Abstract
Background
The association between a robust or depressed antibody response and clinical severity of SARS remains unknown.
Objectives
To study seroconversion and the magnitude of IgG responses in a SARS cohort with different disease severities.
Study design and method
A retrospective analysis of all acute and convalescent-phase sera collected from a cohort of laboratory-confirmed SARS cases. Anti-SARS-CoV IgG antibody was detected using indirect immunofluorescence technique and quantified by two-fold serial dilutions. Characteristics of patients who seroconverted “early” (<median interval) were compared to those documented to remain sero-negative during the same time interval. Median IgG levels in convalescent-phase sera (collected within 30 days) were compared among patients with different disease severities. Correlations between IgG levels and important laboratory parameters were assessed.
Results
A total of 325 laboratory-confirmed SARS cases were analyzed; of which 301 (92.6%) had anti-SARS-CoV IgG detected in their sera at the time of sampling. IgG was first detected on day 4 of illness; seroconversion occurred at a median of 16 days (range 4–35 days), and IgG peak levels were reached in the fourth week. Early seroconversion (<day 16) occurred more frequently among patients who required ICU-admission (χ2; p = 0.011). Higher IgG levels were detected in patients who required supplemental oxygen (Mann–Whitney; p = 0.002), ICU-admission (p = 0.001), had negative pre-discharge fecal RT-PCR results (p = 0.004), and lymphopenia at presentation (p = 0.028). Peak IgG titres also correlated positively with peak LDH levels (Spearman's r = +0.360; p < 0.001) among survivors.
Conclusions
Severe SARS is associated with a more robust IgG response.
Keywords: Anti-SARS-CoV IgG, Seroconversion, SARS, Disease severity
1. Introduction
Severe acute respiratory syndrome (SARS) is a new infectious disease caused by a novel coronavirus, and was associated with significant morbidity and mortality (WHO, 2004). Its pathogenesis appears to involve both a high viral load and an overexuberant immune response (Peiris et al., 2003a, Peiris et al., 2003b). However, it remains unclear as to whether a robust or depressed antibody response is associated with clinical deterioration and a more severe disease course. Such information may improve our understanding of SARS immunopathogenesis and facilitate the design of an appropriate therapeutic strategy. Using indirect immunofluorescence assay (IFA) (Chan et al., 2004a, Hsueh et al., 2004), we have evaluated anti-SARS-CoV IgG responses within the first month of illness among a complete cohort of SARS patients. Rates of “early” seroconversion and magnitude of IgG responses in patients with different disease severities are compared.
2. Methods and data analysis
All cases that fulfilled the CDC case definition of SARS and had been admitted to the medical wards or intensive care unit (ICU) at the Prince of Wales Hospital in Hong Kong during the SARS outbreak in year 2003 were included in the study (Lee et al., 2003b). A total of 325 laboratory-confirmed (by serology, RT-PCR or post-mortem) SARS cases were then retrospectively evaluated for their anti-SARS-CoV IgG response and clinical disease severity. Serum samples were first collected at presentation/admission and convalescent-phase sera were collected either beyond 2 weeks of illness or >10–14 days apart from the acute-phase sera. All serum samples were dated from the day of fever onset. Anti-SARS-CoV IgG was detected by IFA using SARS-CoV-infected Vero cells; and IgG titres were quantified at two-fold serial dilutions starting from 1:40 (Chan et al., 2004a). Routine respiratory specimens collected at presentation, and repeated fecal specimens collected upon discharge at ≥21 days of illness were tested for SARS-CoV by RT-PCR (non-quantitative), according to WHO recommendations (Chan et al., 2004b, WHO, 2004). All serological and RT-PCR assays for SARS-CoV were performed in a BSL-2 containment facility according to CDC recommendations (CDC, 2005). The clinical characteristics and disease progression, investigation methods and details of treatment of this cohort of patients have been described elsewhere (Chan et al., 2004b, Gomersall et al., 2004, Lee et al., 2003a, Lee et al., 2003b, Lee et al., 2004, Sung et al., 2004). SARS patients who required supplemental oxygen therapy during their course of illness or admitted to ICU as a result of severe respiratory failure were regarded to have more severe disease. Supplemental oxygen was prescribed to maintain patient's oxygen saturation >95% when necessary; and severe respiratory failure was defined by failure to maintain an arterial oxygen saturation of >90% despite receiving supplemental oxygen at 50%, and/or a respiratory rate greater than 35 breaths per minute (Gomersall et al., 2004). High-dose corticosteroid ‘rescue’ therapy was given to patients when clinical and radiological progression was noted according to a standard protocol (Sung et al., 2004). Age of 65 years was also used as a cut-off in the comparison between groups since epidemiological data had implicated advanced age as a poor prognostic factor (thus more severe disease) (WHO, 2004). The cut-off value for lymphocyte counts in the comparison groups was determined based on earlier studies (Wong et al., 2003).
A retrospective analysis was conducted. Firstly, clinical and laboratory variables of patients who seroconverted “early” (<median interval) were compared to those cases documented to remain sero-negative during the same time interval using χ 2-test. Variables with p < 0.2 in the univariate analysis were then entered into a model for logistic regression analysis. We excluded patients who had their first serum samples collected on day 0–3 (since IgG was only first detected on day 4 in our cohort), and those who had their first sera collected beyond day 15 (the median interval to seroconvert) from fever onset. Median time of serum collections between all comparative patient groups was not significantly different. Secondly, median anti-SARS-CoV IgG levels in convalescent-phase sera collected within 30 days were compared among patients with different disease severities and laboratory profiles using Mann–Whitney U-test. Variables with p < 0.2 in the univariate analysis were then entered into a nonparametric regression model for analysis. Only the latest serum sample collected from each patient was included in the analysis. Median time of collecting these samples was not significantly different between all comparative patient groups. Thirdly, spearman's rank correlation coefficient was used to assess correlations between IgG titres and LDH levels or lymphocyte counts within 30 days. SPSS for Windows (Release 11.5; SPSS Inc., Chicago, IL, USA) was used for the analyses, and the level of significance was set at 0.05 for all comparisons.
3. Results
A total of 325 laboratory-confirmed SARS cases were analyzed. Of these, 301 (92.6%) had anti-SARS-CoV IgG detected in sera at the time of sampling (of which 211 cases had convalescent-phase sera collected). Most of the remaining cases had only one serum sample collected within the first few days of illness; 21 had their diagnoses confirmed by RT-PCR of SARS-CoV, and three were confirmed at post-mortem. A total of 93 patients had positive RT-PCR results (respiratory specimens: 82.8%, fecal specimens: 17.2%). The median age of the cohort was 37 years (20–96 years), with a male-to-female ratio of 1.4–1. A total of 63 (19.4%) patients had been admitted to the ICU [median age 45 (23–82) years] and 41 (12.6%) patients died (with or without ICU care) [median age 73 (44–96) years]; 239 (73.5%) patients recovered without ICU care. The proportion of patients aged ≥65 years was 19% and 17% in the ICU-admitted patients and those without ICU-admission, respectively. The crude death rates were 3.7% and 54.4% in patients aged <65 and ≥65 years, respectively. Significant co-morbid illnesses (see Table 1 foot notes) were present in 6.3% and 35.1% of cases aged <65 and ≥ 65 years, respectively. A total of 163 (50.2%) patients had required supplemental oxygen at some stage of their illness. High-dose corticosteroid ‘rescue’ therapy was given to 246 (75.7%) cases, at a mean of 8.6 (95% C.I. 4.8–12.4 days) days from fever onset.
Table 1.
Characteristics of “early” (day 4–15) seroconverters as compared to patients who remained sero-negative during the same period
| Patient groups | IgG + n (%) | IgG − n (%) | p-Value (χ2) |
|---|---|---|---|
| Male | 27 (40.9) | 32 (38.6) | 0.770 |
| Female | 39 (59.1) | 51 (61.4) | |
| Age <65 years | 57 (86.4) | 62 (74.7) | 0.078 |
| Age ≥65 years | 9 (13.6) | 21 (25.3) | |
| Co-morbidity | 8 (12.3) | 11 (13.9) | 0.775 |
| No co-morbidity | 57 (87.7) | 68 (86.1) | |
| ICU care | 20 (30.3) | 11 (13.3) | 0.011a |
| No ICU care | 46 (69.7) | 72 (86.7) | |
| Death | 11 (16.7) | 15 (18.1) | 0.822 |
| Survive | 55 (83.3) | 68 (81.9) | |
| O2-therapy | 37 (56.9) | 39 (53.4) | 0.680 |
| No O2-therapy | 28 (43.1) | 34 (46.6) | |
| CS-treated | 52 (78.8) | 58 (69.9) | 0.219 |
| Not CS-treated | 14 (21.2) | 25 (30.1) | |
| Positive RT-PCR | 22 (40.0) | 20 (31.7) | 0.350 |
| Negative RT-PCR | 33 (60.0) | 43 (68.3) | |
| IgG + (all cases) |
IgG − (all cases) |
p-Value (Mann–Whitney) |
|
| Initial lymphocyte (median) × 109/L | 0.74 | 0.73 | 0.824 |
| Lymphocyte nadir (median) × 109/L | 0.23 | 0.28 | 0.315 |
| Peak LDH (median) (IU/L) | 440 | 383 | 0.319 |
IgG: anti-SARS-CoV IgG as detected by indirect immunofluorescence technique. Co-morbidity: malignancy, cirrhosis, chronic renal failure, chronic lung diseases, congestive heart failure, cerebrovascular diseases; ICU: intensive care unit; O2-therapy: oxygen supplement required to maintain oxygen saturation >95%; CS: intravenous high-dose corticosteroid (methylprednisolone); RT-PCR: on respiratory sites specimens and fecal specimens; LDH: lactate dehydrogenase.
Statistically significant.
The anti-SARS-CoV IgG response profile in our cohort has been reported previously (Chan et al., 2004a). IgG was first detected on day 4; seroconversion occurred at a median of 16 days (range 4–35 days), and IgG peak levels were reached in the early fourth week of illness. The lowest and highest IgG titres were 40 and 5120, respectively. So far 15 patients’ late serum samples collected ≥15 months post-infection all showed positive IgG results [median titre = 320, range 80–640]; and the longest interval observed was 542 days (18 months).
Characteristics of patients who seroconverted “early” (day 4–15) were compared to those patients who remained sero-negative during the same period, and the results are shown in Table 1. “Early” seroconversion was observed more frequently among patients who required ICU-admission (χ 2; p = 0.011). Although not reaching statistical significance, there was a trend towards a lower proportion of older patients (≥65 years of age) observed to seroconvert early (p = 0.078). Results from logistic regression analysis indicated that ICU-admission was the only factor associated with early seroconversion (p = 0.015). No significant difference in mortality was observed between the early seroconverters and those who remained sero-negative at day 15.
The median anti-SARS-CoV IgG levels in convalescent-phase sera collected within 30 days were compared among patients with different disease severities and laboratory profiles, and the results are shown in Table 2 . The IgG profiles for patients who had/had not received supplemental oxygen therapy and patients who had/had not been admitted to ICU were illustrated in Fig. 1a and b. Higher IgG levels were detected in patients who had required supplemental oxygen (p = 0.002), ICU-admission (p = 0.001), and had negative pre-discharge fecal RT-PCR results (p = 0.004). Although not reaching statistical significance, there was a trend towards a lower IgG levels detected in cases that were not given high-dose corticosteroid therapy (because of non-progressive/mild diseases). But when the sub-group of patients who received high-dose corticosteroid treatment was analyzed (76% of all cases), higher IgG levels were again detected in patients with more severe diseases (Table 2). By multivariate analysis, higher IgG level was again shown to be associated with ICU-admission (p = 0.006) or supplemental oxygen therapy (p = 0.008), but not age or corticosteroid treatment (p > 0.05). In the corticosteroid-treated sub-group, absolute lymphopenia <1.0 × 109/L at presentation was also noted to associate with higher IgG titres (p = 0.028). Too few death cases had convalescent sera collected to allow a separate analysis.
Table 2.
Comparison of anti-SARS-CoV IgG titres among different patient groups
| Patient groups | All cases [median IgG titre (IQR)] | p-Value (Mann–Whitney) | CS-treated [median IgG titre (IQR)] | p-Value (Mann–Whitney) |
|---|---|---|---|---|
| Male | 640 (320, 640) | 0.286 | 640 (320, 640) | 0.386 |
| Female | 320 (160, 640) | 320 (320, 640) | ||
| Age <65 years | 320 (200, 640) | 0.332 | 320 (320, 640) | 0.347 |
| Age ≥65 years | 640 (320, 640) | 640 (320, 640) | ||
| ICU care | 640 (640, 640) | 0.001* | 640 (640, 640) | 0.002* |
| No ICU care | 320 (160, 640) | 320 (320, 640) | ||
| O2-therapy | 640 (320, 640) | 0.002* | 640 (320, 640) | 0.008* |
| No O2-therapy | 320 (160, 640) | 320 (320, 640) | ||
| CS-treated | 640 (320, 640) | 0.088 | ||
| Not CS-treated | 320 (160, 640) | |||
| Positive RT-PCR (all)a | 320 (160, 640) | 0.325 | 320 (160, 640) | 0.714 |
| Negative RT-PCR (all)a | 640 (320, 640) | 640 (320, 640) | ||
| Positive RT-PCR (fecal)a | 160 (40, 560) | 0.004* | 160 (160, 640) | 0.060 |
| Negative RT-PCR (fecal)a | 480 (320, 640) | 640 (320, 640) | ||
| Initial lymphocyte <1.0 | 640 (320, 640) | 0.058 | 640 (320, 640) | 0.028* |
| Initial lymphocyte ≥1.0 | 320 (160, 640) | 320 (240, 640) | ||
| Lymphocyte nadir <0.5 | 640 (320, 640) | 0.740 | 640 (320, 640) | 0.754 |
| Lymphocyte nadir ≥0.5 | 320 (320, 640) | 640 (320, 640) |
ICU: intensive care unit; O2-therpay: oxygen supplement required to maintain oxygen saturation >95%; CS: intravenous high-dose corticosteroid (methylprednisolone).
“all”: on respiratory and fecal specimens; “fecal”: on fecal specimens collected upon discharge at ≥21 days; included only respiratory specimen RT-PCR negative cases.
Statistically significant.
Fig. 1.
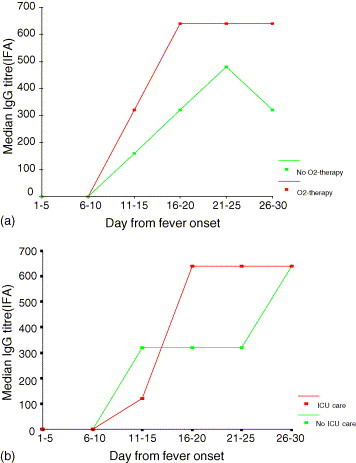
(a and b) Median anti-SARS-CoV IgG titres in patients with different disease severities within first 4 weeks of illness. ICU: intensive care unit; O2-therapy: oxygen supplement required to maintain oxygen saturation >95%; IFA: indirect immunofluorescence assay.
We further examined the correlation between anti-SARS-CoV IgG titres and LDH levels or lymphocyte counts within the first 30 days of illness. A positive correlation between peak LDH levels and peak IgG titres (Spearman's r = +0.360; p < 0.001), and a negative correlation between initial lymphocyte counts and peak IgG titres (Spearman's r = −0.205; p = 0.004) were noted among survivors without ICU-admission. No correlation was found between IgG titre and nadir lymphocyte counts.
4. Discussion
Our study has shown that “early” seroconversion (<day 16) occurred more frequently among patients who required ICU-admission. In addition, higher IgG levels were detected in patients who had more severe diseases as evidenced by need for either supplemental oxygen or ICU-admission (analysis of the ‘corticosteroid-treated’ sub-group showed similar results). Peak IgG titres also correlated positively with peak LDH levels (an indicator of disease severity) among survivors.
The anti-SARS-CoV IgG response profile as described is consistent with serology profiles reported elsewhere (Chen et al., 2004, He et al., 2004, Hsueh et al., 2004, Li et al., 2003). IgG can be detected as early as day 4 by IFA; seroconversion occurs by the end of the second week, and IgG surged to peak levels by the early fourth week. It has been reported that a “second” late rise in antibody titre can occur after week 4, possibly related to the withdrawal of corticosteroid treatment (Woo et al., 2004). Thus our results may represent the initial rate of change of IgG levels within the first month; and they also need to be interpreted in the context of corticosteroid treatment. Our findings of more severe SARS being associated with more robust serological responses are supported by earlier observations. The development of the humoral response coincides temporally with disease progression and clinical deterioration (while viral load starts to decrease) (Hsueh et al., 2003, Nie et al., 2004, Peiris et al., 2003a). Also patients with very mild disease have relatively low antibody titres detected, and a less-sustained response (Ip et al., 2004, Lee et al., 2003a, Tso et al., 2004, Wilder-Smith et al., 2005). Similar phenomena have been observed in other viral diseases (e.g. dengue hemorrhagic fever), in which immunopathogenesis is considered important (Thein et al., 1993).
Our findings have several important clinical implications. Firstly, it is unlikely that disease progression and clinical deterioration result from a depressed humoral response to SARS-CoV. In fact, high titres of anti-SARS-CoV IgG were detected in patients with more severe disease. Naturally occurring neutralizing antibody activity associated with the IgG may have contributed to viral clearance to some extent (Nie et al., 2004, Temperton et al., 2005), but clearly did not confer protection against disease progression in SARS. This is also supported by our finding that higher convalescent-phase IgG titres are associated with negative pre-discharge fecal RT-PCR results. Secondly, it seems possible that a robust humoral response to SARS-CoV is one component of an overall exaggerated immune response in severe SARS, which is associated with cytokine storms (e.g IFN-gamma, IP-10) (Huang et al., 2005, Jiang et al., 2005, Wong et al., 2004). Briefly, marked elevation of Th1 cytokine IFN-γ, inflammatory cytokines IL-1, -6 and -12 was noted during the initial 2 weeks of SARS. The chemokines, including neutrophil chemokine IL-8, monocyte chemoattractant protein-1 (MCP-1), and Th1 chemokine IFN-γ-inducible protein-10 (IP-10) levels were also significantly elevated (Wong et al., 2004). In fact, when patients with detectable and undetectable acute antibody responses were compared, a much higher initial serum IFN-gamma level had been noted in the former (Huang et al., 2005). Our observation of an inverse relationship between the initial lymphocyte counts and IgG levels is also in agreement with a previous report showing a similar inverse relationship between lymphocyte counts and IFN-gamma levels (possibly through cytokine-induced apoptosis) (Huang et al., 2005). Whether the humoral response contributes directly to organ damage is unclear. The exact immunopathogenesis of SARS requires further study. Finally, since higher levels of IgG were associated with more severe disease, the use of convalescent plasma/passive immunity in the treatment of SARS is called into question. It is uncertain whether administration of an exogenous antibody will lead to aggravation of the disease (Hsueh et al., 2003). It has been suggested that if such therapy is contemplated, it should be given only very early in the course of illness or as prophylaxis (Greenough et al., 2005, Jan ter Meulen et al., 2004, Nie et al., 2004). Anecdotal reports have shown that early use of convalescent plasma therapy within the first 2 weeks was associated with lower rates of intubation and death, whereas late administration was associated with worse clinical outcomes (Cheng et al., 2005, Soo et al., 2004).
Our study was limited by its retrospective nature. The issues of delayed seroconversion and changes of anti-SARS-CoV IgM, IgA or neutralizing antibodies in relation to disease severity have not been addressed. The IgG response in patients without corticosteroid treatment remained poorly characterized. Nevertheless, our findings suggest that severe SARS is associated with a more robust IgG response. Further study on SARS immunopathogenesis is warranted.
References
- Centers for Disease Control and Prevention (CDC). Severe acute respiratory syndrome (SARS). http://www.cdc.gov/ncidod/sars/lab/biosafety.htm.
- Chan P.K., Ng K.C., Chan R.C., Lam R.K., Chow V.C., Hui M. Immunofluorescence assay for serologic diagnosis of SARS. Emerg Infect Dis. 2004;10(3):530–532. doi: 10.3201/eid1003.030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.K., To W.K., Ng K.C., Lam R.K., Ng T.K., Chan R.C. Laboratory diagnosis of SARS. Emerg Infect Dis. 2004;10(5):825–831. doi: 10.3201/eid1005.030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhou B., Li M., Liang X., Wang H., Yang G. Serology of severe acute respiratory syndrome: implications for surveillance and outcome. J Infect Dis. 2004;189(7):1158–1163. doi: 10.1086/380397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough T.C., Babcock G.J., Roberts A., Hernandez H.J., Thomas W.D., Jr., Coccia J.A. Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. J Infect Dis. 2005;191(4):507–514. doi: 10.1086/427242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomersall C.D., Joynt G.M., Lam P., Li T., Yap F., Lam D. Short-term outcome of critically ill patients with severe acute respiratory syndrome. Intens Care Med. 2004;30(3):381–387. doi: 10.1007/s00134-003-2143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Dong Q., Zhuang H., Song S., Peng G., Luo G. Kinetics of severe acute respiratory syndrome (SARS) coronavirus-specific antibodies in 271 laboratory-confirmed cases of SARS. Clin Diagn Lab Immunol. 2004;11(4):792–794. doi: 10.1128/CDLI.11.4.792-794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh PR, Hsiao CH, Yeh SH, Wang WK, Chen PJ, Wang JT, et al. SARS Research Group of National Taiwan University College of Medicine and National Taiwan University Hospital. Microbiologic characteristics, serologic responses, and clinical manifestations in severe acute respiratory syndrome, Taiwan. Emerg Infect Dis. 2003; 9(9):1163–7. [DOI] [PMC free article] [PubMed]
- Hsueh P.R., Huang L.M., Chen P.J., Kao C.L., Yang P.C. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10(12):1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip M., Chan P.K., Lee N., Wu A., Ng T.K., Chan L. Seroprevalence of antibody to severe acute respiratory syndrome (SARS)-associated coronavirus among health care workers in SARS and non-SARS medical wards. Clin Infect Dis. 2004;38(12):e116–e118. doi: 10.1086/421019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan ter Meulen J., Bakker A.B., van den Brink E.N., Weverling G.J., Martina B.E., Haagmans B.L. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363(9427):2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J. Characterization of cytokine and chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171(8):850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- Lee H.K., Tso E.Y., Chau T.N., Tsang O.T., Choi K.W., Lai T.S. Asymptomatic severe acute respiratory syndrome-associated coronavirus infection. Emerg Infect Dis. 2003;9(11):1491–1492. doi: 10.3201/eid0911.030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M. A major outbreak of severe acute respiratory syndrome in Hong Kong. New Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Lee N., Allen Chan K.C., Hui D.S., Ng E.K., Wu A., Chiu R.W. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med. 2003;349(5):508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- Nie Y., Wang G., Shi X., Zhang H., Qiu Y., He Z. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J Infect Dis. 2004;190(6):1119–1126. doi: 10.1086/423286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- Soo Y.O., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J.J., Wu A., Joynt G.M., Yuen K.Y., Lee N., Chan P.K. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59(5):414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperton N.J., Chan P.K., Simmons G., Zambon M.C., Tedder R.S., Takeuchi Y. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg Infect Dis. 2005;11(3):411–416. doi: 10.3201/eid1103.040906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein S., Aaskov J., Myint T.T., Shwe T.N., Saw T.T., Zaw A. Changes in levels of anti-dengue virus IgG subclasses in patients with disease of varying severity. J Med Virol. 1993;40(2):102–106. doi: 10.1002/jmv.1890400205. [DOI] [PubMed] [Google Scholar]
- Tso E.Y., Tsang O.T., Lam B., Ng T.K., Lim W., Lai T.S. Natural course of severe acute respiratory syndrome-associated coronavirus immunoglobulin after infection. J Infect Dis. 2004;190(9):1706–1707. doi: 10.1086/424573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Teleman M.D., Heng B.H., Earnest A., Ling A.E., Leo Y.S. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11(7):1142–1145. doi: 10.3201/eid1107.041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.S., Wu A., To K.F., Lee N., Lam C.W., Wong C.K. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326(7403):1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Wong B.H., Chan K.H., Chu C.M., Tsoi H.W. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin Diagn Lab Immunol. 2004;11(4):665–668. doi: 10.1128/CDLI.11.4.665-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO. http://www.who.int/csr/sars/en/.


