Abstract
Background
The incidence of pertussis in the United States has increased in recent years. While characteristics of severe pertussis infection have been described in infants, fewer data are available in older children and adults. In this analysis, we characterize pertussis infections in hospitalized patients of all ages.
Methods
Cases of pertussis with cough onset from 1 January 2011 through 31 December 2015 from 7 US Emerging Infections Program Network states were reviewed. Additional information on hospitalized patients was obtained through abstraction of the inpatient medical record. Descriptive and multivariable analyses were conducted to characterize severe pertussis infection and identify potential risk factors.
Results
Among 15942 cases of pertussis reported, 515 (3.2%) were hospitalized. Three hospitalized patients died. Infants aged <2 months accounted for 1.6% of all pertussis cases but 29.3% of hospitalizations. Infants aged 2–11 months and adults aged ≥65 years also had high rates of hospitalization. Infants aged <2 months whose mothers received acellular pertussis during the third trimester and children aged 2 months to 11 years who were up to date on pertussis-containing vaccines had a 43%–66% reduced risk of hospitalization. Among adolescents aged 12–20 years, 43.5% had a history of asthma, and among adults aged ≥65 years, 26.8% had a history of chronic obstructive pulmonary disease.
Conclusions
Individuals at the extreme ends of life may be the most vulnerable to severe pertussis infections, though hospitalization was reported across all age groups. Continued monitoring of severe pertussis infections will be important to help guide prevention, control, and treatment options.
Keywords: pertussis, hospitalization
Infants aged <2 months are at greatest risk for pertussis-related hospitalization, and severe pertussis infections occur in persons of all ages. Pertussis vaccination remains the most important tool for the prevention of severe infection.
Infection due to Bordetella pertussis affects persons of all ages, with a clinical presentation ranging from a relatively mild-cough illness to a severe illness with pneumonia, seizures, encephalopathy, respiratory failure, and/or death [1]. Despite high coverage with pertussis-containing vaccines among children and adolescents, the incidence of pertussis has increased in the United States since the late 1980s [2–4]. More recently, older children and adolescents have accounted for an increasing proportion of infections, at least in part due to waning immunity following vaccination with acellular pertussis vaccines, which replaced whole-cell vaccines in the United States in the late 1990s [5].
While the risk for severe illness and death is greatest among infants, serious manifestations of pertussis infection are also reported in older children and adults [6]. However, characteristics of severe pertussis infections in these groups remain poorly described. An improved understanding of severe pertussis infections across the life span will help guide prevention, control, and treatment options for those at risk for complications and death from pertussis. In this analysis, we characterize severe pertussis cases, defined as those that require hospitalization, and compare hospitalized and nonhospitalized pertussis patients, identify risk factors for hospitalization, and describe the clinical course and underlying health conditions of hospitalized pertussis patients.
METHODS
Cases of pertussis with cough onset from 1 January 2011 through 31 December 2015 were identified through Enhanced Pertussis Surveillance (EPS) as part of the Emerging Infections Program Network in 7 US states [7]. Cases were reported statewide from Connecticut, Minnesota, and New Mexico and from metropolitan areas in Colorado (5 Denver counties), Georgia (8 Atlanta counties), New York (15 counties in Rochester and Albany), and Oregon (3 Portland counties). Pertussis cases were classified according to the Council of State and Territorial Epidemiologists case definition [8].
Using information obtained from the patient’s diagnosing healthcare provider and through patient interview, public health surveillance personnel completed a standardized case report form that included epidemiologic information, demographics, clinical presentation, and vaccination history on all pertussis cases. Vaccination status for pertussis-containing vaccines was determined by surveillance staff using medical records, state immunization registries, patient vaccination cards, school vaccine records, or, if other sources were unavailable, patient self-report. Starting in 2013, information on the receipt of maternal tetanus, diphtheria, and acellular pertussis (Tdap; tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine) vaccination during pregnancy was collected for infant cases aged <1 year. Patients aged 2 months through 11 years were considered up to date on pertussis-containing vaccines if they received all vaccines recommended for age by the Advisory Committee on Immunization Practices (ACIP). Patients aged ≥12 years were considered up to date if they had received Tdap. For this analysis, only documented vaccine doses administered at least 14 days prior to cough onset and, in the case of maternal Tdap, 14 days prior to infant birth were considered valid.
For hospitalized pertussis patients, defined as those admitted to an inpatient care facility, observation unit, or emergency department for ≥24 hours as a result of pertussis infection, additional information on the patient’s clinical course, treatment, outcomes, and underlying medical conditions was abstracted from the inpatient medical record. For symptoms (eg, cyanosis, apnea) or complications (eg, pneumonia, new-onset seizures), information was collated from the provider or patient interview or documentation in any part of the medical record (eg, admission/discharge diagnoses, progress notes).
State and county-level population denominators by age group (<2 months, 2–11 months, 1–11 years, 12–20 years, 21–64 years, and ≥65 years) were obtained from the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics, and incidence was calculated as the number of confirmed or probable pertussis cases per 100000 population. Significant differences (P < .05) between hospitalized and nonhospitalized pertussis patients were assessed using Pearson χ2 or Fisher exact test statistics. Age-stratified estimates of relative risk for hospitalization and intensive care unit (ICU) admission were calculated by dividing the rate of hospitalization and ICU admission in the age group of interest by that in all other pertussis patients outside the age group.
To assess risk factors for hospitalization among pertussis patients by age group, bivariate log-linear regression with Poisson distribution and robust standard errors for the parameter estimates were used to calculate the relative risk (RR) and 95% confidence intervals (CIs) [9]. Variables associated with hospitalization at the P ≤ .10 level in the bivariate models were retained for inclusion in multivariable models; birth weight of <2500 g was also included in the final model for infants aged <1 year due to the strong a priori suspicion of association with severe pertussis infection. A P value of <.05 was used to determine statistical significance. All analyses were conducted using SAS 9.3.
This evaluation was determined to be public health practice and designated as nonresearch by the CDC Human Research Protection Office and therefore did not require full review by the CDC Institutional Review Board.
RESULTS
Pertussis Epidemiology and Risk of Hospitalization
From 1 January 2011 through 31 December 2015, 15942 cases of pertussis were reported in the surveillance area, with an average annual incidence of 20.3 cases per 100000 population. Pertussis incidence was relatively stable during 2011 and 2013–2015, ranging from 8.5 to 14.8 cases per 100000, and peaked in 2012 with an incidence of 42.0 cases per 100000 population. The average annual incidence by state ranged from 3.5 to 33.0 cases per 100000 population, with a peak in incidence during 2012 reported in all sites. The proportion of pertussis patients who were hospitalized was lowest in 2012 at 2.3% and was relatively stable during 2011 and 2013–2015, ranging from 3.3% to 4.6%.
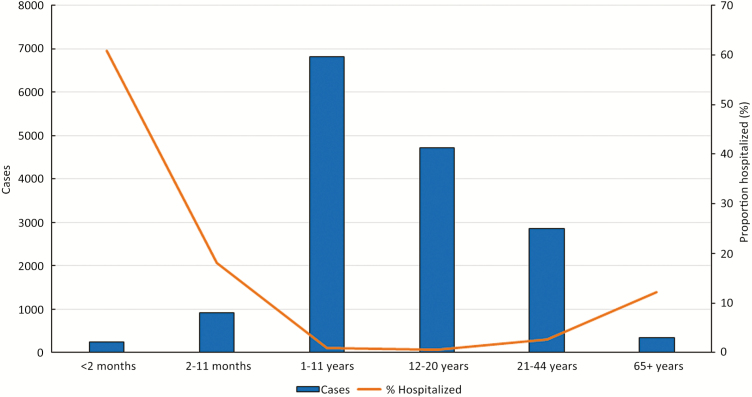
Among these cases, 15427 (96.8%) occurred in patients treated as an outpatient and 515 (3.2%) occurred in patients who were hospitalized; 107 (21.0%) hospitalized patients required admission to the ICU. Although infants aged <2 months accounted for only 1.6% of all pertussis cases, they accounted for 29.3% of all hospitalized cases (Figure 1). Overall, compared to nonhospitalized patients, a significantly higher proportion of hospitalized patients were of a non-white race, of Hispanic ethnicity, received a macrolide antibiotic within 14 days of symptom onset, and had an infection that was laboratory confirmed by culture or polymerase chain reaction (PCR). Fewer hospitalized patients were up to date on pertussis-containing vaccines compared to nonhospitalized patients (Table 1). Three pertussis-related deaths were reported, all in hospitalized patients.
Figure 1.
Number of pertussis cases and the proportion hospitalized by age group at Enhanced Pertussis Surveillance sites, United States, 2011–2015.
Table 1.
Characteristics of Pertussis Patients by Hospitalization Status at Enhanced Pertussis Surveillance Sites, United States, 2011–2015
| Characteristic | All Cases (N = 15942) | Hospitalization Status | ||
|---|---|---|---|---|
| Not Hospitalized (N = 15427) |
Hospitalized (N = 515) |
|||
| [Total Known (%)] N (%) | [Total Known (%)] N (%) | [Total Known (%)] N (%) | P Value | |
| State of residence | [15942 (100.0)] | [15427 (100.0)] | [515 (100.0)] | <.01 |
| Colorado | 3179 (19.9) | 3055 (19.8) | 124 (24.1) | … |
| Connecticut | 492 (3.1) | 439 (2.3) | 53 (10.3) | … |
| Georgia | 278 (1.7) | 246 (1.6) | 32 (6.2) | … |
| Minnesota | 7173 (45.0) | 7047 (45.7) | 126 (24.5) | … |
| New Mexico | 2410 (15.1) | 2306 (15.0) | 104 (20.2) | … |
| New York | 1040 (6.5) | 1010 (6.7) | 30 (5.8) | … |
| Oregon | 1370 (8.6) | 1324 (8.7) | 46 (8.9) | … |
| Age | [15905 (99.8)] | [15390 (99.8)] | [515 (100.0)] | … |
| <2 months | 248 (1.6) | 97 (0.6) | 151 (29.3) | <.01 |
| 2–11 months | 924 (5.8) | 758 (4.9) | 166 (31.9) | … |
| 1–11 years | 6808 (42.8) | 6750 (43.8) | 58 (11.4) | … |
| 12–20 years | 4725 (29.6) | 4702 (30.6) | 23 (4.5) | … |
| 21–64 years | 2864 (18.0) | 2788 (18.1) | 76 (14.8) | … |
| ≥65 years | 336 (2.1) | 295 (1.9) | 41 (8.3) | … |
| Sex | [15902 (99.7)] | [15389 (99.8)] | [513 (99.8)] | … |
| Male | 7312 (46.0) | 7070 (46.0) | 242 (47.2) | .58 |
| Female | 8590 (54.0) | 8319 (54.0) | 271 (52.8) | … |
| Race | [14794 (92.8)] | [14325 (92.9)] | [469 (91.1)] | |
| White | 13056 (88.3) | 12695 (88.6) | 361 (77.0) | <.01 |
| Black | 654 (4.4) | 604 (4.2) | 50 (10.7) | … |
| Othera | 1084 (7.3) | 1026 (7.2) | 58 (12.4) | … |
| Ethnicity | [15026 (94.3)] | [14530 (94.2)] | [496 (96.3)] | … |
| Hispanic | 3108 (20.7) | 2927(20.1) | 181 (36.5) | <.01 |
| Not Hispanic | 11918 (79.3) | 11603 (79.9) | 315 (63.5) | … |
| Birth weight (age <1 year)a | [944 (78.1)] | [668 (74.9)] | [276 (87.1)] | … |
| <2500 g | 88 (9.3) | 60 (9.0) | 28 (10.1) | .58 |
| ≥2500 g | 856 (90.7) | 608 (91.0) | 243 (89.9) | … |
| Maternal age <20 years (age < 1 year)b | [1011(83.60)] | [722 (80.9)] | [289 (91.2)] | … |
| Yes | 114 (11.3) | 73 (10.1) | 41 (14.2) | .06 |
| No | 897 (88.7) | 649 (89.9) | 248 (85.8) | … |
| Maternal tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine during pregnancy (age <2 months)c | [103 (92.8)] | [38 (95.0)] | [65 (91.5)] | … |
| Third trimester | 17 (16.5) | 11 (29.0) | 6 (9.2) | .03 |
| First or second trimester | 6 (5.8) | 2 (5.2) | 4 (6.2) | … |
| No documented pregnancy doses | 80 (77.7) | 25 (65.8) | 55 (84.6) | … |
| Pertussis vaccines up-to-date (age ≥2 months)d | [13202 (84.1)] | [12935 (84.4)] | [267 (73.4)] | … |
| Yes | 9836 (74.5) | 9692 (74.9) | 144 (53.9) | <.01 |
| No | 3366 (25.5) | 3243 (25.1) | 123 (46.1) | … |
| Macrolide antibiotics within 14 days of onset | [15837 (99.4)] | [15327 (99.4)] | [510 (99.0)] | … |
| Yes | 7594 (48.0) | 7284 (47.5) | 310 (60.8) | <.01 |
| No | 8243 (52.0) | 8043 (52.5) | 200 (39.2) | … |
| >1 Physician visit prior to pertussis diagnosis | [14739 (92.5)] | [14224 (92.2)] | [515 (100.0)] | … |
| Yes | 1860 (12.6) | 1670 (11.7) | 190 (36.9) | <.01 |
| No | 12879 (87.4) | 12554 (88.3) | 325 (63.1) | … |
| Laboratory confirmed (culture and/or polymerase chain reaction) | [15942 (100.0)] | [15427 (100.0)] | [515 (100.0)] | … |
| Yes | 11538 (72.4) | 11084 (71.9) | 454 (88.2) | <.01 |
| No | 4404 (27.6) | 4343 (27.2) | 61 (11.8) | … |
| Outcome | [15940 (99.9)] | [15425 (99.9)] | [515 (100.0)] | … |
| Survived | 15937 (99.98) | 15425 (100.0) | 512 (99.4) | <.01 |
| Died | 3 (0.02) | 0 (0.0) | 3 (0.6) | … |
aIncludes Native Americans, Asian/Pacific Islanders, multiple races, and other race (not specified).
bDenominators: all (N = 1209), not hospitalized (N = 892), hospitalized (N = 317).
cDenominators (includes 2013–2015): all (N = 111), not hospitalized (N = 40), hospitalized (n = 71).
dDenominators: all (N = 15694), not hospitalized (N = 15330), hospitalized (N = 364).
Infant patients aged <2 months had 26.2 times the risk of hospitalization (95% CI, 22.7–30.3) and 72 times the risk of ICU admission (95% CI, 50.3–103.0) compared to all other age groups combined. The RR of hospitalization and ICU admission was likewise higher among infants aged 2–11 months (RR, 7.7; 95% CI, 6.5–9.2 and RR, 6.3; 95% CI, 4.2–9.6, respectively) and adult patients aged ≥65 years (RR, 4; 95% CI, 3–5.4 and RR, 1.8; 95% CI, 0.67–4.9, respectively). The RR of hospitalization and ICU admission was low among patients in the 3 age groups between 1 and 64 years, ranging from 0.11 (95% CI, 0.07–0.17) and 0.05 (95% CI, 0.01–0.18), respectively, in 12- to 20-year-olds to 1.2 (95% CI, 0.9–1.7) and 0.8 (95% CI, 0.3–18), respectively, in 45- to 64-year-olds.
Results of the bivariate analyses are shown in Supplementary Tables 1–5. In multivariable analysis, factors associated with hospitalization differed by age group. Of note, infants aged <2 months were less likely to be hospitalized if their mother received a dose of Tdap during the third trimester of pregnancy; similarly, children aged 2 months to 11 years were less likely to be hospitalized if they were up to date with pertussis vaccines. Patients aged 2 months to 20 years of Hispanic ethnicity had an increased risk of hospitalization. Additionally, patients aged ≥2 months with more than 1 physician visit prior to diagnosis were more likely to be hospitalized (Table 2).
Table 2.
Multivariable Analysis of Risk Factors for Hospitalization Among Pertussis Patients by Age Group at Enhanced Pertussis Surveillance Sites, United States, 2011–2015
| Characteristic | Age | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <2 Months | 2–11 Months | 1–11 Years | 12–20 Years | ≥21 Years | |||||||||||
| aRR | 95% CI | P Value | aRR | 95% CI | P Value | aRR | 95% CI | P Value | aRR | 95% CI | P Value | aRR | 95% CI | P Value | |
| Residence in state with high pertussis incidencea,b | 0.83 | 0.62–1.09 | .18 | 0.71 | 0.36–1.37 | .30 | 0.79 | 0.34–1.85 | .59 | 0.29 | 0.11–0.73 | <.01 | – | – | – |
| Male sexc | – | – | – | – | – | – | – | – | – | – | – | – | 1.50 | 1.05–2.14 | .02 |
| Black raced | – | – | – | – | – | – | – | – | – | 6.13 | 1.91–19.67 | <.01 | 1.90 | 0.95–3.82 | .07 |
| Hispanic ethnicitye | – | – | – | 1.94 | 1.11–3.38 | .02 | 1.88 | 1.03–3.42 | .04 | 3.42 | 1.37–8.55 | <.01 | – | – | – |
| Birth weight <2500 gf | 1.92 | 1.23–2.98 | <.01 | 1.98 | 1.01–3.86 | .046 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Maternal age <20 yearsg | – | – | – | 1.20 | 0.54–2.70 | .66 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Maternal Tdap receipth | … | … | … | … | … | … | … | … | … | … | … | … | … | … | … |
| First or second trimester | 0.99 | 0.58–1.68 | .97 | … | … | … | … | … | … | … | … | … | … | … | … |
| Third trimester | 0.51 | 0.28–0.93 | .03 | – | – | – | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Pertussis vaccination up-to-datei | N/A | N/A | N/A | 0.57 | 0.33–0.99 | .046 | 0.34 | 0.20–0.60 | <.01 | – | – | – | – | – | – |
| >1 Physician visit prior to pertussis diagnosisj | 1.08 | 0.79–1.47 | .65 | 2.09 | 1.08–4.03 | .03 | 2.05 | 1.16–3.61 | .01 | 3.94 | 1.57–9.91 | <.01 | 3.52 | 2.38–5.21 | <.01 |
| Macrolide antibiotics within 14 days of onsetk | 1.64 | 1.09–2.45 | .02 | 1.30 | 0.72–2.36 | .38 | – | – | – | – | – | – | – | – | – |
Numbers, proportions, and result bivariate analysis of the relative risk of hospitalization shown in Supplementary Tables 1–5. “–” signifies that the P value on bivariate analysis was ≥.10 and therefore not included in the multivariate analysis. “N/A” signifies that this variable was purposely excluded from the model (ie, maternal Tdap was only assessed for infants aged <2 months, up-to-date status only assessed for persons aged ≥2 months, low birth weight assessed only for infants aged <1 year). Bold indicates that values are significant at P < .05.
Abbreviations: aRR, adjusted relative risk; CI, confidence interval; Tdap, tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine.
aResidence in a state with pertussis incidence above the overall 2011–2015 incidence of 20.3 cases per 100000 population.
Reference level:
bResidence in state with high pertussis incidence.
cFemale sex,
dNon-black race.
eNon-Hispanic ethnicity.
fBirth weight ≥2500 g.
gMaternal age ≥20 years.
hNo maternal Tdap receipt.
iPertussis vaccination status not up-to-date.
jOne physician visit.
kNo receipt of a macrolide antibiotic within 21 days of symptom onset.
Clinical Characteristics of Hospitalized Patients
Overall, the most common hospital admission diagnoses included cough (39.2%), “pertussis” (33.2%), respiratory distress and/or failure (16.1%), bronchiolitis or bronchitis (10.3%), and pneumonia (7.7%), though differences in admission diagnosis were noted by age group (Table 3). Among all hospitalized patients (n = 515), the median length of time from onset to hospitalization was 11 days, ranging from 7 days in infants aged <2 months to 17 days in persons aged ≥65 years. The median length of hospital stay was 3 days, ranging from 1.8 days in children aged 1 to 11 years to 5.9 days among infants aged <2 months. Traditional pertussis clinical symptoms were more commonly reported in hospitalized patients compared to nonhospitalized patients; symptoms included paroxysmal cough (98.1% vs 95.9%, P = .01), post-tussive vomiting (61.2% vs 46.6%, P < .01), whoop (48.4% vs 28.9%, P < .01), cyanosis (44.1% vs 1.7%, P < .01), and apnea (35.7% vs 23.4%, P < .01).
Table 3.
Most Common Admission Diagnoses of Hospitalized Pertussis Patients at Enhanced Pertussis Surveillance Sites, United States, 2011–2015
| Admission Diagnosis | All Ages (n = 515) |
By Age Group | |||||
|---|---|---|---|---|---|---|---|
| <2 Months (n = 151) | 2–11 Months (n = 166) | 1–11 Years (n = 58) | 12–20 Years (n = 23) | 21–64 Years (n = 76) | ≥65 Years (n = 41) | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Cough | 202 (39.2) | 56 (37.1) | 68 (41.0) | 21 (36.2) | 9 (39.1) | 33 (43.4) | 15 (36.6) |
| Pertussis or whooping cough | 171 (33.2) | 63 (41.7) | 76 (45.8) | 18 (31.0) | 2 (8.7) | 6 (7.9) | 6 (14.6) |
| Respiratory distress and/or failure | 83 (16.1) | 28 (18.5) | 17 (10.2) | 9 (15.5) | 3 (13.0) | 18 (23.7) | 8 (19.5) |
| Bronchiolitis or bronchitis | 53 (10.3) | 18 (11.9) | 15 (9.0) | 5 (8.6) | 0 (0.0) | 9 (11.8) | 6 (14.6) |
| Pneumonia | 40 (7.7) | 6 (4.0) | 7 (4.2) | 5 (8.6) | 4 (17.4) | 12 (15.8) | 6 (14.6) |
| Hypoxia | 39 (7.6) | 17 (11.3) | 8 (4.8) | 7 (12.1) | 2 (8.7) | 4 (5.3) | 1 (2.4) |
| Asthma and/or chronic obstructive pulmonary disease exacerbationa | 33 (6.4) | 0 (0.0) | 4 (2.4) | 7 (12.1) | 2 (8.7) | 10 (13.2) | 10 (24.4) |
| Cyanosis | 31 (6.0) | 9 (6.0) | 19 (11.5) | 1 (1.7) | 1 (4.4) | 1 (1.3) | 0 (0.0) |
| Underlying medical conditions | 30 (5.8) | 3 (2.0) | 5 (3.0) | 2 (3.5) | 2 (8.7) | 11 (14.5) | 7 (17.1) |
| Apnea | 29 (5.6) | 18 (11.9) | 9 (5.4) | 2 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Upper respiratory infection | 20 (3.9) | 4 (2.7) | 8 (4.8) | 4 (6.9) | 1 (4.4) | 1 (1.3) | 2 (4.9) |
| Brief resolved unexplained event | 17 (3.3) | 4 (2.7) | 13 (7.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fever | 14 (2.7) | 4 (2.7) | 4 (2.4) | 2 (3.5) | 1 (4.4) | 2 (2.6) | 1 (2.4) |
| Dehydration | 12 (2.3) | 2 (1.3) | 3 (1.8) | 4 (6.9) | 2 (8.7) | 0 (0.0) | 1 (2.4) |
| Syncope | 10 (1.9) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 1 (4.5) | 7 (9.2) | 1 (2.4) |
Numbers do not add up to 100% as multiple admission diagnoses per patient are possible.
aChronic obstructive pulmonary disease is an admission diagnosis only in persons aged ≥21 years.
Among patients who were tested during their hospitalization, 31 of 350 (8.9%) had a maximum white blood cell (WBC) count that surpassed 30 × 109/L (previously associated with an increased risk of pertussis-related infant deaths [10]), and 54 of 271 (19.9%) had a viral codetection (diagnosed through PCR, direct fluorescent antibody testing, culture, or rapid antigen tests). Overall, 127 (24.7%) of hospitalized pertussis patients developed pneumonia, 3.1% developed new-onset seizures, and 1.4% developed encephalopathy (Table 4). In contrast, 254 (1.7%) of all nonhospitalized patients were reported to have pneumonia, with none reporting new-onset seizures and 17 (0.1%) reporting encephalopathy.
Table 4.
Clinical Characteristics and Underlying Conditions in Hospitalized Pertussis Patients by Age Group at Enhanced Pertussis Surveillance Sites, United States, 2011–2015
| Characteristic | All Ages (n = 515) |
By Age Group | |||||
|---|---|---|---|---|---|---|---|
| <2 Months (n = 151) | 2–11 Months (n = 166) | 1–11 Years (n = 58) | 12–20 Years (n = 23) | 21–64 Years (n = 76) | ≥65 Years (n = 41) | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Median delay from onset to hospitalization (days)a | 11 (6–17) | 7 (4–13) | 11 (7–16) | 12 (6–21) | 12 (9–14) | 13 (7–23) | 17 (10–30) |
| Median delay from onset to macrolide antibiotic administration (days)a | 13 (7–21) | 10 (6–16) | 12 (17–19) | 13 (7–20) | 13 (7–20) | 14 (7–22) | 15 (8–26) |
| ICU admission | 107 (21.0) | 57 (38.0) | 30 (18.3) | 5 (8.6) | 2 (8.7) | 9 (12.0) | 4 (10.0) |
| Median length of stay (days)a | |||||||
| Hospitalization | 3 (1.7–6.9) | 5.9 (2.4–10.9) | 2.6 (1.1–5.4) | 1.8 (1–3.9) | 2 (1–3.9) | 3.5 (1.9–6.5) | 3.1 (2–5.9) |
| ICU | 4 (2–9) | 5 (3–11) | 3 (2–7) | 3 (1–3) | 1 (1-1) | 2 (1–3) | 3 (1–13) |
| Symptoms | |||||||
| Paroxysmal cough | 505 (98.1) | 149 (98.7) | 165 (99.4) | 55 (94.8) | 21 (91.3) | 76 (100.0) | 39 (95.1) |
| Whoop | 249 (48.4) | 76 (50.3) | 39 (56.0) | 31 (53.5) | 7 (30.4) | 27 (35.5) | 15 (36.6) |
| Post-tussive emesis | 315 (61.2) | 97 (64.2) | 109 (65.7) | 45 (77.6) | 18 (78.3) | 33 (43.4) | 13 (31.7) |
| Apnea | 184 (35.7) | 93 (61.6) | 65 (39.2) | 11 (19.0) | 1 (4.4) | 9 (11.4) | 5 (12.2) |
| Cyanosis | 227 (44.1) | 102 (67.6) | 101 (60.8) | 16 (27.6) | 5 (21.7) | 3 (4.0) | 0 (0.0) |
| Laboratory findingsb | |||||||
| Maximum white blood cell >30 × 109/L | 31/351 (8.9) | 15/100 (15.0) | 7/86 (8.1) | 5/33 (15.6) | 2/18 (11.1) | 2/74 (2.7) | 0/40 (0.0) |
| Viral codetection | 54/271 (19.9) | 19/92 (20.7) | 21/91 (23.1) | 6/21 (28.6) | 3/14 (21.4) | 3/34 (8.8) | 2/19 (10.5) |
| Respiratory syncytial virus | 22 (8.1) | 11 (12.0) | 10 (11.0) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rhinovirus/enterovirus | 14 (5.2) | 5 (5.4) | 4 (4.4) | 2 (9.5) | 2 (14.3) | 1 (2.9) | 0 (0.0) |
| Adenovirus | 9 (3.3) | 1 (1.1) | 4 (4.4) | 2 (9.5) | 2 (14.3) | 0 (0.0) | 0 (0.0) |
| Human metapneumovirus | 8 (3.0) | 3 (3.3) | 3 (3.3) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 1 (5.3) |
| Otherc | 11 (4.1) | 4 (4.3) | 2 (2.2) | 2 (9.5) | 0 (0.0) | 2 (5.9) | 1 (5.3) |
| Complications | |||||||
| Pneumonia | 127 (24.7) | 34 (22.5) | 28 (16.9) | 13 (22.4) | 8 (34.8) | 29 (38.2) | 15 (36.6) |
| New-onset seizures | 16 (3.1) | 3 (2.0) | 1 (0.6) | 3 (5.2) | 4 (17.4) | 4 (5.3) | 1 (2.4) |
| Encephalopathy | 7 (1.4) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 2 (8.7) | 3 (4.0) | 1 (2.4) |
| Pulmonary hypertensiond | 4 (0.8) | 2 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.6) | 0 (0.0) |
| Underlying medical conditions | |||||||
| At least 1 conditionf | 168 (32.6) | 9 (6.0) | 20 (12.1) | 19 (32.8) | 18 (78.3) | 65 (85.5) | 37 (90.2) |
| Asthma or reactive airway disease | 58 (11.3) | 0 (0.0) | 4 (2.4) | 13 (22.4) | 10 (43.5) | 20 (26.3) | 11 (26.8) |
| Obesity | 58 (11.3) | 0 (0.0) | 0 (0.0) | 1 (1.7) | 1 (4.4) | 40 (52.6) | 16 (39.0) |
| Smoking (current or past) | 37 (7.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 26 (34.2) | 11 (26.8) |
| Immunocompromising condition/medication usee | 35 (6.8) | 0 (0.0) | 2 (1.2) | 2 (3.5) | 8 (34.8) | 19 (25.0) | 4 (9.8) |
| Cardiac condition | 31 (6.0) | 4 (2.7) | 6 (3.6) | 1 (1.7) | 1 (4.4) | 7 (9.2) | 12 (29.3) |
| Diabetes | 28 (5.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 15 (19.7) | 13 (31.7) |
| Other respiratory tract/pulmonary condition | 27 (5.2) | 1 (0.7) | 6 (3.6) | 5 (8.6) | 1 (4.4) | 6 (7.9) | 8 (19.5) |
| Chronic obstructive pulmonary disease | 22 (4.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 (14.5) | 11 (26.8) |
| Neurologic disorder | 18 (3.5) | 1 (0.7) | 2 (1.2) | 2 (3.5) | 5 (21.7) | 4 (5.3) | 4 (9.8) |
| Renal disease | 15 (2.9) | 0 (0.0) | 1 (0.6) | 1 (1.7) | 1 (4.4) | 8 (10.5) | 4 (9.8) |
| Genetic disorder | 10 (1.9) | 1 (0.7) | 1 (0.6) | 4 (6.9) | 3 (13.0) | 1 (1.3) | 0 (0.0) |
| Illicit drug use or perinatal drug exposure | 9 (1.8) | 3 (2.0) | 4 (2.4) | 0 (0.0) | 0 (0.0) | 2 (2.6) | 0 (0.0) |
| Clinical interventions | |||||||
| Mechanical ventilation | 26 (5.1) | 20 (13.3) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 3 (4.0) | 2 (4.9) |
| Extracorporeal membrane oxygenation | 1 (0.2) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Exchange transfusion | 1 (0.2) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Inhaled nitric oxide | 5 (1.0) | 2 (1.3) | 1 (0.6) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 1 (2.4) |
| Inotropic or vasopressor support | 6 (1.2) | 4 (2.7) | 1 (0.6) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Outcome | |||||||
| Died | 3 (0.6) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.3) | 1 (2.4) |
| Discharged with supplemental oxygen | 37 (7.3) | 9 (6.0) | 9 (5.5) | 3 (5.4) | 1 (4.4) | 8 (10.7) | 7 (18.9) |
Abbreviations: ICU, intensive care unit.
aExpressed as median (interquartile range).
Denominators:
bOnly among case-patients in whom testing was performed (N = 350 for white blood cell and N = 271 for viral testing).
cIncludes influenza A and B, coronavirus, parainfluenza, and human herpes virus 6.
dTwenty-seven case-patients underwent echocardiogram or cardiac catheterization.
eNot possible to determine based on chart review whether the case-patient was currently immunosuppressed.
fIncludes other conditions not listed.
At least 1 underlying medical condition was reported in 168 (32.6%) hospitalized patients; this was most commonly observed among adults aged ≥21 years (102/117, 87.2%). Asthma was frequently reported as an underlying condition among patients aged ≥1 year. Among adolescents aged 12 to 20 years, a relatively high proportion had an underlying immune, neurologic, and/or genetic disorder compared to other age groups. A high proportion of adults aged ≥21 years (n = 117) were obese (47.9%), current/former smoker (31.6%), had asthma (26.5%), had diabetes (23.9%), had a potentially immunocompromising condition (19.7%), or had chronic obstructive pulmonary disease (COPD; 18.8%) (Table 4).
Three hospitalized pertussis patients died from their infection: an infant aged 42 days and 2 adults aged 48 years and 76 years. All 3 developed respiratory failure that required mechanical ventilation. The infant was born full term with low birth weight and had a history of neonatal abstinence syndrome and prior history of cardiac arrest. This infant had rhinovirus codetection, a maximum WBC count of 48.5 × 109/L, and required extracorporeal membrane oxygenation (ECMO) for 20 days before death. The patient aged 48 years had a history of human immunodeficiency virus infection and developed pneumonia, sepsis (not further described), and encephalopathy. This patient had a normal WBC of 8.71 × 109/L. The patient aged 76 years had a previous history of asthma and COPD, was reported to have influenza A (though test results not reported through chart review), a maximum WBC count of 19.5 × 109/L, and developed pneumonia (not further described).
DISCUSSION
The reported incidence of pertussis has been increasing in the United States despite high coverage with pertussis-containing vaccines [2–4]. While the changing epidemiology of pertussis and several factors that contribute to the US resurgence have been described, fewer data exist on the current burden and characteristics of severe disease, especially in age groups outside of infancy. Our comprehensive analysis of 5 years of EPS data suggests that individuals at the extreme ends of life may be the most vulnerable to severe pertussis infections; 27% of pertussis patients aged <1 year and 12% of patients aged ≥65 years in our analysis were hospitalized for their pertussis infection. Understanding the spectrum of severe illness across all age groups is necessary for identifying additional populations at increased risk that could benefit from targeted vaccination or post-exposure prophylaxis.
Consistent with previous evaluations, we found that the highest risk of hospitalization and ICU admission occurs in infants aged <2 months, with low birth weight identified as a risk factor for hospitalization [10–14]. Although infants aged <2 months account for less than 2% of all pertussis cases in our analysis overall, they account for approximately 30% of pertussis-related hospitalizations. Among those who are hospitalized, they represent more than 50% of ICU admissions and have the highest rates of advanced medical interventions such as mechanical ventilation, ECMO, or exchange transfusion. Interestingly, we found that infants aged <2 months who had received a macrolide antibiotic within 14 days of symptom onset had an increased risk of hospitalization. While we had hypothesized that delayed initiation of antibiotic treatment may increase the risk of severe disease, this finding, along with the relatively short delay between symptom onset and hospitalization relative to older age groups, may be an indicator of severe symptoms upon presentation at a healthcare facility or a more rapid progression to severe illness in this age group.
While severe pertussis-related morbidity and mortality have been well documented in young infants, pertussis hospitalizations in older children, adolescents, and adults—age groups in which severe pertussis is often not considered—are less well characterized. The association of hospitalization and multiple physician visits prior to a diagnosis of pertussis, along with the increasing delay from symptom onset to hospitalization with age, further suggest missed opportunities for diagnosis and treatment in these age groups, which may have contributed to illness severity. Potentially serious respiratory complications were reported among hospitalized patients across these older age groups, including admission diagnoses that reflected the need for a higher level of care (eg, respiratory distress or failure, hypoxia, apnea), high rates of pneumonia, and viral codetections (eg, respiratory syncytial virus, influenza), that could potentially exacerbate a pertussis infection. However, in some hospitalized patients, the severity of illness was not as clearly perceived from the data abstracted from the chart review. In addition, several of the states with the highest incidence had the lowest hospitalization rates, and vice versa. This suggests that other factors, such as differences in hospitalization practices, thresholds for admission, burden of disease, and differences in surveillance systems’ detection of less severe cases, may all contribute and that use of hospitalization as a proxy for severe illness may overestimate the burden of severe cases across age groups.
Although the role of underlying medical conditions in the development of severe pertussis infections is not well understood, the high proportion of hospitalized adolescent and adult cases with underlying conditions suggests that they may be a contributing factor. Consistent with previous evaluations that have suggested asthma as a risk factor for pertussis [15, 16], we found high rates of asthma in hospitalized patients in our evaluation. Forty-four percent of hospitalized adolescents aged 12 to 20 years and approximately 26% of hospitalized adults aged ≥21 years had a history of asthma compared to approximately 10% of US adolescents and 8% of adults [17]. We also found higher than average rates of COPD, with 19% of hospitalized pertussis patients aged ≥21 years compared to 6.4% among US adults with a history of COPD [18]. Although 1 study demonstrated higher serum levels of anti-B. pertussis immunoglobulin G in persons with COPD [19], the risk of pertussis, including severe pertussis, in persons with COPD is unknown. Not only did a high proportion of patients report a history of asthma or COPD, these conditions appear to have played a role in their clinical severity, as asthma and/or COPD exacerbation were listed as a leading reason for hospital admission in adolescents and adults. In addition, high proportions of hospitalized adolescents aged 12 to 20 years in our evaluation had underlying neurologic, genetic, or potentially immunocompromising conditions, suggesting that of the few adolescents who are hospitalized for their pertussis infection, many have complex medical histories. This high rate of neurologic and genetic underlying conditions may help provide context for the higher rates of certain pertussis-related complications, such as new-onset seizures and encephalopathy, observed in adolescents compared to other age groups. As we did not have a comparison group, we are unable to determine whether certain underlying conditions are risk factors for severe pertussis or, rather, whether persons with these conditions are hospitalized at greater rates due to a perception of increased risk for severe disease.
Pertussis vaccination according to ACIP recommendations is not only effective at preventing disease but has also been shown to reduce the clinical severity of pertussis illness. Consistent with findings from a previous US study [20], our results demonstrate that infants aged <2 months with pertussis infection whose mothers received Tdap during the third trimester of pregnancy had a 49% reduced risk of hospitalization compared to infant pertussis cases with unvaccinated mothers. In addition, a case-control study among pertussis patients age <2 months with matched healthy controls demonstrated that maternal Tdap receipt during the third trimester was 90.5% effective against hospitalization with pertussis [21]. Among older infants and children, the risk of hospitalization in our analysis was reduced by 43% in 2- to 11-month-olds, and 66% in 1- to 10-year-olds who were up to date on pertussis vaccination. Although high amounts of missing vaccination information in adolescents and adults precluded our ability to fully assess this association in older age groups, a recent publication suggested that adolescents and adults vaccinated with Tdap have reduced incidence of post-tussive vomiting, a marker of more clinically significant illness [22]. Thus, although vaccinated persons may still remain at risk for pertussis infection, these data highlight the role that pertussis vaccination plays in the prevention of severe and potentially life-threating infections, suggesting an added benefit of vaccination that goes beyond disease prevention.
In the setting of a pertussis resurgence in the United States and other high-income countries worldwide, emphasis has been placed on the protection of young infants who are at highest risk of severe pertussis-related morbidity and mortality. While new strategies such as maternal immunization offer promise for the prevention of disease and death in the first months of life, our analysis shows that severe pertussis infections that require hospitalization are not limited to infants; certain risk factors, such as key underlying medical conditions, may predispose an individual to more severe infections. Continued monitoring of pertussis hospitalizations through enhanced surveillance systems such as EPS will help better define populations at increased risk of severe disease in order to appropriately target and prioritize current and future pertussis prevention and control strategies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Wendy Baughman (Atlanta Veterans Affairs Medical Center and Georgia Emerging Infections Program), Kari Burzlaff (New York State Department of Health), Paul Cieslak (Oregon Health Authority), Susan Hariri (Centers for Disease Control and Prevention [CDC]), Cynthia Kenyon (Minnesota Department of Health), Juventila Liko (Oregon Health Authority), Roxanne Ryan (Connecticut Department of Public Health), Nong Shang (CDC), Glenda Smith (New York State Department of Health), Kristen Soto (Connecticut Department of Public Health), Salina Torres (New Mexico Department of Health), Amy Tunali (Emory University School of Medicine and Georgia Emerging Infections Program), Yeng Veng (Minnesota Department of Health), Rachel Wester (New York State Department of Health), and Karen Xavier (Colorado Department of Public Health and Environment).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by a CDC cooperative agreement with the Emerging Infections Program Network.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Edwards KM, Decker MD. Pertussis vaccines. In: Plotkin SA, Orenstein WA, Offit PA. Vaccines. 6th ed. Philadelphia, PA: Elsevier Saunders, 2013:467–517. [Google Scholar]
- 2. Centers for Disease Control and Prevention. Pertussis–surveillance and reporting. Available at: https://www.cdc.gov/pertussis/surv-reporting.html. Accessed 25 July 2017. [Google Scholar]
- 3. Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination coverage among children aged 19–35 months—United States, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:1171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheridan SL, Frith K, Snelling TL, Grimwood K, McIntyre PB, Lambert SB. Waning vaccine immunity in teenagers primed with whole cell and acellular pertussis vaccine: recent epidemiology. Expert Rev Vaccines 2014; 13:1081–106. [DOI] [PubMed] [Google Scholar]
- 6. von König CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis 2002; 2:744–50. [DOI] [PubMed] [Google Scholar]
- 7. Skoff TH, Baumbach J, Cieslak PR. Tracking pertussis and evaluating control measures through enhanced pertussis surveillance, emerging infections program, United States. Emerg Infect Dis 2015; 21:1568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Council of State and Territorial Epidemiologists. Revision of the pertussis surveillance case definition to more accurately capture the burden of disease among infants <1 year of age 2013. Available at: https://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/13-ID-15.pdf. Accessed 7 December 2017. [Google Scholar]
- 9. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 10. Winter K, Zipprich J, Harriman K, et al. Risk factors associated with infant deaths from pertussis: a case-control study. Clin Infect Dis 2015; 61:1099–106. [DOI] [PubMed] [Google Scholar]
- 11. Berger JT, Carcillo JA, Shanley TP, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Critical pertussis illness in children: a multicenter prospective cohort study. Pediatr Crit Care Med 2013; 14:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cortese MM, Baughman AL, Zhang R, Srivastava PU, Wallace GS. Pertussis hospitalizations among infants in the United States, 1993 to 2004. Pediatrics 2008; 121:484–92. [DOI] [PubMed] [Google Scholar]
- 13. Marshall H, Clarke M, Rasiah K, et al. Predictors of disease severity in children hospitalized for pertussis during an epidemic. Pediatr Infect Dis J 2015; 34:339–45. [DOI] [PubMed] [Google Scholar]
- 14. Murray EL, Nieves D, Bradley JS, et al. Characteristics of severe Bordetella pertussis infection among infants </=90 days of age admitted to pediatric intensive care units—Southern California, September 2009-June 2011. J Pediatric Infect Dis Soc 2013; 2:1–6. [DOI] [PubMed] [Google Scholar]
- 15. Capili CR, Hettinger A, Rigelman-Hedberg N, et al. Increased risk of pertussis in patients with asthma. J Allergy Clin Immunol 2012; 129:957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu BC, McIntyre P, Kaldor JM, Quinn HE, Ridda I, Banks E. Pertussis in older adults: prospective study of risk factors and morbidity. Clin Infect Dis 2012; 55:1450–6. [DOI] [PubMed] [Google Scholar]
- 17. Prevention CfDCa. National Health Interview Survey (NHIS). Available at: https://www.cdc.gov/asthma/nhis/2015/table4-1.htm. Accessed 12 March 2017. [Google Scholar]
- 18. Centers for Disease Control and Prevention. NCfCDPaHP, Division of Population Health. Chronic Disease Indicators (CDI) Data [online]. Available at: https://nccd.cdc.gov/cdi. Accessed 7 December 2017. [Google Scholar]
- 19. Hashemi SH, Nadi E, Hajilooi M, Seif-Rabiei MA, Samaei A. High seroprevalence of Bordetella pertussis in patients with chronic obstructive pulmonary disease: a case-control study. Tanaffos 2015; 14:172–6. [PMC free article] [PubMed] [Google Scholar]
- 20. Winter K, Cherry JD, Harriman K. Effectiveness of prenatal tetanus, diphtheria, and acellular pertussis vaccination on pertussis severity in infants. Clin Infect Dis 2017; 64:9–14. [DOI] [PubMed] [Google Scholar]
- 21. Skoff TH, Blain AE, Watt J, et al. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants <2 months of age: a case-control evaluation. Clin Infect Dis 2017; 65:1977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McNamara LA, Skoff T, Faulkner A, et al. Reduced severity of pertussis in persons with age-appropriate pertussis vaccination—United States, 2010–2012. Clin Infect Dis 2017; 65:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.