Abstract
Background: Increased levels of monocytic angiotensin-converting enzyme (ACE) found in haemodialysis (HD) patients may directly participate in the pathogenesis of atherosclerosis. We demonstrated recently that uremia triggers the development of highly pro-atherogenic monocytes via an angiotensin II (AngII)–dependent mechanism. Opposing actions of the AngII-degrading ACE2 remain largely unknown. We examined the status of both ACEs and related receptors in circulating leukocytes of HD, not-dialyzed CKD and healthy individuals. Furthermore, we tested the possible impact of monocytic ACEs on atherogenesis and behaviour of the cells under conditions mimicking chronic renal failure.
Methods: Expression of ACE, ACE2, AT1R, AT2R and MASR was investigated on circulating leukocytes from 71 HD (62 ± 14 years), 24 CKD stage 3–5 (74 ± 10 years) patients and 37 healthy control subjects (53 ± 6 years) and isolated healthy monocytes treated with normal and uremic serum. Analyses of ACE, ACE2, ICAM-1, VCAM-1, MCSF and endothelial adhesion were tested on ACE-overexpressing THP-1 monocytes treated with captopril or losartan. ACE2-overexpressing monocytes were subjected to transmigration and adhesion assays and investigated for MCP-1, ICAM-1, VCAM-1, MCSF, AT1R and AT2R expression.
Results: The ACE mRNA level was significantly increased in HD and CKD stage 3–5 leukocytes. Correspondingly, ACE2 was downregulated and AngII as well as MAS receptor expression was upregulated in these cells. Healthy monocytes preconditioned with uremic serum reflected the same expressional regulation of ACE/ACE2, MAS and AngII receptors as those observed in HD and CKD stage 3–5 leukocytes. Overexpression of monocytic ACE dramatically decreased levels of ACE2 and induced a pro-atherogenic phenotype, partly reversed by AngII-modifying treatments, leading to an increase in ACE2. Overexpression of ACE2 in monocytes led to reduced endothelial adhesion, transmigration and downregulation of adhesion-related molecules.
Conclusions: HD and not-dialyzed CKD stage 3–5 patients show enhanced ACE and decreased ACE2 expression on monocytes. This constellation renders the cells endothelial adhesive and likely supports the development of atherosclerosis.
Keywords: ACE2, atherosclerosis, CKD, monocytes, THP-1, uremia
INTRODUCTION
Overactivation of the renin–angiotensin system (RAS) observed under pathophysiological conditions such as chronic kidney disease (CKD) is a common factor leading to cardiovascular complications. Frequently observed chronic inflammation in CKD patients on maintenance intermittent haemodialysis (HD) plays a crucial role in the development of progressive atherosclerosis and related cardiovascular events such as myocardial infarction and stroke [1–5]. Pharmacological blockade of RAS exerts beneficial effects in these patients and slows, but does not prevent, cardiorenal disease progression, suggesting the involvement of other, noncanonical RAS factors [6–8]. The actions of the classical RAS are mediated by angiotensin-converting enzyme (ACE), which generates angiotensin II (AngII), a potent vasoconstrictor and main RAS effector. Pro-inflammatory and pro-proliferative actions of AngII may be counteracted by the recently described ACE2, which degrades AngII to Ang1-7, a potent vasodilator [9, 10].
Both ACEs are abundantly expressed in human cardiac, pulmonary and renal tissues and other organs such as liver, placenta and the central nervous system [11–15]. ACE is also expressed on the surface of monocytes, macrophages and smooth muscle cells [16, 17]. Recently we demonstrated that elevated levels of monocytic ACE in HD patients are associated with increased mortality and cardiovascular morbidity and may also participate in the initial steps of atherosclerosis [5, 18, 19]. Our recent investigations revealed that uremic conditions upregulate the expression of monocytic ACE and drive the monocytes to pro-atherogenic differentiation via an AngII-dependent mechanism [20]. Mouse models demonstrated that ACE deficiency in bone marrow–derived cells decreased hypercholesterolemia-induced atherosclerosis, while macrophages from ACE2-deficient mice promoted atherosclerosis by increased expression and release of inflammatory cytokines and elevated adhesion of these cells to endothelial cells [17, 21]. Conversely adenovirus-mediated ACE2 overexpression in atherogenic animal models was able to decrease pro-atherosclerotic actions and improve endothelial homeostasis by decreasing monocyte adhesion [22, 23].
In this study, we investigated the expression patterns of both ACEs and related receptors in circulating leukocytes obtained from HD and CKD stage 3–5 (CKD3–5) patients and healthy controls (NP). Furthermore, we examined the regulation of ACE2 in primary human monocytes and THP-1 cells under uremic conditions.
MATERIALS AND METHODS
Leukocytic RNA, serum and isolation of primary human monocytes
Blood samples for RNA and serum isolation were obtained from chronic HD patients treated at the Dialysis Ward of the University Clinic Halle, CKD3–5 patients not on dialysis and healthy volunteers. All HD patients were treated thrice weekly (on average 12.7 ± 1.4 h, target Kt/V ≥1.3) by standard bicarbonate HD with ultrapure water (by reverse osmosis and sterile filters) using high-flux polynephron membranes (Nipro Europe). All HD blood samples were obtained prior to a regular HD session from the dialysis access. HD patients were 40–70 years of age, free of acute infectious complications and had no immunosuppressive medication. HD patients were divided into groups taking angiotensin receptor blockers (ARBs; n = 29), ACE inhibitors (ACEis; n = 16) and those without angiotensin-modifying medication (n = 26).
CKD3–5 patients were treated at the Department of Internal Medicine of the University Clinic Halle and classified according to GFR.
All NP had normal renal function as determined by serum creatinine and were not taking any medication. HD, CKD3–5 patients' and NP characteristics are presented in Table 1.
Table 1.
Biometric characteristics of patients in control (NP), HD and CKD3–5 groups
| Parameters | NP (n = 37) | HD (n = 71) | CKD3–5 (n = 24) |
|---|---|---|---|
| Age (years) | 53 ± 6 | 62 ± 14 | 74 ± 10 |
| Male/female (n/n) | 11/26 | 46/25 | 8/16 |
| Body mass index (kg/m2) | 24.35 ± 3.89 | 25.91 ± 4.82 | 28.56 ± 6.42 |
| Hypertension (%) | 0 | 93.24 | 91.67 |
| Diabetes (%) | 2.7 | 38.01 | 33.34 |
| Ever smoker (%) | 43.2 | 39.4 | 41.67 |
| Dialysis vintage HD (years) | 0 | 6.94 ± 4.73 | 0 |
| History of cardiovascular disease (%) | 0 | 36.49 | 66.67 |
| Creatinine (µmol/L) | 69.85 ± 12.71 | 775.17 ± 272.52 | 157.71 ± 67.28 |
| Urea (mmol/L) | 4.52 ± 1.32 | 21.57 ± 6.86 | 15.33 ± 17.48 |
| Albumin (g/dL) | 4.18 ± 0.35 | 3.88 ± 0.47 | N/A |
| C-reactive protein (mg/L) | 1.66 ± 1.28 | 12.77 ± 22.89 | 11.03 ± 14.59 |
| Leukocytes (G/L) | 6.15 ± 1.51 | 7.58 ± 3.36 | 6.71 ± 1.75 |
| Monocytes (% leukocytes) | 8.24 ± 2.39 | 8.50 ± 2.32 | N/A |
| HDL (mmol/L) | 1.76 ± 0.46 | 1.29 ± 0.60 | N/A |
| LDL (mmol/L) | 3.79 ± 0.81 | 2.45 ± 0.88 | N/A |
| Cholesterin (mmol/L) | 6.06 ± 0.91 | 4.86 ± 3.70 | N/A |
| Triglyceride (mmol/L) | 1.2 ± 1.00 | 2.29 ± 2.27 | N/A |
| 25(OH) vitamin D (%) | 0 | 87.8 | 25.00 |
| ACEi (%) | 0 | 22.53 | 33.34 |
| ARB (%) | 0 | 40.84 | 45.83 |
| β-blockers (%) | 0 | 77.27 | 79.17 |
| Statins (%) | 0 | 39.19 | 58.33 |
Blood samples for leukocytic RNA isolation were drawn with the VACUETTE Safety Blood Collection Set (Greiner Bio-One) into Tempus Blood RNA Tubes (Life Technologies). Total RNA was isolated with Tempus Spin RNA Isolation Kits according to the manufacturer's instructions (Life Technologies).
Monocytes for in vitro experiments were isolated from three healthy volunteers with the Pan Monocyte Isolation Kit (Miltenyi) and use of the AutoMacs cell separator (Miltenyi) according to the manufacturer's instructions. This study was approved by the ethical committee of the Martin Luther University, Faculty of Medicine. All patients and persons involved in this study gave written informed consent. The study was performed according to the rules of the Declaration of Helsinki.
Cell culture and treatments of primary human monocytes and THP-1 cells
Of note, 3 × 105 per well primary human monocytes were treated with pooled 10% normal (NS) or HD or CKD5 sera for 72 h in RPMI medium without FCS. Pooled HD sera were obtained from chronic HD patients (n = 10; age range 51–68 years, both males and females) without any angiotensin-modifying medication, treated at the Dialysis Ward of the University Clinic Halle. Pooled uremic sera were isolated from three CKD5 patients not on dialysis (males, 40–70 years of age, not taking any angiotensin-modifying medication) [20]. NS was obtained from healthy volunteers (n = 8; age range 50–67 years, both males and females). All treatments were performed in hydrophobic six-well plates (Greiner bio One) in a standard humidified incubator (37°C, 5% CO2). Total RNA from human primary monocytes was isolated with the ZR RNA MiniPrep Kit (Zymo Research) according to the manufacturer's instructions.
ACE/AngII receptor inhibition studies were performed with captopril (ACEi, 500 nM, Santa Cruz Biotechnology) or losartan [angiotensin II type 1 receptor (AT1R) antagonist, 1 µM, Santa Cruz Biotechnology] on control or THP-1 monocytes stably overexpressing ACE according to Trojanowicz et al. [20]. Captopril and losartan concentrations corresponded to those observed in clinical conditions. Human umbilical vein endothelial cells (HUVECs) were cultured in Medium 200 supplemented with Low Serum Growth Supplement (LSGS; Gibco).
Transfection
Culture and transfection of THP-1 or primary monocytes with the full coding sequence of ACE was described previously [20]. For generation of THP-1 cells stably overexpressing human ACE2, the cells were transfected with hACE2_pcDNA_3.1 plasmid carrying the full coding sequence of ACE2 (received as a gift from Dr E. Lazartigues). Control cells received empty plasmid only. The transfection method was described previously [20].
Microscopic investigations of the living cells in a phase contrast modus were performed with a light/fluorescence microscope (Biozero BZ-9000, Keyence).
RNA isolation and real-time PCR
Total RNA was isolated using the ZR RNA MiniPrep Kit (Zymo Research) according to the manufacturer's instructions. Depending on the experiment, 50 ng–1 µg of total RNA was used as a template for first-strand cDNA synthesis with the High Capacity cDNA Reverse Transcription Kit according to the manufacturer's instructions (Life Technologies). Samples were stored at −20°C.
Amplifications of ACE (Hs00174179_m1), ACE2 (Hs01085333_m1), MASR (Hs00267157_s1), ACTB (Hs99999903_m1), 18S (Hs99999901_s1) and RPL37A (ribosomal protein 37a; Hs01102345_m1) were performed with TaqMan Gene Expression Assays (Life Technologies) and FastStart Universal Probe Master Mix (Roche) in a StepOne plus System. ACTB, 18S and RPL37A were used for normalization of target mRNA expression. Thermal cycling conditions were as follows: hold 10 min at 95°C, followed by 40 cycles of 10 s at 95°C and 30 s at 60°C. Amplifications of macrophage colony-stimulating factor (MCSF), AT1R, angiotensin II type II receptor (AT2R), intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), monocyte chemotactic protein-1/chemokine (C-C motif) ligand 2 (MCP-1/CCL2)) and RPL37A were performed with sequences and conditions according to Trojanowicz et al. [20]. Data evaluation was performed with DataAssist Software (Life Technologies). Dotted lines within the quantitative PCR (qPCR) graphs represent expression of the target transcript in the reference sample and were set as 1 for evaluation purposes.
Western blot
Total protein was lysed with RIPA buffer supplemented with protease inhibitors. Western blot was performed with 40 µg of total protein separated on 7.5% SDS-PAGE and blotted onto polyvinylidene difluoride membranes. Membranes were blocked 1 h in 1% BSA dissolved in Tris-buffered saline and Tween 20 buffer. Thereafter primary ACE2 (1:250, Santa Cruz Biotechnology) and B-actin (1:5000, Cell Signalling) antisera in blocking buffer were applied overnight at 4°C and for 1 h at room temperature, respectively. Finally, secondary anti-rabbit HRP-conjugated antibody (Santa Cruz Biotechnology) was employed. Protein bands were visualized with an ECL kit (Pierce). Analyses of ACE2 and B-actin expression (measurement of the area of the specific protein bands representing pixel intensity) were performed with AlphaView software (ProteinSimple). The area of control bands was set as 100% and compared with the areas of ACE2-specific bands. For normalization purposes, the intensity of the B-actin bands was subtracted from the intensity of ACE2 bands.
Adhesion and transmigration assays
Adhesion of THP-1 monocytes to endothelial HUVEC monolayers and all transmigration assays were performed according to Trojanowicz et al. [20].
Statistics
Each experiment was repeated at least three times. Data are presented as mean ± SD or median with interquartile range. The distribution of quantitative variables was tested using the D'Agostino–Pearson omnibus test. Depending on the data distribution, statistical significance was calculated by Wilcoxon–Mann–Whitney or t-tests. A linear regression model was applied to adjust for age and gender with regard to leukocytic ACE2 mRNA expression (Supplementary data, Table S2). P < 0.05 was considered statistically significant. GraphPad Prism and SPSS software were used for statistical analyses.
RESULTS
Expression of ACE2 is decreased, while MASR, ACE and AngII receptor type I are upregulated in HD leukocytes
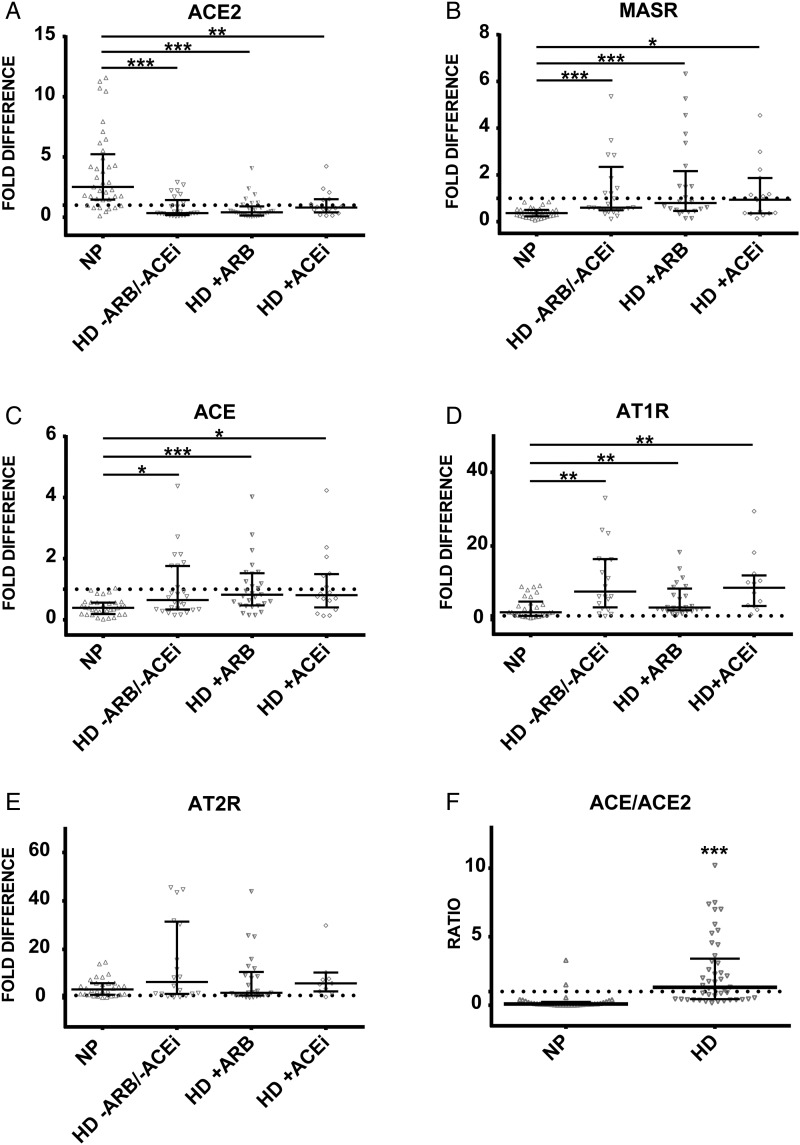
In order to investigate the expression pattern of ACE2, MASR (receptor for Ang1-7) and AngII receptors in circulating cells in patients with chronic renal failure, we subjected the leukocytes obtained from NP and HD patients to qPCR with specific ACE, ACE2 and MASR probes and AT1R and AT2R primers. As demonstrated in Figure 1A, ACE2 expression the HD group was significantly decreased compared with healthy controls. In contrast, levels of MASR were notably increased in the HD group (Figure 1B). Furthermore, expression of ACE was significantly upregulated in HD patients (Figure 1C). AngII receptor I AT1R showed a significantly elevated expression in HD patients compared with NP, while the expression of AT2R did not differ (Figure 1D and E). Investigations of leukocytic balance between both ACEs demonstrated that the expression ratio of ACE/ACE2 was significantly elevated in the HD group compared with the NP group (Figure 1F).
FIGURE 1.
Expression of (A) ACE2, (B) MASR, (C) ACE, (D) AT1R and (E) AT2R in leukocytes obtained from healthy controls (NP) and HD patients on ARB/ACEi and without angiotensin-modifying medication. (F) Expression ratio of ACE/ACE2 obtained by dividing the fold difference values of ACE and ACE2 in NP and all HD patients. Dotted line represents expression of the target transcript in the reference sample and for evaluation purposes was set as 1; medians with IRQs. P-values *<0.01, **<0.001 and ***<0.0001 indicate statistical significance (Bonferroni correction was applied).
Expression of leukocytic AngII receptors and MASR is not affected by angiotensin inhibitors in HD patients
Expression of ACE or ACE2 did not differ between HD patients taking ARBs (n = 29), ACEis (n = 16) or those not treated with angiotensin-modifying medication (n = 26; Figure 1A and C). ARB therapy in HD patients reduced the elevated expression of AT1R and AT2R towards the levels in NP, however, the difference was not significant when compared with patients not treated with angiotensin-modifying medication (Figure 1B, D and E). Investigations of diabetic and nondiabetic HD patients revealed no differences in the expression of ACE, ACE2, AngII receptors and MASR within and between any examined groups (Supplementary data, Table S1).
Uremic stimulus alters leukocytic expression of ACE2, MASR and AngII receptors in primary monocytes
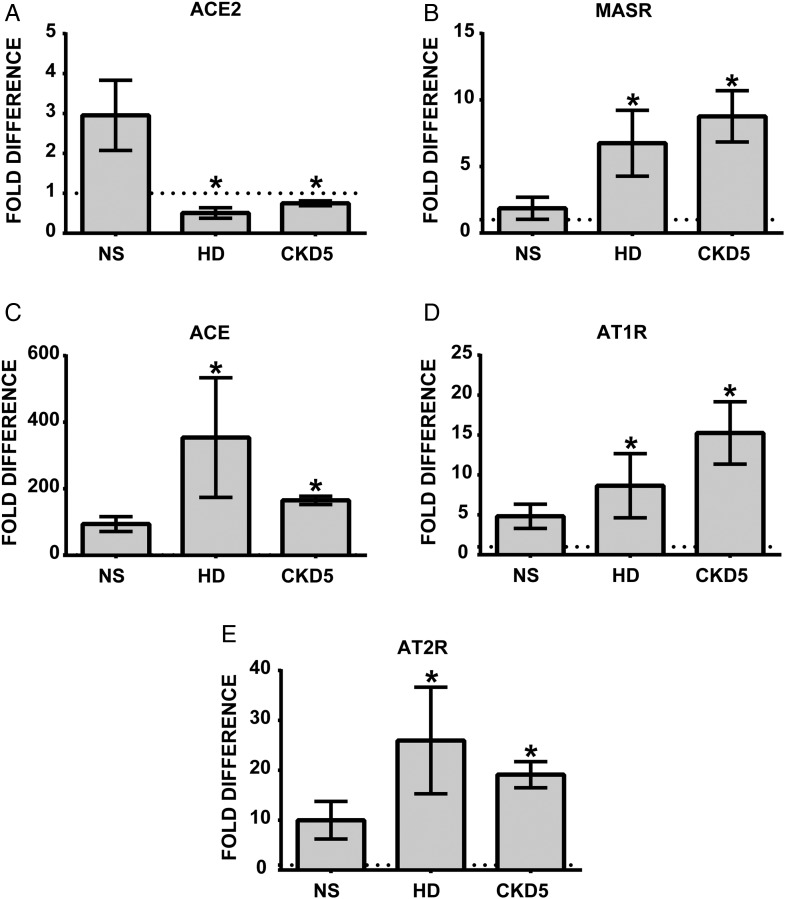
We demonstrated previously that the uremic milieu leads to upregulated expression of monocytic ACE, increased transmigration and endothelial adhesion as compared with treatments with NS [20]. The same ACE expression pattern was observed in leukocytes obtained from HD patients. Here, the influence of serum incubation on the expression of ACE2 as well as the angiotensin receptors was tested. In primary human monocytes, incubation for 72 h with HD or CKD5 serum leads to significantly reduced expression of ACE2 and elevated levels of MASR, AT1R and AT2R (Figure 2A–E) compared with NS. Thus, the uremic milieu induces the complete expression pattern on normal monocytes that is found on cells isolated from dialysis patients.
FIGURE 2.
Expression of (A) ACE2, (B) MASR, (C) ACE, (D) AT1R and (E) AT2R in primary human monocytes conditioned with normal (NS), hemodialysis (HD) and uremic (CKD5) serum obtained from nondialysis patients. Primary human monocytes obtained from three healthy volunteers were treated with 10% NS or HD or CKD5 for 72 h and investigated for mRNA expression. Mean ± SD of three independent experiments. Dotted line represents expression of the target transcript in the reference sample and for evaluation purposes was set as 1. *P < 0.05 as compared with NS.
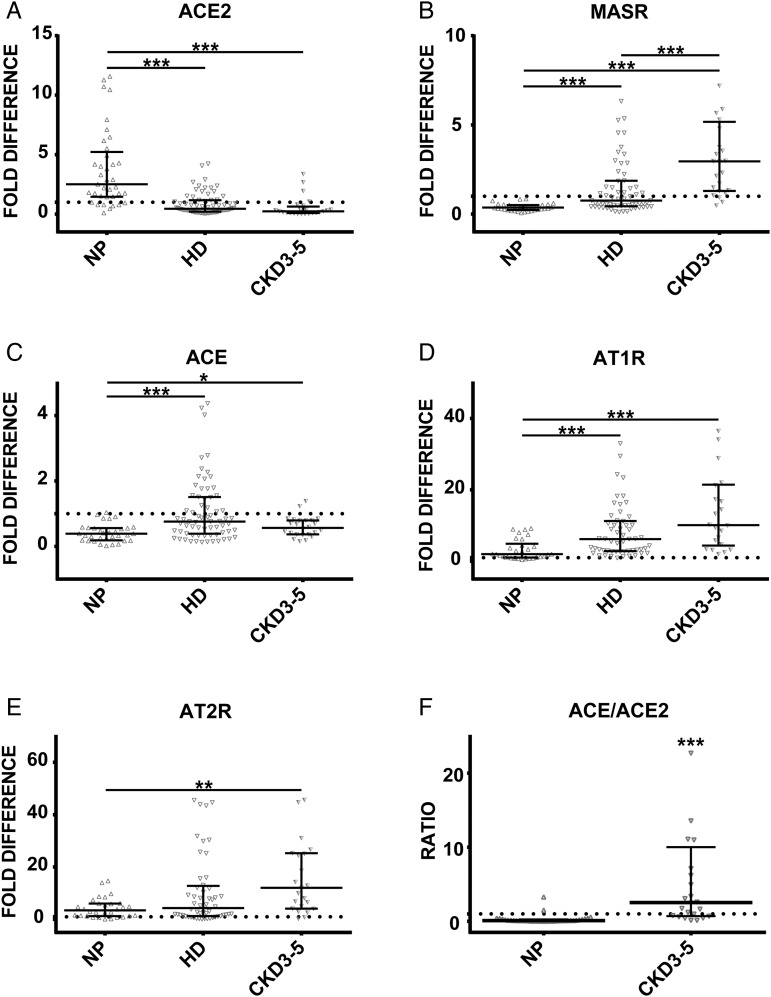
In order to unravel whether HD itself may affect the observed changes in patients' leukocytes, we investigated additionally the expression of target transcripts in CKD3–5 patients not on dialysis. As demonstrated in Figure 3A and C, expression of ACE and ACE2 in CKD3–5 patients was similar to that observed in HD patients and differed significantly from NP. Expression of MASR, AT1R and AT2R in CKD3–5 patients was significantly upregulated compared with NP (Figure 3B, D and E). Additionally, the levels of MASR were significantly higher in CKD3–5 than in HD patients (Figure 3B). Investigations of leukocytic balance between both ACEs demonstrated that the expression ratio of ACE/ACE2 was significantly elevated in the CKD3–5 group compared with NP (Figure 3F).
FIGURE 3.
Expression of (A) ACE2, (B) MASR, (C) ACE, (D) AT1R and (E) AT2R in leukocytes obtained from healthy controls (NP), HD and CKD3–5 patients not on dialysis. (F) Expression ratio of ACE/ACE2 obtained by dividing the fold difference values of ACE and ACE2 in NP and all CKD3–5 patients. Dotted line represents expression of the target transcript in the reference sample and for evaluation purposes was set as 1; medians with IRQs. P-values *<0.01, **<0.001 and ***<0.0001 indicate statistical significance (Bonferroni correction was applied).
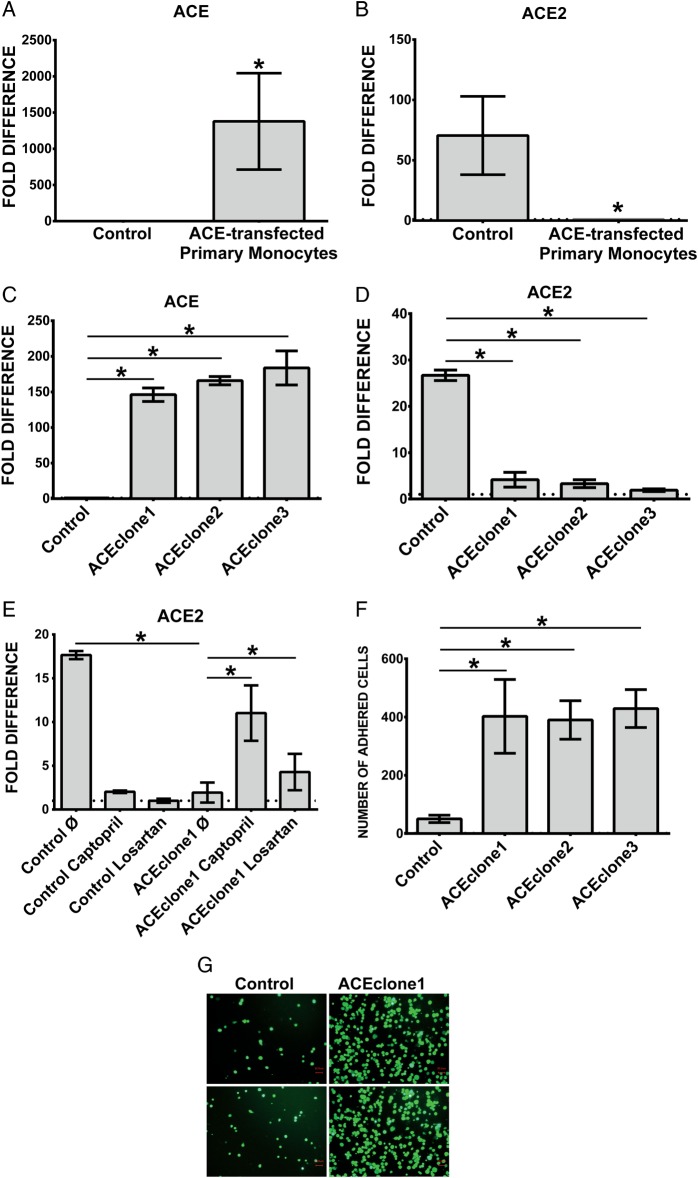
Overexpression of ACE causes suppression of ACE2
Uremia-mediated upregulation of ACE expression correlates with increased adhesion and transmigration of the monocytes as reported in our previous studies. Overexpression of ACE by plasmid-mediated transfection also leads to a marked decrease of ACE2 expression in both primary monocytes and THP-1 cells (Figure 4A–D). This effect can be reversed, at least in part, by the ACEi captopril and to a lesser degree by the ARB losartan (Figure 4E). This indicates that local formation of AngII by monocytic ACE is important for the effect on ACE2 expression. Likely, AT2R, which is not blocked by losartan, plays a role in ACE2 regulation.
FIGURE 4.
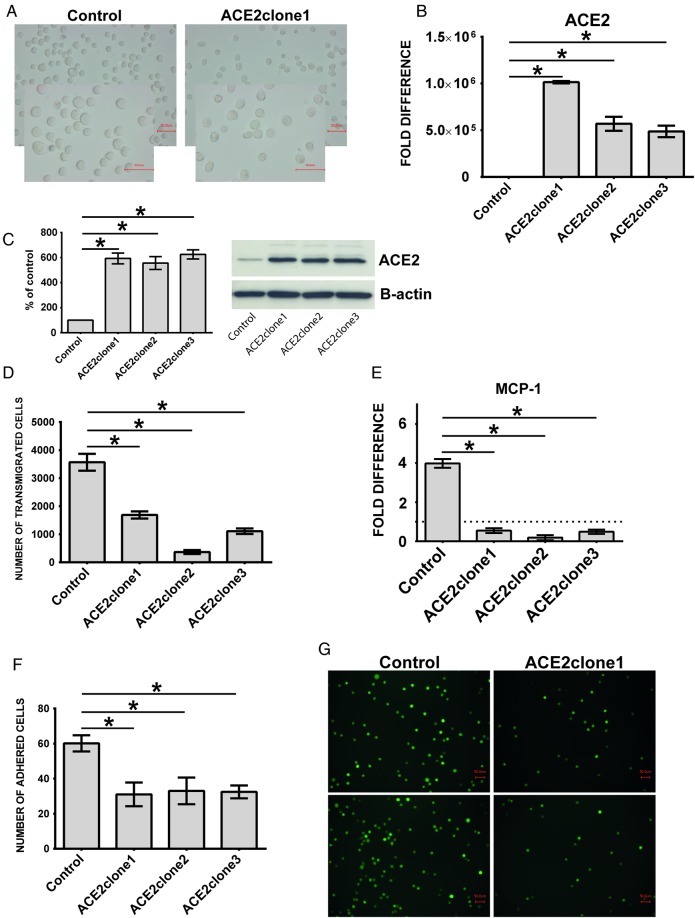
Expression of ACE and ACE2 in (A and B) ACE-transfected primary human monocytes and (C and D) THP-1 cells. Human primary monocytes were transiently transfected with empty or pcDNA3.1− plasmid carrying full coding sequence of ACE. Investigations of (A) ACE-and (B) ACE2 expression were performed 24 h after transfection. (C, D) Empty plasmid (control) and ACE-overexpressing cells (ACEclone1, ACEclone2 and ACEclone3) were investigated for (C) ACE and (D) ACE2 transcripts with specific TaqMan probes. (E) Control and pro-atherogenic ACE-overexpressing cells were treated with 500 nM captopril or 1 µM losartan for 4 h and analyzed for ACE2 expression. Untreated cells are labelled as ø. *P < 0.05 indicates statistical significance. Mean ± SD of three independent experiments. (F and G) Adhesion of THP-1 monocytes overexpressing ACE. Calcein-labelled cells were incubated for 30 min in the presence of endothelial monolayers at the chamber bottom. Adhered cells were visualized with fluorescence microscopy (G) and counted (F). Mean ± SD of cell numbers in 10 microscopic fields in three independent experiments. Representative images of control and ACE-overexpressing cells (ACEclone1) are shown (G). Dotted line within the graphs represents expression of the target transcript in the reference sample and for evaluation purposes was set as 1. *P < 0.05 indicates statistical significance.
Overexpression of ACE affects the expression of adhesion-related transcripts
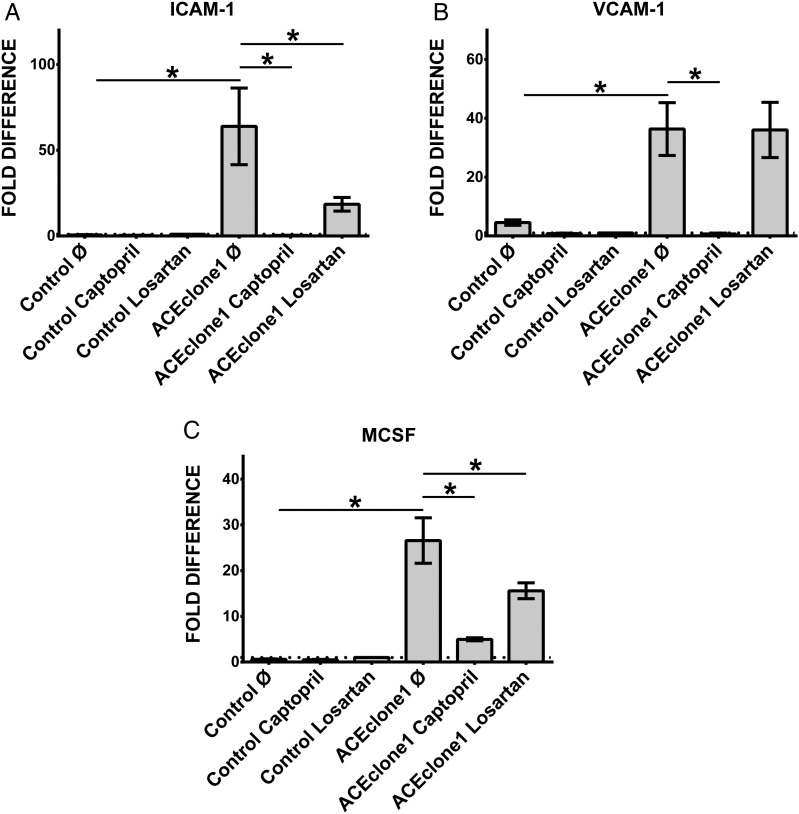
In addition to the effect on ACE2, overexpression of ACE on monocytes also leads to significantly elevated cellular adhesion (Figure 4F and G) and upregulated expression of ICAM-1, VCAM-1 and MCSF, molecules involved in cell–cell interactions and particularly monocyte–endothelium adhesion (Figure 5A–C). As demonstrated in Figure 5A–C, captopril as well as losartan leads to significantly decreased expression of ICAM-1, VCAM-1 and MCSF in ACE-overexpressing cells. The effect of captopril was stronger than that of losartan.
FIGURE 5.
Analysis of adhesion-related transcripts in THP-1 monocytes under ACEi and ARB. Control and pro-atherogenic ACE-overexpressing cells were treated with 500 nM captopril or 1 µM losartan for 4 h and subjected for RT-PCR analysis with primers specific for (A) ICAM-1, (B) VCAM-1 and (C) MCSF. Untreated cells are labelled as ø. *P < 0.05 indicates statistical significance. Mean ± SD of three independent experiments. Dotted line represents expression of the target transcript in the reference sample and for evaluation purposes was set as 1.
Overexpression of ACE2 leads to reduced endothelial adhesion and transmigration
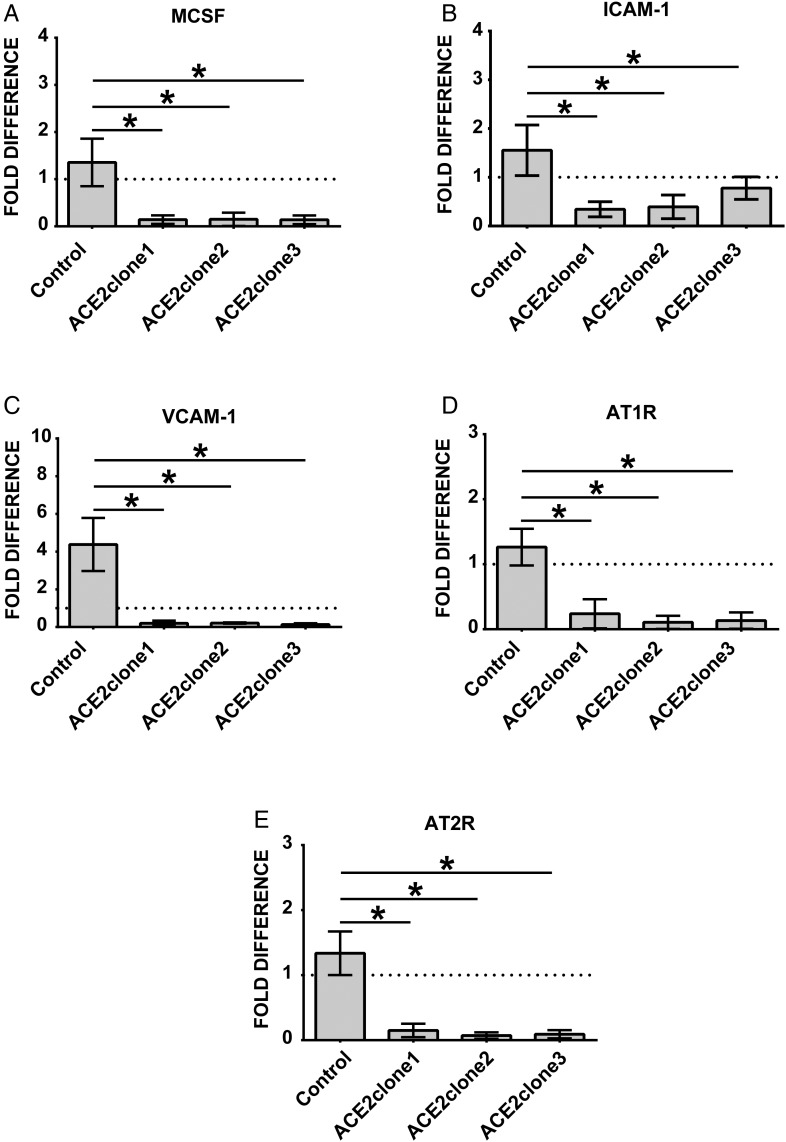
THP-1 monocytes overexpressing ACE2 are smaller than corresponding controls (Figure 6A). Functionally these cells reveal decreased endothelial adhesion (Figure 6F and G), lower transmigration rates and diminished expression of MCP-1 (Figure 6D and E). Further, overexpression of ACE2 leads to inhibition of the adhesion-related molecules ICAM-1 and VCAM-1 as well as reduced expression of angiotensin receptors 1 and 2 (Figure 7A–E).
FIGURE 6.
Morphology and behaviour of THP-1 monocytes overexpressing ACE2. THP-1 monocytes were stably transfected with empty or pcDNA3.1+ plasmid carrying full coding sequence of ACE2. ACE2-overexpressing cells were designated as ACE2clone1, ACE2clone2 and ACE2clone3, and empty plasmid cells as control. (A) Morphology of the cells investigated in contrast phase by conventional light microscopy. Note that ACE2-overexpressing cells (ACE2clone1) are smaller than corresponding controls. (B) Investigations of ACE2-expression performed with TaqMan probes specific for ACE2. (C) Protein expression of ACE2 as demonstrated by Western blot and densitometric analysis. Investigations were performed with specific ACE2 and B-actin antisera, and AlphaView software, respectively. (D) Transmigration of the cells through endothelial monolayers. The cells were transmigrated for 60 min and counted flow cytometrically. (E) Analysis of MCP-1 expression in control and ACE2-overexpressing cells. (F and G) Adhesion of the cells to endothelial monolayers. Calcein-labelled control and ACE2-overexpressing monocytes were incubated for 30 min in the presence of endothelial monolayers at the chamber bottom. Adhered cells were visualized with fluorescence microscopy (G) and counted (F). Mean ± SD of cell numbers in 10 microscopic fields in three independent experiments. Representative images of control and ACE2-overexpressing cells (ACE2clone1) are shown (G). Dotted line within the graphs represents expression of the target transcript in the reference sample and for evaluation purposes was set as 1. *P < 0.05 indicates statistical significance.
FIGURE 7.
RT-PCR analysis of THP-1 monocytes overexpressing ACE2. Empty plasmid (control) and ACE2-overexpressing cells (ACEclone1, ACE2clone2, ACE2clone3) were analyzed for (A) MCSF, (B) ICAM-1, (C) VCAM-1, (D) AT1R and (E) AT2R. Dotted line represents expression of the target transcript in the reference sample and for evaluation purposes was set as 1. *P < 0.05 indicates statistical significance. Mean ± SD of three independent experiments.
DISCUSSION
This study shows that circulating leukocytes obtained from HD and CKD patients express less ACE2, while ACE was detected noticeably stronger when compared with leukocytes from healthy individuals. ACE produces the vasoconstrictive AngII, while ACE2 degrades AngII to Ang1-7, a vasodilator. The altered relation between ACE and ACE2 may contribute to the ubiquitous arterial hypertension in dialysis patients. In addition, the receptors for AngII, AT1R and AT2R and the receptor for Ang1-7, MASR, are upregulated. To our knowledge, this is the first report describing an elevated expression of MASR in dialysis patients. This particular phenotype can be induced in vitro by incubation of monocytes with serum from dialysis patients.
Kovarik et al. [24] showed elevated plasma levels of Ang1-7 in dialysis patients compared with healthy controls. This cannot be explained by the expression pattern of ACE2 on monocytes, thus other sources of Ang1-7 in these patients are likely.
Previous reports revealed that receptors for AngII are detectable on all major leukocyte subsets, with the highest expression on granulocytes and monocytes, and correlate with pathophysiological conditions [25]. In agreement with our data, high-level expression of AT1R has been shown in CKD patients before [26]. Furthermore, in individuals with high risk for vascular events or hyperlipidemic patients, expression of AT1R in circulating leukocytes was noticeably elevated as compared with a healthy low-risk control group [26]. Pharmacological intervention by either RAS blockers or statins led to decreased expression of AT1R and, by interfering with AngII, exerted anti-inflammatory actions in these patients [26–28]. Recently, Chon et al. [26] studied nondialyzed CKD patients and found that despite a relevant antihypertensive effect, angiotensin blockade did not consistently affect AT1R leukocytic expression. The authors postulated that factors involved in uremia are more dominant modulators of leukocytic AT1R expression in these patients. In contrast to these findings, therapy with AngII receptor blockers in our HD patients led to some degree of reduction of leukocytic AT1R and AT2R expression.
Experimental overexpression of ACE on monocytes leads to downregulation of ACE2. This effect is most likely transmitted via formation of AngII and ligation of the AT1R, since it can be inhibited by ACE inhibition, and in part by angiotensin receptor blockade. Such interaction via AT1R has been demonstrated previously [29]. The expression pattern found in dialysis patients is associated with enhanced expression of adhesion molecules ICAM-1 and VCAM-1 as well as the activating cytokine MCSF. In the transfection experiments, overexpression of ACE induces these molecules and the angiotensin receptor blocker losartan reverts the induction. In contrast, overexpression of ACE2 has the opposite effects, with downregulation of the adhesion-promoting molecules.
These findings are in agreement with earlier data that suggested ACE and ACE2 to be a counterregulatory mechanism not only within the kidney, heart or astrocytes, but also within the circulating leukocytes [30–32].
Our results allow speculation that the uremic milieu induces a specific dysregulation of ACE/ACE2 expression that is responsible for the formation of a pro-inflammatory and adhesive monocyte phenotype. This fits well with our earlier data showing high expression of ACE on monocytes in dialysis patients in association with subclinical atherosclerosis [5] or cardiovascular mortality [18]. In comparison with a healthy population with significantly lower leukocytic MASR and up-regulated ACE2 levels, it seems that the uremic environment, and especially uremic toxins, may affect the proper function of a local ACE2–MASR axis as a counterregulatory mechanism. Uremic toxins are not only able to induce the vascular damage of CKD, but also may induce the components of the RAS [33–37]. Shimizu et al. [34] demonstrated that indoxyl sulphate (IS), one of the most representative uremic toxins, upregulated angiotensinogen (AGT) expression in proximal tubular cells. Sun et al. [35] reported that IS and p-cresol sulphate upregulated, in addition to elevated AGT expression, other renin–angiotensin–aldosterone system components such as renin and AT1R. Studies by Vanholder et al. [36] and Pletinck et al. [37] showed clearly that pro-inflammatory effects triggered by protein-bound uremic toxins contribute to vascular damage and renal disease progression by stimulating crosstalk between leukocytes and the vessel wall. Our data cannot rule out an additional participation of the systemic RAS, particularly since Kovarik et al. [24] demonstrated elevated Ang1-7 levels in HD plasma that may strongly upregulate leukocytic MASR. It is also worth noting that human macrophages react to inflammatory stimuli such as LPS with increased MASR transcripts [38]. On the other hand, it has been reported that oral administration of IS to CKD rats led to decreased levels of MASR in proximal tubular cells [39]. Such a contrary expression as compared with increased MASR in uremic leukocytes could be explained by different roles of the Ang1-7–MASR axis in mesangial and tubular cells within the kidney and other cells such as circulating leukocytes.
Recently, there has been emerging evidence that microRNAs may trigger the silencing of distinct transcripts and cause the development and/or progression of renal disease [40]. As miR-421 is able to bind and regulate ACE2 mRNA, the effects of the uremic milieu on the expression of particular microRNAs must be taken into consideration [41].
It is well established that immunological mechanisms and inflammation strongly contribute to the pathogenesis of atherosclerosis. This is particularly suggested by the accumulation of leukocytes and monocyte-derived macrophages in atherosclerotic lesions [42, 43]. Systemic chronic inflammation commonly observed in dialysis patients is one of the main factors associated with plaque-forming atherosclerosis [5].
The components of the local angiotensin system may interact with the systemic RAS and are believed to exert an impact on clinical outcomes, not least atherosclerosis [17].
Our transcriptional data are confirmed by functional experiments. Overexpression of ACE in monocytes leads to a phenotype with decreased ACE2 and upregulated angiotensin receptors. These cells showed dramatically augmented endothelial adhesion and transmigration.
We found that monocytes overexpressing ACE2 revealed not only significantly decreased transmigratory and adhesive properties, but also diminished levels of AngII receptors.
The importance of such anti-atherogenic and antihypertensive actions of the local angiotensin system is reflected in studies performed in anephric patients. It has been suggested that in such patients systemic blood pressure maintenance is regulated in a local tissue- or cell-specific fashion, independent from the systemic RAS [44–46]. Given that anephric patients and individuals with remaining renal function both suffer from progressive atherosclerosis, systemic and local RAS may be pro-atherogenic in these patients to a similar extent.
In conclusion, we demonstrate that uremic conditions promote overactivation of monocytic ACE and inhibition of ACE2, thus contributing to enhanced endothelial adhesion and transmigration. This may elucidate an important mechanism contributing to the progression of atherosclerosis in patients with chronic renal failure.
SUPPLEMENTARY MATERIAL
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
All the authors declared no competing interests.
Supplementary Material
ACKNOWLEDGEMENTS
We are very grateful to Manuela Hajri and Beate Heinze for their excellent technical assistance.
REFERENCES
- 1. Cheung AK, Sarnak MJ, Yan G et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 2000; 58: 353–362 [DOI] [PubMed] [Google Scholar]
- 2. Vanholder R, Massy Z, Argiles A et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 2005; 20: 1048–1056 [DOI] [PubMed] [Google Scholar]
- 3. Matsushita K, van der Velde M, Astor B et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 12: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guerin AP, Blacher J, Pannier B et al. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 2001; 103: 987–992 [DOI] [PubMed] [Google Scholar]
- 5. Ulrich C, Seibert E, Heine GH et al. Monocyte angiotensin converting enzyme expression may be associated with atherosclerosis rather than arteriosclerosis in hemodialysis patients. Clin J Am Soc Nephrol 2011; 6: 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan UA, Garg AX, Parikh CR et al. Prevention of chronic kidney disease and subsequent effect on mortality: a systematic review and meta-analysis. PLoS One 2013; 8: e71784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parving HH, Lehnert H, Bröchner-Mortensen J et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. N Engl J Med 2001; 345: 870–878 [DOI] [PubMed] [Google Scholar]
- 8. Mann JF, Anderson C, Gao P et al. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31: 414–421 [DOI] [PubMed] [Google Scholar]
- 9. Donoghue M, Hsieh F, Baronas E et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000; 87: E1–E9 [DOI] [PubMed] [Google Scholar]
- 10. Santos RA, Simoes e Silva AC, Maric C et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 2003; 100: 8258–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ye M, Wysocki J, William J et al. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 2006; 17: 3067–3075 [DOI] [PubMed] [Google Scholar]
- 12. Soler MJ, Wysocki J, Batlle D. ACE2 alterations in kidney disease. Nephrol Dial Transplant 2013; 28: 2687–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamming I, Timens W, Bulthuis ML et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imai Y, Kuba K, Rao S et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436: 112–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao L, Haack KK, Zucker IH. Angiotensin II regulates ACE and ACE2 in neurons through p38 mitogen-activated protein kinase and extracellular signal-regulated kinase 1/2 signaling. Am J Physiol Cell Physiol 2013; 304: C1073–C1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitazono T, Padgett RC, Armstrong ML et al. Evidence that angiotensin II is present in human monocytes. Circulation 1995; 91: 1129–1134 [DOI] [PubMed] [Google Scholar]
- 17. Chen X, Lu H, Zhao M et al. Contributions of leukocyte angiotensin-converting enzyme to development of atherosclerosis. Arterioscler Thromb Vasc Biol 2013; 33: 2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ulrich C, Heine GH, Seibert E et al. Circulating monocyte subpopulations with high expression of angiotensin-converting enzyme predict mortality in patients with end-stage renal disease. Nephrol Dial Transplant 2010; 25: 2265–2272 [DOI] [PubMed] [Google Scholar]
- 19. Ulrich C, Heine GH, Garcia P et al. Increased expression of monocytic angiotensin-converting enzyme in dialysis patients with cardiovascular disease. Nephrol Dial Transplant 2006; 21: 1596–1602 [DOI] [PubMed] [Google Scholar]
- 20. Trojanowicz B, Ulrich C, Seibert E et al. Uremic conditions drive human monocytes to pro-atherogenic differentiation via an angiotensin-dependent mechanism. PLoS One 2014; 9: e102137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thatcher SE, Zhang X, Howatt DA et al. Angiotensin-converting enzyme 2 deficiency in whole body or bone marrow-derived cells increases atherosclerosis in low-density lipoprotein receptor-/- mice. Arterioscler Thromb Vasc Biol 2011; 31: 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong B, Zhang C, Feng JB et al. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol 2008; 28: 1270–1276 [DOI] [PubMed] [Google Scholar]
- 23. Lovren F, Pan Y, Quan A et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol 2008; 295: 1377–1384 [DOI] [PubMed] [Google Scholar]
- 24. Kovarik JJ, Antlanger M, Domenig O et al. Molecular regulation of the renin–angiotensin system in haemodialysis patients. Nephrol Dial Transplant 2015; 30: 115–123 [DOI] [PubMed] [Google Scholar]
- 25. Rasini E, Cosentino M, Marino F et al. Angiotensin II type 1 receptor expression on human leukocyte subsets: a flow cytometric and RT-PCR study. Regul Pept 2006; 134: 69–74 [DOI] [PubMed] [Google Scholar]
- 26. Chon H, Neumann J, Boer P et al. Enhanced angiotensin II type 1 receptor expression in leukocytes of patients with chronic kidney disease. Eur J Pharmacol 2011; 666: 205–210 [DOI] [PubMed] [Google Scholar]
- 27. Guasti L, Marino F, Cosentino M et al. Prolonged statin-associated reduction in neutrophil reactive oxygen species and angiotensin II type 1 receptor expression: 1-year follow-up Eur. Heart J 2008; 29: 1118–1126 [DOI] [PubMed] [Google Scholar]
- 28. Chon H, Gaillard CA, van der Meijden BB et al. Broadly altered gene expression in blood leukocytes in essential hypertension is absent during treatment. Hypertension 2004; 43: 947–951 [DOI] [PubMed] [Google Scholar]
- 29. Tikellis C, Cooper ME, Bialkowski K et al. Developmental expression of ACE2 in the SHR kidney: a role in hypertension? Kidney Int 2006; 70: 34–41 [DOI] [PubMed] [Google Scholar]
- 30. Ferrario CM, Jessup J, Gallagher PE et al. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int 2005; 68: 2189–2196 [DOI] [PubMed] [Google Scholar]
- 31. Gallagher PE, Chappell MC, Ferrario CM et al. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol 2006; 290: 420–426 [DOI] [PubMed] [Google Scholar]
- 32. Ferrario CM, Jessup J, Chappell MC et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111: 2605–2610 [DOI] [PubMed] [Google Scholar]
- 33. Schepers E, Meert N, Glorieux G et al. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 2007; 22: 592–596 [DOI] [PubMed] [Google Scholar]
- 34. Shimizu H, Saito S, Higashiyama Y et al. CREB, NF-κB, and NADPH oxidase coordinately upregulate indoxyl sulfate-induced angiotensinogen expression in proximal tubular cells. Am J Physiol Cell Physiol 2013; 304: 685–692 [DOI] [PubMed] [Google Scholar]
- 35. Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One 2012; 7: e34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vanholder R, Schepers E, Pletinck A et al. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol 2014; 25: 1897–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pletinck A, Glorieux G, Schepers E et al. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol 2013; 24: 1981–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Souza LL, Costa-Neto CM. Angiotensin-(1-7) decreases LPS-induced inflammatory response in macrophages. J Cell Physiol 2012; 227: 2117–2122 [DOI] [PubMed] [Google Scholar]
- 39. Ng HY, Yisireyili M, Saito S et al. Indoxyl sulfate downregulates expression of Mas receptor via OAT3/AhR/Stat3 pathway in proximal tubular cells. PLoS One 2014; 9: e91517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dwivedi RS, Herman JG, McCaffrey TA et al. Beyond genetics: epigenetic code in chronic kidney disease. Kidney Int 2011; 79: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lambert DW, Lambert LA, Clarke NE et al. Angiotensin-converting enzyme 2 is subject to post-transcriptional regulation by miR-421. Clin Sci (Lond) 2014; 127: 243–249 [DOI] [PubMed] [Google Scholar]
- 42. Galkina E, Ley K. Leukocyte influx in atherosclerosis. Curr Drug Targets 2007; 8: 1239–1248 [DOI] [PubMed] [Google Scholar]
- 43. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 2011; 12: 204–212 [DOI] [PubMed] [Google Scholar]
- 44. Krop M, de Bruyn JH, Derkx FH et al. Renin and prorenin disappearance in humans post-nephrectomy: evidence for binding? Front Biosci 2008; 13: 3931–3939 [DOI] [PubMed] [Google Scholar]
- 45. Wenting GJ, Blankestijn PJ, Poldermans D et al. Blood pressure response of nephrectomized subjects and patients with essential hypertension to ramipril: indirect evidence that inhibition of tissue angiotensin converting enzyme is important. Am J Cardiol 1987; 59: 92–97 [DOI] [PubMed] [Google Scholar]
- 46. Campbell DJ, Kladis A, Skinner SL et al. Characterization of angiotensin peptides in plasma of anephric man. J Hypertens 1991; 9: 265–274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.