1. Introduction
Human metapneumovirus (hMPV) is a paramyxovirus recently recognized as the first human pathogen within the genus Metapneumovirus (subfamily: Pneumovirinae: Family: Paramyxoviridae) (Van den Hoogen et al., 2001). The virus was first identified in The Netherlands in 2001, and shown to cause acute respiratory tract infections in children (Van den Hoogen et al., 2001). Since then, surveillance studies have demonstrated that virtually all children are infected with hMPV between the ages 5 and 10 years (Ebihara et al., 2003, Leung et al., 2005, Wolf et al., 2003). hMPV has subsequently been identified in Asia (Peiris et al., 2003), Australia (Nissen et al., 2002), North America (Bastien et al., 2003, Esper et al., 2003, Peret et al., 2002) and Brazil (Cuevas et al., 2003), where it has been linked to respiratory illness ranging from upper respiratory tract infection to severe bronchiolitis and pneumonia, in patients of all age groups. Sequence analysis has revealed two major subgroups (A and B), each with two minor genetic clusters (A1, A2 and B1, B2) (Van den Hoogen et al., 2004). Infections with hMPV may be severe in the young, the elderly (Falsey et al., 2003) and the immune-compromised (Kumar et al., 2005, Larcher et al., 2005, Martino et al., 2005, William et al., 2005).
Here, we assess the prevalence and severity of hMPV infections in adults in the West of Scotland, quoting a series of cases.
2. Methods
In the 19 months since August 2004 when we introduced hMPV PCR testing as part of our respiratory screen, 9274 respiratory samples were screened using real time RT-PCR for hMPV and 13 other viruses. This survey of respiratory samples, the largest to date, included influenza virus (A, B and C), corona virus (NL63, OC42 and 229E), rhinovirus, RSV A and B and para-influenza virus 1, 2, 3 and 4. The hMPV real time RT-PCR amplified a 70 bp fragment from the hMPV fusion protein gene (Jane et al., 2005).
3. Results
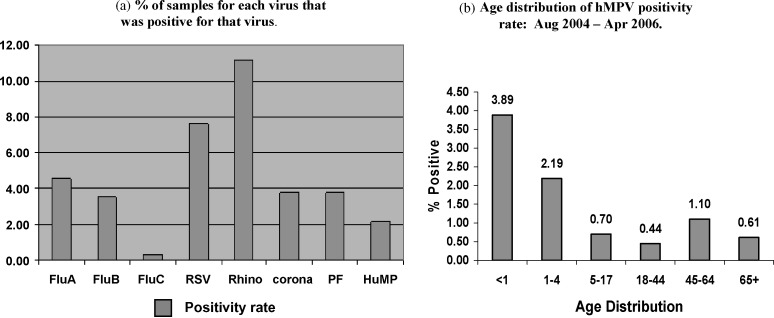
The percentage of samples positive for hMPV and other respiratory virus during the study period is shown in Fig. 1a. hMPV was detected in 206 (2.2%) samples, with a detection rate of 3.89% in patients <1 year of age, 2.19% in those 1–4 years old, and 0.7–1.1% in older age patients (Fig. 1b). The majority of the respiratory samples positive for hMPV (n = 87; 44%) were in children <1 year of age. Thirty-three (0.6%) of 5500 respiratory samples from adults (>18 years old) were positive for hMPV. Within this group, eight (24%) had severe lower respiratory tract infections (LRTI) and four (12%) were managed in an intensive therapy unit (ITU), two of whom died. Mixed infections involving hMPV were found in five samples, with corona virus being the major co-pathogen.
Fig. 1.
(a) % of samples for each virus that was positive for that virus. (b) Age distribution of hMPV positivity rate: August 2004–April 2006.
3.1. Case report of the four patients treated in the ITU for severe hMPV infection
Patient 1. A 50-year-old woman with an underlying common variable immunodeficiency disorder and a long history of Crohn's disease (for which she received steroids and azathioprine), developed a severe lower respiratory tract infection when she was hospitalized for an exacerbation of Crohn's disease in mid-January 2006. The cough and difficulty in breathing, which was initially mild, gradually worsened; she then rapidly de-saturated and required supportive ventilation. Chest X-ray initially showed right middle lobe consolidation that progressed to involve both lung fields diffusely within 48 h. hMPV was strongly positive by RT-PCR on the broncho-alveolar lavage (BAL). She also had a low positive rhinovirus by RT-PCR in the same sample. She recovered completely after withdrawal of azathioprine and reduction in the dosage of steroids.
Patient 2. A 51-year-old man developed B-cell lymphoma in 2002 for which he underwent allogeneic haematopoietic stem cell transplantation (SCT) from a matched unrelated donor in 2003. The post-transplant period was complicated by acute renal failure and graft versus host disease, and a right lung aspergilloma that required middle lobe wedge resection, in September 2005. He took ill while being repatriated from Hong Kong to the UK in January 2006, requiring oxygen on the flight. On arrival in Glasgow his condition deteriorated quite rapidly, needing ITU admission for ventilation support. A chest X-ray showed marked diffuse bilateral alveolar shadowing with significant consolidation in the left lower zone. He showed increasing signs of sepsis and his respiratory function worsened. A BAL sample, taken on the sixth day of ITU admission was positive for hMPV by RT-PCR and immuno-fluorescent staining. He succumbed to worsening respiratory failure, and died on the 21st day of his stay in ITU. Subsequent autopsy revealed the presence of hMPV in the lung tissue, both by RT-PCR and by immune staining (Fig. 2 ).
Fig. 2.
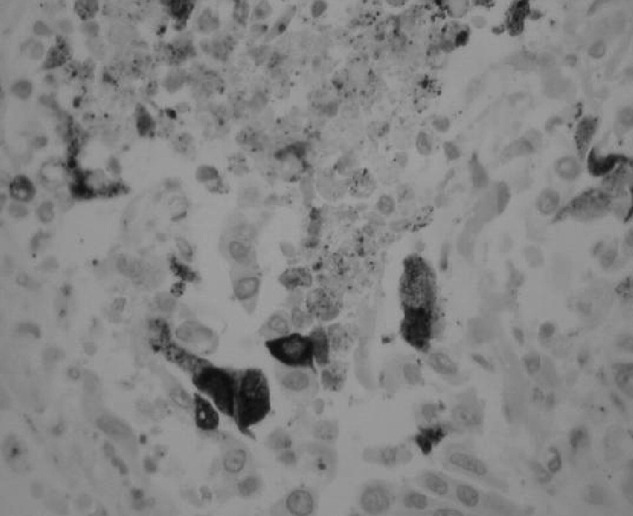
Immunohistochemistry of the lung specimen; the majority of the stained cells appeared to be associated with the airways, either in the abnormal looking epithelial lining or in the lumen as shed cells and debris. There is marked mismatch between the low levels and patchy nature of the viral antigen and the severe, widely distributed pathology of the lung. A striking finding is the relatively low level of lymphocyte infiltration.
Patient 3. A 62-year-old woman had an autologous SCT for lymphoma in December 2005. Within weeks, she developed leucopenia and worsening respiratory signs. She was admitted to ITU for ventilation support. A chest X-ray showed bilateral consolidation. An endo-tracheal aspirate was negative for bacterial culture, but was strongly positive for human metapneumovirus by RT-PCR. She died in ITU after a week of supportive care.
Patient 4. A 75-year-old woman who had longstanding COPD was admitted with a chest infection. She had prior autoimmune neutropenia, rheumatoid arthritis, osteoporosis and pulmonary valvular stenosis. Her respiratory failure worsened within 24 h of hospital admission. hMPV was detected in the tracheal aspirate sample. She required a week of ITU management with ventilation support after which she recovered.
4. Discussion
The overall incidence of hMPV infections in our study was 2.2%, which was higher than the 1.1% reported by Stockton et al. (2002), based on 711 throat and nasal swabs from all ages. Among the 9 hMPV infections in their study, none had institutional treatment; 4 had mild LRTI and all recovered. In contrast, in our study 25 of the 33 adults with respiratory samples positive for hMPV also had LRTI. Chest X-ray changes ranged from infiltration to collapse and consolidation. Four developed respiratory failure, requiring ITU admissions, and two died. This might reflect some selection bias, in that; we are more likely to receive samples from patients with severe disease and varying degrees of immune suppression. Nevertheless, our findings are in contrast to that of an Irish study (Carr et al., 2005), which retrospectively examined 168 BAL samples from adults: four had hMPV infection; none were severe, including in two patients with underlying immunosuppressive disorders.
There have been previous reports of hMPV infection in immunosuppressed patients. The bone-marrow transplant unit in Bristol reported the isolation of hMPV from the nasopharyngeal aspirate of a 33-year-old SCT recipient, who subsequently died of respiratory failure (Cane et al., 2003). Testing for hMPV by RT-PCR was done retrospectively on a stored nasopharyngeal-aspirate sample. A lung-transplant unit in Austria detected hMPV in the BAL samples of nine lung-transplant recipients in 2003–2004 (Larcher et al., 2005). Two of them developed fatal obliterative bronchiolitis and four had acute rejection. The two fatal cases in our report were post-SCT, in whom hMPV was demonstrated in the BAL and endo-tracheal aspirate samples by RT-PCR and by direct immunofluorescent antibody staining in the BAL sample. We were also able to demonstrate hMPV in the autopsy lung specimen of one patient, both by RT-PCR and by immune staining (Fig. 2).
Among the 25 adult patients in our report, there were 3 post-cardiac transplants, in whom hMPV infection was restricted to a mild upper respiratory infection. There were a further two SCTs, in whom the LRTI caused by hMPV was less severe. In another study, it was shown that in a child with lymphoblastic leukaemia, hMPV infection caused repeated respiratory infections in two different seasons, but without significant morbidity (Pelletier et al., 2002). Whether these differences in clinical presentation of hMPV-associated respiratory infections in the different patient groups are due to the biological properties of the different hMPV genotypes or varying degrees of immune suppression in the affected persons, needs further elucidation.
In conclusion, hMPV can cause severe and sometimes fatal respiratory infections in immune compromised adult patients, emphasising the need to include hMPV in the viral screening panel in such patients. The quite dramatic onset of respiratory failure in two of the patients who needed ITU admission further highlights the need for rapid diagnosis and follow-up in order to have appropriate management strategies in place to avert untoward morbidity and mortality.
References
- Bastien N., Ward D., Van Caeseele P., Brandt K., Lee S.H.S., McNabb G. Human metapneumovirus infection in the Canadian population. J Clin Microbiol. 2003;41(10):4642–4646. doi: 10.1128/JCM.41.10.4642-4646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane P.A., van den Hoogen B.G., Chakrabarti S., Fegan C.D., Osterhaus A.D. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transpl. 2003;31(4):309–310. doi: 10.1038/sj.bmt.1703849. [DOI] [PubMed] [Google Scholar]
- Carr M.J., McCormack G.P., Crowley B. Human metapneumovirus-associated respiratory tract infections in the Republic of Ireland during the influenza season of 2003–2004. Clin Microbiol Infect. 2005;11(5):366–371. doi: 10.1111/j.1469-0691.2005.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas L.E., Ben Nasser A.M., Dove W., Gurgel R.Q., Greensill J., Hart C.A. Human metapneumovirus and respiratory syncytial virus in Brazil. Emerg Infect Dis. 2003;9(12):1626–1628. doi: 10.3201/eid0912.030522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T., Endo, Kikuta H., Ishiguro N., Yoshioka M., Kobayashi K. Seroprevalence of human metapneumovirus in Japan. J Med Virol. 2003;70(2):281–283. doi: 10.1002/jmv.10391. [DOI] [PubMed] [Google Scholar]
- Esper F., Boucher D., Weibel C., Martinello R.A., Kahn J.S. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111(6 I):1407–1410. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Erdman D., Anderson L.J., Walsh E.E. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187(5):785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- Kuypers J., Wright N., Corey L., Morrow R. Detection and quantification of human metapneumovirus in paediatric specimens by real-time PCR. J Clin Virol. 2005;33:299–305. doi: 10.1016/j.jcv.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Erdman D., Deshavjee S. Clinical impact of community acquired respiratory viruses in bronchiolitis obliterans after lung transplant. Am J Transpl. 2005;5:2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher C., Geltner C., Fischer H. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transpl. 2005;24:1891–1901. doi: 10.1016/j.healun.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Leung J., Esper F., Weibel C., Kahn J.S. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme linked immuno sorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J Clin Microbiol. 2005;43(3):1213–1219. doi: 10.1128/JCM.43.3.1213-1219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino R., Porras R.P., Rabella N., Williams J.V., Ramila E., Margall N. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract nfections by respiratory viruses in adult recipients of haematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transpl. 2005;11(10):781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen M.D., Siebert D.J., Mackay I.M., Slootsl T.P., Withers S.J. Evidence of human metapneumovirus in Australian children. Med J Aust. 2002;176(4):188. doi: 10.5694/j.1326-5377.2002.tb04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Tang W.-H., Chan K.-H., Khong P.-L., Guan Y., Lau Y., Chiu S.S. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9(6):628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G., Dery P., Abed Y., Boivin G. Respiratory tract reinfections by the new Human Metapneumovirus in an immunocompromised child. Emerg Infect Dis. 2002;8(9):976–978. doi: 10.3201/eid0809.020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peret T.C.T., Boivin G., Li Y., Couillard M., Humphrey C., Osterhaus A.D.M.E. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–1663. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton J., Stephenson I., Fleming D., Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis. 2002;8(9):897–901. doi: 10.3201/eid0809.020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoogen B.G., de Jong J.C., Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoogen B.G., Herfst S., Sprong L., Cane P.A., Forleo-neto E., de Swart R.L. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10:658–666. doi: 10.3201/eid1004.030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D.G., Zakay-Rones Z., Fadeela A., Greenberg D., Dagan R. High seroprevalence of human metapneumovirus among young children in Israel. J Infect Dis. 2003;188(12):1865–1867. doi: 10.1086/380100. [DOI] [PubMed] [Google Scholar]
- William J.V., Martino R., Rabella N. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with Haematological malignancies and respiratory tract infections. J Infect Dis. 2005;192:1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]