Abstract
Background
Acute bacterial sinusitis is a frequent complication of viral upper respiratory infection (URI). We describe the clinical and virologic features of URIs that remain uncomplicated and those that precede an episode of sinusitis. We hypothesize that certain viruses are more likely to lead to acute sinusitis, and we compare viruses identified at the time of diagnosis of sinusitis with those identified early in the URI.
Methods
Children aged 48–96 months were followed longitudinally for 1 year. Nasal samples were obtained at surveillance visits, on Day 3–4 of the URI, and on Day 10, when sinusitis was diagnosed. Molecular diagnostic testing was performed on nasal washes for common respiratory viruses and pathogenic bacteria. A standardized score was used to quantify symptom severity.
Results
We evaluated 519 URIs, and 37 illnesses in 31 patients met the criteria for sinusitis. Respiratory syncytial virus was detected more frequently in URI visits that led to sinusitis, compared to in uncomplicated URIs (10.8% vs 3.4%; P = .05). New viruses were detected in 29% of sinusitis episodes, and their pattern was different than those patterns observed at surveillance. The median number of URIs per subject per year was 1 (range 0–9) in uncomplicated URI subjects and 3 (range 1–9) in sinusitis subjects (P < .001).
Conclusions
Children who developed sinusitis experienced more frequent URIs, compared to children whose URIs remained uncomplicated. When nasal samples were obtained on the day of diagnosis of acute sinusitis, nearly 30% of children had a new virus identified, suggesting that some children deemed to have sinusitis were experiencing sequential viral infections.
Keywords: sinusitis, sinus, virus, upper respiratory infection, children
Children who developed sinusitis experienced more frequent upper respiratory infections (URIs) and higher symptom scores than children whose URIs remained uncomplicated. When nasal samples were obtained on the day sinusitis was diagnosed, 30% of children had a new virus identified.
Acute bacterial sinusitis is usually preceded by a viral respiratory infection. A viral upper respiratory infection (URI) causes mucosal inflammation within the nose and nasopharynx that promotes the obstruction of the sinus ostia [1]. The virus-induced proliferation of pathogenic bacteria in the nasopharynx can set the stage for the development of complications, such as acute sinusitis and acute otitis media [2, 3]. The diagnosis of acute sinusitis is made on clinical criteria alone and is based on the presence and pattern of symptoms that differentiate acute sinusitis from an uncomplicated viral URI [4, 5]. Our previous investigations described virus identification and bacterial colonization in asymptomatic children and in those with uncomplicated URIs [2, 3, 6]. Here, we focus on the subpopulation of children with acute bacterial sinusitis and compare the clinical and virologic features of viral URIs that remain uncomplicated to those that precede an episode of sinusitis in an expanded cohort of children. This study was designed to test the hypothesis that infections with certain respiratory viruses are more likely to predispose children to acute sinusitis than other viruses. In addition, in a post hoc analysis, we questioned whether some episodes of presumed acute sinusitis actually represent sequential infections with unique viruses. We conducted a prospective study of young children, which included monitoring clinical respiratory symptoms and the repeated collection of samples of nasal mucus and analysis with viral molecular diagnostics.
METHODS
Enrollment and Inclusion Criteria
Healthy children aged 48–96 months were recruited throughout the study period (2012–2016) from 2 pediatric practices in Madison, Wisconsin, and were followed for 1 year, as previously described and as detailed in the Supplementary Methods [2, 3].
Procedures
Nasal samples were obtained at entry and during 4 surveillance visits when children were asymptomatic, as verified by the study nurses. Nasal samples were also obtained on Day 3–4 of acute URIs and on Day 10, if and when acute sinusitis was diagnosed. A final “recovery” sample was retrieved from those children with uncomplicated URIs when their symptoms were completely resolved, at approximately Day 15. Parents were instructed to call the study nurses at the first signs of an upper respiratory illness; a symptom survey was filled out on Day 3–4 and was subsequently administered by telephone on Days 7, 10, and 15, as previously described in detail and as described in the Supplementary Methods [7].
Classification of Respiratory Episodes
Each respiratory episode was classified as either an uncomplicated viral URI or sinusitis. The diagnosis of sinusitis was based on 1 of the following clinical criteria: (1) persistent symptoms—nasal discharge, cough, or both—that lasted more than 10 days without improvement, or (2) worsening symptoms, as evidenced by the sudden renewal of respiratory symptoms (nasal discharge or cough) or fever after an apparent improvement, usually beyond the sixth day of illness [5]. Radiographs were not performed to diagnose sinusitis, in accordance with national guidelines [5].
Collection of Samples
Samples of nasal mucus were obtained using an established nose-blowing technique [8–10]. Saline nasal solution was sprayed into each of the child’s nostrils. The study nurse then held a plastic “baggie” to the nose and occluded 1 of the nostrils, taking care to not touch the inside of the baggie. If a child was unable to blow their nose, a sufficient sample was obtained by allowing the sprayed saline to drip into the plastic baggie.
Virus Identification
Diagnostic virology was performed on nasal samples by multiplex polymerase chain reaction (PCR), as previously described [2, 3]. Nasal specimens were also analyzed by partial sequencing, to determine which rhinovirus types were present and differentiate closely related enterovirus from rhinovirus [11].
Bacterial Polymerase Chain Reaction
Nasal samples were also analyzed for Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis using quantitative real-time PCR. DNA was extracted with the BiOstic Bacteremia DNA Isolation Kit (Mo Bio laboratories, Carlsbad, CA) as previously described [2]. Each assay included a standard curve, derived from bacteria of known concentrations, and results are reported as colony-forming unit equivalents/mL (cfue/mL).
Statistical Analysis
Statistical methods are provided in detail in the Supplementary Materials.
RESULTS
Study Population
During the 5-year study period, 4516 letters of invitation were sent to families with eligible children; 1878 children were excluded due to an underlying condition, 323 patients were enrolled, and 237 completed 1 year of follow-up (Supplementary Figure 1). Subjects who developed sinusitis had similar demographic characteristics as those with uncomplicated URIs, except were slightly more likely to report their race as American Indian or Alaska Native (Table 1). There were 519 reported URIs in all subjects during the study period; 37 illnesses in 31 distinct patients met the diagnostic criteria for acute sinusitis. The rate of sinusitis-complicating URIs was 7.1% (95% confidence interval [CI] 5.2–9.7%).
Table 1.
Subject Demographics
| URI Subjects | Sinusitis Subjects | P Value | |
|---|---|---|---|
| (n = 292) | (n = 31) | ||
| Age, in years | |||
| Mean | 5.2 | 5.1 | NS |
| Range | 4–8.0 | 4–8.0 | NS |
| Gender, % female | 46.9 | 48 | NS |
| Race, % | |||
| American Indian or Alaska Native | 0 | 3 | 0.02 |
| Asian | 5.5 | 0 | NS |
| African-American | 6.8 | 6.4 | NS |
| Other | 6.5 | 0 | NS |
| Unknown or not reported | 0.3 | 0 | NS |
| Caucasian | 80.5 | 90.3 | NS |
| Ethnicity | |||
| Hispanic, % | 7.3 | 6.4 | NS |
| Attends day care, % | 84 | 83 | NS |
| Tobacco exposure, % | 3.8 | 0 | NS |
| Maternal education level, % | |||
| Graduate/professional | 39.8 | 25.8 | NS |
| College degree | 35.6 | 45.1 | NS |
| Some college | 12.1 | 22.6 | NS |
| Vocational/tech | 5.5 | 0 | NS |
| High School | 6.6 | 5.5 | NS |
| No or public insurance (%) | 17 | 19 | NS |
Abbreviations: NS, not significant; URI, upper respiratory infection.
Viruses Detected in Acute Upper Respiratory Infections and at Diagnosis of Acute Sinusitis
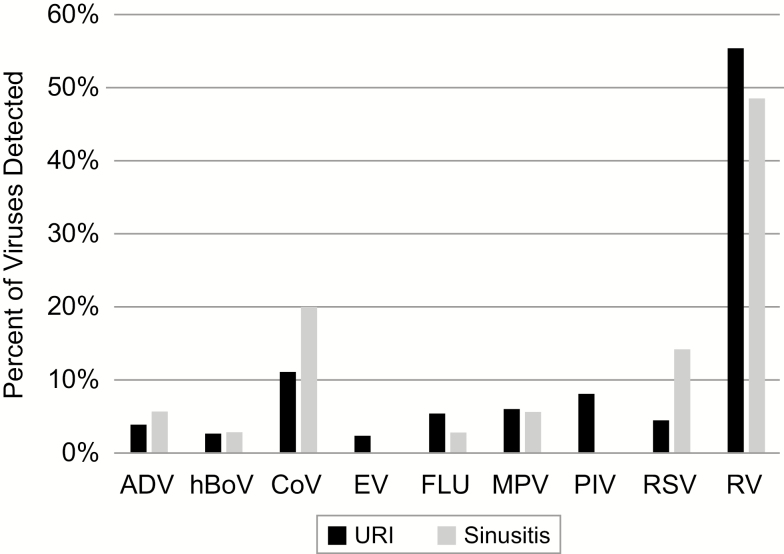
A virus was detected on Day 3 in the nasal washes of 81% and 76% of uncomplicated URIs and sinusitis episodes, respectively (Figure 1). Respiratory syncytial virus (RSV) was detected significantly more often in URI visits that led to sinusitis (10.8% vs 3.4%; P = .05); the distribution of the remaining viruses was similar between uncomplicated URI and sinusitis events. No viruses were detected in 19.3% and 24.3% of uncomplicated URIs and sinusitis visits, respectively (P = .5).
Figure 1.
Viruses detected in Day 3 nasal washes for URIs that remained uncomplicated and those that progressed to sinusitis. Abbreviations: ADV, adenovirus; CoV, coronavirus; EV, enterovirus; FLU, influenza virus; hBoV, human bocavirus; MPV, metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, rhinovirus; URI, upper respiratory infection.
To determine whether the clinical characteristics that defined an episode of acute sinusitis might, in some cases, be caused by sequential viral infections, we compared the viral PCRs performed on the nasal washes obtained at Days 3 and 10 after the onset of an illness. Of the 37 episodes of sinusitis, 6 did not have a second nasal wash on the tenth day of illness, leaving 31 of the 37 sinusitis events available for analysis. There were 18 cases of sinusitis diagnosed based on persistent symptoms and 13 based on worsening symptoms (Table 2). Compared to the results of their Day 3 sample, 78% of children with persistent symptoms and 62% of children with worsening symptoms had a Day 10 nasal sample that was negative for a virus or had the same virus as at Day 3. A new virus was detected in 22% of children with persistent and 38% with worsening symptoms, respectively. When taken together, a new virus was detected in the Day 10 nasal sample in 29% of all 31 episodes. There was no difference in the type of respiratory viruses between children with worsening vs persistent symptoms. On Day 10, 55% of subjects with sinusitis had a virus detected in their nasal wash samples. This compares to 31% of recovery nasal washes taken within the same time frame from children with uncomplicated URIs and 34% taken during surveillance visits (P < .01; Table 3). When nasal wash samples from subjects with sinusitis were compared with those obtained at surveillance visits, the new viruses detected on Day 10 were more likely to be adenovirus, influenza, or respiratory syncytial virus and were less likely to be rhinovirus. In comparison, those sinusitis subjects with the same virus detected on Day 10 as on Day 3 showed a similar virus distribution as observed at surveillance visits (Table 4).
Table 2.
Virus Identification at Day 10 Nasal Sample Compared to Day 3 Sample, According to Clinical Presentation
| Presentation | Clinical | ||
|---|---|---|---|
| Virus Identification on Day 10 | Persistent, N (%) | Worsening, N (%) | All cases, N (%) |
| Negative or same as Day 3 | 14 (78) | 8 (62) | 22 (71) |
| New virus | 4a (22) | 5b (38) | 9 (29) |
| Total | 18 (58) | 13 (42) | 31 (100) |
aIn 1 subject, the same virus (metapneumovirus) was detected in addition to a new virus (influenza virus).
bIn 1 subject, the same virus (respiratory syncytial virus) was detected in addition to a new virus (adenovirus).
Table 3.
Virus Detection in Nasal Samples Performed on Day 3 and Day 10 in 31 Patients With Sinusitis Compared With Uncomplicated URI and Asymptomatic Surveillance Visits
| Virus | Sinusitis | Sinusitis | URI | URI | Surveillance |
|---|---|---|---|---|---|
| Day 3 Sample | Day 10 Sample | Day 3 Sample | Recovery | ||
| (n = 31) | (n = 31) | (n = 352) | (n = 274) | ||
| (n = 869) | |||||
| ADV | 0% | 3% | 0% | 1% | 0% |
| hBoV | 0% | 0% | 1% | 1% | 1% |
| CoV | 13% | 6% | 6% | 4% | 3% |
| EV | 3% | 3% | 2% | 0% | 0% |
| FLU | 0% | 6% | 3% | 0%a | 0%a |
| MPV | 3% | 0% | 3% | 0% | 1% |
| PIV | 0% | 0% | 6% | 1% | 1% |
| RSV | 10% | 3% | 3% | 1% | 0% |
| RV | 35% | 19% | 45% | 19% | 24% |
| Mixed | 23% | 13% | 11% | 3%a | 2%a |
| Negative | 13% | 45% | 19% | 69%a | 66%a |
Abbreviations: ADV, adenovirus; CoV, coronavirus; EV, enterovirus; FLU, influenza virus; hBoV, human bocavirus; MPV, metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, rhinovirus; URI, upper respiratory infection.
a P < .01 for comparison with Sinusitis Day 10 Sample.
Table 4.
Comparison of Virus Detection Rates on Day 10 of Sinusitis Visits Versus Surveillance Visits
| Virus | Sinusitis | Sinusitis | |||
|---|---|---|---|---|---|
| New Virus Day 10 | Same Virus Day 10 | Surveillance | |||
| (n = 13) | (n = 7) | (n = 314) | |||
| N (%) | N (%) | N (%) | P Valuea | P Valueb | |
| ADV | 2 (15.4) | 0 (0.0) | 7(2.2) | .04 | NS |
| BoV | 1 (7.7) | 0 (0.0) | 20 (6.4) | NS | NS |
| CoV | 1 (7.7) | 1 (14.3) | 33 (10.5) | NS | NS |
| EV | 0 (0.0) | 0 (0.0) | 5 (1.6) | NS | NS |
| FLU | 2 (15.4) | 1 (14.3) | 6 (1.9) | .03 | NS |
| MPV | 1 (7.7) | 0 (0.0) | 9 (2.9) | NS | NS |
| PIV | 0 (0.0) | 0 (0.0) | 9 (2.9) | NS | NS |
| RSV | 3 (23.1) | 0 (0.0) | 5 (1.6) | <.01 | NS |
| RV | 3 (23.1) | 5 (71.4) | 220 (70.1) | <.01 | NS |
Abbreviations: ADV, adenovirus; BoV, bocavirus; CoV, coronavirus; EV, enterovirus; FLU, influenza virus; MPV, metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, rhinovirus.
a P value for comparison rates between New Virus Day 10 vs Surveillance.
b P value for comparison rates between Same Virus Day 10 vs Surveillance.
Bacterial Colonization in Patients With Acute Sinusitis
As shown in Supplementary Tables 1 and 2, nasal bacterial colonization rates and bacterial densities on Day 3 during URI visits were similar to the sinusitis visits for S. pneumoniae, H. influenzae, and M. catarrhalis (Supplementary Tables 1 and 2). We next compared the detection rates of bacterial pathogens in subjects diagnosed with sinusitis, according to the virus identified in the 10-day sample (ie, new virus vs negative or same virus). The absence of M. catarrhalis on Day 3 (P < .02) and Day 10 (P = .11) appeared to be a risk factor for the acquisition of a new virus on Day 10 (Table 5). There were no differences between those subjects with a new virus and those with negative or the same virus samples for the density or presence of S. pneumoniae or H. influenzae in either the 3- or 10-day samples.
Table 5.
Detection Rates of Bacterial Pathogens at Sinusitis Visits
| Sinusitis Subjects With New Virus on Day 10 | Sinusitis Subjects With Negative or Same Virus on Day 10 | |||
|---|---|---|---|---|
| (n = 9) | (n = 22) | |||
| N (%) | N (%) | P Valuea | ||
| Streptococcus pneumoniae | Day 3 | 5 (55.6) | 14 (63.6) | .70 |
| Day 10 | 5 (55.6) | 16 (72.7) | .41 | |
| Haemophilus influenzae | Day 3 | 2 (22.2) | 7 (31.8) | .68 |
| Day 10 | 2 (22.2) | 5 (22.7) | .99 | |
| Moraxella catarrhalis | Day 3 | 2 (22.2) | 16 (72.7) | .02 |
| Day 10 | 3 (33.3) | 15 (68.2) | .11 | |
| No bacteria | Day 3 | 3 (33.3) | 2 (9.1) | .13 |
| Day 10 | 3 (33.3) | 2 (9.1) | .13 |
aComparison of sinusitis subjects with new virus on Day 10 vs sinusitis subjects with negative or same virus on Day 10.
Frequency and Severity of Upper Respiratory Infections in Patients With Sinusitis
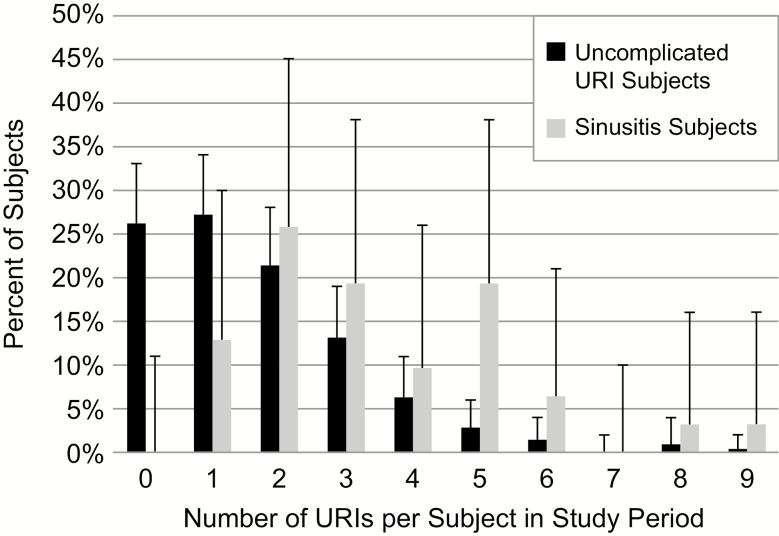
The rate of URIs in the 209 subjects who had only uncomplicated URIs was compared to the rate in the 31 subjects who had sinusitis. The median number of URIs per subject per year was 1 (range 0–9) in the uncomplicated URI subjects and 3 (range 1–9) in the sinusitis subjects (P < .001; Figure 2).
Figure 2.
Frequency of URIs in subjects with uncomplicated URIs and subjects with sinusitis (error bars indicate 95% confidence intervals). Abbreviation: URI, upper respiratory infection.
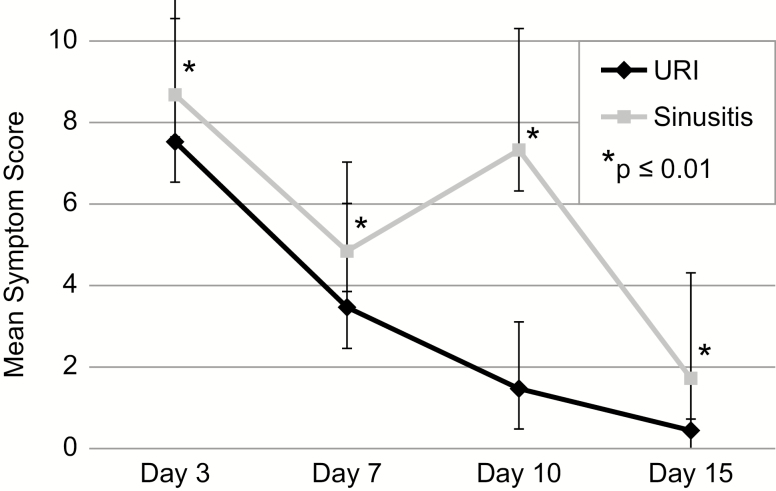
To test the hypothesis that URIs leading to sinusitis would be associated with more severe clinical symptoms than URIs which remained uncomplicated, symptom scores were compared on Days 3, 7, 10, and 15 of illness (Figure 3). Symptom scores were indeed higher on Day 3 (P = .03) and Day 7 (P = .005) for URIs leading to sinusitis compared to uncomplicated URIs (Supplementary Table 3). As expected, the symptom scores were also significantly higher on Days 10 and 15, by definition.
Figure 3.
Mean symptom score by day of illness. Error bars indicate 95% confidence intervals.
To determine which individual symptoms were increased during the acute URIs that led to sinusitis, we compared the area under the curve from Days 0 to 15 for each symptom (Supplementary Table 3). Congestion, cough, and nasal discharge showed the greatest differences between the uncomplicated URIs, compared to the URIs complicated by sinusitis. Impaired appetite, sleep, and activity were also significantly higher at visits for URI that were complicated by sinusitis. Reports of facial pain or swelling, fever, and headache were not different between URI events. When considering Day 7 as the single time point with the greatest differences, the mean (95% CI) scores for the symptoms of congestion, discharge, and cough were 1.4 (95% CI 1.2–1.8), 1.3 (95% CI 1.1–1.6), and 1.2 (95% CI 0.9–1.5), respectively, at URI visits that became complicated with sinusitis and 1.0 (95% CI 0.9–1.1), 1.0 (95% CI 0.9–1.0), and 0.8 (95% CI 0.7–0.9), respectively, at URI visits that remained uncomplicated. These scores were significantly different between the 2 visit types (P < .01 for all 3 symptoms), but were not different for the other symptoms (data not shown).
DISCUSSION
This is the first study to describe the clinical and virologic features of episodes of acute sinusitis, compared with URIs, in a population of children followed longitudinally over a 1-year time period. Children who developed an episode of sinusitis as a complication of URI experienced more frequent URIs and had higher symptom scores early in their illnesses, compared to children whose URIs remained uncomplicated. Most importantly, when nasal samples were obtained on the day of diagnosis of acute sinusitis, nearly 30% of children had a new virus identified, with a different pattern than that observed during surveillance or recovery, strongly suggesting that some children deemed to have sinusitis were actually experiencing sequential viral URIs.
Sinusitis as a Complication of Upper Respiratory Infections
The rate of complications of URIs with sinusitis, of 7.1%, is slightly lower than the 8.8% figure that we reported in an interim analysis of this study population [3], but remains similar to that reported by Marom et al [12]. This decrease may reflect our efforts in the last 24 months of the study to enhance our reporting of URIs. As we have now demonstrated that the rate of sinusitis is related to the severity of the initial URI, increased reporting of milder URIs would be expected to result in a decreased rate of complications. In this study, RSV was detected more frequently at URI events that became complicated by sinusitis, compared to uncomplicated URI visits, suggesting a more prominent pathogenic role for this virus. Although no other studies correlating sinusitis with virology have been reported, similar studies have been done for acute otitis media. Chonmaitree et al [13] followed 362 infants from birth to 12 months of age and performed viral PCRs on nasopharyngeal swabs. The development of acute otitis media was associated with increasing age and with infection with RSV, rhinovirus, enterovirus, or bocavirus. Although we did not find an association with the other viruses, it is pertinent that RSV was associated with complications in both studies. RSV is known to elicit a robust immune response in the nose and lower respiratory tract [14]. It is probable that this virus produces more local inflammation in the nasal mucosa, thereby affecting the sinus ostia.
Viruses Detected at Diagnosis of Sinusitis
National guidelines for the diagnosis of acute sinusitis have relied on clinical criteria to conclude that a child is experiencing an episode of sinusitis: the persistence of respiratory symptoms for 10 or more days without improvement or the worsening of symptoms after a period of improvement [4, 5]. An important problem related to the clinical diagnosis of sinusitis is that persistent or worsening upper respiratory symptoms may result from the development of a classic, secondary bacterial infection or a second viral infection that is closely spaced to the first. We found that, when using the criteria of persistent or worsening symptoms, 71% of patients had the same or no virus detected on the 10-day nasal sample, supporting the diagnosis of bacterial sinusitis in these patients. However, nearly 30% had a new virus identified, suggesting that sequential viral infections may explain the symptoms. This observation is supported by data from several clinical trials, demonstrating that about one-third of children clinically diagnosed to have sinusitis lack evidence of a bacterial infection [7, 15, 16]. In addition, in a randomized, double-blind, controlled trial of placebo vs amoxicillin/clavulanic acid in children with clinically diagnosed sinusitis, the non-response rate to the antibacterial was 36%, while the spontaneous cure rate in recipients of the placebo was 32%; each of these correspond closely to the percentage of patients in our study who had sequential viral infections [7]. This is also close to the number of children (20%) who have normal radiographs despite meeting the diagnostic criteria for acute sinusitis [17].
When surveillance samples of nasal wash are examined in asymptomatic children, about 30% will show the presence of a virus [3]. Accordingly, although it is likely, there is no certainty that the virus identified at Day 10 (at the time of diagnosis of acute sinusitis) is the cause of the respiratory symptoms, since asymptomatic detection is common. Furthermore, the identification of a new virus does not preclude the possibility of a dual infection caused by both a virus and bacteria. However, the compelling data show that when a new virus was detected in subjects with sinusitis on Day 10, it was statistically more likely to be adenovirus, influenza, or RSV (rather than rhinovirus), as compared to the viruses identified during surveillance. Furthermore, a virus was identified in 55% of the 31 patients with sinusitis on Day 10, a frequency of detection significantly different than the identification of viruses during surveillance or recovery.
Bacterial Detection During Sinusitis
To assess the relative role of the bacteria and virus at the 10-day visit, we compared bacterial densities in the nasal washes of children with the same or no virus with those in whom a new virus was identified, using washes from Day 3 and Day 10 for each category and between categories. We found no significant differences in either the bacterial presence or density. Of note, the absence of M. catarrhalis was associated with the acquisition of a new virus. In contrast, Xu et al [18] showed higher rates of colonization with bacterial pathogens in the nasopharynx in otitis media–prone children, compared with those with uncomplicated URIs. However, otitis-prone children may not be comparable to previously healthy children with an episode of sinusitis, with regard to the intensity of their previous respiratory infections.
Overall, these data support the supposition that patients diagnosed with sinusitis using current guidelines who have a new virus detected on the tenth day of illness actually have sequential viral infections. An alternative supposition is that subjects diagnosed with sinusitis with a new virus on Day 10 have both a new viral infection and bacterial sinusitis. Further studies that assess responses to antimicrobials are necessary to fully explore these hypotheses.
Clinical Characteristics of Upper Respiratory Infections Preceding Sinusitis
Children who developed sinusitis as a complication of URI at least once during the 1-year study had more than 3 times the annual rate of URIs than those children who had only uncomplicated URIs. This may be explained by the observation that complete histologic recovery of the mucosa after a viral URI is much slower than clinical recovery [19]. Therefore, even a mild infection could become symptomatic when imposed on a recently damaged respiratory mucosa.
We demonstrated that symptom scores during the early phase of the URI were higher for episodes complicated by sinusitis vs uncomplicated URIs. However, the overlap of scores was substantial, and this finding alone cannot distinguish those patients with URIs who will go on to develop sinusitis.
The strengths of this study include a large sample size, multiple respiratory seasons, and a longitudinal, observational cohort design with extensive, prospective monitoring of both symptoms and microbiology during periods of wellness, URIs, and sinusitis. Limitations of this study include a narrow age range (4–7 years). However, this is the peak age group for children with acute sinusitis and avoids infants and toddlers, who experience a much higher rate of acute otitis media. Children with acute otitis media are likely to receive antibiotics earlier in a URI, which could blunt the appearance of sinusitis. Samples were obtained using nasal washes, which may not represent the microbial conditions in the nasopharynx but are more tolerable in longitudinal studies than repeated nasopharyngeal swabs [20]. Our rate of detection of viruses is similar to other studies, suggesting that—at least for viruses—this method was reliable [8, 21–23]. Finally, despite the substantial sample size, the number of children who developed sinusitis was relatively small. The low prevalence of sinusitis limits the power of the study to detect specific risk factors.
The clinical implication of this study is that sinusitis may be overdiagnosed using current guidelines. While viral PCR on nasal samples is not feasible or sufficient to exclude bacterial sinusitis at the time of a clinical diagnosis, the measurement of nasal cytokines (adaptable to a point-of-care assay) may reflect a host response ascribable to a viral infection [24–28].
CONCLUSIONS
Acute bacterial sinusitis is a common complication of upper respiratory tract infections in children. This study supports the use of published clinical criteria for the diagnosis of acute sinusitis in children and in distinguishing uncomplicated URIs from sinusitis. However, the data presented provide preliminary evidence suggesting that some children deemed to have sinusitis are, instead, experiencing sequential viral infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (grant number R01 AI097172).
Potential conflicts of interest. J. C. G. has received grants from the NIH/NIAID, during the conduct of the study; has received personal fees from PREP Biopharm Inc, Regeneron, and MedImmune, outside the submitted work; has received other support from Meissa Vaccines Inc, outside the submitted work; and has patents pending on Methods of Propagating Rhinovirus C in Previously Unsusceptible Cell Lines and on Adapted Rhinovirus C. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. DeMuri GP, Wald ER. Clinical practice. Acute bacterial sinusitis in children. N Engl J Med 2012; 367:1128–34. [DOI] [PubMed] [Google Scholar]
- 2. DeMuri GP, Gern JE, Eickhoff JC, Lynch SV, Wald ER. Dynamics of bacterial colonization with Streptococcus pneumoniae, haemophilus influenzae, and moraxella catarrhalis during symptomatic and asymptomatic viral upper respiratory tract infection. Clin Infect Dis 2018; 66:1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeMuri GP, Gern JE, Moyer SC, Lindstrom MJ, Lynch SV, Wald ER. Clinical features, virus identification, and sinusitis as a complication of upper respiratory tract illness in children ages 4–7 years. J Pediatr 2016; 171:133–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chow AW, Benninger MS, Brook I, et al. ; Infectious Diseases Society of America. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012; 54:e72–e112. [DOI] [PubMed] [Google Scholar]
- 5. Wald ER, Applegate KE, Bordley C, et al. ; American Academy of Pediatrics Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics 2013; 132:e262–80. [DOI] [PubMed] [Google Scholar]
- 6. Santee CA, Nagalingam NA, Faruqi AA, et al. Nasopharyngeal microbiota composition of children is related to the frequency of upper respiratory infection and acute sinusitis. Microbiome 2016; 4:34 Available at: https://www.ncbi.nlm.nih.gov/pubmed?term=Nasopharyngeal+microbiota+composition+of+children+is+related+to+the+frequency+of+upper+respiratory+infection+and+acute+sinusitis.+Microbiome&TransSchema=title&cmd=detailssearch [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wald ER, Nash D, Eickhoff J. Effectiveness of amoxicillin/clavulanate potassium in the treatment of acute bacterial sinusitis in children. Pediatrics 2009; 124:9–15. [DOI] [PubMed] [Google Scholar]
- 8. Olenec JP, Kim WK, Lee WM, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol 2010; 125:1001–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powell KR, Shorr R, Cherry JD, Hendley JO. Improved method for collection of nasal mucus. J Infect Dis 1977; 136:109–11. [DOI] [PubMed] [Google Scholar]
- 10. Hayden FG, Herrington DT, Coats TL, et al. ; Pleconaril Respiratory Infection Study Group Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis 2003; 36:1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol 2014; 52:2461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marom T, Alvarez-Fernandez PE, Jennings K, Patel JA, McCormick DP, Chonmaitree T. Acute bacterial sinusitis complicating viral upper respiratory tract infection in young children. Pediatr Infect Dis J 2014; 33:803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chonmaitree T, Alvarez-Fernandez P, Jennings K, et al. Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development. Clin Infect Dis 2015; 60:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabarani CM, Bonville CA, Suryadevara M, et al. Novel inflammatory markers, clinical risk factors and virus type associated with severe respiratory syncytial virus infection. Pediatr Infect Dis J 2013; 32:e437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wald ER, Reilly JS, Casselbrant M, et al. Treatment of acute maxillary sinusitis in childhood: a comparative study of amoxicillin and cefaclor. J Pediatr 1984; 104:297–302. [DOI] [PubMed] [Google Scholar]
- 16. Wald ER, Milmoe GJ, Bowen A, Ledesma-Medina J, Salamon N, Bluestone CD. Acute maxillary sinusitis in children. N Engl J Med 1981; 304:749–54. [DOI] [PubMed] [Google Scholar]
- 17. Shaikh N, Hoberman A, Kearney DH, et al. Signs and symptoms that differentiate acute sinusitis from viral upper respiratory tract infection. Pediatr Infect Dis J 2013; 32:1061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu Q, Wischmeyer J, Gonzalez E, Pichichero ME. Nasopharyngeal polymicrobial colonization during health, viral upper respiratory infection and upper respiratory bacterial infection. J Infect 2017; 75:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carson JL, Collier AM, Hu SS. Acquired ciliary defects in nasal epithelium of children with acute viral upper respiratory infections. N Engl J Med 1985; 312:463–8. [DOI] [PubMed] [Google Scholar]
- 20. Goggin RK, Bennett CA, Bassiouni A, et al. Comparative viral sampling in the sinonasal passages; different viruses at different sites. Front Cell Infect Microbiol 2018; 8:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gern JE, Martin MS, Anklam KA, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol 2002; 13:386–93. [DOI] [PubMed] [Google Scholar]
- 22. Lemanske RF Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 2005; 116:571–7. [DOI] [PubMed] [Google Scholar]
- 23. Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008; 178:667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alper CM, Li-Korotky HS, Lo CY, Doyle AP, Winther B, Doyle WJ. Nasal secretion concentrations of IL-5, IL-6, and IL-10 in children with and without upper respiratory tract viruses. Arch Otolaryngol Head Neck Surg 2010; 136:281–6. [DOI] [PubMed] [Google Scholar]
- 25. Burke TW, Henao R, Soderblom E, et al. Nasopharyngeal protein biomarkers of acute respiratory virus infection. EBioMedicine 2017; 17:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landry ML, Foxman EF. Antiviral response in the nasopharynx identifies patients with respiratory virus infection. J Infect Dis 2018; 217:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noah TL, Henderson FW, Wortman IA, et al. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis 1995; 171:584–92. [DOI] [PubMed] [Google Scholar]
- 28. Patel JA, Nair S, Revai K, Grady J, Chonmaitree T. Nasopharyngeal acute phase cytokines in viral upper respiratory infection: impact on acute otitis media in children. Pediatr Infect Dis J 2009; 28:1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.