Abstract
The present study aimed to investigate the clinical relevance of circulating tumor cells (CTCs) in patients with locally advanced head and neck squamous cell carcinoma (LA-HNSCC), particularly in patients with nasopharyngeal and hypopharyngeal squamous cell carcinoma. CTCs were isolated using negative immunomagnetic bead enrichment and were identified by fluorescence in situ hybridization. Youden's index and the receiver operating characteristic (ROC) curve were used to select the optimal CTC baseline value. χ2 test or Fisher's test were used to determine the association between CTC counts and clinical parameters, curative effects and prognosis. The Kaplan-Meier estimator was used to analyze overall survival (OS) and progression-free survival (PFS). In the present study, 356 peripheral blood samples (178 pretreatment samples and 178 post-treatment samples) from 178 patients were examined. The results revealed that the pretreatment CTC detection rate was 73.8%. The minimum, maximum and median CTC counts were 1, 22 and 2/3.2 ml, respectively. The number of polyploid CTCs was associated with distant metastasis (P=0.026). In addition, patients with undetectable CTCs, and decreasing or negative CTCs post-treatment tended to have a good prognosis (P<0.05). For nasopharyngeal squamous cell carcinoma, the PFS of patients with increased CTCs and CTCs ≥2/3.2 ml after treatment was significantly lower (P<0.05). For hypopharyngeal squamous cell carcinoma, it was suggested that CTCs with a cutoff value of 3 may be used to evaluate PFS and OS before and after treatment. In conclusion, CTCs may be used to monitor disease progression and the response to chemoradiotherapy for patients with LA-HNSCC. Furthermore, CTCs are a better predictor of the prognosis of hypopharyngeal squamous cell carcinoma than that of nasopharyngeal squamous cell carcinoma.
Keywords: circulating tumor cells, head and neck squamous cell carcinoma, negative immunomagnetic bead enrichment, fluorescence in situ hybridization, receiver operating characteristic curve, clinical significance, prognosis
Introduction
Globally, head and neck cancers account for 5–10% of all malignancies and >500,000 new cases are diagnosed each year (1,2). Among them, ~95% of head and neck cancers are classified as squamous cell carcinoma. The poor prognosis associated with this type of cancer is related to local recurrence and distant metastasis. It has previously been reported that 50–60% of patients exhibit recurrence and regional lymph node metastasis following treatment, and 20% of patients exhibit distant metastasis (3). Even if the surgical margin appears negative, as determined by histopathology, recurrence still occurs in 20% of patients (2,4,5).
In the past, the diagnosis of recurrence and metastasis was mainly based on imaging, serum tumor marker levels and histopathology. However, these methods are often limited by tumor size and location, low compliance rate, and the inability to achieve real-time monitoring. Previous studies have revealed that circulating tumor cells (CTCs) are reliable indicators that may be used for the early prediction of tumor recurrence and metastasis, thereby facilitating clinical intervention, and improving patient survival and quality of life (6–10).
Liquid biopsies, specifically for CTC detection, can make up for the deficiencies of tissue biopsy. For example, tissue biopsy specimens must be solid lesions, which are unable to respond to the current state of the disease; however, CTC specimens are obtained from peripheral blood and can reflect the current state of the disease. The advantages of CTC detection are its safety, non-invasiveness and reliability (11). Numerous studies have reported the usefulness of CTCs in the evaluation of recurrence, metastasis and prognosis of breast cancer (12,13), prostate cancer (14), colorectal cancer (15), esophageal cancer (16), etc.; therefore, CTCs may be used as an independent predictor of tumor prognosis (12,17–23). In addition, CTCs were defined as a tumor marker by the American Society of Clinical Oncology in 2007 (24), and in 2010, the American Joint Committee on Cancer designated CTCs as a novel M-segment (remote metastasis) standard, which appeared between M0 and M1 as cM0 (i+) (25). In 2017, CTCs were included in the TNM staging system in accordance with the breast cancer guidelines of the National Comprehensive Cancer Network (26).
Tumor recurrence and metastasis are the leading causes of death in patients with head and neck squamous cell carcinoma (HNSCC). To date, only a few studies have focused on the detection of CTCs in this type of cancer (1,2,27–32). Furthermore, these studies have several limitations, including few cases analyzed (1,2,27), low technical detection rate (2,28–31) and difficulty in obtaining specimens (32). To investigate the prognostic effect of CTCs on locally advanced HNSCC (LA-HNSCC), as well as the association between CTCs and clinical tumor features, the CTCs detection rate of patients with LA-HNSCC was studied and changes in CTC detection before and after treatment were analyzed.
Materials and methods
Study population and sample collection
Between October 2015 and September 2018, 264 patients that were admitted to the Chinese PLA General Hospital (Beijing, China), and were histopathologically diagnosed with LA-HNSCC via an endoscopic biopsy, were recruited to the present study. Notably, 86 patients were excluded; therefore, 178 patients were assessed. All patients had an Eastern Cooperative Oncology Group performance status score (33) of 0–1. A complete review of their medical history, as well as a thorough physical examination, was conducted for each patient prior to treatment. CTC counts were determined within 3 days prior to chemoradiotherapy and 1 month after radiotherapy (at first follow-up). All patients were followed prospectively, and all patients read and signed informed consent forms.
Inclusion and exclusion criteria
Initially, 264 patients were recruited and 86 patients were excluded, including patients that had undergone relevant treatment before CTC detection and patients with some types of cancer of which there were few cases (including 5 patients that had undergone chemoradiotherapy, 27 postoperative patients, 12 patients with non-squamous cell carcinoma,6 patients with unknown primary sites, 13 patients with laryngeal squamous cell carcinoma, 12 patients with nasal sinus squamous cell carcinoma and 11 patients with oropharyngeal squamous cell carcinoma). Finally, 178 patients were included in the present analysis. Inclusion criteria were as follows: i) Squamous cell carcinoma; ii) expected survival of >3 months; and iii) no treatment prior to CTC detection. Exclusion criteria were as follows: i) Non-squamous cell carcinoma; ii) cachexia or serious medical disease; and iii) treatments were performed prior to CTC detection.
Treatment protocols
All patients received induction chemotherapy with concurrent chemoradiotherapy. The induction chemotherapy regimen consisted of cisplatin + docetaxel + 5-fluorouracil/cisplatin + docetaxel. The concurrent chemoradiotherapy regimen consisted of cisplatin (nidaplatin) + nimotuzumab (cetuximab)/docetaxel + nimotuzumab. Patients with increased CTCs and patients whose CTCs changed from negative to positive post-treatment, and who did not exhibit disease progression or succumb to the disease were treated with thymopentin (TP5). Subsequently, CTCs were reanalyzed.
Detection of CTCs
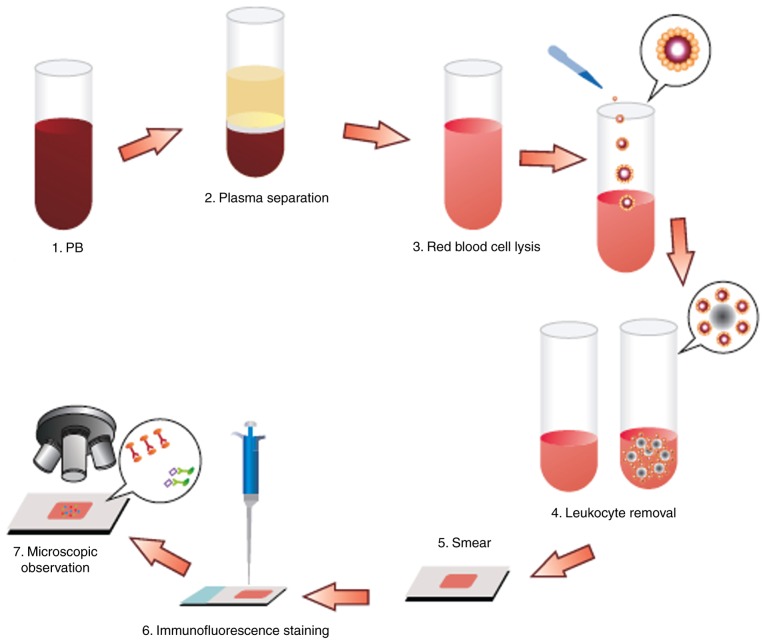
The CTCs were enriched and identified as described previously (34). Briefly, a 3.2-ml peripheral blood sample was drawn into an acid citrate dextrose anticoagulant tube (BD Biosciences) and centrifuged (650 × g; 5 min; room temperature) to separate the cells from the plasma. The red blood cells were lysed with CS2 buffer (Cyttel), followed by resuspension of cell particulates in CS1 buffer (Cyttel) and incubation with an anti-CD45 antibody conjugated to magnetic beads (Cyttel) for 20 min at 15–30°C. The immunomagnetic beads were collected using a magnetic stand (Promega Corporation) and the resulting CTC sample was applied to a glass microscope slide for observation.
The enriched cells (30–100 CTCs/µl) were then fixed in CF1 buffer (Cyttel) for 8 min at 15–30°C. The slides were immersed in saline-sodium citrate buffer for 10 min at 37°C and dehydrated in a gradient series of ethanol baths (75, 85 and 100%) for 2 min each. The slides were then incubated with hybridization solution containing chromosome 8 centromere probe (200–1,000 bp; Abbott Laboratories) at 76°C for 5 min and 37°C for 1.5 h, and placed in a hybridizer (Dako; Agilent Technologies, Inc.) for 1.5 h at 37°C. The CTCs were then immunostained for 1 h at room temperature with an anti-CD45 antibody conjugated to Alexa Fluor® 594 (Invitrogen; Thermo Fisher Scientific, Inc.). After staining the nuclei with 4,6-diamidino-2-phenylindole (DAPI; Invitrogen; Thermo Fisher Scientific, Inc.), the slides were mounted for image analysis under a fluorescence microscope. For image analysis, samples underwent double-probe staining with fluorescence in situ hybridization (FISH)-probe A and FISH-probe B (Abbott Laboratories) at 76°C for 5 min and 37°C for 1.5 h. This protocol was conducted by Cyttel Biosciences, Inc. All assessments were performed by investigators who were blinded to the clinical characteristics of the patients (Fig. 1).
Figure 1.
Flow chart of the Cyttel™-circulating tumor cell negative enrichment procedure. PB, peripheral blood.
Comparison of the enrichment and identification methods adopted in this study with other methods
The negative immunomagnetic bead enrichment method used in the present study can enrich all CTCs, whereas other enrichment methods, such as positive immunomagnetic bead enrichment and two-dimensional electrophoresis, are unable to capture CTCs that no longer possess epithelial cell adhesion molecules after undergoing epithelial-mesenchymal transition. In addition, centrifugation may result in loss of CTCs that have migrated to the plasma, red cell and granulocyte layers, and filtration is not advisable to detect tumor cells <8 µm.
The FISH identification methods adopted in this study are non-radioactive, safe, fast and sensitive, and can be used for analysis of metaphase chromosomes and interphase cells. In addition, the probes used can be detected simultaneously on the same specimen and stored for a long time. Conversely, other identification methods are radioactive, the probes used must be relabeled for each test and the labeled probe is unstable. In addition, when observing the results, more cell divisions are required for statistical analysis.
Follow-up
Follow-up was conducted through outpatient interviews. In some cases, telephone interviews were conducted. Survival was defined as the interval from the time of diagnosis to the time of death or last follow-up. The last follow-up was conducted in November 2018.
Classification of changes in CTC counts
Changes in the CTC counts were classified into three categories: Increasing, stable and decreasing. A positive CTC count (above the threshold) at first follow-up compared with a negative CTC count at baseline was considered an increase in CTCs. A negative CTC count (below the threshold) at first follow-up compared with a positive CTC count at baseline was considered a decrease in CTCs. A negative CTC count at baseline and at first follow-up was considered stable. A positive CTC count at baseline and at first follow-up with an increase in CTC count >2/ml was considered increasing, whereas a decrease in CTC count >2/ml was considered decreasing, and a change in CTC count <2/ml was considered stable (35).
Statistical analysis
Statistical analysis was performed using SPSS software (version 22; IBM Corp.). Youden's index and the receiver operating characteristic (ROC) curve were used to determine the best diagnostic cutoff value. The associations between CTC detection rate, CTC ploidy number at different cutoff values (CTCs ≥1, CTCs ≥2, CTCs ≥3, CTCs ≥4 and CTCs ≥5), CTC count (tumor load) and clinical characteristics were evaluated. Progression-free survival (PFS) and overall survival (OS) was assessed in the groups stratified according to selected CTC cutoff values for all patients with different types of cancer, and the association between changes in CTC count and treatment response and prognosis was evaluated. χ2 test or Fisher's exact test was used to analyze associations. The log-rank test and Cox proportional hazards model were used to identify prognostic factors independently associated with survival. Survival rates were assessed using the Kaplan-Meier method. PFS and OS were defined as the time from the collection of blood to the time of confirmed disease progression or last follow-up, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
Identification of CTCs
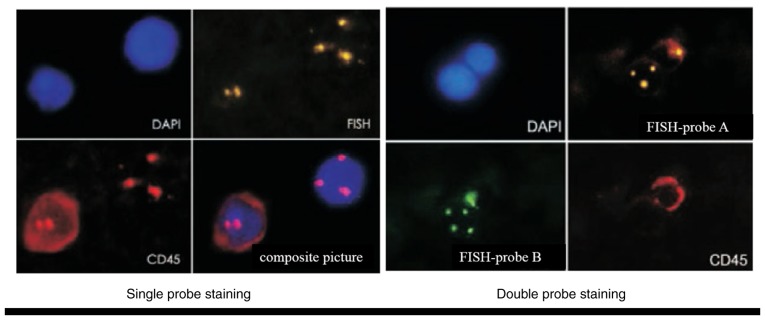
The clinical characteristics of the patients, including cancer type, differentiation and clinical stage are shown in Table I. Abnormally proliferating and CD45-negative CTCs with ≥3 nuclear chromosome enumeration probe signals were identified. The nuclei of CTCs were stained with DAPI (Fig. 2).
Table I.
Clinical characteristics of the patients recruited to the present study.
| Clinical staging type | |||||
|---|---|---|---|---|---|
| Cancer type | Ratio of male to female | Most common degree of differentiation | III | IV | Total number of cases |
| Nasopharyngeal squamous cell carcinoma | 2.65:1 | Low | 75 | 60 | 135 |
| Hypopharyngeal squamous cell carcinoma | 42:1 | Medium and high | 12 | 31 | 43 |
| Total | 3.68:1 | Medium and low | 87 | 91 | 178 |
Figure 2.
FISH was used to detect circulating tumor cells (magnification, ×400). DAPI, 4,6-diamidino-2-phenylindole; FISH, fluorescence in situ hybridization.
CTC detection rate in patients with LA-HNSCC
Before treatment, the CTC detection rate was 73.8%. The minimum, maximum and median CTC counts were 1, 22 and 2/3.2 ml, respectively. The overall distribution was skewed.
Association between CTCs or the CTC detection rate and clinicopathological variables
No significant associations were observed between CTC count or CTC detection rate and clinical characteristics, including sex, age, clinical stage, cancer type and degree of differentiation (data not shown). However, an association was observed between polyploid CTC number and metastasis (P<0.05; Table II).
Table II.
Clinical association between the number of polyploid CTCs before treatment and locally advanced head and neck squamous cell carcinoma.
| Trisomic CTCs | Tetrasomic CTCs | Multibody CTCs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | ||||||||||
| Variable | n | % | n | % | P-value | n | % | n | % | P-value | n | % | n | % | P-value |
| M stage | 0.777 | 0.705 | 0.026a | ||||||||||||
| M0 | 75 | 44.9 | 92 | 55.1 | 115 | 68.9 | 52 | 31.1 | 158 | 94.6 | 9 | 5.4 | |||
| M1 | 4 | 50.0 | 4 | 50.0 | 5 | 62.5 | 3 | 37.5 | 6 | 75.0 | 2 | 25.0 | |||
P<0.05. CTC, circulating tumor cell.
Association between CTCs and the survival rate of patients with LA-HNSCC
Five patients were lost during follow-up; therefore, a total of 173 patients were followed-up, with a follow-up rate of 97.2%. The median follow-up time was 16 months (range, 2–37 months); over the follow-up period, 30 cases exhibited recurrence or metastasis and 12 patients died. According to Youden's index and the receiver operating characteristic (ROC) curve, the best diagnostic cutoff value was determined (Figs. S1 and S2; Tables SI and SII). All patients had a cutoff value of 2 before treatment and 3 after treatment. There was no significant difference in the PFS and OS of all patients with CTCs above the cutoff value compared to those with CTCs below the cutoff value (P>0.05; Figs. S3 and S4). For patients with nasopharyngeal squamous cell carcinoma, the PFS was significantly lower for those with ≥2 CTCs than those with <2 CTCs after treatment (P<0.05 for a threshold of 2 CTCs/3.2 ml blood; Fig. 3), but the OS before and after treatment were not statistically significant (P>0.05; Fig. S5). For patients with hypopharyngeal squamous cell carcinoma, the PFS and OS of patients with ≥3 CTCs were significantly lower than those with <3 CTCs before and after treatment (P<0.05 for a threshold of 3 CTCs/3.2 ml blood; Figs. 4 and 5).
Figure 3.
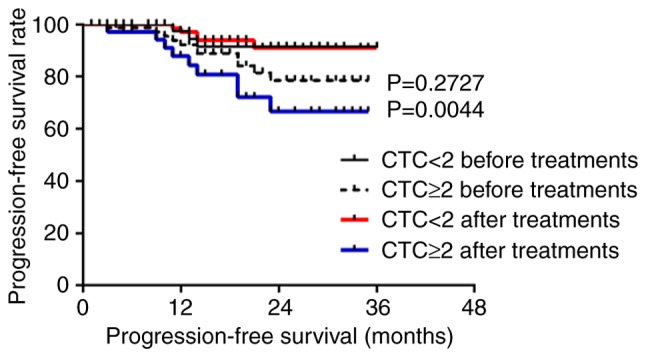
Progression-free survival of patients with nasopharyngeal squamous cell carcinoma with ≥2 CTCs before and after treatment compared with those with <2 CTCs. CTC, circulating tumor cell.
Figure 4.
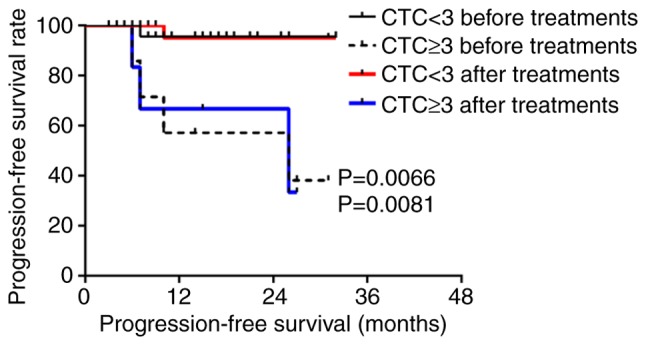
Progression-free survival of patients with hypopharyngeal squamous cell carcinoma with ≥3 CTCs before and after treatment compared with those with <3 CTCs. CTC, circulating tumor cell.
Figure 5.
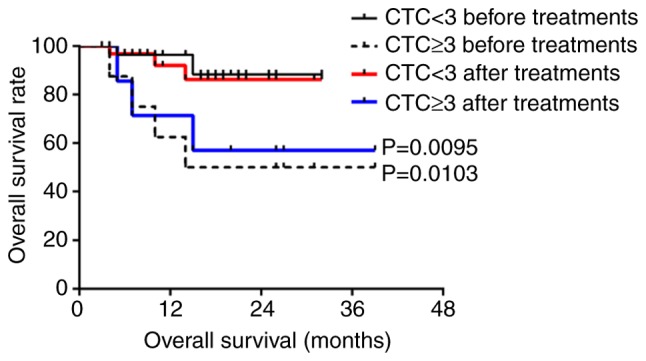
Overall survival of patients with hypopharyngeal squamous cell carcinoma with ≥3 CTCs before and after treatment compared with those with <3 CTCs. CTC, circulating tumor cell.
Association between CTC changes and the survival rate of patients with LA-HNSCC before and after treatment
Blood samples were obtained from 178 patients following treatment. The CTCs of 140 out of 178 patients decreased or remained unchanged after treatment, whereas the CTCs of 38 out of 178 patients increased. In addition, the CTCs of 90 patients were positive before and after treatment, 46 patients were positive before treatment but negative after treatment, and 42 patients were negative before and after treatment. The results revealed that patients with negative CTCs after treatment lived longer than those with positive CTCs after treatment (P<0.05; Figs. S6 and S7). The PFS of these patients was 1.21-fold higher than that of patients with positive CTCs (93.31 vs. 77.40%). The 3-year OS was also significantly reduced by 1.17 times (95.75 vs. 81.70%). In addition, PFS and OS were shorter in patients whose CTCs increased after treatment compared with in patients whose CTCs decreased or remained unchanged (P<0.0001 and P=0.0301; Figs. S8 and S9).
Association between CTCs and survival in patients with nasopharyngeal squamous cell carcinoma and hypopharyngeal squamous cell carcinoma
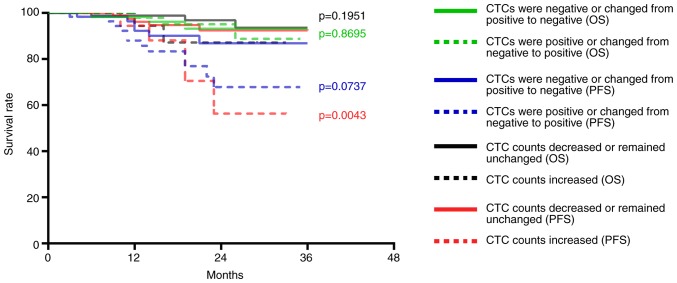
The CTC counts of 114 patients with nasopharyngeal squamous cell carcinoma were decreased or remained unchanged after treatment; of these 114 patients, disease progression occurred in eight patients and three patients died. The CTC counts of 21 patients with nasopharyngeal squamous cell carcinoma were increased; of these 21 patients, disease progression occurred in seven patients and two patients died. Compared with patients with decreased or unchanged CTC counts following treatment, the PFS of patients with increased CTC counts was significantly decreased (P=0.0043; Fig. 6), whereas no significant change was observed for OS (P=0.1951; Fig. 6). In addition, the CTCs of 67 patients were positive post-treatment; of these 67 patients disease progression occurred in nine patients and three patients died. The CTCs of 68 patients were negative post-treatment; of these 68 patients, six exhibited disease progression and two patients died. No differences were observed in the PFS (P=0.0737) and OS (P=0.8695; Fig. 6).
Figure 6.
Association of CTCs with the PFS and OS of patients with nasopharyngeal squamous cell carcinoma. PFS and OS of patients with nasopharyngeal squamous cell carcinoma whose CTC counts increased was compared with those whose CTC counts decreased or remained unchanged (P=0.0043 and 0.1951). PFS and OS of patients with nasopharyngeal squamous cell carcinoma who presented with negative CTCs or whose CTCs changed from positive to negative compared with those who presented with positive CTCs or whose CTCs changed from negative to positive (P=0.0737 and 0.8695). CTC, circulating tumor cell; OS, overall survival; PFS, progression-free survival.
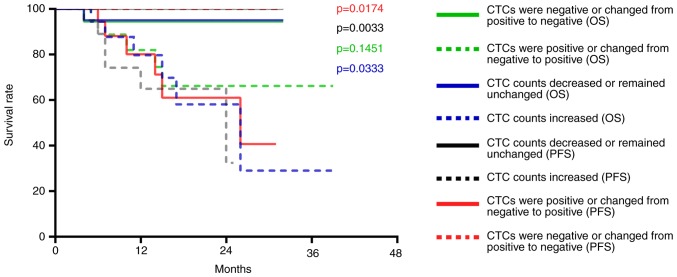
The CTC counts of 26 patients with hypopharyngeal squamous cell carcinoma were decreased or remained unchanged after treatment; no disease progression was observed in this group and one patient died. The CTC counts of 17 patients with hypopharyngeal squamous cell carcinoma were increased; of these 17 patients, six exhibited disease progression and five patients died. Compared with patients with decreased or unchanged CTC counts post-treatment, the PFS (P=0.0033) and OS (P=0.0333) of patients with increased CTCs was significantly decreased (Fig. 7). In addition, the CTCs of 23 patients were positive after treatment; of these 23 patients, six exhibited disease progression and five patients died. The CTCs of 20 patients were negative after treatment; no disease progression was observed in this group and one patient died. Compared with patients with negative CTCs or whose CTCs changed from positive to negative post-treatment, PFS was significantly decreased (P=0.0174) in patients whose CTCs were positive after treatment, whereas OS was not (P=0.1451) (Fig. 7).
Figure 7.
Association of CTCs with the PFS and OS of patients with hypopharyngeal squamous cell carcinoma. PFS and OS of patients with hypopharyngeal squamous cell carcinoma whose CTC counts increased was compared with those whose CTC counts decreased or remained unchanged (P=0.0033 and 0.0333). PFS and OS of patients with hypopharyngeal squamous cell carcinoma who presented with negative CTCs or whose CTCs changed from positive to negative compared with those who presented with positive CTCs or whose CTCs changed from negative to positive (P=0.0174 and 0.1451). CTCs, circulating tumor cells; OS, overall survival; PFS, progression-free survival.
Multivariate analysis of predictors of overall survival for patients with nasopharyngeal and hypopharyngeal squamous cell carcinoma
Univariate analysis revealed that clinical stage, baseline CTC counts, and CTC count at first follow-up were clinical factors affecting OS. Multivariate analysis revealed all of these factors to be independent prognostic markers of OS in patients with hypopharyngeal squamous cell carcinoma (Table III), but not for patients with nasopharyngeal squamous cell carcinoma (data not shown).
Table III.
Multivariate Cox regression analysis for overall survival prediction of patients with hypopharyngeal squamous cell carcinoma.
| Variable | Univariate P-value | Multivariate P-value | Hazard ratio | 95% CI |
|---|---|---|---|---|
| Sex | ||||
| M | 0.770 | |||
| F | ||||
| Age, years | ||||
| ≥60 | 0.240 | |||
| <60 | ||||
| PS, n | ||||
| 0 or 1 | 0.372 | |||
| ≥2 | ||||
| Stage at diagnosis | ||||
| Limited | 0.027a | 0.031a | 1.021 | 1.004 |
| Extensive | ||||
| CTC count at baseline | ||||
| ≥3 | 0.010a | 0.014a | 0.127 | 0.016 |
| <3 | ||||
| CTC count at first follow-up | ||||
| ≥3 | 0.009a | 0.010a | 6.992 | 3.781 |
| <3 |
P<0.05. CTC, circulating tumor cell.
TP5 treatment
TP5 is a synthetic pentapeptide that corresponds to position 32–36 of thymopoietin, and exhibits similar biological activity to thymopoietin, which is responsible for phenotypic differentiation of T cells and regulation of the immune system. TP5 has been clinically used for the treatment of patients with immunodeficiency diseases, including rheumatoid arthritis, cancer, hepatitis B virus infection, and acquired immunodeficiency syndrome (36).
Patients with increased CTCs or patients whose CTCs changed from negative to positive post-treatment, and did not exhibit disease progression or succumb to the disease were treated with TP5. Subsequently, CTCs were measured again and were decreased (data not shown). These findings indicated that increased CTCs or positive CTCs post-treatment, which did not result in disease progression, were caused by low immunity.
Discussion
In the present study, the number of polyploid CTCs was associated with distant metastasis (P=0.026). Furthermore, patients with undetectable CTCs, and decreasing CTCs or negative CTCs after treatment tended to have a good prognosis (P<0.05). For nasopharyngeal squamous cell carcinoma, the PFS of patients with increased CTCs and CTCs ≥2/3.2 ml after treatment was significantly lower (P<0.05). For hypopharyngeal squamous cell carcinoma, CTCs with a cutoff value of 3 may be used to evaluate PFS and OS before and after treatment. The present findings suggested that CTCs may be used to monitor disease progression and the response to chemoradiotherapy for patients with LA-HNSCC. Notably, the results indicated that CTCs are a better predictor of the prognosis of hypopharyngeal squamous cell carcinoma than nasopharyngeal squamous cell carcinoma.
During tumor metastasis, tumor cells interact with their surrounding microenvironment and undergo epithelial to mesenchymal transition (EMT). EMT causes epithelial cells to lose their epithelial cell phenotype and to acquire a mesenchymal cell phenotype, leading to various morphological and functional changes, thereby promoting the migration and invasion of tumor cells. Tumor cells can leave the primary site, and enter vascular or lymphatic systems as CTCs to induce metastasis (37). CTCs can also return to the bone marrow reserve pool in a resting state; under certain conditions, CTCs can again enter the vascular or lymphatic systems, and travel to other organs to form distant metastases (38,39).
CTCs are tumor cells that can be used to screen and classify high-risk tumors (40). Studies have shown that CTC counts are variable in different subtypes and stages of breast cancer, and that CTCs are more frequently observed in advanced stages compared with in early stages (41,42). In addition, CTC counts in patients with non-small cell lung cancer are significantly increased from stages I–II to III–IV (43). Conversely, this study demonstrated that there was no association between CTC count or CTC detection rate and clinical stage in LA-HNSCC.
CTC counts are lower in patients with LA-HNSCC than other types of cancer. Notably, there are few comparative studies on the clinical relevance of CTCs in LA-HNSCC, and these studies have reported different conclusions (1,2,27–32) (Table IV). It may be hypothesized that the relevance of CTCs is associated with cancer type, number of cases and the technology used.
Table IV.
Association between the detection rate of CTCs and clinical risk parameters and outcome of patients with head and neck squamous cell carcinoma in previous studies.
| Author, year | CTC-positive patients/total number of patients | Sampling site/volume | Sampling time | Tumor stage (UICC) | Detection method | Type of tumor markers | Associations | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Buglione et al, 2012 | 11/73 | PB/≥7.5 ml | Before TM | I–IV | CellSearch | EpCAM, CD45, CK, DAPI | Tumor stage, tumor burden, progression | (27) |
| Wollenberg et al, 2004 | 54/176 | BM/- | Before TM | I–IV | IHC-APAAP | CK19 | Progression | (32) |
| Nichols et al, 2012 | 6/15 | PB/10 ml | Before TM | III–IV | CellSearch | EpCAM, CD45, CK, DAPI | Prognosis, treatment outcome and efficacy of adjuvant treatments | (28) |
| Tinhofer et al, 2012 | 42/144 | PB/7.5 ml | Before TM | III–IV | PCR | EGFR | DFS, OS | (30) |
| Bozec et al, 2013 | 8/49 | PB/7.5 ml | Before TM | III–IV | CellSearch | EpCAM, CD45, CK, DAPI | No association detected | (31) |
| Jatana et al, 2010 | 34/48 | PB/10–18 ml | Before TM | I–IV | ICC | CK, CD45, DAPI | PFS | (1) |
| Hristozova et al, 2012 | 18/42 | PB/7.5 ml | Before TM | I–IV | Flow cytometry | EpCAM, CK | N stage | (29) |
CK, cytokeratin; CTCs, circulating tumor cells; DAPI, 4,6-diamidino-2-phenylindole; DFS, disease-free survival; EGFR, epidermal growth factor receptor; EpCAM, epithelial cell adhesion molecule; ICC, immunocytochemistry; IHC-APAAP, immunohistochemistry-alkaline phosphatase-anti-alkaline phosphatase; OS, overall survival; PB, peripheral blood; PCR, polymerase chain reaction; PFS, progression-free survival; TM, treatment.
CellSearch is the most common method used to isolate CTCs from the blood samples of patients with epithelial carcinoma, using epithelial cell adhesion molecule (EpCAM) as the target for cell capture. However, this technique cannot capture CTCs that no longer express EpCAM after they undergo EMT, resulting in a reduced CTC detection rate (44). Buglione et al (27) studied 73 patients with oropharyngeal, hypopharyngeal, nasopharyngeal, laryngeal and nasal sinus cancer using the CD45− + CellSearch approach. The results revealed that the CTC detection rate was 15.1%, and more CTCs were detected at stage IV than at stages I–III. The decrease or complete disappearance of CTCs during treatment meant that the progression of the disease was halted. The present results revealed that decreased or unchanged CTC counts after treatment was associated with a good prognosis, which was consistent with the aforementioned study. However, CTC detection rate was not associated with clinical stage, which may due to the fact that only stage III and IV cases were studied; therefore, the difference in detection rate was not apparent.
Jatana et al (1), studied 48 patients with oral, oropharyngeal, laryngeal and hypopharyngeal carcinoma at stages I–IV using the CD45− + DAPI approach with a detection rate of 70.8%. The results revealed that the CTC detection rate was not associated with clinical stage, tumor site and lymphatic metastasis, which was consistent with this study. Wollenberg et al (32) studied 176 patients with stage I–IV HNSCC using the immunohistochemistry-alkaline phosphatase-anti-alkaline phosphatase + cytokeratin 19 (CK19) method. The results revealed that individual CK19-expressing tumor cells were detected in the bone marrow of 30.7% of patients, and there was an association between occult tumor cells in the bone marrow and recurrence. Univariate and multivariate analyses indicated that metastases in locoregional lymph nodes and disseminated tumor cells in the bone marrow were all important predictors of prognosis. Notably, none of the aforementioned studies investigated the association between CTC counts and prognosis of different HNSCC subtypes. In the present study, polyploid CTCs were associated with distant metastases, indicating that highly proliferating tumor cells may be more likely to metastasize.
In this study, CTC detection rate was increased, compared with in other studies (1,27–32), by increasing the number of cases and improving the detection technology used. The best diagnostic threshold value for the different types of cancer was determined according to the ROC curve. An increase in CTCs post-treatment was associated with a poor prognosis, which often indicates drug resistance and the use of ineffective treatments, whereas a decrease or no change in the CTCs was associated with a better prognosis, which often indicates the use of effective treatments. The 3-year PFS of patients with positive CTCs post-treatment was significantly lower than that of patients with negative CTCs post-treatment. The PFS of patients with negative CTCs was 1.21-fold higher than that of patients with positive CTCs (93.31% vs. 77.40%). The 3-year OS was also significantly reduced by 1.17 times (95.75% vs. 81.70%).
Notably, there were still patients with increased CTCs or patients whose CTCs changed from negative to positive post-treatment that did not exhibit disease progression or succumb to the disease. After TP5 was administered to these patients, CTCs were measured again and were decreased. This finding may be associated with a decline in immunity; after using TP5 to improve immunity, the CTC counts may decrease (45).
For patients with nasopharyngeal carcinoma, CTC counts <2/3.2 ml post-treatment were associated with a significantly higher PFS than in patients with CTC counts ≥2/3.2 ml (P=0.0044). For patients with hypopharyngeal carcinoma, CTC counts ≥3/3.2 ml before and after treatment were associated with a significantly reduced PFS and OS compared with patients with CTC counts <3/3.2 ml (P<0.05). CTCs were related to PFS and OS before and after treatment for hypopharyngeal squamous cell carcinoma; however, CTCs were only associated with PFS after treatment for nasopharyngeal squamous cell carcinoma.
The PFS of patients with nasopharyngeal squamous cell carcinoma and decreased or unchanged CTCs post-treatment was 1.64 times higher than that of patients with increased CTCs (93.62% vs. 87.18%); however, no difference was observed with regards to OS. In addition, PFS and OS were significantly lower in patients with nasopharyngeal squamous cell carcinoma whose CTCs remained positive after treatment than those whose CTCs remained negative. The PFS of patients with hypopharyngeal squamous cell carcinoma and decreased or unchanged CTCs post-treatment was 3.08 times higher than that of patients with increased CTCs (100% vs. 32.47%), and the OS was 3.27 times higher (95% vs. 29.07%); these findings were significant. When CTCs changed from positive to negative or remained negative, the PFS of patients with hypopharyngeal squamous cell carcinoma was 2.46 times higher than that of patients whose CTCs changed from negative to positive or remained positive after treatment (100% vs. 40.7%); however, the OS was not significantly decreased. These findings indicated that CTCs may be a better predictor of the prognosis of hypopharyngeal squamous cell carcinoma than nasopharyngeal squamous cell carcinoma.
Notably, the present study had many novel aspects compared with previous studies; in particular: i) This study used negative enrichment of immunomagnetic beads, meaning all CTCs could be enriched, combined with fluorescence in situ hybridization, which is a non-radioactive, safe, fast and sensitive technique, the probe for which can be stored for a long time, to detect CTCs. The detection rate was 73.8%. ii) This study investigated the clinical significance of CTCs in patients with nasopharyngeal squamous cell carcinoma and hypopharyngeal squamous cell carcinoma with different prognoses. iii) Youden's index and the ROC curve were used to select the optimal CTC baseline value. iv) The associations between increased/decreased CTCs, positive/negative CTCs and prognosis before and after treatment were analyzed. v) It was demonstrated that after using TP5 to improve immunity, the CTC counts were decreased; thus, the present study analyzed the relationship between immunity and CTCs. vi) Polyploid CTCs were revealed to be associated with distant metastases, indicating that highly proliferating tumor cells are more likely to metastasize. This study provided an explanation as to why CTCs are associated with metastasis.
Conversely, in previous studies: i) The detection rate was 6.0–89.0% (46,47), and in the majority of studies the detection rate was <40%. ii) Numerous cancer species were studied with no separate subtype analysis (1,2,27–32). iii) Youden's index and the ROC curve were not used to select the optimal CTC baseline value (1,2,27–32). iv) Only the relationship between increased/decreased CTCs and prognosis was analyzed (28). v) The relationship between immunity and CTC was not assessed (1,27–32). vi) The fact that CTCs are related to metastasis was mentioned, but the underlying mechanism was not discussed (34).
In conclusion, this study revealed that the number of polyploid CTCs was associated with distant metastasis (P=0.026). In addition, patients with undetectable CTCs, and decreasing or negative CTCs post-treatment tended to have a good prognosis (P<0.05). For nasopharyngeal squamous cell carcinoma, the PFS of patients with increased CTCs and CTCs ≥2/3.2 ml after treatment was significantly lower (P<0.05). For hypopharyngeal squamous cell carcinoma, it was suggested that CTCs with a cutoff value of 3 may be used to evaluate PFS and OS before and after treatment. These findings indicated that CTCs may be used to monitor disease progression and the response to chemoradiotherapy for patients with LA-HNSCC. Furthermore, CTCs are a better predictor of the prognosis of hypopharyngeal squamous cell carcinoma than that of nasopharyngeal squamous cell carcinoma.
In future research, lymphocyte subsets will be examined and the changes in immune function will be evaluated by changes in CD4, CD8, B cells and T cells prior to chemoradiotherapy and 1 month after radiotherapy. In conclusion, a large prospective multi-institutional validation study is required to confirm these results.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
KL, SY and XZ contributed to the conception of this study and performed the preliminary documentation. All authors participated in the design of the study and implemented the research. KL, NC, JW and LM examined the archives and identified the cases included in the study, examined the slides and collected the pathological information. KL and JW enrolled patients in the study, performed clinical diagnosis and collected clinical data. All authors participated in the statistical analysis and contributed to the interpretation of the results, as well as the writing of the study. All authors reviewed the data and approved the final manuscript.
Ethics approval and consent to participate
This research abides by international and national regulations in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of the Chinese PLA General Hospital. All patients provided written informed consent before being included in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jatana KR, Balasubramanian P, Lang JC, Yang L, Jatana CA, White E, Agrawal A, Ozer E, Schuller DE, Teknos TN, Chalmers JJ. Significance of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: Initial results. Arch Otolaryngol Head Neck Surg. 2010;136:1274–1279. doi: 10.1001/archoto.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fanelli MF, Oliveira TB, Braun AC, Corassa M, Abdallah EA, Nicolau UR, da Silva Alves V, Garcia D, Calsavara VF, Kowalski LP, Chinen LTD. Evaluation of incidence, significance, and prognostic role of circulating tumor microemboli and transforming growth factor-β receptor I in head and neck cancer. Head Neck. 2017;39:2283–2292. doi: 10.1002/hed.24899. [DOI] [PubMed] [Google Scholar]
- 3.Denaro N, Merlano MC, Russi EG. Follow-up in head and neck cancer: Do more does it mean do better? A systematic review and our proposal based on our experience. Clin Exp Otorhinolaryngol. 2016;9:287–297. doi: 10.21053/ceo.2015.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Economopoulou P, Kotsantis I, Kyrodimos E, Lianidou ES, Psyrri A. Liquid biopsy: An emerging prognostic and predictive tool in head and neck squamous cell carcinoma (HNSCC). Focus on circulating tumor cells (CTCs) Oral Oncol. 2017;74:83–89. doi: 10.1016/j.oraloncology.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Tognela A, Spring KJ, Becker T, Caixeiro NJ, Bray VJ, Yip PY, Chua W, Lim SH, de Souza P. Predictive and prognostic value of circulating tumor cell detection in lung cancer: A clinician's perspective. Crit Rev Oncol Hematol. 2015;93:90–102. doi: 10.1016/j.critrevonc.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Mateo J, Gerlinger M, Rodrigues DN, de Bono JS. The promise of circulating tumor cell analysis in cancer management. Genome Biol. 2014;15:448. doi: 10.1186/s13059-014-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Liu J, Song D, Zhang Q, Ding N, He X. Circulating tumor cells in the blood of poorly differentiated nasal squamous cell carcinoma patients: Correlation with treatment response. Acta Otolaryngol. 2016;136:1164–1167. doi: 10.1080/00016489.2016.1201861. [DOI] [PubMed] [Google Scholar]
- 8.Fu X, Shen C, Wang H, Chen F, Li G, Wen Z. Joint quantitative measurement of hTERT mRNA in both peripheral blood and circulating tumor cells of patients with nasopharyngeal carcinoma and its clinical significance. BMC Cancer. 2017;17:479. doi: 10.1186/s12885-017-3471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu SH, Tsai WS, Chang YH, Chou TY, Pang ST, Lin PH, Tsai CM, Chang YC. Identifying cancer origin using circulating tumor cells. Cancer Biol Ther. 2016;17:430–438. doi: 10.1080/15384047.2016.1141839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang HD, Yu ZK. Enrichment and detection of circulating tumor cells and its application in head and neck squamous cell carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;52:147–151. doi: 10.3760/cma.j.issn.1673-0860.2017.02.020. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 11.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez JM, Fehm T, Orsini M, Cayrefourcq L, Maudelonde T, Pantel K, Alix Panabières C. Prognostic relevance of viable circulating tumor cells detected by EPISPOT in metastatic breast cancer patients. Clin Chem. 2014;60:214–221. doi: 10.1373/clinchem.2013.215079. [DOI] [PubMed] [Google Scholar]
- 14.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 16.Reeh M, Effenberger KE, Koenig AM, Riethdorf S, Eichstädt D, Vettorazzi E, Uzunoglu FG, Vashist YK, Izbicki JR, Pantel K, Bockhorn M. Circulating tumor cells as a biomarker for preoperative prognostic staging in patients with esophageal cancer. Ann Surg. 2015;261:1124–1130. doi: 10.1097/SLA.0000000000001130. [DOI] [PubMed] [Google Scholar]
- 17.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW, Uhr JW. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci USA. 1998;95:4589–4594. doi: 10.1073/pnas.95.8.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiltermann TJN, Pore MM, Van Den Berg A, Timens W, Boezen HM, Liesker JJ, Schouwink JH, Wijnands WJ, Kerner GS, Kruyt FA, et al. Circulating tumor cells in small-cell lung cancer: A predictive and prognostic factor. Ann Oncol. 2012;23:2937–2942. doi: 10.1093/annonc/mds138. [DOI] [PubMed] [Google Scholar]
- 19.Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, et al. Evaluation and prognostic significance of cir-culating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 20.Matsusaka S, Chin K, Oqura M, Suenaga M, Shinozaki E, Mishima Y, Terui Y, Mizunuma N, Hatake K. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci. 2010;101:1067–1071. doi: 10.1111/j.1349-7006.2010.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao C, Bui T, Connelly M, Doyle G, Karydis I, Middleton MR, Clack G, Malone M, Coumans FA, Terstappen LW. Circulating melanoma cells and survival in metastatic melanoma. Int J Oncol. 2011;38:755–760. doi: 10.3892/ijo.2011.896. [DOI] [PubMed] [Google Scholar]
- 22.Gazzaniga P, Gradilone A, de Berardinis E, Busetto GM, Raimondi C, Gandini O, Nicolazzo C, Petracca A, Vincenzi B, Farcomeni A, et al. Prognostic value of circulating tumor cells in nonmuscle invasive bladder cancer: A cellsearch analysis. Ann Oncol. 2012;23:2352–2356. doi: 10.1093/annonc/mdr619. [DOI] [PubMed] [Google Scholar]
- 23.Poveda A, Kaye SB, McCormack R, Wang S, Parekh T, Ricci D, Lebedinsky CA, Tercero JC, Zintl P, Monk BJ. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol. 2011;122:567–572. doi: 10.1016/j.ygyno.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Harris L, Firtsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC, Jr, American Society of Clinical Oncology American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 25.Edge SB, Compton CC. The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, Liu J, Pantel K. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012;18:5701–5710. doi: 10.1158/1078-0432.CCR-12-1587. [DOI] [PubMed] [Google Scholar]
- 27.Buglione M, Grisanti S, Almici C, Mangoni M, Polli C, Consoli F, Verardi R, Costa L, Paiar F, Pasinetti N, et al. Circulating tumour cells in locally advanced head and neck cancer: Preliminary report about their possible role in predicting response to non-surgical treatment and survival. Eur J Cancer. 2012;48:3019–3026. doi: 10.1016/j.ejca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Nichols AC, Lowes LE, Szeto CCT, Basmaji J, Dhaliwal S, Chapeskie C, Todorovic B, Read N, Venkatesan V, Hammond A, et al. Detection of circulating tumor cells in advanced head and neck cancer using the CellSearch system. Head Neck. 2012;34:1440–1444. doi: 10.1002/hed.21941. [DOI] [PubMed] [Google Scholar]
- 29.Hristozova T, Konschak R, Budach V, Tinhofer I. A simple multicolor flow cytometry protocol for detection and molecular characterization of circulating tumor cells in epithelial cancers. Cytometry A. 2012;81:489–495. doi: 10.1002/cyto.a.22041. [DOI] [PubMed] [Google Scholar]
- 30.Tinhofer I, Hristozova T, Stromberger C, Keilhoiz U, Budach V. Monitoring of circulating tumor cells and their expression of EGFR/phospho-EGFR during combined radiotherapy regimens in locally advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;83:e685–e690. doi: 10.1016/j.ijrobp.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Bozec A, Ilie M, Dassonville O, Long E, Poissonnet G, Santini J, Chamorey E, Ettaiche M, Chauvière D, Peyrade F, et al. Significance of circulating tumor cell detection using the cellsearch system in patients with locally advanced head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2013;270:2745–2749. doi: 10.1007/s00405-013-2399-y. [DOI] [PubMed] [Google Scholar]
- 32.Wollenberga B, Walza A, Kolbow K, Pauli C, Chaubal S, Andratschke M. Clinical relevance of circulating tumour cells in the bone marrow of patients with SCCHN. Onkologie. 2004;27:358–362. doi: 10.1159/000079088. [DOI] [PubMed] [Google Scholar]
- 33.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Xiao Y, Zhao J, Chen M, Xu Y, Zhong W, Xing J, Wang M. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology. 2016;21:519–525. doi: 10.1111/resp.12696. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M, Takeuchi H, Osaki Y, Hiraiwa K, Nakamura R, Oyama T, Takahashi T, Wada N, Kawakubo H, Saikawa Y, et al. Prognostic significance of circulating tumor cells in patients with advanced esophageal cancer. Esophagus. 2015;12:352–359. doi: 10.1007/s10388-014-0482-0. [DOI] [Google Scholar]
- 36.Cao Q, Gao X, Lin Y, Yue C, Wang Y, Quan F, Zhang Z, Liu X, Lu Y, Zhan Y, et al. Thymopentin ameliorates dextran sulfate sodium-induced colitis by triggering the production of IL-22 in both innate and adaptive lymphocytes. Theranostics. 2019;9:7490–7505. doi: 10.7150/thno.35015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Li X, Fu Q, Cao Q, Chen X, Wang M, Yu J, Long J, Yao J, Liu H, et al. AKR1B1 promotes basal-like breast cancer progression by a positive feedback loop that activates the EMT program. J Exp Med. 2017;214:1065–1079. doi: 10.1084/jem.20160903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C, Wang L, Guan Y, Sun Y, Liu X, Zhu D, Guo Q. Progress of circulating tumor cells in cancer management. Technol Cancer Res Treat. 2016;15:509–516. doi: 10.1177/1533034615583762. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Wei L, Li J, Zheng J, Zhang S, Zhou J. Epithelial-mesenchymal transition phenotype of circulating tumor cells is associated with distant metastasis in patients with NSCLC. Mol Med Rep. 2019;19:601–608. doi: 10.3892/mmr.2018.9684. [DOI] [PubMed] [Google Scholar]
- 40.Castro J, Sanchez L, Nuñez MT, Lu M, Castro T, Sharifi HR, Ericsson C. Screening circulating tumor cells as a noninvasive cancer test in 3388 individuals from high-risk groups (ICELLATE2) Dis Markers. 2018;2018:4653109. doi: 10.1155/2018/4653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bidard FC, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–414. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Liu Y, Zhang S, Wang T, Bian L, Wu S, Song S, Liu B, Jiang Z. Detection of circulating tumor cells and its clinical value for different stages and various subtypes of breast cancer. Zhonghua Yi Xue Za Zhi. 2014;94:2812–2815. (In Chinese) [PubMed] [Google Scholar]
- 43.Bu XM, Xu FF, Ma J, Jiang B. The expression of circulating tumor cells in peripheral blood of patients with non-small cell lungcancer and its detection. J Biol Regul Homeost Agents. 2018;32:843–849. [PubMed] [Google Scholar]
- 44.Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T, von Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin SL, Liu FK, Cai DM, et al. Effect of perioperative administration of thymopentin on cellular immunity and cytokine level following cardiopulmonary bypass. Xinfei Xueguanbing Zazhi. 2011;30:116–118, 121. (In Chinese) [Google Scholar]
- 46.Tinhofer I, Staudte S. Circulating tumor cells as biomarkers in head and neck cancer: Recent advances and future outlook. Expert Rev Mol Diagn. 2018;18:897–906. doi: 10.1080/14737159.2018.1522251. [DOI] [PubMed] [Google Scholar]
- 47.McMullen KP, Chalmers JJ, Lang JC, Kumar P, Jatana KR. Circulating tumor cells in head and neck cancer: A review. World J Otorhinolaryngol Head Neck Surg. 2016;2:109–116. doi: 10.1016/j.wjorl.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.