Abstract
Background
Outpatient antibiotic prescribing for acute upper respiratory infections (URIs) is a high-priority target for antimicrobial stewardship that has not been described for cancer patients.
Methods
We conducted a retrospective cohort study of adult patients at an ambulatory cancer center with URI diagnoses from 1 October 2015 to 30 September 2016. We obtained antimicrobial prescribing, respiratory viral testing, and other clinical data at first encounter for the URI through day 14. We used generalized estimating equations to test associations of baseline factors with antibiotic prescribing.
Results
Of 341 charts reviewed, 251 (74%) patients were eligible for analysis. Nearly one-third (32%) of patients were prescribed antibiotics for URIs. Respiratory viruses were detected among 85 (75%) of 113 patients tested. Antibiotic prescribing (P = .001) and viral testing (P < .001) varied by clinical service. Sputum production or chest congestion was associated with higher risk of antibiotic prescribing (relative risk [RR], 2.3; 95% confidence interval [CI], 1.4–3.8; P < .001). Viral testing on day 0 was associated with lower risk of antibiotic prescribing (RR, 0.4; 95% CI 0.2–0.8; P = .01), though collinearity between viral testing and clinical service limited our ability to separate these effects on prescribing.
Conclusions
Nearly one-third of hematology–oncology outpatients were prescribed antibiotics for URIs, despite viral etiologies identified among 75% of those tested. Antibiotic prescribing was significantly lower among patients who received an initial respiratory viral test. The role of viral testing in antibiotic prescribing for URIs in outpatient oncology settings merits further study.
Keywords: antimicrobial stewardship, upper respiratory infection, respiratory viral testing, oncology, outpatient
Nearly one-third of hematology–oncology outpatients were prescribed antibiotics for upper respiratory infections (URIs), despite viral etiologies identified in 75% of patients tested. Antibiotic prescribing was significantly lower among patients who received a respiratory viral test and was not associated with subsequent upper respiratory infection-related healthcare visits.
Antibiotics are frequently prescribed for upper respiratory tract infections (URIs) despite viral etiologies for the majority of these illnesses [1, 2]. In the United States, the estimated annual rate of outpatient antibiotic prescriptions for acute respiratory conditions is 221 per 1000 people; of these, approximately 50% are considered inappropriate [3]. As such, the Centers for Disease Control and Prevention and other national organizations have highlighted antibiotic use for acute URIs as a key target for antimicrobial stewardship [4–6]. Most studies that describe antibiotic prescribing for acute URIs have focused on general medical or pediatric populations [3, 7–13]. Little is known about antibiotic prescribing practices for URIs among ambulatory cancer patients.
As cancer care increasingly shifts from inpatient to outpatient [14], it is important to understand how antibiotics are being used in the ambulatory setting for common clinical syndromes. Although it is well established that most acute URIs are due to viral causes, diagnostic uncertainty remains an important driver of antibiotic use [15]. Several studies in immunocompetent outpatient populations suggest that molecular respiratory viral diagnostics may help influence appropriate antimicrobial use [16, 17]. Early detection of respiratory viral infections is essential in cancer and hematopoietic cell transplant (HCT) recipients in order to direct antiviral therapy, inform infection control practices, and guide decisions about timing of chemotherapy and transplantation [18–20]. It is unknown whether respiratory viral diagnostic testing affects antibiotic prescribing practices among hematology–oncology outpatients with acute URIs.
In this study, we evaluated outpatients who sought care for acute URIs at an ambulatory cancer center in order to characterize antibiotic prescribing patterns, the use of respiratory viral diagnostic tests, and clinical outcomes associated with URIs in an immunocompromised population.
METHODS
Study Design
We conducted a retrospective cohort study of outpatients aged ≥18 years who presented for care at the Seattle Cancer Care Alliance (SCCA) with an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) code consistent with acute URI or acute bronchitis (Supplementary Methods) from 1 October 2015 to 30 September 2016. Outpatient care at SCCA comprises 1 large ambulatory clinic and 2 off-site satellite clinics. Electronic medical record data including patient demographics, clinical data, antibiotic and antiviral prescriptions, and viral diagnostic testing were obtained. Chart review was conducted to verify the URI diagnosis code and identify the index date of the first clinical encounter for the URI (day 0). Patients without documented active URI symptoms or with evidence of lower respiratory tract infection (LRTI) on day 0 were excluded. For patients with more than 1 URI during the study period, the first episode was selected for analysis. We captured the provider responsible for decision making at day 0 and the patient-reported URI symptom start date, and we classified antibiotic prescriptions in days 0–14 as URI-related or non–URI-related. Steroid use, other immunosuppressive medications, and chemotherapy were recorded. Clinical outcomes in days 0–14 were collected, including healthcare visits, hospitalizations, and clinical and/or radiographic evidence of LRTI. The Fred Hutch Institutional Review Board approved the study.
Definitions and Laboratory Methods
LRTI was defined by clinical signs or symptoms of respiratory tract infection and pulmonary infiltrate on radiographic imaging compatible with a bacterial or viral pneumonia (eg, consolidation, interstitial infiltrate, or ground-glass opacities). Healthcare visits and hospitalizations were classified as URI-related if URI symptoms were addressed in the encounter, regardless of the primary reason for the encounter. Peak flu season was defined as December through March [21]. Nasal swabs were submitted for respiratory viral testing at the clinicians’ discretion. Testing was generally performed using a laboratory-developed multiplex polymerase chain reaction (PCR) test that can detect 12 respiratory viruses [22–24]. Additional tests, including the Biofire FilmArray Respiratory Panel or Influenza-specific tests (Cepheid Xpert Flu), were also captured. Clostridioides difficile testing was performed using PCR (Xpert C. difficile; Cepheid).
Statistical Analyses
For each patient, the first antibiotic and antiviral prescription indicated for the URI and the first viral diagnostic test in days 0–14 were selected for the main analyses. Poisson generalized estimating equation (GEE) regression models were used to test associations between baseline factors and risk of any antibiotic prescribing for the URI in days 0–14. The Poisson model was chosen to provide relative risk (RR) model estimates [25], and GEE was used to account for correlation among patients seen by the same provider. Candidate explanatory variables were chosen a priori based on factors previously identified as important in antibiotic prescribing for URIs in general populations [8, 12] and factors specific to our cancer population. We limited candidate variables to ensure a minimum of approximately 10 events per covariate. These variables included viral testing performed on day 0, clinical service, peak flu season, patient age, symptoms at presentation, comorbidities, steroids or other immunosuppressive medications in the 2 weeks before day 0, and chemotherapy in the 30 days before day 0. Symptoms at presentation were defined using the following mutually exclusive categories: fever, no fever with either sputum production or chest congestion, and any other respiratory viral symptom in the absence of fever, sputum production, and chest congestion. We first ran univariable models, then constructed parsimonious multivariable models using the purposeful variable selection approach (Supplementary Methods) [26]. Because viral testing and clinical service were highly related, we considered these variables in separate models.
Poisson regression with robust standard errors was used to compare the binary outcome of any URI-related healthcare visit in days 0–14 between patients with and without an antibiotic prescription on day 0. Patients were considered independent for this outcome. SAS, version 9.4 (SAS Institute, Cary, NC), was used for all analyses.
RESULTS
Cohort Description
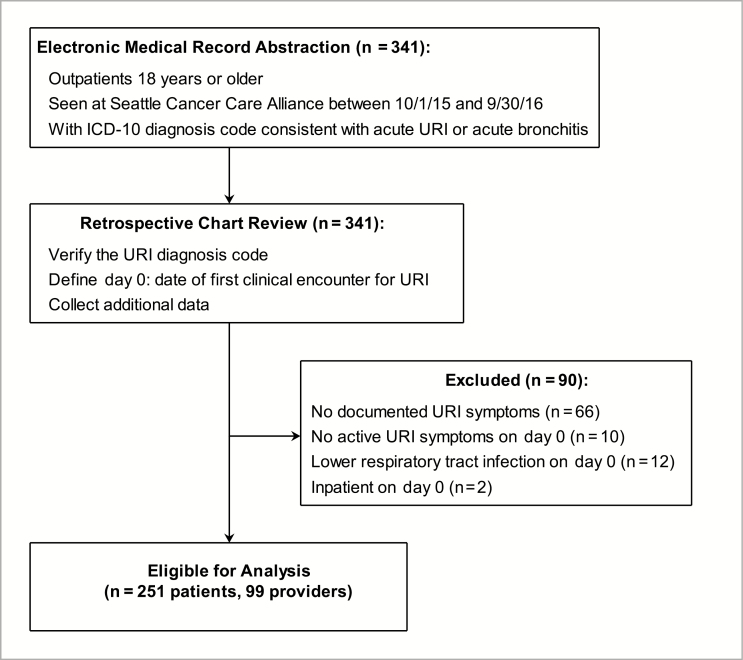
Of 341 patients selected for chart review based on ICD-10 diagnosis codes, 90 (26%) were excluded, mostly due to lack of documented URI symptoms at presentation (Figure 1). There were 251 (74%) patients seen by 99 providers eligible for analysis. Baseline characteristics are shown in Table 1. Most (162 [65%]) patients had a hematological malignancy or disorder; however, of those seen at the satellite clinics, 43 (63%) had a solid tumor malignancy. Cough (154 [61%]) and nasal congestion (113 [45%]) were the most commonly reported presenting symptoms. Approximately one-quarter of patients (65 [26%]) presented for care within 2 days of symptom onset, 80 (32%) within 3–7 days, and 34 (14%) waited longer than 1 week before seeking care; time from symptom onset to day 0 was unknown for 72 (29%) patients.
Figure 1.
Study flowchart outlining the number of patients included in electronic medical record abstraction, chart review, and analysis. Abbreviations: ICD-10, International Classification of Diseases, Tenth Revision, Clinical Modification; URI, upper respiratory infection.
Table 1.
Baseline Characteristics of Outpatients With Upper Respiratory Infections
| Characteristic at First Clinical URI Encounter | Total Cohorta (N = 251 Patients) | Patients With Antibiotic Prescribedb (n = 81 Patients) |
|---|---|---|
| Clinical service | ||
| Bone marrow transplant | 71 (28%) | 8 (11%) |
| Hematology | 69 (27%) | 24 (35%) |
| Solid tumors | 43 (17%) | 16 (37%) |
| Satellite clinics | 68 (27%) | 33 (49%) |
| Viral test on day 0 | ||
| No | 154 (61%) | 64 (42%) |
| Yes | 97 (39%) | 17 (18%) |
| Patient demographics | ||
| Sex | ||
| Women | 123 (49%) | 45 (37%) |
| Men | 128 (51%) | 36 (28%) |
| Age, y | 59 (18–90) | 61 (20–90) |
| Race | ||
| White | 210 (84%) | 68 (32%) |
| Nonwhite | 30 (12%) | 11 (37%) |
| Unknown | 11 (4%) | 2 (18%) |
| Peak flu season | ||
| No (April–November) | 129 (51%) | 38 (29%) |
| Yes (December–March) | 122 (49%) | 43 (35%) |
| Patient clinical characteristics | ||
| Symptoms, all categoriesc | ||
| Fever | 39 (16%) | 15 (38%) |
| Cough | 154 (61%) | 57 (37%) |
| Sputum production | 62 (25%) | 33 (53%) |
| Runny nose | 71 (28%) | 19 (27%) |
| Nasal congestion | 113 (45%) | 40 (35%) |
| Sinus pain | 7 (3%) | 4 (57%) |
| Chest congestion | 14 (6%) | 9 (64%) |
| Sore throat | 81 (32%) | 27 (33%) |
| URI symptoms not otherwise specified | 52 (21%) | 13 (25%) |
| Symptoms, mutually exclusive categoriesd | ||
| Fever | 39 (16%) | 15 (38%) |
| Sputum production or chest congestion, without fever | 51 (20%) | 28 (55%) |
| Any other respiratory symptoms | 161 (64%) | 38 (24%) |
| Days from reported symptom onset to index clinical encounter | ||
| 0–2 | 65 (26%) | 15 (23%) |
| 3–7 | 80 (32%) | 28 (35%) |
| 8–22 | 34 (14%) | 15 (44%) |
| Unknown | 72 (29%) | 23 (32%) |
| Primary diagnosise | ||
| Hematological malignancy or disorder | 162 (65%) | 43 (27%) |
| Solid tumor | 86 (34%) | 38 (44%) |
| Other | 3 (1%) | 0 (0%) |
| Any previous hematopoietic cell transplantation | ||
| No | 169 (67%) | 61 (36%) |
| Yes, >100 days before URI | 60 (24%) | 18 (30%) |
| Yes, ≤100 days before URI | 22 (9%) | 2 (9%) |
| Any comorbiditiesf | ||
| No | 151 (60%) | 49 (32%) |
| Yes | 100 (40%) | 32 (32%) |
| Absolute neutrophil count,g thousands/µL | 3.31 (0.00–31.32) | 3.67 (0.00–15.50) |
| Absolute neutrophil counth <500/µL | ||
| No | 203 (96%) | 65 (32%) |
| Yes | 9 (4%) | 3 (33%) |
| Absolute lymphocyte count,g thousands/µL | 1.02 (0.03–14.00) | 1.10 (0.20–14.00) |
| Immunosuppressive medications in previous 14 daysh | ||
| No | 153 (61%) | 50 (33%) |
| Yes | 98 (39%) | 31 (32%) |
| Chemotherapy within previous 30 days | ||
| No | 113 (45%) | 32 (28%) |
| Yes | 138 (55%) | 49 (36%) |
| Antibiotics for non-URI indication at day 0i | ||
| No | 229 (91%) | 74 (32%) |
| Yes | 22 (9%) | 7 (32%) |
Summaries are n (%) for categorical variables and median (range) for continuous variables.
Abbreviation: URI, upper respiratory infection.
aPercentages are column percentages.
bPatients with antibiotic prescribed for URI in days 0–14. Percentages are row percentages.
cPatients may appear in more than 1 category.
dMutually exclusive categories.
eHematological disorders include myelofibrosis (n = 4), thrombocytopenia (n = 1), aplastic anemia (n = 2), hemolytic anemia (n = 1), amyloidosis (n = 1), acute intermittent porphyria (n = 1), chronic pancytopenia (n = 1), and 1 patient seen for possible Castleman’s disease. Other category includes 2 stem cell donors and 1 patient with an autoimmune disease.
fIncludes any asthma, chronic obstructive pulmonary disease, structural lung disease, chronic kidney disease, cardiac disease, or diabetes.
gMost recent count in 14 days before time zero. Counts only available for 212 patients.
hIncludes antithymocyte globulin, azathioprine, cyclosporine, mycophenolate mofetil, sirolimus, tacrolimus, ruxolitinib, dexamethasone, methylprednisolone, and prednisone.
iExcludes antibiotics used for Pneumocystis jiroveci prophylaxis.
Antibiotic and Antiviral Prescribing
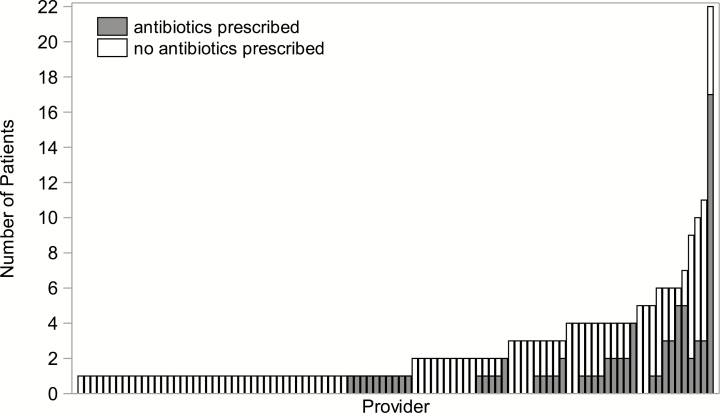
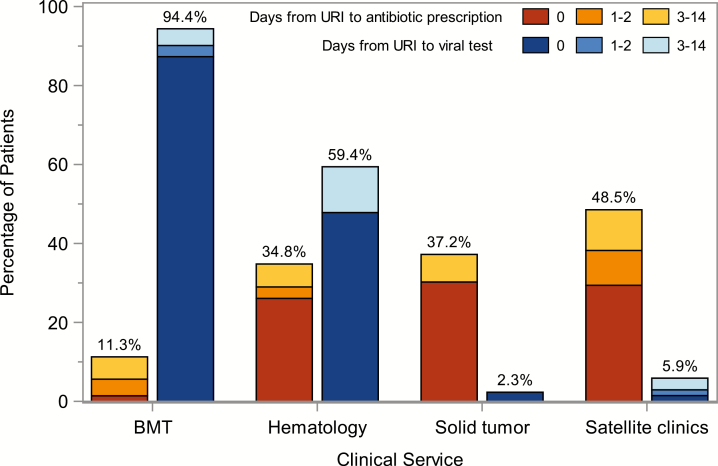
Of the 251 eligible patients, 81 (32%) were prescribed an antibiotic for URI symptoms, with 52 (64%) prescriptions ordered on day 0, 11 (14%) on days 1–2, and 18 (22%) on days 3–14. Of the 81 patients prescribed an antibiotic, only 7 (9%) received their first prescription on or after a visit for worsening URI symptoms or LRTI, and an additional 5 (6%) patients received antibiotics for presumed bacterial sinusitis. Azithromycin (40 [49%]) and fluoroquinolones (19 [23%]) were the most commonly prescribed antibiotics (Supplementary Figure 1). Antibiotic prescribing varied by provider (Figure 2). One provider who saw 22 patients with URIs prescribed antibiotics for 17 (77%) of these patients. When patients seen by this provider were excluded, the antibiotic prescribing rate for the entire cohort was 28%. Antibiotic prescribing also varied by clinical service, with the least use among patients managed by the bone marrow transplant (BMT) service (8 [11%]) and the most use among patients who visited the satellite clinics (33 [49%]) (Figure 3; P = .001).
Figure 2.
Number of patients with and without an antibiotic prescription for upper respiratory infection (URI) by provider. Each bar along the x-axis represents a provider, and the height of each bar shows the total number of patients seen by each provider. The shaded area corresponds to the number of patients for whom the provider prescribed antibiotics, and the unshaded area shows the number of patients for whom the provider did not prescribe antibiotics. Among providers seeing 3 or more URI patients, the top 5 prescribers (each prescribed an antibiotic to ≥67% of the patients they saw), included 2 from the satellite clinics, 2 from the hematology service, and 1 from the solid tumor service.
Figure 3.
Frequency of antibiotic prescriptions and viral testing for the URI in days 0–14 by clinical service. The total height of the bars reflects the percentages of patients in each category who were prescribed an antibiotic (orange bars) and who received a viral test (blue bars); these values are shown on top of the bars. Shading within the bars shows the relative timing of these prescriptions; tests with the darkest portions represent prescriptions or tests ordered on day 0 and lighter portions represent prescriptions or tests ordered later in the study period. Abbreviations: BMT, bone marrow transplant service; URI, upper respiratory infection.
Twenty-six patients (10%) were prescribed an antiviral, with oseltamivir given in most cases; ribavirin was prescribed to 3 patients. In contrast to antibiotics, antivirals were less likely to be prescribed on day 0 (6 [23%]) but more often prescribed on days 1–2 (16 [62%]), with the remaining 4 (15%) patients being prescribed on days 3–14. Of the 26 patients prescribed an antiviral, 6 were also prescribed an antibiotic: 4 were prescribed both drugs on the same day, and 2 were prescribed antivirals before antibiotics.
Viral Diagnostic Testing
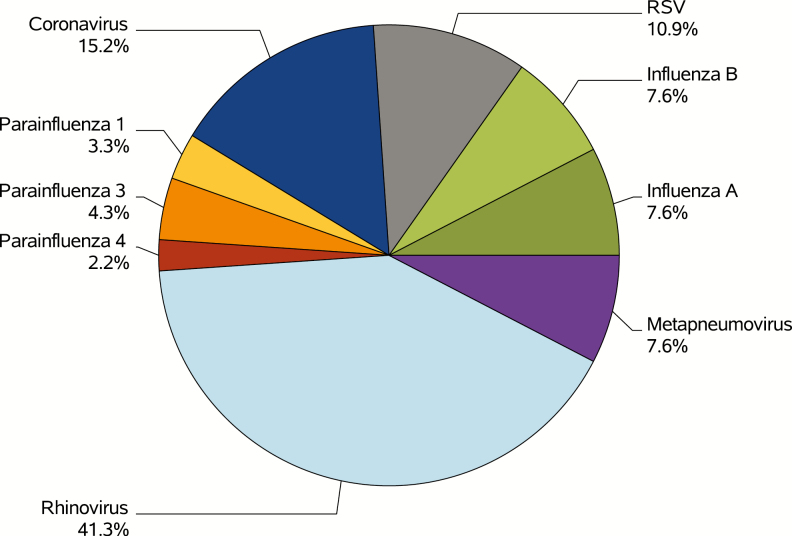
Viral testing was performed among 113 (45%) patients; 110 were tested using the local multiplex PCR test, 2 using the flu-specific test, and 1 using the Biofire FilmArray test. Testing was most frequently done by the BMT teams followed by the hematology service but was rare among solid tumor services and satellite clinics (Figure 3; P < .001). Respiratory viral testing was usually performed on day 0 (97 [86%]). Test results were available on the same day the test was performed for 20% of patients, 1 day later for 79%, and 2 days later in 1 case. Among those tested, 85 (75%) had at least 1 virus detected. Rhinovirus (41%), influenza (15%), and coronavirus (15%) were the most commonly detected viruses (Figure 4).
Figure 4.
Distribution of respiratory virus detected among positive tests using the first viral test per patient. All positive tests shown here used the local polymerase chain reaction test. Patients with more than 1 virus detected appear in more than 1 category. Abbreviation: RSV, respiratory syncytial virus.
Patients with a viral test performed on day 0 were less likely to receive an antibiotic prescription in days 0–14 (17 [18%]) compared to those without a viral test on day 0 (64 [42%], P = .01). However, among the subset of 89 patients with a test on day 0 and without antibiotics prescribed before the test results were available, the percentage of patients who received an antibiotic prescription on or after the test result date did not vary by test result (P = .90), as shown in Table 2. Antivirals were always prescribed following a positive test for influenza and rarely prescribed following a nonflu positive or negative test (P < .001).
Table 2.
Antibiotic and Antiviral Prescribing Among Patients With Viral Testing Performed on Day 0
| Prescription Summary | Viral Test Positive for Flu | Viral Test Positive for Virus Other than Flu | Viral Test Negative | P Valuea |
|---|---|---|---|---|
| Antibiotics | ||||
| Number of patientsb | 12 | 57 | 20 | … |
| Antibiotic prescribed | 1 (8%) | 7 (12%) | 2 (10%) | .90 |
| Days from viral test result to first prescriptionc | ||||
| 0–1 | 0 (0%) | 3 (43%) | 1 (50%) | … |
| 2–10 | 1 (100%) | 4 (57%) | 1 (50%) | … |
| Antivirals | ||||
| Number of patientsd | 12 | 60 | 21 | … |
| Antiviral prescribede | 12 (100%) | 4 (7%) | 2 (10%) | <.001 |
| Days from viral test result to first prescriptionc | ||||
| 0–1 | 11 (92%) | 3 (75%) | 1 (50%) | … |
| 2–5 | 1 (8%) | 1 (25%) | 1 (50%) | … |
aUsing generalized estimating equations regression model.
bExcluding 154 patients without a test on day 0 (64 of whom received an antibiotic prescription), 7 patients with a test on day 0 but an antibiotic prescription before the viral test result was available, and 1 patient with a test on day 0 but test result date unknown.
cAmong patients with a prescription on or after viral test result.
dExcluding 154 patients without a test on day 0 (4 of whom received an antiviral prescription), 3 patients with a test on day 0 but an antiviral prescription before the viral test result was available, and 1 patient with a test on day 0 but test result date unknown.
eAll were oseltamivir except for 2 patients who received a ribavirin prescription, 1 with rhinovirus and respiratory syncytial virus and 1 with respiratory syncytial virus only.
Factors Associated With Antibiotic Prescribing
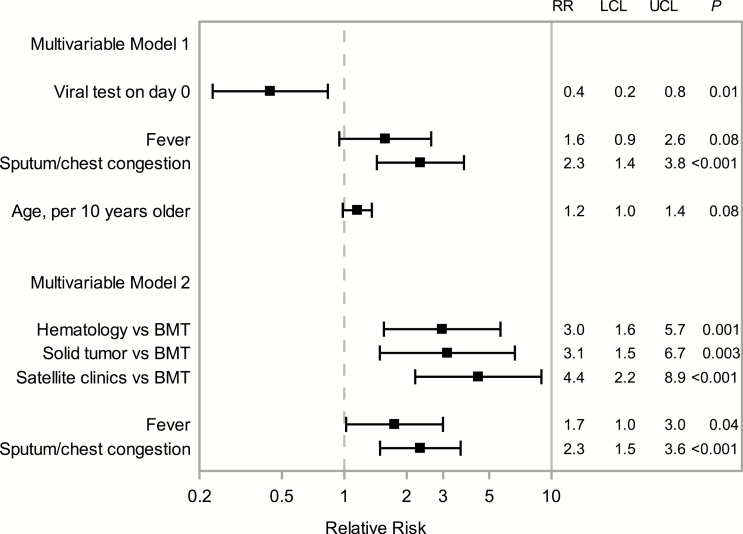
In univariable analysis, viral testing on day 0 was associated with less than half the risk of antibiotic prescribing compared to no testing on day 0 (Supplementary Figure 2). Non-BMT clinical service, older age, and sputum production or congestion were associated with an increased risk of antibiotic prescribing for URI. Due to the collinear relationship between viral testing and clinical services, 2 multivariable models for antibiotic use were generated. In the model that included viral testing rather than clinical service as a potential factor, viral testing and symptoms of sputum production or chest congestion remained significantly associated with antibiotic prescribing, with relative risks similar to unadjusted estimates; age was no longer statistically significant (Figure 5). In a separate model including clinical service rather than viral testing as a candidate variable, clinical service and symptoms at presentation remained significantly associated with antibiotic prescribing; age did not reach the threshold for inclusion in this model. Relative risk estimates were similar to unadjusted estimates, though fever reached statistical significance in this model.
Figure 5.
Multivariable generalized estimating equation regression model estimates for the RR of antibiotic prescribing for the upper respiratory infection in days 0–14. Filled squares represent the RR, and bars connect the LCL and the UCL of the 95% confidence interval for each baseline variable. Multivariable model 1 included viral test on day 0, symptoms at presentation, and age. Multivariable model 2 included clinical service and symptoms at presentation. Abbreviations: BMT, bone marrow transplant service; LCL, lower confidence limit; RR, relative risk; UCL, upper confidence limit.
Clinical Outcomes
Though 103 (41%) patients had a chest X ray or computed tomography scan during the 2-week follow-up period, only 3 (1%) developed evidence of LRTI. One patient who had an autologous HCT 10 days before the URI progressed from upper to lower respiratory tract respiratory syncytial virus infection, while 2 patients had radiographic and clinical concerns for bacterial pneumonia. Two patients tested positive for C. difficile within 30 days of day 0; neither received antibiotics for URI. Within 14 days after the initial URI encounter, 156 (62%) patients had at least 1 clinic visit, emergency department visit, or hospital admission, for a total of 305 healthcare visits; 147 (48%) of those visits were considered URI-related (Supplementary Table 1). Healthcare visits were most common among BMT patients (Supplementary Figure 3). Receipt of an antibiotic prescription on day 0 was not associated with risk of a URI-related follow-up visit in days 0–14 in a model adjusted for age, clinical service, comorbidities, immunosuppressive medications in the previous 2 weeks, and chemotherapy in the previous 30 days (RR, 1.0; 95% confidence interval, 0.6, 1.6; P = .89).
DISCUSSION
To our knowledge, we are the first to describe antibiotic prescribing patterns for acute URIs in an ambulatory oncology setting. We found that nearly 1 in 3 patients received an antibiotic prescription for a URI diagnosis. Antibiotic prescribing varied substantially by clinical service and was significantly less common among patients in whom multiplex respiratory viral testing was performed, though collinearity between clinical service and viral testing limited our ability to separate these effects on prescribing.
Our findings are similar to those reported in a national survey, which estimated that antibiotics are prescribed in 30% of outpatient visits for viral URIs [3]. We included respiratory diagnoses for which antibiotics would generally be considered inappropriate according to national guidelines [6]. Multiplex respiratory viral testing in nearly half of our patients further supports viral etiologies among the majority tested and for which antibiotics are ineffective. Furthermore, we demonstrated in an adjusted analysis that URI-related follow-up visits in the subsequent 2 weeks were not significantly different among patients prescribed antibiotics at the initial encounter compared to those who were not. Serious outcomes related to URIs such as LRTIs and hospitalizations were rare.
We found that antibiotic prescribing was significantly less common for patients who received a respiratory viral test and that viral testing also influenced oseltamivir prescribing. Few studies have evaluated the effect of respiratory viral molecular diagnostic tests on antibiotic prescribing in an outpatient setting. A recent large study of outpatients with acute respiratory infections found that patients with a clinical diagnosis code of influenza had a decreased odds of receiving an antibiotic prescription [12]. However, in that study, only about one-quarter of patients who had research laboratory–confirmed influenza received a clinical diagnosis code for influenza. Antibiotics were prescribed in 29% of patients who had research laboratory–confirmed influenza, suggesting that improved access to rapid molecular diagnostics may be helpful in reducing antibiotic prescribing. An open-label, randomized, controlled trial found that access to multiplex respiratory viral PCR reduced antibiotic prescribing at the initial outpatient visit, although this effect was not sustained at the follow-up visit 8 to 12 days later [17]. A retrospective study of patients from a Veterans Administration medical center found fewer antibiotic prescriptions among patients who tested positive for influenza by on-demand Biofire FilmArray Respiratory Panel, but no difference in antibiotic prescribing between patients with a noninfluenza respiratory virus and patients with no respiratory virus detected [16].
Though we observed significantly lower rates of antibiotic prescribing among patients who received respiratory viral testing, antibiotic prescribing did not vary by test result. The small sample size may have limited our ability to detect a difference by test result. As others have described, it is possible that access to viral testing in and of itself along with the prospect of achieving a diagnosis could have influenced prescribing behavior [17]. Viral testing may also fulfill providers’ desire to meet perceived patient expectations of an intervention for the management of their URI. The turnaround time (TAT) for test results was about 24 hours; it is unknown whether more rapid TAT, which is available with point-of-care tests, could impact prescribing. Finally, some providers may have prescribed antibiotics despite a positive respiratory virus test due to concern for bacterial coinfection among these vulnerable patient populations.
Because the practice of viral testing was highly correlated with clinical service, it is difficult to know whether the lower rates of antibiotic prescribing among patients who received a viral test were attributable to the testing itself or due to service-specific cultural differences in provider prescribing behavior. Interestingly, antibiotic prescribing was lowest among BMT patients who are often considered among the highest risk; however, these patients are seen in the clinic more frequently than other hematology–oncology patients, and providers may have felt more comfortable deferring antibiotics due to closer follow-up. The highest rates of antibiotic prescribing were observed at our satellite clinics, which manage a mix of patients with solid tumor and hematologic malignancies. These clinics as well as several high-volume prescribers that we identified present an opportunity for antimicrobial stewardship program outreach and consideration of strategies previously described, including peer comparison [27]. We did not examine specific provider factors beyond clinical service. However, previous studies have found that physician specialty, age or level of career, gender, patient volume, and country of training were associated with antibiotic prescribing for URIs [8, 9, 11, 28].
Consistent with previous studies [8], patients who presented with symptoms of sputum production or chest congestion were more likely to receive antibiotic prescriptions. We also found a weak association with fever, similar to other studies that demonstrated an association with magnitude generally lower than that of sputum production [8, 11, 12]. Although antibiotic prescribing may be warranted in some cases where these symptoms are present, this result identifies opportunities to clarify such nuances in clinical presentation through targeted education.
This study has several limitations. First, our results may not be generalizable to other hematology–oncology settings where outpatient viral diagnostic testing may not be readily available and the variation in physician culture across clinical services may differ. The high correlation we observed between clinical service and viral diagnostic testing limited our ability to separate these effects on antibiotic prescribing and may also be unique to our institution. Because most viral testing occurred within the BMT or hematology service, it is difficult to draw conclusions about the role of viral testing and antibiotic prescribing among solid tumor patients. Second, the modest sample size limited our ability to evaluate additional factors for associations with antibiotic prescribing. However, the smaller sample size enabled us to address the limitations of billing data by performing a detailed chart review to verify the ICD-10 diagnosis codes. Notably, nearly 20% of patients with ICD-10 codes consistent with URI had no chart documentation of URI symptoms. Larger studies that rely on diagnosis codes from billing data may be unable to account for these discrepancies. Finally, we did not formally review each antibiotic prescription for the URI to assess appropriateness. However, we chose diagnosis codes that correspond to conditions for which antibiotics would generally be considered inappropriate, which is consistent with the approach taken in other studies [3, 7–13]. In addition, we reviewed diagnoses of sinusitis and found that appropriate use [6, 29] in these cases contributed minimally to total antibiotic use in the cohort.
In conclusion, we found that nearly 1 in 3 patients seen at an ambulatory cancer center were prescribed antibiotics for URIs. Viral etiologies were identified in the majority of patients who were tested, and testing itself was associated with decreased antibiotic usage. Antibiotic prescription was not linked to subsequent URI-related healthcare visits. These findings highlight the need for further research to explore the role and cost-effectiveness of molecular respiratory viral testing in limiting unnecessary antibiotic use among hematology–oncology patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Cancer Institute at the National Institutes of Health (grant number P30 CA15704).
Potential conflicts of interest. E. M. K. has received research funding from Global Life Technologies Corp. S. A. P. has received research funding from Global Life Technologies Corp., and has participated in clinical trials with Chimerix, Inc. S. M. has received research funding from Global Life Technologies Corp. All other authors reported no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: This work was presented, in part, at IDWeek, San Francisco, California, from 3–7 October 2018.
References
- 1. van Gageldonk-Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. A case-control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis 2005; 41:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monto AS. Epidemiology of viral respiratory infections. Am J Med 2002; 112(Suppl 6A):4S–12S. [DOI] [PubMed] [Google Scholar]
- 3. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Antibiotic prescribing and use in doctor’s offices—adult treatment recommendations. Available at: https://www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html. Accessed 2 November 2018. [Google Scholar]
- 5. American Board of Internal Medicine Foundation Choosing Wisely. Colds, flu, and other respiratory illnesses in adults. When you need antibiotics and when you don’t. Available at: http://www.choosingwisely.org/patient-resources/colds-flu-and-other-respiratory-illnesses-in-adults/. Accessed 2 November 2018. [Google Scholar]
- 6. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med 2016; 164:425–34. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother 2014; 69:234–40. [DOI] [PubMed] [Google Scholar]
- 8. McKay R, Mah A, Law MR, McGrail K, Patrick DM. Systematic review of factors associated with antibiotic prescribing for respiratory tract infections. Antimicrob Agents Chemother 2016; 60:4106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silverman M, Povitz M, Sontrop JM, et al. Antibiotic prescribing for nonbacterial acute upper respiratory infections in elderly persons. Ann Intern Med 2017; 166:765–74. [DOI] [PubMed] [Google Scholar]
- 10. Schroeck JL, Ruh CA, Sellick JA Jr, Ott MC, Mattappallil A, Mergenhagen KA. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother 2015; 59:3848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163:73–80. [DOI] [PubMed] [Google Scholar]
- 12. Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Netw Open 2018; 1:e180243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chua KP, Fischer MA, Linder JA. Appropriateness of outpatient antibiotic prescribing among privately insured US patients: ICD-10-CM based cross sectional study. BMJ 2019; 364:k5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Journal of Healthcare Contracting. Cancer care migrates to outpatient setting. Available at: http://www.jhconline.com/cancer-care-migrates-to-outpatient-setting-2.html. Accessed 7 February 2019. [Google Scholar]
- 15. Pavia AT. Reducing diagnostic uncertainty to improve treatment of respiratory infections. Lancet Respir Med 2017; 5:364–5. [DOI] [PubMed] [Google Scholar]
- 16. Green DA, Hitoaliaj L, Kotansky B, Campbell SM, Peaper DR. Clinical utility of on-demand multiplex respiratory pathogen testing among adult outpatients. J Clin Microbiol 2016; 54:2950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brittain-Long R, Westin J, Olofsson S, Lindh M, Andersson LM. Access to a polymerase chain reaction assay method targeting 13 respiratory viruses can reduce antibiotics: a randomised, controlled trial. BMC Med 2011; 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waghmare A, Englund JA, Boeckh M. How I treat respiratory viral infections in the setting of intensive chemotherapy or hematopoietic cell transplantation. Blood 2016; 127:2682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013; 56:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2014; 59(Suppl 5):S344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. The flu season. Available at: https://www.cdc.gov/flu/about/season/flu-season.htm. Accessed 5 February 2019. [Google Scholar]
- 22. Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol 2004; 31:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuypers J, Wright N, Corey L, Morrow R. Detection and quantification of human metapneumovirus in pediatric specimens by real-time RT-PCR. J Clin Virol 2005; 33:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol 2006; 44:2382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol 2011; 174:984–92. [DOI] [PubMed] [Google Scholar]
- 26. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eggermont D, Smit MAM, Kwestroo GA, Verheij RA, Hek K, Kunst AE. The influence of gender concordance between general practitioner and patient on antibiotic prescribing for sore throat symptoms: a retrospective study. BMC Fam Pract 2018; 19:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chow AW, Benninger MS, Brook I, et al. ; Infectious Diseases Society of America IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012; 54:e72–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.