Middle East respiratory syndrome (MERS) coronavirus was found by reverse-transcription polymerase-chain-reaction from viral cultures of 4 of 7 air samples and 15 of 68 surface swabs from 3 MERS patients' rooms, calling for epidemiologic investigation for contact and airborne transmission.
Keywords: MERS, transmission, contamination
Abstract
Background. The largest outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) outside the Middle East occurred in South Korea in 2015 and resulted in 186 laboratory-confirmed infections, including 36 (19%) deaths. Some hospitals were considered epicenters of infection and voluntarily shut down most of their operations after nearly half of all transmissions occurred in hospital settings. However, the ways that MERS-CoV is transmitted in healthcare settings are not well defined.
Methods. We explored the possible contribution of contaminated hospital air and surfaces to MERS transmission by collecting air and swabbing environmental surfaces in 2 hospitals treating MERS-CoV patients. The samples were tested by viral culture with reverse transcription polymerase chain reaction (RT-PCR) and immunofluorescence assay (IFA) using MERS-CoV Spike antibody, and electron microscopy (EM).
Results. The presence of MERS-CoV was confirmed by RT-PCR of viral cultures of 4 of 7 air samples from 2 patients' rooms, 1 patient's restroom, and 1 common corridor. In addition, MERS-CoV was detected in 15 of 68 surface swabs by viral cultures. IFA on the cultures of the air and swab samples revealed the presence of MERS-CoV. EM images also revealed intact particles of MERS-CoV in viral cultures of the air and swab samples.
Conclusions. These data provide experimental evidence for extensive viable MERS-CoV contamination of the air and surrounding materials in MERS outbreak units. Thus, our findings call for epidemiologic investigation of the possible scenarios for contact and airborne transmission, and raise concern regarding the adequacy of current infection control procedures.
Many factors are thought to have contributed to the large outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) in South Korea in 2015: the unfamiliarity of physicians with MERS-CoV, suboptimal infection control measures in some hospitals, overcrowding in emergency rooms, patients occupying rooms with many beds, the habit of seeking medical advice from multiple healthcare facilities, and visits to hospitalized patients by friends and family members [ 1 ]. Added to these was the initial failure to trace contacts. Although the transmission routes of MERS are not completely understood [ 2 ], US Centers for Disease Control and Prevention guidelines define close contact as being within 6 feet of an infected patient or within the room or care area of such a patient for a long time [ 3 ]. The Korea Centers for Disease Control and Prevention (KCDC) initially quarantined and followed up, by personal interview or closed-circuit television review, only those who had been in close contact (6 feet) with the index patient or who had shared the same room. However, many patients and guardians became infected and were later recognized to have been >6 feet away from the index patient, though in the same ward [ 4 ]. Eventually, the 4 hospital outbreak clusters (91, 36, 14, and 11 cases, respectively) accounted for 82% of all the cases that occurred [ 5 , 6 ].
Therefore, identifying the possible transmission routes for the distant contacts of index patients is important for our ability to reduce the spread of MERS. The distant transmission could be explained by 2 scenarios: (1) contaminated environmental surfaces including fomites, and/or (2) airborne transmission. A previous study revealed that most of the accessible surfaces in MERS units were contaminated by MERS-CoV, as determined by reverse transcription polymerase chain reaction (RT-PCR) and culture [ 7 ]. Although viral RNA was detected on inaccessible surfaces such as the entrance of air-ventilating equipment [ 7 ], the authors did not test air samples. Therefore, we investigated whether contamination of the air or of accessible/inaccessible surfaces in 3 MERS-CoV–infected patient rooms could explain the transmission of MERS-CoV.
METHODS
Study Sites and Patient Data
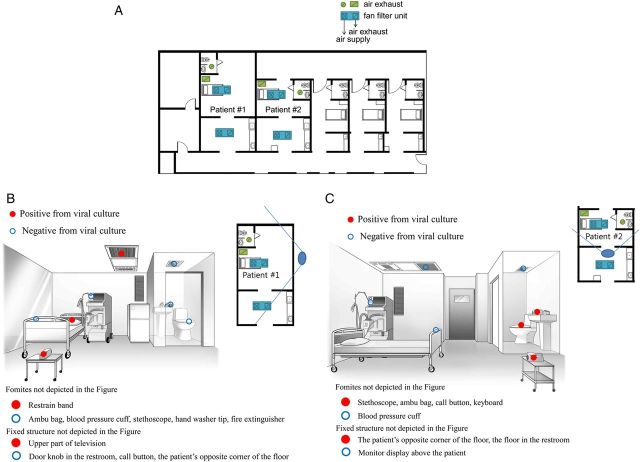
On 1 July 2015, during the 2015 MERS outbreak in South Korea, air and environmental samples were collected from 2 MERS-CoV–infected patients in hospital A and 1 patient in hospital B. The MERS-designated wards in hospital A had been newly constructed in 2012 and were specially designed for highly pathogenic respiratory viral pathogens. Each negative-pressure room had an anteroom (Figure 1A ). In hospital A, 1 room was occupied by patient 1, a 69-year-old man with pneumonia who received mechanical ventilation and extracorporeal membrane oxygenation on day 22 from the onset of symptoms (Figure 1B ) and whose respiratory specimens (tracheal aspirates) persistently tested positive for MERS-CoV by RT-PCR up to the time of environmental sampling. The other room in hospital A was occupied by patient 2, a 54-year-old man with pneumonia who received mechanical ventilation on day 16 from the onset of symptoms (Figure 1C ); his respiratory specimens (tracheal aspirates) also persistently tested positive for MERS-CoV by RT-PCR up to the time of environmental sampling. The MERS-designated wards in hospital B were switched to isolation wards during the MERS outbreak. The rooms lacked anterooms, had portable negative-pressure devices (Cleanroom H13, IQAir, Goldach, Switzerland), and shared a common corridor (Figure 2A ). One room was occupied by patient 3, a 74-year-old man with pneumonia who was not using any mechanical ventilator on day 19 from the onset of symptoms; his respiratory specimens (sputum samples) tested positive for MERS-CoV by RT-PCR 6 days before the time of environmental sampling. At the time of environmental sampling, on day 19, his respiratory symptoms persisted but further respiratory samples were not taken because the attending physician thought that continued positive results from his respiratory specimens would not alter any clinical decisions about management and isolation in this resource-limited hospital. The patient was bedridden and had not used the restroom (Figure 2B ). Environmental sampling of the rooms occupied by patient 1 and 2 was performed 6–7 hours after the daily routine cleaning, and environmental sampling of patient 3 was performed 3–4 hours after the daily routine cleaning.
Figure 1.
A , Floor plan of the well-equipped Middle East respiratory syndrome–designated hospital where each negative-pressure room had an anteroom and postroom. The “x” and slash (/) indicate the air supply and air exhaust, respectively. Patient 1 was a 69-year-old man with pneumonia who received mechanical ventilation and extracorporeal membrane oxygenation on day 22 from the onset of symptoms. B , Results of viral culture of air and swabs from patient 1's room. Patient 2 was a 54-year-old man with pneumonia who received mechanical ventilation on day 16 from the onset of symptoms. C , Results of viral cultures of air and swabs from patient 2's room. The solid blue lines radiating from the large blue ovals indicate the angles of observation used for drawing the illustrations of the patients’ rooms.
Figure 2.

A , Floor plan of the Middle East respiratory syndrome (MERS)–designated hospital that was switched to isolation wards in the MERS outbreaks where each room had a portable negative-pressure device and no anteroom and shared a common corridor. The “x” and slash (/) indicate air supply and air exhaust, respectively. Patient 3 was a 54-year-old man with pneumonia who received mechanical ventilation on day 19 from the onset of symptoms. B , Results of viral culture of air and swabs from patient 3's room. The solid blue lines radiating from the large blue oval indicate the angle of observation used to draw the illustration of the patient's room.
Sample Collection
Air was sampled using an MD8 airscan sampling device (Sartorius, Goettingen, Germany) and sterile gelatin filters (80-mm diameter and 3-µm pores; Sartorius). Air was sampled twice at a speed of 50 L/minute for 20 minutes in the negative-pressure room and its associated restrooms. The filters were dissolved aseptically in 30 mL viral transport medium (sterile phosphate buffer with 10% fetal calf serum, 10 000 U/mL penicillin, 10 mg streptomycin, 25 µg amphotericin B) and stored at −80°C until analyzed.
Dacron swabs premoistened with viral transport medium were used to swab surfaces aseptically. The following types of surface were swabbed: (1) fixed structures in the elevators (ie, buttons, guardrails, and doors); (2) fomites (ie, stethoscopes, bag valve masks, blood pressure cuffs, nasal prongs, pillows, and keyboards); (3) fixed structures in the rooms and their associated restrooms (ie, doorknobs, bed guardrails, toilet seats, and hand soap dispensers); and (4) the ventilation exits on the restroom ceiling and the ventilation exits on the ceilings and walls of the negative-pressure rooms. All the environmental samples were collected after daily cleaning and disinfection of the rooms. Surface swabbing was focused especially on surfaces such as ventilator exits and the tops of television sets, which are easily missed by daily cleaning.
Laboratory Procedures
The MERS-CoV Korea isolate MERS-CoV/KOR/KNIH/002_05_2015 (accession number KT029139.1) for use as a positive control was kindly provided by Dr Sung Soon Kim, Division of Respiratory Viruses, Korea National Institute of Health. Vero cells (ATCC CCL-81) were grown in T-75 flasks, inoculated with MERS-CoV, and incubated at 37°C in a CO 2 incubator. Three days after inoculation, the MERS-CoV–infected Vero cells were harvested.
The detailed procedure for RT-PCR and sequencing of environmental samples are described in the online Supplementary Material . Air and surface swab samples were filtered through 0.1-µm pore syringe filter units (Pall Corporation, Port Washington, New York) to minimize bacterial contamination. Vero E6 (ATCC, CRL-1586) cells were incubated with the filtered samples in Dulbecco's minimal essential medium (Welgen, Korea) supplemented with 100 IU/mL penicillin and 100 µg/mL streptomycin at 37°C in a CO 2 incubator, and checked daily for cytopathic changes. Fourteen days after inoculation, culture supernatants and lysates of Vero E6 cells were harvested and used for detecting MERS-CoV by RT-PCR. The harvested cells were centrifuged for 3 minutes at 1000 rpm to remove cellular debris. The pellets were resuspended in washing buffer (0.1 M phosphate buffer) and centrifuged for 5 minutes at 3000 rpm. After thoroughly removing washing buffer, the cells were fixed with 2.5% glutaraldehyde at 4°C overnight and photographed with a transmission electron microscope (JEOL model GEM-1400, Tokyo, Japan). The same culture supernatants were used to infect Vero cells for immunofluorescence analysis. Immunofluorescence antibody test was conducted at the tissue culture cells on 2 dpi for MERS-CoV/KOR/KNIH/002_05_2015 and 7 dpi for environmental samples, respectively. Anti-MERS-CoV Spike antibody was purchased from Sino Biological Inc (Beijing, China). All images were acquired using the Operetta High-Content Imaging System (PerkinElmer, Waltham, Massachusetts) at ×20 magnification.
All experiments were done at the Institut Pasteur Korea in compliance with the guidelines of the Korea National Institute of Health using enhanced Biosafety Level 3 containment procedures in laboratories approved for use by the KCDC.
RESULTS
RT-PCR Procedure
The RT-PCR procedure was optimized using the control MERS-CoV Korea isolate. Primers specific for the spike gene (nt 22300-22628) were used for RT-PCR ( Supplementary Figure 1A ). Using the optimized RT-PCR procedure, a single DNA band of the expected size (328 bp) was detected from Vero cells infected with the MERS-CoV Korea isolate ( Supplementary Figure 1B ). Sequencing of the amplification product confirmed the expected sequence.
Air Samples
A summary of the patient case status and environmental test results in the 2 MERS-designated hospitals is given in Table 1 . To examine the possibility of airborne transmission of MERS-CoV, air samples collected at hospitals A and B were subjected to RT-PCR. All were positive for MERS-CoV (Table 1 ; Supplementary Table 1 ).
Table 1.
Patient Case Status and Environmental Test Results in 2 Middle East Respiratory Syndrome–Designated Hospitals, Republic of Korea
| Patient Data |
Environmental Data |
||||||
|---|---|---|---|---|---|---|---|
| Hospital | No. | Case Status | Time of Sampling for PCR (Days After Symptom Onset) | MERS-CoV PCR Results | Environmental Sampling | RT-PCR From Samples | RT-PCR From Viral Culture |
| A a | 1 | Pneumonia on mechanical ventilation and ECMO | 22 | (+) at the time of sampling | Air sampling b | 2/2 | 1/2 |
| Fomites swab | 4/6 | 2/6 | |||||
| Fixed-structure swab | 7/13 | 2/13 | |||||
| 2 | Pneumonia on mechanical ventilation | 16 | (+) at the time of sampling | Air sampling b | 2/2 | 2/2 | |
| Fomites swab | 4/4 | 3/4 | |||||
| Fixed-structure swab | 12/12 | 5/12 | |||||
| Elevator | Fixed-structure swab | 1/5 | 0/5 | ||||
| B c | 3 | Pneumonia and bedridden | 19 | (–) at the time of sampling | Air sampling d | 3/3 c | 1/3 |
| Fomites swab | 5/6 | 2/6 | |||||
| Fixed-structure swab | 8/17 | 0/17 | |||||
| Elevator | Fixed-structure swab | 1/5 | 1/5 | ||||
Data are presented as No. of samples with a positive test result/No. of samples tested, unless otherwise indicated.
Abbreviations: (+), positive result; (–), negative result; ECMO, extracorporeal membrane oxygenation; MERS-CoV, Middle East respiratory syndrome coronavirus; RT-PCR, reverse transcription polymerase chain reaction.
a Hospital A was a well-equipped MERS-designated hospital specially designed for highly pathogenic respiratory virus pathogens.
b Air samples were obtained from each patient's room and its affiliated restroom.
c Hospital B was a general hospital with wards that were switched to isolation wards in the MERS outbreaks.
d Three air samples were obtained from the patient's room, its affiliated restroom, and the corridor as a common anteroom.
Next, the presence of viable MERS-CoV was tested by viral culture. MERS-CoV was cultured in Vero E6 cells from 4 of the 7 air samples, from 2 of the patients' rooms, 1 patient's restroom, and 1 common corridor (Figures 1B , 1C , 2A , and 2B ; Supplementary Figure 2B ; Table 1 ; Supplementary Table 1 ). RT-PCR results of viral cultures and subsequent sequencing confirmed RT-PCR amplification of the MERS-CoV spike gene in 14-day culture material from all 4 air samples (Table 1 ; Supplementary Table 1 ; Supplementary Figure 2 ). In addition, high-resolution electron microscopic (EM) images of the cultured virus revealed intact virus particles compatible with MERS-CoV ( Supplementary Figure 3A ), and immunofluorescence assay (IFA) using MERS-CoV Spike antibody on the tissue cultures revealed green granular intracytoplasmic reaction ( Supplementary Figure 3B ).
Surface Swab Samples
Of the 68 swab samples collected at hospitals A and B, 42 samples tested positive for MERS-CoV by RT-PCR and sequencing (Table 1 ; Supplementary Table 1 ). In particular, 13 of 16 fomite swabs and 29 of 52 fixed-structure swabs were positive. Furthermore, MERS-CoV was cultured from 15 swabs comprising 7 of 16 swabs from fomites including a stethoscope, and 8 of 52 swabs obtained from fixed structures including doorknobs and bed guardrails (Figures 1B , 1C , and 2B ; Table 1 ; Supplementary Table 1 ). Interestingly, 1 of 5 swabs obtained from the air exhaust damper and 1 of 10 swabs obtained from elevators were positive for MERS-CoV, as determined by viral culture (Table 1 ; Supplementary Table 1 ; Supplementary Figure 2 ). In addition, EM images of the cultured virus from swab samples revealed intact virus particles compatible with MERS-CoV (data not shown), and IFA using MERS-CoV Spike antibody on the tissue cultures revealed green granular intracytoplasmic reaction ( Supplementary Figure 3B ).
DISCUSSION
Our data demonstrate the presence of MERS-CoV in the hospital environment including air, fomites, and environmental surfaces, although they do not provide direct insight into the routes of transmission. However, the presence of MERS-CoV on the environmental surfaces and in the air of a MERS-CoV–infected patient's room that was routinely disinfected by standard procedures suggests that MERS-CoV can be transmitted via contact and aerosols. Therefore, our findings provide important evidence for the possible routes for distant transmission of MERS-CoV. In addition, our findings call for epidemiologic investigation of the possible scenarios for remote transmission, and raise concern regarding the adequacy of current infection control procedures.
As mentioned above, transmission beyond 6 feet from the index patient could be explained by 2 scenarios; (1) contaminated fomites or environmental structures, and/or (2) airborne transmission. It has been proposed that the severe acute respiratory syndrome (SARS) outbreak in the Hong Kong M Hotel was due to contaminated environmental surfaces [ 8 ], and that those in Amoy Garden in Hong Kong, in airplanes, and in the Prince of Wales Hospital in Hong Kong were due to airborne transmission [ 9–11 ]. Roy and Milton have suggested that SARS-CoV is not transmitted by either droplet transmission or airborne transmission, but rather by a process lying somewhere between the two [ 12 ]. Given that we found that air and surrounding surfaces including accessible and inaccessible areas were all contaminated by MERS-CoV, we agree that the transmission mode of MERS-CoV differs from droplet transmission. Further studies are needed to clarify this question.
There is evidence supporting aerosol transmission of MERS. Experimental aerosolization of MERS-CoV did not decrease its stability at 20°C and 40% relative humidity [ 13 ], and MERS-CoV RNA was detected in an air sample from the barn of an infected camel that transmitted MERS-CoV to a patient who died [ 14 ]. In addition, a recent study demonstrated that the human lung parenchyma has abundant dipeptidyl peptidase 4 (DPP4) receptors for MERS-CoV whereas the human nasal mucosa has few DPP4 receptors for MERS-CoV [ 15 ]. Hence, we assume that aerosol transmission of MERS-CoV is possible, and that an aerosol with a high concentration of infectious particles, and contamination of the surrounding environment, might mimic that expected of large-droplet sprays and surface contact [ 12 ]. We therefore cautiously recommend that contact tracing and infection control precautions equivalent to those involved in cases of airborne transmission are needed in hospitals where patients who are severely ill with MERS stay. It is worth noting that many environmental swab samples contained MERS-CoV despite daily cleaning and disinfection of the patients' rooms. These findings are consistent with previous studies that demonstrated survival of MERS-CoV for 2 days on plastic and steel surfaces [ 13 ], survival of SARS-CoV for 3 days on various surfaces [ 16 ], and survival of human CoV for 6 days in air [ 17 ]. Therefore, the extensive environmental contaminations and prolonged environmental presence of MERS-CoV may partially explain why MERS is easily spread in healthcare settings. In addition, our data emphasize the importance of strict adherence to infection control precautions, including hand hygiene.
An independent research group in South Korea has isolated viruses from swab samples in the environment of MERS-CoV–infected patients and demonstrated that most accessible surfaces in MERS units were contaminated by MERS-CoV, confirmed by RT-PCR and viral culture [ 7 ]. On the other hand, a group in China conducted environmental sampling of the room of a MERS-infected patient on days 13 and 15 after symptom onset, and the swabs were negative for viral RNA [ 18 ]. Our study results partially overlap with those of the study of Bin et al [ 7 ], who performed environmental sampling of the 4 rooms of 4 MERS-infected patients between days 18 and 30 after symptom onset [ 7 ]. However, the positive rates of RT-PCR and viral culture in the previous study [ 7 ] were 20% (30/148) and 4% (6/148), respectively, whereas those in the present study were 65% (49/75) and 25% (19/75), respectively. Caution is needed when comparing the results of our study to those of the previous study [ 7 ], because ours was more focused on airborne transmission. Actually, Bin et al showed that MERS-CoV was detected by RT-PCR but not by viral culture on the entrance to air-ventilating equipment in one patient's room, and they suggested the existence of airborne virus particles. However, they did not perform air sampling. We tested air samples and swabbed surfaces such as the ventilator exit and the top of televisions, which were inaccessible, and areas remote from the patients as well as areas easily missed by daily cleaning. So, it is possible that MERS-CoV particles could have been concentrated in exhaust air grills and the corners of rooms that were not routinely disinfected.
This study has a few limitations. First, it was performed late in the Korean MERS outbreak. The 3 patients included in the study were at similar stages of disease progression, between 16 and 22 days after symptom onset, and patients at a stage <1 week after symptom onset were not included. In addition, it would be interesting to examine the environmental contamination surrounding less severely ill patients. Second, some may question the isolation of viable virus from the surroundings of patient 3, in whom the last positive RT-PCR for MERS-CoV was in a respiratory specimen taken 6 days prior to the date of the environmental sampling. Actually, because a respiratory sample was not taken at the time of the environmental sampling, we cannot know whether the results of RT-PCR of a respiratory specimen would have been positive or not. However, in the previous study [ 7 ], the patient's room and medical equipment were positive for virus up to 5 days after the patient's last positive PCR for a respiratory specimen. We thus assume that virus can still be detected several days after negative PCR conversion of respiratory specimens. Hence, the absence of results for a respiratory specimen from patient 3 at the time of environmental sampling does not significantly affect the interpretation of our results. Finally, the experimental data indicating extensive surface and air contamination only provide some insight into the possible routes of transmission; they do not fully identify the route(s) of transmission. Further epidemiologic and experimental studies are urgently needed in this area.
In conclusion, these data provide experimental confirmation for extensive viral contamination of the air and materials surrounding patients with MERS, pointing to the possibility of airborne and contact transmission during the 2015 MERS outbreak in Korea. Our demonstration that MERS-CoV can be shed into the air and surface environment will no doubt guide the response to future MERS outbreaks.
Supplementary Data
Supplementary materials are available here. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. We thank Chan-Sik Park and Mee Kyeung Gong (Department of Pathology, Asan Medical Center, Seoul, South Korea) for electron microscopic images; Hye-Won Jung (e-medical contents team, Asan Medical Center, Seoul, South Korea) for the excellent artwork; and the Suwon Medical Center for administrative support.
Financial support. This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI15C2774). This work was also supported by the National Research Foundation of Korea (NRF grant number 2014K1A4A7A01074646) funded by the Korea government and by the Gyeonggi Institute of Science & Technology Promotion Middle East respiratory syndrome grant funded by Gyeonggi-do.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ki M . 2015 MERS outbreak in Korea: hospital-to-hospital transmission . Epidemiol Health 2015. ; 37 : e2015033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF , Lau SK , To KK , Cheng VC , Woo PC , Yuen KY . Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease . Clin Microbiol Rev 2015. ; 28 : 465 – 522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . MERS. Interim guidance for healthcare professionals . Available at : http://www.cdc.gov/coronavirus/mers/interim-guidance.html . Accessed 15 September 2015 .
- 4. Park YS , Lee C , Kim KM et al. . The first case of the 2015 Korean Middle East respiratory syndrome outbreak . Epidemiol Health 2015. ; 37 : e2015049 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oh MD , Choe PG , Oh HS et al. . Middle East respiratory syndrome coronavirus superspreading event involving 81 persons, Korea 2015 . J Korean Med Sci 2015. ; 30 : 1701 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korea Centers for Disease Control and Prevention . Middle East respiratory syndrome coronavirus outbreak in the Republic of Korea, 2015 . Osong Public Health Res Perspect 2015. ; 6 : 269 – 78 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bin SY , Heo JY , Song MS et al. . Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea . Clin Infect Dis 2016. ; 62 : 755 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braden CR , Dwell SF , Jernigan DB , Hughes JM . Progress in global surveillance and response capacity 10 years after severe acute respiratory syndrome . Emerg Infect Dis 2013. ; 19 : 864 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu IT , Li Y , Wong TW et al. . Evidence of airborne transmission of the severe acute respiratory syndrome virus . N Engl J Med 2004. ; 350 : 1731 – 9 . [DOI] [PubMed] [Google Scholar]
- 10. Olsen SJ , Chang HL , Cheung TY et al. . Transmission of the severe respiratory syndrome on aircraft . N Engl J Med 2003. ; 349 : 2416 – 22 . [DOI] [PubMed] [Google Scholar]
- 11. Wong TW , Lee CK , Tam W et al. . Cluster of SARS among medical students exposed to single patient, Hong Kong . Emerg Infect Dis 2004. ; 10 : 269 – 76 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy CJ , Milton D . Airborne transmission of communicable infection—the elusive pathway . N Engl J Med 2004. ; 350 : 1710 – 2 . [DOI] [PubMed] [Google Scholar]
- 13. van Doremalen N , Bushmaker T , Munster VJ . Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions . Euro Surveill 2013. ; 18 : 20590 . [DOI] [PubMed] [Google Scholar]
- 14. Azhar EI , Hashem AM , El-Kafrawy SA et al. . Detection of the Middle East respiratory syndrome coronavirus genome in an air sample originating from a camel barn owned by an infected patient . mBio 2014. ; 5 : e01450 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyerholz DK , Lambertz AM , McCray PB Jr . Dipeptidyl peptidase 4 distribution in the human respiratory tract: implications for the Middle East respiratory syndrome . Am J Pathol 2016. ; 186 : 78 – 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duan SM , Zhao XS , Wen RF et al. . SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation . Biomed Environ Sci 2003. ; 16 : 246 – 55 . [PubMed] [Google Scholar]
- 17. Ijaz MK , Brunner AH , Sattar SA , Nair R , Johnson-Lussenburg CM . Survival characteristics of airborne human coronavirus 229E . J Gen Virol 1985. ; 66 : 2743 – 8 . [DOI] [PubMed] [Google Scholar]
- 18. Da Guan W , Mok CK , Chen ZL et al. . Characteristics of traveler with Middle East respiratory syndrome, China, 2015 . Emerg Infect Dis 2015. ; 21 : 2278 – 80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.