This case-control study of hospitalized patients compared underlying conditions, symptoms, signs, laboratory data, and radiographic presentations between Middle East respiratory syndrome coronavirus (MERS-CoV)–positive and –negative patients. Those with MERS-CoV were more likely to be overweight and to have diabetes mellitus, end-stage renal disease, tachypnea, and a normal white blood cell count on bivariate analysis.
Keywords: MERS, coronavirus, case-control, radiographic characteristics
Abstract
Background. There is a paucity of data regarding the differentiating characteristics of patients with laboratory-confirmed and those negative for Middle East respiratory syndrome coronavirus (MERS-CoV).
Methods. This is a hospital-based case-control study comparing MERS-CoV–positive patients (cases) with MERS-CoV–negative controls.
Results. A total of 17 case patients and 82 controls with a mean age of 60.7 years and 57 years, respectively (P = .553), were included. No statistical differences were observed in relation to sex, the presence of a fever or cough, and the presence of a single or multilobar infiltrate on chest radiography. The case patients were more likely to be overweight than the control group (mean body mass index, 32 vs 27.8; P = .035), to have diabetes mellitus (87% vs 47%; odds ratio [OR], 7.24; P = .015), and to have end-stage renal disease (33% vs 7%; OR, 7; P = .012). At the time of admission, tachypnea (27% vs 60%; OR, 0.24; P = .031) and respiratory distress (15% vs 51%; OR, 0.15; P = .012) were less frequent among case patients. MERS-CoV patients were more likely to have a normal white blood cell count than the control group (82% vs 52%; OR, 4.33; P = .029). Admission chest radiography with interstitial infiltrates was more frequent in case patients than in controls (67% vs 20%; OR, 8.13; P = .001). Case patients were more likely to be admitted to the intensive care unit (53% vs 20%; OR, 4.65; P = .025) and to have a high mortality rate (76% vs 15%; OR, 18.96; P < .001).
Conclusions. Few clinical predictors could enhance the ability to predict which patients with pneumonia would have MERS-CoV. However, further prospective analysis and matched case-control studies may shed light on other predictors of infection.
Middle East respiratory syndrome coronavirus (MERS-CoV) is a novel coronavirus initially identified in Saudi Arabia in September 2012 [1]. The first described case resulted in acute pneumonia and subsequent renal failure and death, and had similarities with the clinical presentation of SARS [1]. In a retrospective testing, the first hospital outbreak of MERS-CoV infection was linked to a hospital in Zarqa, Jordan [2]. MERS-CoV causes sporadic infections and intrafamilial and healthcare-associated infections [3, 4]. Since the first case was identified, a total of 162 cases with a fatality rate of 39.5% have been reported from Saudi Arabia [5]. Asymptomatic and mildly symptomatic cases were documented among family and healthcare worker contacts of confirmed cases [6]. Patient-to-patient transmission and intrafamilial transmissions were also reported [7–9].
As of 18 March 2014, a total of 198 cases worldwide had been reported to the World Health Organization [10]. Initial cases appeared to be sporadic in nature and were epidemiologically linked to the Middle East. Between 1 April and 23 May 2013, a total of 23 cases of MERS-CoV infection were reported in a hospital outbreak in the Eastern Province of Saudi Arabia [11]. The clinical presentation and characteristics of MERS-CoV patients have been well described [12]. Because MERS-CoV is still a fairly new disease, there is a paucity of data regarding the characteristics of and differences between suspected patients whose tests were subsequently negative, and laboratory-confirmed cases. Such a study would be useful in triaging patients into risk categories to determine the likelihood of MERS-CoV infection. We undertook this study to identify possible clinical characteristics that may differentiate MERS-CoV–positive patients from MERS-CoV–negative patients with community-acquired pneumonia (CAP).
METHODS
In this case-control study, “cases” were defined as hospitalized patients who tested positive for MERS-CoV between 1 April 2013 and 3 June 2013. The controls were selected from the pool of patients admitted to the same facility during the same timeframe who met the case definition of suspected MERS-CoV and tested negative for MERS-CoV. The case definition of suspected MERS-CoV was an acute febrile respiratory illness (fever, cough, or dyspnea) with radiographic evidence of pneumonia. The study was conducted at a 350-bed general hospital that also accepts referred patients. The hospital provides medical care for about 370 000 individuals eligible for medical care. The hospital has 5 intensive care units (cardiac, medical, surgical, pediatric, and neonatal).
MERS-CoV Testing
Patients suspected to have MERS-CoV infection had either Dacron-flocked nasopharyngeal swabs or tracheal aspirates. These specimens were submitted to the Saudi Ministry of Health MERS-CoV laboratory and the clinical samples were screened with real-time reverse-transcription polymerase chain reaction as described previously [12, 13]. The test amplified both the upstream E protein (upE gene) and ORF1a for MERS-CoV. A positive case was determined if both assays were positive, and controls were classified when the MERS-CoV test was negative, as described previously [12].
Data Collection
We collected data for all patients using a standard Microsoft Excel spreadsheet. The paper chart and electronic medical record reviews were conducted by practicing physicians. A second review of 20% of the charts was done by a different investigator to ensure concordance of the abstracted data. Interrater agreement was high for all variables. The investigators were not blinded to the MERS-CoV status (positive or negative). We collected epidemiological, demographic, clinical, radiographic, and laboratory data. Radiographic features of chest radiographs were extracted from the radiographic reports based on the search for keywords such as lobar, unilateral, and interstitial infiltrate.
Statistical Analysis
Statistical analysis was done using SPSS software for Windows, version 11 (SPSS, Chicago, Illinois). Descriptive analyses were done for demographic, clinical, and laboratory data. Bivariate analysis of association of MERS-CoV status and different parameters was done. Continuous data, such as complete blood count, lactate dehydrogenase (LDH), hepatic panel, and platelet count, were converted into categorical variables (normal, low, or high levels). Multivariate analysis was not performed due to the small sample size. The odds ratio (OR) was obtained for each variable as well. A P value of <.05 was considered to indicate statistical significance. The Kaplan-Meier survival curve was calculated for MERS-CoV–positive cases vs controls. Date of onset of symptoms was used as the starting date. Endpoint was either death or survival as of 30 November 2013. We conducted a death certificate search of medical records to determine if any patients who were alive at discharge subsequently died, and these data were included in our analysis when applicable.
RESULTS
During the study period, a total of 99 patients were admitted and met the case definition of suspected MERS-CoV. There were 17 cases and 82 controls. The mean age was 60.7 years for the cases, and 57 years for the controls (P = .553). No statistical differences existed between cases and controls when looking at sex, age, the presence of a fever or cough on admission, and whether the patient had a single or multilobar infiltrate on chest radiography (Table 1). The MERS-CoV cases were more likely to be overweight than the controls (mean body mass index, 32.02 ± 6.78 kg/m2 vs 27.78 ± 7.6 kg/m2; P = .03; Table 1).
Table 1.
Comparison of Various Characteristics on Admission
| Characteristic | Cases (n = 17) | Controls (n = 82) | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Male sex | 11 (65) | 46 (36) | 1.43 | .48–4.25 | .596 |
| Age, y, median (range) | 62 (14–87) | 59 (5–92) | n/a | n/a | .553 |
| Weight, kg, mean (SD) | 82.25 (14.4) | 71.84 (23.7) | n/a | n/a | .085 |
| Body mass index, mean (SD) | 32.02 (6.78) | 27.78 (7.6) | n/a | n/a | .036 |
| Comorbidities | |||||
| Diabetes | 13 (87) | 35 (47) | 7.24 | 1.53–34.37* | .015 |
| Cardiac disease | 8 (53) | 32 (42) | 1.57 | .52–4.78 | .423 |
| Pulmonary disease (any) | 6 (40) | 30 (40) | 0.98 | .32–3.03 | .96 |
| Constructive pulmonary disease | 4 (27) | 13 (18) | 1.62 | .45–5.91 | .46 |
| Congestive heart failure | 3 (20) | 18 (24) | 0.79 | .20–3.12 | .73 |
| End-stage renal disease (on dialysis) | 5 (33) | 5 (7) | 7 | 1.72–28.55* | .012 |
| Cancer | 1 (7) | 11 (15) | 0.41 | .05–3.43 | .11 |
| Symptoms | |||||
| Onset to admission, d, median (range) | 3 (0–45) | 3 (0–69) | n/a | n/a | n/a |
| Fever | 6 (40) | 47 (63) | 0.4 | .13–1.23 | .1 |
| Shivering | 1 (7) | 10 (17) | 0.34 | .04–2.85 | .29 |
| Dyspnea | 10 (67) | 55 (75) | 0.65 | .20–2.17 | .75 |
| Chest pain | 1 (7) | 22 (32) | 0.16 | .02–1.31 | .056 |
| Wheezing | 2 (14) | 11 (17) | 0.82 | .16–4.18 | .809 |
| Cough | 12 (86) | 57 (77) | 1.79 | .36–8.79 | .469 |
| Hemoptysis | 1 (7) | 3 (5) | 1.45 | .14–15.03 | .753 |
| Sore throat | 1 (7) | 8 (13) | 0.46 | .05–4.03 | .477 |
| Headache | 1 (7) | 2 (3) | 1.96 | .17–23.25 | .586 |
| Myalgias | 1 (7) | 11 (19) | 0.31 | .04–2.63 | .261 |
| Vomiting | 1 (7) | 3 (4) | 1.55 | .15–16.00 | .712 |
| Diarrhea | 1 (7) | 4 (6) | 1.14 | .12–11.02 | .908 |
| Tachypnea | 4 (27) | 45 (60) | 0.24 | .07–.83* | .031 |
| Respiratory distress | 2 (15) | 38 (51) | 0.15 | .03–.69* | .012 |
| Hypoxia, oxygen saturation <95% | 4 (27) | 35 (47) | 0.42 | .12–1.42 | .059 |
| Laboratory findings | |||||
| Leukocytosis | 2 (12) | 34 (42) | 0.18 | .04–.86* | .025 |
| Normal white blood cell count | 14 (82) | 42 (52) | 4.33 | 1.16–16.24* | .029 |
| Lymphopenia | 6 (35) | 14 (17) | 2.57 | .81–8.12 | .103 |
| Elevated ALT | 3 (18) | 7 (8) | 3.61 | .75–17.24 | .107 |
| Elevated AST | 9 (53) | 26 (32) | 2.31 | .73–7.25 | .152 |
| Elevated LDH | 8 (47) | 24 (29) | 1.95 | .63–6.06 | .247 |
| Chest radiography findings | |||||
| Single infiltrate | 6 (40) | 27 (35) | 1.21 | .39–3.76 | .79 |
| Multiple infiltrates | 9 (60) | 34 (45) | 1.85 | .60–5.72 | .495 |
| Interstitial infiltrate | 10 (67) | 15 (20) | 8.13 | 2.42–27.36* | .001 |
| Cardiomegaly | 8 (53) | 16 (21) | 4.29 | 1.35–13.60* | .025 |
| Treatment received | |||||
| Admitted to ICU | 8 (53) | 15 (20) | 4.65 | 1.46–14.84* | .025 |
Data are No. (%) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval: ICU, intensive care unit; LDH, lactate dehydrogenase; n/a, not applicable; SD, standard deviation.
*Represents significant variables.
Bivariate Analysis
The results of bivariate analysis revealed no difference in many underlying comorbidities between cases and controls (Table 1). The only significant comorbidities were diabetes mellitus and end-stage renal disease (ESRD). Of the cases, 5 had ESRD requiring chronic hemodialysis compared with 5 of the controls (33% vs 7%; OR, 7; P = .012). The presence of cardiac disease, pulmonary disease, or active cancer was not statistically different between the 2 groups (Table 1). The median time from symptom onset to hospitalization was 3 days in both the cases and controls. The median duration of hospitalization to either discharge or death was 18 days for cases, and 5 days for controls.
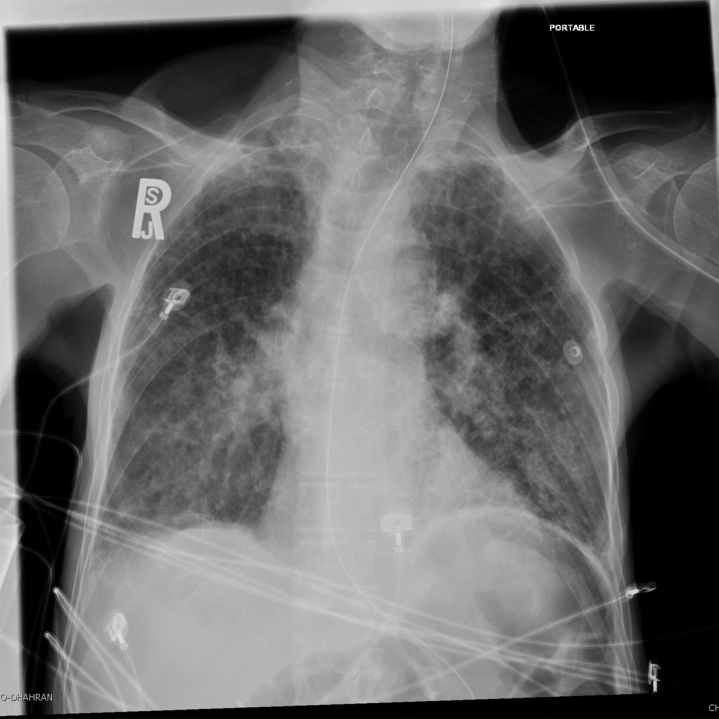
At the time of admission, tachypnea (27% vs 60%; OR, 0.24; P = .031) and respiratory distress (15% vs 51%; OR, 0.15; P = .012) were less frequently seen among cases than controls (Table 1). The presence of fever, shortness of breath, wheezing, chest pain, cough, hemoptysis, sore throat, headache, myalgia, vomiting, and diarrhea was not statistically different between the 2 groups. A comparison of the mean of laboratory data for the cases and the controls is shown in Table 2. On admission, patients with MERS-CoV were more likely to have a normal white blood cell count (WBC) than the controls (82% vs 52%; OR, 4.33; P = .029). Cases were less likely to have leukocytosis than controls (12% vs 42%; OR, 0.18; P = .025) (Table 1). The level of lymphocytosis did not differ statistically between groups. Admission chest radiography showed the presence of interstitial infiltrates more frequently in cases than in controls (67% vs 20%; OR, 8.13; P = .001). An illustrative radiograph showing an interstitial infiltrate in a MERS-CoV–positive patient is shown in Figure 1. Blood and sputum cultures did not reveal any specific etiology of CAP in the MERS-CoV–negative patients.
Table 2.
Comparison of the Mean of Laboratory Data
| Laboratory Finding | MERS-CoV Status |
95% CI of the Difference Between Means |
P Value | ||||
|---|---|---|---|---|---|---|---|
| Positive (n = 17) |
Negative (n = 82) |
||||||
| No. | Mean | No. | Mean | Minimum | Maximum | ||
| WBC count within 1 d of onset | 15 | 8.97 | 75 | 12.17 | −6.24 | −0.16 | .02* |
| Neutrophil % | 15 | 72.07 | 74 | 68.27 | −2.63 | 10.23 | .87 |
| Band % | 15 | 1.13 | 74 | 3.82 | −5.27 | −0.11 | .02* |
| Lymphocyte % | 15 | 18.67 | 74 | 17.3 | −2.75 | 5.49 | .74 |
| Lymphocyte count | 15 | 1.55 | 74 | 1.93 | −0.91 | 0.15 | .08 |
| Eosinophil % | 15 | 0.33 | 74 | 1.11 | −1.43 | −0.13 | .01* |
| Hematocrit | 15 | 36.51 | 74 | 35.88 | −3.7 | 4.96 | .61 |
| Platelet count | 15 | 242.73 | 75 | 251.84 | −68.85 | 50.63 | .38 |
| Creatinine | 15 | 3.29 | 71 | 1.46 | −0.1 | 3.76 | .08 |
| Alanine aminotransferase | 13 | 58.08 | 62 | 42.19 | −21.57 | 53.35 | .79 |
| Aspartate aminotransferase | 13 | 94.31 | 62 | 50.24 | −44.14 | 132.28 | .82 |
| Lactate dehydrogenase | 13 | 936.69 | 61 | 646.43 | −180.96 | 761.48 | .87 |
| aPTT | 13 | 39.62 | 36 | 32 | 0.14 | 15.1 | .03* |
| International normalized ratio | 13 | 1.85 | 39 | 1.17 | −0.17 | 1.53 | .07 |
| C-reactive protein | 5 | 10.16 | 19 | 11.06 | −9.48 | 7.68 | .41 |
Abbreviations: aPTT, activated partial thromboplastin time; CI, confidence interval; MERS-CoV, Middle East respiratory syndrome coronavirus; WBC, white blood cell.
*Represents significant variables.
Figure 1.
A portable anterior–posterior chest radiograph showing interstitial infiltrate in a patient with Middle East respiratory syndrome coronavirus infection.
Kaplan-Meier Analysis
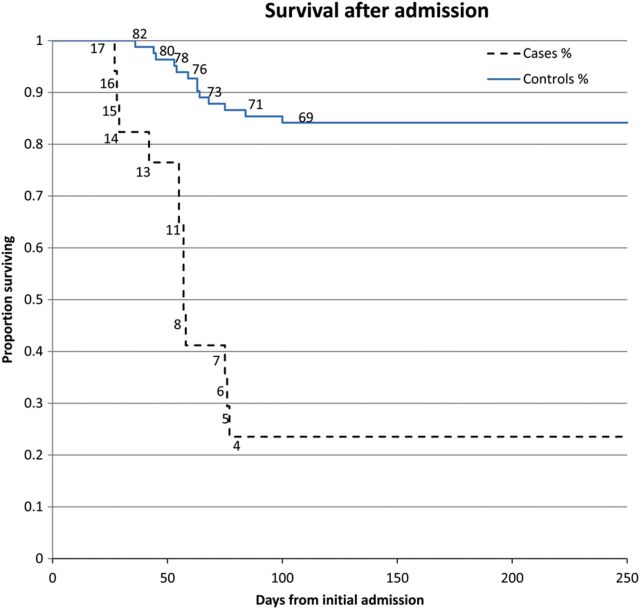
The mortality rate among cases was 76% compared with 15% among controls (P < .001). The Kaplan-Meier survival curve, with right-censoring of patients who were alive at the end of the study period, is shown in Figure 2. At day 77, only 23% of cases were alive compared with 86% of the controls.
Figure 2.
Kaplan-Meier survival curve. Numbers on the lines represent the number of patients still alive at each day.
DISCUSSION
In this study, we identified a total of 17 patients who tested positive for MERS-CoV. To look for potential risk factors or predictors of the disease, we identified an additional 82 patients who met the case definition for suspected MERS-CoV but who ultimately tested negative for the disease. The 2 groups were similar in age and sex. The median age was 62 years for cases; the previously reported median age for cases was 50 years [14]. Male predominance of patients (65% in the cases vs 36% in the control group) did not reach statistical significance. Earlier studies showed male predominance, as in the current study. Most recent analysis showed a larger proportion of younger female cases with a reduction of the male-to-female ratio [15, 16]. One of the reasons for such a change in the epidemiology of the disease is the enhanced surveillance and detection of mildly symptomatic cases.
The clinical presentation of MERS-CoV infection was initially described to be severe, leading to pneumonia with acute respiratory distress syndrome (ARDS), septic shock, and multiorgan failure resulting in death [11, 12]. Subsequently, patients with mild or no symptoms were reported [16]. The initial presentation of the disease is not different than other causes of CAP [2, 7, 11, 12]. Some patients may have sore throat, chills, arthralgia, or myalgia [7, 11, 12, 17, 18]. In the current study, these symptoms were present in MERS-CoV–positive and MERS-CoV–negative patients at equal rates. Thus, these symptoms have poor discriminating power for MERS-CoV infection. At the time of admission, tachypnea and respiratory distress were less frequently seen among cases than controls (27% vs 60% and 15% vs 51%, respectively; Table 1). The exact reason for this difference is not known. Should this finding be confirmed in subsequent studies, it would be an important distinguishing characteristic.
Underlying comorbidities such as ESRD were identified as a risk factor for MERS-CoV infection [11, 12]. Diarrhea and vomiting were observed in 21%–33% of patients [7, 11, 12, 18]. In the current study, the presence of diarrhea or vomiting was present in 4%–7% of cases and controls. The observed rate of gastrointestinal symptoms was lower than those from previous studies and may be related to the small number of the included patients. The presence of vomiting and diarrhea in MERS-CoV patients has a significant impact on infection control measures [12, 19].
On admission, patients with MERS-CoV were more likely to have a normal WBC (82% vs 52%) than the control group and less likely to have leukocytosis than the cases (42% vs 12%).Thus, a normal WBC count on admission of patients with CAP may help in predicting MERS-CoV positivity. This suggests that many of the controls may have had bacterial pneumonia. However, blood and sputum cultures did not reveal any specific etiology of CAP in those patients.
Previously, lymphopenia was observed among MERS-CoV patients [1, 2, 7, 18]. We found no difference in the presence of lymphopenia among cases and controls. Elevation of LDH, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were observed in 49%, 15%–23%, and 11% of MERS-CoV patients, respectively [11, 12]. Consistent with these observations, we also found that MERS-CoV patients had elevation in LDH (47%), AST (53%), and ALT (18%). These findings were not specific for MERS-CoV patients and were observed similarly in the controls.
Admission chest radiography showed the presence of interstitial infiltrates more frequently in cases than in controls. Previous radiographic characteristics included bilateral infiltrates, or unilateral infiltrates [7, 8, 12, 17, 20]. The initial chest radiography of patients in the Zarqa, Jordan, hospital outbreak showed single-lobar, bilateral, or multilobar pneumonias [2]. On CT scan, characteristics of ARDS including interstitial infiltrates were seen in severe cases [7, 12]. The presence of interstitial infiltrates is compatible with the severe presentation of the cases and may be a marker for cardiac disease, as cardiomegaly was identified as a potential risk factor in the bivariate analysis.
In this study, the mortality rate of MERS-CoV patients was significantly higher than that in the control group. This finding is consistent with early findings of higher mortality among MERS-CoV patients [11, 12]. Subsequent analysis of the initial 133 cases revealed that the case fatality rate in the early period of the disease (April–June 2013) was higher than the fatality rate in the second period of the disease [16]. In another analysis, 53 of the 114 (46.5%) hospitalized patients died [14]. This difference is related to the identification of mild cases and asymptomatic cases. The mortality rate in MERS-CoV infections is also related to the number of underlying risk factors [12], 54% in those with 2 underlying conditions compared with 80% in those with 3–4 underlying conditions [12]. Of the 17 patients, 8 were linked epidemiologically to the previously described Al-Hasa outbreak [11]. This fact might explain the higher proportion of cases with underlying medical conditions.
MERS-CoV infection may result in mild to severe and fulminant infections, leading to ARDS requiring hospitalization [11, 12, 18, 21]. In the current study, the median time from symptom onset to hospitalization was 3 days in both the cases and controls. On the other hand, the median duration of hospitalization to discharge or death was 18 days for cases and 5 days for controls. In a previous analysis, the median time from symptom onset to hospitalization was 4 days, and the median time from admission to an intensive care unit or to death was 5 and 11.5 days, respectively [14]. One patient was treated with extracorporeal membrane oxygenation and died after 298 days of symptom onset [17]. There is no proven effective therapy for MERS-CoV infection. The available therapies were based on the analysis of treatment of patients with severe acute respiratory syndrome [22, 23]. Recently, the use of interferon and ribavirin combination in MERS-CoV did not result in an improved outcome, as many patients presented late in their illness [24].
There are several limitations to our study. First, the small number of cases limits our ability to detect discriminant factors on presentation. Second, the retrospective nature of the study may also limit the power of the study to identify clinical predictors. Third, the inclusion of patients from the Al-Hasa outbreak may have contributed to the absence of significance of predictors of MERS-CoV and may explain why there were high proportions of ESRD and diabetic patients among our cases. One of the strengths of our study is ascertainment of cases and controls through the master list that included all cases admitted to rule out MERS-CoV infection during the study period. In conclusion, the results suggest that few clinical predictors could enhance the ability to predict which patients with CAP would have MERS-CoV. However, further prospective analysis and matched case-control studies may shed light on the possible predictors of infection.
Notes
Acknowledgments. The authors acknowledge the use of Saudi Aramco Medical Services Organization (SAMSO) facilities for the data and study, which resulted in this article.
Disclaimer. Opinions expressed in this article are those of the authors and not necessarily of SAMSO.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Hijawi B, Abdallat M, Sayaydeh A, et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19(suppl 1):S12–8. [PubMed] [Google Scholar]
- 3.Joint Kingdom of Saudi Arabia/WHO mission. Riyadh, Saudi Arabia: 2013. Middle East respiratory syndrome coronavirus. Available at: http://www.who.int/csr/disease/coronavirus_infections/MERSCov_WHO_KSA_Mission_Jun13u.pdf. Accessed 12 December 2013. [Google Scholar]
- 4.Al-Tawfiq JA, Assiri A, Memish ZA. Middle East respiratory syndrome novel corona MERS-CoV infection. Epidemiology and outcome update. Saudi Med J. 2013;34:991–4. [PubMed] [Google Scholar]
- 5.Saudi Ministry of Health—novel coronavirus - media statements. Available at: http://www.moh.gov.sa/en/CoronaNew/PressReleases/Pages/default.aspx . Accessed 20 November 2013.
- 6.Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369:884–6. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- 7.Guery B, Poissy J, el Mansouf L, et al. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–72. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omrani AS, Matin MA, Haddad Q, Al-Nakhli D, Memish ZA, Albarrak AM. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17:e668–72. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulland A. Novel coronavirus spreads to Tunisia. BMJ. 2013;346:f3372. doi: 10.1136/bmj.f3372. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV)—update. Available at: http://www.who.int/csr/don/2013_12_02/en/index.html . Accessed 18 March 2014.
- 11.Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–16. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–61. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corman VM, Müller MA, Costabel U, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17:49. doi: 10.2807/ese.17.49.20334-en. [DOI] [PubMed] [Google Scholar]
- 14.The WHO MERS-CoV Research Group. State of knowledge and data gaps of middle east respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. 5. pii: ecurrents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Hajjar S, Memish ZA, McIntosh K. Middle East respiratory syndrome coronavirus (MERS-CoV): a perpetual challenge. Ann Saudi Med. 2013;33:427–36. doi: 10.5144/0256-4947.2013.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penttinen PM, Kaasik-Aaslav K, Friaux A, et al. Taking stock of the first 133 MERS coronavirus cases globally—is the epidemic changing? Euro Surveill. 2013;18:39. doi: 10.2807/1560-7917.es2013.18.39.20596. [DOI] [PubMed] [Google Scholar]
- 17.Bermingham A, Chand MA, Brown CS, et al. Euro Surveill; 2012. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Available at: http://www.eurosurveillance.org/images/dynamic/EE/V17N40/art20290.pdf. Accessed 9 December 2013. [PubMed] [Google Scholar]
- 18.Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–94. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 19.Memish ZA, Al-Tawfiq JA, Assiri A. Hospital-associated Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;369:1761–2. doi: 10.1056/NEJMc1311004. [DOI] [PubMed] [Google Scholar]
- 20.Drosten C, Seilmaier M, Corman VM, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13:745–51. doi: 10.1016/S1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albarrak AM, Stephens GM, Hewson R, Memish ZA. Recovery from severe novel coronavirus infection. Saudi Med J. 2012;33:1265–9. [PubMed] [Google Scholar]
- 22.Momattin H, Mohammed K, Zumla A, Memish ZA, Al-Tawfiq JA. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV), possible lessons from a systematic review of SARS-CoV therapy. Int J Infect Dis. 2013;17:e792–8. doi: 10.1016/j.ijid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Severe Acute Respiratory and Emerging Infection Consortium. Clinical decision making tool for treatment of MERS-CoV version 1.0. 2013. Available at: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317139281416. Accessed 15 February 2014.
- 24.Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–6. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]