used formulations of E. purpurea is the pressed juice of the above-ground parts of the herb (EchinaGuard; known as “Echinacin” in Germany; Madaus Aktiengesellschaft). This preparation has previously been studied by Grimm and Muller [3] for the prevention of natural colds. We conducted a double-blind, randomized, placebo-controlled study to evaluate the efficacy of this preparation of echinacea to reduce the rate of infection and illness in volunteers when administered for 7 days before and 7 days after challenge with rhinovirus type 39 (RV-39).
Subjects and Methods
Subjects. Forty-eight healthy adult volunteers aged 18–65 years with serum neutralizing antibody titers of ⩽1 : 2 to RV-39 were recruited. The study was approved by an independent institutional review board, and all volunteers gave written informed consent for participation. Individuals with conditions likely to affect susceptibility to colds or the severity or duration of cold symptoms were excluded from the study. Individuals who had received medication known to affect rhinorrhea, cough, or nasal congestion within 7 days (4 weeks for cromolyn sodium and long-acting antihistamines) before study initiation were excluded. Pregnant or breast-feeding women and participants who reported sensitivity to any of the ingredients in the study product were also excluded. Participants received financial compensation.
Treatments. One group of participants received a formulation containing the pressed juice of the above-ground plant parts of E. purpurea placed in a 22% alcohol base (EchinaGuard), and another group received a matching placebo. The active medication and placebo were identical in appearance, taste, and smell and were packaged in identical 100-mL bottles.
Experimental design. Participants were randomized to receive either echinacea or placebo, 2.5 mL 3 times per day (every ∼6–8 h) for 14 days. After 7 days, participants returned in the early morning (virus inoculation day 1 [V1]) for inoculation with RV-39 administered intranasally via pipette in 2 inocula provided ∼30 min apart (total dose, 0.25 mL per nostril), with the participant in a supine position. Each participant was asked not to blow his or her nose for 30 min after viral challenge. The virus originated from a clinical isolate and was kindly provided by A. M. Before use, the virus pool was tested for safety. The total virus inoculum was equivalent to ∼300 TCID50 per volunteer.
Beginning 24 h after virus inoculation (i.e., on V2) and continuing through V4, participants were isolated in individual hotel rooms for assessment. During their hotel stay, the participants continued treatment with echinacea or placebo as previously instructed. On V5–V7, participants completed treatment at home.
Identification of infection. Serological assessments of serum neutralizing antibody titer to RV-39 were made on V1 (before virus inoculation) and at V21–V25, as described elsewhere [24]. Specimens for viral culture were obtained by nasal lavage during the subject's hotel stay (V2–V4) to identify the presence of rhinovirus. Infection was defined as at least a 4-fold increase in RV-39 neutralizing antibody titer and/or recovery of rhinovirus on viral culture.
Clinical measurements of illness. The occurrence and severity of symptoms were recorded 3 times daily on a diary card beginning on V1 (the day of virus inoculation) and continuing through V7 using a 4-point severity rating scale (0, absent; 1, mild; 2, moderate; and 3, severe). The symptoms assessed were rhinorrhea, congestion, sneezing, cough, sore throat, headache, malaise, and chilliness. Thereafter, symptoms were assessed once per day until the completion of the study (V21–V25). The maximum scores of the 3 daily assessments for each of the 8 individual symptoms on V1–V5 after rhinovirus inoculation were added to give a 5-day total symptom score. Clinical illness—that is, the presence of a cold—was defined as a 5-day total symptom score of ⩾5 and 1 or both of the following: 3 successive days of rhinorrhea or a positive response to the query on whether the subject felt he or she had developed a cold since virus inoculation. This method of symptom scoring and of diagnosing illness is a modification of the methods of Jackson et al. [25] and Gwaltney et al. [26] described elsewhere.
Data analysis. Demographic parameters were tested for treatment group differences by Student's t test or χ2 analysis, as appropriate. Ninety-five percent CIs were constructed on the proportion of each treatment group who met the criteria for infection, as well as the difference in proportions between the 2 treatment groups. The 95% CIs for differences were based on the normal approximation to the binomial distribution. The treatment proportions were also compared using Fisher's exact test and χ2 analysis.
The study was designed as the first stage of a 2-stage adaptive design based on the methodology described by Bauer and Köhne [27]. The primary end point determined a priori was development of a cold, defined separately as laboratory infection and clinical illness. When ∼50 subjects had completed the study and clinical and virologic end points were known for each subject, an adaptive interim statistical analysis of the data was performed to determine a final sample size for the study and to redefine the primary efficacy parameter. The critical P level for rejection of the null hypothesis at completion of stage 1 was .0087. If the P value at stage 1 (P1) exceeded the critical level, the primary outcome criterion for stage 2 could be revised on the basis of the outcome showing the greatest sensitivity for resolving treatment differences in stage 1. The power of a χ2 test for stage 2 was to be computed on the basis of a sample size of 75 subjects per treatment group, the outcome rates as observed in stage 1, and an α level of 0.0087/P1. Otherwise, the study was to be terminated at stage 1.
When infection was used as the primary end point, the power for stage 2 was estimated to be 5%. When the clinical criterion of illness was used, the power for stage 2 was estimated to be 94%. In view of the primary importance assigned by the study sponsor to the infection criterion, the sponsor decided to terminate the study at stage 1. On the basis of the estimates of the 7-day area-under-the-curve values of the total symptom scores, which showed mean symptom scores of 9.34 for echinacea and 12.17 for placebo, and a pooled SD of 9.45, a sample size of 175 subjects per treatment group would have been required to provide 80% power to detect a statistically significant difference at the 5% level of significance.
Results
Participants. A total of 48 volunteers, 24 in each treatment group, were enrolled and randomized to receive a study drug. All participants completed the study. Two participants, both of whom were from the placebo group, were excluded from the efficacy analysis. One participant had an entry of moderate sneezing in her daily diary before virus inoculation, and 1 participant had a positive serum neutralizing antibody titer at inoculation. There were no significant differences between the echinacea and placebo groups with regard to sex (50% vs. 42% male subjects) or mean age (±SD) (33 ± 13 vs. 33 ± 12 years).
Assessment of infection. Fourfold or greater increases in serum neutralizing antibody titers to RV-39 occurred in 58% of echinacea recipients and in 55% of placebo recipients. Rhinovirus was recovered from 88% and 95% of the volunteers in the echinacea and placebo groups, respectively. The frequency of virus recovery was the same in both groups (82% of cultures were positive for rhinovirus). Overall, the proportion of participants who demonstrated laboratory evidence of infection was 92% for echinacea recipients (95% CI, 73–99) and 96% for placebo recipients (95% CI, 77–100).
Assessment of illness. Colds developed in 58% of the echinacea recipients (95% CI, 37–78) and 82% of the placebo recipients (95% CI, 60–94) (P = .114, by Fisher's exact test). The difference in rates was 24% (range, -2 to 49). The total 7-day symptom score (±SD) was 9.34 ± 9.43 for the recipients of echinacea and 12.17 ± 9.56 for placebo recipients. Similarly, daily symptom scores tended to be lower in echinacea recipients on days 2–7 after RV-39 inoculation than they were in placebo recipients, but the differences were not significant (figure 1). Individual symptom scores were not significantly different between treatment groups (table 1). Of those infected with RV-39, 59% of the echinacea recipients developed colds, compared with 86% of the placebo recipients (P = .0883, by Fisher's exact test).
Figure 1.

Mean total symptom score (+SD) by day after inoculation with rhinovirus type 39 (RV-39) in volunteers treated with either echinacea (black) or placebo (cross-hatched).
Table 1.
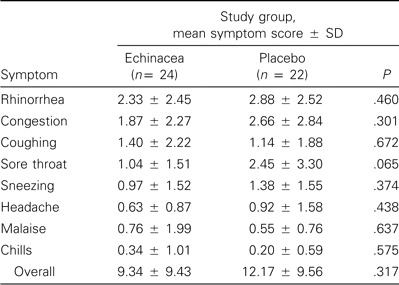
Seven-day individual and total symptom scores after challenge with rhinovirus type 39 in volunteers who received either echinacea or placebo.
Tolerance. Six participants (4 in the placebo group and 2 in the echinacea group) reported a total of 8 adverse events. There were no treatment-limiting adverse events. The 2 adverse events reported by subjects treated with echinacea were sleeplessness and severe oral aphthous ulcers, which resolved spontaneously while receiving treatment. Both events were evaluated to be not or improbably related to the study treatment.
Discussion
E. purpurea, which is one of the most commonly used herbal remedies in the United States, is often ingested to prevent or ameliorate the course of the common cold. In this controlled trial, we used a challenge model to study the effects of the pressed juice of the above-ground plant parts of E. purpurea, administered for 7 days before and for 7 days after RV-39 inoculation, on rhinovirus colds. The results of the study suggest that echinacea was not effective for preventing rhinovirus infection as defined by laboratory criteria. Among those who were infected and receiving echinacea, there was a trend toward reduction in the number of clinical colds, compared with those who were infected and received placebo (59% vs. 86%; P = .0883).
A number of studies that used varying study designs and a variety of plant parts, such as root or above-ground components from different species of echinacea alone or in combination with other herbs, have reported various effects—although mostly not significant—on the prevention of natural colds. Forth and Beuscher [4] studied the effects of a tablet and liquid product containing the roots of E. angustifolia and E. pallida with other extracts on the self-reported incidence and severity of natural colds. Tablet recipients had 38% fewer nasal symptoms than did placebo recipients, but other outcomes were similar [5]. Schmidt et al. [6] studied a preparation of E. angustifolia herb and root and other extracts that was administered to 646 college students for 8 weeks to prevent upper respiratory tract infection and flulike illness. They reported a 15% reduction in illness, which did not achieve statistical significance [5]. A 3-arm study conducted by Melchart et al. [7] compared 12-week regimens of extracts of the roots of E. purpurea and E. angustifolia with placebo for prevention of colds in 302 volunteers. They observed no significant differences in the rate of infection or time to first infection. Grimm and Muller [3] reported a trial of the pressed juice of the above-ground parts of E. purpurea ingested for 8 weeks to prevent natural colds. There were no significant differences in incidence, severity, or duration of colds between echinacea and matched placebo in 108 subjects. Schoneberger [28] had previously reported results from the same trial. In the only previously published study to evaluate echinacea in experimental colds, Turner et al. [2] administered echinacea for 14 days before and 5 days after challenge with RV-23 and observed no benefit.
Numerous studies have evaluated echinacea for the treatment of established colds [8–18]. Varying definitions of respiratory illness, end points, methods of data collection, and differences in the time of initiation of treatment in the course of illness make comparisons of studies difficult. Furthermore, preparations of echinacea used in various studies may have significantly different amounts of active ingredient [20, 21]. Clinical efficacy (although modest in some cases) has been reported in many of the treatment studies, suggesting echinacea is associated with a greater benefit for treating established colds than for preventing infection. A recently published controlled trial by Barrett et al. [18] reported no beneficial effect of a mixture of unrefined E. purpurea herb and root and E. angustifolia root in the treatment of natural colds. In our study, we observed trends toward reduction in the total symptom score (by 23%) and the frequency of illnesses meeting the definition of a cold (by 29%–31%) in echinacea recipients. One explanation for the trends toward improvement in clinical illness without an effect on the rate of infection is that these observations may have been the result of a beneficial effect of echinacea associated with the treatment of established infections rather than with prevention, because therapy was continued for 7 days after virus inoculation, at a time when subjects were symptomatic. Use of the experimental cold model may have allowed for early symptom assessment that was more accurate than that for trials involving natural colds [23].
Our study was unfortunately compromised by its small sample size. The results are consistent with most of the previously reported data with regard to the lack of efficacy of echinacea to prevent natural or experimental colds. Further investigation of echinacea for treatment of experimental rhinovirus infections, with a larger number of subjects and with specific standardized preparations of echinacea of known potency, should clarify the efficacy of echinacea in the treatment of colds.
Acknowledgments
We thank Dr. Gary B. Munk for technical expertise and Dr. Lisa A. Goldman for assistance in the preparation of the manuscript.
Footnotes
Financial support: Madaus Aktiengesellschaft.
References
- 1.Herbal Rx: the promises and pitfalls. Consumer Rep. 1999;64:44–8. [PubMed] [Google Scholar]
- 2.Turner RB, Riker DK, Gangemi JD. Ineffectiveness of echinacea for prevention of experimental rhinovirus colds. Antimicrob Agents Chemother. 2000;44:1708–9. doi: 10.1128/aac.44.6.1708-1709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm W, Muller HH. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am J Med. 1999;106:138–43. doi: 10.1016/s0002-9343(98)00406-9. [DOI] [PubMed] [Google Scholar]
- 4.Forth H, Beuscher N. Beeinflussing der Haufigkeit banaler Erkaltungsinfekte durch Esberitox. ZFA (Stuttgard) 1981;57:2272–5. [PubMed] [Google Scholar]
- 5.Barrett B, Vohmann M, Calabrese C. Echinacea for upper respiratory infection. J Fam Pract. 1999;48:628–35. [PubMed] [Google Scholar]
- 6.Schmidt U, Albrecht M, Schenk N. Pflanzliches Immunstimulans senkt Haufigkeit grippalen Infekte: Plazebokontrollierte Doppelblindstudie mit einem kombinierten Echinacea-Praparat mit 646 Studenten der Kolner Universitat. Nat Genzheits Med. 1990;3:277–81. [Google Scholar]
- 7.Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch Fam Med. 1998;7:541–5. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 8.Lindenmuth GF, Lindenmuth EB. The efficacy of echinacea compound herbal tea preparation on the severity and duration of upper respiratory and flu symptoms: a randomized, double-blind, placebo-controlled study. J Altern Complement Med. 2000;6:327–34. doi: 10.1089/10755530050120691. [DOI] [PubMed] [Google Scholar]
- 9.Schulten B, Bulitta M, Ballering-Bruhl B, Koster U, Schafer M. Efficacy of Echinacea purpurea in patients with a common cold: a placebo-controlled, randomized, double-blind clinical trial. Arzneimittelforschung. 2001;51:563–8. doi: 10.1055/s-0031-1300080. [DOI] [PubMed] [Google Scholar]
- 10.Brinkeborn RM, Shah DV, Degenring FH. Echinaforce and other echinacea fresh plant preparations in the treatment of the common cold: a randomized, placebo controlled, double-blind clinical trial. Phytomedicine. 1999;6:1–5. doi: 10.1016/S0944-7113(99)80027-0. [DOI] [PubMed] [Google Scholar]
- 11.Hoheisel O, Sandberg M, Bertram S, Bulitta M, Schafer M. Echinagard treatment shortens the course of the common cold: a double-blind, placebo-controlled clinical trial. Eur J Clin Res. 1997;9:261–8. [Google Scholar]
- 12.Braunig B, Dorn M, Limburg E, Knick E, et al. Bausendorf: Echinaceae purpureae radix: zur Starkung der korpereigenen Abwehr bei grippalen Infekten. Z Phytother. 1992;13:7–13. [Google Scholar]
- 13.Braunig B, Knick E. Therapeutische Erfahrungen mit Echinaceae pallidae bei grippalen Infekten. Naturheilpraxis. 1993;1:72–5. [Google Scholar]
- 14.Dorn M. Milerung grippaler Effeckte durch ein pflanzliches Immunstimulans. Nutur Ganzheitsmed. 1989;2:314–9. [Google Scholar]
- 15.Vorberg G, Schneider B. Pflanzliches Immunstimulans verkurzt grippalen Infeckt: Doppelblindstudie belegt die Steigerung der unspezifischen Infektabwehr. Arztliche Forschung. 1989;36:3–8. [Google Scholar]
- 16.Vorberg G. Bei Erkaltung unspezifische Immunabwehr stimulieran: Doppelblindstudie zeigt: Das bewahrte Phytotherapeutikum Esberitox verkurzt die Symptommatik. Arztliche Praxis. 1984;36:97–8. [Google Scholar]
- 17.Reitz HD. Immunmodulatoren mit pflanzlichen Wirkstoffen: eine wissenschaftliche Studie am Beispiel Esberitox N. Notabene Med. 1990;20:362–6. [Google Scholar]
- 18.Barrett BP, Brown RL, Locken K, Maberry R, Bobula JA, D'Alessio D. Treatment of the common cold with unrefined echinacea: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:939–46. doi: 10.7326/0003-4819-137-12-200212170-00006. [DOI] [PubMed] [Google Scholar]
- 19.Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2000;2:CD000530. doi: 10.1002/14651858.CD000530. [DOI] [PubMed] [Google Scholar]
- 20.Turner RB. Echinacea for the common cold: can alternative medicine be evidence-based medicine [editorial]? Ann Intern Med. 2002;137:1001–2. doi: 10.7326/0003-4819-137-12-200212170-00015. [DOI] [PubMed] [Google Scholar]
- 21.Gilroy CM, Steiner JF, Byers T, Shapiro H, Georgian W. Echinacea and truth in labeling. Arch Intern Med. 2003;163:699–704. doi: 10.1001/archinte.163.6.699. [DOI] [PubMed] [Google Scholar]
- 22.Gwaltney JM, Jr, Buier RM, Rogers JL. The influence of signal variation, bias, noise and effect size on statistical significance in treatment studies of the common cold. Antiviral Res. 1996;29:287–95. doi: 10.1016/0166-3542(95)00935-3. [DOI] [PubMed] [Google Scholar]
- 23.Gwaltney JM, Jr, Hendley JO, Patrie JT. Symptom severity patterns in experimental common colds and their usefulness in timing onset of illness in natural colds. Clin Infect Dis. 2003;36:714–23. doi: 10.1086/367844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monto AS, Cavallaro JJ. The Tecumseh study of respiratory illness. IV. Prevalence of rhinovirus serotypes, 1966–69. Am J Epidemiol. 1972;96:352–60. doi: 10.1093/oxfordjournals.aje.a121466. [DOI] [PubMed] [Google Scholar]
- 25.Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. Arch Intern Med. 1958;101:267–78. doi: 10.1001/archinte.1958.00260140099015. [DOI] [PubMed] [Google Scholar]
- 26.Gwaltney JM, Jr, Moskalski PB, Hendley JO. Interruption of experimental rhinovirus transmission. J Infect Dis. 1980;142:811–5. doi: 10.1093/infdis/142.6.811. [DOI] [PubMed] [Google Scholar]
- 27.Bauer P, Ksöhne K. Evaluations of experiments with adaptive interim analyses. Biometrics. 1994;50:1029–41. [PubMed] [Google Scholar]
- 28.Schoneberger D. The influence of immune-stimulating effects of pressed juice from Echinacea purpurea on the course and severity of colds. Forum Immunol. 1992;8:2–12. [Google Scholar]


