Severe acute respiratory syndrome (SARS) is the first pandemic of the 21st century (1). Since its recognition, 8437 individuals have been affected and 813 have died (2). Approximately 20–30% of patients required intensive care admission (1). Although there was a slight predominance of female SARS patients, possibly because of the overrepresentation of female healthcare workers (1), male SARS patients were more likely to suffer poor outcomes (3). In a major hospital outbreak in Hong Kong (4), 32% of male and 15% of female SARS patients required intensive care or died. Remarkably, similar demographic data were seen among SARS patients in the greater Toronto area, Canada, where 32% of males and 14% of females with SARS required intensive care or died (5). Karlberg et al. (3) studied the case fatality rates among all confirmed SARS patients documented in the Hong Kong SARS epidemic in 2003. The authors concluded that the mortality rates differed significantly between males and females, being 21.9% and 13.2%, respectively. The relative risk for death in males was 1.62 after adjustment for age. It is thus an intriguing coincidence that ACE2, the gene for the newly identified functional receptor for the SARS coronavirus, angiotensin-converting enzyme 2, maps to the X-chromosome (Xp22) (6).
ACE2 was first identified as a homolog of angiotensin-converting enzyme with zinc metalloproteinase activity (7). Many of its activities differ from those of angiotensin-converting enzyme (8). ACE2 has been found to be an important regulator of cardiac function (9). Since the identification of ACE2 as the functional receptor for the SARS coronavirus (6), efforts have been spent on characterizing its molecular interaction with the virus (10)(11). On the other hand, studies on mouse hepatitis virus, a group 2 coronavirus (12), demonstrated that allelic variants of viral receptor were associated with altered virus-binding activity, which mediated host susceptibility (13). Hence, it is plausible that genetic variants of ACE2 may moderate the effects of SARS coronavirus infection and, possibly, gender-specific effects.
For this study, we obtained institutional ethics approval. We identified 103 single-nucleotide polymorphisms (SNPs) in ACE2, using the University of Washington and Fred-Hutchinson Cancer Research Center Variant Discovery Resource, SeattleSNPs (14). Two of the identified SNPs were located within the coding regions [dbSNP identification nos. rs4646116 and rs4646179 (15)], whereas the remainder were located within the introns of ACE2. SNP validation by direct sequencing of the 101 noncoding SNPs was conducted with use of buffy coat DNA obtained from 10 female Chinese volunteers. This validation strategy allows the detection of SNPs with minor allele frequencies of at least 10% at 90% power. The two coding SNP loci were verified on buffy coat DNA from 20 female Chinese. Forty-eight pairs of primers were designed to facilitate direct sequencing of ACE2 regions spanning all of the SNPs. Buffy coat DNA was extracted according to the Blood and Body Fluid Spin protocol in manufacturer’s instructions for the QIAamp DNA Blood Mini Kit (Qiagen). DNA sequencing was performed by the dideoxy dye terminator method on an automated DNA sequencer (3100 Genetic Analyzer; Applied Biosystems) based on capillary electrophoresis. Sequences were edited and aligned, and comparisons were made with the SeqScape software (Applied Biosystems).
SNP validation confirmed sequence variations at five sites (Table 1 ). All five SNP loci were noncoding. Both of the coding SNPs were shown to be nonpolymorphic among the 20 females. The positions and orientations of the five verified SNPs are illustrated in Fig. 1 . A case–control study was conducted to compare the frequencies of the five polymorphisms among 168 SARS patients (81 males and 87 females, among whom 30 males and 16 females had poor outcomes) from the Prince of Wales Hospital, Hong Kong (4), and 328 healthy volunteers (174 males and 154 females). All of the individuals studied were unrelated individuals of Chinese ethnicity. Genotype characterization was performed with TaqMan (Applied Biosystems) allelic discrimination assays on an ABI Prism 7900HT sequence detection system (Applied Biosystems). Each assay consisted of two allele-specific minor groove binding probes with different fluorescent labels, i.e., 6-carboxyfluorescein (FAM) or VIC™, designed for the discrimination of the two respective alleles at each SNP locus. The assays were set up according to the manufacturer’s instructions (TaqMan Core PCR Kit; Applied Biosystems) in a reaction volume of 10 μL. The primers and fluorescent probes were used at concentrations of 900 and 200 nM, respectively. We used 10 ng of buffy coat DNA for amplification. The thermal profile consisted of an initial denaturation period for 10 min at 95 °C, followed by 40 cycles of denaturation at 92 °C for 15 s, and 1 min of combined annealing and extension at either 62 °C (SNPs rs2285666, rs4646142, and rs714205) or 65.5 °C (SNPs rs2106809 and rs2074192; Table 1 ). The genotypes were scored with the SDS2.1 software.
Table 1.
Allele and genotype frequencies for the studied groups.
| A. Allele frequencies and χ2 analyses (df = 1) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP locus1 (dbSNP ID) | |||||||||||||||||||
| 1848 (rs2106809) | 95701 (rs2285666) | 16854 (rs4646142) | 360242 (rs714205) | 37138 (rs2074192) | |||||||||||||||
| Frequency, n (%) | χ2 | Frequency, n (%) | χ2 | Frequency, n (%) | χ2 | Frequency, n (%) | χ2 | Frequency, n (%) | χ2 | ||||||||||
| Minor allele | T | G | C | G | A | ||||||||||||||
| Controls (174 M/154 F) | 228 (47) | 224 (46) | 223 (46) | 215 (45) | 206 (43) | ||||||||||||||
| SARS patients3 (81 M/87 F) | 114 (45) | χ2 = 0.354; P = 0.552 | 111 (44) | χ2 = 0.470; P = 0.493 | 110 (43) | χ2 = 0.539; P = 0.463 | 111 (44) | χ2 = 0.047; P = 0.840 | 105 (41) | χ2 = 0.109; P = 0.741 | |||||||||
| SARS patients with poor outcome3 (30 M/16 F) | 30 (48) | χ2 = 0.000; P = 0.979 | 27 (44) | χ2 = 0.090; P = 0.765 | 27 (44) | χ2 = 0.072; P = 0.788 | 26 (42) | χ2 = 0.069; P = 0.793 | 25 (40) | χ2 = 0.051; P = 0.821 | |||||||||
| Male SARS patients with poor outcome4 (n = 30) | 13 (43) | χ2 = 0.590; P = 0.443 | 12 (40) | χ2 = 0.488; P = 0.485 | 12 (40) | χ2 = 0.410; P = 0.522 | 11 (37) | χ2 = 0.746; P = 0.388 | 11 (37) | χ2 = 0.401; P = 0.527 | |||||||||
| Male controls (n = 174) | 92 (53) | 85 (49) | 84 (48) | 82 (47) | 78 (45) | ||||||||||||||
| Female SARS patients4 (n = 87) | 78 (45) | χ2 = 0.002; P= 0.962 | 77 (44) | χ2 = 0.008; P = 0.928 | 77 (44) | χ2 = 0.008; P = 0.928 | 78 (45) | χ2 = 0.064; P = 0.799 | 73 (42) | χ2 = 0.000; P = 0.991 | |||||||||
| Female controls (n = 154) | 136 (44) | 139 (45) | 139 (45) | 133 (43) | 128 (42) | ||||||||||||||
| B. Genotype frequencies and χ2 analyses (df = 2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | CC/CT/TT | AA/AG/GG | GG/GC/CC | CC/GC/GG | GG/AG/AA | |||||||
| Female controls (n = 154) | 48/76/30 | χ2 = 0.280; P = 0.878 | 44/81/29 | χ2 = 0.099; P = 0.952 | 44/81/29 | χ2 = 0.099; P = 0.952 | 44/87/23 | χ2 = 1.102; P = 0.576 | 48/84/22 | χ2 = 2.315; P = 0.314 | ||
| Female SARS patients4 (n = 87) | 25/46/16 | 25/47/15 | 25/47/15 | 26/44/17 | 1/39/17 | |||||||
The SNP loci are identified according to the University of Washington ACE2 sequence coordinates (14), and the relevant identification numbers at the SNP database (15) are in parentheses.
Genotyping performed with Applied Biosystems Assays-on-Demand™, C2551616_1 and C2551626_1.
The combined male (M) and female (F) control group is used as the comparison group.
The control group of the same gender is used as the comparison group. Because males are hemizygous for ACE2, the number of alleles is equivalent to the number of males. Because females have two X chromosomes, the number of alleles is twice the number of females. At each SNP locus, only the minor allele frequency is listed.
Figure 1.
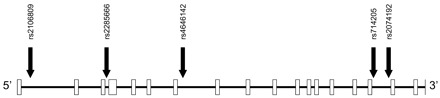
Schematic illustration of the genomic organization of ACE2 and positions of the studied SNP loci.
ACE2 contains 18 exons. Each exon is represented by an open box. The arrows mark the positions of the five verified SNPs. All five SNPs are located within noncoding regions of ACE2. The individual SNPs are named according to their identification numbers registered at the SNP database (dbSNP) (15).
The allele frequencies for the SARS and control groups are listed in Table 1 . When we used the allele frequencies obtained from the control group, the group sample size provided a power of at least 80% for the determination of a genetic factor that contributes 50% increased likelihood toward the development of SARS or poor outcome with 95% confidence. Statistical significance among groups was examined by the χ2 test for each SNP locus (SigmaStat, Ver. 3.0; SPSS). Statistical significance was denoted by a two-tailed P <0.05. No significant difference was observed in the allele distributions between the female and male controls (data not shown), between the SARS cases and controls, between SARS cases with poor outcomes and controls, between the male SARS patients with poor outcome and the male controls, or between the female SARS patients and female controls (Table 1 ). The observed genotype distributions for each of the five loci among the female controls did not deviate significantly from those expected from the Hardy–Weinberg equilibrium. The genotype frequencies for each of the five SNP loci were not statistically significantly different between the female SARS patients and the female controls. Because males are hemizygous for ACE2, the genotype frequency is equivalent to the allele frequency.
We therefore conclude that although ACE2 serves functionally as the receptor for entry of the SARS coronavirus into human host cells, the evidence provided by this study does not support an association between its common genetic variants and SARS susceptibility or outcome. Despite its X-chromosome location, poor outcomes in male SARS patients do not appear to be related to genetic variants of ACE2.
Acknowledgments
The project team is supported by the Research Fund for the Control of Infectious Diseases (RFCID) from the Health, Welfare and Food Bureau of the Hong Kong SAR Government.
References
- 1.Peiris JS, Yuen KY, Osterhaus AD, Stohr K. The severe acute respiratory syndrome. N Engl J Med 2003;349:2431-2441. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Cumulative number of reported probable cases of SARS. http://www.who.int/csr/sars/country/2003_07_11/en/ (accessed May 2004).. [Google Scholar]
- 3.Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do?. Am J Epidemiol 2004;159:229-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003;348:1986-1994. [DOI] [PubMed] [Google Scholar]
- 5.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003;289:2801-2809. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000;275:33238-33243. [DOI] [PubMed] [Google Scholar]
- 8.Danilczyk U, Eriksson U, Crackower MA, Penninger JM. A story of two ACEs. J Mol Med 2003;81:227-234. [DOI] [PubMed] [Google Scholar]
- 9.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002;417:822-828. [DOI] [PubMed] [Google Scholar]
- 10.Wong SK, Li W, Moore MJ, Choe H, Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem 2004;279:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabakaran P, Xiao X, Dimitrov DS. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem Biophys Res Commun 2004;314:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rest JS, Mindell DP. SARS associated coronavirus has a recombinant polymerase and coronaviruses have a history of host-shifting. Infect Genet Evol 2003;3:219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohtsuka N, Taguchi F. Mouse susceptibility to mouse hepatitis virus infection is linked to viral receptor genotype. J Virol 1997;71:8860-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.University of Washington and Fred Hutchinson Cancer Research Center. UW-FHCRC Variation Discovery Resource (SeattleSNPs). ACE2: angiotensin I converting enzyme (peptidyl-dipeptidase A) 2. http://pga.gs.washington.edu/data/ace2/ (accessed December 2003).. [Google Scholar]
- 15.National Center for Biotechnology Information. SNP database. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=snp (acceseed December 2003).. [Google Scholar]


