Abstract
Background
An understanding of immune responses against the Middle East respiratory syndrome (MERS) is important for the development of treatments and preventive measures. Here, we investigated the spectrum of immune responses occurring in patients with MERS during the early period of infection.
Methods
We obtained peripheral blood samples from 27 hospitalized patients recruited during the epidemic that occurred in 2015 in South Korea. Plasma cytokines/chemokines and antibodies were quantified. Virus-specific T cells were examined by intracellular cytokine staining after stimulation of peripheral blood mononuclear cells with overlapping peptides spanning whole virus structural proteins.
Results
At the acute phase of infection, elevated levels of plasma proinflammatory cytokines/chemokines were detected in proportion to the severity of the disease. Distinctively high frequencies of MERS coronavirus–reactive CD8+ T cells were also observed in patients with severe/moderate illness, whereas antibody and CD4+ T-cell responses were minimally detected at this stage. At the convalescent phase, disease severity–dependent antibody responses emerged and antigen-reactive cells were identified in both T-cell subsets. These T cells belonged to the T-helper 1 or type 1 cytotoxic T cell subtypes. While CD8+ T cells responded preferentially to the viral S protein compared with E/M/N proteins, especially at the acute stage, slightly more CD4+ T cells recognized E/M/N proteins compared with S protein at the convalescent phase.
Conclusions
Our findings show an association between the early CD8+ T-cell response and the severity of the infection, and also provide basic information that may help to prepare effective control strategies for MERS in humans.
Keywords: MERS coronavirus, immune response, T lymphocytes, acute phase of infection
Our study examined immune responses to MERS coronavirus at the acute stage of human infection, and shows an association between the early CD8+ T-cell response and the severity of the infection.
The Middle East respiratory syndrome coronavirus (MERS-CoV) mainly causes respiratory illness with a wide range of clinical severity varying from asymptomatic to severe pneumonia with respiratory failure [1]. While the clinical characteristics of MERS and the biology of the causative virus are well documented [2], the pathogenesis and host immune response during MERS-CoV infection have been poorly investigated. This has hampered the development of therapeutics and preventive measures. Recent studies have demonstrated that a strong antibody response develops in most patients after 2–3 weeks of illness and that this antibody response is not likely to be correlated with the elimination of the virus from the body [3, 4]. The elevated serum levels of proinflammatory cytokines and chemokines, such as interleukin (IL) 6 and CXCL-10, were also observed in patients during the early period of severe infection [5–8]. Furthermore, T-cell responses to MERS-CoV have been recently measured in MERS survivors at the late convalescent period, and their association with disease course was analyzed [9]. However, information is lacking regarding the T-cell responses in patients at the acute stage of infection. In vitro and animal studies showed that MERS-CoV preferentially infects respiratory epithelial cells and inhibits the type 1 interferon response [10, 11]. CD8+ T cells and antibodies were revealed to participate in clearing the invading MERS-CoV virions and to protect against subsequent infection, respectively [12, 13]. It was also reported that the local immune response likely plays a role in pulmonary pathology severity [14]. However, it remains to be seen whether the information obtained from animal studies also applies to humans.
In this study, we examined various immunological features, especially T-cell responses, using blood samples obtained from patients during the acute and early convalescent stages of MERS-CoV infection. Our data provide basic information to understand the role of immune responses on the disease process of MERS.
MATERIALS AND METHODS
Patients and Clinical Samples
We recruited 27 patients with MERS who were hospitalized at the National Medical Center (NMC) in Seoul during the 2015 outbreak in South Korea. MERS-CoV infection was confirmed by real-time reverse-transcription polymerase chain reaction (RT-PCR). Clinical information including laboratory data of individual patients is provided in Supplementary Table 1. Peripheral blood was collected from patients at the acute and convalescent phases of infection. In this study, the acute phase was defined as the period after the onset of symptoms but before the peak of illness, usually within 2 weeks after the onset of symptoms; the convalescent phase was defined as the period immediately after the negative conversion of real-time RT-PCR, usually between 2 and 5 weeks after symptom onset. The study was approved by the NMC Ethical Committee.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by density gradient centrifugation using Ficoll-Paque solution (GE Healthcare, Sweden) and stored in liquid nitrogen. Paired plasma samples were collected to determine cytokine concentrations and MERS-CoV–specific antibody responses. Control PBMCs were obtained from 3 healthy persons at the NMC.
Peptide Library
Although some viral peptides that trigger T-cell responses in MERS-CoV infection were recently identified [9], the entire spectrum of viral antigens linked with diverse major histocompatibility complex molecules remains to be determined. The study of T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV), which is a member of the same coronavirus family and elicits a similar respiratory infection in humans, demonstrated that its structural proteins (spike, envelope, membrane, and nucleocapsid) were the most immunogenic to T cells, as compared with the nonstructural proteins [15]. We thus used overlapping synthetic peptides spanning all the 4 structural proteins of MERS-CoV (KOREA/Seoul/014-1-2015, accession number KT374052) as viral antigen to analyze T-cell responses to MERS-CoV. The peptide library comprised 507 peptides consisting of 15-mers overlapping at 11 amino acid residues; the peptides, with >80% purity as determined by mass spectrometry and high-performance liquid chromatography, were manufactured by Mimotopes (Australia). We dissolved each peptide in dimethyl sulfoxide (DMSO) at a concentration of 80 mg/mL and pooled those encompassing the viral S protein into 2 sets (S1: 168 N-terminal peptides; S2: 168 C-terminal peptides) and those of the E, M, and N proteins into 1 set (E/M/N: 171 peptides).
Cytokine Assays
The concentration of plasma cytokines/chemokines (IL-1β, IL-1RA, IL-6, IL-8, IL-10, tumor necrosis factor alpha [TNF-α], interferon gamma-induced protein 10 [IP-10], monocyte chemoattractant protein 1 [MCP-1], macrophage inflammatory protein-1β, and regulated on activation, normal T cell expressed and secreted [RANTES]) was quantified using Bio-Plex Multiplex Immunoassay Systems (Bio-Rad Laboratories, Hercules, California), according to the manufacturer’s instructions. Plasma interferon (IFN) α was measured by enzyme-linked immunosorbent assay (ELISA) using Verikine-HS ELISA kit (PBL Assay Science, Piscataway, New Jersey).
Antibody Assays
Anti–MERS-CoV immunoglobulin M (IgM) and immunoglobulin G (IgG) plasma titers were determined using an indirect immunofluorescence test and ELISA kit (Euroimmun AG, Lubeck, Germany), respectively, following the manufacturers’ instructions. Anti–MERS-CoV IgM titer was defined as the greatest dilution showing the identifiable specific fluorescence when 2-fold serial dilutions (starting from 1:10) of plasma samples were analyzed. The amount of plasma IgG was evaluated semiquantitatively by calculating the ratio of the extinction value of the patient sample over that of the calibrator, as suggested by the manufacturer. Neutralizing antibody (Ab) against spike protein was measured by a pseudotype retrovirus-based neutralization assay as described previously [16, 17]. Neutralizing Ab titers were presented as the highest plasma dilution yielding >50% inhibition of luciferase activity.
Flow Cytometric Analysis
PBMCs were cultured in complete RPMI 1640 medium containing 10% (v/v) human serum (Biowest, Nuaille, France), and stimulated with 1 µg/mL of each peptide in the presence of 1 µg/mL each of anti-CD28 and anti-CD49d monoclonal Abs (mAbs) (BD Biosciences) for 1 hour at 37°C. For negative and positive control cultures, PBMCs were incubated with DMSO alone and with anti-CD3 mAbs (BD Biosciences), respectively. After the addition of 1 µg/mL brefeldin A (eBiosciences) and 0.7 µg/mL monensin (BD Biosciences), cells were further incubated for an additional 5 hours. After staining dead cells using LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Molecular Probes), cells were subjected to surface staining with anti-CD3 BV421, anti-CD4 PerCP-Cy5.5, and anti-CD8 allophycocyanin (APC)-H7 mAbs (BD Biosciences). For the detection of antigen-reactive T cells with degranulation activity, anti-CD107a fluorescein isothiocyanate (FITC) mAb (BioLegend) was added to the stimulation cultures, and also used for surface staining. After fixation and permeabilization using the Intracellular Fix and Perm Set (eBiosciences), cells were stained with either set 1 (anti–IFN-γ PE-Cy7, anti–IL-2 PE, and anti–TNF-α APC) or set 2 (anti–IFN-γ phycoerythrin-cyanine 7 (PE-Cy7), anti–IL-4 PE, anti–IL-10 eFluor660, and anti–IL-17 FITC) mAbs. At least 100000 stained cells per sample were acquired using a FACSverse Flow Cytometer (BD Biosciences), and analyzed using FlowJo software (Tree Star, Ashland, Oregon). The data presented correspond to background-subtracted results using the negative control culture and the sum of responses against different peptide pools unless otherwise specified.
Statistical Analysis
Statistical analysis was performed using Prism version 5.04 software (GraphPad, San Diego, California). The nonparametric Mann-Whitney U test and Wilcoxon signed-rank test were used to compare the 2 groups with independent and paired samples, respectively. Correlation analysis was carried out using the Spearman test. Differences were considered statistically significant at P < .05.
RESULTS
Study Patients
A total of 27 patients were enrolled in this study. As shown in Table 1, these patients were divided into 3 groups, depending on the severity of illness. Severe disease (n = 12) included fatalities (n = 5) and patients who required mechanical ventilation to relieve respiratory failure (n = 7). Moderate disease (n = 7) comprised patients with radiological evidence of pneumonia without respiratory failure. Mild disease (n = 8) encompassed patients who were asymptomatic or who reported symptoms such as fever, headache, cough, and malaise, without distinctive pulmonary lesions. As reported previously [18, 19], older age was linked with severe disease (Table 1; P < .05). In addition, the decrease in lymphocytes was observed in patients with severe and moderate disease, especially at the acute phase of infection (Supplementary Figure 1).
Table 1.
Characteristics of Clinical Samples
| Group | No. of Patients | M/F, No. | Age, y, Mean ± SD | Clinical Findings | No. of Samples | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fatal or MV | Development of Pneumonia | Other Mild Symptoms | Acute | Convalescent | Paired | ||||
| Severe | 12 | 10/2 | 63 ± 16 | + | + | + | 9 | 7 | 4 |
| Moderate | 7 | 4/3 | 54 ± 20 | – | + | + | 3 | 7 | 3 |
| Mild | 8 | 3/5 | 44 ± 25 | – | – | +/– | 5 | 7 | 4 |
Abbreviations: MV, mechanical ventilation; SD, standard deviation.
Plasma Cytokine Profiles
To estimate the role of cytokines and chemokines in infection with MERS-CoV, we measured their plasma levels by cytokine bead array or ELISA. The plasma concentrations of IL-6, IL-1RA, IP-10, and MCP-1 were significantly elevated at the acute phase of infection (10.7-, 6.5-, 17.3-, and 4.0-fold increase in severe/moderate illness, respectively), the extent of which was correlated with disease severity, and declined to basal levels at the convalescent phase (Figure 1). However, IL-1 and TNF-α were not detected at either phase in most of the patients. The plasma IFN-α level increased in most patients at the acute stage, but this increase was not proportional to the disease severity. The anti-inflammatory cytokine IL-10 was rarely detected.
Figure 1.
Plasma cytokine/chemokine levels of patients at the acute and convalescent phases of Middle East respiratory syndrome coronavirus infection. Interferon α was measured by enzyme-linked immunosorbent assay, and the others by cytometric bead arrays. Each dot represents an individual patient and the lines indicate paired samples from the same patient. Abbreviations: Conval, convalescent; IFN, interferon; IL, interleukin; IP, interferon gamma-induced protein; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T cell expressed and secreted; TNF, tumor necrosis factor.
Antibody Responses
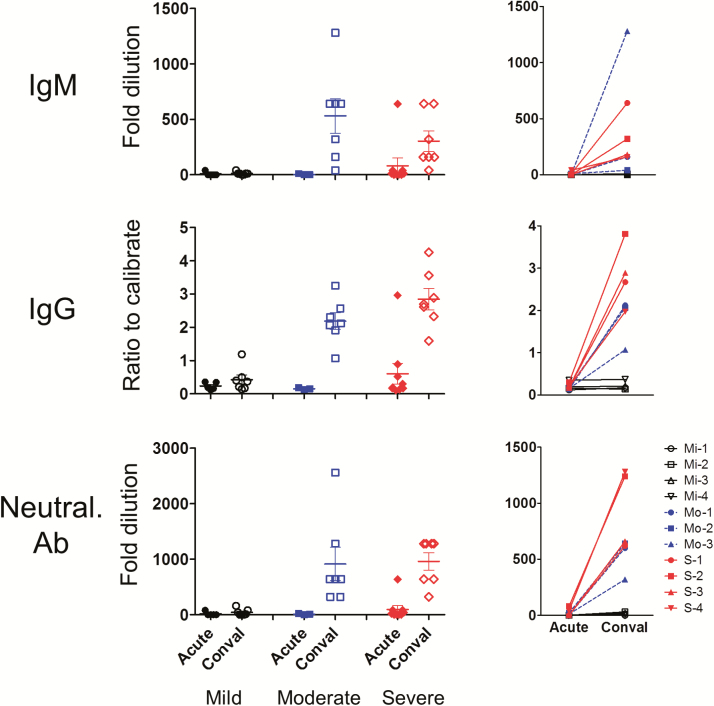
Anti–MERS-CoV Abs—including IgM, IgG, and neutralizing Abs—were not detected in patients except for a case of severe disease within the first 2 weeks of illness. In the convalescent phase, patients with severe and moderate illness developed a neutralizing Ab response, which was also seen with IgM and IgG. Overall, the more severe the illness, the greater the antibody response detected in patients in the convalescent phase; mild or asymptomatic patients rarely develop antibody responses (mean ± standard deviation fold dilution of neutralizing Ab in severe vs moderate vs mild: 960 ± 413.1 vs 914 ± 793.1 vs 41.4 ± 60.1, respectively) (P < .05; Figure 2).
Figure 2.
Middle East respiratory syndrome coronavirus (MERS-CoV)–specific antibody responses in patients at the acute and convalescent phases of infection. The magnitude of MERS-CoV–specific immunoglobulin M (IgM), immunoglobulin G (IgG), and neutralizing antibody responses was measured in patient plasma samples collected at the acute and convalescent phases of infection by immunofluorescence assay, enzyme-linked immunosorbent assay, and pseudotype retrovirus-based neutralization assay, respectively. Fold dilutions ≥10 for IgM and neutralizing Ab, and ratio to calibrate ≥1.1 for IgG were considered to be positive. Each dot shown in the graphs at left represents an individual patient and the lines in the graphs at right indicate paired samples from the same patient. Abbreviations: Conval, convalescent; Mi, mild; Mo, moderate; Neutral. Ab, neutralizing antibody; S, severe.
T-Cell Immune Responses to Viral Antigens
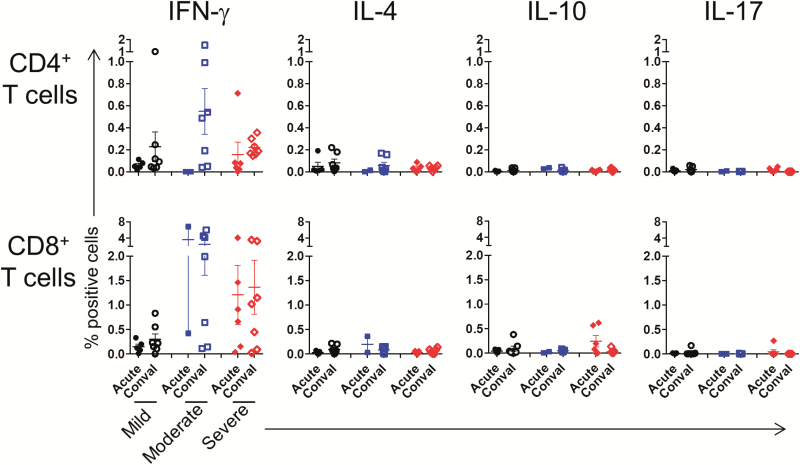
To assess T-cell responses to MERS-CoV, PBMCs of patients were stimulated with 3 pooled viral peptides and analyzed for intracellular cytokines via flow cytometry (Supplementary Figure 2). As shown for 1 representative patient and a healthy donor, virus-reactive cells were detected in T cells from patients, but not in those from healthy donors (Figure 3A). Details of IFN-γ–secreting T-cell frequencies relative to the days after virus exposure and symptom onset in individual patients are provided in Supplementary Figure 3. In summary, at the acute stage, virus-reactive CD4+ T cells—as determined by the secretion of IFN-γ, IL-2, or TNF-α upon antigenic stimulation—were detected in 2 of the 9 patients in the severe group, but not in the moderate and mild groups (Figure 3B and 3C). At the convalescent phase, these CD4+ T cells appeared in most (18/21) of the patients regardless of the severity of infection. Unlike CD4+ T cells, high frequencies (>0.3%) of CD8+ T cells secreting IFN-γ and/or TNF-α and expressing CD107a were seen in most (9/12) of the severe and moderate subjects (median for IFN-γ secretion, 0.94% [range, 0.32%–5.44%]) but not in mild cases at the acute stage of infection. By the convalescent phase, the frequency of these antigen-reactive CD8+ T cells had diminished in a portion (4/7) of the patients with severe or moderate infection (Figure 3B and 3C). T cells secreting a cytokine triplet of IFN-γ, IL-2, and TNF-α are considered more potent than those producing 1 or 2 cytokines [20], and were mostly identified only in CD4+ T cells at the convalescent phase (Figure 3C). We also addressed whether MERS-CoV–reactive T cells produced cytokines other than T-helper 1 (Th1) or type 1 cytotoxic T cell (Tc1) types. As expected, IFN-γ–secreting T cells were predominant, but IL-4, IL-10, or IL-17-secreting T cells were very rare in both CD4+ and CD8+ T-cell populations (Figure 4).
Figure 3.
T-cell responses to Middle East respiratory syndrome coronavirus (MERS-CoV) structural protein antigens in patients at the acute and convalescent phases of infection. Peripheral blood mononuclear cells from patients were stimulated with MERS-CoV structural protein-peptide pools, and virus-reactive T cells were examined by intracellular cytokine staining. A, Staining profiles of T cells from a representative patient (at the acute stage of infection) and a healthy donor. B, Frequency of interferon γ–producing T cells in response to 3 pools of viral peptides. C, Summary of T-cell responses producing T-helper 1– or type 1 cytotoxic T cell–type cytokines and molecules against all 3 peptide pools. Each dot represents an individual patient and the lines indicate paired samples from the same patient. Abbreviations: Conval, convalescent; IFN, interferon; IL, interleukin; MERS, Middle East respiratory syndrome; TNF, tumor necrosis factor.
Figure 4.
Frequency of various functional T-cell subsets responding to Middle East respiratory syndrome coronavirus (MERS-CoV) antigens in patients with MERS-CoV. Peripheral blood mononuclear cells from patients were stimulated with MERS-CoV structural protein-peptide pools, and the frequency of T cells secreting interferon γ, interleukin 4, interleukin 10, or interleukin 17 was measured by intracellular cytokine staining. Each dot represents an individual patient. Abbreviations: Conval, convalescent; IFN, interferon; IL, interleukin.
Next, we analyzed the reactivity of T cells to different viral proteins. While more CD8+ T cells reacted to S protein than to E/M/N proteins, especially at the acute phase of infection (median, 0.26% [interquartile range {IQR}, 0.03%–0.77%] vs 0.07% [IQR, 0.02%–0.33%]; P < .05), slightly more CD4+ T cells recognized E/M/N than S proteins at the convalescent phase (0.10% [IQR, 0.04%–0.25%] vs 0.09% [IQR, 0.02%–0.21%]; P = .057) (Figure 5).
Figure 5.
Reactivity of T cells to different viral proteins in individual patients. Lines indicate the paired frequencies of interferon γ–producing T cells responding to the S1 or S2 pool of peptides and the E/M/N peptide pool in individual patients. The 2-tailed Wilcoxon signed-rank test was used to compare the paired samples.
Finally, when we analyzed the relationship between serologic and T-cell responses in individual patients at the convalescent phase, there was a significant correlation between IgG titers and virus-reactive CD4+ or CD8+ T-cell frequencies. A significant correlation was also found between the frequencies of virus-reactive CD4+ and CD8+ T cells (Figure 6).
Figure 6.
Correlation between T cells and antibody responses. Plasma immunoglobulin G titers and the frequencies of interferon γ–producing CD4+ and CD8+ T cells in the same patients were plotted. Spearman rank correlation coefficient, the corresponding P value, and the line of best fit are shown. Abbreviation: IgG, immunoglobulin G.
DISCUSSION
In this study, we measured T-cell responses together with antibody and proinflammatory cytokine responses in patients at the early period of MERS. Our data revealed that an initial severity-proportional, proinflammatory cytokine/chemokine secretion is followed by antibody and T-cell responses in patients, which is a typical host response to many other acute viral infections. However, one peculiar finding was that extraordinarily high frequencies of MERS-CoV–reactive CD8+ Tc1-type T cells were observed in a large proportion of patients with severe and moderate illness at the acute stage prior to the detection of humoral and CD4+ T-cell responses. At the convalescent phase, the magnitude of the CD8+ T-cell response was not greatly augmented further, and actually contracted in some cases. On the basis of these data, we can infer that inefficient control of invading MERS-CoV brings about robust inflammatory and cytotoxic T lymphocyte (CTL) responses, which clear the invading virions and also destroy lung tissue yielding to pneumonia.
The association of a strong inflammatory response with a severe form of MERS-CoV infection has been revealed by histological and serologic findings in human and animal studies [5, 7, 8, 21, 22]. Our observation reinforces this association. Thus, the elevated serum levels of IL-6, IP-10, and MCP-1, which are associated with inflammatory process and recruitment of inflammatory cells, could reflect the ongoing inflammation at the infection site. In our study, 2 deceased patients who did not show any detectable T-cell responses had a very high level of plasma proinflammatory cytokines and chemokines at their acute phase of infection. Therefore, it is highly probable that the inflammatory reaction would damage diseased lung tissue in the absence of CTL responses in these 2 patients. Interestingly, the plasma level of RANTES was detected to be increased at the convalescent phase of infection irrespective of disease severity. We speculate that this late increase of RANTES might be associated with the secretion of this chemokine by activated virus-reactive T lymphocytes [23].
A few reports explored the role of T-cell responses in MERS-CoV infection using animal models. Examination of infected mice transiently expressing the human DPP-4 receptor revealed that T-cell responses are required for viral clearance [12]. Interestingly, Coleman et al reported that, using a mouse model in which hDPP4 is expressed under the endogenous mDPP4 promoter, CD8+ T cells may contribute to MERS-CoV–induced lung pathology [24]. The potential pathogenic contribution of strong CTL responses at the acute phase of infection has also been demonstrated in animal influenza virus infection [25]. Our data also suggest the pathogenic role of CD8+ T cells in human MERS-CoV infection. We posit 2 pathways for inducing a strong CD8+ T-cell response at the acute phase of MERS-CoV infection. Antigenic stimulation caused by inefficient removal of invading virus and/or a large inoculum of virus could induce a rapid proliferation and differentiation of naive CD8+ T cells in the absence of CD4+ T cells [26]. Otherwise, it could be due to heterologous immunity: memory CD8+ T cells—activated by prior infection with unrelated viruses—cross-reacting with MERS-CoV antigens [27]. Further studies will be needed to address these possibilities.
The spectrum of immune responses against MERS-CoV infection appears to be very similar to that against SARS-CoV infection. As reported in SARS [28], lymphodepletion occurs in patients with severe MERS at the acute phase of infection. Moreover, the magnitude of the innate immune response—represented by the serum levels of inflammatory cytokines and chemokines—and humoral immune response was revealed to increase proportionally with the disease severity in both SARS-CoV and MERS-CoV infection [29]. Our study further demonstrates that, similar to B-cell responses, T-cell responses against MERS-CoV infection are also elevated in severe/moderate cases compared with mild infections. While there is a lack of data on the kinetics of T-cell responses during SARS-CoV infection, numerous studies have investigated the memory T-cell responses in recovered SARS-CoV patients [15, 30–35]. Among them, a comprehensive study of T-cell responses against all the SARS-CoV proteins showed the dominance of CD8+ T-cell responses over CD4+ T cells [15], which is in line with our findings in MERS-CoV, though the observations were made at different time points. However, the major protein recognized by CD4+ T cells seems to be different between SARS-CoV and MERS-CoV. While CD4+ T-cell responses in SARS-CoV were clustered mainly in the spike protein [15], CD4+ T cells in patients with MERS-CoV responded slightly more to E/M/N rather than S protein. This difference is not likely due to the different time points at which CD4+ T-cell responses were analyzed because the same recognition pattern of memory CD4+ T cells was observed in MERS-CoV patients at 1 year postinfection (unpublished data).
According to a recent study conducted by Zhao et al, which examined T-cell responses in patients at 6 or 24 months post–MERS-CoV infection [9], virus-specific neutralizing antibodies and CD4+ T-cell responses correlated with severe disease. The same correlations were also observed at the early convalescent phase of the infection in our study. However, there is a different interpretation regarding CD8+ T-cell responses. Based on the finding that patients with mild or subclinical illness develop prominent virus-specific CD8+ T-cell responses, Zhao et al proposed that measurements of MERS-CoV-specific T-cell responses may be useful for making prognoses [9]. Our study, which used acute stage samples, suggests a poor prognosis if a virus-specific CD8+ T-cell response is detected at the acute phase of infection. Nevertheless, as the presence of virus-specific CD8+ T cells at the acute phase of infection was not shown in some patients with severe and moderate illness, it is not a reliable biomarker for poor outcomes.
Our patient cohort provided a unique opportunity to study immune responses in the early period of MERS-CoV infection. All of the patients were believed to have been exposed to MERS-CoV antigens for the first time in their lives. Thus, we assumed that the anti-MERS-CoV immune responses measured in this study would be a primary immune response. Moreover, the time course of antigen exposure and symptom onset was relatively well defined in these patients due to a sudden outbreak of MERS-CoV in South Korea. The limited number of patients recruited in this study, however, was an obstacle to reaching a conclusion. Another weakness of our study is the absence of viral load data. Consequently, we could not define the relationship between antigen dose and the magnitude of the cellular immune response. Nevertheless, our study provides valuable information about the cellular immune response against MERS-CoV infection in humans at the early period of infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by a grant from the Korean Healthcare Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI15C3227).
Acknowledgments. We thank Professor Nam-Hyuk Cho (Department of Microbiology, Seoul National University College of Medicine) for providing the MERS-CoV S protein–expressing lentiviral vector and 293T/DPP4 cells.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fehr AR, Channappanavar R, Perlman S. Middle East respiratory syndrome: emergence of a pathogenic human coronavirus. Annu Rev Med 2017; 68:387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet 2015; 386:995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park WB, Perera RA, Choe PG, et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis 2015; 21:2186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis 2016; 62:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faure E, Poissy J, Goffard A, et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside?PLoS One 2014; 9:e88716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WD, Mok CK, Chen ZL, et al. Characteristics of traveler with Middle East respiratory syndrome, China, 2015. Emerg Infect Dis 2015; 21:2278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim ES, Choe PG, Park WB, et al. Clinical progression and cytokine profiles of Middle East respiratory syndrome coronavirus infection. J Korean Med Sci 2016; 31:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 2016; 6:25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao J, Alshukairi AN, Baharoon SA, et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T cell responses. Sci Immunol 2017; 2:eaan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu P, Xu Y, Deng W, et al. Comparative pathology of rhesus macaque and common marmoset animal models with Middle East respiratory syndrome coronavirus. PLoS One 2017; 12:e0172093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niemeyer D, Zillinger T, Muth D, et al. Middle East respiratory syndrome coronavirus accessory protein 4a is a type I interferon antagonist. J Virol 2013; 87:12489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao J, Li K, Wohlford-Lenane C, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A 2014; 111:4970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao J, Zhao J, Mangalam AK, et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity 2016; 44:1379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baseler LJ, Falzarano D, Scott DP, et al. An acute immune response to Middle East respiratory syndrome coronavirus replication contributes to viral pathogenicity. Am J Pathol 2016; 186:630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol 2008; 181:5490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perera RA, Wang P, Gomaa MR, et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill 2013; 18 pii:20574. [DOI] [PubMed] [Google Scholar]
- 17. Kim Y, Cheon S, Min CK, et al. Spread of mutant Middle East respiratory syndrome coronavirus with reduced affinity to human CD26 during the South Korean outbreak. MBio 2016; 7:e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alsahafi AJ, Cheng AC. The epidemiology of Middle East respiratory syndrome coronavirus in the kingdom of Saudi Arabia, 2012–2015. Int J Infect Dis 2016; 45:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 2008; 8:247–58. [DOI] [PubMed] [Google Scholar]
- 21. Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med 2013; 19:1313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng DL, Al Hosani F, Keating MK, et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol 2016; 186:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wagner L, Yang OO, Garcia-Zepeda EA, et al. Beta-chemokines are released from HIV-1-specific cytolytic T-cell granules complexed to proteoglycans. Nature 1998; 391:908–11. [DOI] [PubMed] [Google Scholar]
- 24. Coleman CM, Sisk JM, Halasz G, et al. CD8+ T cells and macrophages regulate pathogenesis in a mouse model of Middle East respiratory syndrome. J Virol 2016; 91 pii:e01825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med 1998; 188:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu Z, Molloy MJ, Usherwood EJ. CD4(+) T-cell dependence of primary CD8(+) T-cell response against vaccinia virus depends upon route of infection and viral dose. Cell Mol Immunol 2016; 13:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med 1998; 188:1705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong RS, Wu A, To KF, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 2003; 326:1358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004; 136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H, Hou J, Jiang X, et al. Response of memory CD8+ T cells to severe acute respiratory syndrome (SARS) coronavirus in recovered SARS patients and healthy individuals. J Immunol 2005; 175:591–8. [DOI] [PubMed] [Google Scholar]
- 31. Peng H, Yang LT, Wang LY, et al. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology 2006; 351:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang L, Peng H, Zhu Z, et al. Persistent memory CD4+ and CD8+ T-cell responses in recovered severe acute respiratory syndrome (SARS) patients to SARS coronavirus M antigen. J Gen Virol 2007; 88:2740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan PK, Ma S, Ngai SM. Identification of T-cell epitopes of SARS-coronavirus for development of peptide-based vaccines and cellular immunity assessment methods. Hong Kong Med J 2011; 17:S26–30. [PubMed] [Google Scholar]
- 34. Tang F, Quan Y, Xin ZT, et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol 2011; 186:7264–8. [DOI] [PubMed] [Google Scholar]
- 35. Ng OW, Chia A, Tan AT, et al. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 2016; 34:2008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.