Abstract
A dimeric 40-kDa Kunitz-type trypsin inhibitor was isolated from seeds of the Chinese black soybean Glycine max cv. ‘Dull Black’. The purification protocol comprised ion exchange chromatography on Q-Sepharose, SP-Sepharose, and Mono Q, and gel filtration on Superdex 75. The trypsin inhibitor inhibited chymotrypsin, albeit to a lesser extent than it inhibited trypsin. Its trypsin-inhibitory activity was unaffected after exposure to pH 1–14, or to temperatures up to 80 °C. The trypsin inhibitor was inhibited by dithiothreitol in a dose-dependent (from 2.5 to 50 mM) and a time-dependent (from 5 to 120 min) manner. Besides inhibiting serine proteases, the trypsin inhibitor demonstrated additional biological activities including stimulation of nitric oxide production by macrophages. It inhibited HIV-1 reverse transcriptase, cell-free translation and proliferation of liver cancer cells and breast cancer cells, with an IC50 value 9.4, 14, 39 and 70 μM, respectively. However, it did not exhibit antifungal, antibacterial or mitogenic activity.
Keywords: Kunitz-type trypsin inhibitor, Black soybean, Anti-proliferative, Isolation, Characterization, Seeds
1. Introduction
Protease inhibitors have drawn the attention of many investigators due to their potential value. For instance, HIV protease inhibitors and SARS coronavirus proteinase inhibitors may be used to combat HIV and SARS virus, respectively. Plant protease inhibitors may have anti-insect and antifungal activities. They may be involved in the regulation of programmed cell death in plants. They can cause inhalant allergies and also food allergies [1]. One of the common types of protease inhibitors is trypsin inhibitors which have been isolated from animal tissues and also from plant tissues [1], [2]. There are several types of plant trypsin inhibitors. Kunitz-type trypsin inhibitors have a molecular mass of about 20 kDa, a low cysteine content and a single reactive site while Bowman–Birk trypsin inhibitors are approximately 8 kDa in size and possess a high cysteine content and two reactive sites [3], [4], [5], [6], [7], [8]. The conformation in Kunitz-type trypsin inhibitors is mainly β-sheet with a small amount of regular sheet. The insecticidal activity of Kunitz-type trypsin inhibitors has been demonstrated using transgenic plants [2]. Kunitz-type trypsin inhibitors have been ***isolated from the seeds of Erythrina species [9], Psophocarpus tetragonolebus [10], Prosopis juliflora [11], Acacia confusa [12], Enterolobium contortisiliquum [13], Bauhinia variegata [14], Delonix regia [15], Crotalaria paulina [16], Mucuna pruriens [17], Dimorphandra mollis [18], Copaifera langsdorfii [19], Phaseolus vulgaris [20], Pithecelobium dumosum [21], Veronica hederifolia [22] and Mirabilis jalapa [23]. Kunitz-type trypsin inhibitors also inhibit other enzymes such as chymotrypsin, α-amylase and human plasmin, and block the conversion of prothrombin to thrombin [1]. The formation, degradation and gene expression of Kunitz-type trypsin inhibitor in the soybean have been reported [1]. Some seeds, e.g., those of Glycine max produce both Bowman–Birk and Kunitz-type trypsin inhibitors [24]. The seeds of bitter gourds, sponge gourds, wax gourds and Momordica cochinchinensis produce squash-type trypsin inhibitors with a molecular mass of about 3 kDa [25].
Chinese dull black soybean is a special cultivar of G. max. It has a different usage in traditional Chinese medicine from the yellow soybean. The intent of the present study was to purify and characterize a trypsin inhibitor from Chinese dull black soybean.
2. Materials and methods
2.1. Isolation of trypsin inhibitor
Chinese dull black soybeans (G. max cv. ‘Dull Black Soybean’) from China (100 g) were extracted with distilled water (10 ml/g) at room temperature in a blender for 10 min followed by centrifugation at 13,000 rpm and 4 °C for 30 min. Tris–HCl buffer (1 M, pH 7.4) was added to the resulting supernatant until the final concentration of Tris attained 10 mM. The supernatant was then loaded on a 5 cm × 20 cm column of Q-Sepharose (GE Healthcare) in 10 mM Tris–HCl buffer (pH 7.4). After removal of unadsorbed proteins, the column was eluted successively with 0.1 M, 0.4 M and 1 M NaCl added to the Tris–HCL buffer. The fraction eluted with 0.1 M NaCl was dialyzed against distilled water and then its concentration was adjusted to 10 mM Tris–HCl (pH 7.4) before ion exchange chromatography on a 2.5 cm × 20 cm column of SP-Sepharose (GE Healthcare) in the same buffer. After removal of unadsorbed proteins, the column was eluted sequentially with 0.2 M NaCl and 1 M NaCl added to the Tris–HCl buffer. The fraction desorbed with 0.2 M NaCl was dialyzed prior to FPLC-ion exchange chromatography on Mono Q (GE Healthcare). After unadsorbed proteins had been eluted, the column was eluted, first with a linear gradient of 0–0.3 M NaCl in the starting buffer, and then with a linear gradient of 0.3–1 M NaCl. Fraction S3, the adsorbed fraction desorbed with 0.2 M NaCl, was finally purified on an FPLC-gel filtration Superdex 75 HR10/30 column (GE Healthcare) using an AKTA Purifier (GE Healthcare). The single peak obtained represented black soybean trypsin inhibitor (BSKTI).
2.2. Electrophoresis, molecular mass determination, and N-terminal sequence analysis
The purified protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for molecular mass determination in accordance with the method of Nielsen and Reynolds [26]. After electrophoresis the gel was stained with Coomassie Brilliant Blue. The molecular mass of the isolated protein was determined by comparison of its electrophoretic mobility with those of molecular mass marker proteins from GE Healthcare. Gel filtration on an FPLC-Superdex 75 column, which had been calibrated with molecular mass markers (GE Healthcare), was conducted to determine the molecular mass of the protein. The N-terminal sequence of the protein was determined by using a Hewlett-Packard HP G1000A Edman degradation unit and an HP 1000 HPLC System.
2.3. Assays for trypsin-inhibitory and chymotrypsin-inhibitory activities
Trypsin activity was determined by using N-α-benzoyl-l-arginine ethyl ester hydrochloride (BAEE) from Sigma as substrate [25]. Ten microliters of a bovine pancreatic trypsin (USB Corporation, OH, USA) solution (250 μg/ml) in assay buffer (50 mM Tris–HCl, pH 8, containing 20 mM CaCl2) were added to 980 μl of assay buffer, and then 10 μl of BAEE in assay buffer was added to give a final concentration of 0.6 mM. The reaction rate was determined by monitoring the absorbance change at 253 nm for 1 min.
To assay for trypsin-inhibitory activity, 10 μl test sample in assay buffer was added to trypsin, and incubated at 25 °C for 15 min before addition of substrate (BAEE) to initiate the reaction. Trypsin-inhibitory activity was calculated as follows:
where Abs control is absorbance change in absence of sample, Abs sample is absorbance change in presence of sample, trypsin (mg) is the amount of trypsin in assay mixture. One unit of trypsin-inhibitory activity refers to the activity capable of inhibiting 1 mg trypsin.
Chymotrypsin activity was determined by using N-α-benzoyl-l-tyrosyl ethyl ester hydrochloride (BTEE) as substrate and bovine pancreatic chymotrypsin (USB, Corporation, OH, USA) [27]. Chymotrypsin-inhibitory activity was assayed as described above for trypsin-inhibitory activity but with chymotrypsin and BTEE replacing trypsin and BAEE, respectively.
To investigate thermal stability and pH stability, the isolated trypsin inhibitor was exposed to 0–100 °C (0, 10 °C, etc., at 10 °C intervals) or pH 1–14 (pH 1, 2, 3, etc.) and the assay of trypsin-inhibitory activity was then conducted as mentioned above.
2.4. Effect of dithiothreitol (DTT) on trypsin-inhibitory activity
The isolated trypsin inhibitor (2.41 μM) was incubated with dithiothreitol (DTT) at the final concentrations of 2.5, 20 and 50 mM for 5, 20, 60 and 120 min at 37 °C. For comparison, the soybean trypsin inhibitor (Sigma) (2.88 μM) was similarly treated. The reaction was terminated by adding iodoacetamide at twice the amount of thiol functions contained in each DTT concentration. The remaining trypsin-inhibitory activity was measured at pH 8 as described above. The highest iodoacetamide concentration used in the test was devoid of any effect on the activity of trypsin and the trypsin-inhibitory activity on isolated trypsin inhibitor and soybean trypsin inhibitor [27].
2.5. Assay of antibacterial activity
Bacteria (Streptococcus aureus) were incubated in 10 ml of nutrient broth in a thermal shaker for 12 h at 37 °C, and then 5 ml of this bacterial suspension was transferred to 50 ml of nutrient broth and incubated for another 3–6 h in order to shift bacterial growth to the mid-logarithmic phase. The bacterial suspension was then centrifuged at 2000 × g for 10 min, and the bacterial pellet was washed and resuspended in normal saline. A total of 105 or 107 bacteria per ml were obtained by dilution guided by the optical density at 595 nm. In the experiment, every condition was prepared in triplicate; one aliquot of bacterial suspension was mixed with the isolated trypsin inhibitor at 0.5, 0.25 and 0.125 mg/ml and one aliquot was mixed with only bacteria in saline as a control. The samples were then incubated in a shaker and aliquots were obtained at four time points [0, 3, 6 and 12 h], serially diluted with nutrient broth, and spread on agar plates. After incubation at 37 °C for 24 h, the colonies were counted. The number of bacteria for each condition and dilution was determined from the average colony counts for three plates. The leguminous defensin-like peptide sesquin [28] was used as a positive control emperor banana lectin [30] and bovine serum albumin was used as a negative control.
2.6. Assay of mitogenic activity
Four C57BL/6 mice [20–25 g] were killed by cervical dislocation, and the spleens were aseptically removed. Spleen cells were isolated by pressing the tissue through a sterilized 100-mesh stainless steel sieve and resuspended to 5 × 106 cells/ml in RPMI 1640 culture medium supplemented with 10% fetal bovine serum, 100 units penicillin/ml, and 100 μg streptomycin/ml. The cells [7 × 105 cells/100 μl/well] were seeded into a 96-well culture plate and serial dilutions of a solution of the isolated trypsin inhibitor in 100 μl medium were added. After incubation of the cells at 37 °C in a humidified atmosphere of 5% CO2 for 24 h, 10 μl [methyl-3H]-thymidine [0.25 μCi, GE Healthcare] was added, and the cells were incubated for a further 6 h under the same conditions. The cells were then harvested with an automated cell harvester onto a glass fiber filter, and the radioactivity was measured with a Beckman model LS 6000SC scintillation counter. All reported values are the means of triplicate samples. Con A was used as positive control and bovine serum albumin as a negative control [29].
2.7. Assay of nitric oxide production by murine peritoneal macrophages
The assay was conducted as described by Wong and Ng [30]. Macrophages were collected from the peritoneal cavity of mice after an intraperitoneal injection of a 3% thioglycolate solution. The cells were washed and resuspended in RPMI medium containing 10% fetal bovine serum, 100 IU/ml penicillin, and 100 mg/ml streptomycin. Cells [2 × 105 cells/well] were seeded in a 96-well culture plate for 1 h before incubation with the isolated trypsin inhibitor (23.76 μM) or soybean trypsin inhibitor (25 μM) for 24 h. The amount of nitric oxide in the culture medium was determined by the colorimetric method using sodium nitrite as a standard. In the assay, a 100 μl aliquot of cell-free culture medium from each culture well was allowed to react with 50 μl of Griess reagent [1% sulfanilamide in 5% H3PO4–0.1% naphthalene-ethylenediamine dihydrochloride] for 10 min before absorbance was read at 540 nm using a microplate reader. Lipopolysaccharide was used as a positive control and knife bean lectin as a negative control in this assay [29].
2.8. Assay of anti-proliferative activity on tumor cell lines
Breast cancer MCF-7 cells and hepatoma HepG2 cells were suspended in RPMI medium and adjusted to a cell density of 2 × 104 cells/ml. A 100 μl aliquot of this cell suspension was seeded to a well of a 96-well plate, followed by incubation for 24 h. Different concentrations of the trypsin inhibitor in 100 μl complete RPMI medium were then added to the wells and incubated for 72 h at 37 °C. After 72 h, 20 μl of a 5 mg/ml solution of [3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide] [MTT] in phosphate buffered saline was spiked into each well and the plates were incubated for 4 h. The plates were then centrifuged at 324 × g for 5 min. The supernatant was carefully removed, and 150 μl of dimethyl sulfoxide was added in each well to dissolve the MTT-formazan at the bottom of the wells. After 10 min, the absorbance at 590 nm was measured by using a microplate reader [28] and green lentil trypsin inhibitor [31] was used as a negative control in the assay.
2.9. Assay for HIV-1 reverse transcriptase-inhibitory activity
The assay for HIV reverse transcriptase-inhibitory activity was carried out according to instructions supplied with the assay kit from Boehringer Mannhein (Germany). The assay takes advantage of the ability of reverse transcriptase to synthesize DNA, starting from the template/primer hybrid poly (A) oligo (dT) 15. The digoxigenin- and biotin-labeled nucleotides in an optimized ratio are incorporated into one of the same DNA molecule, which is freshly synthesized by the reverse transcriptase (RT). The detection and quantification of synthesized DNA as a parameter for RT activity follows a sandwich ELISA protocol. Biotin-labeled DNA binds to the surface of microtiter plate modules that have been precoated with strepatavidin. In the next step, an antibody to digoxigenin, conjugated to peroxidase, binds to the digoxigenin-labeled DNA. In the final step, the peroxidase substrate is added. The peroxidase enzyme catalyzes the cleavage of the substrate, producing a colored reaction product. The absorbance of the sample at 405 nm can be determined using a microtiter plate (ELISA) reader and is directly correlated to the level of RT activity. A fixed amount (4–6 ng) of recombinant HIV-1 reverse transcriptase was used. The inhibitory activity of the trypsin inhibitor was calculated as percent inhibition as compared to a control without the protein [28]. The leguminous defensin-like protein sesquin was used as a positive control [28] and the antifungal protein mungin [32] as a negative control.
2.10. Assay of ability to inhibit HIV-1 integrase. Expression and purification of recombinant HiV-1 integrase
The plasmid that expressed His-tagged wild-type HIV-1 integrase, pT7-7-His (Y|TX)-HIV-1-IN, was a generous gift from Dr. S.A. Chow (School of Medicine, UCLA). To express the protein, a 1-l culture of E. coli BL21 (DE3) cells containing the expression plasmid was grown at 37 °C until OD600 reached 0.7–0.8. Cells were induced by addition of 0.8 mM IPTG and harvested, after 4 h incubation, by centrifugation at 6000 × g for 10 min at 4 °C. Cells were suspended at a concentration of 10 ml/g wet cell paste in 20 mM Tris–HCl (pH 8.0), containing 0.1 mM EDTA, 2 mM β-mercaptoethanol, 0.5 M NaCl and 5 mM imidazole. Lysozyme was added to a concentration of 0.2 mg/ml. After incubation at 4 °C for 1 h, the lysate was sonicated and centrifuged at 40,000 × g at 4 °C for 20 min. The pellet was homogenized in 50 ml buffer A (20 mM Tris–HCl, pH 8.0, 2 M NaCl, 2 mM β-mercapto-ethanol) containing 5 mM imidazole. The suspension was rotated at 4 °C for 1 h and cleared by centrifugation at 40,000 × g at 4 °C for 20 min. The supernatant was loaded onto a 1 ml chelating Sepharose column charged with 50 mM imidazole. The column was washed with five column volumes of buffer A containing 5 mM imidazole and the protein was eluted with three column volumes of buffer A containing 200 mM and 400 mM imidazole, respectively. Protein-containing fractions were pooled and EDTA was added to a final concentration of 5 mM. The protein was dialyzed against buffer B (20 mM HEPES, pH 7.5, 1 mM EDTA, 1 M NaCl, 20% glycerol) containing 2 mM β-mercaptoethanol and then against buffer B containing 1 mM dithiothreitol. Aliquots of the protein were stored at −70 °C.
2.11. HIV-1 integrase assay
A non-radioactive ELISA-based HIV-1 integrase assay was performed according to the DNA-coated plates method. In this study, 1 μg of Smal-linearized p Bluescript SK was coated onto each well in the presence of 2 M NaCl as target DNA. The donor DNA was prepared by annealing VU5BR (5′-biotin-GTGTGGAAAATCTCTAGCAGT-3′) and VU5 (5′-ACTGCTAGAGATTTTCCACAC-3′) in 10 mM Tris–HC1, pH 8.0, 1 mM EDTA and 0.1 M NaCl at 80 °C followed by 30 min at room temperature. Integrase reaction was performed in 20 mM HEPES (pH 7.5), containing 10 mM MnCl2, 30 mM NaCl, 10 mM dithiothreitol and 0.05% Nonidet-P40 (Sigma). After the integrase reaction, the biotinylated DNA immobilized on the wells was detected by incubation with streptavidin-conjugated alkaline phosphatase (Boehringer Mannheim) followed by colorimetric detection with 1 mg/ml p-nitrophenyl phosphate in 10% diethanolamine buffer, pH 9.8, containing 0.5 mM MgCl2. The absorbance due to the alkaline phosphatase reaction was measured at 415 nm. The ribosome inactivating protein trichosanthin was used as a positive control [32], [33].
2.12. Screening for inhibitory effect on SARS Coronavious (CoV) protease
The activity of SARS CoV protease was indicated by cleavage of a designed substrate which was composed of two proteins linked by a cleavage site for SARS CoV protease. The reaction was performed in a mixture containing 5 μM SARS CoV protease, 5 μM sample, 20 μM substrate and buffer (20 mM Tris–HCl (pH 7.5), 20 mM NaCl and 10 mM beta-mercaptoethanol) for 40 min at 37 °C. After 40 min, the reaction was stopped by heating at 100 °C for 2 min. Then the reaction mixture was analyzed by SDS-PAGE. If SARS CoV protease is inhibited by the test sample, there is only one band, which is the intact substrate, shown in the SDS-PAGE.
2.13. Assay of antifungal activity
The assay of the isolated trypsin inhibitor for antifungal activity toward Botrytis cinerea, Mycosphaerella arachidicola and Fusarium oxysporum, which are plant pathogens, was carried out using 90 mm × 15 mm Petri plates containing 10 ml of potato dextrose agar. After the mycelial colony had developed, sterile blank paper disks [0.625 cm in diameter] were placed at a distance of 0.5 cm away from the rim of the mycelial colony. An aliquot of a solution of the trypsin inhibitor was added to a disk. The plates were incubated at 25 °C for 72 h until mycelial growth had enveloped disks containing the control and had formed crescents of inhibition around disks containing samples with antifungal activity. The leguminous defensin-like peptide sesquin was employed as a positive control [28] and emperor banana lectin [30] as a negative control.
2.14. Assay of cell-free translation-inhibitory activity
In this assay, the rabbit reticulocyte lysate system was used. The isolated trypsin inhibitor (10 μl) was added to 10 μl of a radioactive mixture [500 mM KCl, 5 mM MgCl2, 130 mM creatine phosphate, and 1 μCi [4,5-3H]leucine] and 30 μl working rabbit reticulocyte lysate containing 0.1 mM hemin and 5 μl creatine kinase. The mixture was incubated at 37 °C for 30 min, and 330 μl of 1 M NaOH containing 1.2% H2O2 was added. Further incubation for 10 min allowed decolorization and tRNA digestion. An equal volume of the reaction mixture was then added to 40% trichloroacetic acid with 2% casein hydrolyzate in a 96-well plate to precipitate radioactively labeled protein. The precipitate was harvested with an automated cell harvester onto a glass fiber filter, and the radioactivity was measured in a Packard Tri-Carb 2900TR low-activity liquid scintillation counter [34]. The ribosome inactivating protein trichosanthin [33] was used as a positive control and emperor banana lectin [30] as a negative control.
3. Results
Ion exchange chromatography of the extract of Chinese dull black soybeans on Q-Sepharose yielded an unadsorbed fraction devoid of trypsin-inhibitory activity and three adsorbed fractions of similar sizes (in terms of protein yields) eluted by 0.1 M NaCl, 0.4 M NaCl and 1 M NaCl in the Tris–HCl buffer, respectively. Trypsin-inhibitory activity resided only in fraction Q1 eluted with 0.1 M NaCl (Fig. 1A). This fraction was next chromatographed on SP-Sepharose to yield an unadsorbed fraction without trypsin-inhibitory activity, a sharp adsorbed fraction SP1 eluted with 0.2 M NaCl, and a broad adsorbed fraction SP2 eluted with 1 M NaCl in the starting buffer (Fig. 1B). Fraction SP1, with trypsin-inhibitory activity eluted with 0.2 M NaCl, was fractionated on Mono Q to yield a small unadsorbed fraction S1 and a broad adsorbed fraction S2, both without trypsin-inhibitory activity. Trypsin-inhibitory activity was located in the sharp and more strongly adsorbed fraction S3 (Fig. 1C). This fraction yielded a single sharp peak with a molecular mass of 40 kDa upon FPLC-gel filtration on a Superdex 75 column which has been calibrated with markers with molecular weights ranging from 67 to 17 kDa (not shown). The peak from Superdex 75 appeared as a single band with a molecular mass of about 20 kDa in SDS-PAGE (Fig. 2 ). The purification of dull black soybean trypsin inhibitor is summarized in Table 1 . There was 65-fold purification. The N-terminal sequence of the purified trypsin inhibitor was highly homologous to those of Kunitz-type trypsin inhibitors from yellow soybean, wild soybean and spinach (Table 2 ). Dull black soybean trypsin inhibitor and soybean trypsin inhibitor more potently inhibited trypsin than they inhibited chymotrypsin (Fig. 3 ). Dithiothreitol dose-dependently and time-dependently inhibited the trypsin-inhibitory activity of dull black soybean and soybean trypsin inhibitors (Fig. 4 ). The trypsin-inhibitory activity of BSKTI was totally preserved after exposure to the entire pH range (1–14) for 2 h, and after exposure to temperatures up to 80 °C for 10 min. Only 80 and 70% activity remained after exposure for 10 min to 90 and 100 °C, respectively (data not shown). The dull black soybean trypsin inhibitor enhanced nitric oxide production by murine macrophages, and so did lipopolysaccharide and Con A (Fig. 5A). The enhancement was in a dose-dependent manner (Fig. 5B). The enhancement caused by soybean trypsin inhibitor was only minimal. The dull black soybean inhibitor inhibited proliferation of HepG2 cells and MCF7 cells with an IC50 of 39 and 70 μM, respectively. The corresponding values for soybean trypsin inhibitor were lower, being 31 and 47 μM, respectively (Fig. 6A and B). Dull black soybean trypsin inhibitor inhibited HIV-1 reverse transcriptase with an IC50 of 9.4 μM (Fig. 7 ). Soybean trypsin inhibitor was inactive. Dull black soybean trypsin inhibitor inhibited translation in the cell-free rabbit reticulocyte system with an IC50 of 14 μM. Soybean trypsin inhibitor did not have translation-inhibitory activity (Fig. 8 ). There was no effect of the two trypsin inhibitors on mitogenic response of splenocytes and the activities of HIV-1 integrase and SARS proteinase (data not shown). Antifungal and antibacterial activities were also absent (data not shown).
Fig. 1.

Purification of dull black soybean trypsin inhibitor (BSKTI) by chromatography on (A) Q-Sepharose, (B) SP-Sepharose, and (C) Mono Q. In (A), the black soybean extract was applied on a Q-Sepharose column (5 cm × 20 cm). After elution of unadsorbed proteins with 10 mM Tris–HCl buffer (pH 7.4), the column was eluted stepwise with 0.1 M NaCl, 0.4 M NaCl, and 1 M NaCl added to the Tris–HCl buffer as indicated by the arrows. In (B), fraction Q1 from the Q-Sepharose column was dialyzed and applied on an SP-Sepharose column (2.5 cm × 20 cm) in 10 mM Tris–HCl buffer (pH 7.4). After elution of unadsorbed proteins, the column was eluted stepwise with 0.2 M NaCl and then with 1 M NaCl added to the buffer as indicated by the arrows. In (C), fraction SP1 from the SP-Sepharose column was loaded on a 1-ml Mono Q column. Following elution of unadsorbed proteins with 10 mM NH4HCO3 buffer (pH 9), adsorbed proteins were eluted sequentially, first with a 0–0.3 M NaCl gradient and then with a 0.3–1 M NaCl gradient.
Fig. 2.

SDS-PAGE of Kunitz-type trypsin inhibitor from dull black soybean (BSKTI) (on the right lane) and GE Healthcare molecular weight markers (on the left lane) including phosphorylase b (94 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrase (30 kDa), soybean trypsin inhibitor (20 kDa), and α-lactalbumin (14.4 kDa).
Table 1.
Summary of purification of dull black soybean (G. max) trypsin inhibitor from 100 g seeds
| Total protein (mg) | Total trypsin-inhibitory activity (U × 104) | Specific activity (U/mg) | Purification fold | Recovery of protein in mass (%) | |
|---|---|---|---|---|---|
| Crude Extract | 14583 | 551 | 378 | 1 | 100 |
| Q1 (After Q-Sepharose) | 4627 | 436 | 857 | 2.3 | 31.7 |
| SP1 (After SP-Sepharose) | 1166 | 314 | 2694 | 7.1 | 8.0 |
| S3 (After Mono Q) | 140 | 161 | 11500 | 30.5 | 0.9 |
| After Superdex 75 | 43 | 105 | 24419 | 64.6 | 0.3 |
Table 2.
N-terminal sequence of dull black soybean (G. max) trypsin inhibitor in comparison with related leguminous trypsin/chymotrypsin inhibitors (identical residues are underscored)
| Residue number | Sequence | Residue number | % Identity to BSKTI | |
|---|---|---|---|---|
| Kunitz-type trypsin inhibitor from dull black soybean (G. max) | 1 | DFVIDNEGNPIEDGG | 15 | 100 |
| Kunitz-type trypsin inhibitor homolog from spinach leaves (Spinacia oleracea) | 1 | DFVLDNEGNPLENGG | 15 | 80 |
| Kunitz-type trypsin inhibitor from wild soybean (G. soja) | 26 | DFVLDNEGNPLENGG | 40 | 80 |
| Trypsin inhibitor subtype B from yellow soybean (G. max) | 26 | DFVLDNEGNPLDSGG | 40 | 73 |
| Kunitz-type trypsin inhibitor from soybean (G. max) | 26 | DIVFDTEGNPIRNGG | 40 | 66 |
| Chymotrypsin inhibitor from winged bean (Psophocarpus tetragonolobus) | 2 | DDLVDAEGNLVENGG | 16 | 53 |
| Acidic trypsin inhibitor 2 from winged bean (P. tetragonolobus) | 1 | ZELVDVEGKTVLNGG | 15 | 33 |
| Basic trypsin inhibitor 1 from winged bean (P. tetragonolobus) | 1 | EPLLDSEGELVRNGG | 15 | 33 |
| Trypsin inhibitor (Erythrina latissima) | 1 | VLLDGNGEVVQNGG | 14 | 27 |
BSKTI = Kunitz-type trypsin inhibitor (dull black soybean).
Fig. 3.
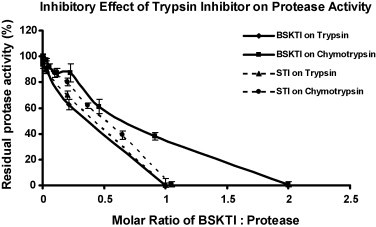
Inhibitory effects of dull black soybean Kunitz-type trypsin inhibitor (BSKTI) and soybean trypsin inhibitor (STI) on the activity of trypsin and chymotrypsin. Results are presented as mean ± S.D. (n = 3).
Fig. 4.
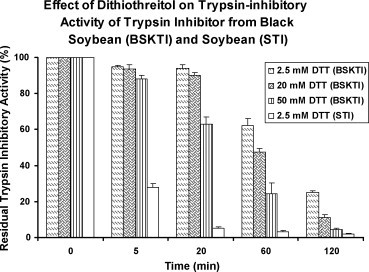
Effect of dithiothreitol on the stability of dull black soybean trypsin inhibitor (BSKTI) and soybean trypsin inhibitor (STI) after incubation at 37 °C for various durations. Results are presented as mean ± S.D. (n = 3).
Fig. 5.
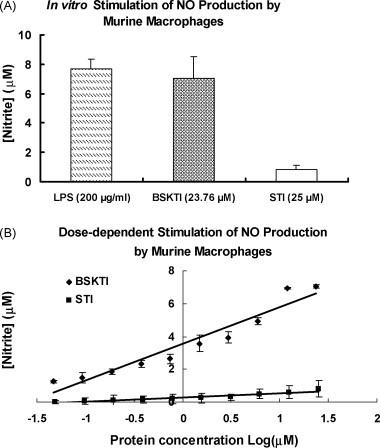
Stimulation of macrophage nitric oxide production after treatment with 23.76 μM dull black soybean trypsin inhibitor (BSKTI) and 25 μM soybean trypsin inhibitor (STI) for 24-h at 37 °C in an atmosphere of 95% CO2–5% O2. Results are presented as means ± S.D. (n = 3). (A) Comparison with lipopolysaccharide (LPS) and STI. (B) Dose–response relationship.
Fig. 6.

Anti-proliferative activity of dull black soybean trypsin inhibitor (BSKTI) and soybean trypsin inhibitor (STI) on (A) HepG2 cells and (B) MCF7 cells after 72 h of treatment at 37 °C in an atmosphere of 95% CO2–5% O2. Results are presented as mean ± S.D. (n = 3).
Fig. 7.
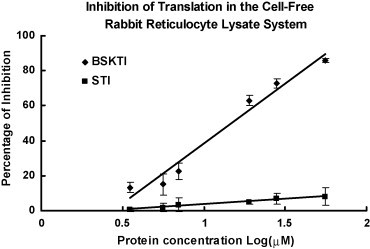
HIV-1 reverse transcriptase inhibition by dull black soybean trypsin inhibitor (BSKTI) and soybean trypsin inhibitor (STI). Results are presented as mean ± S.D. (n = 3).
Fig. 8.

Inhibitory effect of dull black soybean trypsin inhibitor (BSKTI) and soybean trypsin inhibitor (STI) on translation in a cell-free rabbit reticulocyte system. Results are presented as mean ± S.D. (n = 3).
4. Discussion
The Kunitz-type trypsin inhibitor isolated in the present investigation from Chinese dull black soybeans (BSKTI) is similar to its counterpart from yellow soybean (G. max) and wild soybean (Glycine soja) in N-terminal sequence. Similarity to other leguminous trypsin/chymotrypsin inhibitors, however, is less obvious. The difference in molecular mass of the peak derived from BSKTI in gel filtration (40 kDa) and that of the band in SDS-PAGE (20 kDa) suggests that it is a dimeric Kunitz-type trypsin inhibitor like the inhibitor from Leucaena leucocephala [35]. Like G. soja trypsin inhibitor, which is adsorbed on SP-Toyopearl and DEAE-Toyopearl [24], BSKTI is adsorbed on SP-Sepharose, Q-Sepharose and Mono Q. BSKTI is highly purified after three ion-exchange chromatographic steps as evidenced by presence of a single peak in gel filtration on Superdex 75 and a single band in SDS-PAGE. Previously a procedure involving (NH4)2SO4 precipitation, cation exchange chromatography on SP-Sepharose and anion exchange chromatography on Q-Sepharose followed by chromatography on a trypsin affinity column was used to isolate the protease inhibitor bikunin from human urine [36]. The yield of BSKTI is comparable to that from Peltophorum dubium (50 mg/100 g) [37] but much higher than that (1.1 mg/100 g) from broad beans [3]. BSKTI is obtained with a purification fold about 9 times higher than that for the trypsin inhibitor from madeira-vine [38]. Like the sporamin B-like trypsin inhibitor from wampee seeds [39], BSKTI exerts an inhibitory action on cell-free translation in the rabbit reticulocyte system. It is known that some proteins with anti-proliferative activity inhibit translation in the cell-free rabbit reticulocyte lysate system [40].
Unlike some of the protease inhibitors with antifungal activity such as broad bean trypsin inhibitor [3], BSKTI has no activity toward the fungal species studied. Some trypsin inhibitors like lily bulb trypsin inhibitor do not inhibit HIV-1 reverse transcriptase [unpublished data]. The HIV-1 reverse transcriptase-inhibitory potency of BSKTI (IC50 = 9.4 μM) is within the range of potencies reported for anti-HIV compounds [41]. Previously some other trypsin inhibitors have been shown to inhibit HIV-1 reverse transcriptase [3], [41], [42]. The mechanism is probably protein-protein interaction. However, BSKTI is devoid of inhibitory activity toward HIV-1 integrase and SARS proteinase. On the other hand, some of the ribosome inactivating proteins [33], antifungal proteins [43] and milk proteins [44] are capable of inhibiting HIV-1 reverse transcriptase, protease and integrase.
Although broad bean trypsin inhibitor, STI and BSKTI inhibit both trypsin and chymotrypsin, the potency toward trypsin is higher. In this aspect it resembles broad bean trypsin inhibitor [2]. The stability of the trypsin-inhibitory activity of BSKTI despite fluctuations in ambient pH, and its relatively high thermostability, are reminiscent of the pronounced stability of papaya Kunitz-type trypsin inhibitor [27]. The inhibitory effect of dithiothreitol on trypsin-inhibitory activity of BSKTI is indicative of the significance of the disulfide bonds to the activity of the inhibitor. The present findings are in agreement with a previous report on papaya Kunitz-type trypsin inhibitor [27] but at variance with the findings on Erythrina caffra Kunitz-type trypsin inhibitor [2]. Protease inhibitors may exhibit immunomodulatory effects [45]. It is interesting to note that BSKTI stimulates nitric oxide production by macrophages. On the other hand, it fails to evoke a mitogenic response from splenocytes.
Epidemiological evidence suggests that a diet rich in legumes reduces the incidence of cancer probably due to the presence of protease inhibitors [46]. The in vivo antitumor and in vitro anti-proliferative [47], [48], [49], [50], [51], [52], [53], [54], [55], [56] activities of trypsin inhibitors have been demonstrated, but most of the anticarcinogenic activity was shown using Bowman–Birk trypsin inhibitors [2]. It is thus noteworthy that BSKTI reduces proliferation of hepatoma and breast cancer cells with a relatively high potency. Most Kunitz-type trypsin inhibitors are inactivated by heat [2]. The observation that BSKTI has pronounced pH stability and thermostability and serine protease-inhibitory activity adds to the importance of the TI. Leguminous plants produce a variety of proteins including antifungal proteins [28], lectins [29], ribosome inactivating proteins [34], trypsin inhibitors [2], and α-amylase inhibitors. These proteins play a role of defense. It is most likely that BSKTI is also a defense protein.
In summary, the isolated trypsin inhibitor is fairly stable and has some exploitable activities. It has the potential of being developed into an agent for anticancer therapy in view of its potent anti-proliferative activity forward tumor cells and ability to elicit nitric oxide production from macrophages. On the other hand, it may not be useful in helping plants in combating against pathogenic fungi and bacteria since it lacks antifungal and antibacterial activities. It has potent inhibitory activity against HIV-1 reverse transcriptase and hence is a potential antiretroviral agent. However, there is no inhibition of HIV-1 integrase, unlike plant antifungal proteins [43]. The present investigation also reveals that dull black soybean trypsin inhibitor resembles soybean trypsin inhibitor in ability to inhibit activities of trypsin and chymotrypsin and tumor cell proliferation, but differs in ability to stimulate macrophages nitric oxide production and inhibit HIV-1 reverse transcriptase and cell-free translation. It appears that dull black soybean trypsin inhibitor is more versatile than soybean trypsin inhibitor in its biological activity.
Acknowledgment
We thank Miss Kathy Lau for excellent secretarial assistance.
References
- 1.Birk Y. Springer-Verlag; Berlin, Heidelberg, New York: 2003. Plant protease inhibitors. [Google Scholar]
- 2.Zhao M., Naude R.J., Muramoto K., Oelofsen W. Purification and characterization of ostrich pancreatic secretory trypsin inhibitor. Int J Pept Protein Res. 1996;48:174–181. doi: 10.1111/j.1399-3011.1996.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 3.Ye X.Y., Ng T.B., Rao P.F. A Bowman–Birk-type trypsin-chymotrypsin inhibitor from broad beans. Biochem Biophys Res Commun. 2001;289:91–96. doi: 10.1006/bbrc.2001.5965. [DOI] [PubMed] [Google Scholar]
- 4.Dattagupta J.K., Podder A., Chakrabarti C., Sen U., Dutta S.K., Singh M. Structure of a Kunitz-type chymotrypsin from winged bean seeds at 2.95 A resolution. Acta Crystallogr D Biol Crystallogr. 1996;52:521–528. doi: 10.1107/S0907444996000224. [DOI] [PubMed] [Google Scholar]
- 5.do Socorro M., Cavalcanti M., Oliva M.L., Fritz H., Jochum M., Mentele R. Characterization of a Kunitz trypsin inhibitor with one disulfide bridge purified from Swartzia pickellii. Biochem Biophys Res Commun. 2002;291:635–639. doi: 10.1006/bbrc.2002.6436. [DOI] [PubMed] [Google Scholar]
- 6.Kumar P., Rao A.G., Hariharaputran S., Chandra N., Gowda L.R. Molecular mechanism of dimerization of Bowman–Birk inhibitors. Pivotal role of ASP76 in the dimerization. J Biol Chem. 2004;279:30425–30432. doi: 10.1074/jbc.M402972200. [DOI] [PubMed] [Google Scholar]
- 7.Kumar P., Sreerama Y.N., Gowda L.R. Formation of Bowman–Birk inhibitors during the germination of horsegram (Dolichos biflorus) Phytochem. 2002;60:581–588. doi: 10.1016/s0031-9422(02)00178-4. [DOI] [PubMed] [Google Scholar]
- 8.Krauchenco S., Pando S.C., Marangoni S., Polikarpov I. Crystal structure of the Kunitz (STI)-type inhibitor from Delonix regia seeds. Biochem Biophys Res Commun. 2003;312:1303–1308. doi: 10.1016/j.bbrc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 9.Joubert F.J. Purification and properties of the proteinase inhibitors from Erythrina caffra (coast Erythrina) seed. Int J Biochem. 1982;14:187–193. doi: 10.1016/0020-711x(82)90137-9. [DOI] [PubMed] [Google Scholar]
- 10.Shibata H., Hara S., Ikenaka T., Abe J. Purification and characterization of proteinase inhibitors from winged bean (Psophocarpus tetragonolobus (L.) DC.) seeds. J Biochem. 1986;99:1147–1155. doi: 10.1093/oxfordjournals.jbchem.a135578. [DOI] [PubMed] [Google Scholar]
- 11.Negreiros A.N., Carvalho M.M., Xavier Filho J., Blanco-Labra A., Shewry P.R., Richardson M. The complete amino acid sequence of the major Kunitz trypsin inhibitor from the seeds of Prosopsis juliflora. Phytochem. 1991;30:2829–2833. doi: 10.1016/s0031-9422(00)98207-4. [DOI] [PubMed] [Google Scholar]
- 12.Wu H.C., Lin J.Y. The complete amino acid sequence of a Kunitz family trypsin inhibitor from seeds of Acacia confusa. J Biochem. 1993;113:258–263. doi: 10.1093/oxfordjournals.jbchem.a124036. [DOI] [PubMed] [Google Scholar]
- 13.Batista I.F., Oliva M.L., Araujo M.S., Sampaio M.U., Richardson M., Fritz H. Primary structure of a Kunitz-type trypsin inhibitor from Enterolobium contortisiliquum seeds. Phytochem. 1996;41:1017–1022. doi: 10.1016/0031-9422(95)00710-5. [DOI] [PubMed] [Google Scholar]
- 14.de Souza A.F., Torquato R.J., Tanaka A.S., Sampaio C.A. Cloning, expression and characterization of Bauhinia variegata trypsin inhibitor BvTI. Biol Chem. 2005;386:1185–1189. doi: 10.1515/BC.2005.135. [DOI] [PubMed] [Google Scholar]
- 15.Polikarpov I., Golubev A.M., Perles L.A., Pando S.C., Novello J.C., Marangoni S. Purification, crystallization and preliminary crystallographic study of a Kunitz-type trypsin inhibitor from Delonix regia seeds. Acta Crystallogr D Biol Crystallogr. 1999;55 doi: 10.1107/s0907444999009361. 1611-1163. [DOI] [PubMed] [Google Scholar]
- 16.Pando L.A., Di Ciero L., Novello J.C., Oliveira B., Weder J.K., Marangoni S. Isolation and characterization of a new trypsin inhibitor from Crotalaria paulina seeds. UBMB Life. 1999;48:519–523. doi: 10.1080/713803553. [DOI] [PubMed] [Google Scholar]
- 17.Guerranti R., Aguiyi J.C., Ogueli I.G., Onorati G., Neri S., Rosati F. Protection of Mucuna pruriens seeds against Echis carinatus venom is exerted through a multiform glycoprotein whose oligosaccharide chains are functional in this role. Biochem Biophys Res Commun. 2004;323:484–490. doi: 10.1016/j.bbrc.2004.08.122. [DOI] [PubMed] [Google Scholar]
- 18.Mello G.C., Oliva M.L., Sumikawa J.T., Machado O.L., Marangoni S., Novello J.C. Purification and characterization of a new trypsin inhibitor from Dimorphandra mollis seeds. J Protein Chem. 2001;20:625–632. doi: 10.1023/a:1013764118579. [DOI] [PubMed] [Google Scholar]
- 19.Krauchenco S., Nagem R.A., da Silva J.A., Marangoni S., Polikarpov I. Three-dimensional structure of an unusual Kunitz (STI) type trypsin inhibitor from Copaifera langsdorffii. Biochimie. 2004;86:167–172. doi: 10.1016/j.biochi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Morales-de León J.C., Vázquez-Mata N., Torres N., Gil-Zenteno L., Bressani R. Preparation and characterization of protein isolate from fresh and hardened beans (Phaseolus vulgaris L.) J Food Sci. 2007;72:96–102. doi: 10.1111/j.1750-3841.2006.00244.x. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira A.S., Migliolo L., Aquino R.O., Ribeiro J.K., Macedo L.L., Andrade L.B. Purification and characterization of a trypsin-papain inhibitor from Pithecelobium dumosum seeds and its in vitro effects towards digestive enzymes from insect pests. Plant Physiol Biochem. 2007;45:858–865. doi: 10.1016/j.plaphy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Conners R., Konarev A.V., Forsyth J., Lovegrove A., Marsh J., Joseph-Horne T. An unusual helix-turn-helix protease inhibitory motif in a novel trypsin inhibitor from seeds of Veronica (Veronica hederifolia L.) J Biol Chem. 2007;282:27760–27768. doi: 10.1074/jbc.M703871200. [DOI] [PubMed] [Google Scholar]
- 23.Kowalska J., Pszczoła K., Wilimowska-Pelc A., Lorenc-Kubis I., Zuziak E., Ługowski M. Trypsin inhibitors from the garden four o’clock (Mirabilis jalapa) and spinach (Spinacia oleracea) seeds: isolation, characterization and chemical synthesis. Phytochem. 2007;68:1487–1496. doi: 10.1016/j.phytochem.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Deshimaru M., Hanamoto R., Kusano C., Yoshimi S., Terada S. Purification and characterization of proteinase inhibitors from wild soja (Glycine soja) seeds. Biosci Biotechnol Biochem. 2002;66:1897–1903. doi: 10.1271/bbb.66.1897. [DOI] [PubMed] [Google Scholar]
- 25.Wong R.C., Fong W.P., Ng T.B. Multiple trypsin inhibitors from Momordica cochinchinensis seeds, the Chinese drug mubiezhi. Peptides. 2004;25:163–169. doi: 10.1016/j.peptides.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen T.B., Reynolds J.A. Measurements of molecular weights by gel electrophoresis. Methods Enzymol. 1978;48:3–10. doi: 10.1016/s0076-6879(78)48003-6. [DOI] [PubMed] [Google Scholar]
- 27.Azarkan M., Dibiani R., Goormaghtigh E., Raussens V., Baeyens-Volant D. The papaya Kunitz-type trypsin inhibitor is a highly stable beta-sheet glycoprotein. Biochim Biophys Acta. 2006;1764:1063–1072. doi: 10.1016/j.bbapap.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Wong J.H., Ng T.B. Sesquin, a potent defensin-like antifungal peptide from beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides. 2005;26:1120–1126. doi: 10.1016/j.peptides.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Wong J.H., Ng T.B. Isolation and characterization of a glucose/mannose/rhammose-specific lectin from the knife bean Canavalia gladiata. Arch Biochim Biophys. 2005;439:91–98. doi: 10.1016/j.abb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Wong J.H., Ng T.B. Isolation and characterization of a glucose/mannose-specific lectin with stimulatory effect on nitric oxide production by macrophages from the emperor banana. Int J Biochem Cell Biol. 2006;38:234–243. doi: 10.1016/j.biocel.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Cheung A.H., Ng T.B. Isolation and characterization of a trypsin-chymotrypsin inhibitor from the seeds of green lentil (Lens culinaris) Protein Pept Lett. 2007;14:859–864. doi: 10.2174/092986607782110310. [DOI] [PubMed] [Google Scholar]
- 32.Ye X.Y., Ng T.B. Mungin, a novel cyclophilin-like antifungal protein from the mung bean. Biochem Biophys Res Commun. 2000;273:1111–1115. doi: 10.1006/bbrc.2000.3067. [DOI] [PubMed] [Google Scholar]
- 33.Au T.K., Collins R.A., Lam T.L., Ng T.B., Fong W.P., Wan D.C. The plant ribosome inactivating proteins luffin and saporin are potent inhibitors of HIV-1 integrase. FEBS Lett. 2000;471:169–172. doi: 10.1016/s0014-5793(00)01389-2. [DOI] [PubMed] [Google Scholar]
- 34.Lam S.S.L., Wang H.X., Ng T.B. Purification and characterization of novel ribosome inactivating proteins, alpha- and beta-pisavins, from seeds of the garden pea Pisum sativum. Biochem Biophys Res Commun. 1998;253:135–142. doi: 10.1006/bbrc.1998.9764. [DOI] [PubMed] [Google Scholar]
- 35.Sattar R., Ali S.A., Kamal M., Khan A.A., Abbasi A. Molecular mechanism of enzyme inhibition: prediction of the three-dimensional structure of the dimeric trypsin inhibitor from Leucaena leucocephala by homology modelling. Biochem Biophys Res Commun. 2004;314:755–765. doi: 10.1016/j.bbrc.2003.12.177. [DOI] [PubMed] [Google Scholar]
- 36.Yang L., Resnick M.I., Marengo S.R. A simple procedure for isolating microgram quantities of biologically active bikunin from human urine. BJU Int. 2005;96:647–653. doi: 10.1111/j.1464-410X.2005.05700.x. [DOI] [PubMed] [Google Scholar]
- 37.Troncoso M.F., Cerda Zolezzi P., Hellman U., Wolfenstein-Todel C. A novel trypsin inhibitor from Peltophorum dubium seeds, with lectin-like properties, triggers rat lymphoma cell apoptosis. Arch Biochim Biophys. 2003;411:93–104. doi: 10.1016/s0003-9861(02)00726-9. [DOI] [PubMed] [Google Scholar]
- 38.Chuang M.T., Lin Y.S., Hou W.C. Ancordin, the major rhizome protein of madeira-vine, with trypsin inhibitory and stimulatory activities in nitric oxide production. Peptides. 2007;28:1311–1316. doi: 10.1016/j.peptides.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Ng T.B., Lam S.K., Fong W.P. A homodimeric sporamin-type trypsin inhibitor with antiproliferative, HIV reverse transcriptase-inhibitory and antifungal activities from wampee (Clausena lansium) seeds. Biol Chem. 2003;384:289–293. doi: 10.1515/BC.2003.032. [DOI] [PubMed] [Google Scholar]
- 40.Ng T.B., Liu W.K., Sze S.F., Yeung H.W. Action of alpha-momorcharin, a ribosome inactivating protein, on cultured tumor cell lines. Gen Pharmacol. 1994;25:75–77. doi: 10.1016/0306-3623(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 41.Ng T.B., Huang B., Fong W.P., Yeung H.W. Anti-human immunodeficiency virus (anti-HIV) natural products with special emphasis on HIV reverse transcriptase inhibitors. Life Sci. 1997;61:933–949. doi: 10.1016/s0024-3205(97)00245-2. [DOI] [PubMed] [Google Scholar]
- 42.Wang H.X., Ng T.B. Examination of lectins, polysaccharopeptide, polysaccharide, alkaloid, coumarin and trypsin inhibitors for inhibitory activity against human immunodeficiency virus reverse transcriptase and glycohydrolases. Planta Med. 2001;67:669–672. doi: 10.1055/s-2001-17359. [DOI] [PubMed] [Google Scholar]
- 43.Ng T.B., Au T.K., Lam T.L., Ye X.Y., Wan D.C. Inhibitory effects of antifungal proteins on human immunodeficiency virus type 1 reverse transcriptase, protease and integrase. Life Sci. 2002;70:927–935. doi: 10.1016/s0024-3205(01)01458-8. [DOI] [PubMed] [Google Scholar]
- 44.Ng T.B., Ye X.Y. A polymeric immunoglobulin receptor-like milk protein with inhibitory activity on human immunodeficiency virus type 1 reverse transcriptase. Int J Biochem Cell Biol. 2004;36:2242–2249. doi: 10.1016/j.biocel.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 45.Tsoi A.Y., Ng T.B., Fong W.P. Immunomodulatory activity of a chymotrypsin inhibitor from Momordica cochinchinensis seeds. J Pept Sci. 2006;12:605–611. doi: 10.1002/psc.765. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy A.R. The evidence for soybean products as cancer preventive agents. J Nutr. 1995;125:733S–7743S. doi: 10.1093/jn/125.3_Suppl.733S. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi H., Suzuki M., Hirashima Y., Terao T. The protease inhibitor bikunin, a novel anti-metastatic agent. Biol Chem. 2003;284:749–754. doi: 10.1515/BC.2003.083. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy A.R., Billings P.C., Maki P.A., Newberne P. Effects of various preparations of dietary protease inhibitors on oral carcinogenesis in hamsters induced by DMBA. Nutr Canc. 1993;19:191–200. doi: 10.1080/01635589309514249. [DOI] [PubMed] [Google Scholar]
- 49.Banerji A.P., Fernandes A.O. Field bean protease inhibitor preparations, unlike methotrexate, can completely suppress Yoshida sarcoma tumor in rats. Cell Biol Int. 1994;18:1025–1034. doi: 10.1006/cbir.1994.1026. [DOI] [PubMed] [Google Scholar]
- 50.Banerji A., Fernandes A., Bane S. Treatment with field bean protease inhibitor can effectively repress ethylnitrosourea (ENU)-induced neoplasms of the nervous system in Sprague–Dawley rats. Cancer Lett. 1998;130:161–167. doi: 10.1016/s0304-3835(98)00135-9. [DOI] [PubMed] [Google Scholar]
- 51.Banerji A., Fernandes A., Bane S., Ahire S. The field bean protease inhibitor has the potential to suppress B16F10 melanoma cell lung metastasis in mice. Cancer Lett. 1998;129:15–20. doi: 10.1016/s0304-3835(98)00090-1. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes A.O., Banerji A.P. Inhibition of benzopyrene-induced forestomach tumors by field bean protease inhibitor(s) Carcinogenesis. 1995;16:1843–1846. doi: 10.1093/carcin/16.8.1843. [DOI] [PubMed] [Google Scholar]
- 53.Fernandes A.O., Banerji A.P. The field bean protease inhibitor can effectively suppress 7,12-dimethylbenz[a]anthracene-induced skin tumorigenesis in mice. Cancer Lett. 1996;104:219–224. doi: 10.1016/0304-3835(96)04253-x. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi H., Suzuki M., Kanayama N., Terao T. A soybean Kunitz trypsin inhibitor suppresses ovarian cancer cell invasion by blocking urokinase upregulation. Clin Exp Metastasis. 2004;21:159–166. doi: 10.1023/b:clin.0000024751.73174.c2. [DOI] [PubMed] [Google Scholar]
- 55.Troncoso M.F., Biron V.A., Longhi S.A., Retegui L.A., Wolfenskein-Todel C. Peltophorum dubium and soybean Kunitz-type trypsin inhibitors induce human Jurkat cell apoptosis. Int J Immunopharmacol. 2007;7:625–635. doi: 10.1016/j.intimp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 56.von Hofe E., Newberne P.M., Kennedy A.R. Inhibition of N-nitrosomethylbenzylamine-induced esophageal neoplasms by the Bowman–Birk protease inhibitor. Carcinogenesis. 1991;12:2147–2150. doi: 10.1093/carcin/12.11.2147. [DOI] [PubMed] [Google Scholar]


