To the Editor—A global health crisis, the 2014 Ebola outbreak has now struck healthcare workers (HCWs) at unprecedented levels. Whereas historically, Ebola epidemics spread via person-to-person transmission, the current outbreak in West Africa has seen unexpectedly extensive spread of nosocomial disease, despite HCWs’ reliance on previously effective infection control procedures such as patient isolation, barrier nursing procedures, and required personal protective equipment (PPE) [1]. Indeed, infection struck even among HCWs caring for patients with Ebola virus disease (EVD) in Western hospitals equipped with modern facilities and procedures. This has sparked growing concerns regarding how to protect HCWs [2], even those working outside the ill-prepared and overwhelmed regions of West Africa now grappling with Ebola [1].
In our view, the most concerning examples include Dr Khan [3], a Sierra Leonean virologist who contracted Ebola despite his extensive experience and careful adherence to procedures; Dr Spencer [4], a Médecins Sans Frontières physician who became symptomatic upon returning to New York despite working in well-designed isolation units built specifically to protect HCWs from EVD infection; and Dr Sacra, an obstetrician who contracted Ebola without having knowingly cared for any EVD patients [5].
Based on these developments and the knowledge that Ebola may remain viable to a certain degree on dry solid surfaces with fomites for approximately 1 day [6, 7], we hypothesize that fomite transmission of Ebola may best explain some of these unanticipated cases. Fomite transmission is facilitated by the practice of situating patients with acute symptoms and potentially extremely high viral loads outside isolation rooms in environments where adherence to routine disinfection practices is rare [7].
Taiwan's experience with severe acute respiratory syndrome (SARS) in 2003 is instructive. We contend that during the height of the SARS epidemic, HCWs in institutions that failed to identify designated zones of risk simply assumed they were secure from risk as long as they were not in proximity to patients with highly contagious pathogens. However, their confidence in existing barrier precautions and PPE as providing sufficient protection when away from heavily contaminated areas proved unwarranted [8]. As it turned out, consistent use of PPE and negative pressure isolation rooms was insufficient because the main cause of nosocomial SARS transmission was casual contact with fomites in contaminated environments either outside of isolation zones or during removal of PPE [8, 9].
Unlike when dealing with contamination by nuclear or chemical spills, there exist no distinct boundaries delineating contaminated and clean zones when managing biological disasters. Lacking clearly designated zones of risk, fomites far from patient rooms were still found to be positive for SARS coronavirus RNA [10]. In the mistaken belief that they were well away from contaminated areas and because they encountered no visible contamination, HCWs occasionally came into contact with these fomites after removing their PPE. In our view, this same scenario likely explains the infection with Ebola suffered by Drs Khan, Spencer, and Sacra.
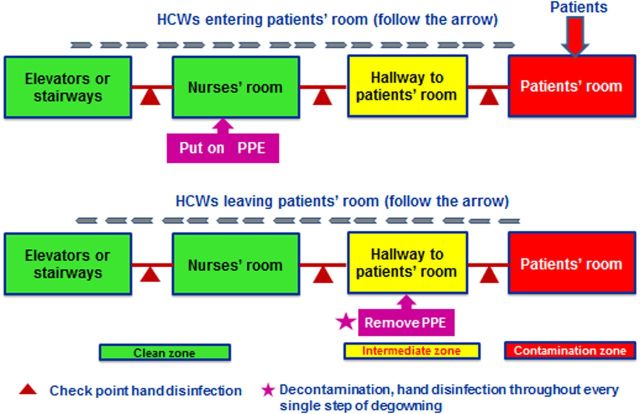
Realizing the threat of nosocomial infection, the Taiwan Centers for Disease Control responded by implementing traffic control bundling (TCB), which included triage and diversion of patients before they enter the hospital; clear delineation of zones of risk between contaminated and clean zones; and gloves-on hand disinfection at checkpoints between zones of risk (Figure 1) [11]. TCB proved critical (P < .05) for protecting HCWs [9]. Indeed, infection rates among HCWs caring for SARS patients dropped to zero following its implementation, ultimately contributing to nationwide SARS control [7].
Figure 1.
Conceptual scheme of traffic control bundling. Following triage outside the hospital entrance, all the way until being hospitalized in the isolation room, the patients remained contained inside a zone of risk (red arrow), which is distinguished from “clean zones” through the “intermediate zone.” Dispensers with 75% alcohol for gloves-on hand sanitation are installed at checkpoints positioned in between zones of risk. Healthcare workers (HCWs) in a clean zone are required to don personal protective equipment (PPE) before entering the zones of risk. When departing a zone of risk, but prior to entering a clean zone, HCWs are required to undergo decontamination and remove PPE in an intermediate zone. Here HCWs disinfect their hands, gloved or not, between every single step of the decontamination process and removal of PPE to avoid casual contact of skin/mucosa with the virus.
A key aspect of successful TCB is installation of alcohol dispensers in all zones to encourage hand disinfection. The dispensers not only demonstrate to HCWs the significance of zones of risk, but also strengthen adherence to, and frequency of, hand disinfection.
During the Taiwan response to SARS, alcohol dispensers were situated along the path from the contaminated zones through the intermediate zones and into the clean zones (Figure 1). As a result, compliance by HCWs with hand disinfection rose to 100% [9, 11]. Having already disinfected their hands, even when HCWs touched their surroundings after leaving contaminated zones and while removing PPE, they were already decontaminated and thus did not make contact with nor left fomites.
In conclusion, we suggest that TCB is a powerful, convenient, and economical tool for protecting HCWs against highly contagious diseases. We recommend that it be implemented as part of ongoing efforts to contain and control the current Ebola outbreak.
Note
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Yusuf I, Adam RU, Ahmad SA, et al. Ebola and compliance with infection prevention measures in Nigeria. Lancet Infect Dis. 2014;14:1045–6. doi: 10.1016/S1473-3099(14)70954-5. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Guidance on personal protective equipment to be used by healthcare workers during management of patients with Ebola virus disease in U.S. hospitals, including procedures for putting on (donning) and removing (doffing). Available at: http://www.cdc.gov/vhf/ebola/hcp/procedures-for-ppe.html?mobile=nocontent . Accessed 28 October 2014.
- 3.Al Jazeera Africa. Sierra Leone's top Ebola doctor gets virus. Available at: http://www.aljazeera.com/news/africa/2014/07/sierra-leone-top-ebola-doctor-gets-virus-2014723144534824780.html . Accessed 28 October 2014.
- 4.Forbes. NYC doctor Craig Spencer followed proper protocol after returning from Ebola-stricken West Africa. Available at: http://www.forbes.com/sites/davidkroll/2014/10/24/ny-doctor-craig-spencer-followed-msf-protocols-for-staff-returning-from-ebola-stricken-west-africa/ . Accessed 28 October 2014.
- 5.Fox News. US doctor with Ebola lands in Nebraska for treatment. Available at: http://www.foxnews.com/health/2014/09/04/us-doctor-infected-with-ebola-lands-in-nebraska-treatment/ . Accessed 12 October 2014.
- 6.Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol. 2010;155:2035–9. doi: 10.1007/s00705-010-0791-0. [DOI] [PubMed] [Google Scholar]
- 7.Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(suppl 2):S142–7. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 8.Yen MY, Lin YE, Su IJ, et al. Using an integrated infection control strategy during outbreak control to minimize nosocomial infection of severe acute respiratory syndrome among healthcare workers. J Hosp infect. 2006;62:195–9. doi: 10.1016/j.jhin.2005.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen MY, Lin YE, Lee CH, et al. Taiwan's traffic control bundle and the elimination of nosocomial severe acute respiratory syndrome among health care workers. J Hosp Infect. 2011;77:332–7. doi: 10.1016/j.jhin.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YC, Huang LM, Chan CC, et al. SARS in hospital emergency room. Emerg Infect Dis. 2004;10:782–8. doi: 10.3201/eid1005.030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yen MY, Chiu AW-H, Schwartz J, et al. From SARS in 2003 to H1N1 in 2009: lessons learned from Taiwan in preparation for the next pandemic. J Hosp Infect. 2014;87:185–93. doi: 10.1016/j.jhin.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]