Abstract
Background
The objective of this study was to evaluate the effect of ribavirin and recombinant interferon (RBV/rIFN) therapy on the outcomes of critically ill patients with Middle East respiratory syndrome (MERS), accounting for time-varying confounders.
Methods
This is a retrospective cohort study of critically ill patients with laboratory-confirmed MERS from 14 hospitals in Saudi Arabia diagnosed between September 2012 and January 2018. We evaluated the association of RBV/rIFN with 90-day mortality and MERS coronavirus (MERS-CoV) RNA clearance using marginal structural modeling to account for baseline and time-varying confounders.
Results
Of 349 MERS patients, 144 (41.3%) patients received RBV/rIFN (RBV and/or rIFN-α2a, rIFN-α2b, or rIFN-β1a; none received rIFN-β1b). RBV/rIFN was initiated at a median of 2 days (Q1, Q3: 1, 3 days) from intensive care unit admission. Crude 90-day mortality was higher in patients with RBV/rIFN compared to no RBV/rIFN (106/144 [73.6%] vs 126/205 [61.5%]; P = .02]. After adjusting for baseline and time-varying confounders using a marginal structural model, RBV/rIFN was not associated with changes in 90-day mortality (adjusted odds ratio, 1.03 [95% confidence interval {CI}, .73–1.44]; P = .87) or with more rapid MERS-CoV RNA clearance (adjusted hazard ratio, 0.65 [95% CI, .30–1.44]; P = .29).
Conclusions
In this observational study, RBV/rIFN (RBV and/or rIFN-α2a, rIFN-α2b, or rIFN-β1a) therapy was commonly used in critically ill MERS patients but was not associated with reduction in 90-day mortality or in faster MERS-CoV RNA clearance.
Keywords: coronavirus, Middle East respiratory syndrome, pneumonia, ribavirin, interferon
In this observational study accounting for baseline and time-varying confounders, ribavirin and recombinant interferon (rIFN-α2a, rIFN-α2b, or rIFN-β1a) therapy was not associated with reduction in 90-day mortality or in faster MERS-CoV RNA clearance.
The Middle East respiratory syndrome (MERS) is a severe respiratory infection caused by a novel coronavirus (MERS-CoV) and is associated with high mortality [1]. To date, there is no specific therapy of proven effectiveness for MERS.
Based on prior experience with severe acute respiratory syndrome and on preclinical data, ribavirin and recombinant interferon (RBV/rIFN) therapy has been used in managing patients with MERS [2–4]. RBV is a guanosine analog that has antiviral activity against multiple RNA viruses [4]. Different preparations of recombinant rIFNs (rIFN-α2a, rIFN-α2b, rIFN-β1a, and rIFN-β1b) are active against MERS-CoV in vitro [5]. RBV and rIFN at relatively high concentrations inhibited MERS-CoV replication in Vero and LLC-MK2 cells, but when used in combination, lower concentrations achieved comparable endpoints [2]. High doses of RBV/rIFN-α2b administered 8 hours after inoculation of rhesus macaques with MERS-CoV resulted in reduced viral loads and was partially effective in preventing progression to pneumonia compared to animals that were untreated [3].
However, clinical data on RBV/rIFN in MERS have been limited to small single-center studies [6–9]. In one report, all 5 patients who received RBV/rIFN-α2b at a median of 19 days from admission died [7]. In a retrospective study (n = 32), patients who received RBV/IFN-α2a had a mortality rate of 85% compared with 64% in those who received RBV/IFN-β1a (P = .24) [8]. Another study (n = 44) showed that RBV/rIFN-α2a therapy compared to control was associated with a significant reduction in 14-day mortality (30% vs 71%; P = .004) but not in 28-day mortality (70% vs 83%; P = .054). Recipients of RBV/rIFN-α2a had a significant reduction (>2 g) in hemoglobin level, raising concerns of a previously described complication of hemolysis [9]. Another study (n = 51) found that MERS therapy using different regimens of rIFN-β, rIFN-α, RBV, and mycophenolate mofetil was associated with lower mortality on univariable analysis but not on multivariable analysis [10].
These studies have not provided clear evidence upon which to base treatment recommendations because of their nonrandomized design, inconsistent results, small sample sizes with limited power, and the lack of adjustment for unbalanced covariates. In addition, because of therapy initiation was not standardized, such studies are prone to 2 sources of bias: immortal time bias and indication bias. Immortal time bias may occur because patients in the therapy group have survived for a period of time before receiving the therapy. Because the outcome (death) could not possibly have occurred during this period in this group, this type of bias systematically underestimates adverse outcomes (eg, death) [11, 12]. Indication bias occurs when the association of the therapy and outcome is caused by the indication for which the therapy was used and not to the therapy itself. If physicians typically prescribe certain therapy, such as RBV/rIFN, only when the clinical condition is not improving or is worsening, standard multivariable analyses may overestimate the association with poor outcome. Addressing both immortal time and indication bias in observational studies requires accounting for baseline and time-varying confounding associated with the decision to initiate treatment.
The objective of this study was to examine the effect of RBV/rIFN therapy in a large cohort of critically ill patients with MERS on the 90-day mortality and MERS-CoV RNA clearance by accounting for baseline and time-varying confounders. Some of these findings have been previously presented in an abstract form [13].
METHODS
Study Setting
This retrospective analysis was conducted on a multicenter database of all critically ill patients with MERS admitted to the intensive care units (ICUs) of 14 hospitals in 5 cities in Saudi Arabia between September 2012 and January 2018 [14]. Details of the cohort have been reported previously [14]. Some of the patients in the current analysis may have been included in previous single-center studies [8–10]. Patient-level informed consent was not required. The institutional review boards (IRBs) of all participating centers approved the study.
Patients
Participating centers followed the Saudi Arabian Ministry of Health guidelines for MERS diagnostic testing. Patients were defined to have MERS if they had a positive MERS-CoV real-time reverse transcription polymerase chain reaction result (rRT-PCR) targeting amplifications of the upstream E protein (upE gene) and open reading frame 1a. Specimens were obtained from nasopharyngeal swabs or sputum in nonintubated patients and from tracheal aspirates or bronchoalveolar lavage in intubated patients [15]. For patients who tested positive for MERS-CoV, follow-up respiratory samples were collected at the discretion of the treating teams approximately 1–2 times per week for infection control purposes. In the current analysis, we excluded patients who were enrolled in a randomized controlled trial (RCT) for MERS antiviral therapy that started enrolling patients in November 2017 (MERS-CoV Infection Treated With a Combination of Lopinavir/Ritonavir and Interferon β-1b [MIRACLE] trial) [16].
RBV/IFN Therapy
The main exposure was RBV/rIFN therapy, defined as the use of RBV/rIFN combination, RBV alone, or rIFN alone. The comparator group was the use of neither RBV nor rIFN. Three different types of rIFNs were used in the current cohort: rIFN-α2a (Pegasys, Hoffmann-La Roche, c/o Genentech, South San Francisco, California); rIFN-α2b (PEG-Intron, Merck Sharp & Dohme, Whitehouse Station, New Jersey); and rIFN-β1a (Rebif, Serono, Rockland, Massachusetts). Commonly used dosing protocols for RBV/rIFN for patients with MERS in the participating hospitals are shown in Supplementary Table 1.
Data Collection
Using standardized case report forms, we documented demographics, clinical presentation, underlying comorbidities, and final outcomes [17]. We collected Sequential Organ Failure Assessment (SOFA) score and physiologic and laboratory parameters on ICU days 1, 3, 7, 14, and 28 [17, 18]. We documented therapeutic interventions, including corticosteroids, mechanical ventilation, oxygen rescue therapies (nitric oxide, prone ventilation, high frequency oscillatory ventilation or extracorporeal membrane oxygenation), packed red blood cell transfusion, vasopressor therapy, and renal replacement therapy. The primary outcome was 90-day mortality.
We also assessed time to MERS-CoV RNA clearance in respiratory samples in patients who had at least 1 follow-up rRT-PCR performed after the diagnostic test. Clearance was defined as the time from ICU admission until the test was negative on 2 occasions, without a positive test afterward. To assess RBV/rIFN safety profile, we evaluated serial levels of hemoglobin, white blood cell (WBC) count, platelet count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, international normalized ratio (INR), lactic acid, and creatinine. Other secondary outcomes were ICU and hospital mortality, and ICU and hospital length of stay.
Statistical Analysis
We compared baseline characteristics, interventions, and outcomes of patients who received RBV/rIFN to those who did not, using χ 2 test or Fisher exact test for categorical variables and Student t test or Mann-Whitney U test for continuous variables. For serial measurements, we tested differences between the 2 groups over time using repeated-measures analysis of variance with Bonferroni correction for multiple comparisons and with no imputation for missing values.
We constructed 3 models to assess the association of RBV/rIFN therapy with 90-day mortality adjusting for baseline characteristics and time-varying confounders. These analytical models were detailed in a previous study [19]. First, we created a logistic regression model adjusting for the following a priori baseline variables of clinical interest and all significant variables at the univariable level (P ≤ .2). These variables included diabetes, liver disease, renal disease, malignancy, SOFA score on the first day of ICU admission, source of infection, and year (before July 2014 and after) by applying the PROC GENMOD procedure (SAS software).
Second, we created a Cox proportional hazards model adjusting for the same covariates and accounting for the RBV/rIFN therapy as a time-varying covariate.
Third, we created a marginal structural model analysis with inverse probability of treatment weighting to account for time-varying confounders that are likely to influence the decision to initiate RBV/rIFN therapy and at the same time may be associated with mortality [20–23]. We calculated stabilized weights for the probability that each subject received the treatment, censored on the day of therapy, ICU discharge, or 28-day mortality, whichever came first. We included in this model selected baseline characteristics as well as time-dependent variables (SOFA on the index day of RBV/rIFN initiation and the previous day, ventilation status on the index day and on the previous day [0: not ventilated, 1: noninvasive ventilation, 2: invasive mechanical ventilation, 3: oxygen rescue therapy], hemoglobin, WBC count, AST and creatinine on the index day, and corticosteroid therapy on the index day). We included corticosteroid therapy as a time-varying covariate, as we have shown previously that corticosteroid therapy in critically ill MERS patients might prolong MERS-CoV RNA clearance although it was not associated with difference in mortality [19]. Because these values were recorded on days 1, 3, 7, 14, and 28, we imputed missing values for the remaining days (Supplementary Methods). We used a weight-trimming approach to deal with extreme weights; weights <5th percentile value were fixed at the 5th percentile value and weights >95th percentile value were fixed at the 95th percentile value. This process continued until the average weight reached approximately 1. Then, we used a weighted regression model using these weights taking in consideration the repeated-measures nature of the data to estimate the association of RBV/rIFN with 90-day mortality.
We carried out sensitivity analyses examining the association of RBV therapy alone compared to no RBV and rIFN therapy alone compared to no rIFN. To account for the possible variation by site, we carried out a sensitivity analysis using a logistic regression model adjusting for clustering by centers in addition to the previously mentioned baseline variables. We further evaluated the association of different types of rIFN (rIFN-α2a, rIFN-α2b, and rIFN-β1a) on 90-day mortality using a similar logistic regression model and adjusting for clustering by center.
We also created 2 models to assess the association of RBV/rIFN therapy with MERS-CoV RNA clearance adjusting for baseline characteristics and time-varying confounders. First, we carried a Cox proportional hazards model adjusting for the same baseline covariates mentioned earlier and accounting for the RBV/rIFN therapy as a time-varying covariate. We censored patients if they never cleared MERS-CoV RNA or at the time of last rRT-PCR test. Second, we created a marginal structural Cox proportional hazards model incorporating the stabilized weights to estimate the effect of RBV/rIFN therapy on MERS-CoV RNA clearance in a similar approach to the marginal structural model used for 90-day mortality. Analyses were conducted using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
Ethical Considerations
The study was approved by the National Guard Health Affairs IRB and by the IRBs of all participating sites. Informed consent was waived by the IRB because of the retrospective nature of the study.
RESULTS
Patient Characteristics
Of the 355 patients with MERS in the cohort, 6 patients were excluded because they were enrolled in the MIRACLE trial. The remaining cohort included 349 patients, of whom 144 (41.3%) received RBV/rIFN therapy (Table 1). Patients in the 2 groups were similar in age, sex, body mass index, and source of admission (Table 1). Comorbidities were common in both those who received RBV/rIFN and those who did not (84.0% vs 78.0%; P = .17). Patients who received RBV/rIFN were more likely to have diabetes (58.3% vs 42.0%; P = .003) and chronic renal disease (36.8% vs 27.3%; P = .06), but less likely to have chronic liver disease (2.1% vs 7.8%; P = .02). Of note, the RBV/rIFN recipients had lower SOFA scores than those not treated (median, 8 [Q1, Q3: 5, 11] vs 10 [Q1, Q3: 6, 13]; P = .01; Table 1).
Table 1.
Baseline Characteristics and Physiological Parameters Among Patients With Middle East Respiratory Syndrome Coronavirus Who Received Ribavirin/Recombinant Interferon and Those Who Did Not on Day 1 of Admission to the Intensive Care Unit
| Variable | RBV/rIFN (n = 144) | No RBV/rIFN (n = 205) | P Value |
|---|---|---|---|
| Age, y, median (Q1, Q3) | 57.5 (47.0, 70.0) | 58.0 (41.0, 70.0) | .98 |
| BMI, kg/m2, median (Q1, Q3) | 28.3 (24.2, 32.9) | 28.5 (24.2, 33.5) | .85 |
| Male sex | 101 (70.1) | 140 (68.3) | .71 |
| Source of infection | |||
| Community-acquired | 68 (47.2) | 117 (57.1) | .15 |
| Healthcare worker, hospital-acquired | 13 (9.0) | 19 (9.3) | |
| Non–healthcare worker, hospital-acquired | 63 (43.8) | 69 (33.7) | |
| Days from onset of symptoms to hospital presentation, median (Q1, Q3) | 5 (3.0, 8.0) | 4 (2.0, 7.0) | .004 |
| Days from onset of symptoms to ICU admission, median (Q1, Q3) | 8 (5.0, 11.0) | 7 (4.0, 11.0) | .13 |
| Days from onset of symptoms to intubation, median (Q1, Q3) | 8.5 (5.0, 12.0) | 7.5 (5.0, 12.5) | .36 |
| Comorbidities | |||
| Any comorbidities | 121 (84.0) | 160 (78.0) | .17 |
| Diabetes with chronic complications | 84 (58.3) | 86 (42.0) | .003 |
| Asthma/chronic pulmonary disease | 17 (11.8) | 29 (14.1) | .52 |
| Moderate to severe liver disease | 3 (2.1) | 16 (7.8) | .02 |
| Chronic renal disease | 53 (36.8) | 56 (27.3) | .06 |
| Chronic cardiac disease | 60 (41.7) | 77 (37.6) | .44 |
| Chronic neurological disease | 15 (10.4) | 23 (11.2) | .81 |
| Obesity | 18 (12.5) | 20 (9.8) | .42 |
| Rheumatological disease | 3 (2.1) | 4 (2.0) | >.99a |
| Any malignancy including leukemia or lymphoma | 9 (6.3) | 25 (12.2) | .07 |
| Physiologic parameters on day 1 | |||
| SOFA score, median (Q1, Q3) | 8 (5.0, 11.0) | 10 (6.0, 13.0) | .01 |
| Tidal volume, mL, median (Q1, Q3) | 400 (350.0, 450.0) | 400 (350.0, 438.0) | .54 |
| PEEP, cm H2O, median (Q1, Q3) | 11 (8.0, 14.0) | 12 (10.0, 14.0) | .46 |
| Plateau pressure, cm H2O, median (Q1, Q3) | 30.0 (26.0, 32.0) | 27 (21.5, 30.0) | .01 |
| PaO2/FiO2 ratio, median (Q1, Q3) | 89.6 (63.0, 151.9) | 115.4 (73.0, 162.0) | .07 |
| Mean arterial pressure, mm Hg, median (Q1, Q3) | 72.5 (63.5, 85.5) | 68 (59.0, 78.0) | .001 |
| Lactate, mmol/L, median (Q1, Q3) | 1.6 (1.1, 2.2) | 2 (1.1, 3.1) | .10 |
| INR, median (Q1, Q3) | 1.1 (1.0, 1.3) | 1.2 (1.0, 1.4) | .002 |
| Creatinine, µmol/L, median (Q1, Q3) | 114.9 (74.0, 314.0) | 132.6 (74.0, 247.0) | .96 |
| Bilirubin level, µmol/L, median (Q1, Q3) | 10.8 (6.8, 19.7) | 14 (8.6, 27.0) | .005 |
| Platelet count, ×109/L, median (Q1, Q3) | 180.5 (119.5, 250.5) | 159.0 (101.0, 231.0) | .09 |
| No. of quadrants with infiltrates on chest radiograph, median (Q1, Q3) | 3 (2.0,4.0) | 2 (2.0, 4.0) | .17 |
| Mechanical ventilation | 84 (58.3) | 130 (63.4) | .34 |
| Vasopressors | 59 (41.0) | 101 (49.3) | .13 |
Data are presented as no. (%) unless otherwise indicated. For continuous variables, Mann-Whitney U test was used to calculate the P value. For categorical variables, χ 2 test was used to calculate the P value.
Abbreviations: BMI, body mass index; FiO2, fraction of inspired oxygen; ICU, intensive care unit; INR, international normalized ratio; PaO2, partial pressure of oxygen in arterial blood; PEEP, positive end-expiratory pressure; RBV/rIFN, ribavirin/recombinant interferon; SOFA, Sequential Organ Failure Assessment.
aFisher exact test.
RBV/IFN THERAPY
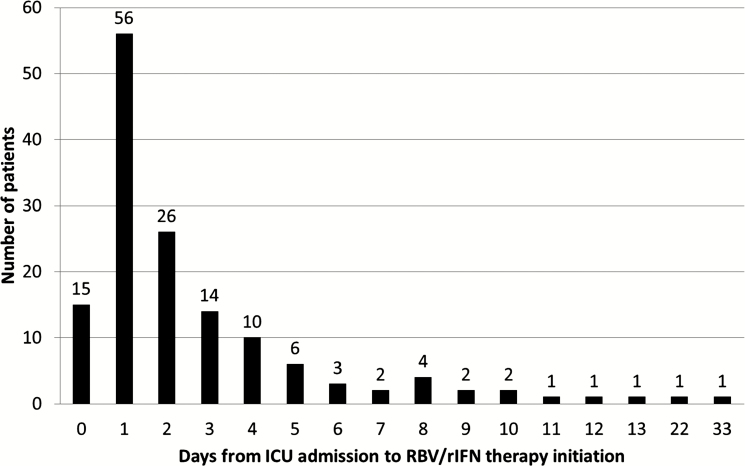
RBV/rIFN therapy was initiated at a median of 2 days (Q1, Q3: 1, 3) from ICU admission, which corresponded to 5.0 days (Q1, Q3: 2.0, 9.0) from hospital admission and 9.0 (Q1, Q3: 6.0, 12.0) from onset of symptoms (Table 2 and Figure 1). Of these patients, 117 (81.3%) patients received RBV/rIFN combination, 18 (12.5%) RBV alone, and 9 (6.3%) rIFN alone. A total of 73 (57.9%) received rIFN-α2a, 22 (17.5%) received rIFN α-2b, 31 (24.6%) received rIFN-β1a, and none received rIFN-β1b (Table 2). The use of RBV/rIFN therapy and the type of rIFN varied by site (Supplementary Figures 1 and 2).
Table 2.
Ribavirin/Recombinant Interferon Therapy in Critically Ill Patients With Middle East Respiratory Syndrome
| Variable | No. (%) or Median (Q1, Q3) |
|---|---|
| RBV and/or rIFN | 144 (100) |
| Combination of RBV and rIFN | 117 (81.3) |
| RBV alone | 18 (12.5) |
| rIFN alone | 9 (6.3) |
| rIFN type (n = 126) | |
| rIFN α-2a | 73 (57.9) |
| rIFN α-2b | 22 (17.5) |
| rIFN-β1a | 31 (24.6) |
| Duration between hospital presentation and RBV/rIFN initiation, d | 5.0 (2.0, 9.0) |
| Duration between ICU admission and RBV/rIFN initiation, d | 2.0 (1.0, 3.0) |
| Duration between onset of ventilation and RBV/rIFN initiation, d | 2.0 (1.0, 3.0) |
| Duration between onset of symptoms and RBV/rIFN initiation, d | 9.0 (6.0, 12.0) |
| Duration of treatment, d | 8 (5, 12) |
| Duration of treatment among survivors, d | 9.5 (7.5, 15.0) |
Abbreviations: ICU, intensive care unit; RBV, ribavirin; rIFN, recombinant interferon.
Figure 1.
Number of days from intensive care unit (ICU) admission to ribavirin/recombinant interferon (RBV/rIFN) therapy initiation.
Cointerventions
During ICU stay, the provision of mechanical ventilation, oxygen rescue therapies, renal replacement therapy, and vasopressor therapy was similar in the 2 groups (Table 3). During the ICU stay, patients who received RBV/rIFN therapy were more likely to receive corticosteroid therapy compared with those who did not receive RBV/rIFN (59.7% vs 44.9%; P = .006; Table 3).
Table 3.
Cointerventions and Outcomes Among Critically Ill Patients With Middle East Respiratory Syndrome Treated With Ribavirin/Recombinant Interferon
| Variable | RBV/rIFN (n = 144) | No RBV/rIFN (n = 205) | P Value |
|---|---|---|---|
| Medications | |||
| Corticosteroids | 86 (59.7) | 92 (44.9) | .006 |
| Oseltamivir | 67 (46.5) | 129 (62.9) | .002 |
| Other interventions | |||
| ECMO | 11 (7.6) | 11 (5.4) | .39 |
| Nitric oxide | 20 (13.9) | 24 (11.7) | .55 |
| Prone positioning | 12 (8.3) | 21 (10.2) | .55 |
| Renal replacement therapy | 74 (51.4) | 100 (48.8) | .63 |
| Vasopressors | 111 (77.1) | 165 (80.5) | .44 |
| Blood transfusion | 58 (40.3) | 58 (28.3) | .02 |
| Noninvasive positive pressure ventilation | 50 (34.7) | 56 (27.3) | .14 |
| Invasive ventilation | 126 (87.5) | 171 (83.4) | .29 |
| Neuromuscular blockade | 46 (31.9) | 87 (42.4) | .047 |
| High-frequency oscillation ventilation | 13 (9.0) | 13 (6.3) | .35 |
| Outcome | |||
| Hospital mortality | 107 (74.3) | 130 (63.4) | .03 |
| 90-d mortality | 106 (73.6) | 126 (61.5) | .02 |
| 28-d mortality | 97 (67.4) | 119 (58.0) | .08 |
| MERS-CoV RNA clearance, d, median (Q1, Q3)a | 28 (17.0, 38.0) | 22 (18.0, 27.0) | .82 |
| Duration of invasive MV, d, median (Q1, Q3) | 10 (5, 17) | 9 (4, 16) | .18 |
| ICU LOS, d, median (Q1, Q3) | 11 (6.5, 20.0) | 8 (5.0, 17.5) | .02 |
| Hospital LOS, d, median (Q1, Q3) | 17 (10, 28) | 20 (10, 36) | .48 |
Data are presented as no. (%) unless otherwise indicated. The cointerventions were recorded throughout the ICU stay irrespective to the timing of RBV/rIFN. For categorical variables, χ 2 test was used to calculate the P value. For continuous variables, Mann-Whitney U test was used to calculate the P value.
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LOS, length of stay; MERS-CoV, Middle East respiratory syndrome coronavirus; MV, mechanical ventilation; RBV/rIFN, ribavirin/recombinant interferon.
aBased on survival analysis. Log-rank test was used to calculate P value. Patients were censored if they never cleared MERS-CoV RNA or at the time of last real-time reverse-transcription polymerase chain reaction test.
Mortality
Crude 90-day mortality was higher in patients who received RBV/rIFN therapy compared to those who did not (106/144 [73.6%] vs 126/205 [61.5%]; P = .02; Table 3). Multivariable logistic regression showed that RBV/rIFN therapy was associated with increased 90-day mortality (adjusted odds ratio [aOR], 2.27 [95% confidence interval {CI}, 1.20–4.32]; P = .01; Table 4). Using Cox proportional hazards analysis accounting for time-varying exposure, RBV/rIFN therapy was also associated with increased 90-day mortality (adjusted hazard ratio [aHR], 1.52 [95% CI, 1.13–2.06]; P = .006). However, using marginal structural model RBV/rIFN therapy was not associated with a significant difference in 90-day mortality (aOR, 1.03 [95% CI, .73–1.44]; P = .87).
Table 4.
Association of Ribavirin/Recombinant Interferon With 90-Day Mortality and Middle East Respiratory Syndrome (MERS) Coronavirus RNA Clearance in Critically Ill Patients With MERS
| Day 90 Mortality | MERS-CoV RNA Clearance | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Logistic Regression | Cox Proportional Hazards Regression Model | Marginal Structural Model | Cox Proportional Hazards Regression Model | Marginal Structural Cox Proportional Hazards Model | |||||||||||
| Variable | No. | aOR (95% CI) | P Value | No. | aHR (95% CI) | P Value | No. | aOR (95% CI) | P Value | No. | aHR (95% CI) | P Value | No. | aHR (95% CI) | P Value |
| RBV/rIFN vs no RBV/ rIFN (ref) | 342 | 2.27 (1.20–4.32) | .01 | 342 | 1.52 (1.13–2.06) | .006 | 342 | 1.03 (.73–1.44) | .87 | 154 | 1.09 (.58–2.04) | .80 | 154 | 0.65 (.30–1.44) | .29 |
| RBV vs no RBV (ref) | 342 | 2.02 (1.09–3.73) | .02 | 342 | 1.41 (1.04–1.90) | .03 | 342 | 1.19 (.84–1.68) | .33 | 154 | 1.03 (.55–1.95) | .92 | 154 | 0.53 (.23–1.23) | .14 |
| rIFN vs no rIFN (ref) | 342 | 2.53 (1.32–4.85) | .005 | 342 | 1.57 (1.18–2.09) | .002 | 342 | 1.05 (.74–1.48) | .80 | 154 | 1.03 (.55–1.92) | .92 | 154 | 0.86 (.38–1.93) | .71 |
Logistic regression and Cox proportional hazards regression models were adjusted for the following variables: diabetes with chronic complications, liver disease, renal disease, any malignancy including leukemia or lymphoma, Sequential Organ Failure Assessment (SOFA) score on day 1, source of infection (community-acquired/healthcare worker, hospital-acquired/non–healthcare worker, hospital-acquired), and year (before 1 July 2014 and after). For logistic regression, Hosmer-Lemeshow goodness-of-fit test was used to assess the model fitness, and P values were not significant for all analyses. For marginal structural model, adjustment was made for the same afore mentioned baseline characteristics and for the following time-varying covariates: SOFA on the index day of RBV/rIFN initiation and the previous day, ventilation status on the index day and on the previous day (0: not ventilated, 1: noninvasive ventilation, 2: invasive mechanical ventilation, 3: oxygen rescue therapy), hemoglobin, white cell count, aspartate aminotransferase and creatinine on the index day, and corticosteroid therapy on the index day. For Cox proportional hazards regression model and marginal structural Cox proportional hazards model, we censored patients if they never cleared MERS-CoV RNA or at the time of last real-time reverse-transcription polymerase chain reaction test.
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; CI, confidence interval; MERS-CoV, Middle East respiratory syndrome coronavirus; RBV, ribavirin; rIFN, recombinant interferon.
MERS-CoV RNA Clearance
Crude analysis showed that MERS-CoV RNA clearance was not different between the 2 groups (median, 28 days [Q1, Q3: 17, 38] vs 22 days [Q1, Q3: 18, 27]; P = .82; Table 3). Using a Cox proportional hazards regression model adjusting for baseline covariates and accounting for the RBV/rIFN therapy as a time-varying covariate, there was no significant association of RBV/rIFN with faster MERS-CoV RNA clearance (aHR, 1.09 [95% CI, .58–2.04]; P = .80). A marginal structural Cox proportional hazards model similarly showed no significant association (aHR, 0.65 [95% CI, .30–1.44]; P = .29).
Subgroup and Sensitivity Analyses
Analyses of RBV therapy vs no RBV and rIFN vs no rIFN were consistent with results to the primary analysis, with no significant association with 90-day mortality or MERS-CoV RNA clearance using marginal structural modeling (Table 4). When the logistic regression model was adjusted for clustering by centers in addition to the previously mentioned baseline variables, there remained no association of RBV/rIFN with 90-day mortality. Examining different types of rIFN on 90-day mortality using a similar logistic regression model and adjusting for clustering by center showed similar results (Supplementary Table 2).
Safety Endpoints
There were no differences between the 2 groups group over time in hemoglobin, WBC count, platelet count, AST, ALT, bilirubin, INR, lactic acid, or creatinine (Supplementary Figure 3). However, patients treated with RBV/rIFN received more blood transfusions compared with those who were not treated with RBV/rIFN (58/144 [40.3%] vs 58/205 [28.3%]; P = .02; Table 3).
DISCUSSION
While benefit of RBV/rIFN was suggested by preclinical studies, our observational study that accounted for baseline and time-varying differences among 349 critically ill patients with MERS treated with RBV/rIFN, or not, demonstrates that RBV/rIFN was not associated with decreased mortality or with faster MERS-CoV RNA clearance.
What are the possible explanations for lack of clinical and virological benefit? First, it has been shown that the RBV concentrations required to inhibit MERS-CoV replication are much higher than clinically achievable concentrations with oral dosing [5]. Second, the lack of rIFN effectiveness may be related to the type used. One study examined the in vitro MERS-CoV susceptibility to different rIFN preparations (rIFN-α2b, rIFN-γ, rIFN-universal, rIFN-α2a, rIFN-β) and found that rIFN-β had the strongest MERS-CoV inhibition, at 41 times lower than the previously reported 50% inhibitory concentration (56.08 U/mL) of rIFN-α2b [5]. Another in vitro study found that serum concentrations achievable at therapeutic doses of rIFN-β-1b were 3–4 times higher than the in vitro inhibitory concentrations of MERS-CoV, whereas those of other rIFN preparations and RBV were lower than inhibitory levels [24]. Of note, none of the patients in the current cohort received rIFN-β-1b. An RCT (MIRACLE) is currently recruiting patients examining the effect of a combination of lopinavir/ritonavir and rIFN-β-1b on mortality of hospitalized patients with MERS [16]. Third, the positive, but modest, effect observed in previous rhesus macaque experiments occurred after very early treatment (8 hours after inoculation of with MERS-CoV) and with the administration of high doses of RBV/rIFN-α2b. In contrast, it took a median of 5 days for patients in our cohort to present to the hospital and another 4 days to start therapy.
To assess safety profile of RBV/rIFN, we compared the levels of hemoglobin, WBC count, platelet count, AST, ALT, bilirubin, INR, lactate, and creatinine and we found no difference between the 2 groups over the ICU stay. However, these data should be interpreted in the context of the potential for time-varying confounding. Of note, we found that patients who were treated with RBV/rIFN received more blood transfusions than patients who were not treated with RBV/rIFN, which is consistent with the findings of a previous study [9]. These changes may be related to hemolysis induced by RBV therapy.
Our study demonstrates that not accounting for time-varying confounding can substantially influence the results of observational studies. Our study found an association of RBV/rIFN with higher crude mortality on crude analysis, with adjustment for baseline characteristics alone (by logistic regression) and with adjustment for baseline characteristics including the time to initiation of RBV/rIFN (by Cox proportional hazards analysis). Ultimately, we did not find evidence that RBV/rIFN therapy was associated with reduced MERS mortality when adjusting for baseline and time-dependent covariates using a marginal structural model. This finding suggests that much of the observed increased mortality may have been related to confounding due to indication bias, and calls for caution when interpreting observational studies that do not account for time-varying confounders.
Our study examined RBV/rIFN therapy in a large multicenter cohort of critically ill patients with MERS. Limitations include its retrospective nature and lack of randomization (and therefore inevitable initial imbalance in potential confounders). Although marginal structural models adjust for time-varying confounding, unmeasured confounders cannot be entirely excluded. Quantitative data on viral loads were not available. Because practices for repeating MERS-CoV rRT-PCR varied among centers and because of competing risk of mortality, sufficient data to assess MERS-CoV RNA clearance were available for only about 50% of the patients. RCTs remain the best approach to derive the most unbiased estimates of treatment effect. Most patients included in this study were diagnosed with MERS prior to the launch of the first therapeutic RCT mentioned earlier (MIRACLE). Since then, efforts have focused on considering eligible patients in this trial.
In conclusion, RBV/rIFN therapy (RBV and/or rIFN-α2a, rIFN-α2b, or rIFN-β1a) is not associated with reduction in 90-day mortality or with faster MERS-CoV RNA clearance. Future studies should test the antiviral and clinical effectiveness of newer antiviral interventions that show more promising results in relevant animal models [25, 26].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Y. A., S. S., E. Q., J. J., H. B., F. G. H., and R. F. were responsible for conception and design, interpretation of data, and drafting of the manuscript. Y. A. was responsible for overall supervision of the study. Y. A. and J. J. were responsible for statistical analysis. Y. A., S. S., Y. M., F. H., A. Al-Omari, E. Q., B. A., A. Almotairi, K. A. K., A. Abdulmomen, I. Q., A. S., A. M., O. S., R. A., K. M., A. R., G. A., H. B., A. Al Harthy, A. K., J. G., A. Al-Aithan, A. Al-Dawood, and L. M. were responsible for acquisition of data and administrative and technical support. Y. A., S. S., Y. M., F. H., A. Al-Omari, E. Q., B. A., A. Almotairi, K. A. K., A. Abdulmomen, I. Q., A. S., A. M., O. S., R. A., K. M., A. R., G. A., H. B., A. Al Harthy, A. K., J. G., A. Al-Aithan, A. Al-Dawood, L. M., F. G. H., and R. F. performed critical revision of the manuscript for important intellectual content.
Acknowledgments. The authors thank the International Severe Acute Respiratory and Emerging Infection Consortium for its support in the database construction.
Potential conflicts of interest. Y. A. is the principal investigator on the MERS-CoV Infection Treated With a Combination of Lopinavir/Ritonavir and Interferon β-1b trial. Y. A. and F. G. H. are nonpaid consultants on therapeutics for Middle East respiratory syndrome for Gilead Sciences, SAB Biotherapeutics, and Regeneron. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Arabi YM, Balkhy HH, Hayden FG, et al. Middle East respiratory syndrome. N Engl J Med 2017; 376:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep 2013; 3:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med 2013; 19:1313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Momattin H, Mohammed K, Zumla A, Memish ZA, Al-Tawfiq JA. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV)—possible lessons from a systematic review of SARS-CoV therapy. Int J Infect Dis 2013; 17:e792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hart BJ, Dyall J, Postnikova E, et al. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol 2014; 95:571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, Butt TS, Al Mutairy E. Ribavirin and interferon-α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antivir Ther 2015; 20:87–91. [DOI] [PubMed] [Google Scholar]
- 7. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis 2014; 20:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother 2015; 70:2129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 2014; 14:1090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al Ghamdi M, Alghamdi KM, Ghandoora Y, et al. Treatment outcomes for patients with Middle Eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect Dis 2016; 16:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008; 167:492–9. [DOI] [PubMed] [Google Scholar]
- 12. Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studies. Am J Respir Crit Care Med 2003; 168:49–53. [DOI] [PubMed] [Google Scholar]
- 13. Arabi YM, Shalhoub S, Al Omari A, et al. Effect of ribavirin and interferon on the outcome of critically ill patients with MERS. Am J Resp Crit Care Med 2017; 195:A6067. [Google Scholar]
- 14. Arabi YM, Al-Omari A, Mandourah Y, et al. Saudi Critical Care Trial Group Critically ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med 2017; 45:1683–95. [DOI] [PubMed] [Google Scholar]
- 15. Corman VM, Muller MA, Costabel U, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill 2012; 17. pii:20334. [DOI] [PubMed] [Google Scholar]
- 16. Arabi YM, Alothman A, Balkhy HH, et al. MIRACLE Trial Group Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials 2018; 19:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). Available at: https://isarictghnorg/. Accessed 25 March 2016.
- 18. Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26:1793–800. [DOI] [PubMed] [Google Scholar]
- 19. Arabi YM, Mandourah Y, Al-Hameed F, et al. Saudi Critical Care Trial Group Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018; 197:757–67. [DOI] [PubMed] [Google Scholar]
- 20. Delaney JW, Pinto R, Long J, et al. Canadian Critical Care Trials Group H1N1 Collaborative The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care 2016; 20:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 11:561–70. [DOI] [PubMed] [Google Scholar]
- 22. Faries DE, Kadziola ZA. Analysis of longitudinal observational data using marginal structural models. In: Analysis of observational health care data using SAS. Cary, NC: SAS Institute Inc; 2010:211. [Google Scholar]
- 23. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–60. [DOI] [PubMed] [Google Scholar]
- 24. Chan JF, Chan KH, Kao RY, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect 2013; 67:606–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Trans Med 2017; 9. doi:10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beigel JH, Voell J, Kumar P, et al. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis 2018; 18:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.