Abstract
Background
The use of oral ribavirin (RBV) for respiratory syncytial virus (RSV) infections is not well studied. With the drastic increase in the cost of aerosolized RBV, we aimed to compare outcomes of hematopoietic cell transplant (HCT) recipients treated with oral or aerosolized RBV for RSV infections.
Methods
We reviewed the records of 124 HCT recipients with RSV infections treated with oral or aerosolized RBV from September 2014 through April 2017. An immunodeficiency scoring index (ISI) was used to classify patients as low, moderate, or high risk for progression to lower respiratory infection (LRI) or death.
Results
Seventy patients (56%) received aerosolized RBV and 54 (44%) oral RBV. Both groups had a 27% rate of progression to LRI (P = 1.00). Mortality rates did not significantly differ between groups (30-day: aerosolized 10%, oral 9%, P = 1.00; 90-day: aerosolized 23%, oral 11%, P = .10). Classification and regression tree analysis identified ISI ≥7 as an independent predictor of 30-day mortality. For patients with ISI ≥7, 30-day mortality was significantly increased overall, yet remained similar between the aerosolized and oral therapy groups (33% for both). After propensity score adjustment, Cox proportional hazards models showed similar mortality rates between oral and aerosolized therapy groups (30-day: hazard ratio [HR], 1.12 [95% confidence interval {CI}, .345–3.65, P = .845).
Conclusions
HCT recipients with RSV infections had similar outcomes when treated with aerosolized or oral RBV. Oral ribavirin may be an effective alternative to aerosolized RBV, with potential significant cost savings.
Keywords: oral ribavirin, aerosolized ribavirin, respiratory syncytial virus, hematopoietic cell transplant, outcome
Among hematopoietic cell transplant recipients, we found no differences in rates of progression from upper to lower respiratory syncytial virus infection or 30-day mortality between those treated with oral ribavirin and those treated with aerosolized ribavirin.
Respiratory syncytial virus (RSV) is a single-stranded RNA virus of the Paramyxoviridae family and a common cause of seasonal respiratory viral infection [1]. Although RSV infection is frequently a self-limiting cause of upper respiratory infection (URI), it may progress to a more severe lower respiratory infection (LRI) in immunocompromised patients, including hematopoietic cell transplant (HCT) recipients. RSV infection in HCT recipients is associated with substantial morbidity and mortality [2–5], and often complicated by respiratory failure [6]. Several risk factors for progression to LRI and/or mortality include myelosuppression, preengraftment or early posttransplantation (ie, within 30 days), age, and mismatched or unrelated donor [6–8].
Ribavirin (RBV), a guanosine analogue active against RNA and DNA viruses, is often used to treat RSV infections. Aerosolized RBV is approved for RSV LRI in pediatric patients, although it is frequently used off-label in other populations [2, 9]. Data on RBV to treat RSV infections in HCT recipients come from small, retrospective studies with heterogeneous treatment combinations. Nevertheless, treatment with RBV was shown to prevent poor outcomes [2]. In the largest evaluation of RBV therapy for RSV in HCT recipients, Shah et al showed that aerosolized RBV at the URI stage reduced progression to LRI (83%) and RSV-associated mortality (87%) compared with no treatment [6]. Most studies on RSV treatment have evaluated aerosolized RBV [2, 6, 10]; information on other formulations is limited [2, 3, 9, 11, 12].
Aerosolized RBV to treat RSV has remained controversial due to lack of randomized controlled trials, occupational exposure concerns, and the high drug cost. Thus, an immunodeficiency scoring index (ISI) was developed to identify patients who would most benefit from RBV based on risk for progression to LRI and RSV-associated mortality [13]. Patients with high ISI (≥7) were shown to have the highest rates of progression (48%) and death (29%), as well as the greatest benefit when aerosolized RBV was administered at the URI stage (6- and 8-fold reduction in progression and mortality, respectively) [13].
A dramatic increase in the cost of aerosolized RBV in 2015 [14] limited its use at many institutions and necessitated the quest for alternative therapies, mainly via oral RBV. In addition to being inexpensive, oral RBV allows for outpatient therapy. Data on use of oral RBV to treat RSV in HCT recipients are scarce [15]. The purpose of this study was to compare progression to LRI and mortality in HCT recipients with RSV infections treated with either oral or aerosolized RBV.
METHODS
We reviewed the records of all HCT recipients who received RBV for RSV infections from 1 September 2014, through 30 April 2017, at The University of Texas MD Anderson Cancer Center. The BioFire FilmArray Respiratory Panel (BioFire Diagnostics, Salt Lake City, Utah) was used to diagnose RSV. Patients aged ≥18 years with a history of HCT were included if they received ≥1 dose of RBV for RSV infection, as an inpatient or outpatient. Patients were excluded if they were enrolled in a clinical trial for RSV, or received RBV for non-RSV indications. No formal restrictions/review of either formulation of RBV was in place at any time during this study.
Patients were identified by accessing pharmacy records for RBV orders/prescriptions. Only the first episode of RSV infection was considered for each patient. Patients were classified into the aerosolized or oral RBV group if they received that formulation for >48 hours at initiation of therapy. If the formulation was switched before 48 hours, they were classified as the formulation to which they were changed.
URI was defined as signs/symptoms of an RSV infection (rhinorrhea, nasal/sinus congestion, pharyngitis, cough) without chest radiographic imaging suggestive of pneumonia. LRI was defined as respiratory symptoms along with chest radiographic imaging suggestive of LRI (ie, ground glass opacities, interstitial infiltrates) with or without isolation of RSV from a lower respiratory sample. RSV was considered community-acquired if symptoms developed before hospitalization or within 5 days after admission and nosocomial if symptoms developed >5 days after admission.
The primary outcome was 30-day all-cause mortality. Secondary outcomes included progression to LRI for patients with URI upon treatment initiation, 90-day all-cause mortality, and need for intensive care unit (ICU) admission or mechanical ventilation. Risk for progression to LRI and RSV-associated death was stratified by ISI [13] (Table 1).
Table 1.
Immunodeficiency Scoring Index Criteria
| Criterion | ISI |
|---|---|
| ANC <500 cells/µL | 3 |
| ALC <200 cells/µL | 3 |
| Age ≥40 y | 2 |
| Myeloablative conditioning regimen used | 1 |
| GVHD, acute or chronic | 1 |
| Corticosteroids within the past 30 d | 1 |
| Recent engraftment (within 30 d) or preengraftment | 1 |
| Risk of progression from URI to LRI and RSV-associated mortality | |
| Low | 0–2 |
| Moderate | 3–6 |
| High | 7–12 |
The overall ISI is the sum of the weighted scores for the immunodeficiency criteria present at the time of RSV diagnosis. Patients with ISI 0–2 were classified as low risk, 3–6 as moderate risk, and 7–12 as high risk for progression to LRI and/or RSV-associated mortality.
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; GVHD, graft-vs-host disease; ISI, immunodeficiency virus scoring index; LRI, lower respiratory infection; RSV, respiratory syncytial virus; URI, upper respiratory infection.
The following information was collected for all patients: demographics, including age, sex, and race/ethnicity; malignancy; HCT and engraftment date; conditioning regimen; graft-vs-host disease (GVHD) status; immunosuppressive medications within 30 days prior to RSV diagnosis; formulation and duration of RBV therapy; creatinine clearance and hemoglobin; respiratory copathogens; radiographic imaging results; ICU admission and/or mechanical intubation, and date and cause of death. This study was approved by the MD Anderson Institutional Review Board and an informed consent waiver was granted.
Statistical Analysis
Comparison of Aerosolized and Oral RBV
Categorical and continuous variables were compared using Fisher exact test and the Wilcoxon-rank sum test, respectively. The primary outcome, 30-day mortality, was assessed visually using Kaplan-Meier curves and multivariate Cox proportional hazards. The final multivariate Cox proportional hazards model incorporated characteristics with a P value <.2 on bivariate analysis with administration of oral RBV forced into the model. The proportional hazards assumption was visually assessed using a plot of the scaled Schoenfeld residuals against time. A propensity score–based analysis was performed to also control for confounding. To create the propensity score, a nonparsimonious logistic regression model incorporating all measured baseline characteristics (sex, race, transplant type, ISI score, site of infection, time from transplantation, GVHD, steroid use, nosocomial infection status) was created to model the probability of oral RBV treatment. This propensity score was used to generate inverse probability of treatment weight (IPTW) and a subsequent IPTW Cox proportional hazards model [16]. Progression from URI to LRI was assessed among patients treated at the URI stage using a Fine-Gray competing risk model, treating death as a competing risk for progression to LRI [17]. A traditional multivariate model and IPTW competing risk model were constructed.
Identification of the Optimal ISI Score for Risk Stratification
Progression to LRI and 30- and 90-day mortality were assessed with previously published ISI risk categories [13]. To identify a single group at highest risk, classification and regression tree (CART) analysis was used to identify the ISI score most predictive of 30-day mortality [18]. Progression to LRI and mortality were compared in CART-identified low- and high-risk patients.
Data were collected using REDCap electronic data capture tools [19]. Statistical analyses were performed using Stata version 14.1 (StataCorp, College Station, Texas) or SAS JMP Pro version 13 (SAS Institute, Cary, North Carolina).
RESULTS
Over 3 consecutive RSV seasons, 127 adult HCT recipients with microbiologically documented RSV infections received RBV therapy. Three patients were excluded because their transplantation was done at outside institutions, and transplantation date and/or the conditioning regimen was unknown. Of the remaining 124 patients, 70 (56%) received aerosolized and 54 (44%) received oral RBV (Table 2). Only 3 patients (2 oral, 1 aerosolized RBV) received the alternate formulation for <48 hours and were classified as the regimen to which they were switched. Both groups had similar baseline demographics. The median age at RSV diagnosis was 59 years (range, 21–80 years), and multiple myeloma (33%) and AML (27%) were the most common underlying malignancies. Median time from HCT to RSV infection was 466 days (range, –1 to 7090 days). Malignancy relapse at time of RSV infection was similar between groups (aerosolized: 24%, oral: 30%, P = .543). Lymphopenia at RSV diagnosis was more common in the aerosolized (27%) than oral RBV group (9%) (P = .021), which was the only significant difference between groups. The number/types of respiratory copathogens were similar between both groups (Supplementary Table 1). Fifty-six patients had URI at RSV presentation (45%), and 68 (55%) presented with LRI.
Table 2.
Baseline Demographic and Clinical Characteristics of Hematopoietic Cell Transplant Recipients Who Received Aerosolized or Oral Ribavirin Therapy for Respiratory Syncytial Virus Infection (n = 124)
| Variable | No. (%) | P Value | ||
|---|---|---|---|---|
| Aerosolized (n = 70) | Oral (n = 54) | Total | ||
| Median age at diagnosis, y (range) | 60 (21–80) | 58 (23–79) | 59 (21–80) | .590 |
| Male sex | 46 (66) | 34 (63) | 80 (65) | .850 |
| Race/ethnicity | .832 | |||
| White | 51 (73) | 40 (74) | 91 (73) | |
| Hispanic | 9 (13) | 9 (17) | 18 (15) | |
| Black | 6 (9) | 4 (7) | 10 (8) | |
| Asian | 2 (3) | 1 (2) | 3 (2) | |
| Other | 2 (3) | 0 (0) | 2 (2) | |
| Type of malignancy | .276 | |||
| AML | 19 (27) | 14 (26) | 33 (27) | |
| ALL | 4 (6) | 4 (7) | 8 (6) | |
| CML | 1 (1) | 4 (7) | 5 (4) | |
| CLL | 0 (0) | 1 (2) | 1 (1) | |
| MDS | 7 (10) | 2 (4) | 9 (7) | |
| Hodgkin lymphoma | 0 (0) | 3 (6) | 3 (2) | |
| Non-Hodgkin lymphoma | 6 (9) | 3 (6) | 9 (7) | |
| Multiple myeloma | 24 (34) | 17 (31) | 41 (33) | |
| Other | 9 (13) | 6 (11) | 15 (12) | |
| Relapsed | 17 (24) | 16 (30) | 33 (27) | .543 |
| Donor relationship | .888 | |||
| Matched related | 15 (21) | 14 (26) | 29 (23) | |
| Matched unrelated | 18 (26) | 12 (22) | 30 (24) | |
| Haploidentical | 6 (9) | 6 (11) | 12 (10) | |
| Mismatched | 2 (3) | 0 (0) | 2 (2) | |
| Cord | 2 (3) | 2 (4) | 4 (3) | |
| Autologous | 27 (39) | 20 (37) | 47 (38) | |
| Myeloablative conditioning regimen | 65 (93) | 50 (93) | 115 (93) | 1.000 |
| Median time from HCT to infection, d (range; IQR) | 359 (–1 to 4571; IQR, 118–903) | 502 (11–7090; IQR, 236–1092) | 466 (–1 to 7090; IQR, 163–1028) | .130 |
| ≤30 d from HCT to RSV infection | 9 (13) | 2 (4) | 11 (9) | .111 |
| ≤90 d from HCT to RSV infection | 15 (21) | 6 (11) | 21 (17) | .153 |
| Acute GVHD | 9 (13) | 6 (11) | 15 (12) | 1.000 |
| Chronic GVHD | 14 (20) | 13 (24) | 27 (22) | .660 |
| Corticosteroid use | 37 (53) | 29 (54) | 66 (53) | 1.000 |
| Neutropeniaa | 5 (7) | 6 (11) | 11 (9) | .531 |
| Lymphopeniab | 19 (27) | 5 (9) | 24 (19) | .021 |
| Hb, g/dL, at baseline, median (IQR) | 9.6 (8.9–11.0)c | 9.7 (8.6–11.7) | 9.6 (8.7–11.3) | .307 |
| Nosocomial infection | 11 (16) | 4 (7) | 15 (12) | .179 |
| Stage at RSV diagnosis | .857 | |||
| URI | 31 (44) | 25 (46) | 56 (45) | |
| LRI | 39 (56) | 29 (54) | 68 (55) | |
| Stage at RBV initiation | .856 | |||
| URI | 30 (43) | 22 (41) | 52 (42) | |
| LRI | 40 (57) | 32 (59) | 72 (58) | |
| CrCl at RBV initiation, mL/min, median (IQR) | 95.12 (68–135)c | 95.06 (60–136) | 95.12 (62–135) | .186 |
| ISI | ||||
| Low risk | 6 (9) | 7 (13) | 13 (10) | .557 |
| Moderate risk | 52 (74) | 41 (76) | 93 (75) | 1.000 |
| High risk | 12 (17) | 6 (11) | 18 (15) | .444 |
| Duration of therapy, d, median (IQR) | 5 (4–5) | 5 (5–8) | ||
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CrCl, creatinine clearance; GVHD, graft-vs-host disease; Hb, hemoglobin; HCT, hematopoietic cell transplant; IQR, interquartile range; ISI, immunodeficiency scoring index; LRI, lower respiratory infection; MDS, myelodysplastic syndrome; RBV, ribavirin; RSV, respiratory syncytial virus; URI, upper respiratory infection.
aAbsolute neutrophil count <500 cells/µL.
bAbsolute lymphocyte count <200 cells/µL.
cn = 68.
Identification of High-risk Patients Based on ISI Score
CART analysis identified ISI ≥7 as the most significant predictor of 30-day mortality (sensitivity 50%, specificity 89%, area under the receiver operating curve 0.70). We found that among patients treated with aerosolized and oral RBV, patients with ISI <7 had similar rates of progression to LRI (24% vs 29%), 30-day mortality (5% vs 6%), and 90-day mortality (16% vs 8%) (Table 3 and Supplementary Tables 2 and 3). Patients with ISI ≥7 had higher 30-day and 90-day mortality rates than those with ISI <7, but rates remained similar between groups (30-day mortality: aerosolized 33%, oral 33%; 90-day mortality: aerosolized 58%, oral 33%).
Table 3.
Outcomes of Hematopoietic Cell Transplant Recipients Treated With Ribavirin for Respiratory Syncytial Virus Infection Stratified by Immunodeficiency Scoring Index
| Outcome | No. (%) | P Value | |
|---|---|---|---|
| ISI <7 | ISI ≥7 | ||
| Progression from URI to LRIa | 13/50 (26) | 1/2 (50) | .470 |
| Death at 30 d | 6/106 (6) | 6/18 (33) | .002 |
| Death at 90 d | 13/106 (12) | 9/18 (50) | .001 |
Abbreviations: ISI, immunodeficiency scoring index; LRI, lower respiratory infection; URI, upper respiratory infection.
aEvaluated in 52 patients treated at the URI stage (30 in the aerosolized group and 22 in the oral group).
Mortality Comparison Between Oral and Aerosolized RBV
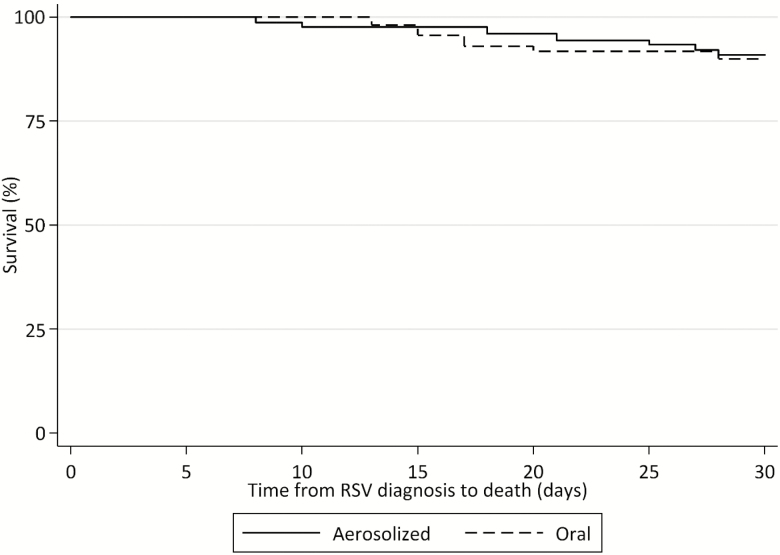
Patient demographic and clinical characteristics stratified by 30-day mortality are presented in Table 4 and outcomes are shown in Tables 5 and 6. Patients who died within 30 days were more likely to receive corticosteroids than survivors (83% vs 50%; P = .034) and to have lymphopenia (50% vs 16%; P = .012), nosocomial infection (50% vs 8%; P = .001), and a high-risk ISI score (50% vs 11%; P = .002). No differences were noted in percentage of patients receiving oral RBV as their initial treatment between survivors (44%) and nonsurvivors (42%) (P = 1.000). Significant predictors of 30-day mortality in the multivariate Cox proportional hazards model were nosocomial infection (adjusted hazard ratio [aHR], 4.75 [95% confidence interval {CI}, 1.13–20.01; P = .034), LRI at diagnosis (aHR, 8.69 [95% CI, 1.24–60.97]; P = .030), and haploidentical transplant (aHR, 10.30 [95% CI, 1.04–102.06]; P = .046). Oral administration did not predict 30-day mortality (aHR, 0.93 [95% CI, .22–4.20]; P = .960) (Figure 1 and Supplementary Figures 1 and 2). Complete results of univariate and multivariate Cox proportional hazards models are depicted in Table 7. In the IPTW Cox proportional hazards model, treatment with oral RBV was not a significant predictor of 30-day mortality (HR, 1.12 [95% CI, .35–3.65]; P = .845).
Table 4.
Baseline Demographic and Clinical Characteristics of Hematopoietic Cell Transplant Recipients Stratified by 30-Day Mortality Status (n = 124)
| Variable | No. (%) | P Value | ||
|---|---|---|---|---|
| Alive (n = 112) | Died (n = 12) | Total | ||
| Median age, y (range) | 59 (21–79) | 62 (28–80) | 59 (21–80) | .384 |
| Male sex | 72 (64) | 8 (67) | 80 (65) | 1.000 |
| Race/ethnicity | .790 | |||
| White | 80 (71) | 11 (92) | 91 (73) | |
| Hispanic | 17 (15) | 1 (8) | 18 (15) | |
| Black | 10 (9) | 0 (0) | 10 (8) | |
| Asian | 3 (3) | 0 (0) | 3 (2) | |
| Other | 2 (2) | 0 (0) | 2 (2) | |
| Type of malignancy | .740 | |||
| AML | 30 (27) | 3 (25) | 33 (27) | |
| ALL | 8 (7) | 0 (0) | 8 (6) | |
| CML | 4 (4) | 1 (8) | 5 (4) | |
| CLL | 1 (1) | 0 (0) | 1 (1) | |
| MDS | 9 (8) | 0 (0) | 9 (7) | |
| Hodgkin lymphoma | 3 (3) | 0 (0) | 3 (2) | |
| Non-Hodgkin lymphoma | 7 (6) | 2 (17) | 9 (7) | |
| Multiple myeloma | 36 (32) | 5 (42) | 41 (33) | |
| Other | 14 (13) | 1 (8) | 15 (12) | |
| Relapsed | 28 (25) | 5 (42) | 33 (27) | .300 |
| Donor relationship | .650 | |||
| Matched related | 28 (25) | 1 (8) | 29 (23) | |
| Matched unrelated | 27 (24) | 3 (25) | 30 (24) | |
| Haploidentical | 10 (9) | 2 (17) | 12 (10) | |
| Mismatched | 2 (2) | 0 (0) | 2 (2) | |
| Cord | 4 (4) | 2 (4) | 4 (3) | |
| Autologous | 41 (37) | 6 (50) | 47 (38) | |
| Myeloablative conditioning regimen | 103 (92) | 12 (100) | 115 (93) | .598 |
| Median time from HCT to infection, d (range) | 480 (–1 to 7090) | 391 (–1 to 2461) | 466 (–1 to 7090) | .710 |
| Acute GVHD | 12 (11) | 3 (25) | 15 (12) | .161 |
| Chronic GVHD | 27 (24) | 0 (0) | 27 (22) | .067 |
| Corticosteroid use | 56 (50) | 10 (83) | 66 (53) | .034 |
| Neutropeniaa | 8 (7) | 3 (25) | 11 (9) | .074 |
| Lymphopeniab | 18 (16) | 6 (50) | 24 (19) | .012 |
| Nosocomial infection | 9 (8) | 6 (50) | 15 (12) | .001 |
| Stage at RSV diagnosis | .064 | |||
| URI | 54 (48) | 2 (17) | 56 (45) | |
| LRI | 58 (52) | 10 (83) | 68 (55) | |
| Stage at RBV initiation | .072 | |||
| URI | 50 (45) | 2 (17) | 52 (42) | |
| LRI | 62 (55) | 10 (83) | 72 (58) | |
| First mode of RBV administration | 1.000 | |||
| Aerosolized | 63 (56) | 7 (58) | 70 (56) | |
| Oral | 49 (44) | 5 (42) | 54 (44) | |
| ISI | ||||
| Low risk | 12 (11) | 1 (8) | 13 (10) | 1.000 |
| Moderate risk | 88 (79) | 5 (42) | 93 (75) | .010 |
| High risk | 12 (11) | 6 (50) | 18 (15) | .002 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; ISI, immunodeficiency scoring index; LRI, lower respiratory infection; MDS, myelodysplastic syndrome; RBV, ribavirin; RSV, respiratory syncytial virus; URI, upper respiratory infection.
aAbsolute neutrophil count <500 cells/µL.
bAbsolute lymphocyte count <200 cells/µL.
Table 5.
Outcomes of Hematopoietic Cell Transplant Recipients Stratified by Treatment With Aerosolized or Oral Ribavirin for Respiratory Syncytial Virus Infection (n = 124)
| Outcome | No. (%) | P Value | ||
|---|---|---|---|---|
| Aerosolized (n = 70) | Oral (n = 54) | Total | ||
| Progression from URI to LRIa | 8 (27) | 6 (27) | 14 (27) | 1.000 |
| Death at 30 d | 7 (10) | 5 (9) | 12 (10) | 1.000 |
| Death at 90 d | 16 (23) | 6 (11) | 22 (18) | .102 |
| Median length of stay, d (IQR) | 7 (5–20) | 7 (4–16) | NA | .150 |
| Transfer to ICUb | 7 (10) | 4 (7) | 11 (9) | .755 |
| Mechanical ventilationb | 7 (10) | 3 (6) | 10 (8) | .511 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; LRI, lower respiratory infection; NA, not available; URI, upper respiratory infection.
aEvaluated in 52 patients treated at the URI stage (30 in the aerosolized group and 22 in the oral group)
bAfter treatment initiation, as assessed by transfer to ICU or initiation of mechanical ventilation 1 calendar day or later after treatment initiation.
Table 6.
Outcomes of Hematopoietic Cell Transplant Recipients Treated With Aerosolized or Oral Ribavirin for Respiratory Syncytial Virus and Stratified by Immunodeficiency Scoring Index
| Outcome | No. (%) | P Value | |
|---|---|---|---|
| ISI <7 | ISI ≥7 | ||
| Progression from URI to LRIa | |||
| Aerosolized | 7/29 (24) | 1/1 (100) | .270 |
| Oral | 6/21 (29) | 0/1 (0) | 1.000 |
| Death at 30 d | |||
| Aerosolized | 3/58 (5) | 4/12 (33) | .010 |
| Oral | 3/48 (6) | 2/6 (33) | .090 |
| Death at 90 d | |||
| Aerosolized | 9/58 (16) | 7/12 (58) | .004 |
| Oral | 4/48 (8) | 2/6 (33) | .130 |
Abbreviations: ISI, immunodeficiency scoring index; LRI, lower respiratory infection; URI, upper respiratory infection.
aEvaluated in 52 patients treated at the URI stage (30 in the aerosolized group and 22 in the oral group).
Figure 1.
Kaplan-Meier 30-day survival curve for hematopoietic cell transplant recipients treated with aerosolized or oral ribavirin for respiratory syncytial virus infection (n = 124). Abbreviation: RSV, respiratory syncytial virus.
Table 7.
Cox Proportional Hazards Regression Model for 30-Day Mortality
| Factor | Bivariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P Value | HR | (95% CI) | P Value | |
| Oral RBV | 0.93 | (.29–2.92) | .90 | 0.96 | (.22–4.20) | .96 |
| Neutropeniaa | 3.88 | (1.05–14.35) | .04 | 0.60 | (.09–4.10) | .61 |
| Lymphopeniab | 4.88 | (1.57–15.14) | <.01 | 0.90 | (.07–11.08) | .94 |
| Acute or chronic GVHD | 0.63 | (.17–2.33) | .49 | … | … | … |
| Age >65 y | 0.75 | (.20–2.79) | .67 | … | … | … |
| Recent corticosteroid use | 4.73 | (1.04–21.58) | .05 | 3.32 | (.55–20.24) | .19 |
| Recent HCT | 0.97 | (.13–7.51) | .98 | … | … | … |
| High ISI risk | 7.39 | (2.38–22.97) | <.01 | 2.60 | (.16–41.47) | .50 |
| Male sex | 0.93 | (.28–3.08) | .90 | … | … | … |
| White race | 4.20 | (.54–32.54) | .17 | 10.08 | (.78–130.60) | .08 |
| AML diagnosis | 0.91 | (.25–3.35) | .89 | … | … | … |
| Relapsed disease | 2.04 | (.65–6.43) | .22 | … | … | … |
| Nosocomial infection | 8.74 | (2.81–27.13) | <.01 | 4.75 | (1.13–20.01) | .03 |
| LRI at diagnosis | 4.49 | (.98–20.47) | .05 | 8.69 | (1.24–60.97) | .03 |
| HCT type | ||||||
| MRD | Ref | … | … | … | … | … |
| MUD | 3.00 | (.31–28.94) | .34 | … | … | … |
| Mismatched | NE | … | … | … | … | … |
| Haploidentical | 4.94 | (.45–54.63) | .19 | 10.30 | (1.04–102.06) | .05 |
| Cord | NE | … | … | … | … | … |
| Autologous | 3.94 | (.47–32.69) | .21 | … | … | … |
Abbreviations: AML, acute myeloid leukemia; CI, confidence interval; GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; HR, hazard ratio; ISI, immunodeficiency scoring index; LRI, lower respiratory infection; MRD, matched related donor; MUD, matched unrelated donor; NE, not estimated; RBV, ribavirin.
aAbsolute neutrophil count <500 cells/µL.
bAbsolute lymphocyte count <200 cells/µL.
Ninety-day mortality was significantly lower in oral RBV recipients compared with aerosolized RBV via multivariate Cox proportional hazards model (aHR, 0.27 [95% CI, .08–.95]; P = .04); however, no significant difference was observed in the IPTW model (HR, 0.52 [95% CI, .19–1.41]; P = .20). Other significant predictors of 90-day mortality generally reflected underlying comorbidities, including relapsed disease and high-risk transplant types (Supplementary Figures 3–5). Full demographic and clinical characteristics of patients and multivariate Cox proportional hazards model for 90-day mortality are presented in Supplementary Tables 4 and 5.
For patients with LRI on presentation, there was no difference in 30- and 90-day mortality between oral and aerosolized therapy (30-day mortality: oral, 4/29 [13.8%] vs aerosolized, 6/39 [15.4%], P = 1.0; 90-day mortality: oral, 5/29 [17.2%] vs aerosolized, 12/39 [30.8%], P = .263).
Progression From URI to LRI
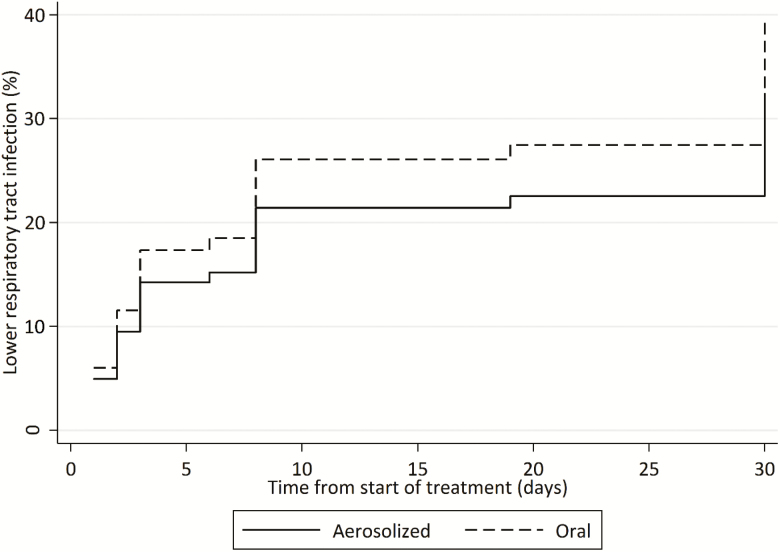
Fifty-two patients were treated with RBV at the URI stage and 17 (33%) progressed. Oral RBV was not a significant predictor of progression to LRI in the traditional multivariate Fine-Gray competing risk model (adjusted subhazard ratio [SHR], 0.67 [95% CI, .25–1.83]; P = .44) (Figure 2) or in the IPTW Fine-Gray competing risk model (SHR, 0.72 [95% CI, .24–2.14]; P = .55). The full Fine-Gray competing risk model is presented in Supplementary Table 6.
Figure 2.
Cumulative incidence function for progression to lower respiratory infection in hematopoietic cell transplant recipients treated with aerosolized or oral ribavirin for upper respiratory syncytial virus infection (n = 52).
ICU Transfer and Need for Mechanical Ventilation
The ICU transfer rate and need for mechanical ventilation in oral vs aerosolized RBV patients was similar (ICU transfer: 7% vs 10%, P = .76; mechanical ventilation: 6% vs 10%, P = .511).
Development of Grade 3 or Greater Anemia
Hemoglobin levels were similar between aerosolized and oral groups at baseline, day 7, and day 14, as was new-onset grade 3 anemia classified by the Common Terminology Criteria for Adverse Events (Supplementary Table 7).
DISCUSSION
In this study, HCT recipients with RSV infections had similar mortality and progression to LRI when treated with aerosolized or oral RBV. Despite the use of multiple statistical methods including propensity score–based modeling, no signals of increased efficacy of aerosolized RBV were observed. Moreover, oral RBV was not associated with increased rates of grade 3 or greater anemia. These findings suggest that oral RBV may be a safe and effective alternative to aerosolized RBV for the treatment of RSV in HCT recipients and have important implications for HCT programs given the significant aerosolized RBV costs.
Treatment options for RSV infection in HCT recipients are limited. RBV is the only commonly used pharmaceutical agent and is marketed in 3 formulations: aerosolized, oral, and intravenous. The intravenous formulation is not approved by the US Food and Drug Administration (FDA) and is not routinely available for clinical use [20]. Although aerosolized RBV is only FDA-approved for the treatment of RSV in children, several retrospective studies suggest that treatment with aerosolized RBV is associated with reduced mortality in HCT recipients and is thus broadly used for this indication [13, 21–23]. Due to teratogenicity, aerosolized RBV must be administered while inpatient via a scavenging tent, and the 6- to 18-hour daily administration time is challenging for patients and providers [9].
In 2015, the cost of aerosolized RBV increased by 400% to roughly $30000 per day, placing the cost of a 5-day treatment course at a staggering $150000 [14]. As a result, aerosolized RBV is no longer viable for many institutions, leaving oral RBV (~$25/day) as the only available treatment [14, 15]. At our institution, aerosolized RBV remained unrestricted, but given the widespread publicity over the increased cost of therapy and ease of oral administration, a shift in use toward oral RBV occurred naturally over the last 3 RSV seasons. However, the paucity of data on oral RBV for RSV in HCT recipients has limited its widespread acceptability as an equivalent alternative to aerosolized RBV.
Prior studies of oral RBV in HCT patients provide limited, mixed results [3, 9, 11, 12, 24, 25]. To our knowledge, ours is the largest study to date that compared outcomes of oral and aerosolized RBV for RSV in HCT recipients. Thirty-day mortality rates were similar between groups, while 90-day mortality was significantly lower in recipients of oral RBV in the multivariate Cox proportional hazards model. As several other significant predictors of 90-day mortality reflect underlying high-risk transplants and relapse of the underlying malignancy, it is more likely that this reflects a degree of residual selection bias rather than a true efficacy signal. Although the number of patients at high risk (ISI ≥7) was relatively low, no indicators of increased mortality were observed in patients treated with oral RBV. Additionally, 30- and 90-day mortality was the same with oral or aerosolized RBV for patients with LRI, suggesting that the oral formulation is as effective as aerosolized therapy for lower respiratory disease.
Patients with URI had a similar progression rates to LRI regardless of treatment with oral or aerosolized RBV (both 27%). This finding was robust in both a standard Fine-Gray competing risk model and a propensity score–based analysis. As with mortality, patients at high risk for progression did not appear to be at any additional risk if treated with oral RBV. These findings contrast with those of Casey et al, who evaluated 13 patients treated with oral RBV for RSV infection after allogeneic HCT (7 URI) [11]. In that cohort, URI progression was documented in every case and 3 patients (23%) died from respiratory failure. However, those patients were treated a median of 14 days following transplantation, so were high risk for progression regardless of treatment, whereas our cohort included the entire posttransplantation spectrum.
Our study did not include a comparator group that did not receive RBV and there are limited data comparing oral RBV to no therapy. Fifty-six patients at the University of Heidelberg were treated with oral RBV during an RSV outbreak, 32 of whom were HCT recipients and received oral RBV [25]. Multivariable analysis identified that treatment with RBV was a protective factor against fatal outcomes (P = .02). Marcelin et al assessed oral RBV administered to immunocompromised patients with RSV infection, including 25 recipients of HCT, with no patient deaths attributable to RSV [12]. More recently, Gorcea et al examined the effectiveness of oral RBV treatment of RSV in 23 allogeneic HCT recipients. No patients had a high-risk ISI score, and 4 patients were escalated to aerosolized therapy. After a median 17 months of follow-up, 17 of 23 patients (74%) were alive and only 1 death was RSV related [9].
At our institution, aerosolized RBV is most commonly dosed intermittently at 2 g over 3 hours 3 times per day. Two common dosing strategies for oral RBV are used: (1) a fixed dose of 600 mg every 8 hours or (2) a loading dose of 10 mg/kg followed by 20 mg/kg/day divided into 3 doses, both with adjustments for renal dysfunction [26–28]. Both of the doses used are based on those reported in the literature; however, the optimal dosing of RBV for RSV has not been established [7, 15]. Similarly, the optimal duration of therapy is unknown and substantial heterogeneity in practice is reported [3, 7, 9, 11, 12, 14, 24, 25]. Assessment of the optimal dose and duration of oral RBV is an area of ongoing research for our group.
Strengths of our study include comparisons of outcomes by both RBV formulation and disease severity after controlling for many cofounders. Additionally, the size of our institution and inclusion of 3 consecutive RSV seasons allowed for the inclusion of a large number of patients with different HCT types and risk strata, including relapsed disease. However, our study has some limitations. This was a single-center study that evaluated only HCT recipients; therefore, generalizability to nontransplant recipients and other institutions may be limited. Our study included low-risk HCT patients (ISI = 0–2) that may not require treatment with RBV; however, this group comprised a small portion (10%) of our cohort. Similarly, the number of patients in the high-risk group (ISI = 7–12) was low (15% of the cohort); thus, the impact of oral RBV in these high-risk patients needs further study. Last, our study was retrospective in nature and there were significantly more patients with lymphopenia in the aerosolized group, although this was not found to be independently associated with mortality on the multivariate analysis (Table 7).
In summary, our study provides evidence supporting the use of oral RBV in place of aerosolized RBV for the treatment of RSV infection in HCT recipients, including patients at high risk for progression and mortality. These findings still require validation, preferably via prospective trial. As many institutions have already adopted this practice, our findings may address some of the remaining concerns over the use of oral instead of aerosolized RBV in HCT patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge Erica Goodoff of the Department of Scientific Publications at MD Anderson for assistance with this manuscript.
Potential conflicts of interest. R. F. C. has received personal fees from Ablynx, JNJ, ADMA Biologics, and grants from Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 2000; 13:371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011; 117:2755–63. [DOI] [PubMed] [Google Scholar]
- 3. Khanna N, Widmer AF, Decker M, et al. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis 2008; 46:402–12. [DOI] [PubMed] [Google Scholar]
- 4. Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant 2005; 11:781–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raboni SM, Nogueira MB, Tsuchiya LR, et al. Respiratory tract viral infections in bone marrow transplant patients. Transplantation 2003; 76:142–6. [DOI] [PubMed] [Google Scholar]
- 6. Shah DP, Ghantoji SS, Shah JN, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 2013; 68:1872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013; 56:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim YJ, Guthrie KA, Waghmare A, et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis 2014; 209:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gorcea CM, Tholouli E, Turner A, et al. Effective use of oral ribavirin for respiratory syncytial viral infections in allogeneic haematopoietic stem cell transplant recipients. J Hosp Infect 2017; 95:214–7. [DOI] [PubMed] [Google Scholar]
- 10. Boeckh M, Englund J, Li Y, et al. ; NIAID Collaborative Antiviral Study Group Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin Infect Dis 2007; 44:245–9. [DOI] [PubMed] [Google Scholar]
- 11. Casey J, Morris K, Narayana M, Nakagaki M, Kennedy GA. Oral ribavirin for treatment of respiratory syncitial virus and parainfluenza 3 virus infections post allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 2013; 48:1558–61. [DOI] [PubMed] [Google Scholar]
- 12. Marcelin JR, Wilson JW, Razonable RR; Mayo Clinic Hematology/Oncology and Transplant Infectious Diseases Services Oral ribavirin therapy for respiratory syncytial virus infections in moderately to severely immunocompromised patients. Transpl Infect Dis 2014; 16:242–50. [DOI] [PubMed] [Google Scholar]
- 13. Shah DP, Ghantoji SS, Ariza-Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood 2014; 123:3263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chemaly RF, Aitken SL, Wolfe CR, Jain R, Boeckh MJ. Aerosolized ribavirin: the most expensive drug for pneumonia. Transpl Infect Dis 2016; 18:634–6. [DOI] [PubMed] [Google Scholar]
- 15. Beaird OE, Freifeld A, Ison MG, et al. Current practices for treatment of respiratory syncytial virus and other non-influenza respiratory viruses in high-risk patient populations: a survey of institutions in the Midwestern Respiratory Virus Collaborative. Transpl Infect Dis 2016; 18:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 18. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med 2003; 26:172–81. [DOI] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riner A, Chan-Tack KM, Murray JS. Original research: Intravenous ribavirin--review of the FDA’s Emergency Investigational New Drug Database (1997–2008) and literature review. Postgrad Med 2009; 121:139–46. [DOI] [PubMed] [Google Scholar]
- 21. Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2014; 59(Suppl 5):S344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah DP, Ghantoji SS, Shah JN, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 2013; 68:1872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seo S, Campbell AP, Xie H, et al. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant 2013; 19:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gueller S, Duenzinger U, Wolf T, et al. Successful systemic high-dose ribavirin treatment of respiratory syncytial virus-induced infections occurring pre-engraftment in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis 2013; 15:435–40. [DOI] [PubMed] [Google Scholar]
- 25. Lehners N, Schnitzler P, Geis S, et al. Risk factors and containment of respiratory syncytial virus outbreak in a hematology and transplant unit. Bone Marrow Transplant 2013; 48:1548–53. [DOI] [PubMed] [Google Scholar]
- 26. K Gupta S, Kantesaria B, Glue P. Exploring the influence of renal dysfunction on the pharmacokinetics of ribavirin after oral and intravenous dosing. Drug Discov Ther 2014; 8:89–95. [DOI] [PubMed] [Google Scholar]
- 27. Gupta SK, Kantesaria B, Glue P. Pharmacokinetics, safety, and tolerability of ribavirin in hemodialysis-dependent patients. Eur J Clin Pharmacol 2012; 68:415–8. [DOI] [PubMed] [Google Scholar]
- 28. Gupta SK, Kantesaria B, Glue P. Pharmacokinetics and safety of single-dose ribavirin in patients with chronic renal impairment. Drug Discov Ther 2013; 7:158–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.