Among healthcare workers with influenza, half were afebrile. There was no significant difference in the rate of fever among individuals with influenza who had been previously vaccinated compared with those who had not been vaccinated (55% vs 39%; P = .33).
Keywords: influenza, vaccination, healthcare workers
Abstract
Background. To prevent transmission of influenza from healthcare workers (HCWs) to patients, many hospitals exclude febrile HCWs from working, but allow afebrile HCWs with respiratory symptoms to have contact with patients. During the 2013–2014 influenza season at our hospital, an influenza-positive HCW with respiratory symptoms but no fever was linked to a case of possible healthcare-associated influenza in a patient. Therefore, we implemented a temporary policy of mandatory influenza testing for HCWs with respiratory symptoms.
Methods. From 3 January through 28 February 2014, we tested HCWs with respiratory symptoms for influenza and other respiratory pathogens by polymerase chain reaction of flocked nasopharyngeal swabs. HCWs also reported symptoms and influenza vaccination status, and underwent temperature measurement. We calculated the proportion of influenza-positive HCWs with fever and prior influenza vaccination.
Results. Of 449 HCWs, 243 (54%) had a positive test for any respiratory pathogen; 34 (7.6%) HCWs tested positive for influenza. An additional 7 HCWs were diagnosed with influenza by outside physicians. Twenty-one (51.2%) employees with influenza had fever. Among influenza-infected HCWs, 20 had previously received influenza vaccination, 18 had declined the vaccine, and 3 had unknown vaccination status. There was no significant difference in febrile disease among influenza-infected employees who had received the influenza vaccine and those who had not received the vaccine (45% vs 61%; P = .32).
Conclusions. Nearly half of HCWs with influenza were afebrile prior to their diagnosis. HCWs with respiratory symptoms but no fever may pose a risk of influenza transmission to patients and coworkers.
Nosocomial transmission of influenza is an important cause of morbidity and mortality among patients during the influenza season each year [1]. Indeed, 17% of influenza cases are acquired in a healthcare setting [2]. Sick healthcare workers (HCWs) serve as a reservoir for influenza and may transmit the virus to vulnerable patients [3, 4]. To prevent transmission of influenza and other respiratory viruses, the Centers for Disease Control and Prevention (CDC) recommends that HCWs with fever and respiratory symptoms be excluded from work until at least 24 hours after they are afebrile without the use of antipyretics [5]. In contrast, the CDC suggests that HCWs with respiratory symptoms but no fever be allowed to work, provided that they wear a face mask during patient care activities and adhere to proper respiratory etiquette and standard precautions. Such afebrile HCWs are generally considered to be at low risk of transmitting influenza to patients. However, a recent event that occurred at our 600-bed hospital in Chicago raised concern for influenza transmission by an afebrile HCW.
In December 2013, our hospital's Infection Control Program was alerted to a case of possible healthcare-associated influenza in an inpatient. A patient was diagnosed with influenza after being hospitalized for 11 days. Further investigation of all potential contacts found that a HCW with respiratory symptoms but no fever had cared for the patient in the days before the potential case. This HCW tested positive for influenza and was the only identified source of infection for the affected patient. In accordance with the CDC's guidelines, our hospital's routine sick policy at the time prohibited febrile HCWs from working, but not those with respiratory symptoms in the absence of fever.
Given the risk of influenza transmission to patients from afebrile employees with influenza, we implemented a temporary mandatory influenza-testing policy for all HCWs with respiratory symptoms.
METHODS
From 3 January through 28 February 2014, the following policy for mandatory influenza testing was in place. HCWs without fever but with respiratory symptoms (including cough, sore throat, runny nose, or congestion) were required to undergo influenza testing to continue working. HCWs with fever and respiratory symptoms were not allowed to work, in accordance with the usual sick policy, but were also given the option of being tested for influenza.
To test for influenza, flocked nasal swabs were collected from both nares. Swabs were then analyzed by the FilmArray Respiratory Panel (Biofire, Salt Lake City, Utah), a multiplex polymerase chain reaction (PCR) assay that tests for respiratory viral and bacterial pathogens, including influenza, adenovirus, coronavirus, parainfluenza, and respiratory syncytial virus (RSV), among others.
At the time of testing, HCWs also completed a screening questionnaire describing their symptoms and influenza vaccination history and had their temperature measured to assess for fever.
HCWs whose tests were positive for influenza or who did not undergo testing were required to refrain from work for 7 days or until symptoms resolved, whichever was longer. Work restrictions were also implemented for employees who tested positive for other viruses depending on work area—for example, HCWs with RSV were not allowed to work in the neonatal intensive care unit. HCWs with any respiratory symptoms were not allowed to work in the stem cell transplantation unit until symptoms completely resolved, regardless of test result. Afebrile employees with negative tests were allowed to continue to work in all other units if they felt well enough to do so, but were required to use a mask at work at all times until their respiratory symptoms had resolved. Febrile employees with negative tests for influenza were allowed to return to work when they had been without fever for >24 hours without the use of antipyretics.
The χ2 test was used to compare results. Stata 13 statistical software was used for analyses (StataCorp, College Station, Texas).
RESULTS
Over the 2-month screening period, 449 HCWs underwent 458 respiratory virus panel tests; 243 (54%) HCWs had a positive test for any respiratory pathogen. The most common viruses isolated were coronavirus (142 positive results), influenza (35 positive results), and RSV (33 positive results). Fourteen HCWs were coinfected with 2 respiratory viruses. Eighty (18%) HCWs reported fever or had fever measured during their evaluation. See Table 1 for the frequency of symptoms present among individuals infected with the most frequently identified respiratory pathogens.
Table 1.
Symptoms Associated With Most Frequently Isolated Respiratory Viruses
| Symptom | Influenza A (n = 34) | Coronavirus 229E (n = 37) | Coronavirus HKU (n = 44) | Coronavirus NL63 (n = 16) | Coronavirus OC43 (n = 36) | Human Metapneumovirus (n = 10) | Rhinovirus/Enterovirus (n = 29) | RSV (n = 31) | Negative Test (n = 203) | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Fever | 42.4% | 13.5% | 11.4% | 31.3% | 8.3% | 0% | 17.2% | 12.9% | 19.2% | .005 |
| Cough | 100% | 56.8% | 40.9% | 87.5% | 55.6% | 50.0% | 65.5% | 96.8% | 58.6% | <.001 |
| Rhinorrhea | 63.6% | 94.6% | 90.9% | 87.5% | 83.3% | 90.0% | 82.8% | 67.7% | 64.0% | <.001 |
| Sneezing | 51.5% | 86.5% | 70.5% | 87.5% | 83.3% | 50.0% | 62.1% | 51.6% | 44.3% | .001 |
| Congestion | 63.6% | 83.8% | 65.9% | 87.5% | 75.0% | 60.0% | 69.0% | 74.2% | 55.7% | .010 |
| Sore throat | 60.6% | 56.8% | 54.5% | 50.0% | 55.6% | 70.0% | 65.5% | 71.0% | 54.2% | .725 |
Abbreviation: RSV, respiratory syncytial virus.
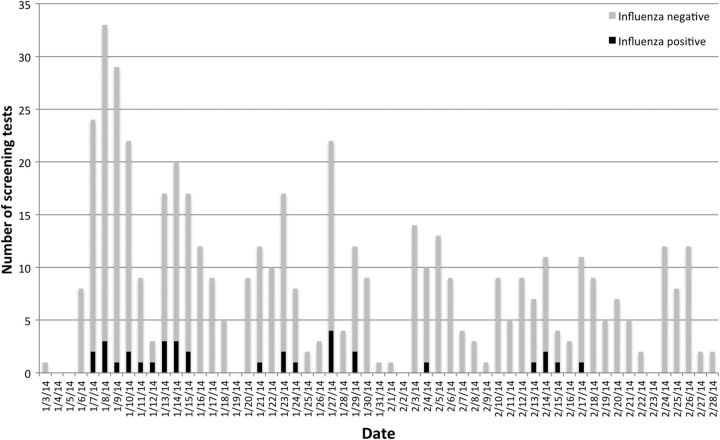
Among the HCWs with influenza, 33 tested positive for influenza A(H1N1), and 1 tested positive for the H3 subtype of influenza A. One of the HCWs with H1N1 simultaneously tested positive for influenza B. Figure 1 illustrates the proportion of positive influenza tests over time.
Figure 1.
Results of screening tests for influenza over time.
An additional 7 HCWs were diagnosed with influenza A(H1N1) via PCR testing performed by their primary physicians outside the employee screening program, bringing the total number of influenza-infected employees to 41. These additional HCWs were also asked about symptoms of fever and influenza vaccination status. Only 21 (51.2%) employees with influenza reported history of fever or were found to be febrile during evaluation. Among influenza-infected HCWs, 20 had received the influenza vaccine for the 2013–2014 season prior to their influenza diagnosis, 18 had declined the vaccine, and 3 had unknown vaccination status. There was a trend toward fever being more common among influenza-infected employees who had not received influenza vaccination compared with employees who had received influenza vaccination, but this result was not statistically significant (61% [11/18] vs 45% [9/20]; P = .32). Of note, our institutional policy expects HCWs to receive yearly influenza vaccination, but does not mandate it. Overall influenza vaccination compliance among staff at our institution was 68% for the 2013–2014 influenza season.
DISCUSSION
We have characterized the symptoms associated with a variety of respiratory viruses in the context of a mandatory influenza screening program for symptomatic HCWs. Although a higher percentage of individuals with influenza experienced fever compared with individuals with other respiratory viruses, fever was present in only half of influenza-infected employees. Previous studies have similarly reported that a sizeable proportion of individuals infected with influenza A are afebrile, ranging from 32% to 56% [6–8].
The absence of fever among many influenza-infected individuals raises serious concern about the current practice of using fever as the criteria for excluding HCWs from work. Fever is often used as a proxy for possible influenza in HCWs with respiratory symptoms. In accordance with the CDC's recommendations, many hospitals allow afebrile employees with respiratory symptoms to continue to have contact with patients. Because fever is only present half of the time among employees with influenza, using fever as the main exclusion criteria for work is not sufficient to prevent employees with influenza from caring for patients.
It is possible that afebrile HCWs with influenza may be less contagious than those who have fever. The magnitude of influenza viral shedding is lower in infected individuals with fewer symptoms compared with more highly symptomatic individuals [7]. One study found that the higher a person's temperature, the higher the rate of influenza viral shedding [7]. However, it is not known if the level of viral shedding perfectly correlates with the risk of influenza transmission. Afebrile employees with influenza may still shed virus and pose a risk of influenza transmission to patients and coworkers [9].
Theoretically, HCWs with respiratory symptoms should wear masks and practice hand hygiene, and so the risk of transmission of respiratory viruses to patients should be limited. However, HCW compliance with face masks and other personal protective equipment is self-reported to be around 60% but often observed to be less than this [10]. It is unlikely that HCWs with respiratory viral illnesses would have a much higher compliance with this policy. Until better hand hygiene and personal protective equipment compliance is demonstrated across multiple healthcare settings, it would be inadvisable to rely solely on these measures to preclude the spread of influenza in hospitals.
To prevent healthcare-associated influenza, hospitals should consider more stringent infection control measures for HCWs with respiratory symptoms, even if no fever is present. A mandatory influenza testing program for all HCWs with respiratory symptoms is one such measure, but is admittedly expensive and labor-intensive. The FilmArray Respiratory Panel alone can cost the laboratory up to $200 per panel, including labor and equipment. A more limited screening program may be sufficient, only testing employees with direct patient care or those with certain symptoms, such as cough. We found that 100% of employees with influenza disclosed having a cough. During the screening program at our hospital, 276 employees reported coughing; if we had only screened HCWs with cough, we could have reduced the number tested for influenza by 40%.
Many HCWs with influenza had been vaccinated for influenza in the months prior to their diagnosis. Although not statistically significant, a higher percentage of HCWs who had not received the influenza vaccine were febrile than HCWs who had received the influenza vaccine (61% vs 45%). The influenza vaccine has been shown to reduce the incidence of influenza among healthy adults, and may reduce the severity of illness among vaccinated individuals who do develop influenza [11–14]. If vaccination predisposes to subclinical or less severe influenza, it may actually contribute to HCWs working with influenza because they have mild illness. Although several studies have shown that vaccination of HCWs may decrease the risk of nosocomial influenza [15–18], our findings highlight the importance of not relying solely on influenza vaccination of HCWs for prevention of nosocomial influenza transmission. Other infection control precautions are necessary, such as careful evaluation of sick employees and use of masks and hand hygiene.
Our study does have limitations. The temporary policy required influenza testing for afebrile HCWs with respiratory symptoms, but not for febrile HCWs as they were expected to stay home from work regardless of their test results. HCWs with fever and more severe symptoms may not have chosen to be tested, and so we may have underestimated the proportion of influenza-positive HCWs with fever and severe symptoms. Conversely, there were likely asymptomatic HCWs or those with mild symptoms who were not tested, in which case we would have overestimated the proportion of HCWs with influenza with fever. Other HCWs may have been diagnosed with influenza or other respiratory viruses by outside clinicians and not have reported their results to their employer. An additional limitation is that vaccination status was collected based on self-report. It is possible that sick HCWs' self-report of vaccination status was not entirely accurate, but there is no reason to believe that febrile HCWs with influenza would systematically report vaccination status differently than afebrile HCWs with influenza. Another potential limitation is that the determination of “fever” was also partially based on self-report. Some HCWs who reported fever may not have had an objectively measured temperature >37.8°C (100.0°F). However, hospitals' sick policies rely on HCWs’ self-assessment of fever to determine whether or not they are eligible to work, and so self-report of fever more accurately reflects the true condition of fever identification among HCWs in the workplace. PCR may not be 100% sensitive for detection of influenza, and we may have missed some cases of influenza if individuals had a low viral load or if specimens were not properly collected. However, PCR is more sensitive than other influenza diagnostic tests including viral culture [19, 20]. Finally, these data were collected only during the 2013–2014 influenza season when the H1N1 strain was the predominant circulating strain; it is unknown if our findings are generalizable to other strains of influenza.
We have described the symptoms associated with respiratory viruses among HCWs in a large urban hospital. Most strikingly, we found that afebrile employees with respiratory symptoms, including those previously vaccinated, are potential sources of nosocomial influenza transmission. These findings can inform infection control practices and sick leave policies during the influenza season.
Notes
Acknowledgments. We thank the University of Chicago Microbiology Laboratory, Respiratory Therapy, and Occupational Medicine for their assistance in implementing the employee influenza surveillance program.
Financial support. This work was supported by the University of Chicago Medicine.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Salgado CD, Farr BM, Hall KK, Hayden FG. Influenza in the acute hospital setting. Lancet Infect Dis 2002; 2:145–55. [DOI] [PubMed] [Google Scholar]
- 2. Taylor G, Mitchell R, McGeer A, et al. Healthcare-associated influenza in Canadian hospitals from 2006 to 2012. Infect Control Hosp Epidemiol 2014; 35:169–75. [DOI] [PubMed] [Google Scholar]
- 3. Oguma T, Saito R, Masaki H, et al. Molecular characteristics of outbreaks of nosocomial infection with influenza A/H3N2 virus variants. Infect Control Hosp Epidemiol 2011; 32:267–75. [DOI] [PubMed] [Google Scholar]
- 4. Maltezou HC, Drancourt M. Nosocomial influenza in children. J Hosp Infect 2003; 55:83–91. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Prevention strategies for seasonal influenza in healthcare settings, 2013. Available at: http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm Accessed 28 July 2014.
- 6. Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000; 160:3243–7. [DOI] [PubMed] [Google Scholar]
- 7. Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis 2010; 201:1509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med 2009; 361:2507–17. [DOI] [PubMed] [Google Scholar]
- 9. Foy HM, Cooney MK, Allan ID, Albrecht JK. Influenza B in households: virus shedding without symptoms or antibody response. Am J Epidemiol 1987; 126:506–15. [DOI] [PubMed] [Google Scholar]
- 10. Daugherty EL, Perl TM, Needham DM, Rubinson L, Bilderback A, Rand CS. The use of personal protective equipment for control of influenza among critical care clinicians: a survey study. Crit Care Med 2009; 37:1210–6. [DOI] [PubMed] [Google Scholar]
- 11. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44. [DOI] [PubMed] [Google Scholar]
- 12. Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2010: CD001269. [DOI] [PubMed] [Google Scholar]
- 13. Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir Viruses 2011; 5:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Treanor JJ, Kotloff K, Betts RF, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 1999; 18:899–906. [DOI] [PubMed] [Google Scholar]
- 15. Shugarman LR, Hales C, Setodji CM, Bardenheier B, Lynn J. The influence of staff and resident immunization rates on influenza-like illness outbreaks in nursing homes. J Am Med Dir Assoc 2006; 7:562–7. [DOI] [PubMed] [Google Scholar]
- 16. Potter J, Stott DJ, Roberts MA, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis 1997; 175:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lemaitre M, Meret T, Rothan-Tondeur M, et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster-randomized trial. J Am Geriatr Soc 2009; 57:1580–6. [DOI] [PubMed] [Google Scholar]
- 18. Benet T, Regis C, Voirin N, et al. Influenza vaccination of healthcare workers in acute-care hospitals: a case-control study of its effect on hospital-acquired influenza among patients. BMC Infect Dis 2012; 12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol 2004; 42:1564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruest A, Michaud S, Deslandes S, Frost EH. Comparison of the Directigen flu A+B test, the QuickVue influenza test, and clinical case definition to viral culture and reverse transcription-PCR for rapid diagnosis of influenza virus infection. J Clin Microbiol 2003; 41:3487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]