Abstract
Background
Hospital-based studies identify parechovirus (PeV), primarily PeV-A3, as an important cause of severe infections in young children. However, few community-based studies have been published and the true PeV infection burden is unknown. We investigated PeV epidemiology in healthy children participating in a community-based, longitudinal birth cohort study.
Methods
Australian children (n = 158) enrolled in the Observational Research in Childhood Infectious Diseases (ORChID) study were followed from birth until their second birthday. Weekly stool and nasal swabs and daily symptom diaries were collected. Swabs were tested for PeV by reverse-transcription polymerase chain reaction and genotypes determined by subgenomic sequencing. Incidence rate, infection characteristics, clinical associations, and virus codetections were investigated.
Results
PeV was detected in 1423 of 11 124 (12.8%) and 17 of 8100 (0.2%) stool and nasal swabs, respectively. Major genotypes among the 306 infection episodes identified were PeV-A1 (47.9%), PeV-A6 (20.1%), and PeV-A3 (18.3%). The incidence rate was 144 episodes (95% confidence interval, 128–160) per 100 child-years. First infections appeared at a median age of 8 (interquartile range, 6.0–11.7) months. Annual seasonal peaks changing from PeV-A1 to PeV-A3 were observed. Infection was positively associated with age ≥6 months, summer season, nonexclusive breastfeeding at age <3 months, and formal childcare attendance before age 12 months. Sole PeV infections were either asymptomatic (38.4%) or mild (32.7%), while codetection with other viruses in stool swabs was common (64.4%).
Conclusions
In contrast with hospital-based studies, this study showed that diverse and dynamically changing PeV genotypes circulate in the community causing mild or subclinical infections in children.
Parechovirus can cause severe illnesses in children. However, studies focus mainly on hospitalized populations. True disease burden in the community remains largely unknown. From our community-based cohort, we found diverse parechovirus genotypes in the community, causing mild or subclinical infections in children.
Keywords: parechovirus, clinical epidemiology, viral infection, healthy children, longitudinal study
Parechovirus A (PeV) is a small, nonenveloped, single-stranded RNA virus within the Picornaviridae family that replicates within the human gastrointestinal and respiratory tracts and is transmitted by the fecal-oral or respiratory routes [1, 2]. It is distributed worldwide and circulates year-round with annual peaks in summer and autumn months [2]. Of the 19 known genotypes, the most common are PeV types 1, 3, 4, and 6. Generally, PeV types 1, 4, and 6 are detected in children <2 years of age where they may be associated with mild respiratory or gastrointestinal illnesses [3]. In contrast, PeV-A3, first reported in 2004, has caused severe outbreaks of meningoencephalitis, myocarditis, and sepsis-like illnesses in infants aged <3–6 months [4–8]. These PeV-A3 outbreaks occur biennially, arising in odd and even years in the southern and northern hemispheres, respectively. Furthermore, a recent Australian study reported that almost 20% of infants hospitalized with a PeV-A3 infection had impaired neurodevelopment 12 months later [9].
Much of what is known of PeV epidemiology, including PeV-A3 outbreaks, relies upon hospital-based studies, and uncertainty exists over the actual disease burden within the community [3, 6]. Seroprevalence studies from Europe and Japan suggest that infection is common and occurs early in life with PeV-A1 antibodies present in 25%–30% of infants by age 1 year, increasing to 70%–90% by age 2–5 years, with high antibody levels maintained in older children and throughout adulthood [10, 11]. Recently, PeV-A3 seroepidemiology in Australia, the Netherlands, and the United States was shown to be similar [12]. The overall prevalence of neutralizing antibodies to PeV-A3 increased from nearly 33% in children aged 1–2 years, to 65% in those aged 5–9 years, peaking at 78% in adults aged 20–29 years, and then declining to 42% in older age groups [12]. The lower PeV-A3 seroprevalence in older adults may indicate waning childhood immunity or the relatively recent emergence and circulation of PeV-A3 globally.
Beyond seroprevalence, few studies describe the epidemiology and clinical characteristics of PeV infections in the community [13–18]. These have included secondary analyses of monthly stool samples collected from 3 months until 1–3 years of age in participants of 3 case-control studies from Norway and Finland. These studies were designed originally to determine the incidence of type 1 diabetes in genetically at-risk children [13–15]. By age 12 months, 43% of 108 Norwegian children recruited between 2001 and 2006 had at least 1 PeV detection, which increased to 86% by 2 years of age [13]. A similar pattern of PeV infection, but with lower incidence rates, was observed in 200 Finnish children enrolled between 1996 and 2007 where by age 12 months 22% had PeV detected in their stools on at least 1 occasion, which increased to 48% approaching their second birthday [15]. In these longitudinal cohort studies, PeV-A1 was the most commonly observed genotype (76%–93%) with PeV-A3 and PeV-A6 detected only occasionally [13–15]. Limited symptom and epidemiological risk data were reported by the 2 Norwegian studies, both of which found no association between PeV detection and respiratory or gastrointestinal symptoms [13, 14]. Other studies assessed upper airway samples for various viruses, including PeV, in healthy older children and adults, but either lacked sufficient numbers or clinical or sociodemographic data to determine risk factors for PeV infections or only sampled infrequently [16–18].
Longitudinal, community-based studies employing sensitive molecular diagnostic assays with regular and frequent sampling, irrespective of illness, are best suited to explore the true nature and disease burden of PeV infections in young children. We therefore aimed to describe the epidemiology of PeV in the first 2 years of life by the means of an unselected community-based birth cohort whose recruitment coincided with the first reported outbreak of severe PeV-A3 disease in Australia [5, 19], and to investigate the risk factors and symptoms associated with acquiring PeV in these young children.
METHODS
Study Design and Sample Collections
The Observational Research in Childhood Infectious Diseases (ORChID) project (ClinicalTrials.gov identifier NCT01304914) is a community-based, longitudinal, birth cohort study of acute respiratory infections (ARIs) and acute gastroenteritis (AGE) in unselected, healthy Australian children in their first 2 years of life [20–23]. Recruitment was progressive over 2 years; participants needed to be healthy, born at term (36–42 weeks), and without congenital or underlying chronic disorders. The Children’s Health Queensland, Royal Brisbane and Women’s Hospital, and The University of Queensland human research ethics committees approved the study.
At enrollment, baseline sociodemographic and health data were collected by parental interview. Parents maintained daily symptom diaries related to ARIs and AGE and a separate illness impact diary healthcare visits for these episodes. Diaries were returned monthly by mail. Telephone interviews were conducted to collect data on feeding and childcare attendance every 3 months. Parents collected from their child weekly anterior nasal swabs and diaper stool swabs from birth until age 2 years. Swabs were mailed to the laboratory where they were processed and stored at –80°C (Supplementary Methods).
Definitions
Table 1 lists definitions for PeV episodes and ARI and AGE symptoms [22, 23].
Table 1.
Definitions of Parechovirus A Episodes, Acute Respiratory Infection, and Acute Gastroenteritis
| Term | Definition |
|---|---|
| PeV episode | ≥1 consecutive swab(s) where PeV was detected. A new episode was determined when a different PeV genotype was detected or the same genotype was identified after ≥2 negative swabs or at least 30 d after the last positive swab [22]. |
| ARI symptoms | Presence of nasal congestion/discharge, dry or wet-sounding cough, wheezing, shortness of breath, or doctor-diagnosed otitis media or pneumonia [22] |
| AGE symptoms | ≥3 loose stools recorded during a 24-h period [23] |
| Symptomatic episode | ARI, AGE, or fever only symptoms present within 7 d before or after the first positive PeV detection [22, 23]. |
Abbreviations: AGE, acute gastroenteritis; ARI, acute respiratory infection; PeV, parechovirus A.
Parechovirus Detection and Genotyping
Nucleic acid was extracted from the swabs using previously described protocols implemented with quality control [22, 23] (Supplementary Methods). PeV in extracts were detected using a previously published reverse-transcription polymerase chain reaction (RT-PCR) assay (Supplementary Table 1) [24]. Stool swabs were screened for 8 additional enteric viruses (Supplementary Table 2) and nasal swabs for 17 respiratory viruses [22] using published assays [20]. A selection of specimens positive for PeV within the same episode (Figure 1 and Supplementary Methods) were genotyped using published methods targeting the VP3/1 and VP1 regions (Supplementary Methods) [25–27]. One representative sequence per PeV episode was selected for the phylogenetic analyses.
Figure 1.
Submission of swabs, symptom diaries, human parechovirus detections, detection episodes, symptoms, and genotyping in the Observational Research in Childhood Infectious Diseases birth cohort. Abbreviations: AGE, acute gastroenteritis; ARI, acute respiratory infection; Ct, cycle threshold; ERV3, endogenous retrovirus 3; PeV-A, parechovirus A.
Statistical Analysis
Incidence rates with 95% confidence intervals (CIs) were assessed using Poisson regression, including the natural logarithm of the number of swabs returned as an offset. Each swab returned was defined as representing 1 week of study time. The time to first detection was calculated using life tables. Infants were censored at either the date of the last swab submitted if the next swab was not returned for >42 days, or at 730 days, whichever came first. The association between potential risk factors and PeV detection was examined using mixed-effects logistic regression with the child entered as a random effect to account for repeated measurements. Risk factors considered were age (categorized as 0–<3 months, 3–<6 months, 6–<12 months, 12–24 months), sex, exclusive breastfeeding, older siblings in the household, childcare attendance (none/informal/formal), season of PeV detection, and season of birth. Given the strong association between breastfeeding and childcare with age, both breastfeeding-by-age and childcare-by-age interaction terms were included in the models. All variables (except sex) were analyzed as time-varying variables. Univariable and multivariable analyses were conducted. In multivariable analyses, all variables were included in the regression models.
A linear regression model was used to investigate the association between symptoms and PeV cycle threshold (Ct) values, with the latter being inversely proportional to the amplified target nucleic acid in the sample, representing a semiquantitative estimate of viral load. Ct values from the first PeV detection in each episode were analyzed. Data were analyzed using Stata version 15.1 software (StataCorp, College Station, Texas).
RESULTS
Cohort Characteristics
Of 165 infants enrolled, 7 were excluded: 1 due to preterm birth (born <36 weeks), and 6 due to failure to provide any swabs. The remaining 158 children (75 male) provided 11 208 stool and 11 192 nasal swabs, of which 11 124 and 8100, respectively, met the inclusion criteria (Figure 1). Symptom diaries were submitted for 154 children, constituting 88 811 child-days of observation. Cohort characteristics are summarized in Supplementary Table 3.
Parechovirus A Detection and Genotyping
PeVs were detected in 1423 (12.8%) stool and 17 (0.2%) nasal weekly swabs (Figure 1). Based on stool swab results, 306 distinct PeV episodes were identified during the study, including 16 (5.2%) episodes that coincided with the 17 positive nasal swab detections.
Of the 295 of 306 (96.4%) episodes meeting genotyping inclusion criteria (Figure 1), 284 (96.2%) were typed successfully (Supplementary Table 4). PeV-A1 episodes predominated (n = 136 [47.9%]), followed by PeV-A6 (n = 57 [20.1%]) and PeV-A3 (n = 52 [18.3%]). All 17 paired positive stool and nasal genotypes matched (PeV-A, 1 n = 10; PeV-A3, n = 6; PeV-A6, n = 1). Twenty-six occurrences of different genotypes were detected in consecutive PeV-positive swabs (Supplementary Figure 1).
Phylogenetic analysis confirmed the genotyping results and revealed the high diversity of multiple PeV genotypes co-circulating in the cohort (Figure 2 and Supplementary Figure 2). Among PeV-A3 detected in the cohort, samples collected during the same period from different children formed unique clusters in the VP3/1 region, including alignment with local strains from hospitalized infants with sepsis during the 2013–2014 outbreak (Supplementary Figure 3) [28, 29].
Figure 2.
Phylogenetic analysis of VP3/1 sequences based on MUSCLE alignment of 255 sequences from the Observational Research in Childhood Infectious Diseases (ORChID) cohort with 58 GenBank reference sequences. ORChID sequences were depicted as filled triangles (▲) and GenBank reference sequences as circles (◯). The evolutionary relationships of taxa were inferred using the neighbor-joining method with bootstrap tests of 1000 replicates; only values ≥70% are shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distance method and are in the units of the number of base differences per site. All ambiguous positions were removed for each sequence pair. A total of 289 positions were in the final dataset. Evolutionary analyses were conducted in MEGA6. Abbreviations: MUSCLE, MUltiple Sequence Comparison by Log- Expectation; ORChID, Observational Research in Childhood Infectious Diseases; PeV-A, parechovirus A.
Parechovirus A Infection Characteristics
The overall PeV incidence rate was 144 (95%CI, 128–161) episodes per 100 child-years. Incidence rates for the 3 major types (PeV-A types 1, 3, and 6) were 64 (95% CI, 54–75), 24 (95% CI, 19–32), and 27 (95% CI, 21–35) episodes per 100 child-years, respectively. After the first 3 months of life, the incidence of new PeV infections rose steadily until age 8 months before plateauing and then gradually declining in the second year (Supplementary Figure 4). The maximum episode number detected was 6 in 2 children (Supplementary Results and Supplementary Figure 5). The mean individual episode duration was 4.8 (standard deviation, 3.5) weeks with a maximum shedding duration of 16 weeks. In the 26 occurrences with different PeV genotype detections from sequential swabs, the maximum duration of combined genotype shedding was 23 weeks (Supplementary Figure 1).
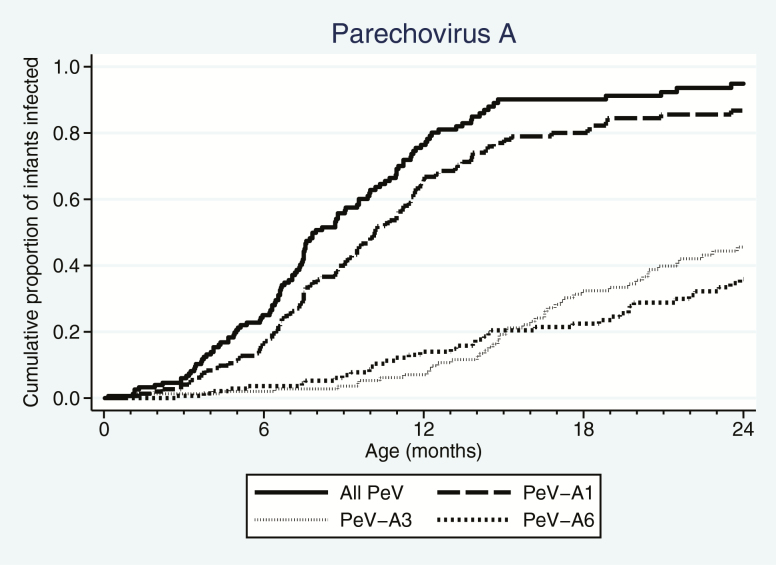
The first PeV infection appeared at a median of 8.0 months of age (interquartile range [IQR], 6.0–11.7; Supplementary Table 5) with the earliest detection at day 5 of life in a stool swab. By 12 months, 76.4% had experienced at least 1 PeV infection, increasing to 94.9% by their second birthday. First infections by PeV-A1 occurred typically much earlier than for PeV-A3 and PeV-A6 (Figure 3).
Figure 3.
Timing of first parechovirus A (PeV) infections by any PeVs and genotypes 1, 3, and 6. PeV first infections occurred in all age groups between 0 and 24 months (solid line), but 76.4% of the cohort had acquired their first infection before 12 months of age.
PeVs were detected throughout each year, but peaked in summer and early autumn (December through March; Supplementary Figure 6). Seasonality of the 3 main genotypes varied in magnitude between years, with dominant genotypes alternating or sharing seasonal peaks (Figure 4). Of particular note was PeV-A3, whose magnitude rose sharply and became the dominant genotype during the spring/summer months of 2013–2014 (Figure 4).
Figure 4.
Seasonality of total episodes and episodes per enrolled child each month of all parechovirus A (PeV-A) genotypes as a function of enrolled subjects.
Characteristics independently associated with PeV infections included increasing age (particularly >6 months), summer season, not exclusively breastfeeding at a younger age (0–3 months), and attending formal childcare at a younger age (<12 months) (Supplementary Table 6). Sex, season of birth, and presence of older siblings in household were not associated with PeV infections.
Clinical Association With Symptoms and Viral Codetections
Of 306 discrete PeV episodes (Figure 1), 138 (45.1%) were asymptomatic, 120 had ARI symptoms (39.2%), 7 had AGE (2.3%), 17 had both (5.6%), 6 had an undifferentiated febrile illness (2.0%), and 18 lacked symptom data (5.9%). When PeV was the sole detected virus in stools, 38.4% of episodes were asymptomatic (Table 2), whereas if respiratory viruses in nasal swabs were also absent, the proportion without symptoms increased to 65.9% (Table 3). Similar findings were observed when PeV-A3 episodes were considered alone (Supplementary Table 7). Of the 96 of 150 symptomatic episodes with recorded burden information, 63 (65.6%) received a primary care consultation, but none needed hospitalization. Overall, no association was observed between PeV viral load and symptomatic episodes (symptomatic vs asymptomatic mean Ct difference, 0.6 [95% CI, –0.5 to 1.6]). This result remained the same after censoring all initial PeV-positive swabs codetected with other viruses (Supplementary Table 8).
Table 2.
Frequency of Parechovirus A Detection, Codetection of Other Viruses in Stool Swabs, and Associated Symptomatic Episodes
| Codetectionsa (n = 197 [64.4%]) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathogenic Enteric Viruses, No. (%)b | Other Commonly Shed Enteric Viruses, No. (%) | ||||||||
| PeV Detection Episodes | PeV Sole Detection | Sapovirusc | Human Norovirus 1 & 2 | Human Rotavirusd | Human Classical Astrovirus | Adenovirus 40 & 41 | Adenovirus–Generic | Human Bocavirus 1 | Enterovirus |
| Total (N = 306) | 109 (35.6) | 36 (11.8) | 34 (11.1) | 23 (7.5) | 14 (4.6) | 7 (2.3) | 103 (33.7) | 62 (20.3) | 48 (15.7) |
| Type of infection | |||||||||
| Asymptomatic (n = 138) | 53 (38.4) | 11 (8.0) | 15 (10.9) | 18 (13.0) | 7 (4.1) | 1 (0.7) | 40 (29.0) | 25 (18.1) | 11 (8.0) |
| Symptomatic (n = 150) | 49 (32.7) | 22 (14.7) | 17 (11.3) | 5 (3.3) | 7 (4.7) | 5 (3.3) | 56 (37.3) | 35 (23.3) | 34 (22.7) |
| ARI (n = 120) | 40 (33.3) | 18 (15.0) | 13 (10.8) | 5 (4.2) | 6 (5.0) | 2 (1.7) | 46 (38.3) | 26 (21.7) | 25 (20.8) |
| ARI + AGE (n = 17) | 6 (35.3) | 3 (17.6) | 1 (5.9) | 0 (0.0) | 1 (5.9) | 2 (11.8) | 7 (41.2) | 3 (17.6) | 4 (23.5) |
| AGE (n = 7) | 1 (14.3) | 1 (14.3) | 2 (28.6) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 3 (42.9) | 4 (57.1) | 3 (42.9) |
| Fever alone (n = 6) | 2 (33.3) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 2 (33.3) |
| No symptom datae (n = 18) | 7 (38.9) | 3 (16.7) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 7 (38.9) | 2 (11.1) | 3 (16.7) |
Abbreviations: AGE, acute gastroenteritis; ARI, acute respiratory infection; PeV, parechovirus A.
aAn episode can be codetected with ≥1 other virus(es).
bEpisode numbers (row percentage) for each type of infection that was detected with each enteric virus.
cSapovirus detection included genogroups I, II, IV, and V.
dNineteen of 23 (82.6%) human rotavirus–positive swabs were from live attenuated multivalent human-bovine reassortant vaccine strains (RotaTeq, Merck and Co) [23].
eSymptom diaries not recorded by parents at the time a PeV swab tested positive.
Table 3.
Frequency of Parechovirus A Detection, Codetection of Other Viruses in Nasal Swabs, and Associated Symptomatic Episodes
| Respiratory Virus Codetections in Associated Nasal Swabsa (155 [50.7%]) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RNA Virusesb, No. (%)c | DNA Viruses, No. (%) | ||||||||
| PeV Detection Episodes | PeV Sole Detection | Human Rhinovirusd | Human CoV (OC43, NL63, HKU1, & 229E) | RSV A & B | Parainfluenza Virus 1–3 | Influenza Viruses A & B | Human Polyomavirus WU and KI | Human Bocavirus 1 | Adenovirus |
| Total (N = 306) | 151 (49.3) | 124 (40.5) | 14 (4.6) | 10 (3.3) | 6 (2.0) | 1 (0.3) | 15 (4.9) | 10 (3.3) | 4 (1.3) |
| Type of infection | |||||||||
| Asymptomatic (138) | 91 (65.9) | 37 (26.8) | 2 (1.4) | 4 (2.9) | 2 (1.4) | 0 (0.0) | 4 (2.9) | 2 (1.4) | 1 (0.7) |
| Symptomatic (150) | 51 (34.0) | 78 (52.0) | 12 (8.0) | 6 (4.0) | 4 (2.7) | 1 (0.7) | 11 (7.3) | 8 (5.3) | 3 (2.0) |
| ARI (120) | 38 (31.7) | 64 (53.3) | 9 (7.5) | 6 (5.0) | 4 (3.3) | 1 (0.8) | 10 (8.3) | 6 (5.0) | 2 (1.7) |
| ARI + AGE (17) | 3 (17.6) | 12 (70.6) | 3 (17.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.9) | 1 (5.9) | 1 (5.9) |
| AGE (7) | 6 (85.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Fever alone (6) | 4 (66.7) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No symptom datae (n = 18) | 9 (50.0) | 9 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: AGE, acute gastroenteritis; ARI, acute respiratory infection; CoV, coronavirus; PeV, parechovirus A; RSV, respiratory syncytial virus.
aAn episode can be co-detected with ≥1 other virus(es).
bHuman metapneumovirus was not codetected with PeV.
cEpisode numbers (row percentage) in each type of infection that was detected with each enteric virus.
dHuman rhinovirus detection included all 3 species (RV-A, RV-B, and RV-C).
eSymptom diaries not recorded by parents at the time a PeV swab tested positive.
Overall, in 197 (64.4%) PeV-associated episodes, at least 1 other virus was detected in stool swabs collected within 7 days of the onset of the PeV infection (Table 2). Indeed, shedding of non-PeV viruses was also relatively common in the stools of ORChID subjects (Supplementary Table 9). In 137 PeV-associated ARI episodes, 96 (70.1%) had at least 1 respiratory virus detected in nasal swabs during the 7-day window (Figure 1 and Table 3); these were primarily rhinoviruses, which were present in 76 of 137 (55.5%) symptomatic ARI episodes.
DISCUSSION
The ORChID study provides high-resolution representation of PeV infection in the first 2 years of life in healthy Australian children. We observed frequent PeV detections in stools, but not in nasal swabs, from these young children. Diverse PeV genotypes circulated in the community, with distinct seasonal peaks and genotype transitioning during summer and early autumn. Prolonged PeV shedding was from sequential infections with different genotypes rather than single infections, suggesting limited heterotypic protection. A sharp increase in PeV-A3 detections in cohort children coincided with a PeV-A3–related sepsis outbreak in Australian infants where both sets of strains clustered phylogenetically [19]. However, infections in cohort children were either community-managed or asymptomatic in nature with symptom association confounded by high viral codetection rates.
Primary PeV infection occurred early in ORChID subjects with >75% affected by their first birthday, compared with 22% [15] and 43% [13] of children with PeV detections by the same age in 2 other community-based studies. However, neither study recruited before 3 months of age, and both sampled less frequently (monthly) and could sequence fewer (30%–73%) positive samples, meaning short or sequential episodes may have been missed [13, 15]. By age 24 months, 94.9% of ORChID children had at least 1 PeV infection, agreeing with the Norwegian (86%), but not Finnish (48%) studies [10, 13, 15], and aligning with seroprevalence surveys [10, 11, 30]. In ORChID, primary PeV-A1 detections occurred earlier than PeV-A3, agreeing with seroprevalence findings [10, 11], but conflicting with RT-PCR–based studies [11, 31]. However, the RT-PCR studies relied upon clinical samples, which may bias the PeV detection ages as PeV-A3 is associated primarily with disease before age 3 months [2, 3, 5, 7, 11].
Unlike stools, PeV was detected rarely in nasal swabs (0.2%) and was always associated with a PeV-positive stool of the same genotype, supporting fecal-oral as one transmission route. Previous community-based studies reported higher PeV detection rates (4%–9%) in respiratory samples from asymptomatic subjects [16, 18], possibly resulting from different sampling techniques (gargle and nasopharyngeal swabs). In contrast, infants hospitalized in Brisbane with PeV-A3–associated sepsis had high and comparable positive nasopharyngeal swab (86.2%) and stool (88.9%) detection rates [19]. Given the asymptomatic or mild nature of cases in our study, this observed discrepancy with hospitalized infant PeV detection rates is likely a reflection of more severe systemic disease.
Across the study, PeV-A3 was detected infrequently until the 2013–2014 spring/summer when it predominated. This coincided with the outbreak of PeV-A3–associated sepsis in Australian infants [5, 19], and aligns with the predicted timing of a genomic recombination event leading to a more virulent phenotype [28]. Nevertheless, in our cohort PeV-A3 was not associated with severe symptoms, even when detected during the outbreak period or within the high-risk first 3 months of life. Case-control studies [32] and the sharp increase in PeV infections after age 3 months in our cohort suggest maternal antibody protection against PeV-related disease early in life. Thus, emergence of a novel recombinant PeV-A3 strain and declining PeV-A3 neutralizing antibodies in women of childbearing age at a population level may contribute to the biennial outbreaks in Australian infants [12, 32].
PeV infection risk increased during summer, in agreement with some [2, 3, 7, 11, 15], but not all studies as late autumn peaks are also reported [6, 11, 13, 15]. Presence of older household siblings was not associated with PeV or PeV-A3 infections within ORChID, a finding contrasting with hospital-based studies focusing mainly on PeV-A3 cases [33, 34]. It however, agrees with the Norwegian community-based study where a sibling age gap >2 years increased the likelihood of infection, possibly originating from similar-aged peers attending kindergarten [13].
Almost half the sole PeV episodes within ORChID were asymptomatic, while virus codetection was common in the remaining symptomatic episodes, primarily from rhinovirus-associated ARIs, but also other enteric viruses. However, we may have underestimated the etiologic role of PeV in ARIs as rhinoviruses themselves have also been shown to be detected frequently in asymptomatic cases from the same cohort [22]. Nevertheless, these results highlight the overall mild nature of PeV infections within the community with none of the participants hospitalized, including infants infected with PeV-A3.
The strength of our study lies in the systematic, high-frequency sample and symptom data collection from an unselected community-based birth cohort, a combination not previously reported in PeV epidemiology studies. Weekly samples allowed us to observe infections <4 weeks’ duration (45.4% of all episodes) that would not be captured by sampling less frequently [13, 15]. In the Finnish study, 8 (10.3%) children had secondary episodes, with only 1 genotyped [15]. This contrasts with our findings where 90.7% of subsequent infections were typed successfully allowing multiple samples within the same month being available for sequencing and potentially allowing identification of sequential infections.
However, several limitations should be considered. First, the ORChID study was conducted in an urban, subtropical setting and most enrolled families were socioeconomically advantaged with higher rates of early age childcare attendance [22]. While the findings are valid, they may not be entirely generalizable to other populations. Second, the study relied upon parents diligently recording symptoms and collecting samples. Although completion rates were excellent for such a demanding study, not all diaries and swabs were returned. Finally, the 2 genotyping regions used in this study are highly conserved within the genome and may not reflect the true genetic diversity shown by the PeV-A3 recombinant variant associated with sepsis [28]. The sharp increase in ORChID PeV-A3 cases observed during the first PeV-A3 outbreak [19] suggests this recombinant variant was circulating within the community.
In summary, ORChID extends previous limited seroprevalence and community-based surveys by confirming that PeV infections from multiple genotypes are common during the first 2 years of life, with virus shedding primarily through the fecal route. Prolonged PeV shedding is from sequential infections with different genotypes rather than a single virus episode. Annual outbreaks occurred in the summer/autumn months with the severe disease–associated PeV-A3 succeeding PeV-A1 as the predominant strain in the 2013–2014 season, but it and other PeV infections were typically asymptomatic or associated with mild ARI or AGE symptoms. Despite participants being predominantly from advantaged families, which may limit the extrapolation of findings to lower socioeconomic settings, these observations illustrate the value of unselected, high-resolution longitudinal community-based cohort studies helping to identify the overall epidemiology, risk factors, and clinical features associated with infection rather than focusing exclusively upon the few cases with severe disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study concept and design: K. G., S. B. L., R. S. W., S. B., C. Y. W. Analysis and interpretation of data: C. Y. W., S. B., R. S. W., K. G. Laboratory analysis: C. Y. W., L. M., S. T., R. D. Statistical analysis: C. Y. W., R. S. W. Manuscript drafting: C. Y. W., K. G., S. B., R. S. W., S. B. L. All authors reviewed and approved the manuscript.
Acknowledgments. The authors acknowledge the generosity of the study families who participated in the study; the efforts of the recruitment nurses and volunteer staff for administrative assistance; and Minda Sarna for her assistance in data analyses.
Financial support. This work was supported by the Australian National Health and Medical Research Council (grant number GNT615700) and Children’s Hospital Foundation Queensland (CHFQ; grant number 5006). S. T. was supported by a CHFQ Sakzewski Translational Research grant (number 10416). S. B. L. is the recipient of a National Health and Medical Research Council Early Career Fellowship and a CHFQ Midcareer Fellowship.
Potential conflicts of interest. All authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sharp J, Bell J, Harrison CJ, Nix WA, Oberste MS, Selvarangan R. Human parechovirus in respiratory specimens from children in Kansas City, Missouri. J Clin Microbiol 2012; 50:4111–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Esposito S, Rahamat-Langendoen J, Ascolese B, Senatore L, Castellazzi L, Niesters HG. Pediatric parechovirus infections. J Clin Virol 2014; 60:84–9. [DOI] [PubMed] [Google Scholar]
- 3. Kadambari S, Harvala H, Simmonds P, Pollard AJ, Sadarangani M. Strategies to improve detection and management of human parechovirus infection in young infants. Lancet Infect Dis 2019; 19:e51–8. [DOI] [PubMed] [Google Scholar]
- 4. Harvala H, Robertson I, Chieochansin T, McWilliam Leitch EC, Templeton K, Simmonds P. Specific association of human parechovirus type 3 with sepsis and fever in young infants, as identified by direct typing of cerebrospinal fluid samples. J Infect Dis 2009; 199:1753–60. [DOI] [PubMed] [Google Scholar]
- 5. Cumming G, Khatami A, McMullan BJ, et al. Parechovirus genotype 3 outbreak among infants, New South Wales, Australia, 2013–2014. Emerg Infect Dis 2015; 21:1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olijve L, Jennings L, Walls T. Human parechovirus: an increasingly recognized cause of sepsis-like illness in young infants. Clin Microbiol Rev 2018; 31:e00047–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreras Antolin L, Kadambari S, Braccio S, et al. Increased detection of human parechovirus infection in infants in England during 2016: epidemiology and clinical characteristics. Arch Dis Child 2018; 103:1061–6. [DOI] [PubMed] [Google Scholar]
- 8. Midgley CM, Jackson MA, Selvarangan R, et al. Severe parechovirus 3 infections in young infants—Kansas and Missouri, 2014. J Pediatric Infect Dis Soc 2018; 7:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Britton PN, Khandaker G, Khatami A, et al. High prevalence of developmental concern amongst infants at 12 months following hospitalised parechovirus infection. J Paediatr Child Health 2018; 54:289–95. [DOI] [PubMed] [Google Scholar]
- 10. Westerhuis B, Kolehmainen P, Benschop K, et al. Human parechovirus seroprevalence in Finland and the Netherlands. J Clin Virol 2013; 58:211–5. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe K, Hirokawa C, Tazawa T. Seropositivity and epidemiology of human parechovirus types 1, 3, and 6 in Japan. Epidemiol Infect 2016; 144:3451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karelehto E, Brouwer L, Benschop K, et al. Seroepidemiology of parechovirus A3 neutralizing antibodies, Australia, the Netherlands, and United States. Emerg Infect Dis 2019; 25:148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tapia G, Cinek O, Witsø E, et al. Longitudinal observation of parechovirus in stool samples from Norwegian infants. J Med Virol 2008; 80:1835–42. [DOI] [PubMed] [Google Scholar]
- 14. Tapia G, Cinek O, Rasmussen T, Grinde B, Stene LC, Rønningen KS. Longitudinal study of parechovirus infection in infancy and risk of repeated positivity for multiple islet autoantibodies: the MIDIA study. Pediatr Diabetes 2011; 12:58–62. [DOI] [PubMed] [Google Scholar]
- 15. Kolehmainen P, Oikarinen S, Koskiniemi M, et al. Human parechoviruses are frequently detected in stool of healthy Finnish children. J Clin Virol 2012; 54:156–61. [DOI] [PubMed] [Google Scholar]
- 16. Morikawa S, Hiroi S, Kase T. Detection of respiratory viruses in gargle specimens of healthy children. J Clin Virol 2015; 64:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cristanziano VD, Bottcher S, Diedrich S, et al. Detection and characterization of enteroviruses and parechoviruses in healthy people living in the south of Cote d’Ivoire. J Clin Virol 2015; 71:40–3. [DOI] [PubMed] [Google Scholar]
- 18. Moe N, Pedersen B, Nordbø SA, et al. Respiratory virus detection and clinical diagnosis in children attending day care. PLoS One 2016; 11:e0159196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joseph L, May M, Thomas M, et al. Human parechovirus 3 in infants: expanding our knowledge of adverse outcomes. Pediatr Infect Dis J 2019; 38:1–5. [DOI] [PubMed] [Google Scholar]
- 20. Lambert SB, Ware RS, Cook AL, et al. Observational research in childhood infectious diseases (ORChID): a dynamic birth cohort study. BMJ Open 2012; 2:e002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarna M, Ware RS, Sloots TP, Nissen MD, Grimwood K, Lambert SB. The burden of community-managed acute respiratory infections in the first 2-years of life. Pediatr Pulmonol 2016; 51:1336–46. [DOI] [PubMed] [Google Scholar]
- 22. Sarna M, Lambert SB, Sloots TP, et al. Viruses causing lower respiratory symptoms in young children: findings from the ORChID birth cohort. Thorax 2018; 73:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ye S, Whiley DM, Ware RS, Kirkwood CD, Lambert SB, Grimwood K. Multivalent rotavirus vaccine and wild-type rotavirus strain shedding in Australian infants: a birth cohort study. Clin Infect Dis 2018; 66:1411–8. [DOI] [PubMed] [Google Scholar]
- 24. Benschop K, Molenkamp R, van der Ham A, Wolthers K, Beld M. Rapid detection of human parechoviruses in clinical samples by real-time PCR. J Clin Virol 2008; 41:69–74. [DOI] [PubMed] [Google Scholar]
- 25. Harvala H, Robertson I, McWilliam Leitch EC, et al. Epidemiology and clinical associations of human parechovirus respiratory infections. J Clin Microbiol 2008; 46:3446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pham NT, Trinh QD, Khamrin P, et al. Diversity of human parechoviruses isolated from stool samples collected from Thai children with acute gastroenteritis. J Clin Microbiol 2010; 48:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benschop K, Thomas X, Serpenti C, Molenkamp R, Wolthers K. High prevalence of human parechovirus (HPeV) genotypes in the Amsterdam region and identification of specific HPeV variants by direct genotyping of stool samples. J Clin Microbiol 2008; 46:3965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexandersen S, Nelson TM, Hodge J, Druce J. Evolutionary and network analysis of virus sequences from infants infected with an Australian recombinant strain of human parechovirus type 3. Sci Rep 2017; 7:3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McNeale D, Wang CYT, Arden KE, Mackay IM. HPeV-3 predominated among parechovirus A positive infants during an outbreak in 2013–2014 in Queensland, Australia. J Clin Virol 2018; 98:28–32. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka S, Aoki Y, Matoba Y, et al. Seroepidemiology of human parechovirus types 1, 3, and 6 in Yamagata, Japan, in 2014. Microbiol Immunol 2016; 60:854–8. [DOI] [PubMed] [Google Scholar]
- 31. Nielsen NM, Midgley SE, Nielsen AC, Christiansen CB, Fischer TK. Severe human parechovirus infections in infants and the role of older siblings. Am J Epidemiol 2016; 183:664–70. [DOI] [PubMed] [Google Scholar]
- 32. Karelehto E, Wildenbeest JG, Benschop KSM, et al. Human parechovirus 1, 3 and 4 neutralizing antibodies in Dutch mothers and infants and their role in protection against disease. Pediatr Infect Dis J 2018; 37:1304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aizawa Y, Yamanaka T, Watanabe K, Oishi T, Saitoh A. Asymptomatic children might transmit human parechovirus type 3 to neonates and young infants. J Clin Virol 2015; 70:105–8. [DOI] [PubMed] [Google Scholar]
- 34. Izumita R, Deuchi K, Aizawa Y, et al. Intrafamilial transmission of parechovirus A and enteroviruses in neonates and young infants. J Pediatric Infect Dis Soc 2018. doi:10.1093/jpids/piy079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.