Abstract
Background
Influenza antigenic point-of-care (POC) tests are too insensitive for individual reliable diagnosis of influenza virus infections without additional laboratory confirmation. Molecular POC tests could be a valuable alternative.
Objectives
To evaluate the first influenza molecular POC test commercially available, the Cepheid Xpert Flu A Panel designed to simultaneously detect influenza A virus and subtype A(H1N1) 2009 pandemic virus, and compare it with in-house real-time RT-PCR (qRT-PCR).
Study design
Clinical specimens positive for influenza virus and influenza virus isolates with different viral loads and of different type and subtype were used to determine the analytical reactivity and sensitivity. A panel of pathogen negative specimens and isolates of 19 different respiratory pathogens were used to determine the analytical specificity.
Results
Except A(H9N2) virus the Xpert Flu A Panel detected A(H1N1) seasonal and 2009 pandemic, A(H3N2), A(H5N2), A(H5N1) and A(H7N7) viruses and correctly subtyped A(H1N1) 2009 virus. Analytical sensitivity was similar to qRT-PCR in the range of 400–5000 viral particles per ml. However, of most subtypes some specimens with cycle threshold values greater than 30 in qRT-PCR and A(H1N1) 2009 specimens with inconsistent results in the qRT-PCR due to primer or probe mismatches were not detected in the Xpert Flu A Panel. Analytical specificity was 100%.
Conclusions
The Xpert Flu A Panel is the first commercially available POC molecular test for detection of influenza A virus and determination of the H1 2009 subtype and is analytically reasonable sensitive compared with qRT-PCR and highly specific and therefore a welcome alternative to antigenic POC tests.
Abbreviations: CLIA, Clinical Laboratory Improvement Amendments; Ct, cycle threshold; EM, electron microscopy; EQA, External Quality Assessment; FDA, US Food and Drug Administration; GLY, Glucose-Lactalbumin-Yeast viral transport medium; POC, point-of-care; QCMD, Quality Control for Molecular Diagnostics; qRT-PCR, in-hous real-time RT-PCR; RT-PCR, reverse transcriptase polymerase chain reaction; SPC, sample processing control; UTM, Universal Transport Medium
Keywords: Diagnostics, Point-of-care, Molecular, Influenza, Pandemic, Seasonal
1. Background
For diagnosis of influenza virus infections a variety of techniques is being used, of which the application depends on the clinical setting and purpose.1 Time consuming virus isolation is required to determine the antigenic match with the influenza vaccine whilst point-of-care (POC) antigenic tests are used for rapid determination of infection to be able to timely implement therapy for the patient or preventive measures to limit spread in outbreak situations.1, 2, 3, 4 A major drawback, however, of POC antigenic tests is their relative insensitivity and hence unsuitability for individual patient diagnosis without additional (confirmatory) tests.2, 3 Therefore, a need for more sensitive POC tests arose, in particular for diagnosing infections with the highly fatal A(H5N1) influenza virus.5 Although real-time RT-PCR (qRT-PCR) is rapid it needs expensive equipment, a well suited laboratory and trained personnel for RNA purification and RT-PCR reagent preparation and still takes about 4 hours.1, 3 The main bottle-neck in developing POC qRT-PCR has been sample preparation.6 Several prototype POC qRT-PCR systems for diagnosing influenza and other respiratory virus infections have been developed, e.g. the Liat Tube real-time (RT-)PCR from IQuum Inc. (http://www.iquum.com), the Enigma ML real-time PCR from Enigma Diagnsotics Ltd. (http://www.enigmadiagnostics.com), the FilmArray Respiratory Panel from Idaho Technology Inc. (http://www.idahotech.com/FilmArray) and the Xpert Flu A Panel from Cepheid (http://www.cepheid.com/tests-and-reagents/xpert-flu-a-panel). They all combine sample preparation with single or multiplex qRT-PCR in a closed system and an assay can be completed within an hour. A(H1N1) 2009 pandemic virus was included in these assays as POC qRT-PCR systems could be particular useful for on-site triage and for rapid and easy diagnosis during off-office hours. The first commercial available POC qRT-PCR test for detecting influenza A and subtyping A(H1N1) 2009 virus on the market was the Xpert Flu A Panel which is evaluated in this study.
2. Objectives
Evaluation of the Xpert Flu A Panel POC test and comparison with in-house real-time RT-PCR (qRT-PCR) assays used for influenza A virus detection and subtyping of the A(H1N1) 2009 pandemic virus.
3. Study design
3.1. Xpert Flu A Panel
The primers en probes in the Xpert Flu A Panel are designed for the generic detection of the type A influenza virus matrix gene and for the specific detection of the haemagglutinin gene of the A(H1N1) 2009 pandemic virus. The panel consists of a single-use cartridge with freeze-dried reagents for RNA extraction and real-time RT-PCR to which nucleic acid binding reagent in a squeeze ampoule and the specimen using a disposable pipette provided in the kit has to be added. A 4-site GeneXpert Dx System (Cepheid, Sunnyval, CA) attached to a laptop computer with a barcode reader was used. Assays were performed according to manufacturer's instructions.
3.2. qRT-PCR
Briefly, nucleic acid was purified from specimens using a MagnaPure LC system with the MagnaPure LC total nucleic acid isolation kit (Roche Diagnostics, Almere, The Netherlands). qRT-PCR was performed on a LightCycler 480 (Roche Diagnostics). Primary qRT-PCR diagnostics of clinical specimens was initially done using the Taqman Master kit for two-step qRT-PCR (Roche Diagnostics, Almere, The Netherlands), replaced during the 2009 A(H1N1) pandemic by the TaqMan EZ RT-PCR core reagents kit for one-step qRT-PCR (Applied Biosystems, Nieuwerkerk aan den IJssel, The Netherlands). For optimal comparison, influenza virus containing specimens were repeated using the one-step qRT-PCR in parallel with the Xpert Flu A Panel on the same day. Detailed information about the (qRT-)PCR assays for the pathogens listed in Table 1, Table 2 are available upon request.
Table 1.
Reactivity Xpert Flu A Panel.
| Species | N | Ct values q(RT)-PCR (range)a | Xpert Flu A Panel positive |
|
|---|---|---|---|---|
| Influenza A | H1 2009 | |||
| Influenza A(H1N1) seasonal, clinical specimens (2007/2008) | 6 | 23.49–32.51 | 5 | 0 |
| Influenza A(H3N2), clinical specimens (2007/2008 and 2008/2009) | 6 | 29.05–32.62 | 5 | 0 |
| Influenza A(H1N1) 2009, clinical specimens, normal results in qRT-PCR (matrix, H1v and N1v positive) | 7 | 26.61–34.19 | 5 | 5 |
| Influenza A(H1N1) 2009, clinical specimens, inconsistent results in qRT-PCR (2 with very low viral load, 1 with only H1v positive, 1 with matrix negative but H1v and N1v positive) | 4 | NAb | 0 | 0 |
| Influenza B/Victoria/2/87 lineage clinical specimen | 1 | 30.79c | 0 | 0 |
| Influenza B/Yamagata/16/88 lineage clinical specimen | 1 | 25c | 0 | 0 |
| Influenza A/Chicken/Pennsylvania/2152/1983 (H5N2) | 4 | 24.39–33.82 | 2 | 0 |
| Influenza A/Netherlands/33/2003 (H7N7) | 3 | 31.6–34.47 | 3 | 0 |
| Influenza A/Chicken/Saudi-Arabia/569017/2000 (H9N2) | 3 | 32.15–36.12 | 0 | 0 |
| Influenza A/Duck/Vietnam/TG24-01/2005 (H5N1) | 1 | 24.18 | 1 | 0 |
| QCMD A/Netherlands/344/2006 (H3N2) | 2 | 35.03–35.16 | 2 | 0 |
| QCMD A/Netherlands/602/2009 (H1N1) 2009 | 2 | 32.99–33.01 | 2 | 2 |
| QCMD A/Netherlands/361/2006 (H1N1) seasonal | 2 | 32.2–32.34 | 2 | 0 |
| QCMD A/Hong Kong/213/03 (H5N1) | 2 | 34.69–34.85 | 0 | 0 |
| QCMD B/Netherlands/207/2006 (B/Victoria/2/87 lineage) | 1 | 33.66c | 0 | 0 |
Matrix qRT-PCR values of parallel retesting unless otherwise indicated.
NA: not applicable.
Original qRT-PCR result.
Table 2.
Specificity Xpert Flu A Panel.
| Species | N | Ct values q(RT)-PCR (range)a | Xpert Flu A Panel negative |
|
|---|---|---|---|---|
| Influenza A | H1 2009 | |||
| Respiratory Syncytial Virus type A | 1 | 25.74 | 1 | 1 |
| Respiratory Syncytial Virus type B | 1 | 23.98 | 1 | 1 |
| Rhinovirus (different types) | 3 | 22.60–26.05 | 3 | 3 |
| Enterovirus (different types) | 3 | 12.38–29.63 | 3 | 3 |
| Human Coronavirus OC43 | 1 | 25.06 | 1 | 1 |
| Human Coronavirus 229E | 1 | 28.43 | 1 | 1 |
| Human Coronavirus NL63 | 1 | 24.22 | 1 | 1 |
| Adenovirus | 1 | 22.07 | 1 | 1 |
| Human Metapneumovirus | 1 | 28.31 | 1 | 1 |
| Chlamydophila pneumoniae | 1 | Conv. PCR | 1 | 1 |
| Mycoplasma pneumoniae | 1 | Conv. PCR | 1 | 1 |
| Parainfluenzavirus Type 1 | 1 | 25.98 | 1 | 1 |
| Parainfluenzavirus Type 2 | 1 | 28.76 | 1 | 1 |
| Parainfluenzavirus Type 3 | 1 | 26.89 | 1 | 1 |
| Parainfluenzavirus Type 4 | 1 | 28.38 | 1 | 1 |
| Virus negative specimens (3 clinical and 1 QCMD) | 4 | – | 4 | 4 |
Based on average original results of eight tests per pathogen in the in-house q(RT-)PCR assays, unless otherwise indicated.
3.3. Specimens
For determination of the analytical reactivity, clinical specimens (combined nose and throat swabs in Glucose-Lactalbumin-Yeast viral transport medium [GLY]) containing different influenza viruses with a variety of viral loads or with inconsistent qRT-PCR results, several influenza virus isolates in different dilutions in GLY and the Quality Control for Molecular Diagnostics (QCMD) 2009 influenza virus haemagglutinin typing External Quality Assessment (EQA) panel consisting of various dilutions of influenza virus isolates in virus transport medium were used (Table 1). For determination of the analytical specificity, a respiratory panel and negative specimens were used (Table 2). For determination of the analytical sensitivity, qRT-PCR cycle threshold (Ct) values were used as a semi-quantitative measure whereas for determination of the exact viral load in virus particles per ml an electron microscopy (EM) counted standard of human influenza A/Puerto Rico/8/1934 (H1N1) was used. All specimens that were parallel tested with the Xpert Flu A panel and the qRT-PCR had the same freeze/thaw cycle. As Universal Transport Medium (UTM) (Copan Italia S.p.A, Italy) is the preferred specimen transport medium for the Xpert Flu A Panel and we use GLY transport medium, we compared performance of both transport media by diluting clinical specimens in UTM and GLY and analysing the diluted specimens by both assays.
4. Results
4.1. Analytical reactivity
Except for the A(H9N2) and the QCMD A(H5N1) virus specimens, all influenza A virus subtypes were correctly detected in the Xpert Flu A Panel matrix test, and for the A(H1N1) 2009 virus in the Xpert Flu A Panel H1 2009 test (Table 1). The Xpert Flu A Panel H1 2009 test did not show any cross-reactivity with the H genes of A(H1N1) seasonal, A(H3N2), A(H5N2), A(H5N1), A(H7N7) and A(H9N2) virus containing specimens. However, there were some issues. One seasonal A(H1N1) clinical specimen, one A(H3N2) clinical specimen and two A(H5N2) virus isolate specimens were not detected in the Xpert Flu A Panel matrix test. In addition, the three A(H1N1) 2009 specimens with inconsistent results in the qRT-PCR were neither detected in the matrix nor in the H1 2009 specific Xpert Flu A Panel tests.
4.2. Analytical specificity
None of the 19 specimens of the respiratory panel containing viruses other than influenza A virus and bacteria commonly detected in specimens from patients with influenza-like illness were positive in the Xpert Flu A Panel tests (Table 2). In addition, four virus negative specimens were also negative in the Xpert Flu A Panel tests.
4.3. Analytical sensitivity
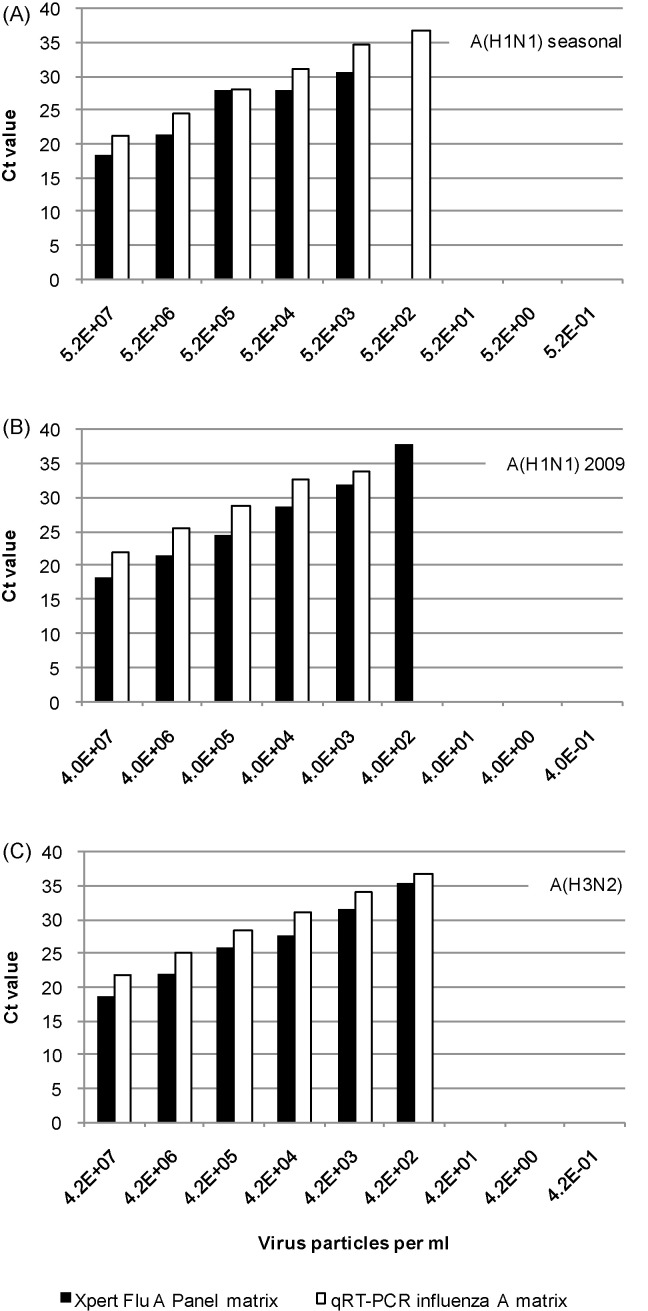
Using the EM counted standard the viral load of virus isolates were determined, for A/Bilthoven/4310800347/2008 (H1N1) seasonal 5.2E + 08 ± SD 3.2E + 07 virus particles per ml, for A/Bilthoven/4310902347/2009 (H1N1) 2009 4.0E + 08 ± SD 2.7E + 07 virus particles per ml and for A/Bilthoven/4310801070/2008 (H3N2) 4.2E + 08 ± SD 1.8E + 07 virus particles per ml. Both qRT-PCR and Xpert Flu A Panel matrix tests performed similar for all three viruses with an analytical sensitivity of about 400–5000 viral particles per ml (Fig. 1 ). The Xpert Flu A Panel H1 2009 test was one tenfold dilution step less sensitive than the Xpert Flu A Panel matrix test with the A(H1N1) 2009 virus. All influenza A viruses not detected with the Xpert Flu A Panel as shown in Table 1 were specimens with lowest viral load tested for a given subtype; Ct values in the qRT-PCR matrix assay greater than 30.
Fig. 1.
Analytical sensitivity of the Xpert Flu A Panel matrix test and our qRT-PCR matrix test for: (A) A(H1N1) seasonal, (B) A(H1N1) 2009 and (C) A(H3N2) influenza virus detection.
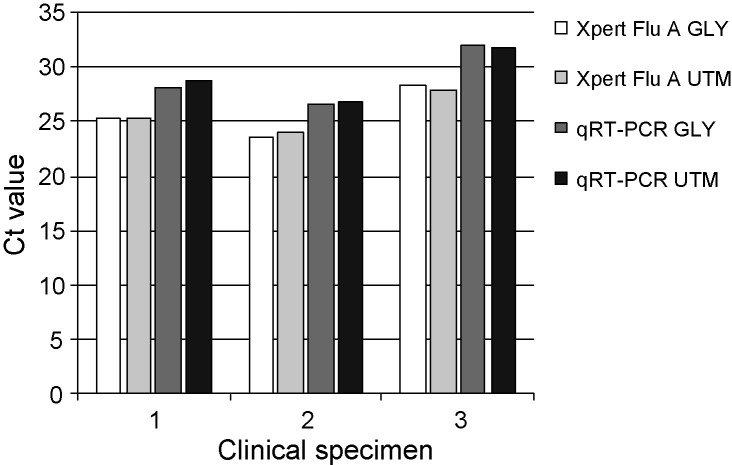
4.4. Comparison transport media
Preliminary results from other users of the Xpert Flu A Panel assay suggested that freeze-thawing of nose or throat swabs collected in UTM might result in clogging of the cartridge. With none of the freeze-thawed clinical specimens in GLY analysed in this study we noticed any reduced performance due to clogging of the cartridge. No significant differences were found between the Xpert Flu A Panel and the qRT-PCR with three original clinical specimens diluted 1:10 in GLY or UTM and subsequently freeze-thawed (Fig. 2 ).
Fig. 2.
Performance of Xpert Flu A Panel matrix and in-house qRT-PCR matrix tests with different virus transport media after a freeze–thaw cycle.
5. Discussion
The US Food and Drug Administration (FDA) granted Emergency Use Authorization for the use of the Cepheid Xpert Flu A Panel (granted December 2009) and the Iquum Liat Influenza A/2009 H1N1 Assay (granted May 2010) tests for detection of influenza A virus and identification of the H1N1 2009 subtype in laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) to perform “moderate complexity” testing, which enables the tests to be performed in hospital near-patient, aka POC, settings. For POC use the GeneXpert system is available as a 1-site apparatus linked to a laptop and barcode reader. The Iquum system is an all-in-one system and does not need additional equipment. However, compared to the Iquum system the GeneXpert system offers already other useful rapid diagnostics, e.g. methicillin-resistant Staphylococcus aureus and enterovirus in cerebrospinal fluid.7, 8 Nevertheless, most promising for rapid diagnosis of respiratory illness is the FilmArray Respiratory Panel which combines sample preparation with real-time (RT-)PCR detection of 18 viruses, including the A(H1N1) 2009 variant, and three bacteria commonly found in acute respiratory infections within one hour (http://www.idahotech.com/FilmArray).9 However, this system is currently still in its clinical trial phase for FDA clearance.
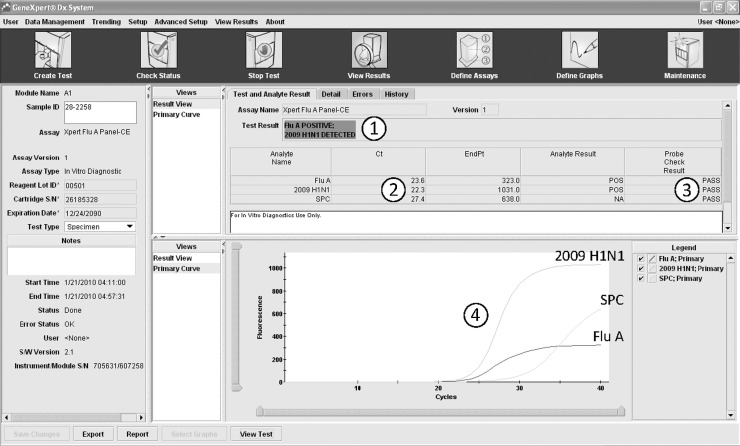
The Xpert Flu Panel A test was easy to perform and took only one hour from start to result with less than one minute hands-on time. Results were easy to interpret with clear positive/negative information, for e.g. clinicians and information in Ct values and amplification curves for users with qRT-PCR experience (Fig. 3 ). Validity of the test was clearly indicated by the results of the sample processing control and the probe-checks.
Fig. 3.
Example results screen of the Xpert Flu A Panel. (1) Clear-cut colour coded (and in writing) results for none laboratory experienced person, red for positive, green for negative. (2) Ct values for moderately experienced laboratory person giving semi-quantitative information. (3) Probe-check information confirming validity of the results. (4) Amplification curves for laboratory expert interpretation of results. SPC: sample processing control.
Our analytical evaluation shows the potential of the Xpert Flu A Panel to become a real POC molecular test, however, some limitations were determined. The Xpert Flu A Panel does detect human influenza A viruses including the A(H1N1) 2009 variant with an analytical sensitivity similar to our qRT-PCR, however, some low viral load specimens were not detected. In addition, viral load limitation was particularly noticed for avian influenza viruses A(H5N1) and A(H5N2), whilst A(H9N2) was not detected at all. This is an important limitation as there is an urgent need for molecular POC tests detecting avian influenza viruses.5
The Xpert Flu A Panel H1 2009 test was found specific for the H gene of the A(H1N1) 2009 virus and was able to subtype all A(H1N1) 2009 virus containing specimens that were found positive in the matrix test of the Xpert Flu A Panel. However, all specimens with repeatable inconsistent A(H1N1) 2009 results in the qRT-PCR were not detected in the Xpert Flu A Panel. Sequencing of the primer and probe targets of the qRT-PCR of these viruses revealed that there were some mismatches explaining the inconsistent results (not shown). As the primer and probe binding targets included in the Xpert Flu A Panel were not revealed by Cepheid it was impossible to determine whether lack of performance with some specimens was caused by mismatching of primers and/or probes. Drift of influenza viruses necessitates regular updating of primers and probes and especially quick updating is needed when a virus with mutated primer or probe binding site becomes the dominant virus in a season. Emergency use authorisation of commercial influenza molecular tests might overcome this problem, however, even emergency development and validation might take longer than a season lasts. Therefore, molecular POC can never fully replace in-house qRT-PCR for detection and identification of influenza viruses.
In contrast to findings of others we could not confirm clogging problems due to the use of UTM and freeze/thawing of clinical specimens. All our historical specimens have been vigorously vortex on arrival disrupting large particles and mucus flocks which might play a role in clogging when omitted in POC settings.
Although the analytical sensitivity of the Xpert Flu A Panel exceeds the published analytical sensitivity of antigenic A(H1N1) 2009 tests10 by at least 1000-fold, the purchase of a 1-site GeneXpert machine and the costs per test (approximately 45 euros per cartridge) will be still a bottle-neck compared to the antigenic tests (approximately 15 euros per test) for real POC use. However, the Xpert Flu A Panel is a welcome alternative to antigenic rapid tests in large practices and to qRT-PCR in laboratories for emergency diagnostics during off-office hours. To become really useful as POC test, especially in areas where avian influenza viruses infecting humans are endemic, the Xpert Flu A Panel should be improved for detection of all influenza A viruses and for determination of relevant subtypes.
In conclusion, the Xpert Flu A Panel is the first commercially available POC molecular test for detection of influenza A virus and determination of the H1 2009 subtype, is analytically reasonable sensitive compared with qRT-PCR and highly specific and therefore a welcome alternative to antigenic POC tests.
Funding
None.
Competing interest
None declared.
Ethical approval
Not required.
Acknowledgements
The authors thank Ngoc Hoa Chung and the Tuberculosis Reference Laboratory, RIVM, for excellent technical assistance, Martin Schutten, Erasmus University Medical Center, Rotterdam, The Netherlands for EM counted A(H1N1) standard, Guus Koch, Central Veterinary Institute, Lelystad, The Netherlands for A(H5N2) and A(H9N2) strains, Brunhilde Schweiger, RKI, Berlin, Germany for A/Duck/Vietnam/TG24-01/2005 virus, QCMD for granting permission to use the influenza subtyping EQA panel 2009 and information in the report for this study, general practitioners of the Continuous Morbidity Registry coordinated by NIVEL Netherlands Institute for Health Services Research for providing clinical specimens, and Cepheid for help in setting up the GeneXpert Dx System.
References
- 1.Dwyer D.E., Smith D.W., Catton M.G., Barr I.G. Laboratory diagnosis of human seasonal and pandemic influenza virus infection. Med J Aust. 2006;185(10 Suppl.):S48–53. doi: 10.5694/j.1326-5377.2006.tb00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2005. WHO recommendations on the use of rapid testing for influenza diagnosis. Available from: http://www.who.int/csr/disease/avian_influenza/guidelines/RapidTestInfluenza_web.pdf. [Google Scholar]
- 3.Foo H., Dwyer D.E. Rapid tests for the diagnosis of influenza. Aust Prescr. 2009;32:64–67. [Google Scholar]
- 4.Meijer A. Importance of rapid testing to combat the global threat of bird flu. Expert Rev Mol Diagn. 2006;6:1–4. doi: 10.1586/14737159.6.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Chung Expert consultation on diagnosis of H5N1 avian influenza infections in humans. Influenza Other Respir Viruses. 2007;1:131–138. doi: 10.1111/j.1750-2659.2007.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dineva M.A., MahiLum-Tapay L., Lee H. Sample preparation: a challenge in the development of point-of-care nucleic acid-based assays for resource-limited settings. Analyst. 2007;132:1193–1199. doi: 10.1039/b705672a. [DOI] [PubMed] [Google Scholar]
- 7.Laurent C., Bogaerts P., Schoevaerdts D., Denis O., Deplano A., Swine C. Evaluation of the Xpert MRSA assay for rapid detection of methicillin-resistant Staphylococcus aureus from nares swabs of geriatric hospitalized patients and failure to detect a specific SCCmec type IV variant. Eur J Clin Microbiol Infect Dis. May 29, 2010 doi: 10.1007/s10096-010-0958-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Kost C.B., Rogers B., Oberste M.S., Robinson C., Eaves B.L., Leos K. Multicenter beta trial of the GeneXpert enterovirus assay. J Clin Microbiol. 2007;45:1081–1086. doi: 10.1128/JCM.01718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvarangan R, Metzler G, Nilsson K. Comparison of two multiplex respiratory pathogen PCR panels: The FilmArray™ 20-Pathogen Panel versus Luminex xTAG® 12-Pathogen Panel. Poster session presented at: annual meeting Pan American Society for Clinical Virology; 2010. Available from: http://www.idahotech.com/Support/posters.html.
- 10.Chan K.H., Lai S.T., Poon L.L., Guan Y., Yuen K.Y., Peiris J.S. Analytical sensitivity of rapid influenza antigen detection tests for swine-origin influenza virus (H1N1) J Clin Virol. 2009;45:205–207. doi: 10.1016/j.jcv.2009.05.034. [DOI] [PubMed] [Google Scholar]