Understanding of the microbiology of pneumonia has evolved. The role of pneumococcus has greatly declined. “Atypical” agents cause only a very small proportion of cases. Viruses are prominent. Intensive investigations fail to identify a causative organism in more than 50% of cases.
Keywords: community-acquired pneumonia, Streptococcus pneumoniae, pneumococcus, etiology, antibiotic stewardship
Abstract
Before 1945, Streptococcus pneumoniae caused more than 90% of cases of pneumonia in adults. After 1950, the proportion of pneumonia caused by pneumococcus began to decline. Pneumococcus has continued to decline; at present, this organism is identified in fewer than fewer10%–15% of cases. This proportion is higher in Europe, a finding likely related to differences in vaccination practices and smoking. Gram-negative bacilli, Staphylococcus aureus, Chlamydia, Mycoplasma, and Legionella are each identified in 2%–5% of patients with pneumonia who require hospitalization. Viruses are found in 25% of patients, up to one-third of these have bacterial coinfection. Recent studies fail to identify a causative organism in more than 50% of cases, which remains the most important challenge to understanding lower respiratory infection. Our findings have important implications for antibiotic stewardship and should be considered as new policies for empiric pneumonia management are developed.
Pneumonia, at first called “the special enemy of old age” [1] by Osler and later, by a more mature Osler, “the friend of the aged” [2], has long been with humankind and shows little likelihood of going away. In the preantibiotic era, Streptococcus pneumoniae was the overwhelmingly predominant cause of pneumonia, but this is clearly no longer the case. Despite its prevalence and importance—pneumonia affects about 2% of all elderly persons each year [3]—and despite newly available techniques for diagnosis, at the present time and in the majority of cases, the causative organism(s) remain(s) uncertain.
How does such an evolution occur? For recognized or unrecognized reasons, there may be changes in the actual incidence of a disease. Rheumatic fever began to decline in the late 1920s, well before the introduction of antibiotics, and auxotrophic strains of Neisseria gonorrhoeae that made acute arthritis an everyday occurrence in public hospitals in the early 1980s have largely disappeared. Newly discovered organisms may cause disease, and new diagnostic techniques may increase recognition of already-identified diseases. Raised awareness of a condition may lead to more diagnostic testing and increased documentation.
Here, we document the declining role of S. pneumoniae and the increasing recognition of other pathogens in the etiology of community acquired pneumonia (CAP) in adults.
LITERATURE ANALYSIS
A PubMed search of the English-language literature through December 2015 was performed using the following search term: (pneumonia[title]) AND (etiolog* OR aetiolog* OR cause OR microbiolog* OR “Streptococcus pneumoniae”[Mesh] OR bacteria OR culture*) NOT (children OR infant* OR pediatric* OR randomized OR nosocomial[title] OR organizing[title] OR interstitial[title] OR healthcare[title] OR ventilator[title] OR Case Reports[ptyp]). The references of included studies were also searched for candidate articles. We included studies that applied validated pneumococcal tests to ≥50% of patients. The following were considered evidence of pneumococcal infection: S. pneumoniae cultured from a sterile site or from a lower respiratory tract specimen (ie, not a nasopharyngeal specimen), including recovery after mouse intraperitoneal inoculation; pneumococcal antigen detection in urine; and polymerase chain reaction (PCR) testing of pleural fluid. Studies that focused on a specific population (eg, the elderly or immunocompromised) were excluded. See Supplementary Material for details. We identified 31 studies (21120 patients) from the United States and Canada published between 1917 and 2015 and 37 studies (21166 patients) from Europe. We included all publications that met our inclusion criteria [4–71] (Table 1).
Table 1.
Frequency of Streptococcus pneumoniae in Community-Acquired Pneumonia in Included Studies
| Study | Location | Year(s) ofStudy | No. ofPatientsa | Pneumococcal Community-Acquired Pneumonia | |
|---|---|---|---|---|---|
| n | % | ||||
| United States/Canada | |||||
| Avery 1917 [4] | United States | NR | 529 | 454 | 85.8 |
| Cecil 1922 [5] | United States | 1920 | 1000 | 834 | 83.4 |
| Sutliff 1933 [6] | United States | NR | 1067 | 996 | 93.3 |
| Bullowa 1937 [7] | United States | 1928–1936 | 4416 | 3591 | 81.3 |
| Cecil 1938 [8] | United States | 1928–1938 | 560 | 531 | 94.8 |
| Warner 1945 [9] | United States | 1943–1944 | 150 | 139 | 92.7 |
| Warner 1948 [10] | United States | 1944–1946 | 202 | 172 | 85.1 |
| Dowling 1948 [11] | United States | NR | 2500 | 2453 | 98.1 |
| Tillotson 1969 [12] | United States | 1967 | 149 | 84 | 56.4 |
| Fiala 1969 [13] | United States | 1966–1967 | 193 | 107 | 55.4 |
| Fekety 1971 [14] | United States | 1965–1966 | 100 | 62 | 62.0 |
| Dorff 1973 [15] | United States | 1969–1970 | 148 | 79 | 53.4 |
| Moore 1977 [16] | United States | 1971–1972 | 154 | 65 | 42.2 |
| Oseasohn 1978 [17] | United States | 1971–1972 | 187 | 171 | 91.4 |
| 1972–1973 | |||||
| Miller 1978 [18] | United States | 1975 | 54 | 26 | 48.1 |
| Ebright 1980 [19] | United States | 1973 | 106 | 38 | 35.8 |
| Boerner 1982 [20] | United States | NR | 89 | 32 | 36.0 |
| Dans 1984 [21] | United States | 1979–1980 | 147 | 51 | 34.7 |
| Marrie 1985 [22] | Canada | 1981–1982 | 138 | 13 | 9.4 |
| Larsen 1984 [23] | United States | 1981–1982 | 217 | 52 | 24.0 |
| Fang 1990 [24] | United States | 1986–1987 | 359 | 55 | 15.3 |
| Farr 1991 [25] | United States | 1984–1986 | 245 | 45 | 18.4 |
| Bates 1992 [26] | United States | 1985 | 154 | 9 | 5.8 |
| Mundy 1995 [27] | United States | 1990 | 205 | 31 | 15.1 |
| Marston 1997 [28] | United States | 1991 | 2776 | 351 | 12.6 |
| Fine 1999 [29] | United States, Canada | 1991–1994 | 1551 | 158 | 10.2 |
| Park 2001 [30] | United States | 1994–1996 | 389 | 46 | 11.8 |
| Yang 2005 [31] | United States | 2001–2003 | 131 | 30 | 22.9 |
| Restrepo 2008 [32] | United States | 1999–2002 | 730 | 60 | 8.2 |
| Musher 2013 [33] | United States | 2011–2012 | 215 | 19 | 8.8 |
| Jain 2015 [34] | United States | 2010–2012 | 2259 | 115 | 5.1 |
| Europe | |||||
| Hug 2001 [35] | Switzerland | 1998–1998 | 293 | 30 | 10.2 |
| Garbino 2002 [36] | Switzerland | 1997–1999 | 318 | 40 | 12.6 |
| Genne 2006 [37] | Switzerland | 1999–2000 | 67 | 25 | 37.3 |
| Piso 2012 [38] | Switzerland | 2007–2008 | 139 | 39 | 28.1 |
| Stralin 2010 [39] | Sweden | 1999–2002 | 235 | 74 | 31.5 |
| Johansson 2010 [40] | Sweden | 2004–2005 | 184 | 53 | 28.8 |
| Pareja 1992 [41] | Spain | NR | 165 | 12 | 7.3 |
| Almirall 1993 [42] | Spain | 1990–1991 | 105 | 13 | 12.4 |
| Lorente 2000 [43] | Spain | 1996–1998 | 110 | 40 | 36.4 |
| Almirall 2000 [44] | Spain | 1993–1995 | 232 | 27 | 11.6 |
| Gutierrez 2005 [45] | Spain | 1999–2001 | 493 | 101 | 20.5 |
| Andreo 2006 [46] | Spain | 2000 | 107 | 35 | 32.7 |
| Briones 2006 [47] | Spain | 2000–2004 | 959 | 290 | 30.2 |
| Fernandez Alvarez 2007 [48] | Spain | 1995–1998 | 258 | 14 | 5.4 |
| 2003–2006 | 229 | 17 | 7.4 | ||
| 1999–2002 | 268 | 11 | 4.1 | ||
| Falguera 2009 [49] | Spain | 1995–2005 | 3272 | 972 | 29.7 |
| Cilloniz 2011 [50] | Spain | 1996–2008 | 3523 | 613 | 17.4 |
| Sorde 2011 [51] | Spain | 2007–2008 | 474 | 177 | 37.3 |
| Capelastegui 2012 [52] | Spain | 2006–2007 | 700 | 170 | 24.3 |
| Viasus 2013 [53] | Spain | 2010–2011 | 747 | 118 | 15.8 |
| Tudose 2010 [54] | Romania | 2008–2010 | 120 | 11 | 9.2 |
| Roysted 2016 [55] | Norway | 2007–2008 | 374 | 75 | 20.1 |
| Boersma 1991 [56] | Netherlands | 1987–1989 | 87 | 34 | 39.1 |
| Bohte 1995 [57] | Netherlands | 1991–1993 | 334 | 90 | 26.9 |
| Endeman 2008 [58] | Netherlands | 2004–2006 | 201 | 60 | 29.9 |
| Huijts 2014 [59] | Netherlands | 2008–2009 | 1057 | 332 | 31.4 |
| Ruf 1989 [60] | Germany | 1984–1985 | 442 | 68 | 15.4 |
| Kothe 2008 [61] | Germany | 2003–2005 | 2647 | 130 | 4.9 |
| Levy 1988 [62] | France | 1983–1984 | 116 | 30 | 25.9 |
| Lehtomaki 1988 [63] | Finland | 1983–1984 | 106 | 26 | 24.5 |
| Hohenthal 2008 [64] | Finland | 1999–2004 | 384 | 107 | 27.9 |
| Leesik 2006 [65] | Estonia | 1996–1998 | 439 | 54 | 12.3 |
| White 1981 [66] | England | 1974–1980 | 210 | 24 | 11.4 |
| Bewick 2012 [67] | England | 2008–2010 | 920 | 366 | 39.8 |
| Ostergaard 1993 [68] | Denmark | NR | 254 | 39 | 15.4 |
| Marekovic 2008 [69] | Croatia | NR | 80 | 23 | 28.8 |
| Blasi 1993 [70] | Italy | 1991–1992 | 108 | 10 | 9.3 |
| Logroscino 1999 [71] | Italy | 1994–1996 | 409 | 36 | 8.8 |
Abbreviation: NR, not reported.
aSee Supplementary Material for details on inclusion criteria.
PREANTIBIOTIC ERA
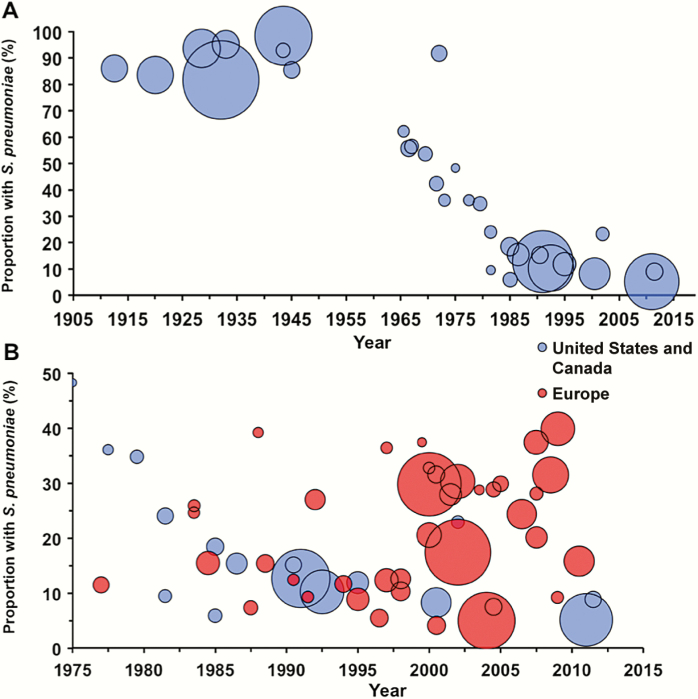
In the preantibiotic era, S. pneumoniae caused approximately 90% of all cases of pneumonia [4, 72–74] (Figure 1A). Streptococcus pyogenes, Klebsiella, Haemophilus influenzae, and Staphylococcus aureus were identified in the remaining cases; in fewer than 5%, no bacterial cause could be found. In the preantibiotic era, no antibiotic would have interfered with a bacteriologic diagnosis, and, compared to current practice, clinicians made greater efforts to obtain sputum samples for culture. Of course, pneumonia due to influenza virus had been recognized for quite some time [75]. Modern studies of patients who died in the 1918 influenza epidemic suggest that most of those deaths were, in fact, associated with secondary bacterial infection [76]. Outbreaks of pneumonia, thought to be viral and later identified as adenoviral [77], occurred in young adults, especially in military settings where crowding and physical and mental exhaustion were prevalent.
Figure 1.
A, Frequency of Streptococcus pneumoniae as a cause of community-acquired pneumonia, United States/Canada. B, Comparison of the frequency of S. pneumoniae between the United States/Canada (blue) and Europe (red). The area of each circle is proportional to the number of patients in each study. For both figures, the year is the reported (or estimated) midyear of investigation (see Supplementary Material for details).
In the late 1930s, a pneumonia that behaved differently from pneumococcal pneumonia was attributed to an “atypical agent” [78, 79], subsequently called Eaton agent [80, 81] and eventually identified as Mycoplasma pneumoniae [82]. Recognized cases, documented by culture, nearly always occurred in children, teenagers, and young adults (eg, military recruits [80]). Older adults were largely unaffected [83, 84], an observation that later came to be overlooked when diagnosis began to rely on serologic techniques. As a result, Mycoplasma pneumonia came to be greatly overdiagnosed. During World War II, an Armed Forces Commission, established to investigate outbreaks of pneumonia in military forces, identified the following 4 kinds of pneumonia: pneumococcal, mycoplasmal, influenzal, and acute respiratory disease of recruits, later shown to be adenoviral [85]. Nevertheless, pneumococcus was still regarded as the principal cause. In fact, through its 12th (1967) edition, the Cecil-Loeb Textbook of Medicine covered the subject of pneumonia under the rubric “Bacterial diseases: pneumococcal pneumonia” [86]. Although other causes were cited, “pneumococcal pneumonia … [remained] the most important of all pneumonias, not only in numerical terms, but because … [it was] the prototype of all the bacterial pneumonias” [86].
EARLY ANTIBIOTIC ERA THROUGH 1990
Pneumococcus
Our literature search revealed no pneumonia studies reporting the etiologic spectrum from 1947 through 1964. In 1965–1966, a report from Johns Hopkins Hospital [14] implicated pneumococcus in 62% of cases. No cause was found in 34%, and no case was attributed to H. influenzae, but chocolate agar was not used routinely to culture sputum until the 1970s. In 1971–1972, at the same hospital [16], an “aggressive” approach to diagnosis identified pneumococcus in 30% of cases. Haemophilus influenzae was cultured from sputum in 29%. However, because only 1 patient was bacteremic, the authors stated that the “others can not critically be called Haemophilus pneumonia.” By 1979–1980 and 1990–1991, reports from Johns Hopkins Hospital implicated pneumococcus in 35% [21] and 15% [27] of cases, respectively.
Anaerobic Flora
In the 1970s attention was directed toward anaerobic flora of the mouth and upper respiratory tract as a potential cause of pneumonia. In 1898 Veillon implicated anaerobic bacteria in lung infection [87]; their importance as the most common agents in lung abscess was subsequently emphasized by Smith [88] and confirmed by Cohen [89]. Further development of anaerobic bacteriology by Bartlett, Gorbach, and Finegold [90], together with the use of transtracheal aspiration, showed the importance of these organisms as a cause of pneumonia, especially in patients whose lifestyle suggested aspiration and who presented with a subacute syndrome of respiratory infection characterized by putrid sputum, lung abscess, and/or empyema.
Normal Respiratory Flora
Some adults who present with acute bacterial pneumonia were also shown to be infected by normal facultative or anaerobic organisms [90–98]. More recent reports on the etiology of pneumonia fail to mention these organisms, probably reflecting the failure to use transtracheal aspiration and/or anaerobic microbiology. Microscopic examination of good-quality sputum from patients with pneumonia may show large numbers of gram-positive cocci together with other organisms. Such findings suggest that so-called normal respiratory flora, including the Streptococcus anginosus group, Streptococcus mitis, and/or mixed respiratory flora, may cause a substantial proportion of cases of pneumonia when no recognized respiratory pathogen is identified.
Legionella
An outbreak of pneumonia in 1977 [99] led to identification of Legionella [100]. Implication of Legionella in other outbreaks [101] was soon followed by recognition of this organism as a cause of sporadic pneumonia, both in hospitals [102] and in the community [103, 104]. The relation to water sources [105, 106] and the association with geographical locations [104] was also documented.
Haemophilus influenzae
In 1983, Musher et al [107] showed that H. influenzae commonly caused pneumonia in older men, identifying 30 cases at a single hospital in a 2-year period. Haemophilus from 26 of these patients was nontypable; only 1 of these had associated bacteremia. Four patients had H. influenzae type b in their sputum, of whom 3 were bacteremic.
Chlamydia
In 1986, Grayston et al identified Chlamydia pneumoniae as an important cause of pneumonia in university students [108, 109]. As with Mycoplasma, this organism was not found commonly in older adults with pneumonia, raising question about more recent studies that have used only serologic techniques to implicate it as a common cause of pneumonia in hospitalized adults.
Viruses
During the 1980s, viral cultures of respiratory secretions increasingly documented a causative role for respiratory viruses in pneumonia. The potential importance of parainfluenza [14] and respiratory syncytial viruses [110] in adults was recognized; rhinovirus, coronavirus [111], and human metapneumovirus [112] were added later. Thus, by the end of the 1980s, most microorganisms that are currently known to cause CAP had been identified.
A landmark prospective study of CAP patients admitted to a private hospital, a university hospital, and a veterans’ hospital in Pittsburgh in 1986–1987 [24] used classic bacteriologic techniques and identified pneumococcus in only 15% of cases, followed by Haemophilus in 11%. Serologic studies were positive for Mycoplasma or Chlamydia in 8%. Legionella was detected by antibody rise, immunofluorescence, and/or culture in 7%. Viruses were not sought. No cause was found in one-third of cases. The authors emphasized the decline of pneumococcus as a cause of pneumonia in the antibiotic era.
ANTIBIOTIC ERA WITH NEW TECHNIQUES, 1990–2010
After 2000, new techniques greatly increased the ability to identify respiratory pathogens. Tests to detect antigens of S. pneumoniae and Legionella in urine reliably increased recognition of the role played by these agents [67, 113–117]. Detection of capsular polysaccharides [118] or other pneumococcal constituents such as DNA that encode lytA in sputum or nasal secretions [119] appeared to increase the diagnostic yield. However, these and other nonstandardized techniques may also lead to overdiagnosis [120], and further validation is required before they can be used in diagnosis. There is, however, little question that the availability of PCR has revolutionized our understanding of the role of respiratory viruses in pneumonia in adults.
More recent studies in the United States, that were designed to select a convenience sample of patients or to include patients in a pharmaceutical study (but not a complete evaluation of successive admissions), detected pneumococcus in 10%–14% of adults hospitalized for CAP [121,122] (Figure 1A). During the same period, a greater proportion of cases have been attributed to pneumococcus in Europe than in the United States (Table 2). Lim et al [123] reported an etiologic agent in 75% of cases, including pneumococcus in 48% and “atypical organisms,” principally Chlamydia and Mycoplasma, in 18%. Nearly one-third of pneumococcal diagnoses were made serologically by rises in antibody to various pneumococcal constituents or by counterimmunoelectrophoresis (CIE) to detect capsular polysaccharides in sputum. However, these serologic tests have not been validated, and CIE is regularly positive in sputum that does not contain pneumococci (D. Musher and C. Stager, unpublished). If these serologic results are excluded, pneumococcus was found in 34% of cases. Diagnoses of Chlamydia and Mycoplasma were only made serologically. Interestingly, an earlier study by these same investigators [124] in which no special serologic techniques were used identified an etiologic agent in 54% of cases, with pneumococcus in 30%, Haemophilus in 8%, and “atypical organisms” in 1%. European investigators [125, 126] used only traditional microbiology and detection of pneumococcal cell wall antigen in urine to implicate S. pneumoniae in 37%–38% of cases of CAP.
Table 2.
Etiology of Community-Acquired Pneumonia in Europe, 2001–2010
| Pathogen | Percentage of Patients | ||
|---|---|---|---|
| Englanda [123] | Swedenb [40] | Netherlandsc [126] | |
| Streptococcus pneumoniae | 48 | 38 | 37 |
| Haemophilus | 7 | 11 | 0 |
| Staphylococcus aureus | 1 | 1 | 1 |
| Gram-negative rods | 1 | 1 | 4 |
| Legionella | 3 | 1 | 4 |
| Mycoplasma or Chlamydia | 18 | 8 | 7 |
| Virus | 18 | 29 | 5 |
| No pathogen | 25 | 11 | 44 |
aStandard microbiology, urine pneumococcal antigen (UAg), and serologic tests for pneumococcus and “atypical” organisms.
bStandard microbiology, UAg, polymerase chain reaction on nasopharyngeal swabs and sputum.
cStandard microbiology, UAg, serology for “atypical” organisms.
Our present study shows a marked decline in the frequency with which pneumococcus causes pneumonia in the United States/Canada but no such decline in Europe (Figure 1B). An important limitation of our findings is between-study heterogeneity in patient population, severity of illness, microbiologic tests performed, and the thoroughness of diagnostic testing. We attempted to minimize the latter 2 factors by including only studies that performed pneumococcal testing in a significant proportion of cases. For studies that provided sufficiently granular data, we calculated the frequency of pneumococcal pneumonia by limiting the denominator to only those patients who underwent such testing. Also, we chose a case definition of pneumococcal pneumonia that emphasizes validated microbiologic tests that have been in clinical practice for decades. While an in-depth qualitative analysis is beyond the scope of this article, we provide preliminary evidence to suggest differences between the United States/Canada and Europe with respect to the relative frequency of pneumococcus as a cause of pneumonia.
Factors that contribute to this difference might include the widespread use of pneumococcal polysaccharide vaccine [127] and the decreased rate of cigarette smoking among adults in the United States [128]. The nearly universal administration of pneumococcal conjugate vaccine to US children beginning in 2000 further explains the decline [3]. In Europe and other parts of the world where pneumococcal vaccines have been recommended for routine use for adults and where the incidence of cigarette smoking remains high, pneumococcus remains responsible for a higher proportion of cases of CAP (Figure 1B) [40, 123, 126, 129–131]. Reliance on serologic techniques has probably led to overdiagnosis of Mycoplasma and Chlamydia [132]. As noted above, earlier studies that used culture for diagnosis suggested that these organisms only occasionally infect older adults. These findings have important implications for comparing results of CAP studies or following guidelines for management written on either side of the Atlantic.
STUDIES AFTER 2010
An intensive investigation of the causes of pneumonia included all patients hospitalized for pneumonia at a Veterans Affairs (VA) medical center from July 5, 2011 to June 30, 2012 [33]. Blood culture, urine pneumococcal and Legionella antigen, PCR for 15 respiratory viruses (but not Mycoplasma or Chlamydia), and serum procalcitonin were each performed in more than 95% of cases. Sputum was studied in 70%. Of 259 patients admitted from the emergency department with CAP, 44 (17%) were thought to be uninfected, an observation with important implications for reconciling quality improvement measures that relate to promptness of antibiotic therapy. Of the remaining 215 patients (Table 3), 29% had documented bacterial infection; S. pneumoniae was detected in only 9% of the 215 patients. PCR identified a respiratory virus in 23%. Bacterial and viral coinfection occurred in 6% of cases. Despite an intense search for an etiologic agent, the cause remained unknown in 55%.
Table 3.
Etiology of Community-Acquired Pneumonia, Studies Since 2010
| Pathogen | Percentage of Patients | ||
|---|---|---|---|
| Houston [33] | Centers for Disease Control and Prevention [34] | Netherlands [136] | |
| Bacteria | 29 | 15 | 30 |
| Streptococcus pneumoniae | 9 | 5 | 16 |
| Haemophilus | 6 | <1 | 7 |
| Staphylococcus aureus | 5 | 2 | 3 |
| Pseudomonas | 3 | <1 | 2 |
| Legionella | 1 | 1 | 1 |
| Mycoplasma, Chlamydia | – | <3 | 1 |
| Other | 6 | 3 | 3 |
| Nocardia | 1 | 0 | 0 |
| Mycobacteria | 2 | 1 | <1 |
| Fungi (Pneumocystis) | 3 | 1 | 2 |
| Viruses | 20 | 27 | 3 |
| Rhinovirus | 13 | 9 | – |
| Coronavirus | 3 | 2 | – |
| Human metapneumovirus | 2 | 4 | – |
| Influenza | 1 | 6 | 3 |
| Parainfluenza | 2 | 3 | – |
| Respiratory syncytial virus | 2 | 3 | – |
| No pathogen | 55 | 62 | 66 |
Two subsequent studies (Table 3) yielded similar results. The Centers for Disease Control and Prevention’s EPIC study [34] of more than 2000 patients at 8 medical centers reported an even lower proportion of pneumococcal pneumonia (5% vs 9%) and all bacterial pneumonia (15% vs 29%) than Musher et al [33]. Routine use of PCR for Mycoplasma and Chlamydia confirmed [132] that these organisms are very uncommon causes of CAP leading to hospitalization of adults, suggesting that serologic tests have contributed to marked overdiagnosis. Two more recent studies identified Mycoplasma as a cause of pneumonia in 6% of cases; however, 1 included 50% [133] and the other [134] included 25% of patients with a PORT (Patient Outcomes Research Team) score of ≤2. Such patients are generally not hospitalized and would not have been included in the VA study or the EPIC study.
Using a serotype-specific enzyme-linked immunosorbent assay (ELISA) on urine [135], a Dutch pneumococcal vaccine trial [136] identified pneumococcus in 16% of CAP in the control (nonvaccinated) group, consistent with the higher incidence of pneumococcus in Europe, although lower than some of the other European studies cited above [40, 123, 126]. The finding in these 3 studies [33, 34, 136] of Legionella, Pseudomonas, or S. aureus as a cause of 1%, ≤3%, or ≤5% of pneumonias, respectively, has important implications for the selection of empiric antibiotics used to treat CAP.
Perhaps the most important observation in these 3 studies was the failure to identify a cause for pneumonia in more than 50% of cases. The VA study [33] attempted to use clinical scenarios with support from laboratory and radiologic studies to distinguish bacterial from nonbacterial (largely viral) pneumonia. The investigators defined a likely bacterial pneumonia as 3 or more of the following: hyperacute presentation; septic shock; white blood cell count >15000 or <6000 with increased band forms; dense segmental or lobar consolidation; absence of upper respiratory symptoms; biphasic illness with upper respiratory symptoms followed by a sudden deterioration; or an elevated procalcitonin level. Likely nonbacterial pneumonia had none of these findings plus 2 or more of the following: exposure to sick contact(s); upper respiratory symptoms; patchy infiltrates; and a low serum procalcitonin level. About 58% of patients with no identifiable cause stratified to likely bacterial disease and 15% to likely viral disease; 27% could not be categorized. The authors hypothesized that so-called normal respiratory flora play a prominent etiologic role in patients whose syndrome suggested likely bacterial pneumonia and in whom all other tests failed to reveal a recognized pathogen.
TECHNIQUES TO IDENTIFY S. PNEUMONIAE CURRENTLY UNDER STUDY
A serotype-specific urinary ELISA increases the diagnostic yield for pneumococcus by 15%–20% [135]. Unfortunately, the available test only detects strains in the 13-valent conjugate pneumococcal vaccine, many of which have greatly declined in incidence [137]. Therefore, a new set of antigens needs to be studied and the method validated before it will provide useful information in the future.
Albrich et al [138] used a quantitative PCR on nasopharyngeal swabs to detect pneumococcal lytA in human immunodeficiency virus (HIV)–infected South African patients with pneumonia. Patients who met criteria for pneumococcal infection had 1000-fold greater counts than patients without nonpneumococcal CAP and 100000-fold greater counts than HIV-infected controls without CAP. To our knowledge, this work has not been validated in a study from a developed country. A quantitative, multiplex PCR detects 40 pneumococcal serotypes [139] but has yet to be studied as a diagnostic tool for CAP.
A recent study from the United Kingdom identified an etiologic agent by quantitative PCR in 87% of CAP patients, including S. pneumoniae in 36%, H. influenzae in 40%, Moraxella in 14%, S. aureus in 10%, Klebsiella in 4%, Pseudomonas in 3%, and Mycoplasma or Legionella in <2% each [140]. This technique appears to be either overly sensitive or nonspecific since 2 or more bacterial pathogens were found in 32% of cases and Moraxella was found in 14%. A respiratory virus was detected in 30% of cases but alone (without a bacterial respiratory pathogen) in only 6%.
CONCLUSION
In conclusion, principal trends in determining etiologic agents in pneumonia include the following: a marked but unexplained decline in the prevalence of pneumococcal disease; continued recognition of pneumococcus as the most commonly identified bacterial pathogen, especially in critically ill patients; a greater frequency of pneumococcus in Europe compared to the United States; a greatly increased role for respiratory viruses; detection of Mycoplasma and Legionella in a far smaller proportion of cases than had been reported in 1990–2010; and, perhaps most importantly, failure to establish an etiologic diagnosis in more than 50% patients. The principal challenges for the future appear to be to create a balance between overly sensitive and sufficiently sensitive diagnostic techniques, to identify an etiologic agent in the one-half of cases that now go undiagnosed, and to investigate the role of so-called normal respiratory flora in causing pneumonia. All these factors need to be considered as guidelines for management of community acquired pneumonia are formulated.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Note
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Osler W. The Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine. First ed Edinburgh & London: Young J. Pentland, 1892. [Google Scholar]
- 2. Osler W. The Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine. 8th ed. New York and London: D. Appleton and Company, 1914. [Google Scholar]
- 3. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avery OT, Chickering HT, Cole R, Dochez AR. Acute lobar pneumonia: prevention and serum treatment. Monographs of the Rockefeller Institute for Medical Research. New York: Rockefeller Institute for Medical Research, 1917. [Google Scholar]
- 5. Cecil RL, Larsen NP. Clinical and bacteriologic study of one thousand cases of lobar pneumonia. JAMA 1922; 79:343–9. [Google Scholar]
- 6. Sutliff WD, Finland M. The significance of the newly classified types of pneumococci in disease: types IV to XX inclusive. JAMA 1933; 101:1289–95. [Google Scholar]
- 7. Bullowa JGM. The Management of the Pneumonias: For Physicians and Medical Students. New York: Oxford University Press, 1937. [Google Scholar]
- 8. Cecil RL, Lawrence EA. Pneumonia in private practice: a study of 911 patients. JAMA 1938; 111:1889–94. [Google Scholar]
- 9. Warner GF, Li JG, Farber SM. A study of pneumonia in San Francisco, 1943–1944. Cal West Med 1945; 62:161–2. [PMC free article] [PubMed] [Google Scholar]
- 10. Warner GF, Gardner FH. A further study of pneumonia in San Francisco, 1944–1946. Calif Med 1948; 68:32–4. [PMC free article] [PubMed] [Google Scholar]
- 11. Dowling HF, Sweet LK, Hirsh HL. The Acute Bacterial Diseases. Philadelphia: W.B. Saunders Co, 1948. [Google Scholar]
- 12. Tillotson JR, Finland M. Bacterial colonization and clinical superinfection of the respiratory tract complicating antibiotic treatment of pneumonia. J Infect Dis 1969; 119:597–624. [DOI] [PubMed] [Google Scholar]
- 13. Fiala M. A study of the combined role of viruses, mycoplasmas and bacteria in adult pneumonia. Am J Med Sci 1969; 257:44–51. [DOI] [PubMed] [Google Scholar]
- 14. Fekety FR Jr, Caldwell J, Gump D et al. Bacteria, viruses, and mycoplasmas in acute pneumonia in adults. Am Rev Respir Dis 1971; 104:499–507. [DOI] [PubMed] [Google Scholar]
- 15. Dorff GJ, Rytel MW, Farmer SG, Scanlon G. Etiologies and characteristic features of pneumonias in a municipal hospital. Am J Med Sci 1973; 266:349–58. [DOI] [PubMed] [Google Scholar]
- 16. Moore MA, Merson MH, Charache P, Shepard RH. The characteristics and mortality of outpatient-acquired pneumonia. Johns Hopkins Med J 1977; 140:9–14. [PubMed] [Google Scholar]
- 17. Oseasohn R, Skipper BE, Tempest B. Pneumonia in a Navajo community: a two-year experience. Am Rev Respir Dis 1978; 117:1003–9. [DOI] [PubMed] [Google Scholar]
- 18. Miller J, Sande MA, Gwaltney JM Jr, Hendley JO. Diagnosis of pneumococcal pneumonia by antigen detection in sputum. J Clin Microbiol 1978; 7:459–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebright JR, Rytel MW. Bacterial pneumonia in the elderly. J Am Geriatr Soc 1980; 28:220–3. [DOI] [PubMed] [Google Scholar]
- 20. Boerner DF, Zwadyk P. The value of the sputum gram’s stain in community-acquired pneumonia. JAMA 1982; 247:642–5. [PubMed] [Google Scholar]
- 21. Dans PE, Charache P, Fahey M, Otter SE. Management of pneumonia in the prospective payment era. A need for more clinician and support service interaction. Arch Intern Med 1984; 144:1392–7. [PubMed] [Google Scholar]
- 22. Marrie TJ, Haldane EV, Faulkner RS, Durant H, Kwan C. Community-acquired pneumonia requiring hospitalization. Is it different in the elderly? J Am Geriatr Soc 1985; 33:671–80. [DOI] [PubMed] [Google Scholar]
- 23. Larsen RA, Jacobson JA. Diagnosis of community-acquired pneumonia: experience at a community hospital. Compr Ther 1984; 10:20–5. [PubMed] [Google Scholar]
- 24. Fang GD, Fine M, Orloff J et al. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore) 1990; 69:307–16. [DOI] [PubMed] [Google Scholar]
- 25. Farr BM, Sloman AJ, Fisch MJ. Predicting death in patients hospitalized for community-acquired pneumonia. Ann Intern Med 1991; 115:428–36. [DOI] [PubMed] [Google Scholar]
- 26. Bates JH, Campbell GD, Barron AL et al. Microbial etiology of acute pneumonia in hospitalized patients. Chest 1992; 101:1005–12. [DOI] [PubMed] [Google Scholar]
- 27. Mundy LM, Auwaerter PG, Oldach D et al. Community-acquired pneumonia: impact of immune status. Am J Respir Crit Care Med 1995; 152:1309–15. [DOI] [PubMed] [Google Scholar]
- 28. Marston BJ, Plouffe JF, File TM Jr et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. The Community-based Pneumonia Incidence Study Group. Arch Intern Med 1997; 157:1709–18. [PubMed] [Google Scholar]
- 29. Fine MJ, Stone RA, Singer DE et al. Processes and outcomes of care for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team (PORT) cohort study. Arch Intern Med 1999; 159:970–80. [DOI] [PubMed] [Google Scholar]
- 30. Park DR, Sherbin VL, Goodman MS et al. ; Harborview CAP Study Group The etiology of community-acquired pneumonia at an urban public hospital: influence of human immunodeficiency virus infection and initial severity of illness. J Infect Dis 2001; 184:268–77. [DOI] [PubMed] [Google Scholar]
- 31. Yang S, Lin S, Khalil A et al. Quantitative PCR assay using sputum samples for rapid diagnosis of pneumococcal pneumonia in adult emergency department patients. J Clin Microbiol 2005; 43:3221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Restrepo MI, Mortensen EM, Velez JA, Frei C, Anzueto A. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest 2008; 133:610–7. [DOI] [PubMed] [Google Scholar]
- 33. Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect 2013; 67:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jain S, Self WH, Wunderink RG et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hug B, Rossi M. A year’s review of bacterial pneumonia at the central hospital of Lucerne, Switzerland. Swiss Med Wkly 2001; 131:687–92. [DOI] [PubMed] [Google Scholar]
- 36. Garbino J, Sommer R, Gerber A et al. Prospective epidemiologic survey of patients with community-acquired pneumonia requiring hospitalization in Switzerland. Int J Infect Dis 2002; 6:288–93. [DOI] [PubMed] [Google Scholar]
- 37. Genné D, Siegrist HH, Lienhard R. Enhancing the etiologic diagnosis of community-acquired pneumonia in adults using the urinary antigen assay (Binax NOW). Int J Infect Dis 2006; 10:124–8. [DOI] [PubMed] [Google Scholar]
- 38. Piso RJ, Iven-Koller D, Koller MT, Bassetti S. The routine use of urinary pneumococcal antigen test in hospitalised patients with community acquired pneumonia has limited impact for adjustment of antibiotic treatment. Swiss Med Wkly 2012; 142:w13679. [DOI] [PubMed] [Google Scholar]
- 39. Strålin K, Olcén P, Törnqvist E, Holmberg H. Definite, probable, and possible bacterial aetiologies of community-acquired pneumonia at different CRB-65 scores. Scand J Infect Dis 2010; 42:426–34. [DOI] [PubMed] [Google Scholar]
- 40. Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010; 50:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pareja A, Bernal C, Leyva A, Piedrola G, Maroto MC. Etiologic study of patients with community-acquired pneumonia. Chest 1992; 101:1207–10. [DOI] [PubMed] [Google Scholar]
- 42. Almirall J, Morató I, Riera F et al. Incidence of community-acquired pneumonia and Chlamydia pneumoniae infection: a prospective multicentre study. Eur Respir J 1993; 6:14–8. [PubMed] [Google Scholar]
- 43. Lorente ML, Falguera M, Nogués A, González AR, Merino MT, Caballero MR. Diagnosis of pneumococcal pneumonia by polymerase chain reaction (PCR) in whole blood: a prospective clinical study. Thorax 2000; 55:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Almirall J, Bolíbar I, Vidal J et al. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J 2000; 15:757–63. [DOI] [PubMed] [Google Scholar]
- 45. Gutiérrez F, Masiá M, Rodríguez JC et al. Epidemiology of community-acquired pneumonia in adult patients at the dawn of the 21st century: a prospective study on the Mediterranean coast of Spain. Clin Microbiol Infect 2005; 11:788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andreo F, Domínguez J, Ruiz J et al. Impact of rapid urine antigen tests to determine the etiology of community-acquired pneumonia in adults. Respir Med 2006; 100:884–91. [DOI] [PubMed] [Google Scholar]
- 47. Briones ML, Blanquer J, Ferrando D, Blasco ML, Gimeno C, Marín J. Assessment of analysis of urinary pneumococcal antigen by immunochromatography for etiologic diagnosis of community-acquired pneumonia in adults. Clin Vaccine Immunol 2006; 13:1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fernández Alvarez R, Suárez Toste I, Rubinos Cuadrado G et al. Community-acquired pneumonia: aetiologic changes in a limited geographic area. An 11-year prospective study. Eur J Clin Microbiol Infect Dis 2007; 26:495–9. [DOI] [PubMed] [Google Scholar]
- 49. Falguera M, Carratalà J, Ruiz-Gonzalez A et al. Risk factors and outcome of community-acquired pneumonia due to gram-negative bacilli. Respirology 2009; 14:105–11. [DOI] [PubMed] [Google Scholar]
- 50. Cillóniz C, Ewig S, Polverino E et al. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 2011; 66:340–6. [DOI] [PubMed] [Google Scholar]
- 51. Sordé R, Falcó V, Lowak M et al. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Intern Med 2011; 171:166–72. [DOI] [PubMed] [Google Scholar]
- 52. Capelastegui A, España PP, Bilbao A et al. ; Poblational Study of Pneumonia (PSoP) Group Etiology of community-acquired pneumonia in a population-based study: link between etiology and patients characteristics, process-of-care, clinical evolution and outcomes. BMC Infect Dis 2012; 12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Viasus D, Marinescu C, Villoslada A et al. ; Influenza A (H1N1) Study Group of the Spanish Network for Research in Infectious Diseases (REIPI) Community-acquired pneumonia during the first post-pandemic influenza season: a prospective, multicentre cohort study. J Infect 2013; 67:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tudose C, Moisoiu A, Bogdan M. Mortality risk and etiologic spectrum of community-acquired pneumonia in hospitalized adult patients. Maedica (Buchar) 2010; 5:258–64. [PMC free article] [PubMed] [Google Scholar]
- 55. Røysted W, Simonsen Ø, Jenkins A et al. Aetiology and risk factors of community-acquired pneumonia in hospitalized patients in Norway. Clin Respir J 2016; 10:756–64. [DOI] [PubMed] [Google Scholar]
- 56. Boersma WG, Löwenberg A, Holloway Y, Kuttschrütter H, Snijder JA, Koëter GH. Pneumococcal capsular antigen detection and pneumococcal serology in patients with community acquired pneumonia. Thorax 1991; 46:902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bohte R, van Furth R, van den Broek PJ. Aetiology of community-acquired pneumonia: a prospective study among adults requiring admission to hospital. Thorax 1995; 50:543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Endeman H, Schelfhout V, Voorn GP, van Velzen-Blad H, Grutters JC, Biesma DH. Clinical features predicting failure of pathogen identification in patients with community acquired pneumonia. Scand J Infect Dis 2008; 40:715–20. [DOI] [PubMed] [Google Scholar]
- 59. Huijts SM, Boersma WG, Grobbee DE et al. ; CAP Diagnostics Investigators Predicting pneumococcal community-acquired pneumonia in the emergency department: evaluation of clinical parameters. Clin Microbiol Infect 2014; 20:1316–22. [DOI] [PubMed] [Google Scholar]
- 60. Ruf B, Schürmann D, Horbach I, Fehrenbach FJ, Pohle HD. The incidence of legionella pneumonia: a 1-year prospective study in a large community hospital. Lung 1989; 167:11–22. [DOI] [PubMed] [Google Scholar]
- 61. Kothe H, Bauer T, Marre R, Suttorp N, Welte T, Dalhoff K; Competence Network for Community-Acquired Pneumonia Study Group Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J 2008; 32:139–46. [DOI] [PubMed] [Google Scholar]
- 62. Lévy M, Dromer F, Brion N, Leturdu F, Carbon C. Community-acquired pneumonia. importance of initial noninvasive bacteriologic and radiographic investigations. Chest 1988; 93:43–8. [DOI] [PubMed] [Google Scholar]
- 63. Lehtomäki K, Leinonen M, Takala A, Hovi T, Herva E, Koskela M. Etiological diagnosis of pneumonia in military conscripts by combined use of bacterial culture and serological methods. Eur J Clin Microbiol Infect Dis 1988; 7:348–54. [DOI] [PubMed] [Google Scholar]
- 64. Hohenthal U, Vainionpää R, Meurman O et al. Aetiological diagnosis of community acquired pneumonia: utility of rapid microbiological methods with respect to disease severity. Scand J Infect Dis 2008; 40:131–8. [DOI] [PubMed] [Google Scholar]
- 65. Leesik H, Ani U, Juhani A, Altraja A. Microbial pathogens of adult community-acquired pneumonia in Southern Estonia. Medicina (Kaunas) 2006; 42:384–94. [PubMed] [Google Scholar]
- 66. White RJ, Blainey AD, Harrison KJ, Clarke SK. Causes of pneumonia presenting to a district general hospital. Thorax 1981; 36:566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bewick T, Sheppard C, Greenwood S et al. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax 2012; 67:540–5. [DOI] [PubMed] [Google Scholar]
- 68. Ostergaard L, Andersen PL. Etiology of community-acquired pneumonia. Evaluation by transtracheal aspiration, blood culture, or serology. Chest 1993; 104:1400–7. [DOI] [PubMed] [Google Scholar]
- 69. Mareković I, Plecko V, Boras Z et al. Evaluation of PCR as rapid microbiological method in diagnosis of pneumococcal pneumonia. Scand J Infect Dis 2008; 40:843–5. [DOI] [PubMed] [Google Scholar]
- 70. Blasi F, Cosentini R, Legnani D, Denti F, Allegra L. Incidence of community-acquired pneumonia caused by Chlamydia pneumoniae in Italian patients. Eur J Clin Microbiol Infect Dis 1993; 12:696–9. [DOI] [PubMed] [Google Scholar]
- 71. Logroscino CD, Penza O, Locicero S et al. Community-acquired pneumonia in adults: a multicentric observational AIPO study. Monaldi Arch Chest Dis 1999; 54:11–7. [PubMed] [Google Scholar]
- 72. Cecil RL, Baldwin HS, Larsen NP. Clinical and bacteriological study of 2000 typed cases of lobar pneumonia. Arch Intern Med 1927; 40: 253–80. [Google Scholar]
- 73. Cole RI. Acute pulmonary infections, Delamar Lectures. Baltimore: Williams and Wilkins Co, 1927. [Google Scholar]
- 74. Heffron R. Pneumonia with special reference to pneumococcus lobar pneumonia. New York: The Commonwealth Fund, 1939. [Google Scholar]
- 75. Francis T., Jr Influenza. In: Rivers TM, Horsfall FL Jr. Viral and Rickettsial Diseases of Man. Third ed Philadelphia: J. B. Lippincott:633–72. [Google Scholar]
- 76. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hilleman MR, Werner JH. Recovery of new agent from patients with acute respiratory illness. Proc Soc Exp Biol Med 1954; 85:183–8. [DOI] [PubMed] [Google Scholar]
- 78. Reimann HA. An acute infection of the respiratory tract with atypical pneumonia. A disease entity probably caused by a filterable virus. JAMA 1938; 111: 2377–84. [PubMed] [Google Scholar]
- 79. Reimann HA. Pneumococcal and “virus” pneumonia. Bull N Y Acad Med 1941; 17:187–94. [PMC free article] [PubMed] [Google Scholar]
- 80. Chanock RM, Mufson MA, Bloom HH, James WD, Fox HH, Kingston JR. Eaton agent pneumonia. JAMA 1961; 175:213–20. [DOI] [PubMed] [Google Scholar]
- 81. Eaton MD, Van Herick W. Serological and epidemiological studies on primary atypical pneumonia and related acute upper respiratory disease. Am J Hyg 1947; 45:82–95. [DOI] [PubMed] [Google Scholar]
- 82. Chanock RM, Rifkind D, Kravetz HM, Kinght V, Johnson KM. Respiratory disease in volunteers infected with Eaton agent: a preliminary report. Proc Natl Acad Sci USA 1961; 47:887–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mufson MA, Chang V, Gill V, Wood SC, Romansky MJ, Chanock RM. The role of viruses, mycoplasmas and bacteria in acute pneumonia in civilian adults. Am J Epidemiol 1967; 86:526–44. [DOI] [PubMed] [Google Scholar]
- 84. Foy HM, Kenny GE, McMahan R, Mansy AM, Grayston JT. Mycoplasma pneumoniae pneumonia in an urban area. Five years of surveillance. JAMA 1970; 214:1666–72. [PubMed] [Google Scholar]
- 85. Clinical patterns of undifferentiated and other acute respiratory diseases in Army recruits. Medicine (Baltimore) 1947; 26:441–64. [DOI] [PubMed] [Google Scholar]
- 86. Wood WBJ. Pneumonia. Pneumococcal pneumonia. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. Twelfth ed Philadelphia and London: W. B. Saunders Co, 1967:145–59. [Google Scholar]
- 87. Veillon A, Zuber A. Recherches sur quelques microbes strictement anaerobies et leur role en pathologie. Arch MedExper d’Anat Path 1898; 10:517–45. [Google Scholar]
- 88. Smith DT. Fusospirochetal disease of the lungs, its bacteriology, pathology and experimental reproduction. Amer Rev Tuberc 1927; 16: 584–98. [Google Scholar]
- 89. Cohen J. The bacteriology of abscess of the lung and methods for its study. Arch Surg 1932; 24:171–88. [Google Scholar]
- 90. Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med 1974; 56:202–7. [DOI] [PubMed] [Google Scholar]
- 91. Bartlett JG. Anaerobic bacterial infections of the lung and pleural space. Clin Infect Dis 1993; 16Suppl 4:S248–55. [DOI] [PubMed] [Google Scholar]
- 92. Hahn HH, Beaty HN. Transtracheal aspiration in the evaluation of patients with pneumonia. Ann Intern Med 1970; 72:183–7. [DOI] [PubMed] [Google Scholar]
- 93. Hoeprich PD. Etiologic diagnosis of lower respiratory tract infections. Calif Med 1970; 112:1–8. [PMC free article] [PubMed] [Google Scholar]
- 94. Bartlett JG, Rosenblatt JE, Finegold SM. Percutaneous transtracheal aspiration in the diagnosis of anaerobic pulmonary infection. Ann Intern Med 1973; 79:535–40. [DOI] [PubMed] [Google Scholar]
- 95. Ries K, Levison ME, Kaye D. Transtracheal aspiration in pulmonary infection. Arch Intern Med 1974; 133:453–8. [PubMed] [Google Scholar]
- 96. Thorsteinsson SB, Musher DM, Fagan T. The diagnostic value of sputum culture in acute pneumonia. JAMA 1975; 233:894–5. [PubMed] [Google Scholar]
- 97. Bartlett JG. Anaerobic bacterial infections of the lung. Chest 1987; 91:901–9. [DOI] [PubMed] [Google Scholar]
- 98. Bartlett JG. Diagnostic accuracy of transtracheal aspiration bacteriologic studies. Am Rev Respir Dis 1977; 115:777–82. [DOI] [PubMed] [Google Scholar]
- 99. Fraser DW, Tsai TR, Orenstein W et al. Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med 1977; 297:1189–97. [DOI] [PubMed] [Google Scholar]
- 100. McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, Dowdle WR. Legionnaires’ disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med 1977; 297:1197–203. [DOI] [PubMed] [Google Scholar]
- 101. Macrae AD, Lewis MJ. Legionnaires’ disease in Nottingham. Lancet 1977; 2:1225–6. [DOI] [PubMed] [Google Scholar]
- 102. Gorman GW, Yu VL, Brown A et al. Isolation of Pittsburgh pneumonia agent from nebulizers used in respiratory therapy. Ann Intern Med 1980; 93:572–3. [DOI] [PubMed] [Google Scholar]
- 103. Muder RR, Yu VL, Zuravleff JJ. Pneumonia due to the Pittsburgh pneumonia agent: new clinical perspective with a review of the literature. Medicine (Baltimore) 1983; 62:120–8. [DOI] [PubMed] [Google Scholar]
- 104. Yu VL, Kroboth FJ, Shonnard J, Brown A, McDearman S, Magnussen M. Legionnaires’ disease: new clinical perspective from a prospective pneumonia study. Am J Med 1982; 73:357–61. [PubMed] [Google Scholar]
- 105. Wadowsky RM, Yee RB, Mezmar L, Wing EJ, Dowling JN. Hot water systems as sources of Legionella pneumophila in hospital and nonhospital plumbing fixtures. Appl Environ Microbiol 1982; 43:1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stout J, Yu VL, Vickers RM et al. Ubiquitousness of Legionella pneumophila in the water supply of a hospital with endemic Legionnaires’ disease. N Engl J Med 1982; 306:466–8. [DOI] [PubMed] [Google Scholar]
- 107. Musher DM, Kubitschek KR, Crennan J, Baughn RE. Pneumonia and acute febrile tracheobronchitis due to Haemophilus influenzae. Ann Intern Med 1983; 99:444–50. [DOI] [PubMed] [Google Scholar]
- 108. Grayston JT, Kuo CC, Wang SP, Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med 1986; 315:161–8. [DOI] [PubMed] [Google Scholar]
- 109. Marrie TJ, Grayston JT, Wang SP, Kuo CC. Pneumonia associated with the TWAR strain of Chlamydia. Ann Intern Med 1987; 106:507–11. [DOI] [PubMed] [Google Scholar]
- 110. Dowell SF, Anderson LJ, Gary HE Jr et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis 1996; 174:456–62. [DOI] [PubMed] [Google Scholar]
- 111. Couch RB, Englund JA, Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med 1997; 102:2–9; discussion 25–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stockton J, Stephenson I, Fleming D, Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis 2002; 8:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gutiérrez F, Masiá M, Rodríguez JC et al. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin Infect Dis 2003; 36:286–92. [DOI] [PubMed] [Google Scholar]
- 114. Rosón B, Fernández-Sabé N, Carratalà J et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis 2004; 38:222–6. [DOI] [PubMed] [Google Scholar]
- 115. Boulware DR, Daley CL, Merrifield C, Hopewell PC, Janoff EN. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect 2007; 55:300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ishida T, Hashimoto T, Arita M, Tojo Y, Tachibana H, Jinnai M. A 3-year prospective study of a urinary antigen-detection test for Streptococcus pneumoniae in community-acquired pneumonia: utility and clinical impact on the reported etiology. J Infect Chemother 2004; 10:359–63. [DOI] [PubMed] [Google Scholar]
- 117. Sinclair A, Xie X, Teltscher M, Dendukuri N. Systematic review and meta-analysis of a urine-based pneumococcal antigen test for diagnosis of community-acquired pneumonia caused by Streptococcus pneumoniae. J Clin Microbiol 2013; 51:2303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sheppard CL, Harrison TG, Smith MD, George RC. Development of a sensitive, multiplexed immunoassay using xMAP beads for detection of serotype-specific Streptococcus pneumoniae antigen in urine samples. J Med Microbiol 2011; 60:49–55. [DOI] [PubMed] [Google Scholar]
- 119. Strålin K, Herrmann B, Abdeldaim G, Olcén P, Holmberg H, Mölling P. Comparison of sputum and nasopharyngeal aspirate samples and of the PCR gene targets lytA and Spn9802 for quantitative PCR for rapid detection of pneumococcal pneumonia. J Clin Microbiol 2014; 52:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Musher DM, Mediwala R, Phan HM, Chen G, Baughn RE. Nonspecificity of assaying for IgG antibody to pneumolysin in circulating immune complexes as a means to diagnose pneumococcal pneumonia. Clin Infect Dis 2001; 32:534–8. [DOI] [PubMed] [Google Scholar]
- 121. File TM Jr, Low DE, Eckburg PB et al. Integrated analysis of FOCUS 1 and FOCUS 2: randomized, doubled-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin Infect Dis 2010; 51:1395–405. [DOI] [PubMed] [Google Scholar]
- 122. Sherwin RL, Gray S, Alexander R et al. Distribution of 13-valent pneumococcal conjugate vaccine Streptococcus pneumoniae serotypes in US adults aged ≥50 years with community-acquired pneumonia. J Infect Dis 2013; 208:1813–20. [DOI] [PubMed] [Google Scholar]
- 123. Lim WS, Macfarlane JT, Boswell TC et al. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax 2001; 56:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Macfarlane JT, Colville A, Guion A, Macfarlane RM, Rose DH. Prospective study of aetiology and outcome of adult lower-respiratory-tract infections in the community. Lancet 1993; 341:511–4. [DOI] [PubMed] [Google Scholar]
- 125. Johansson N, Kalin M, Hedlund J. Clinical impact of combined viral and bacterial infection in patients with community-acquired pneumonia. Scand J Infect Dis 2011; 43:609–15. [DOI] [PubMed] [Google Scholar]
- 126. Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med 2010; 181:975–82. [DOI] [PubMed] [Google Scholar]
- 127. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013; 1:CD000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2011. MMWR Morb Mortal Wkly Rep 2012: 889–94. [PubMed] [Google Scholar]
- 129. Rozenbaum MH, Pechlivanoglou P, van der Werf TS, Lo-Ten-Foe JR, Postma MJ, Hak E. The role of Streptococcus pneumoniae in community-acquired pneumonia among adults in Europe: a meta-analysis. Eur J Clin Microbiol Infect Dis 2013; 32:305–16. [DOI] [PubMed] [Google Scholar]
- 130. Huijts SM, Pride MW, Vos JM et al. Diagnostic accuracy of a serotype-specific antigen test in community-acquired pneumonia. Eur Respir J 2013; 42:1283–90. [DOI] [PubMed] [Google Scholar]
- 131. Karhu J, Ala-Kokko TI, Vuorinen T, Ohtonen P, Syrjälä H. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis 2014; 59:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014; 371:1619–28. [DOI] [PubMed] [Google Scholar]
- 133. Barrera CM, Mykietiuk A, Metev H et al. ; SOLITAIRE-ORAL Pneumonia Team Efficacy and safety of oral solithromycin versus oral moxifloxacin for treatment of community-acquired bacterial pneumonia: a global, double-blind, multicentre, randomised, active-controlled, non-inferiority trial (SOLITAIRE-ORAL). Lancet Infect Dis 2016; 16:421–30. [DOI] [PubMed] [Google Scholar]
- 134. File TM Jr, Rewerska B, Vucinic-Mihailovic V et al. SOLITAIRE-IV: a randomized, double-blind, multicenter study comparing the efficacy and safety of intravenous-to-oral solithromycin to intravenous-to-oral moxifloxacin for treatment of community-acquired bacterial pneumonia. Clin Infect Dis 2016; 63:1007–16. [DOI] [PubMed] [Google Scholar]
- 135. Leeming JP, Cartwright K, Morris R, Martin SA, Smith MD; South-West Pneumococcus Study Group Diagnosis of invasive pneumococcal infection by serotype-specific urinary antigen detection. J Clin Microbiol 2005; 43:4972–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bonten MJ, Huijts SM, Bolkenbaas M et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 137. Kobayashi M, Bennett NM, Gierke R et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015; 64:944–7. [DOI] [PubMed] [Google Scholar]
- 138. Albrich WC, Madhi SA, Adrian PV et al. Use of a rapid test of pneumococcal colonization density to diagnose pneumococcal pneumonia. Clin Infect Dis 2012; 54:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Messaoudi M, Milenkov M, Albrich WC et al. The relevance of a novel quantitative assay to detect up to 40 major Streptococcus pneumoniae serotypes directly in clinical nasopharyngeal and blood specimens. PLoS One 2016; 11:e0151428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gadsby NJ, Russell CD, McHugh MP et al. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis 2016; 62:817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.