Abstract
Accumulating evidence suggests that lncRNAs are involved in almost all normal physiological processes and that aberrant expression of lncRNAs may be involved in the development of diseases, including non-small cell lung cancer (NSCLC). However, the roles of lncRNA-TPTE pseudogene 1 (TPTEP1) in lung cancer and the underlying molecular mechanisms have remained elusive. In the present study, significant downregulation of TPTEP1 in tumors compared with normal tissues from patients with NSCLC was observed. Overexpression of TPTEP1 inhibited cell proliferation and induced apoptosis in NSCLC cells. A bioinformatics analysis based on miRDB predicted microRNA (miR)-328-5p as a potential binding miRNA for TPTEP1. Using a dual-luciferase reporter assay and western blot analysis, it was further validated that TPTEP1 sponged miR-328-5p to upregulate Src kinase signaling inhibitor 1 (SRCIN1) in NSCLC cells. Through regulation of SRCIN1, TPTEP1 was indicated to inactivate the Src and STAT3 pathways in NSCLC cells. Notably, silencing of SRCIN1 reversed the TPTEP1 overexpression-induced inhibition of cell proliferation and increase of the apoptotic rate in NSCLC cells. Pearson correlation analysis revealed a significant positive correlation between TPTEP1 and SRCIN1 mRNA levels in NSCLC tumors. The present results provided insight into the roles of TPTEP1 in NSCLC and the underlying mechanisms.
Keywords: long non-coding RNA TPTE pseudogene 1, microRNA-328-5p, non-small cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer-associated mortality for males and females worldwide. According to statistics, >2 million new cases were diagnosed in 2018 and ~1.7 million patients succumbed to lung cancer (1). According to the pathological features, lung cancer may be categorized into two major types: Non-small cell lung cancer (NSCLC) and SCLC (2,3). Compared with SCLC, NSCLC is a common lung cancer subtype, accounting for >80% of diagnosed cases (4). NSCLC is insensitive to chemotherapy and the prognosis of patients with NSCLC is unsatisfactory with a five-year overall survival rate of <50% (5,6). Further investigation of the molecular mechanisms of NSCLC is imperative to provide valuable targets for the development of novel therapeutic approaches for patients with NSCLC (7).
Non-coding RNAs are single-stranded RNAs with no protein-coding function (8). Long non-coding RNAs (lncRNAs) are non-coding RNAs of >200 nucleotides in length (9). Previously considered as ‘junk’ RNAs, recent studies revealed that dysregulation of lncRNAs is relevant to human diseases, including tumorigenesis (10,11). Mechanistically, lncRNAs may interact with micro (mi)RNAs, mRNAs or proteins to regulate gene expression and protein localization (12,13). The competing endogenous (ce)RNA hypothesis suggests that the expression of lncRNAs controls the mRNAs levels via sponging miRNAs (14). Several lncRNAs have been reported to be oncogenes or tumor suppressors in NSCLC (15–17). For instance, Yang et al (18) indicated that lncRNA insulin-like growth factor binding protein 4-1 was significantly upregulated in lung cancer and promoted tumor cell metabolism to facilitate cancer cell proliferation. lncRNA-HIT interacted with E2F transcription factor 1 to regulate target gene expression and promoted cell proliferation of NSCLC cells (19). lncRNA TPTE pseudogene 1 (TPTEP1) was identified as one of most significantly downregulated lncRNAs in NSCLC via a bioinformatics analysis of The Cancer Genome Atlas (TCGA) dataset (20). However, the roles of TPTEP1 in NSCLC have remained elusive.
Src kinase signaling inhibitor 1 (SRCIN1), also known as p140CAP, is an adapter protein that binds to Src and inactivates Src kinase through C-terminal Src kinase (21). Non-receptor protein tyrosine kinase Src is a well-characterized oncogene and its activity is associated with the progression of cancer (22,23). Src is known to mediate several oncogenic signaling pathways in cancer cells, including the PI3K and STAT3 pathways (24,25). Via inactivation of Src, SRCIN1 functions as a tumor suppressor in multiple cancer types (26,27). However, it has remained elusive how SCRIN1 expression is regulated in NSCLC.
The present study aimed to investigate the clinicopathological significance and prognosis of TPTEP1 as well as its functional role in NSCLC. A bioinformatics analysis, reverse transcription-quantitative (RT-q)PCR, western blot analysis and dual-luciferase reporter assays were performed to explore the molecular mechanisms of TPTEP1 in NSCLC cells. The results demonstrated a tumor suppressor role of TPTEP1 in NSCLC.
Materials and methods
Patients and samples
Human NSCLC tumors and matched normal tissues were collected from 56 patients (41 males and 15 females; age range, 35–76 years) with NSCLC who underwent surgery at Shangqiu First People's Hospital and the First Affiliated Hospital of Henan University between June 2015 and July 2016. The information of sex, age and smoking history was obtained from patients. Written informed consent was obtained from all participants prior to the study. The patients did not receive any chemotherapy or radiotherapy prior to surgery. The NSCLC samples were staged according to surgical and pathological results, which were based on the guidelines described by the 7th edition of the American Joint Committee on Cancer/Union for International Cancer Control (28). All experiments were approved by the Ethics Committee of Shangqiu First People's Hospital and the First Affiliated Hospital of Henan University. Tissues were stored in liquid nitrogen at the time of surgery and stored in a −80°C refrigerator.
Cell lines and culture
Human NSCLC cell lines (A549 and NCI-H1299) and the human lung epithelial cell line BEAS-2B were purchased from the American Type Culture Collection. These cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator with 5% CO2.
RNA extraction and RT-qPCR
Total RNA was extracted from BEAS-2B, A549, NCI-H1299 cells and tissue samples with the RNeasy Mini Kit (Qiagen) following the manufacturer's protocol. The RNA concentration was measured with a NanoDrop 2000 (Thermo Fisher Scientific, Inc.). First-strand complementary (c) DNA was synthesized with a SuperScript III First-Strand kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Realtime qPCR was performed using TB Green Premix Ex Taq (Takara Bio, Inc.) with the following protocol: Initial pre-denaturation at 98°C for 30 sec, followed by 35 cycles of denaturation at 98°C for 5 sec and elongation/annealing at 60°C for 30 sec. GAPDH and U6 were used as internal controls for mRNA and miRNA, respectively. The relative expression of genes were calculated with the 2−ΔΔCq method (29). The primer sequences were listed as follows: Stem-loop, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCTGA-3′; miR-328-5p-forward, 5′-GCCGAGGGGGGGGCAGGAGG-3′ and reverse, 5′-CTCAACTGGTGTCGTGGA-3′; TPTEP1 forward, 5′-CTGGGAGAAGTGCCCTTGC-3′ and reverse, 5′-CACCTCATCAGTCATTTGCTCA-3′; SRCIN1 forward, 5′-GAGGCTCGCAACGTCTTCTAC-3′ and reverse, 5′-GCGATGCGTACACCATCTCTC-3′; GAPDH forward, 5′-TCAACAGCAACTCCCACTCTTCCA-3′ and reverse, 5′-ACCCTGTTGCTGTAGCCGTATTCA-3′.
Overexpression of TPTEP1 and silencing of SRCIN1
Full-length TPTEP1 was amplified by PCR (TPTEP1 forward, 5′-GTGAATTCCTCGAGACTAGTTCTGCCTCTCCCGGTACCTGCT-3′ and reverse, 5′-GGATCCGCGGCCGCTCTAGCACTAGTTTTTGATGGAATTTTTAGTTT-3′) from A549 cDNA and ligated into pcDNA3.1 plasmid. pcDNA3.1 or pcDNA3.1-TPTEP1 was transfected into A549 or NCI-H1299 cells with Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. SRCIN1 siRNA and control siRNA were purchased from GenePharma Co., Ltd. SRCIN1 siRNA (5′-GCCCGCUGAGCGCCUCCAGAC-3′) or control siRNA (5′-UUCUCCGAACGUGUCACGU-3′) was transfected into A549 or NCI-H1299 cells with Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. After 72 h, the cells were collected and the RNA or proteins were extracted for the subsequent experiments.
Knockdown and overexpression of miR-328-5p
miR-negative control (NC) mimics (5′-UUCUCCGAACGUGUCACGU-3′), miR-328-5p mimics (5′-AGGGGGGGCAGGAGGGGCUCAGGG-3′), miR-NC inhibitor (5′-UUCUCCGAACGUGUCACGU-3′) and miR-328-5p inhibitor (5′-CCCUGAGCCCCUCCUGCCCCCCCU-3′) were synthesized and provided by GenePharma Co., Ltd. miR-NC mimics, miR-328-5p mimics, miR-NC inhibitor or miR-328-5p inhibitor was transfected into A549 or NCI-H1299 cells with Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. At 72 h after transfection, the cells were collected and subjected to RT-qPCR or western blot analysis.
Dual-luciferase reporter assay
Full-length TPTEP1 and the full length of 3′untranslated region (3′UTR) of SRCIN1 were amplified from A549 cDNA and ligated into pGL3-basic plasmid. Two site mutations were introduced into pGL3-TPTEP1 or pGL3-SRCIN1 3′UTR plasmid with a Quick Site-directed mutation kit (Agilent Technologies, Inc.). For the dual-luciferase reporter assay, cells were transfected with a combination of pGL3-TPTEP1 or pGL3-SRCIN1 3′UTR or pGL3-TPTEP1 mutated (Mut) or pGL3-SRCIN1 3′UTR-Mut with miR-NC mimics or miR-328-5p mimics by using Lipofectamine 3000. At 48 h after transfection, the relative luciferase activity was measured with a Dual-Luciferase Reporter Assay System (Promega Corp.).
Protein extraction and western blot analysis
Primary antibodies of SRCIN1 (product number 3757; dilution 1:1,000), P-SRC (TYR416) (product number 6943; dilution 1:1,000), SRC (product number 2108; dilution 1:1,000) p-STAT3 (product number 9145; dilution 1:1,000) and STAT3 (product number 9139; dilution 1:1,000) were purchased from Cell Signaling Technology, Inc. GAPDH (product code ab8245; dilution 1:5,000) antibody was obtained from Abcam. HRP-conjugated secondary antibodies of mouse (cat. no. SA00001-1; dilution 1:5,000) and rabbit (cat. no. SA00001-2; dilution 1:5,000) were purchased from ProteinTech Group. Radioimmunoprecipitation assay lysis buffer (RIPA; Thermo Fisher Scientific, Inc.) was used to prepare protein lysates from cells following the manufacturer's protocol. Subsequently, the protein concentration was determined with a BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). Lysates containing 25 µg protein were loaded in each well of 8% SDS gels and separated by SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes (EMD Millipore) and blocked with non-fat milk for 30 min at room temperature. The membrane was then washed and incubated with a primary antibody for 1 h at room temperature. Subsequently, the membranes were washed and incubated with secondary antibodies for 1 h at room temperature. Finally, the blots were developed with ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc.). The relative expression of protein was quantified with ImageJ software (v. 1.52r; National Institutes of Health).
Bioinformatics analysis
The expression of TPTEP1 in multiple cancer types and normal tissues of different organs was analyzed using the Gene Expression Profiling Interactive Analysis database (GEPIA; http://gepia.cancer-pku.cn/) based on TCGA datasets. To study the association between TPTEP1 expression and prognosis of patients with NSCLC, the gene expression data and patient survival information from the dataset GSE30219 (30) comprising 307 patients with lung cancer were downloaded. The samples were divided into two groups based on the expression of TPTEP1 (above or below the median expression level of TPTEP1 (relative expression of TPTEP1=4.20554) (n=141 for each group) and were analyzed using Kaplan-Meier analysis with log-rank testing. The miRNAs containing putative binding sites for TPTEP1 were predicted with miRDB software (http://mirdb.org/). The potential target genes of miR-328-5p were predicted with TargetScan software (http://www.targetscan.org/vert_72/).
Cell proliferation assay
The proliferation ability of cells was determined with a CCK-8 kit (Dojindo Molecular Technologies, Inc.) following the manufacturer's protocol. On the first day, 5,000 cells were seeded in each well of 96-well plates. On the second day, cells were transfected with plasmids and/or miRNA inhibitor. At 0, 24, 48 and 72 h after transfection, 10 µl CCK-8 solution was added to each well, followed by incubation for 2 h. The medium containing CCK-8 was transferred to another 96-well plate and the absorbance at 450 nM was detected with a microplate reader (Bio-Rad Laboratories, Inc.) to determine the number of cells.
Cell apoptosis assay
The cell apoptosis rate was detected with a Dead Cell Apoptosis Kit with Annexin V Alexa Fluor™ 488 and propidium iodide (PI) (Thermo Fisher Scientific, Inc.). In brief, at 48 h after transfection, cells were harvested and resuspended in 1X Binding Buffer provided with the kit. Subsequently, 5 µl PI and 1 µl Annexin V was added to the cells, followed by incubation at room temperature for 15 min. The cells were immediately subjected to flow cytometric analysis on a MACSQuant Analyzer X (Miltenyi Biotec, Inc.). The data were analyzed using FlowJo 10 software (FlowJo LLC).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software, Inc.). Student's t-test (paired test for specimens and unpaired test for cell-based assays) was applied to analyze differences between two groups. For comparison of three groups, one-way analysis of variance followed by Student-Neuman-Keuls analysis was performed. The association between TPTEP1 expression and characteristics of patients was analyzed by Chi-square test. All statistical analyses were two-tailed and P<0.05 was considered to indicate a statistically significant difference.
Results
lncRNA TPTEP1 is downregulated in NSCLC and associated with good prognosis
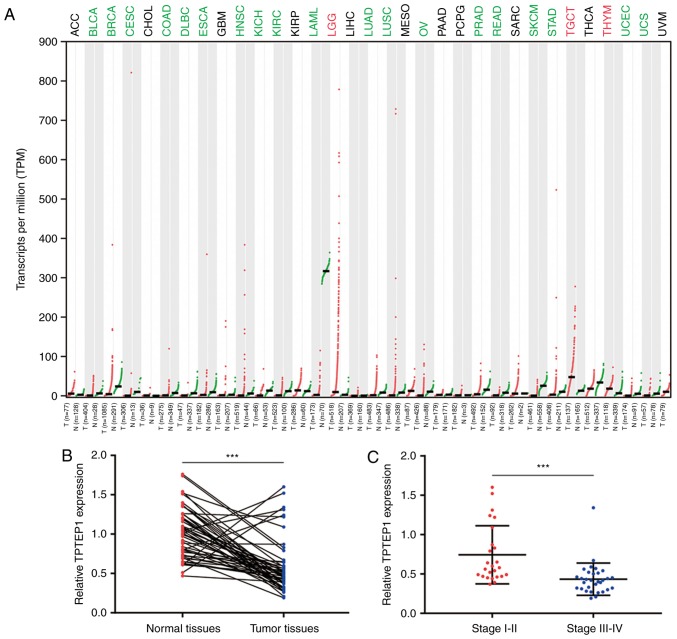
To investigate the expression pattern of TPTEP1 in NSCLC, the GEPIA database was used to explore its expression in cancer and normal tissues of various origins from TCGA datasets. TPTEP1 was downregulated in a majority of cancer types, including bladder urothelial carcinoma and breast invasive carcinoma. Notably, TPTEP1 was significantly downregulated in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) (Fig. 1A). For confirmation, RT-qPCR was performed to detect TPTEP1 expression in 56 pairs of tumors and adjacent normal tissues from patients with NSCLC. The results revealed that TPTEP1 was downregulated in most tumors (Fig. 1B). It was also indicated that TPTEP1 expression was lower in high-grade (stage III–IV) tumors compared with that in low-grade tumors (stage I–II; Fig. 1C). Low expression of TPTEP1 was associated with high-grade tumors (stage III–IV; P=0.006), but no significant association was determined between TPTEP1 expression and sex, age and smoking history (Table I). The prognostic value of TPTEP1 in patients with NSCLC was then analyzed. For the dataset GSE30219, the Kaplan-Meier plot indicated that high expression of TPTEP1 was associated with a favorable overall survival for patients with NSCLC (log-rank P=0.001; Fig. 1D). In addition, in the tumor tissues collected from patients with NSCLC, high expression of TPTEP1 was further confirmed to be associated with a relatively good prognosis (log-rank P=0.036; Fig. 1E). Next, TPTEP1 expression was analyzed in a panel of NSCLC cell lines and lung epithelial cells. TPTEP1 was ~2-fold decreased in NSCLC cells (A549 and NCI-H1299) compared with that in lung epithelial cells (BEAS-2B) (Fig. 1F).
Figure 1.
TPTEP1 is downregulated in NSCLC and predicts a favorable prognosis. (A) According to scatter diagram depiction of TPTEP1 expression in multiple cancers and normal tissues generated from GEPIA database, TPTEP1 was downregulated in several cancer types including LUAD and LUSC. (B) RT-qPCR was performed to detect TPTEP1 expression in 56 pairs of tumors and matched normal tissues from patients with NSCLC. TPTEP1 was significantly downregulated in NSCLC tumors. (C) Expression of TPTEP1 was decreased in high grade (grade III–IV) NSCLC tumors compared to those of low grade (grade I–II). (D) Using data from GSE30219 (n=307), the Kaplan-Meier analysis revealed that the TPTEP1 high-expression group exhibited a prolonged overall survival compared with those with low expression. (E) Kaplan-Meier analysis based on 56 patients with NSCLC revealed that patients with high expression of TPTEP1 exhibited a prolonged overall survival compared to those with low expression. (F) RT-qPCR revealed that TPTEP1 was downregulated in NSCLC cells (A549 and NCI-H1299) compared to lung epithelial cells (BEAS-2B). **P<0.01; ***P<0.001. TPTEP1, TPTE pseudogene 1; NSCLC, non-small cell lung cancer; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma.
Table I.
Association between clinicopathological characteristics and TPTEP1 expression in patients with NSCLC.
| TPTEP1 expression | ||||
|---|---|---|---|---|
| Characteristics | Total cases | High expression | Low expression | P-value |
| Sex | 0.227 | |||
| Male | 41 | 23 | 18 | |
| Female | 15 | 5 | 10 | |
| Age | 0.391 | |||
| <60 | 18 | 7 | 11 | |
| ≥60 | 38 | 21 | 17 | |
| Smoking history | 0.503 | |||
| No | 11 | 7 | 4 | |
| Yes | 45 | 21 | 24 | |
| TNM stage | 0.006 | |||
| I–II | 23 | 17 | 6 | |
| III–IV | 33 | 11 | 22 | |
TPTEP1, TPTE pseudogene 1; NSCLC, non-small cell lung cancer.
Overexpression of TPTEP1 inhibits NSCLC cell proliferation and induces apoptosis
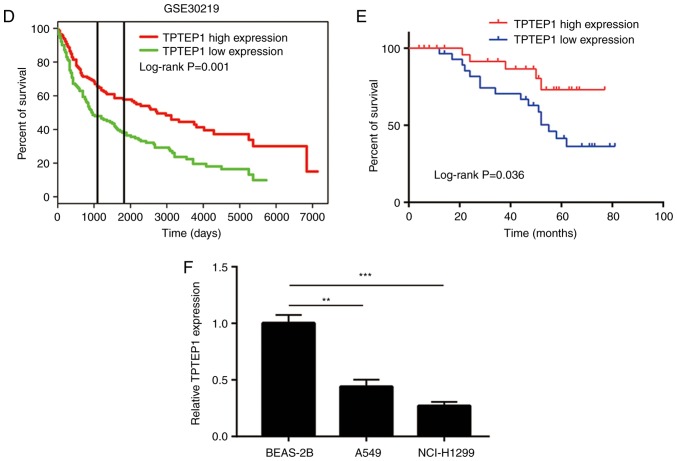
To study the role of TPTEP1 in NSCLC, pcDNA3.1-TPTEP1 with full-length TPTEP1 was constructed and transfected into NSCLC cells. Transfection of TPTEP1 led to a >4-fold increase of TPTEP1 in A549 and NCI-H1299 cells (Fig. 2A and B). In the cell proliferation assay, overexpression of TPTEP1 led to a significant decrease in the proliferation rate of A549 and NCI-H1299 cells (Fig. 2C and D). The strong cell growth-inhibitory effect of TPTEP1 suggested that cell apoptosis may be involved in this process. Flow cytometry was used to determine the ratio of apoptotic cells following overexpression of TPTEP1. The results indicated that overexpression of TPTEP1 significantly increased the apoptotic ratio of A549 cells (Fig. 2E). Similarly, a significant increase of cell apoptosis was observed in NCI-H1299 cells transfected with TPTEP1 (Fig. 2F). The results collectively indicated that TPTEP1 was pivotal for resistance to apoptosis of NSCLC cells.
Figure 2.
TPTEP1 overexpression inhibits cell proliferation and induces cell apoptosis in NSCLC cells. (A) Transfection of pcDNA3.1-TPTEP1 increased TPTEP1 expression in A549 cells. (B) Transfection of pcDNA3.1-TPTEP1 increased TPTEP1 expression in NCI-H1299 cells. (C) Overexpression of TPTEP1 inhibited cell proliferation in A549 cells. (D) Overexpression of TPTEP1 inhibited cell proliferation in NCI-H1299 cells. (E) Overexpression of TPTEP1 induced cell apoptosis in A549 cells. (F) Overexpression of TPTEP1 induced cell apoptosis in NCI-H1299 cells. ***P<0.001. TPTEP1, TPTE pseudogene 1; NSCLC, non-small cell lung cancer.
TPTEP1 sponges miR-328-5p in NSCLC cells
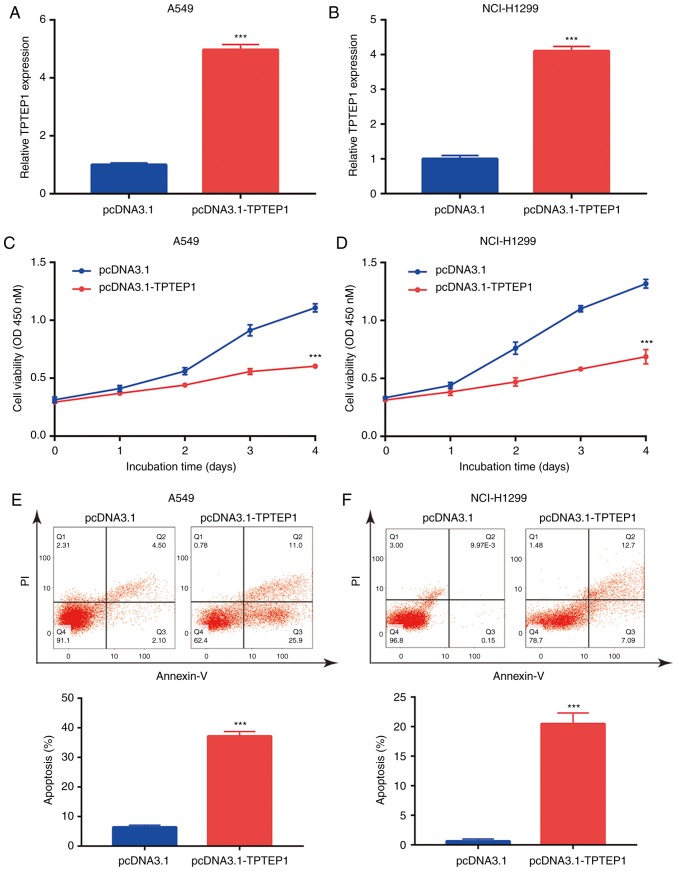
lncRNAs may function as ceRNAs to sponge certain miRNAs and thereby regulate cancer progression (31). By using miRDB, 38 miRNAs containing a putative binding site for TPTEP1 were predicted. Among them, miR-328-5p was previously reported as an upregulated miRNA in NSCLC (32). As indicated in Fig. 3A, a putative binding site for miR-328-5p on TPTEP1 was predicted. In the 56 NSCLC tumors, a significant negative correlation between miR-328-5p levels and TPTEP1 expression was observed (r=−0.416, P<0.01; Fig. 3B). To explore the regulatory association between miR-328-5p and TPTEP1, miR-328-5p was knocked down in A549 and NCI-H1299 cells by transfection of miR-328-5p inhibitor (Fig. 3C and D). Downregulation of miR-328-5p led to an increase of TPTEP1 expression in A549 cells (Fig. 3E). A similar effect was also observed in NCI-H1299 cells (Fig. 3F). Conversely, overexpression of TPTEP1 decreased miR-328-5p levels in A549 and NCI-H1299 cells (Fig. 3G and H). Transfection of miR-328-5p mimics was confirmed to increase miR-328-5p levels in A549 and NCI-H1299 cells (Fig. 3I and J). In the dual-luciferase reporter assay, overexpression of miR-328-5p was indicated to decrease the relative luciferase activity of pGL3-TPTEP1-wild-type (WT) plasmid but did not influence the relative luciferase activity of pGL3-TPTEP1-Mut in A549 cells (Fig. 3K). Similarly, miR-328-5p mimics also suppressed the relative luciferase activity of pGL3-TPTEP1-WT in NCI-H1299 cells (Fig. 3L). These results indicated that TPTEP1 directly interacts with miR-328-5p in NSCLC cells.
Figure 3.
TPTEP1 sponges miR-328-5p in NSCLC cells. (A) Sequence alignment of TPTEP1 to miR-328-5p is revealed. Two site mutations were introduced into putative binding sites of miR-328-5p in TPTEP1 sequence. (B) Pearson correlation analysis revealed a negative correlation between TPTEP1 and miR-328-5p levels in NSCLC tumors. (C) Transfection of miR-328-5p inhibitor decreased miR-328-5p levels in A549 cells. (D) Transfection of miR-328-5p inhibitor decreased miR-328-5p levels in NCI-H1299 cells. (E) Transfection of miR-328-5p inhibitor increased TPTEP1 levels in A549 cells. (F) Transfection of miR-328-5p inhibitor increased TPTEP1 levels in NCI-H1299 cells. (G) Overexpression of TPTEP1 decreased miR-328-5p expression in A549 cells. (H) Overexpression of TPTEP1 decreased miR-328-5p expression in NCI-H1299 cells. (I) Transfection of miR-328-5p mimic increased miR-328-5p levels in A549 cells. (J) Transfection of miR-328-5p mimic increased miR-328-5p levels in NCI-H1299 cells. (K) Transfection of miR-328-5p mimic decreased the relative luciferase activity of pGL3-TPTEP1-WT not pGL3-TPTEP1-Mut in A549 cells. (L) Transfection of miR-328-5p mimic decreased the relative luciferase activity of pGL3-TPTEP1-WT not pGL3-TPTEP1-Mut in NCI-H1299 cells. ***P<0.001. TPTEP1, TPTE pseudogene 1; NSCLC, non-small cell lung cancer.
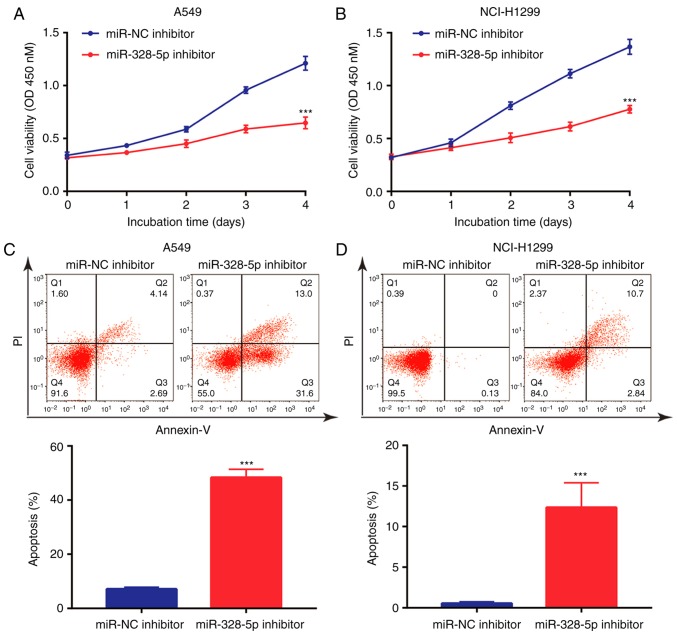
Downregulation of miR-328-5p inhibits NSCLC cell proliferation and induces cell apoptosis
Although it has been previously indicated that miR-328-5p was overexpressed in NSCLC and may serve as a diagnostic biomarker (32), the function of miR-328-5p in NSCLC has remained elusive. The present results indicated that in A549 and NCI-H1299 cells, downregulation of miR-328-5p inhibited cell proliferation (Fig. 4A and B). In addition, miR-328-5p inhibitor induced apoptosis in A549 cells (Fig. 4C), which was also observed in NCI-H1299 cells (Fig. 4D). Collectively, similar to the effect of overexpression of TPTEP1, downregulation of miR-328-5p inhibited cell proliferation and induced apoptosis in NSCLC cells.
Figure 4.
miR-328-5p promotes cell proliferation and survival of NSCLC cells. (A) Transfection of miR-328-5p inhibitor inhibited cell proliferation of A549 cells. (B) Transfection of miR-328-5p inhibitor inhibited cell proliferation of NCI-H1299 cells. (C) Transfection of miR-328-5p inhibitor induced cell apoptosis of A549 cells. (D) Transfection of miR-328-5p inhibitor induced cell apoptosis of NCI-H1299 cells. ***P<0.001. TPTEP1, TPTE pseudogene 1; NSCLC, non-small cell lung cancer.
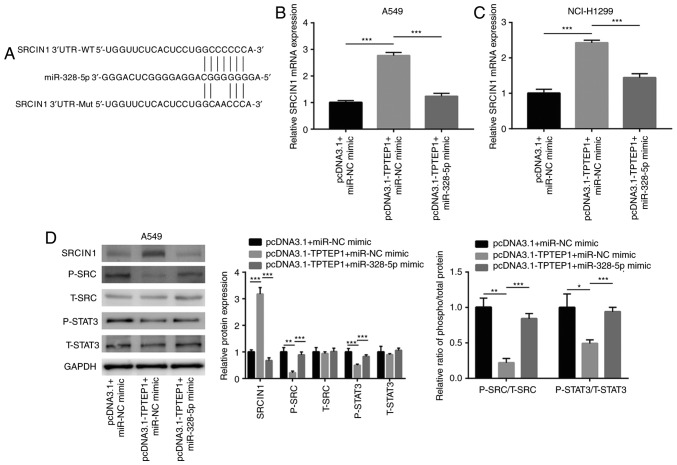
TPTEP1 causes upregulation of SRCIN1 via sponging miR-328-5p
Using TargetScan, several target genes of miR-328-5p were predicted, and among them, SRCIN1 is a well-characterized tumor suppressor (33) which was indicated to share a putative binding site for miR-328-5p (Fig. 5A). Overexpression of TPTEP1 increased SRCIN1 mRNA levels, and transfection of miR-328-5p mimics attenuated the upregulation of SRCIN1 mRNA in A549 and NCI-H1299 cells (Fig. 5B and C). Previous studies reported that SRCIN1 directly inactivated Src signaling and indirectly inactivated STAT3 signaling in cancer cells (34,35). In the present study, western blot analysis revealed that TPTEP1 overexpression increased SRCIN1 protein expression and decreased p-Src and p-STAT3 protein levels in A549 cells (Fig. 5D). The decrease of the p-SRC/t-SRC and p-STAT3/t-STAT3 ratios indicated inactivation of SRC and STAT3 signaling in response to TPTEP1 overexpression in A549 cells. Similar to the effect in A549 cells, TPTEP1 overexpression increased SRCIN1 protein expression and decreased p-SRC and p-STAT3 protein levels in NCI-H1299 cells (Fig. 5E). SRC and STAT3 signaling became inactivated in NCI-H1299 after TPTEP1 overexpression. In the dual-luciferase reporter assay, miR-328-5p mimics suppressed the relative luciferase activity of the plasmid driven by the SRCIN1 3′UTR in A549 and NCI-H1299 cells (Fig. 5F and G), revealing that miR-328-5p directly binds to the 3′UTR of SRCIN1 mRNA. Hyperactivation of Src and STAT3 signaling are pivotal for cancer cell survival (36,37). The present results indicated that TPTEP1 sponged miR-328-5p to upregulate SRCIN1 and inactivated Src and STAT3 signaling to promote NSCLC cell apoptosis.
Figure 5.
TPTEP1 promoted SRCIN1 expression via sponging miR-328-5p. (A) There was a putative binding site for miR-328-5p on SRCIN1 3′UTR. Two sites mutations were introduced into SRCIN1 3′UTR on the putative binding site. (B) SRCIN1 mRNA was upregulated after TPTEP1 overexpression which was reversed after miR-328-5p overexpression in A549 cells. (C) SRCIN1 mRNA was upregulated after TPTEP1 overexpression which was reversed after miR-328-5p overexpression in NCI-H1299 cells. (D) Western blotting revealed that TPTEP1 overexpression increased SRCIN1 and decreased p-SRC, p-STAT3 protein expression which was reversed after miR-328-5p overexpression in A549 cells. (E) Western blotting revealed that TPTEP1 overexpression increased SRCIN1 and decreased p-SRC, p-STAT3 protein expression which was reversed after miR-328-5p overexpression in NCI-H1299 cells. (F) The dual luciferase reporter assay revealed that miR-328-5p mimic reduced the relative luciferase activity of SRCIN1 3′UTR-WT not SRCIN1 3′UTR-Mut in A549 cells. (G) The dual luciferase reporter assay revealed that miR-328-5p mimic reduced the relative luciferase activity of SRCIN1 3′UTR-WT not SRCIN1 3′UTR-Mut in NCI-H1299 cells. *P<0.05; **P<0.01; ***P<0.001. TPTEP1, TPTE pseudogene 1; SRCIN1, Src kinase signaling inhibitor 1.
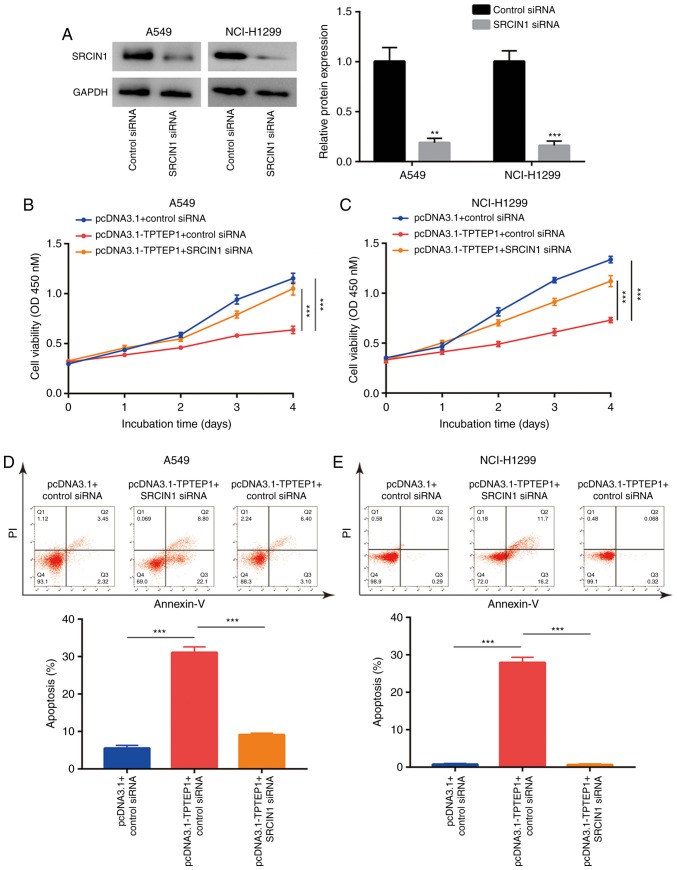
TPTEP1 inhibits cell proliferation and induces apoptosis via upregulation of SRCIN1
To investigate whether SRCIN1 is involved in TPTEP1-meditated cell growth arrest and cell apoptosis, SRCIN1 siRNA was transfected into A549 and NCI-H1299 cells to knock down SRCIN1 expression. Western blot analysis confirmed that transfection of SRCIN1 siRNA decreased SRCIN1 protein expression in A549 and NCI-H1299 cells (Fig. 6A). The cell proliferation assay indicated that silencing of SRCIN1 abrogated the inhibitory effect of TPTEP1 overexpression on the proliferation of A549 and NCI-H1299 cells (Fig. 6B and C). Furthermore, flow cytometric analysis revealed that silencing of SRCIN1 inhibited the effect of TPTEP1 overexpression to induce apoptosis in A549 cells (Fig. 6D), which was also observed in NCI-H1299 cells (Fig. 6E).
Figure 6.
TPTEP1 negatively regulates cell proliferation and induces cell apoptosis via upregulation of SRCIN1 in NSCLC cells. (A) Transfection of SRCIN1 siRNA decreased SRCIN1 protein expression in A549 and NCI-H1299 cells. (B) Overexpression of TPTEP1 decreased cell proliferation which was reversed after SRCIN1 silencing in A549 cells. (C) Overexpression of TPTEP1 decreased cell proliferation which was reversed after SRCIN1 silencing in NCI-H1299 cells. (D) Overexpression of TPTEP1 induced cell apoptosis which was reversed after SRCIN1 silencing in A549 cells. (E) Overexpression of TPTEP1 induced cell apoptosis which was reversed after SRCIN1 silencing in NCI-H1299 cells. **P<0.01; ***P<0.001. TPTEP1, TPTE pseudogene 1; SRCIN1, Src kinase signaling inhibitor 1.
TPTEP1 expression is positively correlated with SRCIN1 mRNA levels in NSCLC tumors
To examine the association between SRCIN1 and TPTEP1 in clinical samples, RT-qPCR was performed to detect SRCIN1 mRNA levels in 56 pairs of NSCLC tumors and adjacent normal tissues. Downregulation of SRCIN1 was observed in NSCLC tumors (P<0.001; Fig. 7A), which was consistent with the results of a previous study (33). In addition, SRCIN1 protein expression was detected in 10 pairs of NSCLC tumors and normal tissues by western blot analysis. Consistently, SRCIN1 protein expression was significantly downregulated in tumors (P<0.001; Fig. 7B). Pearson correlation analysis revealed a strong positive correlation between TPTEP1 and SRCIN1 mRNA expression (r=0.591, P<0.001; Fig. 7C) and a negative correlation between SRCIN1 mRNA and miR-328-5p levels (r=−0.436, P<0.001; Fig. 7D) in tumors.
Figure 7.
Expression of TPTEP1 is negatively correlated with SRCIN1 mRNA levels in NSCLC tumors. (A) RT-qPCR revealed that SRCIN1 mRNA was decreased in NSCLC tumors compared to matched normal tissues from 56 patients with NSCLC. (B) Western blotting revealed that SRCIN1 protein was decreased in 10 NSCLC tumors compared to matched normal tissues. The blots of two representative tumors and normal tissues are presented. (C) Pearson Correlation analysis revealed that TPTEP1 expression was positively correlated with SRCIN1 mRNA levels in 56 NSCLC tumors. (D) Pearson Correlation analysis revealed that SRCIN1 mRNA levels were negatively correlated with miR-328-5p levels in 56 NSCLC tumors. ***P<0.001. TPTEP1, TPTE pseudogene 1; SRCIN1, Src kinase signaling inhibitor 1; NSCLC, non-small cell lung cancer.
Discussion
Due to the development of RNA sequencing technology, numerous aberrantly expressed lncRNAs have been identified in NSCLC (38). Several lncRNAs were experimentally identified to function as tumor suppressors or oncogenes during NSCLC (38–40). DiGeorge syndrome critical region gene 5, an upregulated lncRNA in lung adenocarcinoma identified through integrated analysis of TCGA data (20), was indicated to be pivotal for proliferation and metastasis of lung cancer cells via sponging miR-1180 and miR-873-5p (41,42). The present study focused on TPTEP1, another differentially expressed lncRNA in lung adenocarcinoma, and indicated for the first time to the best of our knowledge, that TPTEP1 regulated cell proliferation and apoptosis in NSCLC.
Downregulation of TPTEP1 was first reported in kidney, liver, lung and stomach cancers as a result of DNA methylation (43). In the present study, the expression pattern of TPTEP1 was analyzed in several cancer types and normal tissues according to TCGA datasets, indicating that TPTEP1 was downregulated in a majority of cancers, including kidney renal clear cell carcinoma, LUAD, LUSC and stomach adenocarcinoma. The present RT-qPCR data further confirmed the downregulation of TPTEP1 in NSCLC tumors. In addition, based on an analysis of previously published data (30), it was indicated that the high expression of TPTEP1 was able to predict a prolonged overall survival of patients with lung cancer. The result was verified in the samples collected from 56 patients with NSCLC. Certain lncRNAs are crucial for cancer cell proliferation and survival (44,45). In hepatocellular carcinoma, overexpression of TPTEP1 suppressed cell proliferation and metastasis but did not affect cell apoptosis (46). The present results indicated that overexpression of TPTEP1 significantly inhibited cell proliferation and induced apoptosis in NSCLC cells. These results indicated that TPTEP1 may have a pivotal role by inducing cell death to inhibit overgrowth of NSCLC cells.
The role of miR-328-5p in cancers is controversial. miR-328-5p levels were high in the serum of patients with NSCLC and may serve as an excellent biomarker for early prediction of NSCLC (32). In breast cancer, loss of miR-328-5p expression was observed in tumors and miR-328-5p exerted an anti-proliferative effect on breast cancer cells by targeting RAGE (47). In the present study, miR-328-5p was determined to be overexpressed in NSCLC tumors. This is consistent with the RT-qPCR data from a previous study of NSCLC tumors (48). Similar to the effect in NSCLC cells transfected with TPTEP1, knockdown of miR-328-5p inhibited cell proliferation and induced cell apoptosis in NSCLC cells. The dual-luciferase assay confirmed the direct binding of TPTEP1 and miR-328-5p in NSCLC cells. Aberrant expression of miR-328-5p has been reported in several cancer types, and was negatively regulated by circRNA (circRNA-5692) and lncRNA (LINC00210) (47,49,50). Our data implied that TPTEP1 downregulation may contribute to the increase of miR-328-5p in NSCLC, indicating TPTEP1 as a novel regulator of miR-328-5p.
As a non-receptor kinase, Src drives NSCLC cell proliferation, drug resistance and cell survival by activating pathways including STAT3 signaling (51,52). SRCIN1 suppresses Src activity and according to a previous study, its expression was relatively low in tumors (53). Upregulation of miR-873 and miR-346 were indicated to be responsible for the downregulation of SRCIN1 in various tumor types (33,54). In the present study, a bioinformatics analysis indicated that SRCIN1 is a potential target gene of miR-328-5p. In addition, downregulation of miR-328-5p increased SRCIN1 expression and decreased the phosphorylation levels of Src and STAT3, indicating inactivation of Src and STAT3 signaling. Furthermore, SRCIN1 was confirmed as a target gene of miR-328-5p in the dual-luciferase reporter assay. miR-328-5p targeted mRNA of several genes such as RAGE, PLCE1 and PTEN to exert its role during cancer progression (47,55,56). To the best of our knowledge, the present study was the first to indicate that miR-328-5p promotes NSCLC cell proliferation and sustains cell survival at least partially via targeting of SRCIN1 to activate the Src and STAT3 pathways.
In conclusion, the present results revealed that TPTEP1 was downregulated in NSCLC and predicted a good prognosis for patients with NSCLC. Mechanistically, TPTEP1 inactivated Src and STAT3 signaling via sponging miR-328-5p to upregulate SRCIN1. These results may provide novel insights into the molecular basis of NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was funded by Hebei Keynote Research and Development Plan Self-Financing Project (182777234).
Availability of materials and methods
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
FC, ZW, YF, HZ and MY carried out the experimental studies and analyzed the data. XW, HZ and SZ designed the study, and performed the literature research. XW and SZ also carried out the experimental studies and prepared the manuscript preparation. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Shangqiu First People's Hospital and the First Affiliated Hospital of Henan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Jin X, Chen Y, Chen H, Fei S, Chen D, Cai X, Liu L, Lin B, Su H, Zhao L, et al. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for Early-stage non-small cell lung cancer using next-generation sequencing. Clin Cancer Res. 2017;23:5311–5319. doi: 10.1158/1078-0432.CCR-17-0577. [DOI] [PubMed] [Google Scholar]
- 3.Schrodl K, Oelmez H, Edelmann M, Huber RM, Bergner A. Altered Ca2+-homeostasis of cisplatin-treated and low level resistant non-small-cell and small-cell lung cancer cells. Cell Oncol. 2009;31:301–315. doi: 10.3233/CLO-2009-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh ES, Sun A, Tran TH, Tsang R, Pintilie M, Hodgson DC, Wells W, Heaton R, Gospodarowicz MK. Clinical dose-volume histogram analysis in predicting radiation pneumonitis in Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys. 2006;66:223–228. doi: 10.1016/j.ijrobp.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 5.Xing J, Stewart DJ, Gu J, Lu C, Spitz MR, Wu X. Expression of methylation-related genes is associated with overall survival in patients with non-small cell lung cancer. Br J Cancer. 2008;98:1716–1722. doi: 10.1038/sj.bjc.6604343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakelee H, Kelly K, Edelman MJ. 50 Years of progress in the systemic therapy of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2014:177–189. doi: 10.14694/EdBook_AM.2014.34.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitiello M, Tuccoli A, Poliseno L. Long non-coding RNAs in cancer: Implications for personalized therapy. Cell Oncol (Dordr) 2015;38:17–28. doi: 10.1007/s13402-014-0180-x. [DOI] [PubMed] [Google Scholar]
- 8.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 9.Harries LW. Long non-coding RNAs and human disease. Biochem Soc Trans. 2012;40:902–906. doi: 10.1042/BST20120020. [DOI] [PubMed] [Google Scholar]
- 10.Li DY, Chen WJ, Luo L, Wang YK, Shang J, Zhang Y, Chen G, Li SK. Prospective lncRNA-miRNA-mRNA regulatory network of long non-coding RNA LINC00968 in non-small cell lung cancer A549 cells: A miRNA microarray and bioinformatics investigation. Int J Mol Med. 2017;40:1895–1906. doi: 10.3892/ijmm.2017.3187. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Gao L, Guo X, Shi X, Wu H, Song F, Wang B. A network based method for analysis of lncRNA-disease associations and prediction of lncRNAs implicated in diseases. PLoS One. 2014;9:e87797. doi: 10.1371/journal.pone.0087797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong G, Lou W, Yao M, Du C, Wei H, Fu P. Identification of novel mRNA-miRNA-lncRNA competing endogenous RNA network associated with prognosis of breast cancer. Epigenomics. 2019;11:1501–1518. doi: 10.2217/epi-2019-0209. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Fu H, Wu Y, Zheng X. Function of lncRNAs and approaches to lncRNA-protein interactions. Sci China Life Sci. 2013;56:876–885. doi: 10.1007/s11427-013-4553-6. [DOI] [PubMed] [Google Scholar]
- 14.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: The rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng Q, Ren M, Li Y, Song X. LncRNA-RMRP acts as an oncogene in lung cancer. PLoS One. 2016;11:e0164845. doi: 10.1371/journal.pone.0164845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H, Yan L, Sun K, Sun X, Zhang X, Cai K, Song T. lncRNA BCAR4 increases viability, invasion, and migration of non-small cell lung cancer cells by targeting glioma-associated oncogene 2 (GLI2) Oncol Res. 2019;27:359–369. doi: 10.3727/096504018X15220594629967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng ZH, Wu DM, Fan SH, Zhang ZF, Chen GQ, Lu J. Upregulation of miR-675-5p induced by lncRNA H19 was associated with tumor progression and development by targeting tumor suppressor p53 in non-small cell lung cancer. J Cell Biochem. 2019;120:18724–18735. doi: 10.1002/jcb.29182. [DOI] [PubMed] [Google Scholar]
- 18.Yang B, Zhang L, Cao Y, Chen S, Cao J, Wu D, Chen J, Xiong H, Pan Z, Qiu F, et al. Overexpression of lncRNA IGFBP4-1 reprograms energy metabolism to promote lung cancer progression. Mol Cancer. 2017;16:154. doi: 10.1186/s12943-017-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu L, Fang F, Lu S, Li X, Yang Y, Wang Z. lncRNA-HIT promotes cell proliferation of non-small cell lung cancer by association with E2F1. Cancer Gene Ther. 2017;24:221–226. doi: 10.1038/cgt.2017.10. [DOI] [PubMed] [Google Scholar]
- 20.Sui J, Li YH, Zhang YQ, Li CY, Shen X, Yao WZ, Peng H, Hong WW, Yin LH, Pu YP, Liang GY. Integrated analysis of long non-coding RNA-associated ceRNA network reveals potential lncRNA biomarkers in human lung adenocarcinoma. Int J Oncol. 2016;49:2023–2036. doi: 10.3892/ijo.2016.3716. [DOI] [PubMed] [Google Scholar]
- 21.Di Stefano P, Damiano L, Cabodi S, Aramu S, Tordella L, Praduroux A, Piva R, Cavallo F, Forni G, Silengo L, et al. p140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity. EMBO J. 2007;26:2843–2855. doi: 10.1038/sj.emboj.7601724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/A:1023772912750. [DOI] [PubMed] [Google Scholar]
- 23.Park SI, Shah AN, Zhang J, Gallick GE. Regulation of angiogenesis and vascular permeability by Src family kinases: Opportunities for therapeutic treatment of solid tumors. Expert Opin Ther Targets. 2007;11:1207–1217. doi: 10.1517/14728222.11.9.1207. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y, Wang H, Mills GB. Targeting PI3K-AKT pathway for cancer therapy. Rev Clin Exp Hematol. 2003;7:205–228. [PubMed] [Google Scholar]
- 25.Thakur R, Trivedi R, Rastogi N, Singh M, Mishra DP. Inhibition of STAT3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci Rep. 2015;5:10194. doi: 10.1038/srep10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy S, Clynes M, Doolan P, Mehta JP, Rani S, Crown J, O'Driscoll L. SNIP/p140Cap mRNA expression is an unfavourable prognostic factor in breast cancer and is not expressed in normal breast tissue. Br J Cancer. 2008;98:1641–1645. doi: 10.1038/sj.bjc.6604365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Wang H, Li X, Liu Y, Zhao C, Zhu D. SRCIN1 suppressed osteosarcoma cell proliferation and invasion. PLoS One. 2016;11:e0155518. doi: 10.1371/journal.pone.0155518. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Rousseaux S, Debernardi A, Jacquiau B, Vitte AL, Vesin A, Nagy-Mignotte H, Moro-Sibilot D, Brichon PY, Lantuejoul S, Hainaut P, et al. Ectopic activation of germline and placental genes identifies aggressive metastasis-prone lung cancers. Sci Transl Med. 2013;5:186ra166. doi: 10.1126/scitranslmed.3005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro-Oropeza R, Melendez-Zajgla J, Maldonado V, Vazquez-Santillan K. The emerging role of lncRNAs in the regulation of cancer stem cells. Cell Oncol (Dordr) 2018;41:585–603. doi: 10.1007/s13402-018-0406-4. [DOI] [PubMed] [Google Scholar]
- 32.Ulivi P, Foschi G, Mengozzi M, Scarpi E, Silvestrini R, Amadori D, Zoli W. Peripheral blood miR-328 expression as a potential biomarker for the early diagnosis of NSCLC. Int J Mol Sci. 2013;14:10332–10342. doi: 10.3390/ijms140510332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Y, Xue Q, Wang D, Du M, Zhang Y, Gao S. miR-873 induces lung adenocarcinoma cell proliferation and migration by targeting SRCIN1. Am J Transl Res. 2015;7:2519–2526. [PMC free article] [PubMed] [Google Scholar]
- 34.Portillo JC, Muniz-Feliciano L, Lopez Corcino Y, Lee SJ, Van Grol J, Parsons SJ, Schiemman WP, Subauste CS. Toxoplasma gondii induces FAK-Src-STAT3 signaling during infection of host cells that prevents parasite targeting by autophagy. PLoS Pathog. 2017;13:e1006671. doi: 10.1371/journal.ppat.1006671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao M, Hou D, Liang H, Gong F, Wang Y, Yan X, Jiang X, Wang C, Zhang J, Zen K, et al. miR-150 promotes the proliferation and migration of lung cancer cells by targeting SRC kinase signalling inhibitor 1. Eur J Cancer. 2014;50:1013–1024. doi: 10.1016/j.ejca.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Wang N, Liang H, Zhou Y, Wang C, Zhang S, Pan Y, Wang Y, Yan X, Zhang J, Zhang CY, et al. miR-203 suppresses the proliferation and migration and promotes the apoptosis of lung cancer cells by targeting SRC. PLoS One. 2014;9:e105570. doi: 10.1371/journal.pone.0105570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M, Meng B, Liu Y, Yu J, Chen Q, Liu Y. MiR-124 Inhibits Growth and enhances radiation-induced apoptosis in non-small cell lung cancer by inhibiting STAT3. Cell Physiol Biochem. 2017;44:2017–2028. doi: 10.1159/000485907. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Lin J, Liu T, Chen T, Pan S, Huang W, Li S. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer. 2014;85:110–115. doi: 10.1016/j.lungcan.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Yang T, Li H, Chen T, Ren H, Shi P, Chen M. LncRNA MALAT1 depressed Chemo-sensitivity of NSCLC cells through directly functioning on miR-197-3p/p120 Catenin Axis. Mol Cells. 2019;42:270–283. doi: 10.14348/molcells.2019.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang B, Wang H, Wang Q, Xu J, Jiang P, Li W. Knockout of lncRNA UCA1 inhibits drug resistance to gefitinib via targeting STAT3 signaling in NSCLC. Minerva Med. 2019;110:273–275. doi: 10.23736/S0026-4806.19.05979-2. [DOI] [PubMed] [Google Scholar]
- 41.Chen EG, Zhang JS, Xu S, Zhu XJ, Hu HH. Long non-coding RNA DGCR5 is involved in the regulation of proliferation, migration and invasion of lung cancer by targeting miR-1180. Am J Cancer Res. 2017;7:1463–1475. [PMC free article] [PubMed] [Google Scholar]
- 42.Luo J, Zhu H, Jiang H, Cui Y, Wang M, Ni X, Ma C. The effects of aberrant expression of LncRNA DGCR5/miR-873-5p/TUSC3 in lung cancer cell progression. Cancer Med. 2018 May 23; doi: 10.1002/cam4.1566. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang Q, Ding J, Xu R, Xu Z, Zheng S. The novel human endogenous retrovirus-related gene, psiTPTE22-HERV, is silenced by DNA methylation in cancers. Int J Cancer. 2010;127:1833–1843. doi: 10.1002/ijc.25213. [DOI] [PubMed] [Google Scholar]
- 44.Xie JJ, Guo QY, Jin JY, Jin D. SP1-mediated overexpression of lncRNA LINC01234 as a ceRNA facilitates non-small-cell lung cancer progression via regulating OTUB1. J Cell Physiol. 2019;234:22845–22856. doi: 10.1002/jcp.28848. [DOI] [PubMed] [Google Scholar]
- 45.Yan R, Jiang Y, Lai B, Lin Y, Wen J. The positive feedback loop FOXO3/CASC11/miR-498 promotes the tumorigenesis of non-small cell lung cancer. Biochem Biophys Res Commun. 2019;519:518–524. doi: 10.1016/j.bbrc.2019.08.136. [DOI] [PubMed] [Google Scholar]
- 46.Ding H, Liu J, Zou R, Cheng P, Su Y. Long non-coding RNA TPTEP1 inhibits hepatocellular carcinoma progression by suppressing STAT3 phosphorylation. J Exp Clin Cancer Res. 2019;38:189. doi: 10.1186/s13046-019-1193-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Luo T, Yan Y, He Q, Ma X, Wang W. miR-328-5p inhibits MDA-MB-231 breast cancer cell proliferation by targeting RAGE. Oncol Rep. 2018;39:2906–2914. doi: 10.3892/or.2018.6353. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, Wang S, Ma F, Zhang W. miRNA-328 overexpression confers cisplatin resistance in nonsmall cell lung cancer via targeting of PTEN. Mol Med Rep. 2018;18:4563–4570. doi: 10.3892/mmr.2018.9478. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z, Yu Y, Huang Z, Kong Y, Hu X, Xiao W, Quan J, Fan X. CircRNA-5692 inhibits the progression of hepatocellular carcinoma by sponging miR-328-5p to enhance DAB2IP expression. Cell Death Dis. 2019;10:900. doi: 10.1038/s41419-019-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S, Li P, Zhao L, Xu L. LINC00210 as a miR-328-5p sponge promotes nasopharyngeal carcinoma tumorigenesis by activating NOTCH3 pathway. Biosci Rep. 2018;38(pii):BSR20181168. doi: 10.1042/BSR20181168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson FM, Gallick GE. SRC family nonreceptor tyrosine kinases as molecular targets for cancer therapy. Anticancer Agents Med Chem. 2007;7:651–659. doi: 10.2174/187152007784111278. [DOI] [PubMed] [Google Scholar]
- 52.Byers LA, Sen B, Saigal B, Diao L, Wang J, Nanjundan M, Cascone T, Mills GB, Heymach JV, Johnson FM. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clin Cancer Res. 2009;15:6852–6861. doi: 10.1158/1078-0432.CCR-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen R, Liao JY, Huang J, Chen WL, Ma XJ, Luo XD. Downregulation of SRC Kinase signaling inhibitor 1 (SRCIN1) expression by MicroRNA-32 promotes proliferation and Epithelial-mesenchymal transition in human liver cancer cells. Oncol Res. 2018;26:573–579. doi: 10.3727/096504017X14954923820137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang F, Luo LJ, Zhang L, Wang DD, Yang SJ, Ding L, Li J, Chen D, Ma R, Wu JZ, Tang JH. MiR-346 promotes the biological function of breast cancer cells by targeting SRCIN1 and reduces chemosensitivity to docetaxel. Gene. 2017;600:21–28. doi: 10.1016/j.gene.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 55.Han N, Zhao W, Zhang Z, Zheng P. MiR-328 suppresses the survival of esophageal cancer cells by targeting PLCE1. Biochem Biophys Res Commun. 2016;470:175–180. doi: 10.1016/j.bbrc.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Liang F, Cui ZJ, Liu JD, Liu KP, Li L, Chen YL. Downregulated miR-328 suppressed cell invasion and growth in hepatocellular carcinoma via targeting PTEN. Eur Rev Med Pharmacol Sci. 2018;22:6324–6332. doi: 10.26355/eurrev_201810_16043. [DOI] [PubMed] [Google Scholar]