Abstract
Background
Before kidney transplantation, donors and recipients are routinely screened for viral pathogens using specific tests. Little is known about unrecognized viruses of the urinary tract that potentially result in transmission. Using an open metagenomic approach, we aimed to comprehensively assess virus transmission in living-donor kidney transplantation.
Methods
Living kidney donors and their corresponding recipients were enrolled at the time of transplantation. Follow-up study visits for recipients were scheduled 4–6 weeks and 1 year thereafter. At each visit, plasma and urine samples were collected and transplant recipients were evaluated for signs of infection or other transplant-related complications. For metagenomic analysis, samples were enriched for viruses, amplified by anchored random polymerase chain reaction (PCR), and sequenced using high-throughput metagenomic sequencing. Viruses detected by sequencing were confirmed using real-time PCR.
Results
We analyzed a total of 30 living kidney donor and recipient pairs, with a follow-up of at least 1 year. In addition to viruses commonly detected during routine post-transplant virus monitoring, metagenomic sequencing detected JC polyomavirus (JCPyV) in the urine of 7 donors and their corresponding recipients. Phylogenetic analysis confirmed infection with the donor strain in 6 cases, suggesting transmission from the transplant donor to the recipient, despite recipient seropositivity for JCPyV at the time of transplantation.
Conclusions
Metagenomic sequencing identified frequent transmission of JCPyV from kidney transplant donors to recipients. Considering the high incidence rate, future studies within larger cohorts are needed to define the relevance of JCPyV infection and the donor’s virome for transplant outcomes.
Keywords: kidney transplantation, metagenomic sequencing, virus infection, JC polyomavirus
Kidney transplantation is inevitably associated with the transmission of viral and microbial pathogens. Using metagenomic virome sequencing, we revealed JC polyomavirus (JCPyV) transmission in 20% of cases, despite seropositivity. The relevance of JCPyV infection in kidney transplantation demands further attention.
Donor-derived infections after solid organ transplantation range from rare to very common, and from life-threatening to subclinical [1]. Recommendations for pre-transplant screening of organ recipients and donors for latent or active viral infections have been issued and are included in routine pre-transplant care [2]. This screening aims to identify donors with infections that would disqualify them for donation or require prophylactic strategies in the recipient. The most common example for the latter is cytomegalovirus (CMV), where transmission is considered an acceptable risk, if prevention strategies are in place. BK polyomavirus (BKPyV) has been associated with post-transplant complications causing polyomavirus-associated nephropathy (PyVAN), which increases the risk of allograft failure in 1–15% of transplant recipients [3, 4].
Besides these established viral infections, the role of additional pathogens in the donor’s virome is understudied. This is surprising, as there are ample examples that the transmission of pathogenic viruses, albeit commonly rare, can occur. Prominent examples in renal transplantation are West Nile virus [5], lymphocytic choriomeningitis virus [6], human gammaherpesvirus 8 [7], and rabies lyssavirus [8]. Analyses of the virome thus far have focused solely on the kidney transplant recipients [9, 10]. Urinary tract infections of kidney transplant recipients with BKPyV and JC polyomavirus (JCPyV) were associated with complications occurring after transplantation [9, 11–13]. Whether these viruses were reactivated from latency under immunosuppressive therapy or were transferred from donor to recipient has not been resolved. In 1 study, identical BKPyV genome sequences in donors and recipients indicated transmission via the graft [14].
The growing uncertainty over which viruses need to be monitored and the relevance of the donor as a significant, but rarely studied, source of transmission prompted us to conduct a prospective, systematic characterization of the virome in a cohort of both kidney donors and their corresponding recipients. Participants were monitored at the time of transplantation and up to 1 year post-transplant using an open, metagenomic approach.
MATERIAL AND METHODS
Ethical Statement
Samples were obtained from living kidney transplant donors and recipients within the Viral Metagenome Study of the Clinical Research Priority Program ‘Viral Infectious Diseases’ of the University of Zurich. The Ethics Committee of the Canton of Zurich approved the study, and written informed consent was obtained from all participants (protocol number KEK-ZH-Nr. 2013-0087).
Transplant Cohort
Recipients of kidney grafts and their corresponding living donors were enrolled at the time of transplantation. For kidney graft recipients, consecutive, post-transplant study visits were planned at 4–6 weeks and 1 year after transplantation. For living kidney donors, a single visit at the time of organ donation was scheduled. At each visit, plasma and urine samples were collected and recipients were evaluated for signs of infection or present BK viremia, graft function, and transplant-related complications. The cohort was divided in 2 subsets, based on chronological order.
Immunosuppressive Therapy and Antimicrobial Prophylaxis
Induction immunosuppression therapy was administered to all 30 recipients, consisting of methylprednisolone (500 mg on day of surgery) and either basiliximab (20 mg on day of surgery and on day 4) or anti-thymocyte immunoglobulin (ATG) (1.5 mg/kg body weight on day of surgery and on post-transplant days 1–4). The most common regimen for maintenance immunosuppression consisted of tacrolimus, mycophenolate mofetil, and prednisone.
Kidney transplant recipients received Pneumocystis jirovecii prophylaxis with trimethoprim/sulfamethoxazole at 400/80 mg daily for 6 months post-transplant. Topical antifungal prophylaxis with amphotericine B pastilles at 10 mg 4 times a day was administered until prednisone was tapered below 20 mg daily.
Recipients with intermediate or high-risk CMV constellation and ATG induction immunosuppression received prophylaxis with valganciclovir orally for a duration of 3 months. For all others, a preemptive strategy was followed.
Metagenomic Sequencing
Metagenomic sequencing and data analysis was performed as described previously [15, 16] (Supplementary Methods). Briefly, samples were centrifuged, filtered (0.45 μm), and nuclease-treated. RNA and DNA were amplified using random reverse transcription and anchored PCR in 2 separate workflows. Both workflows were pooled for library construction with NexteraXT (Illumina) prior to sequencing for 150 bp on an Illumina MiSeq system. Reads were analyzed using VirMet (https://github.com/ozagordi/VirMet/releases/tag/v1.1.1). Briefly, reads were quality-filtered, cleaned from non-viral reads, and aligned with Basic Local Alignment Search Tool (BLAST) against an in-house, viral database that contains approximately 68 000 different virus sequences. Raw sequence data and JCPyV consensus sequences have been uploaded to zenodo (doi: 10.5281/zenodo.1344146).
Confirmation of Viral Reads Using Quantitative Polymerase Chain Reaction
Specific PCRs for BKPyV, JCPyV, torque teno virus (TTV), and human pegivirus (HPgV) were performed as described [17–20] (Supplementary Methods; Supplementary Table 1).
JC Polyomavirus Serology
Enzyme-linked immunosorbent assay to assess the JCPyV–immunoglobulin G (IgG) serostatus was performed as described previously [21]. JCPyV-immunoglobulin M (IgM) serology was performed as for IgG, using an IgM-specific secondary antibody [22]. A cut-off value of 0.1 normalized Optical Density (nOD) for sera diluted 1:200 or 1:400 for IgG or IgM, respectively, was used.
RESULTS
Study Characteristics
To define the virome of kidney transplant recipients and their living donors, a total of 30 kidney transplant pairs were included in our study. In 10 out of 30 patients (33.3%), kidney transplantation was preemptive; 20 patients (66.6%) were on renal replacement therapy prior to transplant, with 16 receiving hemodialysis and 4 peritoneal dialysis (Table 1). For 28 transplant recipients, follow-up data for at least 1 year post-transplant was available. No major complications and only few symptomatic episodes occurred during this period. There were 5 recipients who experienced symptoms suggestive of infections, such as fever, gastroenteritis, or inflammatory syndrome (Supplementary Figure 1) at the time points of study visits.
Table 1.
Demographics
| Demographics | Recipients | Donors |
|---|---|---|
| Total, N | 30 | 30 |
| Male, n (%) | 11 (36.6%) | 12 (40%) |
| Ethnicity, n (%) | ||
| Caucasian | 14 (93.3%) | 29 (96.7%) |
| Asian | 2 (6.7%) | 1 (2.3%) |
| Age, in years, median (IQR) | 44.5 (32–55) | 56 (52–64) |
| Underlying disease, n (%) | ||
| Glomerulonephritis | 9 (30.0%) | |
| ADPKD | 4 (13.3%) | |
| Hereditary cause other than ADPKD | 2 (6.7%) | |
| Congenital disease | 2 (6.7%) | |
| Hypertensive nephropathy | 2 (6.7%) | |
| Diabetic nephropathy | 2 (6.7%) | |
| Multifactorial | 2 (6.7%) | |
| Unknown | 2 (6.7%) | |
| Other causes | 5 (16.7%) | |
| Renal replacement therapy, pre-transplant, n (%) | ||
| None | 10 (33.3%) | |
| Hemodialysis | 16 (53.3%) | |
| Peritoneal dialysis | 4 (13.3%) | |
| Induction immunosuppression, n (%) | ||
| Basiliximab/methylprednisolone | 24 (80.0%) | |
| ATG/methylprednisolone | 6 (20.0%) |
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; ATG, anti-thymocyte immunoglobulin; IQR, interquartile range.
Conventional Viral Diagnostics in Kidney Transplant Recipients
In the context of routine post-transplant care, viral quantitative PCR (qPCR) monitoring detected BKPyV and CMV in the blood samples of 20 recipients. Standard laboratory analyses detected several cases of respiratory viruses, such as influenza A and B virus, rhinovirus, or coronavirus, in throat swabs (Figure 1).
Figure 1.
Detected viruses by conventional diagnostic tests. As part of routine post-transplant care, kidney transplant recipients are monitored regarding a number of viral pathogens. Negative, low positive, or positive quantitative polymerase chain reaction tests (ticks, open circles, and closed circles, respectively) in blood samples (left panels) and throat swabs (right panels) are shown for each patient of subset 1 (upper panels) and subset 2 (lower panels), from the time point of transplantation up to 100 weeks after transplantation. Abbreviation: CMV, cytomegalovirus; EBV, Epstein Barr virus.
Metagenomic Sequencing of Blood and Urine Samples of Donors and Recipients
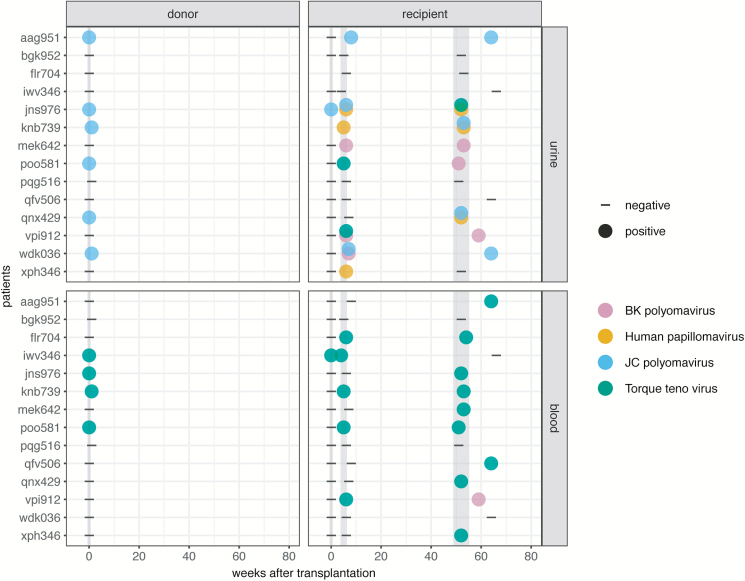
Our goal was to obtain a comprehensive overview of the viral metagenome of kidney donors and corresponding recipients. For 14 living kidney donors and their recipients (subset 1), blood and urine samples from the time of transplantation (donors and recipients), as well as 4–6 and 52 weeks after transplantation (recipients), were analyzed by metagenomic sequencing. In 6 donors, reads for JCPyV were detected in urine samples at the time of transplantation. No other viral reads were identified in the donors, except TTV in blood (n = 4). In the urine of kidney transplant recipients, JCPyV was found in 5 recipients, BKPyV in 4, human papilloma virus (HPV) in 4, and TTV in 3 (Figure 2; Supplementary Table 2). Interestingly, in 5 of 6 cases of JCPyV-positive donors, JCPyV was also detected in the urine samples of the respective recipients at 4–6 weeks or 1 year after transplantation. In the blood of kidney recipients, TTV was found in 11 recipients and BKPyV in 1 recipient.
Figure 2.
Metagenomic sequencing of subset 1 suggests JC polyomavirus transmission. Negative and positive sequencing results (dashes and closed circles, respectively) determined in urine (upper panels) and blood samples (lower panels) of donors (left panels) and respective recipients (right panels) of subset 1 for sampling time points 0, 4–6, and 52 weeks after transplantation.
Sequence-specific Quantitative Polymerase Chain Reaction in Subset 1 Patients
To confirm the findings obtained by metagenomic sequencing, blood and urine samples of all time points of subset 1 donors and recipients were tested by virus-specific PCRs for BKPyV, JCPyV, and TTV. Individual patient-virus specific PCR systems were designed for HPV for suspected cases. PCR analyses confirmed all cases of BKPyV, JCPyV, HPV, and TTV infection reported by metagenomic sequencing, and revealed further positive cases amongst recipients that were not detected by metagenomic sequencing. The additional infections were detected in urine (1 BKPyV, 2 JCPyV; Supplementary Table 3A) and blood (8 BKPyV, 2 JCPyV; Supplementary Table 3B).
Sequence-specific Quantitative Polymerase Chain Reaction in Subset 2 Patients
We next used subset 2 to investigate whether the viruses that were predominantly found by metagenomics analyses in subset 1—namely, BKPyV, JCPyV, and TTV—were generally prevalent. Sequence-specific qPCRs for these 3 viruses were performed in blood and urine samples of the remaining 16 living-donor kidney transplant pairs. We identified 2 additional cases of JCPyV-positive donor-recipient pairs (tvy653, ume111) and 1 case of JCPyV detection that was only in the donor (sjp926). BKPyV was detected in the urine of 4 recipients (Supplementary Table 3A).
Phylogenetic Analysis of JC Polyomavirus Isolates Revealed Clusters of Donor-Recipient Pairs
Combining subsets 1 and 2, we detected JCPyV in 9 of the 30 kidney donors at the time of transplantation. The corresponding recipients were JCPyV-negative at this time point in 8 out of the 9 cases. There were 7 recipients who tested positive for JCPyV in urine, either at 4–6 weeks and/or 1 year post-transplant (Figure 2; Supplementary Table 2), while 2 recipients stayed negative for JCPyV after transplantation, despite positive donors.
To define whether JCPyV in recipients is due to transmission from the donor or to latent reactivation, we constructed consensus sequences of the JCPyV isolates in donors and recipients. For this purpose, candidate samples from subset 2 were sequenced as well (Supplementary Table 2). Full genome coverage was achieved in all cases, except for jns976, where only between 25% and 80% of the genome was covered (Supplementary Figure 4). Phylogenetic analysis revealed a tight clustering of JCPyV sequences in donor-recipient pairs in 6 out of 7 cases (Figure 3), implying that, in these cases, JCPyV infection was contracted from the donor. In the remaining pair (jns976), despite lower coverage, the phylogenetic analysis revealed that more distinct strains infected donor and recipient. Of note, recipient jns976 was already positive for JCPyV at the time of transplantation (Figure 2).
Figure 3.
Phylogenetic analysis confirms transmission of JCPyV. Phylogenetic analysis reveals a close relationship of JCPyV isolates, obtained from donor-recipient pairs in 6 out of 7 suspected cases. JCPyV isolates are indicated by patient/pair-ID, the type of patient (D = donor [open shape], R = recipient [closed shape]) and the sampling time point after transplantation (0w = time of transplantation, 4-6w = 4-6 weeks after transplantation, 52w = 1 year after transplantation). Shapes are used to indicate transmission pairs. The JCPyV reference sequences used for alignments are indicated with GenBank-ID and isolate name. The phylogenetic tree was constructed in MEGA7 using the Maximum Likelihood method, based on the Kimura 2-parameter model. Bootstrap values for 50 resamplings are shown next to the branches. Abbreviation: PyV, polyomavirus.
JC Polyomavirus Serology Detected High Prevalence of JC Polyomavirus–specific Antibodies
In order to confirm or exclude pre-transplant JCPyV infections, we performed IgG-specific serology for all participants at the time of transplantation. There were 21 donors and 27 recipients sero-positive for JCPyV, corresponding to a seroprevalence of 80% among all 60 individuals. In the 6 recipients with JCPyV transmission from the donor, the IgG serology revealed the presence of virus-specific antibodies from before the transplantation (Supplementary Table 4).
An interesting case was recipient jns976, who was seronegative at the time of transplantation, although being positive for JCPyV. Therefore, we additionally performed IgG and IgM serology for 2 time points before (-4 months and -1 day) and 2 time points after transplantation (4–6 weeks and 1 year). JCPyV-specific IgM was negative at all time points. In contrast, JCPyV-specific IgG was low positive at 4 months and 1 day prior to transplantation, as well as 1 year after, but undetectable 4–6 weeks after transplantation (Supplementary Table 5).
Torque Teno Virus Levels Increased After Initiation of Immunosuppressive Therapy
As shown for other cases of transplantation-related immunosuppression, TTV loads increased in all kidney transplant recipients after transplantation. Viral loads reached their highest levels at 4–6 weeks post-transplantation (Supplementary Figure 2). We did not find any significant difference in the viral load of TTV in recipients with or without CMV or BKPyV replication, respectively.
Low Prevalence of Human Pegivirus in Kidney Transplant Cohort
HPgV was recently reported as a predominant and persistent component of the blood virome in immunosuppressed patients after transplantation [18]. As our metagenomic analysis of subset 1 recipients did not pick up HPgV in any of the tested samples, we searched for HPgV by specific PCR in both subset 1 and subset 2 recipients. We detected only a single case of HPgV, in a subset 2 recipient who was not studied by metagenomics before.
Effect of Induction Immunosuppression on JC Polyomavirus Replication
All recipients received induction immunosuppression, with most of them treated with basiliximab/methylprednisolone (80%) and the minority with ATG/methylprednisolone (20%; Table 1). All cases of JCPyV replication were detected in patients receiving induction immunosuppression with basiliximab/methylprednisolone (7 of 24 recipients), whereas none of the recipients with administration of ATG/methylprednisolone tested positive for JCPyV replication (Fisher’s exact test, P = 0.29).
Parameters of Renal Function and JC Polyomavirus Infection
To investigate whether JCPyV infection had an influence on renal function, we compared estimated glomerular filtration rates and proteinuria in JCPyV-positive and -negative kidney transplant recipients. We did not detect any significant differences in estimated glomerular filtration rates (Supplementary Figure 3A) and protein/creatinine ratios (Supplementary Figure 3B) between transplant recipients with or without JCPyV infection.
DISCUSSION
Here, we studied the viral metagenomes of living kidney transplant donors and their corresponding recipients. The analysis of a first subset of 14 patient pairs by our open metagenomic approach identified JCPyV in the urine samples of 6 donors and BKPyV, JCPyV, HPV, and TTV replication in the urine samples of 9 recipients, which were confirmed by specific PCR. PCR screening of a second subset for the same viruses identified JCPyV in the urine samples of 3 donors, with 2 additional cases of JCPyV replication in donors and corresponding recipients. The presence of JCPyV in the urine of kidney donors at the time of transplant and in the corresponding kidney recipients 4–6 or 52 weeks after transplantation strongly suggested transmission of JCPyV via the graft. Phylogenetic analysis confirmed a transmission in 6 of 9 positive donors to the recipient, despite the seropositive immune status of the recipient. Only 1 additional recipient developed JC viruria, supporting the importance of donor-derived JCPyV infection in living donor kidney transplantation.
Notably, JCPyV-specific serology revealed the presence of virus-specific antibodies at the time of transplantation in these 6 cases. This is in line with previous reports [23] and the seroprevalence of 80% among the 60 individuals in this cohort. Nevertheless, prior immune responses did not prevent nor rule out transmission (super infection) during transplantation, especially in kidney transplantation as the organ of JCPyV persistence.
In 1 patient (jns976), the JCPyV sequences of the donor and recipient did not cluster. Here, we initially suspected a recent infection of the recipient, as the individual was IgG negative at the time of transplant but, at the same time, JCPyV reads could be detected, which has been reported before in cases of primary JCPyV infection in kidney transplant patients [22, 24, 25]. Surprisingly, the patient was already IgG positive before transplantation, as well as 1 year thereafter, while IgG levels were undetectable around the time of transplantation. IgM levels were undetectable at all time points. The reasons for these observations remain unclear. This transplant recipient did not receive immunosuppressive therapy—especially, no B-cell–depleting therapy—prior to transplantation, which could have lowered the specific IgG levels assessed at the time of transplantation [26].
The impact of JCPyV replication after renal transplantation is only emerging. Several studies reported the detection of the virus in the urine of kidney transplant recipients [9, 27]. In the context of immunosuppressed individuals, JCPyV has been associated with 2 clinical entities: that is, progressive multifocal leukoencephalopathy and PyVAN [28]. However, in contrast to BKPyV, JCPyV has been rarely observed as a causative pathogen for PyVAN [22, 29]. Different results have been stated in the literature regarding JCPyV viruria and its impact on transplant outcomes. On the one hand, JCPyV urinary shedding was associated with reduced creatinine clearance in kidney and liver transplant patients, and might therefore play a role in renal dysfunction [30]. On the other hand, the finding of JCPyV viruria was shown to correlate with a favorable clinical course, especially if detected early [25]. Interestingly, Cheng et al reported better graft survival and lower rejection rates in kidney recipients with JCPyV viruria [31]. Also, in our study, clinically and on renal function, no impact was seen. In studies with larger numbers, longer periods of observation and, presumably, in deceased donor kidney transplantation, the relevance might be higher.
The literature suggests a frequent donor origin for BKPyV viremia after kidney transplantation [32, 33]. Considering the detection of genetically-identical JCPyV isolates in 6 corresponding kidney donor and recipient pairs, our results strongly underline that transmission from donor to recipient must be considered also for JCPyV. Of note, while JCPyV was detected in the urine of transplant recipients, no detectable deterioration of renal function parameters occurred, suggesting an asymptomatic replication. Further studies will be needed to define whether JCPyV is only excreted from the transplanted kidney, or also disseminates and replicates in the recipient. Considering that we only detected JCPyV in the urine, a restriction to dissemination (eg, through preexisting immunity) needs to be considered.
In line with previous reports, TTV was detected frequently in donors and recipients, and levels were shown to increase in transplant recipients, which is consistent with prior reports on transplant patients receiving immunosuppressive treatment [34–37]. Due to low read numbers, it was not possible to assess whether TTV was also transmitted from positive donors to recipients.
While we detected HPV in several samples by metagenomic sequencing, our cohort did not provide evidence for a clinical correlate of HPV infection. It remains possible that the presence of HPV could either be an indication for skin flora contamination [38, 39] or asymptomatic replication. The HPV types could not be determined accurately due to low coverage.
We could not find a high prevalence of HPgV as a predominant and persistent component of the blood virome in immunosuppressed transplant patients [18]. In the combined metagenomic and specific-PCR analyses, only a single case was HPgV-positive.
Of note, the metagenomic sequencing in plasma and urine recorded BKPyV in the urine of recipients at an early time point of 4–6 weeks (mek642, vpi912, sjp926, utf849). Of those, 3 had their earliest positive test in blood by routine PCR at week 10 (Figure 1). In 1 case (sjp926), the BKPyV infection was not detected in blood by routine qPCR at any time point (up to 52 weeks post-transplant), whereas metagenomic sequencing yielded BKPyV sequences in urine after 5 weeks post-transplantation. On the other hand, metagenomic sequencing also missed viruses identified by PCR in several cases, showing that metagenomic sequencing still lacks sensitivity, as compared to specific PCR.
As our findings highlight, unbiased metagenomic sequencing, a method theoretically capable of detecting any virus in any clinical specimen, empowers routine virus diagnostics. Here, we obtained novel insight into the influence of the donor virome on the recipient in renal transplantation. In conclusion, viral metagenomic sequencing of a cohort of donor-recipient renal transplant pairs revealed the prevalence of JCPyV transmission from kidney transplant donors to recipients in at least 20% of cases. Considering this high prevalence of JCPyV transmission, its relevance for the transplant outcomes, graft losses, and life expectancies of recipients need to be investigated in larger cohorts.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all the participating individuals and the staff of the Department of Nephrology at the University Hospital Zurich. Plasmid pAF121950 was obtained from Laurent Kaiser and Samuel Corday (originally from the National Institutes of Health Acquired Immunodeficiency Syndrome Reagent Program, Division of Acquired Immunodeficiency Syndrome, NIAID, National Institutes of Health, Dr. Jinhua Xiang and Dr. Jack Stapleton).
Financial support. This work was supported by the Clinical Research Priority Program ‘Viral Infectious Diseases’ of the University of Zurich.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ison MG, Grossi P; AST Infectious Diseases Community of Practice Donor-derived infections in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4):22–30. [DOI] [PubMed] [Google Scholar]
- 2. Fischer SA, Avery RK; AST Infectious Disease Community of Practice Screening of donor and recipient prior to solid organ transplantation. Am J Transplant 2009; 9(Suppl 4):S7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirsch HH. BK virus: opportunity makes a pathogen. Clin Infect Dis 2005; 41:354–60. [DOI] [PubMed] [Google Scholar]
- 4. Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice BK polyomavirus in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4):179–88. [DOI] [PubMed] [Google Scholar]
- 5. Kumar D, Prasad GV, Zaltzman J, Levy GA, Humar A. Community-acquired West Nile virus infection in solid-organ transplant recipients. Transplantation 2004; 77:399–402. [DOI] [PubMed] [Google Scholar]
- 6. Fischer SA, Graham MB, Kuehnert MJ, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. New Eng J Med 2009; 354:2235–49. [DOI] [PubMed] [Google Scholar]
- 7. Pietrosi G, Vizzini G, Pipitone L, et al. Primary and reactivated HHV8 infection and disease after liver transplantation: a prospective study. Am J Transplant 2011; 11:2715–23. [DOI] [PubMed] [Google Scholar]
- 8. Zhou H, Zhu W, Zeng J, et al. Probable rabies virus transmission through organ transplantation, China, 2015. Emerg Infect Dis 2016; 22:1348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rani A, Ranjan R, McGee HS, et al. A diverse virome in kidney transplant patients contains multiple viral subtypes with distinct polymorphisms. Sci Rep 2016; 6:1–13. doi: 10.1038/srep33327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burnham P, Dadhania D, Heyang M, et al. Urinary cell-free DNA is a versatile analyte for monitoring infections of the urinary tract. Nat Commun 2018; 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar D. Emerging viruses in transplantation. Curr Opin Infect Dis 2010; 23:374–8. [DOI] [PubMed] [Google Scholar]
- 12. Menter T, Mayr M, Schaub S, Mihatsch MJ, Hirsch HH, Hopfer H. Pathology of resolving polyomavirus-associated nephropathy. Am J Transplant 2013; 13:1474–83. [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez S, Escobar-Serna DP, Suarez O, et al. BK virus nephropathy in kidney transplantation: an approach proposal and update on risk factors, diagnosis, and treatment. Transplant Proc 2015; 47:1777–85. [DOI] [PubMed] [Google Scholar]
- 14. Schmitt C, Raggub L, Linnenweber-Held S, Adams O, Schwarz A, Heim A. Donor origin of BKV replication after kidney transplantation. J Clin Virol 2014; 59:120–5. [DOI] [PubMed] [Google Scholar]
- 15. Lewandowska DW, Capaul R, Prader S, et al. Persistent mammalian orthoreovirus, coxsackievirus and adenovirus co-infection in a child with a primary immunodeficiency detected by metagenomic sequencing: a case report. BMC Infect Dis 2018; 18:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewandowska DW, Zagordi O, Geissberger F-D, et al. Optimization and validation of sample preparation for metagenomic sequencing of viruses in clinical samples. Microbiome 2017; 5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirsch HH, Mohaupt M, Klimkait T. Prospective monitoring of BK virus load after discontinuing sirolimus treatment in a renal transplant patient with BK virus nephropathy. J Infect Dis 2001; 184:1494–5; author reply 5–6. [DOI] [PubMed] [Google Scholar]
- 18. Gibson PE, Knowles WA, Hand JF, Brown DW. Detection of JC virus DNA in the cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. J Med Virol 1993; 39:278–81. [DOI] [PubMed] [Google Scholar]
- 19. Maggi F, Fornai C, Vatteroni ML, et al. Low prevalence of TT virus in the cerebrospinal fluid of viremic patients with central nervous system disorders. J Med Virol 2001; 65:418–22. [DOI] [PubMed] [Google Scholar]
- 20. Vu D-L, Cordey S, Simonetta F, et al. Human pegivirus persistence in the human blood virome after allogeneic haematopoietic stem cell transplantation. Clin Microbiol Infect 2018; S1198-743X(18)30410-5, doi: 10.1016/j.cmi.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 21. Kardas P, Leboeuf C, Hirsch HH. Optimizing JC and BK polyomavirus IgG testing for seroepidemiology and patient counseling. J Clin Virol 2015; 71:28–33. [DOI] [PubMed] [Google Scholar]
- 22. Lautenschlager I, Jahnukainen T, Kardas P, et al. A case of primary JC polyomavirus infection-associated nephropathy. Am J Transplant 2014; 14:2887–92. [DOI] [PubMed] [Google Scholar]
- 23. Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 2009; 199:837–46. [DOI] [PubMed] [Google Scholar]
- 24. Helanterä I, Hirsch HH, Auvinen E, et al. High-level JCPyV viruria after kidney transplantation-Clinical and histopathological findings. J Clin Virol 2016; 85:75–9. [DOI] [PubMed] [Google Scholar]
- 25. Drachenberg CB, Hirsch HH, Papadimitriou JC, et al. Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: a prospective evaluation. Transplantation 2007; 84:323–30. [DOI] [PubMed] [Google Scholar]
- 26. Makatsori M, Kiani-Alikhan S, Manson AL, et al. Hypogammaglobulinaemia after rituximab treatment-incidence and outcomes. QJM 2014; 107:821–8. [DOI] [PubMed] [Google Scholar]
- 27. Rani A, Ranjan R, McGee HS, et al. Urinary microbiome of kidney transplant patients reveals dysbiosis with potential for antibiotic resistance. Transl Res 2016; 181:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Delbue S, Ferraresso M, Ghio L, et al. A review on JC virus infection in kidney transplant recipients. Clin Dev Immunol 2013; 2013:926391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Höcker B, Tabatabai J, Schneble L, et al. JC polyomavirus replication and associated disease in pediatric renal transplantation: an international CERTAIN Registry study. Pediatr Nephrol 2018; 33:2343–52. [DOI] [PubMed] [Google Scholar]
- 30. Kusne S, Vilchez RA, Zanwar P, et al. Polyomavirus JC urinary shedding in kidney and liver transplant recipients associated with reduced creatinine clearance. J Infect Dis 2012; 206:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng XS, Bohl DL, Storch GA, et al. Inhibitory interactions between BK and JC virus among kidney transplant recipients. J Am Soc Nephrol 2011; 22:825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant 2005; 5:2213–21. [DOI] [PubMed] [Google Scholar]
- 33. Andrews CA, Shah KV, Daniel RW, et al. A serological investigation of BK virus and JC virus infections in recipients of renal allografts. J Infect Dis 1988; 158:176–81. [DOI] [PubMed] [Google Scholar]
- 34. Lewandowska DW, Schreiber PW, Schuurmans MM, et al. Metagenomic sequencing complements routine diagnostics in identifying viral pathogens in lung transplant recipients with unknown etiology of respiratory infection. PLOS One 2017; 12:e0177340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young JC, Chehoud C, Bittinger K, et al. Viral metagenomics reveal blooms of anelloviruses in the respiratory tract of lung transplant recipients. Am J Transplant 2015; 15:200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Görzer I, Haloschan M, Jaksch P, Klepetko W, Puchhammer-Stöckl E. Plasma DNA levels of torque teno virus and immunosuppression after lung transplantation. J Heart Lung Transplant 2014; 33:320–3. [DOI] [PubMed] [Google Scholar]
- 37. Nordén R, Magnusson J, Lundin A, et al. Quantification of torque teno virus and Epstein-Barr virus is of limited value for predicting the net state of immunosuppression after lung transplantation. Open Forum Infect Dis 2018; 5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller S, Naccache SN, Messacar K, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. bioRxiv 2018; doi:10.1101/330381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaiser L. Virome and transplantation Available at: https://www.escmid.org/escmid_publications/escmid_elibrary/?q=laurent+kaiser&id=2173&L=0&x=0&y=0&tx_solr%5Bsort%5D=relevance%2Basc&tx_solr%5Bfilter%5D%5B0%5D=main_filter_eccmid%253Atrue&tx_solr%5Bfilter%5D%5B1%5D=pub_date%253A201801010000-201812312359. Accessed 20 December 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.