Abstract
Background: Exaggerated activation of cytokines/chemokines has been proposed as a factor in adverse outcome of severe acute respiratory syndrome (SARS). Previous studies on chemokines have included only small numbers of patients, and the utility of plasma chemokines as prognostic indicators is unclear.
Methods: We studied 255 archival plasma samples collected during the first or second week after disease onset. The chemokines interferon-inducible protein-10 (IP-10), monokine induced by interferon-γ (MIG), interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP-1), and regulated upon activation normal T cell expressed and secreted (RANTES) were measured by cytometric bead array with a 4-color FACSCalibur flow cytometer. Reverse transcription and real-time quantitative PCR and immunohistochemical staining were performed to analyze the production of IP-10 in lung tissue at autopsy. Conditional logistic regression was used to identify independent predictors for adverse disease outcome.
Results: Increases in IP-10, MIG, and IL-8 during the first week after onset of fever were associated with adverse outcome (intensive care unit admission or death) in the univariate analysis. During the second week, only MIG concentration was associated with prognosis. After adjusting for other risk factors, plasma IP-10 concentration at the first week remained as an independent prognostic factor, with an odds ratio for adverse outcome of 1.52 (95% confidence interval, 1.05–2.55) per fold increase in plasma IP-10 concentration above the median. During the second week, chemokines provided little independent prognostic information. IP-10 was increased in lung tissue from patients who died of SARS.
Conclusions: Increased plasma IP-10 during the first week of SARS symptoms is an independent predictor of outcome. Chemokine activation may be an early event in SARS, and an exaggerated host response may produce complications.
A worldwide outbreak of severe acute respiratory syndrome caused by a new coronavirus (SARS-CoV) 1 occurred between November 2002 and July 2003 (1). A total of 8098 probable cases were reported, with a death toll of 774 (2). The clinical course of SARS is characterized by fever, myalgia, and other systemic symptoms that generally improve after a few days, and the majority of patients in that outbreak improved with treatment. Some patients, however, suffered recurrent fever, oxygen desaturation, and progressive pneumonia (3)(4). In addition, 20%–36% of patients required intensive care unit (ICU) admission with acute respiratory distress syndrome (ARDS) necessitating invasive ventilatory support (1)(3)(5), which is associated with a high mortality rate. Lung pathology findings in fatal SARS cases were dominated by diffuse alveolar damage, epithelial cell proliferation, macrophage infiltration in the lung (6)(7)(8)(9), and occasionally, features of bronchiolitis obliterans organizing pneumonia (10).
Several chemokines, such as T-helper (Th)-1 chemokine interferon γ-inducible protein-10 (IP-10) and the neutrophil chemokine interleukin-8 (IL-8), have been implicated in the pathogenesis of ARDS after SAR-CoV infection in mouse models (11). Histopathologic findings suggested that dysregulation of immune response and overactivation of cytokines play key roles in the pathogenesis of lung lesions (6)(12)(13). In keeping with this hypothesis, relatively mild disease is found in immunocompromised hosts, such as HIV and pediatric patients (14)(15).
Specifically, we and others observed increased chemokine concentrations in blood samples collected from SARS patients during the acute febrile illness (12)(16)(17). The first observation of chemokine activation during the first 2 weeks after fever onset was reported in 20 SARS patients by our group (12). Huang et al.(17) studied 88 SARS patients with the same chemokine panel bead array assay and confirmed activation of chemokines, including IP-10, monokine induced by interferon-γ (MIG), and monocyte chemoattractant protein-1 (MCP-1). By univariate analysis, concentrations of these chemokines were associated with risk of fatal outcome. A similar observation was reported in another study of 23 SARS patients (16). However, the sample sizes in these studies were small, and the time of sample collection was not clearly documented.
Only a few independent predictors have been established for disease outcome in SARS. Old age, higher neutrophil count, low CD4 and CD8 counts, and increased serum lactate dehydrogenase (LD) concentrations are the only established markers that have been associated with subsequent ICU admission (1)(5)(18). However, these are likely surrogate markers by themselves; the underlying biological determinants of body immune reactions and disease outcome remain undefined. Early markers, particularly those available within the first week of onset of fever, are important for patient stratification and treatment decisions. In addition, clinically apparent deterioration usually occurs 2 weeks after fever onset. We therefore investigated whether clinical variables and chemokine concentrations obtained early in the course of illness would predict subsequent deterioration leading to adverse outcomes.
Materials and Methods
patients and blood samples
We performed retrospective analysis of blood samples stored during the SARS outbreak from March to June 2003 in the Departments of Microbiology and Chemical Pathology, Prince of Wales Hospital, Hong Kong. All patients had an initial diagnosis of probable SARS according to WHO criteria. Exposure to the virus was subsequently confirmed by laboratory evidence of serologic conversion to SARS-CoV, a positive viral culture, and/or positive SARS-CoV detection by real-time PCR. Because we were looking for early prognostic factors, only archival samples collected during the first 2 weeks after onset of fever were studied. A total of 255 archival plasma samples were available (126 were collected during the first week and the remaining 129 were second-week samples). Adverse disease outcome was defined as either ICU admission or death from SARS-CoV infection (1)(19). All plasma samples had been preserved at −70 °C before analysis. Details of disease course, results of biochemical and hematologic investigations, and comorbidity (including history of diabetes, chronic lung diseases, hypertension, cerebrovascular accident, cancer, ischemic heart disease, chronic renal failure, and chronic liver disease) were retrieved from patients’ clinical notes. All patients had been treated according to a standard protocol as detailed elsewhere (4)(20). This study was approved by our institutional and hospital research ethics committee.
measurement of plasma chemokines
We measured a panel of inflammatory cytokines and chemokines by a cytometric bead array using 4-color FACSCalibur flow cytometer (Becton Dickinson). All sample processing and assays were performed inside a biosafety level 2 laboratory. The cytometric bead array allowed measurements of up to 6 analytes in 1 assay. In each assay, 6 bead populations with distinct fluorescence properties were coated with different capture antibodies for specific cytokines or chemokines. After incubation with patient samples, phycoerythrin-conjugated detection antibodies were used to form sandwich complexes, and the fluorescence data were acquired with the flow cytometer. The concentrations of the chemokines IP-10 (CXCL10), MIG (CXCL9), IL-8, MCP-1 (CCL2), and regulated upon activation normal T cell expressed and secreted (RANTES; CCL5) were measured. The analytical performance of the assays and reference intervals have been described previously (12).
ip-10 in postmortem lung tissue
We studied 13 postmortem lung tissue specimens collected at the time of the SARS outbreak, 7 from patients who died of SARS infection and, for comparison, 6 from patients whose deaths were unrelated to SARS infection or other pulmonary pathology. Reverse transcription and real-time quantitative PCR were performed to assay the expression of IP-10. RNA samples from each of the 13 lung tissues were extracted with the RNeasy Mini Kit (QIAGEN). RNA samples were then reverse-transcribed by SuperScript™ III reverse transcriptase (Invitrogen). The relative expression of the IP-10 gene in lung samples was determined by quantitative PCR with the SYBR® system (Applied Biosystems) and presented as relative expression against the expression of a housekeeping gene, β-actin. The sequences of the IP-10 and β-actin primers were as follows. IP-10 forward, 5′-TCG AAG GCC ATC AAG AAT TT-3′; IP-10 reverse, 5′-GCT CCC CTC TGG TTT TAA GG-3′; β-actin forward, 5′-TAA GGA GAA GCT GTG CTA CGT C-3′; β-actin reverse, 5′-GGA GTT GAA GGT AGT TTC GTG G-3′.
Immunohistochemical staining for IP-10 (R&D Systems) was also performed. The paraffin sections were deparaffinized and subjected to antigen retrieval by microwave heating in EDTA buffer; 30 mL/L H2O2 was then applied to block endogenous peroxidase, followed by rinsing and incubation with CAS-block (Zymed) for nonspecific blocking. After overnight incubation with IP-10 antibodies (1:20 dilution), the sections were washed, and SuperPicture™ horseradish peroxidase polymer conjugate was added. The signal was detected with a liquid DAB Substrate Kit (Zymed).
statistical analysis
Because plasma concentrations of the analytes did not follow a gaussian distribution, we used the nonparametric Mann–Whitney U-test to compare the concentrations of analytes in the 2 groups of patients (those with favorable outcome vs those with adverse outcome). Association between categorical variables was performed with the Fisher exact test. Conditional logistic regression was used to identify independent predictors for adverse disease outcome and determine the adjusted odds ratios of these risk factors. Before performing logistic regression analysis, we normalized the chemokine plasma concentrations by conversion to multiples of the median (MoM). The use of MoM enables future application in other laboratories using different assays for these analytes. To reflect a clinical scenario, we developed 2 logistic regression models for variables that were available at the first week and the second week after fever onset. Specifically, only variables that would be available at the specific time point were entered into the multivariate logistic model to identify independent prognostic features. Statistical and ROC analyses were performed with SPSS (SPSS Inc).
Results
patients and blood samples
The archived blood samples had been collected from 183 patients with a median (range) age of 35.5 (15–100) years. Sixty-three percent were females. Forty-one patients (22%; 20 males and 21 females) had an adverse outcome as defined above. Patients with adverse outcome were older [median (interquartile range, IQR), 49 (40–72) years] than other patients [33 (26–44) years].
changes in circulating chemokine concentrations during the first 2 weeks
The majority of first-week samples (82% of 126 samples) were collected between days 3 and 6 after onset of fever. The median plasma concentrations of IP-10, MCP-1, MIG, IL-8, and RANTES were 4020, 48.8, 540, 11.7, and 29 800 ng/L, respectively (Table 1 ). Increased plasma concentrations occurred most frequently for IP-10 (88% of patients), followed by RANTES (78%) and IL-8 (58%).
Table 1.
Circulating concentrations of chemokines during the first 2 weeks after onset of fever.
| First week (n = 126) | Second week (n = 129) | Range in healthy controls, ng/L | |||||
|---|---|---|---|---|---|---|---|
| Median (IQR) plasma concentration, ng/L | Patients with increased concentration, % | Median (IQR) plasma concentration, ng/L | Patients with increased concentration, % | ||||
| IP-101 | 4020 (2520–6265) | 88 | 2695 (945–4760) | 65 | 202–1480 | ||
| MCP-11 | 48.8 (26.0–94.0) | 45 | 31.6 (14.9–68.0) | 28 | <10 to 57 | ||
| MIG | 540 (375–918) | 55 | 612 (360–1032) | 62 | 48–482 | ||
| IL-8 | 11.7 (6.6–31.7) | 58 | 8.9 (5.4–32.8) | 42 | <0.2 to 10.0 | ||
| RANTES | 29 800 (18 900–33 720) | 78 | 32 500 (16 200–36 600) | 87 | 4400–18 800 | ||
Concentrations during the first 2 weeks showed significant changes (P <0.05, Mann–Whitney test).
Most of the second-week samples (70% of 129 samples) were collected between days 9 and 12 after onset of fever. The plasma concentrations of IP-10 and MCP-1 during the second week were significantly lower than those in the first week (P <0.05, Mann–Whitney U-test), and 65% and 28% of patients, respectively, had an increased concentration of these chemokines. Most patients (87%) had an increased RANTES concentration.
changes in other biochemical and hematologic markers
Most patients had increased plasma LD activity on admission [median (IQR), 215 (178–296) U/L; upper reference limit, 213 U/L; Fig. 1 ]. Both groups of patients (those with favorable and adverse outcomes) showed an increasing trend of LD in the first 2 weeks after onset. However, patients with adverse outcomes had much higher LD concentrations.
Figure 1.
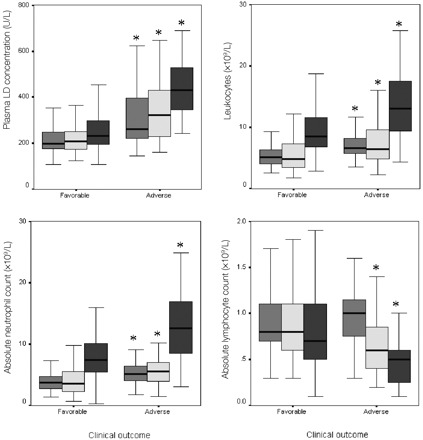
Leukocyte and LD results for patients with favorable (n = 142) or adverse (n = 41) outcomes during the first 2 weeks of SARS.
∗, significantly different between patient groups with different outcomes (P <0.05 by nonparametric Mann–Whitney U-test).  , values on admission; ▦, values at day 5; ▪, values at day 12.
, values on admission; ▦, values at day 5; ▪, values at day 12.
Both the total lymphocyte count and the absolute neutrophil count followed a similar trend of increasing noticeably in the second week. Again, patients with adverse outcomes had significantly higher counts at all 3 time points. Absolute lymphocyte counts showed a downward trend with disease progression. Patients with adverse outcomes had significantly lower counts on days 5 and 12; lymphocyte counts in the 2 groups were not different on admission, however.
prognostic markers in sars patients
Univariate analysis showed significant differences between the 2 groups of patients for most of the routine clinical laboratory values (Fig. 1 ). Among the 9 items of past medical history that were investigated, only history of cerebrovascular accident was associated with poor disease outcome (P <0.05, Fisher exact test). Prevalence of a prior stroke was 12% among patients with more severe disease and only 2% among patients with favorable outcome.
circulating chemokine concentrations as independent prognostic markers
During the first week after onset of fever, concentrations of 3 chemokines (IP-10, IL-8, and MIG) differed between patients in the favorable and adverse outcome groups; thus, these chemokines were potential prognostic markers (Table 2 ). During the first week, an increased IP-10 concentration 1.5 times above the median (≥5250 ng/L) was associated with an odds ratio of 3.7 (95% confidence interval, 1.5–9.2; P <0.005) for subsequent deterioration leading to an adverse outcome. However, in the second week, an increased concentration of only one chemokine, MIG, was associated with poor disease outcome by univariate analysis.
Table 2.
Circulating chemokines associated with disease outcome during the first 2 weeks after infection.
| Median (IQR) | P, Mann–Whitney test | |||
|---|---|---|---|---|
| Favorable outcome | Adverse outcome | |||
| Samples collected the first week after onset (n = 126) | ||||
| n | 93 | 33 | ||
| IP-10, ng/L | 3710 (2435–5862) | 5880 (3395–8610) | <0.01 | |
| MIG, ng/L | 498 (372–792) | 876 (384–1767) | <0.01 | |
| IL-8, ng/L | 4.4 (<0.2 to 8.2) | 13.6 (<0.2 to 32.1) | <0.01 | |
| Samples collected the second week after onset (n = 129) | ||||
| n | 105 | 24 | ||
| MIG, ng/L | 600 (330–1032) | 921 (450–2275) | <0.05 | |
To identify independent predictors for adverse disease outcome, 2 logistic regression models were developed for the first- and second-week biochemical markers. Because the chemokine and LD concentrations did not follow a gaussian distribution, they were normalized as MoM and multiples of the upper reference value, respectively. Day 5 and day 12 routine laboratory results were also entered into the corresponding logistic regression model.
The results of the 2 logistic regression models are shown in Table 3 . Among the variables for which results were available during the first week, 4 risk factors for an adverse outcome were identified. They were patient age, plasma LD concentration on admission, absolute neutrophil count on admission, and plasma IP-10 concentration during the first week. Interestingly, these variables were not useful predictors later in the course of disease when results of laboratory investigations at day 12 were available. At that time, only plasma LD concentration (day 12) and absolute neutrophil count (day 12) were important predictive factors of adverse disease outcome (Table 3 ).
Table 3.
Independent predictive factors of disease outcome.
| Predictive risk factors | Adjusted (95% CI)1 odds ratio |
|---|---|
| During the first week after onset of fever2 | |
| Age (decades) | 1.88 (1.32–2.67) per decade |
| LD on admission (MoU) | 3.69 (1.19–11.41) per fold increase from the MoU |
| Neutrophil count on admission (× 109 cells/L) | 1.33 (1.06–1.68) per 1 × 109 cells/L |
| IP-10 concentration during week 1 (MoM) | 1.52 (1.05–2.55) per fold increase from the group median value |
| During the second week after onset of fever2 | |
| LD on day 12 (MoU) | 8.38 (2.74–25.59) per fold increase from the MoU |
| Neutrophil count at day 12 (× 109 cells/L) | 1.19 (1.05–1.34) per 1 × 109 cells/L |
CI, confidence interval; MoU, multiple(s) of the upper reference limit.
Two logistic regression models were used for the outcome-predicting factors available at the 2 time points after disease onset. Routine laboratory results (plasma LD and blood counts) on days 5 and 12 were used as the predictive factors at the time of the first and second week, respectively.
ROC analysis of the first-week plasma IP-10 concentration confirmed the clinical utility of this marker in prediction of disease outcome. The area under the curve was 0.74, which was significantly >0.5 (P <0.001; Fig. 2 ). At a specificity of 50%, first-week plasma IP-10 concentration provided a sensitivity of 75% for identification of patients with a subsequent adverse clinical outcome, a result that suggests that IP-10 concentration could be used for clinical triage of patients for a more active intervention.
Figure 2.
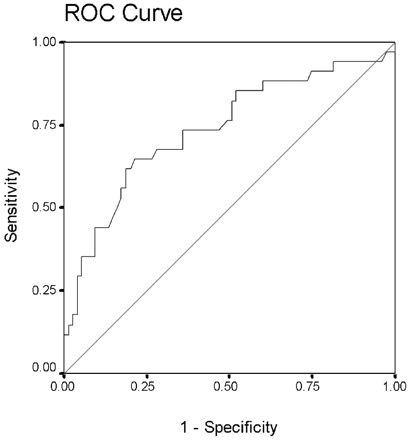
ROC analysis of plasma IP-10 (CXCL-10) concentrations during the first week as a prognostic marker of disease outcome.
Area under curve, 0.74 (95% confidence interval, 0.65–0.85; P <0.001).
study of postmortem lung tissues of patients with SARS-CoV infection
Respiratory failure is an important feature of fatal SARS infection. Postmortem findings for 7 patients have been described previously (10). In brief, the lungs showed diffuse alveolar damage with pulmonary edema and hyaline membrane formation. Focal hemorrhage was seen in some cases.
Expression of the IP-10 gene was significantly increased (P <0.05) in postmortem lung tissue collected from SARS patients compared with 6 lung tissue specimens from non-SARS patients. In autopsy lung tissue from patients with lethal SARS, the relative expression index of IP-10 after normalization by β-actin mRNA was 7.1- to 352-fold, and in the lung tissue from non-SARS patients, it was 0.6- to 1.5-fold. Immunohistochemical staining for IP-10 also confirmed enhanced protein production in both pneumocytes and alveolar macrophages in the lung tissue of SARS patients (Fig. 3 ).
Figure 3.
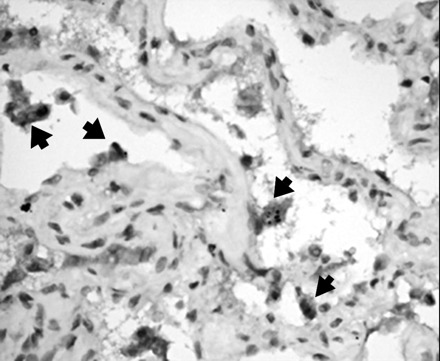
Immunohistochemical staining for IP-10 in postmortem lung tissues of patient with SARS-CoV infection.
The pneumocytes are partially detached from the alveolar wall. Cytoplasmic staining for IP-10 is noted (arrows); the other cells in the alveolar wall were negative.
Discussion
The challenge of SARS infection has generated a worldwide medical response. The virus has been well characterized down to the genomic scale (21), and laboratory diagnosis has been streamlined (22). Rapid diagnosis within 48 h after the onset of fever is achievable with blood reverse transcription-PCR assays (23). The pathogenesis of organ damage and determinants of disease outcome, however, are still not well understood.
Although the majority of SARS patients recovered after treatment (20), more than 20% developed ARDS, requiring invasive mechanical ventilation. Histopathologic examination of lung specimens from SARS victims revealed features of diffuse alveolar damage with marked pulmonary edema and hyaline membrane formation. Intraalveolar organization and interstitial thickening with mild to moderate fibrosis were features at a later stage. Features of bronchiolitis obliterans-organizing pneumonia-like lesions were also noted in some patients (9)(10)(24). These histologic findings signified a dysregulated immune reaction as a key component of the SARS-induced pulmonary lesions.
Cytokines, particularly chemokines, are key mediators for recruitment of leukocytes and other inflammatory cells, leading to pulmonary damage, and have been implicated in the pathogenesis of ARDS/diffuse alveolar damage (25). In a previous study, we showed that plasma concentrations of several chemokines, including IP-10, MCP-1, and IL-8, were significantly increased after SARS infection, whereas other cytokines such as tumor necrosis factor-α and interferon-γ (IFN-γ) were increased in only a small proportion of patients (26). Production of chemokines has been studied in ARDS secondary to other etiologies. IL-8, an α-chemokine, was the most commonly studied chemokine that was found to be increased in both bronchoalveolar lavage fluid and systemic circulation before and after the onset of ARDS (27). Some reports suggested that chemokine concentrations in bronchoalveolar lavage fluid and/or blood predicted the outcome of ARDS (27)(28).
A few studies have investigated the cytokine-chemokine response in SARS infection. Zhang et al. (29) studied serum cytokine concentrations in 60 SARS patients during the symptomatic period, days 3–7. They confirmed that many of the “classic” cytokines, such as tumor necrosis factor-α, IFN-γ, and IL-1α, were not increased during SARS infection. The change in serum cytokine concentrations occurred after the SARS-CoV virus load in the throat peaked, indicating that cytokines were mediators in the induction of lung and systemic inflammation (30). In a study with 23 patients, Jiang et al. (16) found markedly increased IP-10 in SARS patients confirmed by IP-10 expression in lung tissue. The recent study by Huang et al. (17) tried to correlate by univariate analysis clinical outcome with chemokine concentrations in a sample of 88 patients. Although higher concentrations of IP-10, MIG, MCP-1, and IL-18 were associated with adverse clinical course, they were not adjusted for other established clinical variable, including age and plasma LD concentration, which were important prognostic factors.
In this study, we have shown that IP-10 (CXCL10), MIG (CXCL9), and MCP-1 (CCL2) were significantly increased during the early phase of SARS infection. In addition, increased plasma concentrations of both IP-10 and MIG during the first week of fever onset were associated with poor clinical outcome (Table 2 ). Similarly, a high concentration of IL-8 during the first week was also associated with an adverse outcome. Interestingly, univariate analysis showed that increased MIG was the only chemokine associated with poor clinical outcome in the second week. After control for other prognostic factors, IP-10 in the first week was an independent predictive factor for adverse outcome.
Both IP-10 and MIG are lymphocyte-targeting CXC chemokines acting on the common receptor CXCR3, and they are produced at high concentrations by activated bronchial epithelial cells in response to infection (31). Activation of the CXC chemokine–CXCR3 cascade was implicated in the etiology of bronchiolitis obliterans syndrome after lung transplantation (32), lymphocytic alveolitis in AIDS (33), and pulmonary fibrosis (16). SARS-CoV infection in susceptible mice also induced production of IP-10 and MIG together with their receptor, CXCR3, suggesting key participation of this cascade in SAR-CoV infection in mice (11). We have also confirmed high concentrations of both IP-10 mRNA and protein in lung specimens from SARS patients.
Several studies have reported that some routine laboratory findings were predictive of adverse clinical outcome after SARS infection. Our study confirmed that age, plasma LD concentration on admission, and absolute neutrophil count were independent predictors of adverse outcome. These 3 predictors have been consistently identified by different groups studying different patient cohorts (1)(18)(34). In addition, for the first time, we noted that a high IP-10 concentration during the first week of illness was an independent risk factor for adverse outcome. All of these prognostic indicators are readily applicable in the clinical setting and are early markers that can be measured within the first week of illness. On the other hand, second-week chemokine concentrations provided information in addition to that provided by LD activity and neutrophil count obtained at day 12. Persistently increased LD activity after the first week may be the best indicator of poor outcome, which might also be fairly obvious from the clinical status at that moment.
In future applications, particularly in laboratories using other assays, the median IP-10 concentration can be determined from a whole group of patients, such as SARS patients, and those patients with a 1.5-fold increase above the median would have a 3.7-fold increase in the risk of adverse outcomes.
In summary, we identified prognostic factors that can be detected within the first week after onset of SARS symptoms. A high IP-10 concentration was an independent indicator of poor prognosis. Overexpression of the chemokine cascade appears to play an important role in the integrated immune response to SARS-CoV and the subsequent intense immune reactions associated with extensive parenchymal damage and adverse clinical outcome.
Acknowledgments
This project was funded by the Research Grants Council of the Hong Kong SARS, China (Project No. CUHK 4507/03M).
Footnotes
Nonstandard abbreviations: SARS, severe acute respiratory syndrome; CoV, coronavirus; ICU, intensive care unit; ARDS, acute respiratory distress syndrome; IP-10, interferon-γ–inducible protein-10 (CXCL-10); IL, interleukin; MIG, interferon-γ–induced monokine (CXCL9); MCP-1, monocyte chemoattractant protein-1 (CCL2); LD, lactate dehydrogenase; RANTES, regulated upon activation normal T cell expressed and secreted (CCL5); MoM, multiple(s) of the median; IQR, interquartile range; and IFN-γ, interferon-γ.
References
- 1.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003;348:1986-1994. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Summary of probable SARS cases with onset of illness from Nov 1 2002 to 31 July 2003 (http://www.who.int/csr/sars/country/Table2003_09_23/en (accessed May 1, 2005)..
- 3.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui DS, Sung JJ. Treatment of severe acute respiratory syndrome. Chest 2004;126:670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003;289:2801-2809. [DOI] [PubMed] [Google Scholar]
- 6.Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet 2003;361:1773-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 2003;200:282-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franks TJ, Chong PY, Chui P, Galvin JR, Lourens RM, Reid AH, et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol 2003;34:743-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung OY, Chan JW, Ng CK, Koo CK. The spectrum of pathological changes in severe acute respiratory syndrome (SARS). Histopathology 2004;45:119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tse GM, To KF, Chan PK, Lo AW, Ng KC, Wu A, et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J Clin Pathol 2004;57:260-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass WG, Subbarao K, Murphy B, Murphy PM. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol 2004;173:4030-4039. [DOI] [PubMed] [Google Scholar]
- 12.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004;136:95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2005;288:L3-L15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bermejo JF, Munoz-Fernandez MA. Severe acute respiratory syndrome, a pathological immune response to the new coronavirus—implications for understanding of pathogenesis, therapy, design of vaccines, and epidemiology. Viral Immunol 2004;17:535-544. [DOI] [PubMed] [Google Scholar]
- 15.Mandavilli A. Immune response to SARS sets up puzzling paradox. Nat Med 2004;10:1268. [DOI] [PubMed] [Google Scholar]
- 16.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest 2004;114:291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, et al. An interferon-γ-related cytokine storm in SARS patients. J Med Virol 2005;75:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsui PT, Kwok ML, Yuen H, Lai ST. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis 2003;9:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strieter RM, Belperio JA, Keane MP. Host innate defenses in the lung: the role of cytokines. Curr Opin Infect Dis 2003;16:193-198. [DOI] [PubMed] [Google Scholar]
- 20.Sung JJ, Wu A, Joynt GM, Yuen KY, Lee N, Chan PK, et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax 2004;59:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadler K, Masignani V, Eickmann M, Becker S, Abrignani S, Klenk HD, et al. SARS—beginning to understand a new virus. Nat Rev Microbiol 2003;1:209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan PK, To WK, Ng KC, Lam RK, Ng TK, Chan RC, et al. Laboratory diagnosis of SARS. Emerg Infect Dis 2004;10:825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng EK, Hui DS, Chan KC, Hung EC, Chiu RW, Lee N, et al. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem 2003;49:1976-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.To KF, Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J Pathol 2004;203:740-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 2003;14:523-535. [DOI] [PubMed] [Google Scholar]
- 26.Wong CK, Lam CW. Clinical applications of cytokine assays. Adv Clin Chem 2003;37:1-46. [DOI] [PubMed] [Google Scholar]
- 27.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1996;154:602-611. [DOI] [PubMed] [Google Scholar]
- 28.Headley AS, Tolley E, Meduri GU. Infections and the inflammatory response in acute respiratory distress syndrome. Chest 1997;111:1306-1321. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun 2004;72:4410-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang WK, Chen SY, Liu IJ, Kao CL, Chen HL, Chiang BL, et al. Temporal relationship of viral load, ribavirin, interleukin (IL)-6, IL-8, and clinical progression in patients with severe acute respiratory syndrome. Clin Infect Dis 2004;39:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, et al. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol 1999;162:3549-3558. [PubMed] [Google Scholar]
- 32.Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Zisman DA, Xue YY, et al. Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute lung allograft rejection. J Immunol 2003;171:4844-4852. [DOI] [PubMed] [Google Scholar]
- 33.Agostini C, Facco M, Siviero M, Carollo D, Galvan S, Cattelan AM, et al. CXC chemokines IP-10 and mig expression and direct migration of pulmonary CD8+/CXCR3+ T cells in the lungs of patients with HIV infection and T-cell alveolitis. Am J Respir Crit Care Med 2000;162:1466-1473. [DOI] [PubMed] [Google Scholar]
- 34.Choi KW, Chau TN, Tsang O, Tso E, Chiu MC, Tong WL, et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med 2003;139:715-723. [DOI] [PubMed] [Google Scholar]


