Abstract
Background
Few studies have suggested the potential role of respiratory viruses in cystic fibrosis (CF) exacerbation, but their real impact is probably underestimated.
Method
Sixty-four sputum samples collected from 46 adult patients were included in the study: 33 samples were collected during exacerbation of CF, and 31 during the stable phase. After extraction, nucleic acids were tested for the presence of respiratory viruses. When rhinovirus (HRV) was detected, the 5′UTR and VP4/2 regions were sequenced, and phylogenetically analyzed. The characteristics of patients in exacerbation and stable phase were compared.
Results
Viruses were found in 25% of samples. The HRV viruses were the most frequently detected followed by coronaviruses. Only the HRV detection was significantly associated with the occurrence of CF pulmonary exacerbation (p < 0.027). Characterization of 5′UTR and VP4/2 regions of the HRV genome specified that HRV-A, -B, -C were detected. All HRV-C were recombinant HRV-Ca.
Conclusions
HRV were the most frequently detected viruses; their detection was significantly associated with the occurrence of an exacerbation. The reality of viral recombination between HRV was demonstrated in CF patients for the first time, raising the role of viruses in lung microbiota. Further studies are now warranted to decipher virus impact in CF.
Keywords: Cystic fibrosis, Pulmonary exacerbation, Rhinovirus, PCR
1. Introduction
Cystic fibrosis (CF) is the major genetic inherited disease in the European Caucasian population. In addition to bacteria, which are well known to cause recurrent exacerbations of CF-associated pulmonary disease and often determine the vital prognosis of patients [1], many airborne particles such as viral entities or fungal spores can also colonize the respiratory tract of patients [1], [2].
For a long time, the detection of respiratory viruses, especially in exacerbations of chronic lung diseases has been underestimated because of the lack of sensitive methods for virus detection. Since the sensitive molecular methods for detection of viruses are more and more common, several recent studies highlight the clinical importance of respiratory viruses especially during exacerbation of asthma, chronic obstructive pulmonary disease (COPD) or CF [3], [4], [5], [6], [7], [8], [9]. In addition, viruses that were not previously sought are now detected, such as human rhinoviruses (HRVs) within the Picornaviridae family. Among the HRV genus, a new species of viruses named HRV-C has been described recently, is involved in severe respiratory infections in elderly and in immunocompromised patients [5], [10], [11], [12]. Furthermore, HRV-C has been described in patients with CF exacerbation but the real impact in the progression of the lung disease remains unclear [7].
2. Objectives
The primary objective of our study was to investigate the frequency and species of viruses present in sputa collected from CF adult patients with either pulmonary exacerbation or stable disease, and to determine the clinical relevance of viruses. As the most frequently detected viruses were HRVs, we phylogenetically characterized them and identified potential clinical associations, expressly for a significant relationship between HRV detections and occurrence of CF exacerbations.
3. Materials and methods
3.1. Patients and samples
Sixty four sputum samples collected from 46 CF patients in Lille (31 samples, 24 patients), Dunkerque (3 samples, 3 patients), and Grenoble (30 samples, 19 patients) between October 2006 and December 2010 were prospectively screened for respiratory virus carriages (reference number of the institutional ethics committees of Lille Hospital, CPP 06/84 – Written informed consent was obtained from each patient). All patients had a well-documented diagnosis of CF with either the mutations identified in the CFTR gene or an abnormally high sweat chloride test. They were included by physicians according to the same criterion (an annual check-up or an exacerbation situation that required an expectorated sputum sample that allowed us to search also for bacterial and fungal microorganisms). Clinical data including spirometry, therapeutic, radiological and biological data were collected by clinic staff at each time of the visit. At each sampling time, the clinical status of the patients was defined as pulmonary exacerbation or stable period according to the physician defined requirement (i.e. recent changes in clinical parameters and/or modification of the pulmonary function provide criteria of exacerbation according to ERS statement) [13].
3.2. Microbial analysis of sputa
Twenty eight patients had one sputum examined, and 18 patients had two sequential sputa examined, which were considered as independent events since the delay between the samples was at least 6 months [2], [14]. Samples were performed by expectoration into a sterile cup after a water rinse to prevent excessive salivary contamination, analyzed according to a standardized protocol as previously described, and screened for respiratory virus carriage [2].
3.3. Molecular analysis of sputa
Total nucleic acids were obtained after mechanical lysis of sputum sample. Briefly, 200 μL of each sample were subjected to fluidification in a MagNAlyser (Roche Diagnostics) according to the manufacturer's instructions. QIAampMin Elute Virus Spin kit (QIAgen) was used on crude lysate according to the manufacturer's instructions. Purified nucleic acids were frozen at −80 °C until use. Reverse transcription was performed with RevertAid First Strand cDNA Synthesis kit (Fermentas) using 8 μL of previously obtained RNA. Detection of respiratory viruses was performed with Seeplex RV15 ACE Detection kit (Seegene) in three independent PCR reactions targeting influenza viruses A and B, RSV A and B, human adenovirus, human metapneumovirus (hMPV), human coronavirus (HCoV 229E, NL63, OC43 and HKU1), human parainfluenza viruses 1–4, HRV, human enteroviruses, and human bocaviruses in three independent PCR reactions. Amplification products were visualized after semi-automated capillary electrophoresis in a TapeStation Lab 901 (Eurobio) and analysis was performed with Tape DS12 software.
Samples that were positive for HRV were identified, based on sequencing of the VP4/VP2 and 5′UTR regions, according to the previously described nested RT-PCR strategy and the primers listed in Table 1 [15]. The three HRV-specific VP4/VP2, 5′UTR inner sense primers were included in the second amplification step. The amplicons were directly sequenced using ABI Prism 3500XL (Life Technologies) and results were analyzed with the SeqScape 2.7 software (Applied Biosystem).
Table 1.
Primers used for amplification of HRV VP4/VP2 and 5′UTR regions.
| 5′–3′ sequences | Region | Orientation | Positiona |
|---|---|---|---|
| CCGGCCCCTGAATGYGGCTA | VP4/VP2 | Outer sense | 458 |
| ACATRTTYTSNCCAAANAYDCCCAT | VP4/VP2 | Outer antisense | 1125 |
| ACCRACTACTTTGGGTGTCCGTG | VP4/VP2 | Inner sense | 547 |
| TCWGGHARYTTCCAMCACCANCC | VP4/VP2 | Inner antisense | 1087 |
| HCAAGYACTTCTGTYWCCCCSG | 5′UTR | Outer sense | 178 |
| GAAACACGGACACCCAAAGTAGT | 5′UTR | Outer antisense | 573 |
| TTAGCCRCATTCAGGGGCCGG | 5′UTR | Inner antisense | 477 |
The 5′ base position numbered according to the HRV-B serotype 14 genome (GenBank accession number NC_001490).
3.4. Phylogenetic analysis of HRV-A and C genome sequence
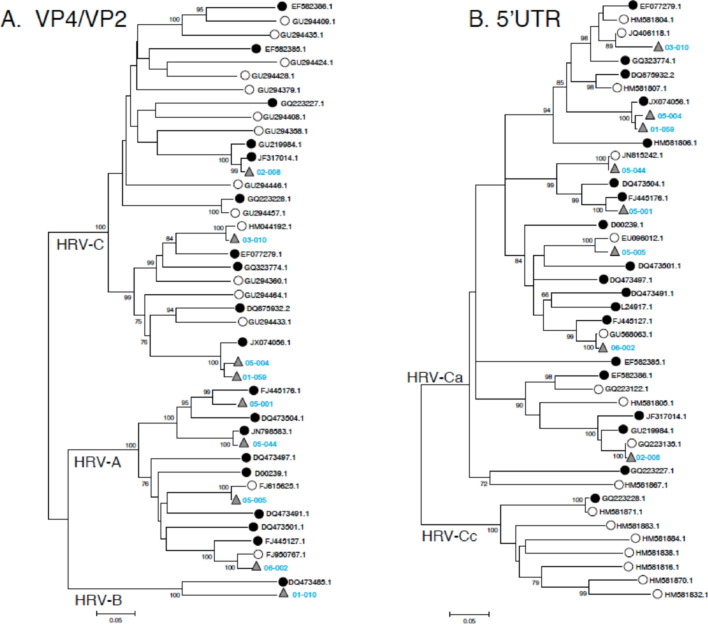
Phylogenetic trees of the VP4/VP2 and 5′UTR regions were constructed with unambiguously aligned sequences (516 and 285 sites for VP4/VP2 and 5′UTR, respectively) using neighbor joining of pairwise maximum composite likelihood method implemented in MEGA5 program [16]. The reliability of internal branches was assessed using the bootstrap method with 1000 replicates. Accession numbers of the sequences included in the dataset are listed in Fig. 2 .
Fig. 2.
Phylogenetic trees of VP4/VP2 gene (A) and 5′UTR region (B) of HRVs. Trees were constructed from alignments of available sequences of complete (white circles), partial genomes (black circles) and sequences obtained from HRV-positive samples (gray triangles). HRV sequences were deposited in GenBank under the accession numbers KC492746–62.
3.5. Statistical analysis
Quantitative variables were described by median, mean and standard deviation; qualitative variables were described by frequency and percentage. The comparison of sub-population regarding HRV carriage were performed using the Mann–Whitney U test for quantitative variables. Qualitative variables were compared using Chi-square test or Fisher's exact test when appropriate. A p value of <0.05 was considered significant. All statistical analyses were performed under SAS software (SAS Institute, Cary, NC, USA; version 9.2).
Interactions between rhinovirus infection and the different variables were investigated with one interaction term with respective subterms in the model (univariate model) and then repeated with adjustments to dyspnea, S-K score, ABPA, segmental or sub-segmental bronchiectasis, fungus detection, intravenous antibiotics or azithromycin treatment or systemic steroids regimens (multivariate model).
4. Results
4.1. Study population characteristics
Forty six patients (23 males and 23 females) were included in this study, with a mean age of 25 years (mean ± SD: 25 ± 11.4). Their characteristics at enrolment and at each sampling time are summarized in Table 2 . A majority of patients (82%) were homozygous or heterozygous for the del-F508 mutation in the CFTR gene associated with an abnormally high sweat chloride test (median ± SD: 112 ± 58.6 mmol/L). Our population was composed of patients with moderate CF disease, during a state of clinical stability (31 episodes) and during acute exacerbation (33 occurrences) according to ERS statement [13]. Most subjects had exocrine pancreatic dysfunction (88.4%), while diabetes mellitus or impaired glucose tolerance was observed in 37.2%, in agreement with published data [17]. All, but one (a 22 year-old woman), patients had microbial colonization in their sputa as determined by culturing. Colonization with Pseudomonas aeruginosa was mainly detected (53.1%); Aspergillus fumigatus and Candida albicans were observed in 45.3% and 68.7% of patients respectively, as previously published [1], [2], [17]. Regarding therapy management, most of the patients received rhDNAse (65.6%) and/or 70.5% inhaled steroids; 70.0% received low dose of azithromycin. Whereas oral antibiotic regimens remained the most often prescribed therapeutics (86.9%), intravenous regimens of antibiotics were prescribed in 75.0% of episodes. Antifungal drugs were prescribed for 11 (18.0%) of cases in agreement with allergic bronchopulmonary aspergillosis (ABPA) diagnosis [18]. In 28 cases (45.9%), patients were under systemic steroids. None other systemic immunosuppressive therapy was used.
Table 2.
Whole population data and comparison of subjects with and without detectable HRV RNA in sputum samples.
| Patient characteristic | Whole population | Comparison of sub-population regarding HRV carriage | ||
|---|---|---|---|---|
| At enrolment | n = 46 | HRV-positive (n = 7) | HRV-negative (n = 38) | pvalue |
| Age (mean; median, SD in years) | 25; 29 (11.4) | 23; 24.8 (5.7) | 26; 29.9 (12.1) | 0.181 |
| Sex (Male) | 23 (50%) | 3 (37.5%) | 20 (52,6%) | 0.699 |
| Sweat chloride test (mean; median, SD in mmol/L) | 112; 117.6 (58.6) | 128; 126 (6.2) | 103.5; 116.2 (63.4) | 0.366 |
| CFTR mutations (ΔF508 homozygous) | 36 (82%) | 6 (85.7%) | 30 (81.1%) | 1 |
| At each sampling time | n = 64 | HRV-positive (n = 9) | HRV-negative (n = 55) | pvalue |
| BMIb (mean; median, SD) | 19.6; 19.7 (2.3) | 19.7; 19.8 (2.6) | 19.4; 19.7 (2.2) | 0.983 |
| S-K scorea (mean; median, SD) | 65.0; 64.2 (16.0) | 66.0; 62.7 (19.8) | 65.0; 64.5 (15.5) | 0.963 |
| FEV1c (mean; median, SD in % of predicted value) | 43.0; 50.7 (25.9) | 53.0; 54.6 (28.5) | 41.0; 50.1 (25.7) | 0.535 |
| FVCd (mean; median, SD in % of predicted value) | 66.0; 69.9 (24.7) | 74.5; 74.1 (25.9) | 63.5; 69.2 (24.7) | 0.586 |
| Dyspnea evaluated using Sadoul scale | 2.0; 1.7 (1.2) | 1.0; 1.8 (1.9) | 2.0; 1.6 (1.0) | 1.0 |
| Pulmonary exacerbation (%) | 33 (51.6) | 8 (88.9) | 25 (45.4) | 0.027 |
| Wheezing (%) | 19 (41.3) | 3 (42.5) | 16 (41.0) | 0.318 |
| Ronchi (%) | 15 (30.0) | 1 (14.3) | 14 (32.6) | 0.244 |
| Crackles (%) | 21 (42.0) | 3 (42.7) | 18 (41.7) | 0.316 |
| Co-morbidities | ||||
| CF-related diabetes (%) | 25 (41.0) | 2 (25.0) | 23 (43.4) | 0.452 |
| ABPAe (%) | 17 (26.6) | 2 (22.2) | 15 (27.3) | 1.000 |
| Microbial colonization at enrolment | ||||
| Staphylococcus aureus (%) | 16 (30.2) | 1 (16.7) | 15 (31.9) | 0.654 |
| Pseudomonas aeruginosa mucoide strain (%) | 29 (54.7) | 3 (50.0) | 26 (55.3) | 1.000 |
| Pseudomonas aeruginosa non-mucoide strain (%) | 16 (30.2) | 1 (16.7) | 15 (31.9) | 0.655 |
| Aspergillus fumigatus (%) | 29 (45.3) | 4 (44.4) | 25 (45.4) | 1.000 |
| Penicillium sp. (%) | 10 (15.6) | 0 (0.0) | 10 (18.2) | 0.333 |
| Candida albicans (%) | 44 (68.7) | 5 (55.6) | 39 (70.9) | 0.443 |
| Other yeast (%) | 46 (71.9) | 5 (55.6) | 41 (74.5) | 0.255 |
| Treatments | ||||
| Nebulized rhDNase (%) | 40 (65.6) | 4 (50.0) | 36 (67.9) | 0.186 |
| Inhaled steroids (continuous) (%) | 43 (70.5) | 5 (62.5) | 38 (71.7) | 0.267 |
| Azithromycin (sub-therapeutic dosage) (%) | 42 (70.0) | 7 (87.5) | 35 (67.3) | 0.190 |
| Oral antifungalf (%) | 11 (18.0) | 2 (25.0) | 9 (17.0) | 0.297 |
| Systemic steroids (continuous or as bolus) (%) | 28 (45.9) | 3 (37.5) | 25 (47.17) | 0.264 |
| Intravenous antibiotics (1–14 regimens) (%) | 33 (75.0) | 5 (71.4) | 28 (75.7) | 1 |
S-K score: Shwachman-Kulczycki score.
BMI: body mass index.
FEV1: forced expiratory volume in 1 second.
FVC: forced vital capacity.
ABPA: allergic bronchopulmonary aspergillosis as referred in [17].
Oral antifungal treatments were: itraconazole in 8 cases, voriconazole in 1 case, and fluconazole in 2 cases.
4.2. Virus detection
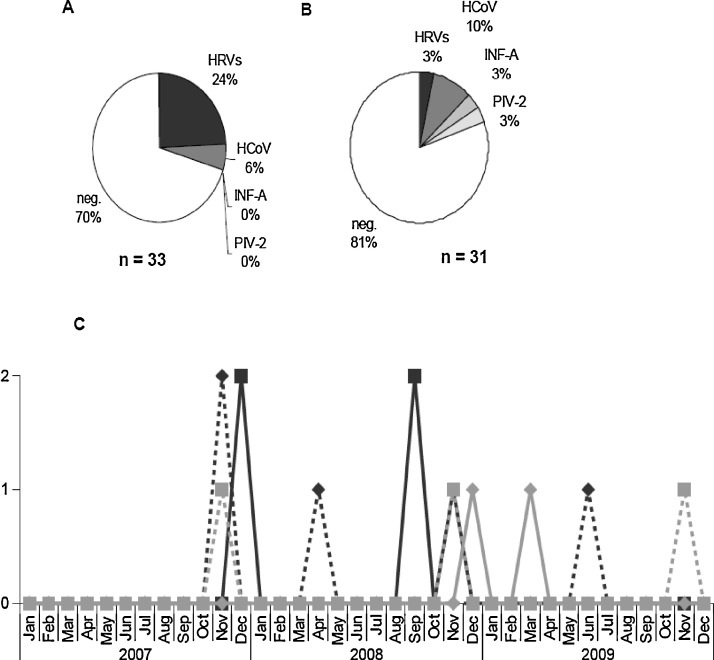
Overall, viruses were identified from 16 out of 64 sputum samples (25%). Rhinoviruses (9/64; 14%) and HCoV (5/64; 8%) were the main identified agents. Eight HRVs were isolated from patients with acute exacerbation (8/33 occurrences; 24%, Fig. 1A) and 1 from a patient during a stable state (1/31 episodes; 3.2%, Fig. 1B). Two (2/33; 6%) OC43/HKU1 or 229E/NL63 HCoVs have been detected from sputa collected during pulmonary exacerbation (Fig. 1A). Three (3/31; 10%) HCoVs, 1 (1/31; 3%) influenza A virus and 1 (1/31; 3%) parainfluenza 2 virus (PIV-2) were detected in sputa collected from patient in stable clinical condition (Fig. 1B). Detection of respiratory viruses occurred mainly in the winter season (Fig. 1C). Indeed, detection of influenza A virus and PIV-2 occurred in October 2008 and February 2009, respectively. Coronaviruses were exclusively detected during the two winter seasons. HRV-A viruses were observed in the autumn 2007 and in springs 2008 and 2009, while detection of other HRVs occurred during the winter 2007 and the autumn 2008 (Fig. 1C), in agreement with the phylogenetic characterization (see Section 4.4). No co-infection with respiratory viruses was detected.
Fig. 1.
Frequency and seasonal distribution of respiratory viruses detected from sputa of CF patients. In each subgroup composed of sputa collected during pulmonary exacerbation (A) or of sputa collected during stable period (B), positive and negative samples are presented as percentages from the total in each subgroup. Seasonal distribution (C) of HRV-A, dotted black line with diamond; of HRV-C, black line with square; HCoV-229E/NL63, gray line with diamond; and of HCoV-OC-43/HKU1, dotted gray line with square.
4.3. Association between respiratory viruses and CF pulmonary exacerbation: importance of HRVs
No significant differences were found in baseline values between the subgroups composed of patients with and without virus detection. Viral respiratory infections in our population of CF patients were not significantly associated with either respiratory symptoms, or deterioration of body mass index, of Shwachman-Kulczycki score, and of spirometry parameters, or comorbidity factors, or microbial colonization, or an increased use of antibiotics, of antifungal or steroids (Table 2). Alterations confirmed by radiology or scanner as proximal bronchiectasis or as segmental/sub-segmental bronchiectasis (identified in 56.4% and 51.3% of the population, respectively) were not significantly associated with virus detection, but missing data were about 39%.
Regarding HRV detection, neither general features of CF patients (such as CFTR mutations or body mass index), nor spirometric values, nor clinical criteria of respiratory function (such as dyspnea, wheezing, ronchi, or crackles), nor radiological and microbial parameters were significantly associated with HRV detection (Table 2). Regarding clinical criteria of respiratory function, missing data were about 23% for dyspnea, and 21.8% for wheezing, ronchi, and crackles, which might explained the absence of statistical significance. Interestingly, detection of rhinoviruses was significantly associated with pulmonary exacerbation (p = 0.027, Table 2).
4.4. Genetic diversity of HRVs
Sequencing the VP4/VP2 region (nucleotide positions 547–1087) was successfully performed in the 9 HRV positive samples. The obtained sequences were aligned with 13 published complete genome sequences and 12 available partial VP4/VP2 sequences (Fig. 2A). Four HRV sequences were identified as belonging to HRV-A species, 4 to HRV-C species and 1 to HRV-B species. The 8 samples that were assigned to HRV-C or HRV-A species were characterized further by sequencing the 5′UTR region (nucleotide position 178–477; Fig. 2B). Using a bootstrap value of 70% or greater to define clades, topologies of the trees constructed from VP4/VP2 and 5′UTR regions were in agreement with previous published results [19], [20], [21]. Surprisingly, the 4 sequences obtained classified as HRV-C by comparative analysis of VP4/VP2 sequences were related more closely to HRV-A strains when their 5′UTR regions were analyzed. All query sequences grouped within the HRV-A clade, with bootstrap value of 90 and 84% (Figure 2), and belong to HRV-Ca variants, according to recent published data [19], [20], [21], [22].
5. Discussion
In the present study, we reported detection of respiratory viruses from adult patients with CF during either routine visit or acute pulmonary exacerbation. We detected respiratory viruses in 25% of 64 sputum samples. This proportion is close to previously published results obtained using conventional diagnosis methods but notably smaller than other recently published data, which might be related to our sampling method [8], [10], [23], [24], [25]. Upper respiratory tract sample such as nasal washes or oropharyngeal swabs are the most frequent sample types used to detect respiratory viruses. Sputa were considered as lower respiratory tract samples and has been recently described as suitable to identify viruses in CF patients [24], [26]. Here, we performed a mechanical pre-treatment of each sputum sample before nucleic acid extraction; this pre-treatment is newly recommended for respiratory virus detection in sputa from patients with asthma or CF [26], [27]. Moreover, sputum analysis has been used for rhinovirus detection in CF patients, who present exacerbation and sputum production [26].
Even if there is only a little data on the incidence of viral pathogens causing exacerbation in adult CF patients, coronavirus, PIV-2, pandemic A/H1N1 were detected in our population as previously reported [6], [8], [25], [28], [29], [30]. Our screening for respiratory viruses showed the presence of coronavirus in samples collected both from patients with exacerbation and from patients during stable disease. However, the presence of coronavirus in samples is not associated with the occurrence of an exacerbation, according to previous study [30], [31]. We detected influenza A virus in only one case, and therefore did not confirm the link between influenza infection and the occurrence of an exacerbation in CF previously described [29]. Due to the potential severity of influenza infection in CF, it is recommended to vaccinate patients against influenza viruses, but the patient positive to A/H1N1 detection did not follow this recommendation and was not vaccinated. The low influenza A virus detection frequency observed here might be linked to the good matching of the influenza vaccine to the circulating influenza viruses during our study period, even if the vaccination status of our CF patient cohort was not documented. Consistent with previous studies, we detected coronaviruses preferentially during the cooler months, HRV-A mainly in spring and autumn, and HRV-C variants preferentially during the autumn and winter months [15], [32], [33].
HRVs were the most commonly detected viruses, as previously reported in CF children [9], [25], [26]. Whereas the physiopathology of virus-induced exacerbation in CF remains unclear, we established a significant association between HRV detection and respiratory exacerbation, confirming the results of recent studies [7], [32], [33]. The detection of HRVs is more frequent in nasal swabs, especially in patients in a stable phase of CF [9]. However, our results show that the detection of HRV in sputum is associated with the occurrence of an exacerbation suggesting that the analysis of sputum could be more relevant than nasal swabs when a viral cause of pulmonary exacerbation is suspected. Some recent studies have shown that HRV-C are involved in severe respiratory infections especially during exacerbations of asthma [34], [35]. Numerous findings underscore the polymicrobial nature of respiratory tract as well as the importance of lung microbiota as a distinctive feature of tissue compartmentalization, but viral microbiota (or virome) has been poorly studied in respiratory tract [22], [36]. Respiratory virome appears composed of three major virus families: Paramyxoviridae, Orthomyxoviridae or Picornaviridae for which a novel type of Rhinovirus C was identified [36].
Here, we performed sequence-based typing of 9 HRVs resulting in 1 HRV-B, 4 HRV-A, and 4 HRV-C. As previously described, HRV-A and HRV-C were the most frequently detected viruses compared to the frequency of HRV-B detection [37]. Recent studies have shown recombination between the 5′UTR region of HRV-C genome and the coding region of HRV-A genome leading the authors to propose a new classification of HRVs in two subspecies, HRV-Ca and -Cc [15], [19], [20], [38]. Occurrence of such recombination was then confirmed, HRV-Ca and HRv-Cc being detected in various upper respiratory diseases [37]. Here, we identified all the HRV-C isolates as belonged to the sub-species HRV-Ca by sequencing both the VP4/VP2 and 5′UTR regions. To our knowledge, the present study describes for the first time the presence of HRV-Ca subtype in patients with CF. Although the clinical significance of such recombination is still matter of debate, our results confirm the well-known recombination ability of enteroviruses (notably HRVs); a fact of particular relevance when phage community represents a key point in the lung microbiota analysis as well as in the improvement of resistosome concept [26], [35], [38], [39]. In Fact, human microbiota represents a huge reservoir of mobilizable genes, as proposed by Rolain and co-workers [39]; some of those genes encoding antimicrobial resistance that the authors named ‘resistome’ [39]. This concept is based on few metagenomic studies that have analyzed viral and bacterial communities in CF sputum samples, revealing the presence of an important bacteriophage community containing genes encoding antimicrobial resistance, and demonstrating these bacteriophages are consistent with the characteristics of multidrug-resistant microbial communities that are commonly observed in CF patients [26], [35], [38], [39].
To conclude, we have reported a relatively high frequency of respiratory viruses in a cohort of CF adult patients from France, and demonstrated for the first time that rhinovirus detection including newly identified HRV-Ca variants are the most frequent and significantly associated with respiratory exacerbations. Our results can be considered as contribution to the characterization the role of viruses in CF pulmonary exacerbation, underscoring the importance of the lung virome that remains poorly understood in the context of CF.
Funding
The authors thank PHRC 1902 – Vaincre la mucoviscidose – Pfizer Laboratories for their financial support.
Competing interests
None.
Ethical approval
Reference number of the institutional ethics committees of Lille Hospital, CPP 06/84. Written informed consent was obtained from each patient.
Acknowledgments
The authors thank all the clinicians from the three hospitals for their cooperation in collecting sputum samples and clinical data. The authors would like to thank Carolyn Engel-Gautier for English editing.
Footnotes
A part of this work was presented as a poster at “Journées Francophones de Virologie”, Paris April 2013.
References
- 1.Sibley C.D., Parkins M.D., Rabin H.R., Duan K., Norgaard J.C., Surette M.G. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci U S A. 2008;105:15070–15075. doi: 10.1073/pnas.0804326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delhaes L., Monchy S., Fréalle E., Hubans C., Salleron J., Leroy S. The airway microbiota in cystic fibrosis: a complex fungal and bacterial community – implications for therapeutic management. PLoS One. 2012;7:e36313. doi: 10.1371/journal.pone.0036313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gangell C., Gard S., Douglas T., Park J., de Klerk N., Keil T. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin Infect Dis. 2011;53:425–432. doi: 10.1093/cid/cir399. [DOI] [PubMed] [Google Scholar]
- 4.Singanayagam A., Joshi P.V., Mallia P., Johnston S.L. Viruses exacerbating chronic pulmonary disease: the role of immune modulation. BMC Med. 2012;10:27. doi: 10.1186/1741-7015-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contoli M., Marku B., Conti V., Saturni S., Caramori G., Papi A. Viral infections in exacerbations of asthma and chronic obstructive pulmonary disease. Minerva Med. 2009;100:467–478. [PubMed] [Google Scholar]
- 6.Wat D., Gelder C., Hibbitts S., Cafferty F., Bowler I., Pierrepoint M. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7:320–328. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Almeida M.B., Zerbinati R.M., Tateno A.F., Oliveira C.M., Romão R.M., Rodrigues J.C. Rhinovirus C and respiratory exacerbations in children with cystic fibrosis. Emerg Infect Dis. 2010;16:996–999. doi: 10.3201/eid1606.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoek R.A.S., Paats M.S., Pas S.D., Bakker M., Hoogsteden H.C., Boucher C.A.B. Incidence of viral respiratory pathogens causing exacerbations in adult cystic fibrosis patients. Scand J Infect Dis. 2013;45:65–69. doi: 10.3109/00365548.2012.708942. [DOI] [PubMed] [Google Scholar]
- 9.Stelzer-Braid S., Johal H., Skilbeck K., Steller A., Alsubie H., Tovey E. Detection of viral and bacterial respiratory pathogens in patients with cystic fibrosis. J Virol Methods. 2012;186:109–112. doi: 10.1016/j.jviromet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Lau S.K.P., Yip C.C.Y., Tsoi H.-W., Lee R.A., So L.-Y., Lau Y-L. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie J.K., Yagi S., Nelson F.A., Kiang D., Glaser C.A., Rosenberg J. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis. 2005;41:262–265. doi: 10.1086/430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ison M.G., Hayden F.G., Kaiser L., Corey L., Boeckh M. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis. 2003;36:1139–1143. doi: 10.1086/374340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilton D., Canny G., Conway S., Dumcius S., Hjelte L., Proesmans M. Pulmonary exacerbation: towards a definition for use in clinical trials, report from the EuroCareCF Working Group on outcome parameters in clinical trials. J Cyst Fibros. 2011;10(Suppl. 2):S79–S81. doi: 10.1016/S1569-1993(11)60012-X. [DOI] [PubMed] [Google Scholar]
- 14.Montes-Cano M.A., de la Horra C., Dapena F.J., Mateos I., Friaza V., Respaldiza N. Dynamic colonisation by different Pneumocystis jirovecii genotypes in cystic fibrosis patients. Clin Microbiol Infect. 2007;13:1008–1011. doi: 10.1111/j.1469-0691.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- 15.Wisdom A., Leitch E.C.M., Gaunt E., Harvala H., Simmonds P. Screening respiratory samples for detection of human rhinoviruses (HRVs) and enteroviruses: comprehensive VP4-VP2 typing reveals high incidence and genetic diversity of HRV species C. J Clin Microbiol. 2009;47:3958–3967. doi: 10.1128/JCM.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blyth C.C., Middleton P.G., Harun A., Sorrell T.C., Meyer W., Chen S.C.-A. Clinical associations and prevalence of Scedosporium spp. in Australian cystic fibrosis patients: identification of novel risk factors? Med Mycol. 2010;48(Suppl. 1):S37–S44. doi: 10.3109/13693786.2010.500627. [DOI] [PubMed] [Google Scholar]
- 18.Stevens D.A., Moss R.B., Kurup V.P., Knutsen A.P., Greenberger P., Judson M.A. Allergic bronchopulmonary aspergillosis in cystic fibrosis – state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003;37(Suppl. 3):S225–S264. doi: 10.1086/376525. [DOI] [PubMed] [Google Scholar]
- 19.Huang T., Wang W., Bessaud M., Ren P., Sheng J., Yan H. Evidence of recombination and genetic diversity in human rhinoviruses in children with acute respiratory infection. PLoS One. 2009;4:e6355. doi: 10.1371/journal.pone.0006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntyre C.L., McWilliam Leitch E.C., Savolainen-Kopra C., Hovi T., Simmonds P. Analysis of genetic diversity and sites of recombination in human rhinovirus species C. J Virol. 2010;84:10297–10310. doi: 10.1128/JVI.00962-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wisdom A., Kutkowska A.E., McWilliam Leitch E.C., Gaunt E., Templeton K., Harvala H. Genetics, recombination and clinical features of human rhinovirus species C (HRV-C) infections; interactions of HRV-C with other respiratory viruses. PLoS One. 2009;4:e8518. doi: 10.1371/journal.pone.0008518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lysholm F., Wetterbom A., Lindau C., Darban H., Bjerkner A., Fahlander K. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PLoS One. 2012;7:e30875. doi: 10.1371/journal.pone.0030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong D., Grimwood K., Carlin J.B., Carzino R., Hull J., Olinsky A. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol. 1998;26:371–379. doi: 10.1002/(sici)1099-0496(199812)26:6<371::aid-ppul1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Olesen H.V., Nielsen L.P., Schiotz P.O. Viral and atypical bacterial infections in the outpatient pediatric cystic fibrosis clinic. Pediatr Pulmonol. 2006;41:1197–1204. doi: 10.1002/ppul.20517. [DOI] [PubMed] [Google Scholar]
- 25.Asner S., Waters V., Solomon M., Yau Y., Richardson S.E., Grasemann H. Role of respiratory viruses in pulmonary exacerbations in children with cystic fibrosis. J Cyst Fibros. 2012;11(September (5)):433–439. doi: 10.1016/j.jcf.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns J.L., Emerson J., Kuypers J., Campbell A.P., Gibson R.L., McNamara S. Respiratory viruses in children with cystic fibrosis: viral detection and clinical findings. Influenza Other Respir Viruses. 2012;6:218–223. doi: 10.1111/j.1750-2659.2011.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim Y.W., Schmieder R., Haynes M., Willner D., Furlan M., Youle M. Metagenomics and metatranscriptomics: Windows on CF-associated viral and microbial communities. J Cyst Fibros. 2012;12(March (2)):154–164. doi: 10.1016/j.jcf.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang E.E., Prober C.G., Manson B., Corey M., Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med. 1984;311:1653–1658. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz J.R., Neuzil K.M., Victor J.C., Wald A., Aitken M.L., Goss C.H. Influenza-associated cystic fibrosis pulmonary exacerbations. Chest. 2010;137:852–860. doi: 10.1378/chest.09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Silva Filho L.V.R.F., Zerbinati R.M., Tateno A.F., Boas L.V., de Almeida M.B., Levi J.E. The differential clinical impact of human coronavirus species in children with cystic fibrosis. J Infect Dis. 2012;206:384–388. doi: 10.1093/infdis/jis274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu S.S., Chan K.H., Chu K.W., Kwan S.W., Guan Y., Poon L.L.M. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savolainen-Kopra C., Al-Hello H., Paananen A., Blomqvist S., Klemola P., Sobotova Z. Molecular epidemiology and dual serotype specificity detection of echovirus 11 strains in Finland. Virus Res. 2009;139:32–38. doi: 10.1016/j.virusres.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Tan C.Y.Q., Ninove L., Gaudart J., Nougairede A., Zandotti C., Thirion-Perrier L. A retrospective overview of enterovirus infection diagnosis and molecular epidemiology in the public hospitals of Marseille, France (1985–2005) PLoS One. 2011;6:e18022. doi: 10.1371/journal.pone.0018022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieninger E., Singer F., Tapparel C., Alves1 M.P., Latzin1 P., Tan H-L. High rhinovirus burden in lower airways of children with cystic fibrosis. Chest. 2013;143(3):782–790. doi: 10.1378/chest.12-0954. [DOI] [PubMed] [Google Scholar]
- 35.Brennan S., Sly P.D., Gangell C.L., Sturges N., Winfield K., Wikstrom M. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur Respir J. 2009;34:655–661. doi: 10.1183/09031936.00178508. [DOI] [PubMed] [Google Scholar]
- 36.Willner D., Furlan M., Haynes M., Schmieder R., Angly F.E., Silva J. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS One. 2009;4:e7370. doi: 10.1371/journal.pone.0007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briese T., Renwick N., Venter M., Jarman R.G., Ghosh D., Köndgen S. Global distribution of novel rhinovirus genotype. Emerg Infect Dis. 2008;14:944–947. doi: 10.3201/eid1406.080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gern J.E. The ABCs of rhinoviruses, wheezing, and asthma. J Virol. 2010;84:7418–7426. doi: 10.1128/JVI.02290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fancello L., Desnues C., Raoult D., Rolain J.M. Bacteriophages and diffusion of genes encoding antimicrobial resistance in cystic fibrosis sputum microbiota. J Antimicrob Chemother. 2011;66:2448–2454. doi: 10.1093/jac/dkr315. [DOI] [PubMed] [Google Scholar]