Abstract
Background
Military personnel are highly susceptible to febrile respiratory illnesses (FRI), likely due to crowding, stress and other risk factors present in the military environment.
Objective
Our objective was to investigate the viral etiological agents responsible for FRI among military recruits training in a tropical climate in Singapore.
Study design
From March 2006 through April 2007, a total of 1354 oropharyngeal (throat) swabs were collected from military recruits who reported sick with an oral temperature of ≥38 °C and a cough and/or sore throat. Real-time polymerase chain reaction (PCR) was used to assay for the presence of influenza A and B viruses and adenoviruses (H-AdV), and conventional PCR used for the remaining respiratory viruses in all specimens.
Results
Influenza A virus was the dominant infection with a laboratory-confirmed incidence of 24% (326/1354) and a predominance of the H3N2 subtype. The temporal pattern for influenza A virus infections coincided with the nation-wide pattern in the civilian community. Detection rates of 12% (159/1354) and 2.7% (5/1354) were obtained for influenza B virus and other respiratory viruses, respectively.
Conclusions
The laboratory findings identified influenza A virus as the primary causative viral agent for FRI in the Singapore military, in strong contrast to findings from temperate countries and countries where recruits are often vaccinated for influenza. Our results suggest that influenza vaccination should be considered as a requirement to reduce the incidence of influenza infections. This is the first report describing respiratory infections in a tropical military setting, in a developed country in Asia.
Keywords: Singapore military, Tropical, Influenza A and B viruses, Febrile respiratory illness, Recruits
1. Background
Febrile respiratory illness (FRI) patterns in military populations are different than those observed in general civilian populations. This increased vulnerability has been ascribed to high population density, extreme physical and psychological challenges during training and wartime operations.1 High rates of FRI were consistently observed throughout the history of the US military.2, 3, 4 Although influenza outbreaks and infections have been documented, human adenovirus (H-AdV) has consistently been the predominant cause of FRI with US military.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 In Asia, influenza outbreaks have been reported with Taiwan military and H-AdV infections with South Korea military, but none reported in the tropics.15, 16 Other surveys in Asia were conducted by US military personnel stationed on active duty in Asian countries.17, 18, 19 These reports suggest that acute respiratory infections can occur in military populations indigenous to Asia, but these are either mostly not surveyed, or remained unreported. Clearly, the spread of these diseases can continue to compromise mission capability and security.
2. Objectives
The objective of our study was to determine the viral FRI burden, especially by influenza viruses and H-AdV, in the military in Singapore, a tropical Asian city-state. The virological data gathered from this study will inform policy makers of the disease impact and can guide the implementation of public health interventions to reduce the disease burden.
3. Study design
3.1. Recruitment of participants
Participants were recruited from a military training camp (Camp X) from March 2006 through April 2007. Personnel who reported sick with FRI symptoms, defined as fever (oral temperature ≥38 °C) with acute respiratory symptoms of cough or/and sore throat were recruited from Monday through Friday throughout the entire year. Oropharyngeal (throat) swabs (Remel, Lenexa, Kansas, USA) were collected from participants who consented to the study, and asked to fill out a simple self-report form including their demographic and clinical details.
3.2. Specimen collection and laboratory processing
Swabs were resuspended in viral transport medium and total nucleic acids were extracted using the RNeasy minikit (Qiagen, Inc., Valencia, CA, USA) according to manufacturer's instructions, and stored at −80 °C before PCR analysis.
3.3. PCR analysis
Real-time PCR tests for influenza A and B viruses and H-AdV were performed in a Lightcycler 1.5 (Roche Diagnostics, Mannheim, Germany).8, 20, 21 Influenza A viruses were subtyped using a modified nested PCR format in an MJ conventional machine (Promega).22, 23 Multiplex PCR tests for the remaining respiratory viruses listed in Table 1 were used on the same conventional PCR machine.24 The amplicons for HMPV were genotyped by DNA sequencing using the BigDye Terminator cycle sequencing (Applied Biosystem, Foster City, CA, USA).
Table 1.
Number of clinical specimens that tested PCR positive for respiratory viruses in Camp X.
| Viral agents tested | Numbera (%) |
|---|---|
| Number of specimens collected | 1354 |
| Adenovirus | 5 (0.4) |
| Influenza A virus | 326 (24) |
| Influenza B virus | 159 (12) |
| Influenza C virus | 4 (0.3) |
| Parainfluenza type 1 | 1 |
| Parainfluenza type 2 | 0 |
| Parainfluenza type 3 | 4 (0.3) |
| Parainfluenza type 4 | 0 |
| Rhinovirus | 15 (1.1) |
| Coronavirus, OC43 | 0 |
| Coronavirus, 229E | 1 |
| Respiratory syncytial virus | 0 |
| Human metapneumovirus | 9 (0.7) |
| Total number tested positive | 524 (38.7) |
The number of clinical specimens that tested PCR positives refer to the specimens that tested positive for each viral agent relative to the total number of specimens collected for the study. The percentage is represented within brackets.
4. Results
4.1. Incidence of viral infections
A total of 1354 specimens were collected, representing all recruits matching the inclusion criteria who reported for medical care during weekdays. Twenty-four percent (326/1354) were PCR positive for influenza A virus, 12% (159/1354) for influenza B virus and 0.4% (5/1354) for H-AdV (Table 1). Of the remaining 864 specimens, only 4 influenza C virus, 1 human coronavirus 229E, 9 human metapneumovirus (HMPV) (7 of the genotype A2), 15 rhinovirus, 1 parainfluenza virus (PIV) type 1, and 4 PIV type 3 (Table 1) were PCR-detected. The remaining 830 (61%) specimens were negative by all tests performed, suggesting that these specimens may be collected in ways that precluded viral identification, or that the collected specimens were not stored properly before transport to the diagnostic laboratory.
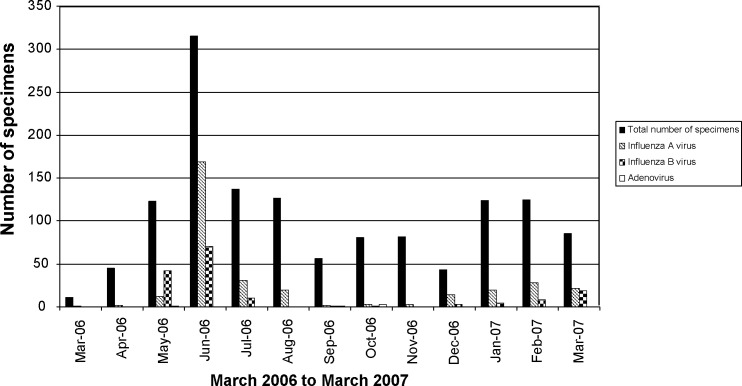
These results indicate that influenza A virus is the leading identifiable cause of FRI among Singapore military recruits. As shown in Fig. 1 , the FRI incidence differed significantly over time. There were 2 distinctive surges of FRI that coincided with the peaks for influenza A virus in the months of June 2006 and January-February 2007. We observed a predominance of H3N2 subtypes at 38.0% (124/326) of all influenza A virus infections, and less H1N1 at 15.6% (51/326) (data not shown). The remaining influenza A-positive samples remained negative from the PCR-subtyping assays, suggesting that these specimens were of relatively low viral load. The pattern for influenza B virus incidence was sporadic throughout the surveillance period (Fig. 1). In May 2006, the incidence of influenza B virus superseded that of influenza A virus, resulting in a noticeable peak (Fig. 1). We speculate that this could represent a small outbreak among the recruits within the same training group, or sharing the same sleeping dormitories.
Fig. 1.
Number of FRI specimens collected showing PCR positive for the laboratory-confirmed incidence of influenza A and B viruses, and H-AdV. The real-time PCR testings were performed on the LightCycler, and the results for the presence of influenza viruses were read on the F1 channel and that for H-AdV were differentiated from non-specific products such as primer-dimers via the different melting points. The laboratory-identified incidences of FRI, measured by the total number of specimens collected, were monitored on a monthly basis together with the specific incidences of influenza viruses and H-AdV. The disease data are presented as incidence, as rate data could not be collected due to the classified nature of recruit population data. (■) represents total number of specimens collected that fulfilled the FRI criterion, ( ) PCR positive for the presence of influenza A virus, (
) PCR positive for the presence of influenza A virus, ( ) for influenza B virus and (□) for H-AdV.
) for influenza B virus and (□) for H-AdV.
4.2. Demographical and clinical details
The symptoms most frequently reported by participants testing positive for influenza viruses or H-AdV are shown in Table 2 . Other than high fever (which was a requirement for inclusion), the most common symptoms were cough, sore throat, headache, body ache and running nose. Participants with influenza A virus infections were more likely to have cough when compared with participants with influenza B virus or H-AdV (P < 0.05). From our results, the clinical impact of FRI can be estimated, with at least 2708 training (work) days lost among the 1354 patients recruited for the study.
Table 2.
Demographic and clinical characteristics of participants.
| Characteristic | Influenza A virus Na = 326 |
Influenza B virus Na = 215 |
Adenovirus Na = 39 |
P-valueb |
|---|---|---|---|---|
| Age | ||||
| Range | 16.8–43.4 years | 16.8–43.4 years | 19.0–21.4 years | f0.635 |
| Median | 20.2 years | 19.8 years | 20 years | |
| Gender | Male | Male | Male | |
| Symptoms durationc | ||||
| Range | 1–16 days | 1–7 days | 1–2 days | f0.11 |
| Median | 2 days | 2 days | 1 day | |
| Highest temperature | ||||
| Range | 36.5–40.3 °C | 37–40.2 °C | 38.2–38.7 °C | f0.2 |
| Median | 38.6 °C | 38.6 °C | 38.6 °C | f0.93 |
| Current temperature | ||||
| Range | 35.4–40.2 °C | 37–40 °C | 37.9–38.6 °C | |
| Median | 38.5 °C | 38.5 °C | 38.4 °C | |
| Sore throat | 235/326 (72.1) | 116/159 (73.0) | 5/5 (100.0) | f0.38 |
| Cough | 305/326 (93.6) | 137/159 (86.2) | 2/5 (40.0) | d, e<0.05 |
| Shortness of breath | 80/326 (24.5) | 41/159 (25.8) | 1/5 (20) | f0.93 |
| Congestion | 216/326 (66.3) | 94/159 (59.1) | 3/5 (60) | f0.30 |
| Headache | 233/326 (71.5) | 115/159 (72.3) | 3/5 (60) | f0.83 |
| Pink eyes | 19/326 (5.8) | 13/159 (8.2) | 1/5 (20) | f0.31 |
| Body ache | 158/326 (48.5) | 78/159 (49.1) | 2/5 (40) | f0.92 |
| Nausea | 83/326 (25.5) | 44/159 (27.7) | 1/5 (20) | f0.83 |
| Asthma | 75/326 (23.0) | 26/159 (16.4) | 1/5 (20) | f0.24 |
| Vaccinated for influenza viruses | 5/326 (1.5) | 6/159 (3.8) | 0/5 (0) | f0.28 |
| Smoking | 75/326 (23) | 33/159 (20.8) | 1/5 (20) | f0.85 |
N represents number of respondents who answered all the questions in the case form, and whose specimens tested PCR positive.
For each viral agent, a positive characteristic was scored and this is expressed relative to the number of respondents. The percentage is represented within brackets. Data analysis was performed using the open-source statistical software R (http://www.r-project.org/) and univariate analysis with the Pearson's Chi-squared test to examine categorical measurements of illness characteristics, physical examination findings and laboratory results.
Refers to the number of days reported sick before recruitment.
Influenza A virus versus influenza B virus.
Influenza A virus versus adenovirus.
NS represents not significant.
5. Discussions
This is the first comprehensive study that provides data on the viral burden in a military population in Asia with a tropical setting. Throughout the current one-year study, the laboratory findings identified influenza A and B viruses in 36% (485/1354) of the FRI cases, with H3N2 as the predominant annual subtype in Singapore. Two peaks were observed, in June 2006 where the proportion of influenza detected rose above 80%, and January-February 2007 (Fig. 1). This bimodal pattern and the predominant H3N2 subtype concurred with reports at the national level from the Singapore Ministry of Health.25, 26, 27 On the other hand, the sporadic pattern for influenza B virus incidence is in contrast with the cluster of infections reported nationally at the beginning of 2006 and 2007.25, 26 The laboratory-confirmed data suggests that the outbreaks in the military were related to the national outbreaks due to the interactions by the military personnel and the community. In addition, the influenza A cases in our study had significantly more symptoms of cough than other FRI cases (Table 2). The participants in Camp X were new recruits who were exposed to military activities for the first time, and we expected them to demonstrate increased vulnerability to respiratory infections. In the US military, it was reported that new recruits were 29 times more likely to be hospitalized for respiratory infections than other military personnel.2, 3, 4 The laboratory-confirmed incidence of H-AdV infections remained low throughout the study period, at 0.4% (5/1364). This is in contrast to more than 50% reported in South Korean and the US military recruits.9, 14, 16 No respiratory syncytial virus (RSV) was detected in the Singapore cohort.28 Instead 9 cases of HMPV (Table 1) were detected and majority were of the A2 genotype, reported to be associated with an increased severity in respiratory infections.29, 30
The data from the current study further suggests that in the event an outbreak happens in the Singapore military, the attack rate is likely to be high because vaccination is not mandatory for the Singapore military. In contrast, influenza outbreaks in US trainees are rare because they are vaccinated against circulating influenza strains.6, 7, 8 Vaccine effectiveness against influenza A virus was reported to be at 92% for the US military, and 38.1% and 41.6% in asymptomatic and symptomatic Israeli soldiers respectively.31, 32 These reports support reductions of influenza-associated illness in young adults by the use of influenza vaccination. In conclusion, this prospective surveillance study demonstrated that influenza viruses are the most common identifiable viral agents causing FRI in the Singapore military. Compulsory vaccination to reduce influenza infections should be considered upon enlistment of personnel for the Singapore military.
Conflict of interest
None.
Funding
This research is funded by Ministry of Defence, Singapore.
Acknowledgements
We thank all personnel at the medical centres for their assistance in facilitating the specimen collections, and other logistic matter. This study was approved by the Joint Medical Committee for Research, Singapore Armed Forces, and supported by the Ministry of Defence, Singapore. The views expressed herein are those of the authors and do not represent the official position of the US Department of Defense or Department of the Navy.
References
- 1.Russell K. Respiratory infections in military recruits. In: Lenhart M.K., editor. Recruit medicine. The Office of the Surgeon General; Washington, DC: 2006. pp. 227–253. Available at: http://www.bordeninstitute.army.mil/published_volumes/recruit_medicine/RM-ch13.pdf [accessed 17.09.06] [Google Scholar]
- 2.Pazzaglia G., Pasternack M. Recent trends of pneumonia morbidity in US Naval personnel. Mil Med. 1983;148:647–651. [PubMed] [Google Scholar]
- 3.Gray G.C., Callahan J.D., Hawksworth A.W., Fisher C.A., Gaydos J.C. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5:379–385. doi: 10.3201/eid0503.990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray G.C., Blankenship T.L., Gackstetter G. History of respiratory illness at the U.S. Naval Academy. Mil Med. 2001;166:581–586. doi: 10.1093/milmed/166.7.581. [DOI] [PubMed] [Google Scholar]
- 5.Kak V. Infections in confined spaces: cruise ships, military barracks, and college dormitories. Infect Dis Clin North Am. 2007;21:773–784. doi: 10.1016/j.idc.2007.06.004. ix–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earhart K.C., Beadle C., Miller L.K., Pruss M.W., Gray G.C., Ledbetter E.K. Outbreak of influenza in highly vaccinated crew of U.S. navy ship. Emerg Infect Dis. 2001;7:463–465. doi: 10.3201/eid0703.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makras P., Alexiou-Daniel S., Antoniadis A., Hatzigeorgiou D. Outbreak of meningococcal disease after an influenza B epidemic at a Hellenic air force recruit training center. Clin Infect Dis. 2001;33:e48–50. doi: 10.1086/322609. [DOI] [PubMed] [Google Scholar]
- 8.Krafft A.E., Russell K.L., Hawksworth A.W., McCall S., Irvine M., Daum L.T. Evaluation of PCR testing of ethanol-fixed nasal swab specimens as an augmented surveillance strategy for influenza virus and adenovirus identification. J Clin Microbiol. 2005;43:1268–1275. doi: 10.1128/JCM.43.4.1768-1775.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudding B.A., Top F.H., Jr., Winter P.E., Bruescher E.L., Lamson T.H., Leibovitz A. Acute respiratory disease in military trainees: the adenovirus surveillance program, 1966–1971. Am J Epidemiol. 1973;97:187–198. doi: 10.1093/oxfordjournals.aje.a121499. [DOI] [PubMed] [Google Scholar]
- 10.Ryan M.A., Gray G.C., Smith B., McKeehan J.A., Hawksworth A.W., Malasig M.D. Large epidemic of respiratory illness due to adenovirus types 7 and 3 in healthy young adults. Clin Infect Dis. 2002;34:577–582. doi: 10.1086/338471. [DOI] [PubMed] [Google Scholar]
- 11.Russell K.L., Broderick M.P., Franklin S.E., Blyn L.B., Freed N.E., Moradi E. Transmission dynamics and prospective environmental sampling of adenovirus in a military recruit setting. J Infect Dis. 2006;194:877–885. doi: 10.1086/507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houng H.S., Clavio S., Graham K., Kuschner R., Sun W., Russell K.L. Emergence of a new human adenovirus type 4 (Ad4) genotype: identification of a novel inverted terminal repeated (ITR) sequence from majority of Ad4 isolates from US military recruits. J Clin Virol. 2006;35:381–387. doi: 10.1016/j.jcv.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Kajon A.E., Moseley J.M., Metzgar D., Huong H.S., Wadleigh A., Ryan M.A.K. Molecular epidemiology of adenovirus type 4 infections in US military recruits in the postvaccination era (1997–2003) J Infect Dis. 2007;196:67–75. doi: 10.1086/518442. [DOI] [PubMed] [Google Scholar]
- 14.Metzgar D., Osuna M., Kajon A.E., Hawksworth A.W., Irvine M., Russell K.L. Abrupt emergence of diverse species B adenoviruses at US military recruit training centers. J Infect Dis. 2007;196:1465–1473. doi: 10.1086/522970. [DOI] [PubMed] [Google Scholar]
- 15.Liu P.Y., Wang L.C., Lin Y.H., Tsai C.A., Shi Z.Y. Outbreak of influenza A and B among military recruits: evidence from viral culture and polymerase chain reaction. J Microbiol Immunol Infect. 2009;42:114–121. [PubMed] [Google Scholar]
- 16.Jeon K., Kang C.I., Yoon C.H., Lee D.J., Kim C.H., Chung Y.S. High isolation rate of adenovirus serotype 7 from South Korean military recruits with mild acute respiratory disease. Eur J Clin Microbiol Infect Dis. 2007;26:481–483. doi: 10.1007/s10096-007-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith T.J., Olson L.C., Kandel G.E., Snitbhan R. Hong Kong influenza in US military airmen in Thailand. Am J Trop Med Hyg. 1970;5:866–871. doi: 10.4269/ajtmh.1970.19.866. [DOI] [PubMed] [Google Scholar]
- 18.Sanford J.P. Acute respiratory disease in the United States Army in the Republic of Vietnam, 1965–1970. Yale J Biol Med. 1975;3:179–184. [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent J.M., Cherry J.D., Nauschuetz W.F., Lipton A., Ono C.M., Costello C.N. Prolonged afebrile nonproductive cough illnesses in American soldiers in Korea: a serological search for causation. Clin Infect Dis. 2000;3:534–539. doi: 10.1086/313707. [DOI] [PubMed] [Google Scholar]
- 20.Spackman E., Senne D.A., Myers T.J., Bulaga L.L., Garber L.P., Perdue M.L. Development of a real-time reverse transcriptase PCR assay for Type A Influenza and the Avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Echavarria M., Forman M., Ticehurst J., Dumler S., Charache P. PCR method for detection of adenovirus in healthy and human immunodeficiency virus-infected individuals. J Clin Microbiol. 1998;36:3323–3326. doi: 10.1128/jcm.36.11.3323-3326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schweiger I., Zadow R., Heckler H., Timm G. Application of a fluorogenic PCR assay for typing and subtyping influenza viruses in respiratory samples. J Clin Microbiol. 2000;38:1552–1558. doi: 10.1128/jcm.38.4.1552-1558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan C.H., Lin K.L., Chan Y., Wang Y.L., Chi Y.T., Tu H.L. Amplification of the entire genome of Influenza A virus H1N1 and H3N2 subtypes by reverse-transcription polymerase chain reaction. J Virol Methods. 2006;136:38–43. doi: 10.1016/j.jviromet.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Bellau-Pujol S., Vabret A., Legrand L., Dina J., Gouarin S., Petitjean-Lecherbonnier J. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods. 2005;126:53–63. doi: 10.1016/j.jviromet.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Health, Singapore . Communicable diseases surveillance in Singapore 2006. 2007. Air/droplet-borne diseases; pp. 3–21. Available at: http://www.moh.gov.sg/mohcorp/publicationsreports.aspx?id=17516 [accessed 15.09.08] [Google Scholar]
- 26.Ministry of Health, Singapore . Communicable diseases surveillance in Singapore 2007. 2008. Air/droplet-borne diseases; pp. 3–18. Available at http://www.moh.gov.sg/mohcorp/publicationsreports.aspx?id=20288 [accessed 30.06.09] [Google Scholar]
- 27.Chow A., Ma S., Ling A.E., Chew S.K. Influenza-associated deaths in tropical Singapore. Emerg Infect Dis. 2006;12:114–121. doi: 10.3201/eid1201.050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Shea M.K., Ryan M.A., Hawksworth A.W., Alsip B.J., Gray G.C. Symptomatic respiratory syncytial virus infection in previously healthy young adults living in a crowded military environment. Clin Infect Dis. 2005;41:311–317. doi: 10.1086/431591. [DOI] [PubMed] [Google Scholar]
- 29.Sugrue R.J., Tan B.H., Loo L.H. The emergence of human metapneumovirus. Fut Rev Virol. 2008;9:1396–1398. [Google Scholar]
- 30.Vicente D., Montes M., Cilla G., Perez-Yarza E.G., Perez-Trallero E. Differences in clinical severity between genotype A and genotype B human metapneumovirus infection in children. Clin Infect Dis. 2006;42:e111–e113. doi: 10.1086/504378. [DOI] [PubMed] [Google Scholar]
- 31.Strickler J.K., Hawksworth A.W., Myers C., Irvine M., Ryan M.A.K., Russell K.L. Influenza vaccine effectiveness among US military basic trainees, 2005–06 season. Emerg Infect Dis. 2007;13:617–619. doi: 10.3201/eid1304.061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grotto I., Mandel Y., Green M.S., Varsano N., Gdalevich M., Ashkenazi I. Influenza efficacy in young healthy adults. Clin Infect Dis. 1998;4:913–917. doi: 10.1086/513934. [DOI] [PubMed] [Google Scholar]