Abstract
Background. Pneumonia is recognized as a leading cause of morbidity in seniors. However, the overall burden of this disease—and, in particular, the contribution of ambulatory cases to that burden—is not well defined. To estimate rates of community-acquired pneumonia and to identify risk factors for this disease, we conducted a large, population-based cohort study of persons aged ⩾65 years that included both hospitalizations and outpatient visits for pneumonia.
Methods. The study population consisted of 46,237 seniors enrolled at Group Health Cooperative who were observed over a 3-year period. Pneumonia episodes presumptively identified by International Classification of Diseases, Ninth Revision, Clinical Modification codes assigned to medical encounters were validated by medical record review. Characteristics of participants were defined by administrative data sources.
Results. The overall rate of community-acquired pneumonia ranged from 18.2 cases per 1000 person-years among persons aged 65–69 years to 52.3 cases per 1000 person-years among those aged ⩾85 years. In this population, 59.3% of all pneumonia episodes were treated on an outpatient basis. In multivariate analysis, risk factors for community-acquired pneumonia included age, male sex, chronic obstructive pulmonary disease, asthma, diabetes mellitus, congestive heart failure, and smoking.
Conclusions. On the basis of these data, we estimate that roughly 915,900 cases of community-acquired pneumonia occur annually among seniors in the United States and that ∼1 of every 20 persons aged ⩾85 years will have a new episode of community-acquired pneumonia each year.
The risk of pneumonia increases markedly with age, and pneumonia is a leading cause of death among seniors [1, 2]. In the United States, an estimated 600,000 hospitalizations for community-acquired pneumonia (CAP) [3] and 59,000 deaths attributed to pneumonia and influenza occur among seniors every year [4]. Despite the importance of CAP in this population, information on the epidemiology of CAP among seniors in the United States is limited. In particular, because many prior studies of pneumonia have included only hospitalized patients, the increasingly important contribution of outpatient disease is not well defined. To better assess the burden of CAP in seniors and to identify risk factors for this disease, we conducted a population-based retrospective cohort study of >46,000 members of a health maintenance organization observed over a 3-year period. We identified and validated cases of pneumonia in hospitalized patients and outpatients and compared characteristics of persons with and those without pneumonia.
Methods
Study population and setting. The study population consisted of 46,237 members of Group Health Cooperative (GHC), a health maintenance organization in Washington State, who were aged ⩾65 years as of the study start date (1 March 1998), who had been enrolled for ⩾1 year prior to that date, and who were not residents of a nursing home. Cohort members were observed until the date of death, disenrollment from GHC, admission to a nursing home, or the study end date (28 February 2001), whichever occurred first.
Data sources. GHC maintains administrative databases that record immunizations, medication prescriptions, radiographic test reports, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)-coded diagnoses associated with outpatient visits and hospitalizations. Each member also has a paper medical record that included copies of hospital admission and discharge summaries and outpatient and emergency department visit notes.
Identification and validation of hospitalized and outpatient pneumonia episodes. Hospitalizations for pneumonia among the study population had been identified and validated as part of a prior study of pneumococcal vaccine effectiveness [5]. In that study, hospitalizations assigned a discharge diagnosis of pneumonia (ICD-9-CM codes 480–487.0) or streptococcal or pneumococcal bacteremia (ICD-9-CM codes 038.0, 038.2, 041.0, 041.2, and 320.1) were selected for chart review. Cases of nosocomial pneumonia, defined as cases in which pneumonia symptoms developed after hospitalization or in which the patient had been hospitalized in the previous 7 days, were excluded. A confirmed episode of CAP involving hospitalization was defined by an indication that, at the conclusion of their clinical evaluation, the treating physicians considered pneumonia to be the etiology of the presenting illness.
Episodes of pneumonia treated on an outpatient basis were presumptively defined by an outpatient or emergency department visit with a pneumonia ICD-9-CM code that was associated with both prescription of antibiotics and obtainment of a chest radiograph within 14 days after the visit. An outpatient episode of CAP was defined as confirmed if the chart review indicated that pneumonia was the most likely diagnosis attributed to the illness by the treating physician and if the patient had not been hospitalized in the prior 7 days.
Definition of baseline covariates. To assess potential risk factors for pneumonia among the study population, a series of baseline covariates was defined from GHC administrative data sources and the regional cancer registry. These variables include ischemic cardiac disease, congestive heart failure, chronic obstructive pulmonary disease (COPD), asthma without COPD, renal disease, stroke, dementia, lung cancer, serious nonlung cancer, other cancer, diabetes mellitus, receipt of prednisone, receipt of other immunosuppressive medications, pneumonia hospitalization in the year prior to cohort entry, use of home oxygen, and receipt of home health care. The full definitions of these variables are provided in table A1 in Appendix A.
Table A1.
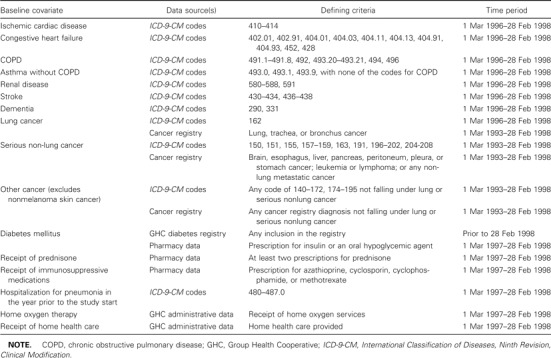
Definition of baseline covariates.
Smoking status was defined on the basis of data routinely collected at GHC during outpatient visits [5]. Information on smoking status was missing for 12% of study subjects. These subjects differed in several characteristics from persons with known smoking status. Alternate analyses were conducted that excluded persons who were missing smoking information, but because the results did not vary substantially from the results of analyses of the entire study population, the results presented include persons with and persons without smoking data.
Rates of pneumonia. Crude event rates were calculated by dividing the number of cases by the cumulative person-time in each age- and sex-specific stratum. Crude event rates were based on all events occurring during the study period and could include multiple events per person.
To examine seasonal variation in pneumonia rates, biweekly crude rates were calculated by dividing the number of cases occurring in 2-week intervals by the cumulative person-time in each interval. We plotted these rates over time and compared the pattern of variation during and outside of influenza seasons with the pattern of proportionate mortality from pneumonia and influenza in the United States, as defined by national vital statistics data [6]. Influenza seasons were defined on the basis of local and national surveillance data [7–10].
Statistical analysis. Differences in rates were assessed using the χ2 test. Multivariate Cox proportional hazards models [11] were used to evaluate the association of baseline covariates with the time to first of each outcome event during the study period. Additional models were fit to test for the presence of an interaction between age or sex and other covariates with the risk of pneumonia. The attributable risk, attributable risk percentage, population-attributable risk, and population-attributable risk percentage of CAP cases associated with smoking were all calculated by comparing the crude rates of CAP in current smokers with the rates of CAP in former smokers and those who have never smoked using standard formulas [12].
Results
Characteristics of the Study Subjects
The cohort consisted of 46,237 persons who contributed 122,605 person-years of observation during the study period. Overall, 42% of the subjects were male, 53% were aged 65–74 years at baseline, and 38% were aged 75–84 years at baseline (table 1).
Table 1.
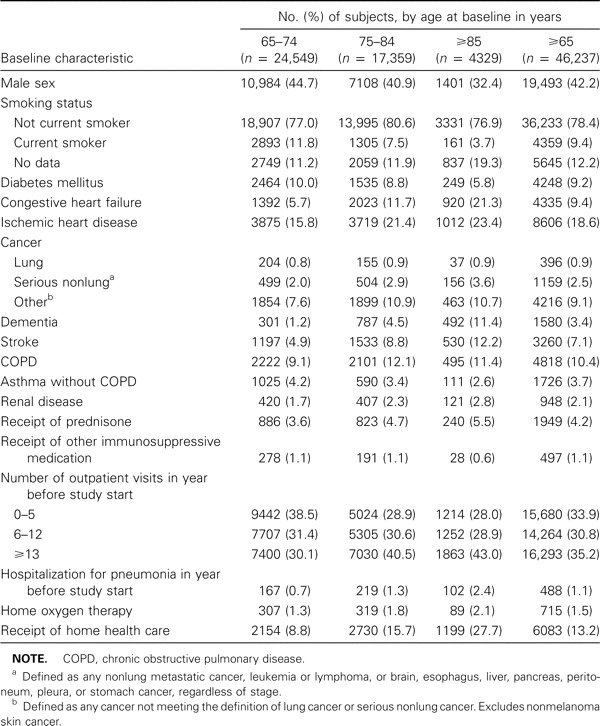
Baseline characteristics of study subjects, by age group
Validation of Pneumonia Outcomes
Hospitalizations for pneumonia. A total of 2486 hospitalizations associated with a pneumonia ICD-9-CM code among 2161 persons were identified. Information on 2371 (95%) of these events was available for review. Of these events, 45 were readmissions for pneumonia and 119 were episodes of nosocomially acquired pneumonia. Of the 2207 remaining hospitalizations, a clinical diagnosis of CAP was confirmed for 1411 (64%); 1264 cohort members accounted for these cases. An additional 2 confirmed hospitalizations for pneumonia were identified by chart review of hospitalizations associated with a bacteremia ICD-9-CM code. Thus, 1413 confirmed CAP-associated hospitalizations among 1266 persons were identified during the study period.
Outpatient visits for pneumonia. A total of 3153 episodes of presumptive outpatient pneumonia among 2736 persons were identified on the basis of administrative data, and records of 3124 (99%) of these events were available for review. Of these events, 86 episodes were excluded because a record of care for a respiratory illness was not verified, and 129 were excluded because the respiratory illness was associated with a hospitalization. Of the remaining 2909 events, a diagnosis of CAP was confirmed for 2063 (71%), which occurred among 1881 cohort members.
Rates of CAP
Rates of CAP and the percentage of cases resulting in hospitalization increased with age (table 2). The rate of pneumonia was consistently higher among men than among women, across all age groups and for both hospitalized patients and outpatients with pneumonia, although the difference was not always statistically significant. Overall, 59.3% of CAP episodes were treated on an outpatient basis. Among persons aged ⩾85 years, the rate of CAP was 52.3 events per 1000 person-years, which is roughly 1 episode of pneumonia for every 20 persons per year.
Table 2.
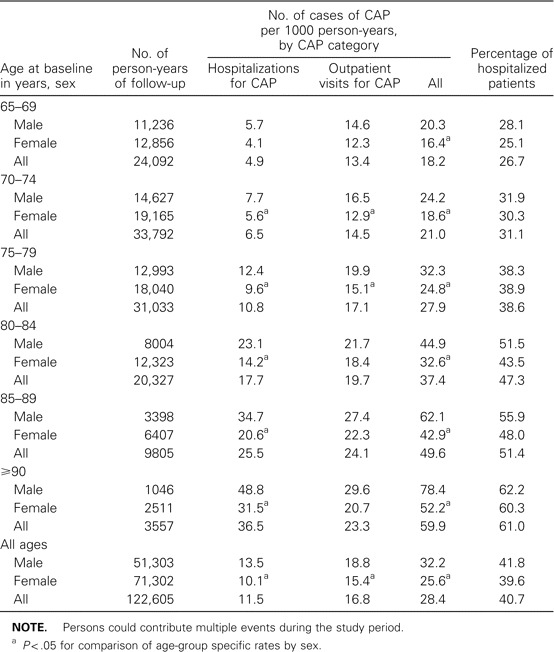
Rates of community-acquired pneumonia (CAP) and the proportion of patients hospitalized, by age group, during the study period.
Among persons hospitalized for CAP, 12.5% died within 30 days after hospital admission. Among outpatients with pneumonia, 0.4% died within 30 days after the first diagnosis. Overall, 3.6% of all the deaths in the cohort during the study period occurred within 30 days after a CAP diagnosis.
Seasonality
We found that the peak rates of all CAP (both hospitalizations and outpatient visits for pneumonia) coincided with periods of influenza viral circulation (figure 1). In addition, the seasonal pattern of CAP in our study population closely mirrored the temporal pattern of the percentage of deaths attributed to pneumonia and influenza nationally. Similar seasonal patterns were seen in analyses restricted to either outpatients or hospitalized patients, and the proportion of pneumonia episodes requiring hospitalization did not vary between influenza and noninfluenza periods, either for the cohort overall or by age group (data not shown).
Figure 1.
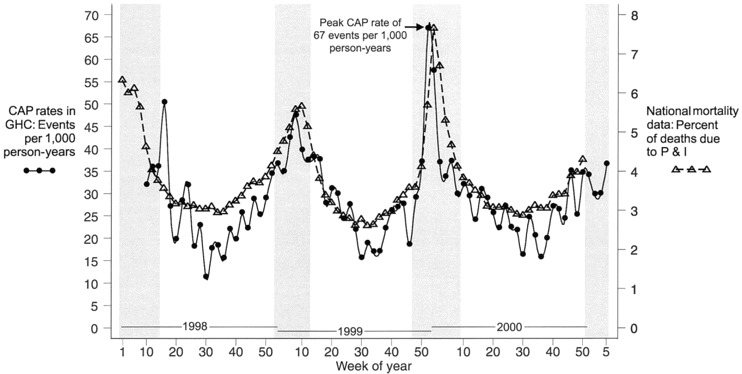
Biweekly rates of all cases of community-acquired pneumonia (CAP) among seniors in Group Health Cooperative (GHC), including hospitalizations and outpatient visits for CAP, during the study period. Periods of influenza virus circulation, as defined by Centers for Disease Control and Prevention surveillance data, are shaded [7–10]. For comparison, the biweekly proportion of all deaths in the United States attributed to pneumonia and influenza (P&I), as reported by national vital statistics data, is also presented [6].
Attributable Risks
The attributable risk for smoking among the study cohort was 12.3 CAP events per 1000 person-years, and the attributable risk percentage for smoking was 30.8%. That is, assuming there is a causal relationship between smoking and CAP, we estimate that ∼30% of episodes of pneumonia among smokers were due to smoking. The population-attributable risk for smoking (among the population of persons with smoking data) was 1.3 CAP events per 1000 person-years, and the population-attributable risk percentage for smoking was 4.4%. That is, among the population of persons with smoking data, ∼4% of pneumonia events were due to smoking. There was no significant difference in the attributable risks of smoking across age groups or between men and women.
Risk Factors for Pneumonia
In multivariate analysis, age, male sex, current smoking, diabetes mellitus, congestive heart failure, lung cancer, serious nonlung cancer, COPD, asthma without COPD, dementia, stroke, receipt of prednisone, use of home oxygen services, greater number of outpatient visits, and hospitalization for pneumonia in the year prior to the study start date were independently associated with risk of all CAP (table 3). In general, indicators of chronic illness at baseline were more strongly associated with risk of hospitalization for pneumonia than of outpatient visits for pneumonia. The variable most strongly associated with all CAP was COPD. There was no significant interaction between age or sex and other covariates on risk of pneumonia.
Table 3.
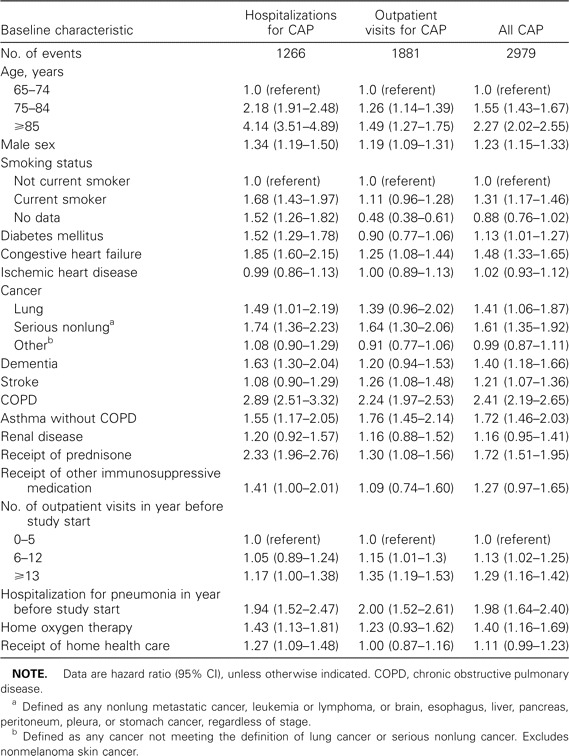
Results of multivariate Cox proportional analyses of time to first event for the outcomes of chart review-confirmed hospitalizations for community-acquired pneumonia (CAP), outpatient visits for CAP, and all CAP.
Discussion
To our knowledge, this is the first contemporary study to assess rates of both hospitalizations and outpatient visits for pneumonia among community-dwelling seniors in the United States, and it provides, to our knowledge, the only population-based assessment of risk factors for CAP among seniors in the United States. Our study builds on previous population-based studies of hospitalizations and outpatient visits for CAP conducted among communities in Finland [13, 14] and among the GHC population in the 1970s and early 1980s [15], each of which had substantially fewer seniors in their study populations. Extrapolating our age-specific rates to the population of seniors in the United States who were not in nursing homes, as reported in 2000 census data [16], we estimate a disease burden of 551,600 cases of CAP treated on an outpatient basis and 364,300 cases of CAP resulting in hospitalization, for a total of 915,900 cases of CAP. These rates also indicate that ∼1 of every 20 persons aged ⩾85 years will have a new episode of CAP each year.
Our rates of hospitalizations for CAP are consistent with those reported by Marston et al. [1] in a Centers for Disease Control and Prevention-sponsored prospective study of radiographically confirmed hospitalization for CAP patients in 2 counties in Ohio in 1991. After adjusting for differences in the age distribution of persons aged ⩾65 years in the 2 study populations, our age-adjusted hospitalization rate of 10.4 cases per 1000 person-years was nearly identical to the rate of 10.1 cases per 1000 person-years among community-dwelling seniors reported by Marston et al. [1]. The Ohio study did not, however, include an assessment of outpatient visits for CAP, which is particularly important, given the trend towards outpatient treatment that has occurred over the past 2 decades. We found that, even among persons aged ⩾80 years, nearly one-half (49.3%) of CAP events were treated on an outpatient basis. Inclusion of outpatient episodes allows a more comprehensive estimate of the burden of disease and a more accurate assessment of risk factors for CAP. Persons with chronic conditions who develop CAP are more likely to be hospitalized for treatment than are persons without chronic conditions. This means that assessments restricted to hospitalizations for CAP will likely overestimate the association of those conditions with the risk of CAP.
The availability of administrative data on the GHC population allowed us to define the presence of underlying conditions consistently across the study population. Because of this, we were able to assess the independent association of those characteristics with risk of pneumonia in multivariate analyses. As previously reported among adults, we found that COPD, immunosuppression, smoking, and congestive heart failure were all independently associated with disease risk among seniors [17–21]. In addition, we also found that diabetes, lung cancer, serious nonlung cancer, and previous hospitalizations for pneumonia were also risk factors for CAP among elderly persons.
In this cohort, rates of CAP were higher in men than in women, and male sex was a risk factor for CAP even after adjusting for age, smoking, and the presence of chronic medical conditions. A survey of hospitalizations associated with a pneumonia ICD-9-CM code reported in Medicare claims [3] found a significantly higher risk among men than among women, as did a follow-up assessment of the risk of a hospitalization associated with a pneumonia diagnosis among participants of the First National Health and Nutrition Examination Survey (NHANES I) [17]. Neither of these studies assessed whether male sex was associated with pneumonia after adjusting for other risk factors, so it was not clear in these studies whether male sex was simply a marker for a higher prevalence of other risk factors. A population-based study involving 4175 elderly persons in Finland found that crude CAP rates did not differ between men and women and that male sex was not significantly associated with CAP after accounting for age and certain chronic medical conditions and risk factors, including asthma, receipt of immunosuppressive therapy, and heart disease [21]. Because our study population was much larger than that of the Finnish study, we have more power to detect an independent risk of CAP associated with male sex if such a risk truly exists. However, it is possible that the difference in risk we observed could be the result of confounding by factors unmeasured in our population.
We found that current smokers were at an increased risk of hospitalization for CAP, which is consistent with previous reports. Previous case-control studies have shown that smoking is associated with an increased risk of pneumonia [19, 22]. One study has reported that, among adults who quit smoking, the excess risk of CAP appears to have decreased 5 years after quitting [22]. We estimate that, among elderly smokers, nearly one-third of pneumonia episodes can be attributed to smoking, suggesting that smoking cessation could potentially reduce the risk of CAP among smokers by an important degree.
Among our study population, the seasonal pattern of pneumonia closely followed the pattern of pneumonia and influenza mortality nationally. Correlations between rates of hospitalization for acute respiratory disease and patterns of influenza activity have been reported in adult populations in other geographic areas [23–25]. The 1999–2000 influenza season is recognized as having been more severe than the several preceding seasons [10], and timing of the peak rate of CAP for the elderly GHC population in this study period closely matched the peak proportional pneumonia and influenza mortality rates in the United States, occurring in late December 1999 and early January 2000. This correlation suggests that data from health management organizations (HMOs) on pneumonia-related hospitalizations and outpatient visits could be an additional method for identifying periods of influenza virus circulation or quantifying the severity of influenza seasons.
Rates of CAP in this population were high, especially among some subgroups of subjects, such as those aged ⩾85 years, and there was close correlation with influenza virus circulation. This is despite the fact that vaccination rates for both influenza and pneumococcal polysaccharide vaccines were high in this population. Annual influenza vaccination rates among the study population were >70%, and >75% of subjects received pneumococcal vaccine either prior to or during the study period, emphasizing the need for other measures for preventing pneumonia in seniors. The burden of illness could potentially be reduced by the availability of other vaccines, such as pneumococcal conjugate vaccines or vaccines effective against human metapneumovirus or respiratory syncytial virus infection. Alternatively, increased emphasis on other strategies to reduce the risk of influenza infection, such as vaccination of caregivers and household contacts, could potentially impact disease risk.
One limitation of this study is the possible underascertainment of pneumonia events, because our case finding was primarily restricted to medical encounters assigned a pneumonia ICD-9-CM code, and so would not capture pneumonia episodes associated exclusively with other diagnosis codes. For outpatient pneumonia, we would also have missed episodes in which a chest radiograph was not ordered. We cannot estimate the extent to which underascertainment may have influenced our results. However, this problem is likely to be more significant for pneumonia treated on an outpatient basis (in which a health care provider may only see a patient early in the course of the illness and assign a diagnosis before the evaluation has been completed) than in cases in the hospital setting (in which the discharge diagnoses are assigned at the end of the hospital stay and should represent a more complete assessment of the illness episode).
A second limitation is that the study population includes enrollees of a single health maintenance organization in a single geographic area, and the findings may not be generalizable to the US population as a whole. As noted above, however, our age-standardized rate of CAP-related hospitalizations was essentially identical to that observed in the study of CAP in Ohio [1]. This similarity, along with the close correspondence between the observed rates over time in our study and the national proportional rates of mortality due to pneumonia and influenza, suggest that our study population is not dissimilar from the elderly population of the United States as a whole.
Our method for detection of both hospitalizations and outpatient visits for CAP among a defined population of HMO enrollees has several advantages for conducting pneumonia surveillance. This type of surveillance can identify high-risk subgroups, can track trends in pneumonia incidence over time, and is suitable for both prospective and retrospective studies. Surveillance of this type would be particularly useful for studying the effect of new vaccines or the introduction of new pathogens, such as severe acute respiratory syndrome coronavirus, on rates of pneumonia.
Acknowledgments
Financial support. Vaccine Safety Datalink contract with the America's Health Insurance Plans, funded by the Centers for Disease Control and Prevention.
Potential conflicts of interest. K.M.N. has received grant support from Aventis-Pasteur. L.A.J. has received grant support from Aventis-Pasteur and has served as a consultant to Chiron Vaccines. All other authors: no conflicts.
References
- 1.Marston BJ, Plouffe JF, File TM, Jr, et al. Incidence of community-acquired pneumonia requiring hospitalization. results of a population-based active surveillance study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med. 1997;157:1709–18. [PubMed] [Google Scholar]
- 2.Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001;49:1–113. [PubMed] [Google Scholar]
- 3.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott Watson R, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165:766–72. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 4.Kochanek KD, Smith B. Deaths: preliminary data for 2002. National Vital Statistics Reports. 2004;52:1–48. [PubMed] [Google Scholar]
- 5.Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–55. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 6.Vital statistics mortality data, multiple cause detail, 1998–2000. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- 7.Centers for Disease Control and Prevention Update: influenza activity—United States and worldwide, 2000–01 season, and composition of the 2001–02 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2001;50:466–79. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Update: influenza activity—United States and worldwide, 1997–98 season, and composition of the 1998–99 influenza vaccine. MMWR Morb Mortal Wkly Rep. 1998;47:280–4. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Update: influenza activity—United States and worldwide, 1998–99 season, and composition of the 1999–2000 influenza vaccine. MMWR Morb Mortal Wkly Rep. 1999;48:374–8. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Update: influenza activity—United States and worldwide, 1999–2000 season, and composition of the 2000–01 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2000;49:375–81. [PubMed] [Google Scholar]
- 11.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–57. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 12.Koepsell T, Weiss N. Epidemiologic methods: studying the occurrence of illness. New York: The University of Chicago Press; 2003. [Google Scholar]
- 13.Jokinen C, Heiskanen L, Juvonen H, et al. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am J Epidemiol. 1993;137:977–88. doi: 10.1093/oxfordjournals.aje.a116770. [DOI] [PubMed] [Google Scholar]
- 14.Koivula I, Sten M, Leinonen M, Makela PH. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am J Med. 1997;103:281–90. doi: 10.1016/s0002-9343(97)00149-6. [DOI] [PubMed] [Google Scholar]
- 15.Foy HM, Cooney MK, Allan I, Kenny GE. Rates of pneumonia during influenza epidemics in Seattle, 1964 to 1975. JAMA. 1979;241:253–8. [PubMed] [Google Scholar]
- 16.Hetzel L, Smith A. Census 2000 briefs: the 65 years of age and older population: 2000. Washington, DC: US Census Bureau; 2001. [Google Scholar]
- 17.LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of US older people: the role of chronic conditions, health behaviors, and nutritional status. Public Health Rep. 1989;104:350–60. [PMC free article] [PubMed] [Google Scholar]
- 18.Almirall J, Bolibar I, Balanzo X, Gonzalez CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J. 1999;13:349–55. doi: 10.1183/09031936.99.13234999. [DOI] [PubMed] [Google Scholar]
- 19.Farr BM, Bartlett CL, Wadsworth J, Miller DL. Risk factors for community-acquired pneumonia diagnosed upon hospital admission. British Thoracic Society Pneumonia Study Group. Respir Med. 2000;94:954–63. doi: 10.1053/rmed.2000.0865. [DOI] [PubMed] [Google Scholar]
- 20.Farr BM, Woodhead MA, Macfarlane JT, et al. Risk factors for community-acquired pneumonia diagnosed by general practitioners in the community. Respir Med. 2000;94:422–7. doi: 10.1053/rmed.1999.0743. [DOI] [PubMed] [Google Scholar]
- 21.Koivula I, Sten M, Makela PH. Risk factors for pneumonia in the elderly. Am J Med. 1994;96:313–20. doi: 10.1016/0002-9343(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 22.Almirall J, Gonzalez CA, Balanzo X, Bolibar I. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest. 1999;116:375–9. doi: 10.1378/chest.116.2.375. [DOI] [PubMed] [Google Scholar]
- 23.Glezen WP. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- 24.Perrotta DM, Decker M, Glezen WP. Acute respiratory disease hospitalizations as a measure of impact of epidemic influenza. Am J Epidemiol. 1985;122:468–76. doi: 10.1093/oxfordjournals.aje.a114128. [DOI] [PubMed] [Google Scholar]
- 25.Neuzil KM, Reed GW, Mitchel EF, Jr, Griffin MR. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281:901–7. doi: 10.1001/jama.281.10.901. [DOI] [PubMed] [Google Scholar]


