Abstract
Civil aviation is fast-growing (about +5% every year), mainly driven by the developing economies and globalisation. Its impact on the environment is heavily debated, particularly in relation to climate forcing attributed to emissions at cruising altitudes and the noise and the deterioration of air quality at ground-level due to airport operations. This latter environmental issue is of particular interest to the scientific community and policymakers, especially in relation to the breach of limit and target values for many air pollutants, mainly nitrogen oxides and particulate matter, near the busiest airports and the resulting consequences for public health. Despite the increased attention given to aircraft emissions at ground-level and air pollution in the vicinity of airports, many research gaps remain. Sources relevant to air quality include not only engine exhaust and non-exhaust emissions from aircraft, but also emissions from the units providing power to the aircraft on the ground, the traffic due to the airport ground service, maintenance work, heating facilities, fugitive vapours from refuelling operations, kitchens and restaurants for passengers and operators, intermodal transportation systems, and road traffic for transporting people and goods in and out to the airport. Many of these sources have received inadequate attention, despite their high potential for impact on air quality. This review aims to summarise the state-of-the-art research on aircraft and airport emissions and attempts to synthesise the results of studies that have addressed this issue. It also aims to describe the key characteristics of pollution, the impacts upon global and local air quality and to address the future potential of research by highlighting research needs.
Keywords: Aviation, Atmospheric pollution, Emissions, LTO cycles, Particulate matter, Oxides of nitrogen
Highlights
-
•
Aviation is globally growing (+5% y−1) mainly driven by developing countries.
-
•
Airport operations cause an increase in ground-level pollution.
-
•
Chemical and physical properties of the emitted gases and particles are reviewed.
-
•
An overview of other additional sources within airports is provided.
-
•
Future research needs on aircraft emissions are highlighted.
List of abbreviations
- AAFEX
Alternative Aviation Fuel Experiment
- AEs
Airport emissions
- APEX
Aircraft Particle Emissions eXperiment
- APU
Auxiliary power unit
- BC
Black carbon
- C∗
Effective saturation concentration
- CIs
Chemi-ions
- CIMS
Chemical ionisation mass spectrometry
- EC
Elemental carbon
- EI
Emission index
- EXCAVATE
EXperiment to Characterise Aircraft Volatile Aerosol and Trace-species Emissions
- F00
Engine thrust expressed as a percentage of maximum rated power
- FGEP
Fixed ground electrical power
- FSC
Fuel sulphur content
- FT
Fischer–Tropsch fuel
- GMD
Geometric number mean diameter
- GPUs
Ground power units
- GRPs
Ground running procedures
- GSEs
Ground service equipments
- ICAO
International Civil Aviation Organization
- LTO
Landing and take-off cycle
- OC
Organic carbon
- NMHC
Non-methane hydrocarbon
- NOx
Nitrogen oxides (NO + NO2)
- NOy
Reactive odd nitrogen (NOx and their oxidation products)
- OA
Organic aerosol
- PAHs
Polycyclic aromatic hydrocarbons
- PM
Particulate matter
- PM1
Particulate matter (aerodynamic diameter less than 1 μm)
- PM2.5
Particulate matter (aerodynamic diameter less than 2.5 μm)
- PM10
Particulate matter (aerodynamic diameter less than 10 μm)
- RF
Radiative forcing
- RPK
Revenue passenger kilometres
- RTK
Revenue tonne kilometres
- SARS
Severe acute respiratory syndrome
- SIA
Secondary inorganic aerosol
- SN
Smoke number
- SOA
Secondary organic aerosol
- SVOCs
Semi-volatile organic compounds
- TC
Total carbon
- TF
Turbofan engine
- TIM
Time-in-mode
- TJ
Turbojet engine
- TP
Turboprop engine
- TS
Turboshaft engine
- UFP
Ultrafine particles (diameter <100 nm)
- UHC
Unburned hydrocarbons
- VOCs
Volatile organic compounds
- ε
Abundance ratio ((•SO3+H2SO4)/total sulphur)
- ξ
Partitioning coefficient
1. Introduction
Among pollution issues, poor air quality attracts a high level of interest within the scientific community and engages public opinion because of the known relationship between exposure to many air pollutants and increased adverse short- and long-term effects on human health (e.g., Schwartz, 1997, Ayres, 1998, Brunekreef and Holgate, 2002, Kampa and Castanas, 2008, Maynard, 2009, Yang and Omaye, 2009, Rückerl et al., 2011). In addition, air pollution can seriously impair visibility (Hyslop, 2009), may damage materials in buildings and cultural heritage (Watt et al., 2009, Screpanti and De Marco, 2009) and has direct and indirect effects upon climate (Ramanathan and Feng, 2009). While air pollution remains a major concern for developing countries (Fenger, 2009, Liaquat et al., 2010) as a result of the rapid growth of population, energy demand and economic growth, developed countries have experienced a significant decline in the concentrations of many air pollutants over the past decade.
Airport emissions (AEs) have received increasing attention in recent years because of the rapid growth of air transport volumes and the expected expansion to meet capacity needs for future years (Amato et al., 2010, Kurniawan and Khardi, 2011, Kinsey et al., 2011). Most studies highlight knowledge gaps (e.g., Webb et al., 2008, Wood et al., 2008a, Lee et al., 2010) which are a matter of concern as the literature indicates that aircraft emissions can significantly affect air quality near airports (Unal et al., 2005, Carslaw et al., 2006, Herndon et al., 2008, Carslaw et al., 2008, Mazaheri et al., 2009, Dodson et al., 2009) and in their surroundings (Farias and ApSimon, 2006, Peace et al., 2006, Hu et al., 2009, Amato et al., 2010, Jung et al., 2011, Hsu et al., 2012). Emission standards for new types of aircraft engines have been implemented since the late 1970s by the International Civil Aviation Organization (ICAO) through the Committee on Aircraft Engine Emissions (CAEE) and the subsequent Committee on Aviation Environmental Protection (CAEP). One of the key actions of the ICAO committees was the provision on engine emissions in Volume II of Annex 16 to the Convention on International Civil Aviation, the so-called “Chicago Convention”, which recommended protocols for the measurement of carbon monoxide (CO), nitrogen oxides (NO + NO2 = NOx), unburned hydrocarbons (UHC) and smoke number (SN) for new engines (ICAO, 2008). Standards were listed on a certification databank (EASA, 2013), which represents a benchmark for engine emissions performance and is used in many regulatory evaluations (ICAO, 2011). This regulation has produced significant improvements in engine and fuel efficiency and technical progress to reduce emissions. However, although these efforts have led to a substantial reduction in direct aircraft emissions over the past two decades, these gains may be offset by the forecast growth of the aviation industry and the resulting increase in airport traffic (ICAO, 2011). Furthermore, the ICAO regulation address only four main generic pollutants and a more detailed chemical and physical characterisation of exhausts is required to quantitatively and qualitatively assess aircraft emissions. An increasing number of studies provide a detailed chemical speciation for many exhaust compounds, including gases and airborne particulate matter (e.g., Anderson et al., 2006, Herndon et al., 2008, Agrawal et al., 2008, Mazaheri et al., 2009, Onasch et al., 2009, Herndon et al., 2009, Kinsey et al., 2011, Mazaheri et al., 2011, Santoni et al., 2011). However, the literature remains very sparse and many questions remain unresolved because of the large differences in measurement strategies, technologies and methods, compounds analysed and environments studied.
Aircraft exhausts are only one of several sources of emission at an airport (ICAO, 2011). Although exhaust plumes from aircraft engines were conventionally considered to account for most of the emissions, other sources are present within modern airports and contribute to air pollution at the local scale. Among these, tyre, brake and asphalt wear and the re-suspension of particles due to the turbulence created by the aircraft movements can account for large fractions of total particulate matter mass (e.g., British Airports Authority, 2006), but their chemical and physical characteristics have been investigated in only a few studies (Bennett and Christie, 2011, Bennett et al., 2011). Moreover, the emissions of the units providing power to the aircraft on the ground have received relatively little consideration despite their potentially high impact on the local air quality (Schäfer et al., 2003, Ratliff et al., 2009, Mazaheri et al., 2011). These units include the auxiliary power units (APUs), which are small on-board gas-turbine engines, and the ground power units (GPUs) provided by airports. In addition, airport ground service equipment (GSEs) further impact the air quality (e.g., Nambisan et al., 2000, Amin, 2001, Schäfer et al., 2003). GSEs include most of the equipment that an airport offers as a service for flights and passengers and includes a large number of vehicles, such as passenger buses, baggage and food carriers, container loader, refilling trucks, cleaning, lavatory services and de/anti-icing vehicles, and tugs, which are used to move any equipment or to push the aircraft between gates and taxiways. Only few studies are available on the air traffic-related emissions produced by ground services such as GSEs, GPUs or APUs (e.g., Webb et al., 2008, Ratliff et al., 2009, Mazaheri et al., 2011, Presto et al., 2011).
Additional sources may also be present at airports, including maintenance work, heating facilities, fugitive vapours from refuelling operations, kitchens and restaurants for passengers and operators, etc. Moreover, as many airports are located far from cities, their emission inventories should also include sources not directly present within a terminal, but on which the airport has an influence. These sources may include intermodal transportation systems or road traffic including private cars, taxis, shuttle buses and trucks for transporting people and goods in and out of the airport.
As most large airports are located near heavily populated urban settlements, in combination they have a potentially significant impact on the environment and health of people living in their vicinity. For example, 150 airports in the USA are located in areas designated to be in non-attainment for one or more criteria air pollutants (Ratliff et al., 2009). In undertaking air quality assessments and the development of successful mitigation strategies, it is therefore fundamental to consider all the aspects associated with the entire “airport system”. However, current information on many aspects of this polluting source is inadequate, including a detailed speciation of hydrocarbons, physicochemical characteristics of particles, volatile and semi-volatile emissions and especially the secondary transformations from the aging of aircraft exhausts and other airport-related emissions. Some of these gaps are well summarised in a US Transportation Research Board report (Webb et al., 2008).
1.1. Aims and outline of the review
Since the scientific literature on AEs remains very sparse and many questions are still open, this review aims to summarise the state-of-the-art of airport emissions research and attempts to synthesise and analyse the published studies. An overview of current information on airport-related emissions is presented and the key characteristics of the pollution and the impacts on the local and global air quality are discussed. This review further summarises the various methodologies used for measurements and attempts to critically interpret the data available in the literature. Finally, this review will highlight priority areas for research.
The next section traces the main stages of the development of civil aviation, by focussing especially on the changes and development strategies of modern airport systems. Recent traffic data and statistics are presented and the trends are also discussed in order to understand the potential future growth of air transport, which is fundamental to forecasting the impacts of aviation in future years. The third section gives an overview of the operation of aircraft engines, briefly discusses the most widely used technologies, describes some fuel characteristics, such as the sulphur content, and analyses the current use and future jet fuel consumption scenarios. The fourth section reviews the current information on aircraft engine exhaust: the landing and take-off cycles are described since they are commonly used to assess aircraft emissions during the operational conditions within an airport and within the atmospheric surface boundary layer; the main gaseous and particulate-phase compounds emitted by aircraft are listed and their key chemical and physical characteristics are described in separate subsections. A summary of data on the emission indices for many pollutants is also provided. The fifth section describes the non-exhaust emissions related to aircraft operations, such as the tyre and brake wear and the re-suspension of runway material, which have been little investigated even though they may have serious impacts on local air quality. The sixth section reviews data on the non-aircraft emissions potentially present within an airport, including the ground service equipment emissions, the auxiliary/ground power units and others. The seventh section presents the results of studies conducted indoors and outdoors at airports to directly assess the impacts of AEs upon human health. Finally, this paper reviews the results of the recent literature on aircraft emissions and other airport-related contributions to highlight the potential role of AEs upon local air quality.
2. Present scenarios and future perspectives of civil aviation and airports
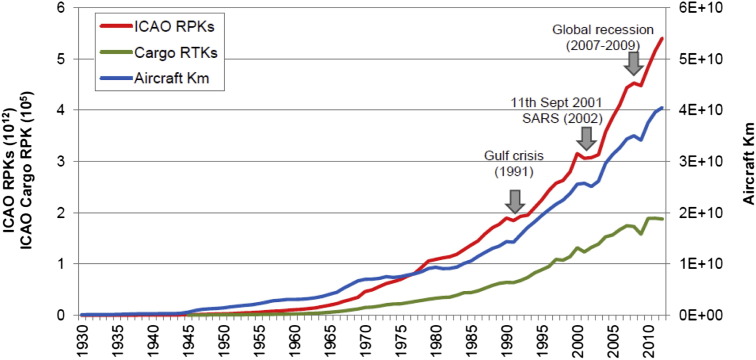
The Airport Council International (ACI, 2013) has reported recent statistics on the air traffic volumes for 2012: more than 79 million aircraft movements carried annually 5.7 billion passengers between 1598 airports located in 159 countries, and reported that the total cargo volume handled by airports was 93 million tonnes. However, these numbers are expected to further increase in the forthcoming decades: in the past half century, the aviation industry has experienced a strong and rapid expansion as the world economy has grown and the technology of air transport has developed (Baughcum et al., 1999). Generally, air traffic has been expressed as revenue passenger kilometres (RPKs) by multiplying the number of revenue-paying passengers aboard the vehicle by the travelled distance, or occasionally in revenue tonne kilometres (RTK). Fig. 1 shows the absolute growth of aviation recorded by ICAO in terms of RPK, RTK and aircraft kilometres from the 1930s to today (ICAO, 2013, Airlines for America, 2013). Despite some global-scale events, such as the Gulf crisis (1991), the terrorist attack of 11th September 2011, the outbreak of severe acute respiratory syndrome (SARS) in 2002–2003 and the recent global economic crisis (2008–2009), an average annual growth rate of 5% was observed and this trend is expected to continue over the next decades mainly driven by the economic growth of emerging regions (ACI, 2007, ACI, 2008, Airbus, 2012, Boeing, 2013). It is anticipated that there will be more than 9 billion passengers globally by 2025 and more than 214 million tonnes of total world freight traffic are forecast over almost 120 million air traffic movements (ACI, 2007). The future growth of air transport will inevitably lead to the growth of airline fleets and route networks and will therefore lead to an associated increase in airport capacity in terms of both passengers and cargo. This poses questions as to the consequent impact on air quality.
Fig. 1.
Absolute growth of aviation (1930–2012) recorded by ICAO in terms of RPK, RTK and aircraft kilometres. Data refers to ICAO (2013) and were taken from Airlines for America (2013).
3. Aircraft: characteristics and in-use technologies
Emissions from aircraft engines are recognised as a major source of pollutants at airports and have been extensively investigated over the past 40 years. Initially, the main historical concern for supersonic aircraft was over stratospheric ozone depletion (Johnston, 1971) and secondarily about the formation of contrails at cruising heights (Murcray, 1970, Schumann, 2005) and indirect effect on the Earth's radiative budgets (Kuhn, 1970). Apart the development of the Concorde and the Tupolev Tu-144, a supersonic fleet flying in the stratosphere was never developed and today all commercial airliners are subsonic equipped with turbofan or turboprop engines. Therefore, the main present issue arising from civil aviation has today shifted to the increased levels of ozone in the upper troposphere and lower stratosphere resulting from the atmospheric chemistry of emitted NOx (Lee et al., 2010 and reference therein). Furthermore, the development of increasingly restrictive legislation on ambient air quality and the implementation of enhanced monitoring networks in many developed countries has highlighted the effects of aircraft emissions at ground-level and the deterioration of air quality near airports.
3.1. Engines
Engines for civil and general aviation are generally classified as gas turbine engines (turbofan and turboprop) fuelled with aviation kerosene (also named jet fuel) and internal combustion piston engines fuelled with aviation gasoline, often referred as avgas (ICAO, 2011). The majority of modern airliners are equipped with turbofan engines. These engines are derived from predecessor turbojet engines developed during World War II. A turbojet is composed of an inlet compressor, a combustion section adding and igniting fuel, one or more turbines extracting energy from the exhaust gas in expansion and driving the compressor. A final exhaust nozzle accelerates the exhaust gas from the back of the engine to generate thrust. Turbofan engines use a turbojet as a core to produce energy for thrust and for driving a large fan placed in front of the compressor. In modern airliners, the fan provides most of the thrust. The “bypass ratio” refers to the ratio of mass flux bypassing the combustor and turbine to the mass flux through the core: high-bypass ratios are preferred for civil aviation for good fuel efficiency and low noise. Some small and regional airliners are instead equipped with turboprop engines, which use a turbine engine core fitted with a reduction gear to power propellers. A simplified diagram of a turbofan engine is provided in Fig. 2 . In August 2013 the ICAO (EASA, 2013) listed a total of 487 in-use turbofan engines (including packages): Table 1 provides a summary of the current engine families mounted in the most popular airliners (75% of total in-use turbofan engines).
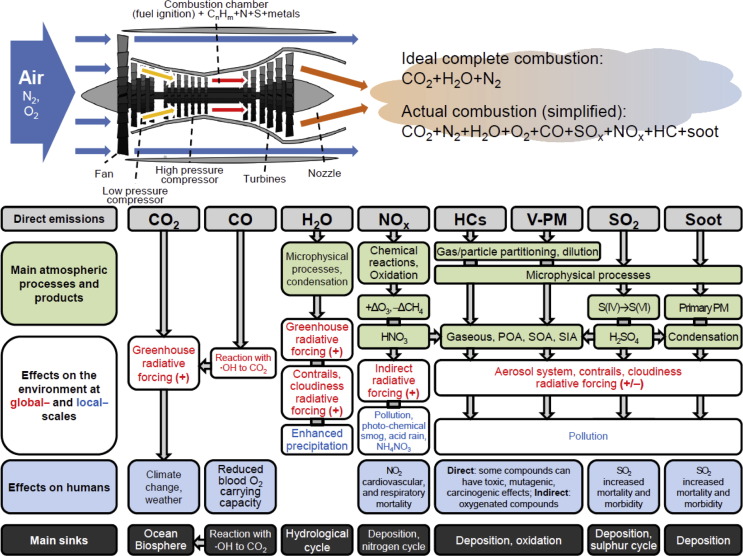
Fig. 2.
Simplified diagram of a turbofan engine (upper left); products of ideal and actual combustion in an aircraft engine (upper right); and related atmospheric processes, products, environmental effects, human health effects and sinks of emitted compounds (bottom). Adapted from Prather et al., 1999, Wuebbles et al., 2007 and Lee et al. (2009).
Table 1.
Engine-family mounted in the most popular aircraft. The number of engines for each aircraft in given within brackets. This list represents ∼75% of total in-use turbofan engines provided by the ICAO databank at August 2013 and does not report data for regional jets. Average data (mean ± standard deviation) for fuel consumption and emissions per LTO cycle are also reported per each engine family.
| Manufacturer | Engine family | Main aircraft and number of engines | Fuel and emissions per LTO cycle (kg) |
|||
|---|---|---|---|---|---|---|
| Fuel | CO | NOx | HC | |||
| General Electric | CF6 series | A300 (2); A310 (2); A330 (2); B747 (4); B767 (2); MD DC-10 (3); MD-11 (3) | 811 ± 76 | 11 ± 5 | 12 ± 2 | 2.3 ± 2.2 |
| GE90 series | B777 (2) | 1159 ± 141 | 14 ± 7 | 25 ± 5 | 1.1 ± 0.8 | |
| GEnx series | B747 (4); B787 (2); replacing CF6 series | 827 ± 74 | 7 ± 1 | 10 ± 3 | 0.2 ± 0.1 | |
| CMF International | CFM56 series | A318 (2); A319 (2); A320 (2); A321 (2); A340 (4); B737 (2): MD DC-8 (4) | 419 ± 46 | 6 ± 2 | 5 ± 1 | 0.6 ± 0.4 |
| Pratt & Whitney | JT8D series | B707 (4); B727 (3); B737 (2); MD DC-9 (2); MD80 (2) | 477 ± 35 | 5 ± 2 | 4 ± 1 | 1 ± 0.9 |
| JT9D series | A300 (2); A310 (2); B747 (4); B767 (2); MD DC-10 (3) | 842 ± 45 | 19 ± 10 | 13 ± 1 | 7 ± 4.8 | |
| PW 4000 series | A300 (2); A310 (2); B747 (4); B767 (2); B777 (2); MD DC-11 (3) | 966 ± 150 | 8 ± 3 | 17 ± 6 | 1 ± 0.8 | |
| Rolls-Royce | RB211 series | B747 (4); B757 (2); B767 (2); L1011 (3); Tu-204 (2) | 852 ± 128 | 15 ± 15 | 15 ± 5 | 7.1 ± 11.1 |
| Trent series | A330 (2); A340 (4); A380 (4); B777 (2); B787 (2) | 817 ± 370 | 5 ± 2 | 19 ± 4 | 0.2 ± 0.3 | |
| BMW Rolls-Royce | BR700 series | B717 (2) | 332 ± 32 | 4 ± 1 | 4 ± 1 | 0.1 ± 0.1 |
| International Aero Engines | V2500 series | A319 (2); A320 (2); A321 (2); MD-90 (2) | 452 ± 35 | 3 ± 0.4 | 6 ± 1 | 0.04 ± 0.01 |
| Aviadvigatel' Solov'ëv | D30 series | Tu-154 (3) | 622 ± 110 | 21 ± 6 | 5 ± 1 | 5.5 ± 2.4 |
B (Boeing); A (Airbus); MD (McDonnell Douglas); L (Lockheed); Tu (Tupolev).
Reciprocating piston engines are predominately fitted in small-sized aircraft typically related to private use, flying clubs, flight training, crop spraying and tourism. Internal piston engines run under the same basic principles as spark ignition engines for cars, but generally require higher performance. Four-stroke-cycle engines are commonly used, more rarely these can be two-stroke and occasionally diesel. The principal difference between jet and piston engines is that combustion is continuous in jet engines and intermittent in piston engines. Other flying vehicles may be present within an airport, such as helicopters. These vehicles are usually less numerous than the airliners in most terminals, but in some circumstances their contribution to the air quality cannot be disregarded. Today, most modern helicopters are equipped with turboshaft engines, whose functioning is similar to a turbojet but are optimised to generate shaft power instead of jet thrust. This review abbreviates turbojet (TJ), turbofan (TF), turboprop (TP) and turboshaft (TS).
3.2. Fuel characteristics
At the current time, almost all aviation fuel (jet fuel) is extracted from the middle distillates of crude oil (kerosene fraction), which distils between the gasoline and the diesel fractions. The kerosene-type fuels most used worldwide in civil aviation are of Jet A and Jet A-1 grades: Jet A is used in most of the world, except North America where Jet A-1 is used. An exhaustive review of jet fuel production processes is given elsewhere (Liu et al., 2013). The specifications of such fuels are addressed by two organizations, the American Society for Testing and Materials (ASTM) and the United Kingdom Ministry of Defence (MOD). Jet A is used for almost all commercial aviation flying within or from the USA and is supplied against the ASTM D1655 specification. It has a flash point minimum of 38 °C and a freeze point maximum of −40 °C. Jet A-1 is widely used outside the USA and follows the UK DEF STAN 91-91 (Jet A-1) and ASTM D1655 (Jet A-1) specifications. It has same flash point as Jet A but a lower freeze point (maximum of −47 °C) and a mean C/H ratio of C12H23 (Lewis et al., 1999, Chevron Corporation, 2006, Lee et al., 2010). Other fuels can be used as an alternative to Jet A-1. Jet B is a wide-cut type fuel covering both the naphtha and kerosene fractions of crude oil and is used in very cold climates, e.g. in northern Canada where its thermodynamic characteristics (mainly lower freeze point and higher volatility) are suitable for handling and cold starting. ASTM publishes a specification for Jet B, but in Canada it is supplied against the Canadian specification CAN/CGSB 3.23. Other specifications also exist such as DCSEA (France) and GHOST (Russia). TS-1 is the main jet fuel grade available in Russian and CIS states, along with T-1, T-2 and RT; it is a kerosene-type fuel with slightly higher volatility (flash point is 28 °C minimum) and lower freeze point (<−50 °C) compared to Jet A and A-1 fuels. Various types of jet fuels are instead regulated by Chinese specifications: RP-1 and RP-2 are kerosene-type fuels similar to Russian TS-1, while RP-4 to Jet B. Nowadays, virtually all jet fuel in China is RP-3, which is quite comparable to Jet A-1 (Shell, 2013). Fuels for military purposes are formulated for high-performances and are regulated separately by many governments; some of these (JP grades for USA and NATO forces) were used in several studies (e.g., Anderson et al., 2006, Chen. et al., 2006, Cowen et al., 2009, Cheng et al., 2009, Cheng and Corporan, 2010, Santoni et al., 2011). The kerosene-based JP-8 grade is currently the primary fuel for NATO aircraft. Corporan et al. (2011) reported some JP-8 characteristics.
Jet fuels are a mixture of thousands of different hydrocarbons. The range of their molecular weights is restricted by the distillation: in kerosene-type fuels (e.g., Jet A and Jet A-1) the carbon number ranges between about 8 and 16, while in wide-cut jet fuels (Jet B), between about 5 and 15. Spicer et al. (1994) reported that jet fuel is primarily composed of species with five or more carbons and 70% of the compounds by weight contain 11–14 carbon atoms. Most of the hydrocarbons in jet fuel are members of the normal paraffins, iso-paraffin, cycloparaffin, aromatic and alkene classes: 20% n-paraffins, 40% iso-paraffin, 20% naphthenes and 20% aromatics are typical (Lindstedt and Maurice, 2000, Liu et al., 2013 and reference therein). Moreover, a series of different additives are required or approved for use by ASTM and DEF STAN specifications to enhance or maintain some fuel properties, improve performance or handling. Among those approved for Jet A and Jet A-1 fuels, some hindered phenols serve as antioxidants, the di-ethylene glycol monomethyl ether acts as icing inhibitor, the N,N′-disalicylidene-1,2-propane diamine is added as chelating agent for many metal ions. Other additives act as electrical conductivity/static dissipaters, corrosion inhibitor and biocides: a summary is listed in Chevron Corporation (2006).
The aviation industry is nowadays investing significant effort towards the use of alternative fuels (Blakey et al., 2011, Williams et al., 2012). Since aircraft emissions are recognised to be closely linked to the fuel composition (Beyersdorf et al., 2013 and reference therein), recently the introduction of synthetic fuels and bio-fuels instead of common oil-derivate jet fuels has been much discussed in terms of beneficial effects upon exhaust emissions (e.g., Corporan et al., 2005, Corporan et al., 2007, DeWitt et al., 2008, Timko et al., 2010a, Corporan et al., 2011, Lobo et al., 2011, Williams et al., 2012, Cain et al., 2013). Among others, the Fischer–Tropsch (FT) fuel seems to be a potential candidate for replacing, or mixing with, oil-derived conventional jet fuels. The FT reaction was developed in the first half of twentieth century and uses a mixture of carbon monoxide and hydrogen to produce a complex product stream of paraffins, olefins, and oxygenated compounds such as alcohols and aldehydes via product upgrading (e.g., cracking, fractionation, and isomerisation). The mechanism is explained in Liu et al. (2013). The FT process leads to a fuel with low aromatic content and no sulphur, which are reported to be beneficial in reduction of emissions of particulate matter and its precursors from aircraft engines (Corporan et al., 2007, Timko et al., 2010a, Lobo et al., 2011). Corporan et al. (2011) report gas chromatograms and hydrocarbon content of JP-8 and various alternative jet fuels. To study the effects of FT fuel usage on aircraft gaseous and particulate emissions the Alternative Aviation Fuel Experiment (AAFEX) was carried out in 2009: results are spread across various papers (e.g., Lee et al., 2011, Santoni et al., 2011, Anderson et al., 2011, Kinsey et al., 2012a, Kinsey et al., 2012b, Beyersdorf et al., 2013).
Avgas for general aviation is distilled separately from the most common motor gasoline and is formulated for stability, safety, and predictable performance under a wide range of environments. Nowadays there are two main grades (100 and 100LL low lead) regulated by the ASTM D910 and UK DEF STAN 91-90 specifications. Tetraethyl Pb is added to avgas for increasing fuel octane and avgas 100LL has a lead content up to 0.56 g Pb L−1. The impact of general aviation is under discussion, since it was reported as one of the largest remaining source of lead emissions to the air in the USA (e.g., Carr et al., 2011). Avgas is principally composed of isoparaffinic and aromatic hydrocarbons and their carbon numbers vary from about 4 (butane) to 10, with the most prevalent carbon number being 8 (Chevron Corporation, 2006). It may include tetraethyl lead as antiknock additive, icing inhibitors, antioxidants and others.
3.3. Sulphur content in fuels
Over the past decades there has been a worldwide trend to decrease sulphur content in fuels and many jurisdictions, including the USA and the European Union, have recently required very low sulphur levels in road and marine fuels to reduce the SOx and particulate matter emissions from the transport sector. A similar reduction has not occurred for jet fuel although at the beginning of the 2000s the IPCC indicated that reducing the sulphur content of kerosene will reduce SOx emissions and sulphate particle formation (IPCC, 1999). The maximum sulphur content of aviation fuel has remained at 3 g S kg Fuel−1, or 3000 ppm by mass (Lewis et al., 1999, Ebbinghaus and Wiesen, 2001, Anderson et al., 2005, Barrett et al., 2012). However, lower values of fuel sulphur content (FSC) have commonly been reported: Fahey et al. (1999) stated that in the world market at the beginnings of the 2000s the FSC was near 400 ppm; Hileman et al. (2010) reported that average FSC in commercial Jet A, Jet A-1 and military JP-8 fuel grades varied between 550 and 750 ppm; Agrawal et al. (2008) reported that FSC in the fuel was 300 ppm. Popovicheva et al. (2004) and Demirdjian et al. (2007) reported that the aviation kerosene TS-1 has a FSC of 1100 ppm and less than 10−4 wt.% of metals.
FSC in jet fuels is directly related to the SO2 emissions in aircraft exhaust (e.g., Arnold et al., 1998a, Schumann et al., 1998, Hunton et al., 2000). Some research projects, such as APEX-1, were designed to study the effects of FSC on aircraft engine emissions (e.g., Wey et al., 2006, Wey et al., 2007, Kinsey, 2009, Onasch et al., 2009). Generally the studies reported that the emissions of both SO2 and sulphates are proportional to S levels in fuels, but no systematic difference between the low and high sulphur fuels in terms of other emitted organic sulphur species (OCS and CS2) were reported (Anderson et al., 2006). The conversion of S(IV) to S(VI) is amply discussed later in this review.
Recently, the impact of ultra-low sulphur jet fuel (15 ppm) upon public health, climate, and economics was examined by Barrett et al. (2012). They reported that the use of ultra-low sulphur fuels on a global-scale will cost 1−4 billion US $ per year, but may prevent 900−4000 air quality-related premature mortalities per year. Moreover, Barrett and co-authors also stated that the radiative forcing (RF) associated with reductions in atmospheric sulphate, nitrate, and ammonium loading can be estimated as +3.4 mW m−2, i.e. equivalent to about 1/10th of the warming due to CO2 emissions from aviation.
3.4. Current use and future jet fuel consumption scenarios
The availability of reliable information on fuel consumption is essential to make robust estimates of aviation emissions at both global and regional scales. Various estimates of aviation fuel consumption are available in the literature and generally refer only to jet fuel, since piston-powered flights were estimated to account for approximately 2% of propeller (piston plus turboprops) and ∼0.05% of total (propeller plus jet) fuel burn (Kim et al., 2007). Gauss et al. (2006) estimated a total of 169 Tg fuel globally burned in 2000, of which 152 Tg is due to civil flights. The AERO2k global aviation emissions inventories reported a total of 176 Tg of kerosene used in 2002 for both civil (156 Tg) and military (19.5 Tg) aviation (Eyers et al., 2004); other studies of the 2000–2005 period estimated that the global aviation industry consumed approximately 170–203 Tg of kerosene per year with an evident decrease in 2001–2002 following the drop of aviation traffic due to the 11th September 2001 and SARS events (Kim et al., 2007); Wilkerson et al., 2010, Whitt et al., 2011 and Olsen et al. (2013) reported that the global commercial aircraft fleet burned 188 Tg of fuel in 2006; Chèze et al. (2011) reported a world consumption of 229 Mt of jet fuel in 2008. These estimates accounted for approximately 3% of current annual fossil fuel energy usage (Barrett et al., 2010, and reference therein). Data from OPEC (Mazraati, 2010) stated that the aviation sector in 2006 was the second major consumer of total oil demand in the transportation sector (11.2%) and accounted for 5.8% of total oil consumed in the world. Given the past and future growth of the aviation industry, this consumption may rise further: AERO2k emission inventories estimated a forecast scenario for 2025 in which the fuel demand for aviation will be 327 Tg y−1 (Eyers et al., 2004); Chèze et al. (2011) reported that the world jet fuel demand is projected to grow by 38% between 2008 and 2025, rising to more than 316 Mt in 2025 at a mean growth rate of 1.9% per year. Owen et al. (2010) estimated the future global aviation emissions under four of the IPCC/SRES (Intergovernmental Panel on Climate Change/Special Report on Emissions Scenarios) marker scenarios and reported a fuel use of 336 Tg in 2020 and varying from 426 to 766 Tg for 2050. This study also reported an estimate of 325 Tg for 2050 if the ambitious technology targets of the Advisory Council for Aeronautical Research in Europe (ACARE, 2002) were to be achieved. Table 2 summarises the yearly global fuel consumption reported in recent studies. However, aviation traffic growth and jet fuel demand have been shown not to be strictly correlated, since the efficiencies of aircraft engines and air traffic management are improving and modern airliners are 75% quieter with consequent fuel consumption reduced by 70% with respect to the 1960s (Baughcum et al., 1999, Nygren et al., 2009, and references therein). In particular, the current average fuel consumption of in-use fleets was estimated to be less than 5 L fuel every 100 RPK, while in most modern aircraft it drops to approximately 3.5 L/100 RPK: Nygren et al. (2009) reported the historical world fleet of aircraft average fuel consumption and found an exponential trend in fuel consumption reduction from 1987 to the present day. Oil prices have driven investment in more efficient aircraft models. Fuel costs exceed those of labour costs for airlines. Fuel costs accounted for ∼13% of total costs in 2002, but today they are closer to 34% (Boeing, 2013).
Table 2.
Total annual fuel burned by aviation and emissions of H2O, CO2, NOx, CO, HC, SOx and soot (when available) provided by recent studies. Forecasts for 2020 and 2025 are also provided. Global emission data for 2008 and forecasts for 2025 were calculated starting from fuel data of Chèze et al. (2011) and emission indices of Lee et al. (2010). Kim et al. (2007) provided fuel burn and NOx emission during LTO for the 2000–2005 period; LTO emissions of H2O, CO2 and SO2 were calculated starting from fuel data of Kim et al. (2007) and emission indices of Lee et al. (2010). Note that all emissions calculated in this review are in italics.
| Year | Fleeta | Fuel |
H2O |
CO2 |
NOxb |
CO |
HC |
SOxc |
Soot |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Tg | Mg | |||||||||
| Global | ||||||||||
| 1999 | Scheduled air traffic which includes turboprops, passenger jets, and jet cargo aircraft | 128 | – | – | 1.7 | 0.685 | 0.189 | – | – | Sutkus et al. (2001) |
| 2000 | Scheduled and non-scheduled commercial aviation | 214d | – | 677 | 2.9 | – | – | – | – | Owen et al. (2010) |
| 2000 | Civil and military aircraft Civil aircraft Military (difference) |
169 152 44 |
– – – |
– – – |
2.15 1.95 0.2 |
– – – |
– – – |
– – – |
– – – |
Gauss et al. (2006) Gauss et al. (2006) Gauss et al. (2006) |
| Commercial aviation | 181 | 224 | 572 | 2.51 | 0.541 | 0.076 | 0.145 | – | Kim et al. (2007) | |
| 2001 | Commercial aviation | 170 | 210 | 536 | 2.35 | 0.464 | 0.063 | 0.136 | – | Kim et al. (2007) |
| 2002 | Commercial aviation | 171 | 211 | 539 | 2.41 | 0.480 | 0.064 | 0.137 | – | Kim et al. (2007) |
| Civil aviation | 156 | 193 | 492 | 2.06 | 0.507 | 0.063 | – | 3.9 | Eyers et al. (2004) | |
| Military aviation | 19.5 | 24.1 | 61 | 0.178 | 0.647 | 0.066 | – | – | Eyers et al. (2004) | |
| Civil + Military aviation | 176 | 217 | 553 | 2.24 | 1.150 | 0.129 | – | >3.9 | Eyers et al. (2004) | |
| 2003 | Commercial aviation | 176 | 218 | 557 | 2.49 | 0.486 | 0.062 | 0.141 | – | Kim et al. (2007) |
| 2004 | Commercial aviation | 188 | 233 | 594 | 2.69 | 0.511 | 0.063 | 0.151 | – | Kim et al. (2007) |
| Commercial aviatione | 174 | 215 | 550 | 2.456 | 0.628 | 0.090f | 0.102g | 6.1 | Wilkerson et al. (2010) | |
| 2005 | Commercial aviation | 203 | 251 | 641 | 2.9 | 0.554 | 0.065 | 0.163 | – | Kim et al. (2007) |
| 2006 | Commercial aviation | 188 | 233 | 595 | 2.656 | 0.679 | 0.098f | 0.111h | 6.8 | Wilkerson et al. (2010) |
| 2008 | From ICAO commercial air carriers—traffic database | 229 | 282 | 725 | 3.21 | 0.688 | 0.092 | 0.183 | 5.7 | Fuel demand by Chèze et al. (2011) |
| Forecasted trend | ||||||||||
| 2020 | Scheduled and non-scheduled commercial aviation | 336 | – | 1062 | 4 | – | – | – | – | Owen et al. (2010) |
| 2025 | – | 317 | 390 | 1001 | 4 | 0.951 | 0.127 | 0.253 | 7.9 | Fuel demand forecast by Chèze et al. (2011) |
| Emission indices | ||||||||||
| EI | Mean emission indices | – | 1230 | 3160 | 14 | 3 | 0.4 | 0.8 | 0.025 | Lee et al. (2010) |
| LTO cycles | ||||||||||
| 2000 | Commercial aviation | 12.9 | 15.9 | 40.8 | 0.197 | – | – | 0.010 | – | Kim et al. (2007) |
| 2001 | Commercial aviation | 12.3 | 15.1 | 38.9 | 0.191 | – | – | 0.010 | – | Kim et al. (2007) |
| 2002 | Commercial aviation | 12.2 | 15.0 | 38.6 | 0.194 | – | – | 0.010 | – | Kim et al. (2007) |
| 2003 | Commercial aviation | 12.4 | 15.3 | 39.2 | 0.199 | – | – | 0.010 | – | Kim et al. (2007) |
| 2004 | Commercial aviation | 12.9 | 15.9 | 40.8 | 0.21 | – | – | 0.010 | – | Kim et al. (2007) |
| 2005 | Commercial aviation | 13.9 | 17.1 | 43.9 | 0.227 | – | – | 0.011 | – | Kim et al. (2007) |
Type of fleet, as specified in different estimates.
NOx is expressed as NO2 in Sutkus et al., 2001, Gauss et al., 2006 and Wilkerson et al. (2010).
SOx expressed as SO2.
Normalized to the IEA total aviation fuel sales figure (see Owen et al., 2010).
Corrected global fuel burn results (see Wilkerson et al., 2010).
HC expressed as CH4.
Expressed as S–SOx, assuming that 96.3% of the SOx–S was partitioned to SO2–S and 3.7% to S(VI)–S (particle).
Expressed as S–SOx, assuming that 98% of the SOx-S was partitioned to SO2–S.
4. Aircraft exhaust emissions
Emissions from aircraft engines are generally considered to be the dominant source at airports and the large majority of studies available in the literature focus on aircraft emissions. Common airliners burning kerosene-type fuels primarily produce carbon dioxide and water (Wahner et al., 1995, Lewis et al., 1999, Anderson et al., 2006, Lee et al., 2010), which are directly related to the burned fuel, with minor variations due to the carbon–hydrogen ratio of the fuel. In this context, it is reported that the fuel flow of common airliner engines is approximately linearly proportional to engine thrust setting (e.g., Anderson et al., 2005, Wey et al., 2006).
The oxidation of atmospheric nitrogen at the very high temperatures in engine combustors drives the formation of nitrogen oxides, while the presence of trace amounts of sulphur, nitrogen and some metals (e.g., Fe, Cu, Zn) in fuels (Lewis et al., 1999) and non-ideal combustion conditions within engines may lead to the production of by-products, including sulphur oxides, additional nitrogen oxides, unburned hydrocarbons and particulate soot. Furthermore, exhausts can also contain species from the combustion and release of lubricant oils (Dakhel et al., 2007, Timko et al., 2010b, Yu et al., 2010, Kinsey et al., 2011, Yu et al., 2012) and from mechanical component wear (Petzold et al., 1998, Demirdjian et al., 2007). Therefore a more realistic, but simplified, combustion scheme in aircraft engines can be summarised as (Lee et al., 2009):
| CnHm + N2 + O2 + S → CO2 + N2 + H2O + O2 + CO + SOx + NOx + HC + soot |
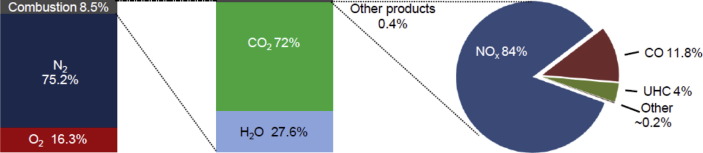
IPCC reported that approximately 99.5–99.9% of the molar content of typical commercial engine exhaust consists of N2, O2, CO2, and H2O (Lewis et al., 1999). Fig. 3 reports a more detailed breakdown of combustion products for a core engine mass flow: the combustion products in aircraft exhausts are mainly made up of CO2 (∼72%), H2O (∼27.6%), while residual products account for less than 1%. Fig. 2 summarises the main exhaust components of aircraft engines and their potential effects on the environment and human health. It is estimated that roughly 90% of aircraft emissions, except hydrocarbons and CO (∼70%), are produced while cruising at altitude, while the remainder is emitted during landing, take-off, and ground level operations (e.g., FAA, 2005).
Fig. 3.
Division of the combustion products from an aircraft engine, adapted from Lewis et al. (1999).
Aircraft emissions have been studied extensively since the late-1960s and initially the interest was mainly driven by their direct and indirect effects on climate and the generation of contrails. For this reason, many early studies focused on emissions at high cruise altitudes (e.g., Reinking, 1968, Kuhn, 1970, Arnold et al., 1992, Fahey et al., 1995a, Fahey et al., 1995b, Wahner et al., 1995, Brasseur et al., 1996, Schumann, 1996, Schumann, 1997, Anderson et al., 1998a, Anderson et al., 1998b). The interest in aviation emissions at airports also dates back many years (e.g., Daley and Naugle, 1979, Naugle and Fox, 1981), but only recently was there an increasing awareness of the effects of aircraft emissions at ground level, or at least within the planetary boundary layer. The recent interest in aircraft emissions at ground-level was initially motivated by public concern, given that more and more often airports are held responsible for air pollution and noise in nearby residential areas (e.g., Mahashabde et al., 2011). Since aircraft emissions are related to engine thrust (e.g., Anderson et al., 2006, Lobo et al., 2007, Whitefield et al., 2008, Timko et al., 2010b, Kinsey et al., 2010, Kinsey et al., 2011) and engines are designed for high performance while cruising at high altitudes, some aircraft operations within airports require that engines operate outside of their optimal regimes, ranging from maximum thrust during take-off to low power settings during operations on the ground. This fact was clearly highlighted during the APEX-1 campaign by Onasch et al. (2009), who reported that a CFM56 engine is less efficient at the low thrust levels usually used at airports. This may result in potentially higher emissions on the ground than that during cruising for those pollutants mainly emitted at low power, such as CO and hydrocarbons.
Early reports of nitrogen oxides, carbon monoxide, hydrocarbons and particulate matter from jet aircraft turbine engines were made by Spicer et al. (1984). Subsequent studies (Spicer et al., 1992, Spicer et al., 1994) added further information and provided detailed information on the organic component of turbine engine emissions. Following from these pioneering studies, the scientific literature now comprises a large number of studies and most have concluded that aircraft exhausts are responsible for significant emissions of a series of gaseous, semi-volatile and non-volatile species. Non-volatile emissions are produced in the combustor and are made up of refractory material such as soot (e.g., Agrawal et al., 2008, Kinsey, 2009, Dodson et al., 2009, Lee et al., 2010, Presto et al., 2011), which is emitted into the atmosphere as particulate matter even at the high engine exit temperatures, but also contains many organic compounds (e.g., Herndon et al., 2006, Anderson et al., 2006, Webb et al., 2008, Wood et al., 2008a, Agrawal et al., 2008, Herndon et al., 2009, Lee et al., 2010, Mazaheri et al., 2011, Presto et al., 2011, Kinsey et al., 2011, Mazaheri et al., 2013).
Volatile emissions include compounds that exists as vapour at engine exit temperature and pressure (Presto et al., 2011) and are made up of gaseous and vapour-phase pollutants, such as CO2, CO, NOx, SO2, O3 and many organic compounds, including alkanes, alkenes, carbonyls, aromatic compounds and a number of other volatile organic species. The least volatile fraction has been shown to range from 10 to 20% of the total organic emissions (Presto et al., 2011) and its presence is particularly challenging, because it can react in the atmosphere and may undergo condensation in the exhaust plumes leading to aerosol particles or volatile coating of pre-existing particles (Lee et al., 2010, Miracolo et al., 2011). This latter component is named volatile PM, however there is today a considerable controversy about its definition (Kinsey, 2009). Such particles may act as condensation nuclei or may interact with soot to form condensation nuclei and thus may have effects on cloud formation, precipitation and climate. In addition, additional compounds may subsequently originate from the aging of exhausts following a chain of oxidation with atmospheric oxidants and gases.
The relative amount of exhaust emissions depends upon combustor temperature and pressure, fuel to air ratio and the extent to which fuel is atomised and mixed with inlet air (Anderson et al., 2006). It is well recognised that the amounts of many pollutants may vary considerably with the engine technology, model and especially with the thrust. For example Slemr et al., 1998, Slemr et al., 2001 and Spicer et al., 1992, Spicer et al., 1994 reported that hydrocarbon emissions can be dependent upon engine type, use and maintenance history as well as fuel composition.
4.1. Geographical and vertical distributions of flights
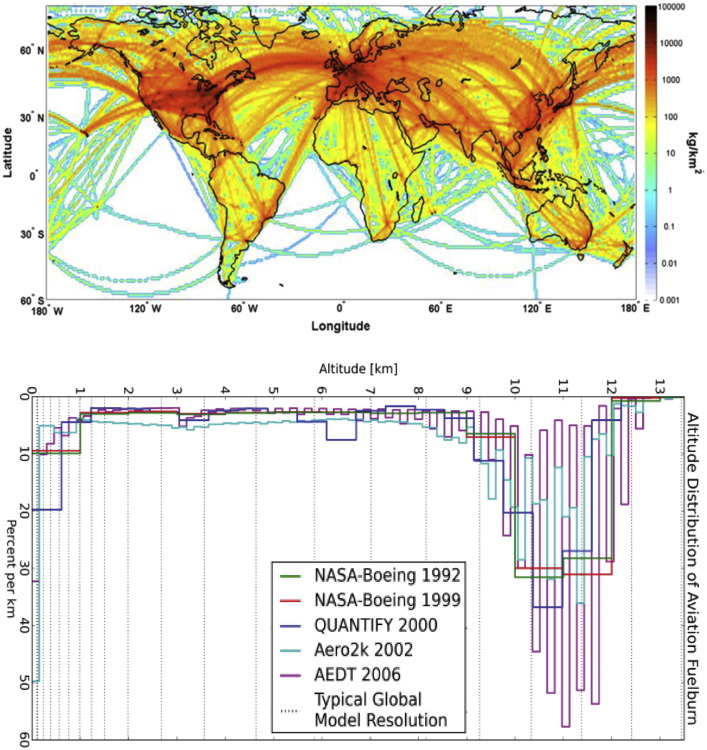
Based upon the main air traffic routes, a series of studies have discussed the geographical and vertical distributions of fuel consumption, which can be used to further assess the relative emissions from aviation (e.g., Kim et al., 2007, Wilkerson et al., 2010, Olsen et al., 2013, Simone et al., 2013). Due to the geographical distribution of civil aviation in the 2000s, the global fuel burn by domestic flights is dominated by the North America and Caribbean regions, while fuel consumed by international flights is dominated by Asia, North America and the Caribbean, and Western Europe and North Atlantic (Kim et al., 2007). Using the Aviation Emissions Inventory Code (AEIC, Stettler et al., 2011) Simone et al. (2013) estimated the fuel burn by country of origin/destination in 2005 and reported that the USA was the most important (59.1 Tg), followed by Japan (9.7 Tg), UK (9.4 Tg), China (8.5 Tg, excluding Hong Kong), Germany (6.7 Tg) and France (5.4 Tg). A map showing the column sum of global fuel burn from scheduled civil aviation in 2005 is provided in Fig. 4 a. Other studies have been carried out to estimate annual fuel consumption and pollutant emissions more locally: for example Fan et al. (2012) assessed the fuel consumption and emissions for each airline in China in 2010.
Fig. 4.
Geographical and vertical distributions of aviation: a) column sum of global fuel burn from scheduled civil aviation in 2005, as reported by Simone et al. (2013) using AEIC model (Stettler et al., 2011); b) annual global vertical distribution of commercial aviation fuel burn for the NASA-Boeing 1992 and 1999 (Baughcum et al., 1996a, Baughcum et al., 1996b; Sutkus et al., 2001), QUANTIFY 2000 (Owen et al., 2010), AERO2k (Eyers et al., 2004) and AEDT 2006 (Roof et al., 2007) datasets, taken from Olsen et al. (2013).
Kim et al. (2007) and Lee et al. (2007) used the System for assessing Aviation's Global Emissions (SAGE) model to estimate the vertical profiles of commercial aviation and pointed out that the highest fuel burn and emissions are between 9 and 12 km, which corresponds to typical cruise altitude. Generally, most studies also reported that about 5–7% of total jet fuel is consumed within 1 km above ground level during airport operations (Kim et al., 2007, Simone et al., 2013), and Olsen et al. (2013) reported a comparison of the annual global vertical distribution of fuel burn by the commercial aviation deriving from different estimates (Fig. 4b). Although most studies have concluded that 5–10% of fuel is burned below 1000 m, aircraft operations within airports may further increase fuel consumption due to the acceleration and deceleration of the engines following airport congestion (Anderson et al., 2005, Nikoleris et al., 2011) or due the unaccounted use of fuel for APUs (Ratliff et al., 2009).
4.2. Emissions at ground
4.2.1. Landing and take-off (LTO) cycles
The emissions of all aircraft engine must comply with applicable standards promulgated by the International Civil Aviation Organization (ICAO, 2008) and measured upon the landing and take-off (LTO) cycles. A LTO cycle refers to all the operations the aircraft carry out below 3000 ft above field elevation (equivalent to 914 m) over a specific range of certifiable operating conditions and includes four stages in terms of both engine thrust settings (expressed as a percentage of maximum rated thrust, or F00) and typical time in each specific mode of operation (time-in-mode, TIM). The 3000 ft height roughly corresponds to the atmospheric mixing height, i.e. the lower part of the troposphere within which pollutants emitted at ground-level mix rapidly (e.g., Schäfer et al., 2006). The LTO cycles are designed for aircraft engines manufactured after 1985 whose rated output is greater than 26.7 kN and aim to guarantee they not exceed certain regulatory environmental limits for a series of pollutants, namely unburned total hydrocarbons, carbon monoxide, nitrogen oxides and smoke number (SN). This latter parameter is roughly representative of the amount of soot an engine generates (e.g., Wayson et al., 2009, Stettler et al., 2013a, Stettler et al., 2013b). In the first LTO phase the aircraft descends from cruising altitude toward the runway and lands at the airport. This phase is named “approach” and is estimated as lasting for 4 min with engines at 30% F00. After landing, the aircraft enters in the “idle” phase which includes all the ground-based operations: it proceeds at a low speed to the gate (taxi-in), remains on stand-by for the loading and unloading operations and again prepares for take-off proceeding towards the runway (taxi-out). Idle lasts 26 min and the engines are required to be at 7% F00. The subsequent operating modes include the “take-off” with engines stressed to the full thrust (100% F00) for 0.7 min, and the “climb” (85% F00 for 2.2 min) up to 3000 ft height. A standardised LTO cycle is shown in Fig. 5 .
Fig. 5.
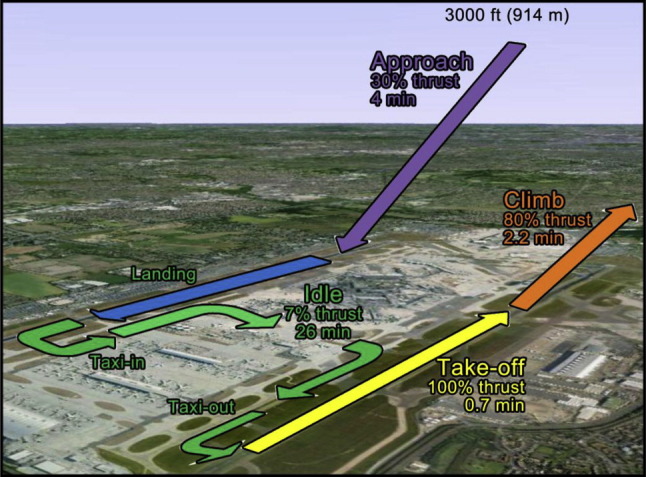
Standard ICAO LTO cycle. Adapted from ICAO (2011).
4.2.2. Engine ground running procedures
In addition to the operations falling within LTO cycles, the ground running procedures (GRPs) may lead to further emission loads from aircraft engines at airports. GRPs refer to the operation of some or all engines carried out on the ground for the purpose of functionally checking the operation of either engines or aircraft systems. GRPs are therefore an essential part of the operation of any airliner prior to the release to service of an aircraft from maintenance. The main reasons for running the engines on the ground are (Buttress and Morris, 2005): (i) check starts after minor maintenance actions; (ii) runs at no more than ground idle to ensure that the engine operates correctly after maintenance action, these include thrust reverser function checks, etc.; (iii) runs at powers greater than ground idle to check the correct operation of certain valves, leak checks, etc. To date, only few studies take into account the emissions from GRPs, but their importance for the atmospheric loads of some pollutants cannot be neglected. For example, Buttress and Morris (2005) showed that GRPs at London Heathrow airport release approximately 15.6 Mg y−1 NOx. Mazaheri et al. (2011) investigated the annual emissions of particle number, particle mass and NOx throughout the LTO cycles and GRP at the Brisbane Airport and showed that annual emissions account for less than 3%. Despite the evidence that GRPs may have a substantial impact on local air quality at airports, up to now they have received only minor consideration. GRPs are not yet regulated internationally and must comply only with local regulatory requirements imposing limitations on the locations, times and engine thrust levels employed during ground running which may differ from one airport to another.
4.2.3. Limitations in the use of standard LTO cycles
The use of standard LTO cycles as a surrogate for typical aircraft operations close to the ground represents an approximation and is not always representative of operations at airports. One limitation is that the ICAO engine emissions standards are applied through national and multi-national certification processes to turbojet and turbofan engines, but not turboprop, turboshaft and piston engines (ICAO, 2011). This limitation may be negligible at large airports, where most traffic is due to common airliners equipped with TF engines, but may represent a major approximation for small and medium-sized airports where small, private, business and regional aircraft account for a large portion of flight traffic. In addition, despite LTO cycles having been designed to model optimally all the operational procedures of aircraft in the vicinity of airports, sometimes they are not well adapted to engine settings and actual TIM, which depend upon pilot' technique, fleets, airport layouts and flight traffic. In fact, default ICAO TIM are not representative of real operations and are for certification purposes. Consequently, although some inventories account for the deviations from the ICAO default TIMs and thrust settings, some deviations from the standardised LTO procedures may occur during actual LTO cycles. This inevitably leads to some differences between actual airport operations and emission inventories used in modelling studies. The main deviations/limitations are:
-
•
reduced thrust during take-off. This practice is often carried out for performance and cost-efficiency reasons (ICAO, 2011) and has been widely observed on operational runways (Carslaw et al., 2008, Herndon et al., 2008); it may depend on aircraft weight and weather factors (Morris, 2002) and is often largely unknown (Carslaw et al., 2008). Since the emissions of some pollutants increase monotonically with the thrust (e.g., NOx), this could lead to an overestimation of emissions from airports;
-
•
lower thrust at idle/taxi mode. It has been reported that most aircraft use a thrust of 3%–4% F00 instead of 7% (Morris, 2005a, Morris, 2005b, Nikoleris et al., 2011 and reference therein) during idle operations. Since most pollutants emitted in exhaust plumes are strongly increased at decreased power settings (CO and generally all hydrocarbons), this may lead to underestimation of emissions at airports. In this context, Wood et al. (2008b) suggested that the thrust used in taxi operations can be split in two modes, i.e. ‘ground idle’ carried out at 4% F00 and ‘taxiway acceleration’ with thrust settings up to 17%. Moreover, higher thrust levels are sometimes used for turning;
-
•
acceleration and deceleration of the engines or stop-and-go situations. This is mainly the result of congestion on taxiways and is known to be responsible for significant increases in fuel consumption and increased emissions (Anderson et al., 2005, Nikoleris et al., 2011). For example Morris (2005a) reported that instant accelerations up to 10% F00 and lasting ∼10 s may occur at London Heathrow airport when aircraft cross an active runway or make a sharp turn. Due to this, the entire taxiway phase of operation using a uniform engine thrust level have been also recognised as problematic for emission inventory estimates because of the nonlinear emission rate of many compounds at low power (Herndon et al., 2009);
-
•
use of a reverse thrust phase during landing. Reverse thrust is applied to assist mechanical brakes in slowing down the landing aircraft and is not generally required for normal operations onto a dry runway (ICAO, 2011). However, it generally occurs with idle thrust power as a prudent safety precaution, and under some circumstances it may also occur at power higher than 10% F00 (Morris and Easey, 2005, Stettler et al., 2011). Generally, reverse thrust is applied for 10–20 s (Fanning et al., 2007, Stettler et al., 2011), but may vary as a function of the landing velocity, runway length and aircraft weight;
-
•
the evident differences between the standard TIM, which is used as part of the ICAO engine emissions certification processes, and the actual TIM used at airports (e.g., Unique, 2004, Watterson et al., 2004, Patterson et al., 2009, Stettler et al., 2011, Mazaheri et al., 2011, Khadilkar and Balakrishnan, 2012). For example, Patterson et al. (2009) and Khadilkar and Balakrishnan (2012) observed that total fuel burn during departures and arrivals at airports is generally overestimated by the ICAO method with respect to emissions computed from real-time aircraft flight data. Other studies have also reported measured TIM at airports: Unique (2004) reported TIM in Zurich airport and detected differences in all the LTO phases: idle (−43%), approach (+10%), climb (−77%) and take-off (+129%) which have been estimated to have a strong impact on the calculation of emissions, resulting in reduced fuel flow (−38%) and NOx emissions (−31%);
-
•
the composition of the fleet that serves an airport and the weight of the aircraft. Since the ICAO certifies the engines and not the full aircraft, some airplane characteristics, mainly the aircraft weight, may have a key role in determining the emissions. Furthermore, in addition to the mass of the aircraft, its load of fuel, passengers and goods affect the overall weight: it is reported that passengers, crew and luggage usually add 6–15% to aircraft weight (Hu et al., 2009). Most of those factors vary from flight to flight, are largely unknown and may have direct implications for reduced thrust during take-off. In fact, it should be inferred that the increase of the aircraft weight has direct effects upon the thrust levels needed for carrying out usual LTO operations. For example, Carslaw et al. (2008) studied the NOx emissions at London Heathrow and found evidence for statistically significant differences in the emissions from the same engine type used on the same aircraft frame. Among other factors, they speculated that the aircraft weight could be a cause. In a study conducted in eight major busy airports, Turgut and Rosen (2010) detected significant differences in the emissions of some pollutants and concluded that every airport has LTO cycles carried out by aircraft with different characteristics and, consequently, emissions. Another recent study by Turgut et al. (2013) showed a good relationship between aircraft mass and the NOx emission during take-off and climb, which supports the concept of an explicit relationship between the aircraft weight and emissions. There is a general lack of knowledge about the relationships between aircraft mass and emissions, although some recent studies have indicated that heavier aircraft also emit more particles (Zhu et al., 2011).
Recent studies assessing airport emissions have proposed and used LTO cycles which are much more complex than those standardised by the ICAO. For example, in a study of the air quality and public health impacts of UK airports, Stettler et al. (2011) used specific TIMs derived from Watterson et al. (2004) and Underwood et al. (2004) composed of 12 phases, namely approach, landing roll, reverse thrust, taxi-in, taxiway acceleration, APU, taxi-out, taxiway acceleration, hold, take-off, initial climb and climb-out. Proposed TIMs were developed by analysing the common procedures of an A320 aircraft at London Heathrow, but may vary by aircraft size category. Other studies (e.g., Ratliff et al., 2009), used models, such as the Emissions and Dispersion Modelling System (EDMS), which also requires jet fuel quality data, main engine and APU specifications, aircraft weight and ground operating time to generate more reliable emission estimates.
4.2.4. The emission indices (EIs)
The emissions during standardised LTO cycles are then reported as emission indices (EIs) expressed as mass of pollutant emitted per unit mass of fuel burned. Fuel-based emission indices for the compound X are calculated according to:
where Fc represents the stoichiometric calculation of CO2 produced per kilogram of fuel consumed (with units g CO2 kg Fuel−1) assuming complete combustion and given a particular hydrogen to carbon ratio (e.g., Herndon et al., 2004). Mx and are the molecular weights of the compound X and CO2, respectively, and ΔX and ΔCO2 are the enhancements of compound X and CO2 within the plume, respectively (e.g., Anderson et al., 2006). Unless specified differently, by convention EI(NOx) is defined in terms of NO2 and therefore the mass of NOx emissions is:
where M(NO2) and M(NO) are the molecular weights of NO2 and NO, respectively. In a similar way it should be specified that EI(hydrocarbons) is often referenced to methane (Wahner et al., 1995). ICAO maintains a databank of engine certification data for commercial aviation reporting EIs for the four selected pollutants (EASA, 2013). Emissions of a pollutant X from an engine can be therefore calculated using three parameters: the first two are provided by the ICAO databank and are the main engine EI(X) and the engine fuel flow, i.e., the burned fuel at a defined power setting (expressed as kg s−1); the third parameter is the time-in-mode (TIM), i.e. the time the engines spend at an identified power setting (ICAO, 2011):
Analogous to the EI for the emitted pollutant, emission indices for the number of particles have been commonly reported in the literature. For convention, they are here reported as EI(#).
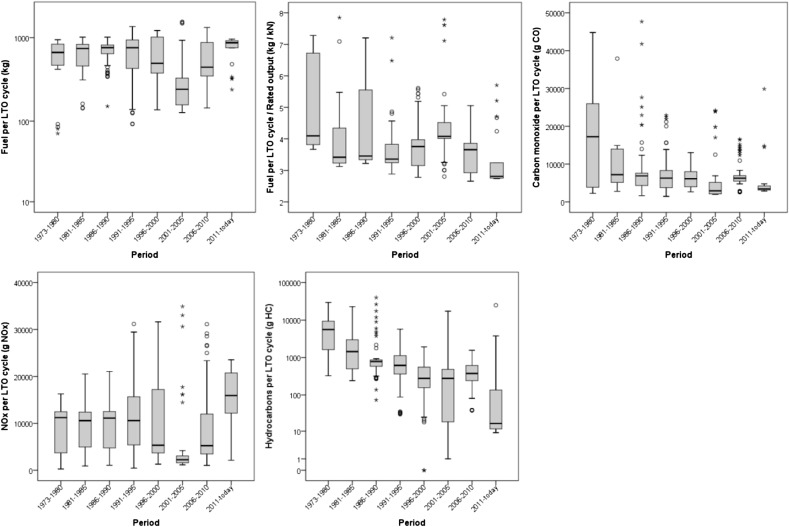
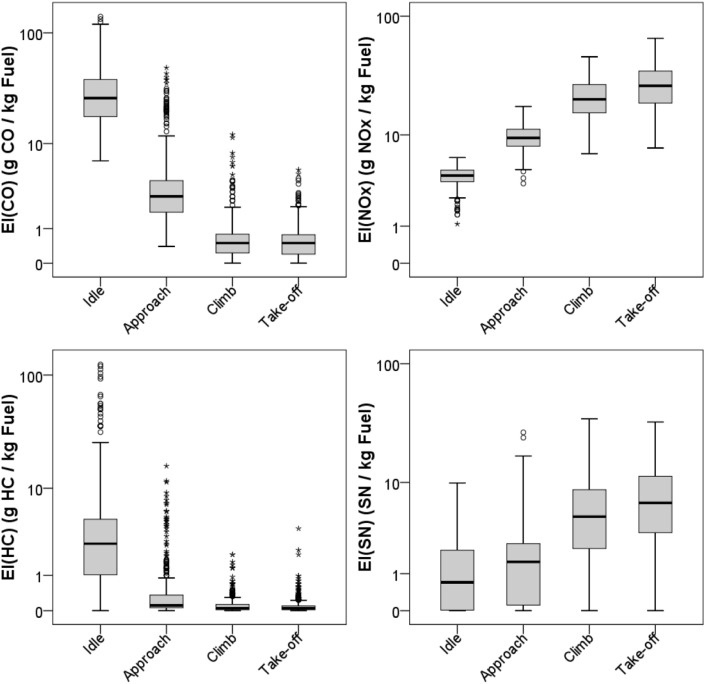
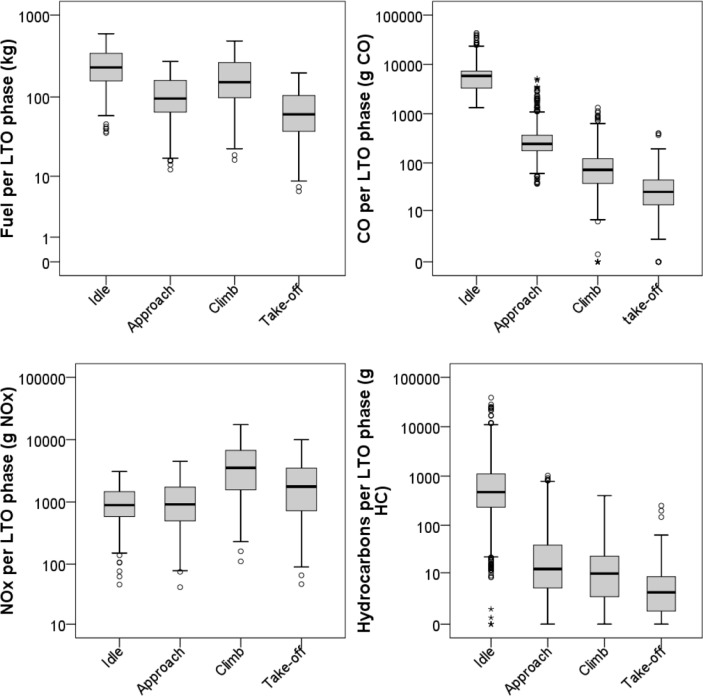
Using ICAO EIs and standardised LTO TIMs, Fig. 6, Fig. 7, Fig. 8 report a reprocessing of the data included in the ICAO databank. In particular, Fig. 6 shows the total burned fuel and the mass of emitted pollutants (CO, NOx and hydrocarbons) during a complete LTO cycle, i.e. the sum of standardised time in each mode per fuel flow per average EI at each of the four power settings (ICAO, 2013); data are organised to show the changes in the ICAO emission data for in-use engines certified from 1973 to present (five year steps). Since different engines have different characteristics, including the thrust force, Fig. 6 also shows the ratios between the fuel burned during complete LTO cycles and the engine maximum rated thrust (in kN) to normalise the fuel consumption of the engine power. Fig. 7 summarises the ICAO EI data (all in-use engines certified from 1976 to today) per each LTO stage, expressed as g pollutant emitted per kg fuel burned. Fig. 8 shows the total burned fuel and emissions per each LTO phase, i.e. the product of EIs per standardised time in each phase per fuel flow. The reprocessing of ICAO data does not take into account the number of units produced for each engine model, but only the different models produced and still in service in April 2013 (and included in the ICAO databank), regardless of manufacturer, type and technology. Moreover, data refer to single engines, and generally conventional aircraft are equipped with 1–4 engines. Therefore the sole purpose of the reprocessing of ICAO data is to report qualitatively the trends in fuel consumption and emissions for in-use TF engines.
Fig. 6.
Burned fuel and emissions for complete standardised LTO cycle. Data from ICAO databank at April 2013 (EASA, 2013). All engines certified in each period were included in the statistics, without distinction of type, manufacturer, model or technology.
Fig. 7.
EIs provided by the ICAO databank (EASA, 2013). All in-use engines certified from 1976 to today (April 2013) are included.
Fig. 8.
Fuel burned and emissions of CO, NOx and total unburned hydrocarbons during the four LTO phases. Data were calculated from the EIs and fuel consumption provided by the ICAO databank (EASA, 2013). All in-use engines certified from 1976 to today (April 2013) were included and reprocessed as a function of LTO stages and standard times (i.e., 0.7 min for take-off, 2.2 min for climb-out, 4 min for approach and 26 min for idle).
Currently, the scientific literature includes several studies aiming to give EIs for comparison with reported ICAO databank certification data and for many other components, including particulate matter, elements, ions and speciated hydrocarbons. However, such data are often sparse and results poorly comparable. Most studies were carried out using single or a few engine types, under certain environmental conditions, without a standardised thrust and/or often using different measurement techniques and instrumental set-up. Table 3 lists the most recent studies available in the literature reporting EIs for various engines in aircraft and helicopters. The table also shows some information (if available) about tested aircraft, engine models, selected thrust, type of fuel, sampling methodologies and analytical techniques. Table 4 provides a list of recent studies which measured EIs during real aircraft operations at airports. Most of the data in such studies (both engine tests and real world operations) are summarised in the Supplemental Information Tables SI1, SI2, SI3 and SI4, which provide detailed information about the EIs for many gaseous pollutants, speciated hydrocarbons, particle number, particle mass (including soot) and species/ions in particulate matter, respectively. Note that specific thrust levels provided in the tables are derived from the literature and are categorised in five groups, named idle, approach, cruise, climb and take-off, on the basis of the engine type. The thrust, expressed as F00, is always provided along with the EIs. Additional tested thrust levels (if available) are also reported, along with fuel and analytical methodologies.
Table 3.
List of recent studies in the literature that measure EIs directly from engine or airplane tests. The table also reports studies on hydrocarbon profiles. Some information about tested aircraft and engine models, selected thrust and sampling methodologies and analytical techniques, type of fuel, date and location of experiments is also given.
| Airframe/Engine | Analysed compounds | Sampling and experimental (Sampling system [analytical methods]) | Tested regimes and [fuels] | References |
|---|---|---|---|---|
| F101 (Military TF with reheat used on the B-1B aircraft); F110 (Military TF with reheat used on the F-16C and F-16D aircraft) | CO2, CO, NOx, total hydrocarbons, individual organic species | Samples collected from each engine using a probe positioned just behind the exhaust nozzle | Four power settings from idle to intermediate power | Spicer et al. (1992) |
| TF-39 (Military TF of Lockheed C-5) and CFM-56 (TF) | CO, NO, NOx, total hydrocarbons, C2 to C17 organics, PAHs, aldehydes | Sampling: sampling rake behind the engine. Experimental: non-dispersive infrared instruments, chemiluminescence, FID, polymeric adsorbent (XAD) and DNPH cartridges[GC/MS, GC/FID], On-Line Cryogenic Trap/GC, canister [GC/MS], Total Hydrocarbon Analyzer | Idle, 30%, 80%; [JP-4; JP-5; JP-8] | Spicer et al., 1984, Spicer et al., 1994 |
| PW 305 (TF in small business jets) | N2O, CH4 | Sampling: gas samples collected in the core of the engine without any bypass air. Experimental: infrared absorption spectroscopy | 5.5%; 23.5%; 33.4%; 71.4%; 95.6% | Wiesen et al. (1994) |
| Various military aircraft: T56-A-7; TF39-GE-1C; GTCP85-180; GTCP-165-1; T700-GE-700; J69-T-25; J85-GE-5A; F110-GE-100; F108-CF-100; TF33-P-7/7A; F101-GE-102; TF33-P-102; F117-PW-100; AFB F118-GE-100; F404-GE-F102/400; F110-GE-129; F100-PW-100; F100-PW-229; T64-GE-100; TF34-GE-100A (All Military) | CO2; CO; NOx; NMHCs; Aldehydes and ketones; VOCs; filterable and condensable particulate | Sampling: various test cells, hush house exhaust rate determined using three methods: carbon balance, tracer gas and F-factor. Experimental: various US-EPA' methods, including continuous emissions monitoring system; canister [GC/MS; GC/FID]; HI-VOL [lab analysis] | Idle; Approach; Intermediate; Military; Afterburner; [JP-8] | Gerstle et al. (1999) |
| Research aircraft: VFW-Fokker 614 ATTAS. Engine: Rolls-Royce/SNECMA M45H Mk501 (TF) | Aerosol size distribution and chemical composition (total carbon, BC) | Sampling: ground-based measurements (also report in-flight measurements). Experimental: filter substrates[thermal technique], PCASP-100X | Different engine thrust levels: idle run and take-off | Petzold and Schröder, 1998, Petzold et al., 1999 |
| Fighter aircraft: F-22 Raptor (Military); Engine: F119-PW-100 (TF with reheat) | CO2; CO; NOx; NMHCs; Filterable and condensable particulate; Aldehydes and ketones; VOCs | Sampling: engine exhaust sampling rake system; augmentor tube slipstream sampling system. Experimental: various US-EPA' methods: continuous emissions monitoring system; canister [GC/MS; GC/FID]; HI-VOL [lab analysis] | Idle (10%); approach (20%); Intermediate (70%); Military (100%); Afterburner (150%); [JP-8] | Gerstle et al. (2002) |
| NASA Boeing 757; Engine: RB-211-535E4 (TF) | CO2, H2O, HONO, HNO3, SO2, SO3, H2SO4, nonmethane hydrocarbons, aerosol size, BC | Sampling: 1 m down steam of the turbine exhaust, aerosol-sampling probe was also affixed to the blast fence 25 m downstream of the engine exhaust plane. Experimental: IR spectrometer, DMA, OPC, aethalometer, grab samples, tunable diode laser, AMS | A range of power settings from idle to near take-off thrust; [JP-5, low and high S (810 and 1820 ppm S)] | EXCAVATE: Anderson et al., 2005, Anderson et al., 2006 |
| Jet trainer: T-38A Talon; Engine: 85-GE-5A (TJ) | CO2, aerosol size, BC, nonmethane hydrocarbons, SO2, CO2, SO3, H2O, HONO, H2SO4, HONO, HNO3 | Sampling: 1 m down steam of the turbine exhaust. Experimental: IR spectrometer, DMA and OPC, aethalometer, grab samples, tunable diode laser, AMS | A range of power settings from idle to near take-off thrust; [JP-5 (810 ppm S)] | EXCAVATE: Anderson et al. (2005) |
| Fighter: F-18 (Military). Engine: F404-GE-400 in twin-engine (TF with reheat) | Particle mass concentration, PAHs, BC | Sampling: Navy jet engine exhaust emissions from tethered aircraft, measurements at a site on the active flightline tarmac, directly from the exhausts of tethered aircraft. Experimental: DustTrak particle mass monitor, PAS, photoacoustic analyzer, Gundel denuder sampler (with PUF/XAD/PUF “sandwich” cartridges), SMPS, MOUDI cascade impactor | Power-setting increases from 65% to 70%, and from 70% to 80% | Rogers et al. (2005) |
| Engine: dismounted T700-GE-401 (TS), which is fitted in Seahawk, Super Cobra, and Jayhawk helicopters (Military) | Particle mass concentration, PAHs, BC | Sampling: Navy jet engine exhaust emissions from engine maintenance test cells, measurements at Aircraft Intermediate Maintenance Department facility. Experimental: DustTrak particle mass monitor, PAS, photoacoustic analyzer, Gundel denuder sampler (with PUF/XAD/PUF “sandwich” cartridges), SMPS, MOUDI cascade impactor | Power-setting increases from idle to 98% | Rogers et al. (2005) |
| NASA Boeing 757; Engine: RB211-535-E4 (TF) | Gaseous carbon species | Sampling: 10 m behind the engine exit plane. Experimental: Canister, analyses of whole air samples [GC/FID, GC/ECD, GC/MS] | 4–7%; 26%; 47%; 61%; [JP-5 low and high S] | EXCAVATE: Anderson et al. (2006) |
| Bell helicopter; UH-1H (TS) | 22 PAHs | Sampling: engine placed in a testing chamber, exhaust samples collected from the stack of the chamber using an isokinetic sampling system. Experimental: GC/MS | Five power settings: idle (50%), fly idle (67%), beed band check (79%), inlet guide vane (95%), and takeoff (100%); [JP-4] | Chen et al. (2006) |
| Military jet fighters: F-15 Eagle and the F-16 Falcon aircraft. Engines: PW F-100-PW-100 (TF with reheat) | Automatic measurements: CO2, CO, NO, NO2, total hydrocarbons | Sampling: extractive sampling at 23 m behind the exhaust exit plane for tests at idle through military power, and at 38 m for afterburner tests; optical remote sensing measurements 23 m behind the engine exit plane. Experimental: automatic measurements; canisters [GC/MS]; DNPH-coated cartridges [HPLC/UV detector]; OP-FTIR; UV-DOAS | Ground idle (65–70%), low intermediate (80%), high intermediate (85%), military (91–93%) and afterburner (reheat); [JP-8+100] | Cowen et al. (2009) |
| Aircraft: Boeing DC-8. Engine: CFM-56-2C1 (TF) | CO, CO2, NO, NO2, HONO, total VOCs, gas-phase speciated hydrocarbons, particle number concentration, particle size distribution, PM2.5 [mass, EC/OC, SVOCs, inorganic ions, elemental composition] | Sampling: the exhaust plume was sampled at 1, 10 and 30 m downstream of the engines. Experimental: continuous and time-integrated instruments: IR absorption, TILDAS, PTR-MS, AMS, canister [GC/MS, GC/FID], DNPH cartridges [HPLC], TEOM, CPC, SMPS, DMA, PM-2.5 cyclones [47 mm PTFE filter], PM-2.5 cyclones [47 mm QFF + PUF], ELPI, aethalometer, PAH analyzer; lab analyses on filters and PUF [GC/MS, TOA@NIOSH, ion chromatography, XRF] | “EPA test matrix” (typical LTO); “NASA test matrix” including 11 power settings; [3 fuels: base fuel, high sulphur (1639 ppm), high aromatic] | APEX-1: Wey et al., 2006, Knighton et al., 2007, Wormhoudt et al., 2007, Yelvington et al., 2007, Wong et al., 2008; Onasch et al. (2009); Kinsey (2009) |
| Aircraft: B737-700; B737-300. Engines: CFM56-7B24, CFM56-3B1, CFM56-3B2 (all TF) | CO2, gas-phase speciated hydrocarbons, particle number concentration, particle size distribution, PM2.5 [mass, EC/OC, SVOCs, inorganic ions, elemental composition, PAHs] | Sampling: on-wing at the ground run-up enclosure; 1, 30 and 54 m from the exhaust nozzle exit. Experimental: continuous and time-integrated instruments: IR absorption, canister [GC/MS, GC/FID], DNPH cartridges [HPLC], TEOM, CPC, SMPS, EEPS, DMA, PM-2.5 cyclones [47 mm PTFE filter, 47 mm QFF + PUF], ELPI, aethalometer, PAH analyzer; lab analyses on filters and PUF [GC/MS, TOA@NIOSH, ion chromatography, XRF], AMS | 4%, 7%, 30%, 40%, 65%, 85%; [Jet-A] | APEX-2: Agrawal et al., 2008, Kinsey, 2009, Timko et al., 2010b, Timko et al., 2010c |
| Aircraft: B737-300, Embraer ERJ-145, A300, B775, plus Learjet Model 25. Engines: CFM56-3B1, AE3007A1E, AE3007A1/1, PW4158, RB211-535E4-B (all TF), plus CJ610-8ATJ (TJ) | CO2, gas-phase speciated hydrocarbons, particle number concentration, particle size distribution, PM2.5 [mass, EC/OC, SVOCs, inorganic ions, elemental composition] | Sampling: the exhaust plume was sampled at a location 1, and 30 m downstream of the engines (sometimes at 15 and 43 m); Sampling was done at the centre-line using a single probe. Experimental: continuous and time-integrated instruments: IR absorption, TILDAS, quantum cascade-TILDAS, canister [GC/MS, GC/FID], DNPH cartridges [HPLC], TEOM, CPC, SMPS, EEPS, DMA, PM-2.5 cyclones [47 mm PTFE filter, 47 mm QFF + PUF], ELPI, aethalometer, PAH analyzer; lab analyses on filters and PUF [GC/MS, TOA@NIOSH, ion chromatography, XRF], AMS | 4%, 7%, 15%, 30%, 45%, 65%, 85%, 100% [slightly varying for some engines, see Kinsey (2009)]; [Jet-A] | APEX-3: Knighton et al., 2007, Kinsey, 2009, Timko et al., 2010b, Timko et al., 2010c |
| Military helicopters: Blackhawk, Apache: T700-GE-700 and T700-GE-701C (TS) | CO2, H2O, CO, NO, and N2O (FTIR); particle number, mass and size distributions, smoke number (automatic); elements, ions, EC, OC (on PM filters) | Sampling: extractive sampling at the engine nozzle, plus extractive sampling (4.14 m) and remote-sensing at a predetermined distance downstream of the engine exhaust plane. Experimental: FTIR, TDLAS, UV DOAS, OP-FTIR; CPC, DMA, SMPS, TEOM, smoke machine, sandwiched PM1 impaction-style sampler [XRF, ion chromatography, TOA@NIOSH] | Idle, 75%, max; [JP-8, FT] | Cheng, 2009, Cheng et al., 2009, Cheng and Corporan, 2010 |
| Military transport (cargo) aircraft: Lockheed C-130 Hercules. Engine: T56-A-15 (TP) | CO2, H2O, CO, NO, and N2O (FTIR); particle number, mass and size distributions, smoke number (automatic); elements, ions, EC, OC (on PM filters) | Sampling: at the engine exit plane and at 5 and 15 m downstream of the engine exit. Experimental: remote sensing: FTIR, TDLAS, UV DOAS, OP-FTIR; Extractive measurements: on-line gas analyzer, cross-filter correlation spectroscopy, chemiluminescence, CPC, SMPS, TEOM, smoke machine, PM1 sampler [XRF, ion chromatography, carbon analyzer] | Low speed ground idle (4%); high speed ground idle (7%); flight idle (20%); cruise (41%); max (100%); [JP-8, FT] | Cheng et al., 2008, Corporan et al., 2008, Cheng, 2009, Cheng and Corporan, 2010 |
| Military bomber: B-52. Engine: TF33-P-3/103 (TF) | CO2, H2O, CO, NO, and N2O (FTIR); particle number, mass and size distributions, smoke number (automatic); elements, ions, EC, OC (on PM filters) | Sampling: extractive sampling at the engine nozzle, plus extractive sampling and remote-sensing at a predetermined distance downstream of the engine exhaust plane. Experimental: FTIR, TDLAS, UV DOAS, OP-FTIR; CPC, SMPS, TEOM, smoke machine, PM1 sampler [XRF, ion chromatography, carbon analyzer] | TF33 (idle, 80%, 90%, 95%); [JP-8, FT] | Cheng, 2009, Cheng and Corporan, 2010 |
| Update and consolidation of the existing HAPs profile using data from Spicer et al. (1994), EXCAVATE and APEXs campaigns | Hydrocarbons, EIs and profiles (mass fraction) | Data analysis | Various | Knighton et al. (2009) |
| Military transport (cargo) aircraft: Lockheed C-130 Hercules. Engine: Allison T56 (TP) | CO2, CO, NOx, total hydrocarbons, organic gases including carbonyls | Experimental: non-dispersive IR, cross-filter correlation spectroscopy, chemiluminescence, FID, PTR-MS, canister [GC/MS], DNPH cartridges [HPLC] | Low speed ground idle, High speed ground idle, Flight idle Cruise, Maximum power; [JP-8] | Spicer et al. (2009) |
| Jet fighter: F-15. Engine: PW F100-PE-100 (TF with reheat) | CO2, CO, NOx, total hydrocarbons, organic gases including carbonyls | Experimental: non-dispersive IR, cross-filter correlation spectroscopy, chemiluminescence, FID, PTR-MS, canister [GC/MS], DNPH cartridges [HPLC] | Idle, Low intermediate, High intermediate, Military, Afterburner; [JP8+100] |
Spicer et al. (2009) |
| Summary of the APEX1‒3 campaigns: CFM56-2C1, CFM56-7B24, CFM56-3B1, CFM56-3B2, AE3007A1E, AE3007A1/1, P&W 4158, RB211-535E4-B (all TF), and CJ610-8ATJ (TJ) | Physical and chemical characterisation of PM; PM mass, particle number concentrations and size, BC, surface-bound PAHs; inorganic ions, EC, OC, SVOCs, elements | As for APEX1‒3 campaigns | LTO and others | Kinsey et al., 2010, Kinsey et al., 2011 |
| Pratt & Whitney; PW three high-bypass TF, representing two different distinct engine model types | Total particulate mass, chemical composition and size distributions of the emitted oil | Sampling: Particulate matter emitted from the lubrication system overboard breather vent with a self-designed collecting and diluting apparatus. Experimental: C-TOF AMS, TEOM, engine exhaust particle sizer, CPC and ultra high sensitivity aerosol spectrometer | Cycles from idle to 65–70% thrust | Yu et al. (2010) |
| NASA DC-8; CFM56-2C1 (TF) | CO2, CO, NOx, SO2, CH4, N2O, HONO, total and speciated hydrocarbons, hazardous air pollutants; particle measurements included number density, size distribution, mass, aerosol chemical composition, and black carbon composition | Sampling: from inlet probes positioned 1 and 30 m downstream of the aircraft's engines; aged plumes at 145 m away from the engine output in the direction of the predominant wind, 1.3 m above the ground. Experimental: NDIR, CPC, SMPS, EEPS, DMS, MAAP, PAS 2000, AMS, CCN, TILDAS, PTR-MS, conventional gas analyzers, TEOM | 7 thrusts: LTO +4%(idle); 45%(approach); 65%(cruise); [JP-8, FT (Shell), FT (Sasol)] | AAFEX: Anderson et al., 2011, Santoni et al., 2011 |
| KC-135T Stratotanker (Military); CFM56-2B1 (TF) | CO2, CO,O2, NOx, total hydrocarbon; PM, particle number concentration and size (after exhausts dilution in smog chamber) | Sampling: exhaust sampled using a rake inlet installed 1 m downstream of the engine exit plane; a dilution sampler and portable smog chamber were also used. Experimental: five-gas exhaust gas analyzer; canister [GC/MS], PM2.5 cyclone[QFF and PTFE filters, Tenax TA sorbent, GC/MS, OC/EC analyzer], SMPS, AMS | 4%, 7%, 30%, 85%; [JP-8] | Presto et al., 2011, Miracolo et al., 2011 |
| Helicopters; Allison T63-A-700 (TS) | CO2, CO, NOx, CH4, and C2H4, unburned hydrocarbons, number and size of particles, BC | Samples were extracted from the engine exit plane via temperature-controlled probes, charcoal tubes, DNPH tubes; NDIR, FTIR, FID, CPC, SMPS, MAAP, GC/MS | 3% (low-speed idle), 7% (high-speed idle), 15% (intermediate), 85% (cruise); [JP-8, a synthetic paraffinic kerosene, and four two-component surrogate mixtures] | Cain et al. (2013) |
Used acronyms: AMS = aerosol mass spectrometer; BAM = beta-attenuation mass monitor; CPC = condensation particle counter; C-TOF AMS = time-of-flight aerosol mass spectrometer; DMA = differential mobility analyser; EEPS = engine exhaust particle sizer; ELPI = electrical low pressure impactor; FTIR = Fourier transform infrared spectroscopy; GC/ECD = gas chromatography/electron capture detector; GC/FID = gas chromatography/flame ionization detector; GC/MS = gas chromatography/mass spectrometry; HI-VOL = high volume PM sampler; LIDAR = laser interferometry detection and ranging; MAAP = multi-angle absorption photometer; NDIR = non-dispersive infrared spectroscopy; OPC = optical particle counting and photometry; OP-FTIR = open-path Fourier transform infrared spectroscopy; PAS = photoelectric aerosol sensor; PTFE = Teflon; PTR-MS = proton-transfer reaction mass spectrometry; QFF = quartz fibre filter; SEM/EDX = scanning electron microscopy/energy-dispersive X-ray spectroscopy; SMPS = scanning mobility particle sizer spectrometer; TDLAS = tunable diode laser absorption spectroscopy; TEOM = tapered element oscillating microbalance; TF = turbofan; TILDAS = tunable infrared differential absorption spectroscopy; TJ = turbojet; TOA = thermo-optical OC–EC analyzer (@used method); TP = turpoprop; TS = turboshaft; UV-DOAS = UV differential optical absorption spectroscopy; VOC = volatile organic compounds; XRF = X-ray fluorescence spectroscopy.
Table 4.
List of recent studies available in the literature reporting EIs during real aircraft operation. The table also reports supplementary information (if available) about the target of the study, period and location of experiments, tested aircraft or engine models, measured pollutants, analysed LTO phases and sampling methodologies. The list of acronyms is provided in Table 3.
| Target; period; airport | Analysed compounds | Sampling; analytical | Engine thrusts (if know) or LTO phases | References |
|---|---|---|---|---|
| In service military and civil aircraft at various airports | CO2, H2O, CO, NO, N2O | Measurements performed at distances of 20–40 m to the nozzle exit perpendicular to the exhaust flow via ground-based FTIR analysis | Various thrusts | Heland and Schäfer, 1997, Heland and Schäfer, 1998 |
| Various (90) in service aircraft: from gulfstream executive jets to Boeing 747-400s at London Heathrow Airport (UK) | CO2, CO, NO, hydrocarbons | The remote sensor positioned at ground level. Experimental: non-dispersive IR spectroscopy, dispersive UV spectrometer | Mix of idle, taxi-out and take-off modes | Popp et al. (1999) |
| Emission indices of different aircraft engines using non-intrusive measurements at Frankfurt/Main (GER), London–Heathrow (UK), Vienna (AT) airports | CO2, CO, NO, NO2, ethene, ethine, formaldehyde | Open paths of 80 up to 150 m length were installed in parallel directly behind the aircraft. Experimental: FTIR with MIDAC spectrometer, FTIR with K300 spectrometer, DOAS | Aircraft operating conditions, idling aircraft | Schäfer et al. (2003) |
| 30 individual planes, ranging from TP to jumbo jets; August 2001; J.F. Kennedy Airport (USA) | CO2, NO, NO2 | Measurements within 350 m of a taxiway and 550 m of a runway. Experimental: automatic (IR), TILDAS | Taxiway thrust and take-offs | Herndon et al. (2004) |
| In-use commercial aircraft; period: 2001–2003; Airports: J.F. Kennedy airport in New York City and Logan airport in Boston (USA) | Particulate matter, number concentration and size distributions | Extractive sampling of the advected plumes of aircraft using a novel approach, 200 m of an active taxiway and runway. Experimental: ELPI, CPC | Several different types of plumes were sampled, including approach (landing) and engine start-up in addition to idle, taxi, and take-off | Herndon et al. (2005) |
| 45 intercepted plumes identified as being associated with specific aircraft: regional jets, B737s, MD88s, and B757s; Period: May 2003; Logan airport in Boston (USA) | CO2; Formaldehyde, acetaldehyde, benzene, and toluene, as well as other hydrocarbon species; NOy | Ambient air is continuously analysed through a sample port located near the roof on the front of the truck. Experimental: IR, PTR-MS; TILDAS; total reactive nitrogen instrument | Idle, taxi, approach (or landing), and take-off, as well as engine-start modes | Herndon et al. (2006) |
| Real time data at Los Angeles International Airport (USA); Period: September 23–29, 2005 | UFPs (diameter <100 nm), black carbon, PM2.5 mass, and chemical species (PAHs, butadiene, benzene, acrolein, formaldehyde) | At blast fence (140 m from the take-off) and five downwind sites up to 600 m from the take-off runway. Experimental: SMPS (DMA/CPC), aethalometers, E-BAM, automatic PAHs analyzer, canister, cartridge | – | Fanning et al. (2007); Zhu et al. (2011) |
| Impact of airport emissions at Zurich–Kloten airport (Switzerland); Period: June 2004 to July 2004 | NO, NO2, CO, CO2, VOCs | Measurements with in-situ and open-path devices; COV samples taken directly within the plume of the engine, about 50–100 m behind an aircraft, at a height of 1 m. Experimental: FTIR; DOAS; canister [GC/FID] | – | Schürmann et al. (2007) |
| Emissions from in-use commercial aircraft engines analysed using continuous extractive sampling and associated with specific engine using tail numbers; Period: September 2004; Location: Hartsfield–Jackson Atlanta International Airport (USA) | CO2, CO, NO, NO2, formaldehyde, particle number, BC, particle size, mass-based composition | Two mobile laboratories located downwind of active runways. Experimental: Automatic (IR); TILDAS; CPC; MAAP; SMPS; DMS; AMS | Various | JETS/APEX-2 campaign: Herndon et al. (2008) |
| Plume characterisation from commercial aircraft at Brisbane Airport (AUS) | CO2, SO2, NOx, particle mass, number concentration and size | Plume capture and analysis system mounted in a four-wheel drive vehicle positioned in the airfield 60–180 m downwind of aircraft operations. Experimental: CPC, SMPS, NOx analyzer, aerosol photometer fitted with a PM2.5 impactor |
Normal airport operations, taxiing phase | Johnson et al. (2008) |
| In-use commercial airfreight and general aviation at Oakland International Airport (USA); Period: August 20–29, 2005; | Formaldehyde, acetaldehyde, ethene, propene, and benzene | At the end of an active taxiway next to the main runway. Data collected on an ambient sampling manifold consisting of a 3.8 cm diameter tube, ∼7 m long drawing ∼150 slpm. Experimental: TILDAS; proton transfer reaction mass spectrometer measurements | Idle (taxiway/runway) | JETS/APEX-2 campaign: Herndon et al. (2009) |
| Real world conditions, 280 individual aircraft at Brisbane Airport (AUS) | Particle number concentration, size and mass (PM2.5), CO2, NOx | 80 m from the aircraft using a novel mobile measurement system. Experimental: CPC, SMPS, NOx analyzer, aerosol photometer fitted with a PM2.5 impactor | Various modes of LTO cycles including idle, taxi, landing, and take-off | Mazaheri et al. (2009) |
| In-use commercial aircraft at Chicago Midway Airport and O'Hare International Airport (USA); Period: February 2010 | CO, NO, NOx, oil leaks | Mobile laboratory located at downwind locations to monitor air advected from the active taxiways (30−150 m). Experimental: TILDAS; HR-ToF AMS; MAAP, CPC | – | Yu et al. (2012) |
| Emission of Roanoke Regional Airport in Virginia (USA); Period: July 2011–February 2012 | CO2, NOx, particle number, BC | A mobile eddy covariance laboratory with a mast extending nearly 15 m above ground level and placed near active runways. Experimental: automatic devices, CPC, aethalometer | Idle/taxi and take-off | Klapmeyer and Marr (2012) |
| Real-time measurements of aircraft engine specific emissions at Oakland International Airport (USA); Period: August 26, 2005 | CO2, particle number concentration, size distributions, PM mass | 100–300 m downwind of an active taxi-/runway. Experimental: Automatic IR, Combustion DMS500, CPC, SMPS, MAAP | Normal LTO operations | Lobo et al. (2012) |
4.2.5. Considerations about the EIs
As indicated by the large number of studies in Table 3, Table 4, most of the literature provides results through the calculation of EIs. When applied to the specific testing studies on engines or airplanes, such methodology has the advantage of giving data easily comparable with EIs reported in the ICAO databank. This may allow a better evaluation of the differences amongst tested engines and technologies or, in case of the use of innovative analytical devices, allows a check the agreement between data obtained and certified values. In contrast, expressing the results as EIs from studies conducted during real-world operations at airports has both advantages and limitations. An advantage of the specific studies may be comparison of the results with the ICAO data to detect changes due to evolution of the exhaust plume, e.g. aging and gas-to-particle partitioning. Carslaw et al. (2008) noticed that EIs do not give a clear indication of the absolute contribution of aircraft emissions to ground-level concentrations, which is important for assessing air quality at airports. Furthermore, they commented that the value of EIs may be substantially affected by limited knowledge of some important aircraft operational factors, such as the aircraft weight and thrust setting at take-off. A list of remaining studies conducted at airports and in their surroundings, which do not report data expressed as EIs, is provided in Table 5 . In summary, Table 3, Table 4, Table 5 provide an overview of the most important studies reported in this review for the characterisation of aircraft emissions in both tests and real operations.
Table 5.
List of recent studies available in the literature conducted at airports or in their surroundings. The table also reports supplementary information (if available) about the target of the study, period and location of experiments, tested aircraft or engine models, measured pollutants, analysed LTO phases and sampling methodologies. The list of acronyms is provided in Table 3.
| Target; period; airport | Analysed compounds | Sampling; analytical | Engine thrusts (if know) or LTO phases | References |
|---|---|---|---|---|
| Air quality data in the vicinity of Hong Kong International Airport (1997–1998) and Los Angeles International Airport (2000–2001) | CO, NOx, SO2, and respirable suspended particles | Data from routine air quality monitoring site and special study | – | Yu et al. (2004) |
| Airport traffic at Heathrow (UK); Period: Jul. 2001–Dec. 2004 | NOx, NO2 | LHR2 site at 180 m north of the northern runway centreline. Experimental: Common automatic devices | – | Carslaw et al. (2006) |
| Ambient air and personal at Fiumicino Airport, Rome (Italy); Period: January–February 2005 | 23 PAHs, urinary 1-hydroxy-pyrene, micronucleus assay, Comet assay, Sister chromatid exchange | Air samples collected from airport apron, airport building and terminal/office area during 5 working days, plus a biomarker of exposure following 5 working day. Experimental: Active ECHO PUF sampler at 35 L/min for the first 20 min and at 120 L/min for the remaining 23 h and 40 min on each day, [GC/MS analysis] | – | Cavallo et al. (2006) |
| Individual plumes from 29 commonly used engines; Period: October 19–November 15, 2005; Location: London Heathrow (UK) | NOx | 180 m from the runway. Experimental: chemiluminescence monitor | – | Carslaw et al. (2008) |
| Analysis of the extent of Los Angeles International Airport emissions on downwind ambient air in a mixed use neighbourhood that includes residences. Period: spring of 2003 |
UFP, BC, NOx, particle-phase PAHs | Data collected at various sites in and around the airport: 500 m upwind of the north runway and downwind of the airport (500 m north and east of the centreline of the north runway; 100 m downwind of the taxiway; 100 m downwind of the south runway; 900 m downwind of the south runway). Experimental: CPC, SMPS, DMA, aethalometer, photoelectric aerosol sensor, NOx analyzer | – | Westerdahl et al. (2008) |
| APEX2-3: Oakland International Airport in August 2005, and Cleveland Hopkins International Airport in Oct–Nov 2005. |
NOx and NOy, including HONO | Panel truck. Experimental: TILDAS; quantum cascade-TILDAS; chemiluminescence analyzer | – | Wood et al. (2008b) |
| Airport traffic at Warwick, Rhode Island (USA); Period: July 2005–September 2006 | BC | Five monitoring sites: 4 close and 1 approx 3.7 km from the airport. Experimental: Continuous with aethalometers | – | Dodson et al. (2009) |
| General aviation and private jets at Santa Monica Airport (USA); Period: Spring and summer 2008 | UFP, PM2.5, BC, particle bound PAHs, CO, NOx, NO, NO2 | Downwind of the airport using an electric vehicle mobile platform equipped with fast response instruments. Experimental: CPC, FMPS, aethalometer, PAS, automatic measurements of gases | Idle/taxi and take-off | Hu et al. (2009) |
| Airport traffic at El Prat, Barcelona (Spain); Period: October 17-November 16, 2007 | PM10, PM2.5 and PM1 continuously; PM10 (EC, OC, SO42−, NO3−, Cl−, NH4+, Al, Ca, K, Mg, Fe, S, Na, As, Ba, Bi, Cd, Ce, Co, Cr, Cs, Cu, Ga, Hf, La, Li, Mn, Mo, Nb, Ni, P, Pb, Rb, Sb, Sc, Se, Sn, Sr, Th, Ti, Tl, U, V, W, Y, Zn, Zr) | Mobile laboratory van at about 130 m from the major runway. Experimental: PM10, PM2.5 and PM1 with laser-spectrometer dust monitors and PM10 on QFF using HI-VOL sampler | Take-off, sometimes landing | Amato et al. (2010) |
| Commercial aircraft; Period: 10–20 May 2005; Airports: Manchester and London Heathrow (UK) | Dispersion of exhaust plumes | Rapid-scanning LIDAR system installed at ground 200–330 m on the sides of runways | All modes were observed: taxiing, take-off, rotation, climb-out, approach, and landing. Landing tyre smoke | Bennett et al., 2010, Bennett and Christie, 2011 |
| Commercial airliners at London Heathrow (UK): A320 232; B757 236; B747 436) | PM elemental composition, particle size spectrum | Samples of dust from the undercarriage. Experimental: SEM/EDX; aerosizer/aerodisperser | – | Bennett et al. (2011) |
| Ambient air and personal at the Teterboro Airport, New York/New Jersey metropolitan area (USA); Period: Summer 2006 and winter 2006–2007; | BTEX | At 15 households located close to the airport (indoor, outdoor, and personal), at the end of airport runways and an out-of-neighbourhood location. Experimental: Passive samplers (48 h) [GC/MS] | – | Jung et al. (2011) |
| High-resolution monitoring and flight activity data to quantify contributions from LTO at T.F. Green Airport in Warwick (USA). Period: 2007–2008 | Particle number concentration | Four stationary monitoring sites around the airport. Experimental: CPC | Various LTO phases, especially departures | Hsu et al. (2012) |
| Aircraft emissions and local air quality impacts from take-off activities at Los Angeles International Airport (USA). Periods: September 2005; Feb–Mar 2006; May 2006 | Particle number concentrations and size distributions, and time integrated black carbon, PM2.5 mass, and chemical species | Data collected at the blast fence (∼140 m from the take-off position) and 5 sites located downwind, up to 600 m from the take-off runway and upwind of a freeway. Experimental: CPC, SMPS, aethalometers, BAM, PAH Tisch Sampler, canister and cartridge samplers[lab analysis] | Taxi-way and take-off operations | Zhu et al. (2011) |
| Contributions of aircraft arrivals and departures to UFP at Los Angeles International Airport (USA). Period: summer 2008 | Particle number concentration | Five sites around the airport. Experimental: Fast Mobility Particle Sizer | LTO phases: aircraft arrivals and departures | Hsu et al. (2013) |
4.3. Emissions at cruise altitudes
Although injected at high altitudes, aircraft cruise emissions have been found to impact surface air quality through the mean meridional streamlines due to the polar, Ferrel, and Hadley cells (Barrett et al., 2010, Barrett et al., 2012) and they are not currently regulated. Consequently, although this review focuses on airport emissions, a brief statement upon the aircraft emissions during cruise (8–12 km) is presented, as the majority of exhaust from aircraft is emitted at high altitudes (e.g., Gardner et al., 1997, FAA, 2005, Wilkerson et al., 2010, Whitt et al., 2011). A more exhaustive summary of the effects of both civil (subsonic) aviation in the upper troposphere and supersonic aircraft in the stratosphere is reported in two reviews by Lee et al., 2009, Lee et al., 2010.
Impacts of aviation during cruising first focused the interest of the scientific community in the late 1960s in relation to contrail generation at high altitudes and the relative effect on climate (Reinking, 1968, Kuhn, 1970). Contrails are formed whenever the requisite conditions of either ice or water supersaturation exist within aircraft exhaust plumes (DeWitt and Hwang, 2005). Subsequently, in the early 1970s, concern grew over a possible role in stratospheric ozone depletion while interest in the impact of nitrogen oxide emissions on the formation of tropospheric ozone began in the late 1980s (Lee et al., 2009, and references therein). Subsequent studies (e.g., Wahner et al., 1995, Brasseur et al., 1996, Schumann, 1997) investigated a number of emissions other than CO2, and effects from aviation with potential effects on climate. To date there are a large number of studies characterising aircraft emissions during cruising (e.g., Fahey et al., 1995a, Fahey et al., 1995b, Busen and Schumann, 1995, Schumann et al., 1996, Schlager et al., 1997, Paladino et al., 1998, Anderson et al., 1998a, Curtius et al., 1998, Brock et al., 2000, Schröder et al., 2000, Schumann et al., 2000, Schumann et al., 2002, Curtius et al., 2002, Jurkat et al., 2011).
The RF of civil aviation emissions has been extensively studied (e.g., Prather et al., 1999, Wuebbles et al., 2007, Lee et al., 2009) and can be summarised in the following emitted compounds and processes, each having positive (+) or negative (−) forcing: H2O (+); CO2 (+); the atmospheric chemistry of NOx causes the formation of tropospheric O3 (+) but also the destruction of methane (−); oxidation of SO2 results in sulphate particles (−); contrails (+); aviation-induced cloudiness (potentially +); soot, mainly composed of black carbon (+). Lee et al. (2009) estimated that aviation-induced RF in 2005 was ∼55 mW m−2, which accounted for 3.5% of global anthropogenic RF. In addition, black carbon emissions generated by aircraft at altitude have been shown to have a role in the formation of contrails (Schumann, 1996) and contrail-induced cirrus clouds, which affect the Earth's radiation balance by reflecting incoming solar radiation and by absorbing and re-emitting long wave radiation. The result is an additional positive RF of a magnitude similar to that of CO2 (IPCC, 1999, Sausen et al., 2005, Lee et al., 2010). Recently, Azar and Johansson (2012) also assessed the non-CO2 climate impact of aviation, including NOx and contrails, and calculated the emissions weighting factors, i.e. the factor by which aviation CO2 emissions should be multiplied to get the CO2-equivalent emissions for annual fleet average conditions. Recently, Gettelman and Chen (2013) reported the climate impact of aviation aerosol. Although such studies highlighted the climate impact of aviation, it should be borne in mind that the magnitude of the total emissions of pollutants from aviation in terms of mass with direct and/or indirect effects on climate are one to two orders of magnitude smaller than from road transport or shipping (Balkanski et al., 2010, Eyring et al., 2010). The study of aircraft emissions at cruise altitudes is very challenging mainly due to the obvious difficulty of sampling. Thus, measurements are commonly performed indirectly or extrapolated from data collected on the ground or in the laboratory. For this reason, the assessment of cruise emissions at altitude offers unique challenges to understanding the impacts of atmospheric emissions and their processing (Herndon et al., 2008, and reference therein). Computational models are available to extrapolate the test stand EI data to cruise altitude conditions (Baughcum et al., 1996b, Sutkus et al., 2001).
4.4. Military aircraft emissions
Despite most attention being given to civil aviation, a number of studies have also addressed emissions from military aircraft (e.g., Spicer et al., 1984, Spicer et al., 1992, Spicer et al., 1994, Heland and Schäfer, 1997, Heland and Schäfer, 1998, Gerstle et al., 1999, Gerstle et al., 2002, Miller et al., 2003, Anderson et al., 2005, Brundish et al., 2007, Corporan et al., 2008, Cheng, 2009, Cowen et al., 2009, Spicer et al., 2009, Cheng et al., 2009, Cheng and Corporan, 2010). Despite the relatively high potential impact of military aircraft emissions under particular circumstances, the task of studying military emissions is very difficult. Unlike civil aviation, military operations generally do not work to set flight profiles and do not follow fixed plans (Wahner et al., 1995). In addition, national and military authorities are reluctant to disclose sensitive information either about operations or in-use technologies. The lack of comprehensive data about military operations makes realistic assessments of the contribution of military aircraft in terms of fuel consumption extremely difficult. In addition, some aircraft may have a dual function, such as the C-130 Hercules, which can be engaged in both military and civilian operations. Henderson et al. (1999) reported a historical breakdown of aviation fuel burn for civil and military aviation: in 1976 fuel burned by civil aviation was 64%, while military was 36%. In 1992 the percentages were 82% and 18%, respectively. Subsequent studies stated that military aviation fleets used 11% (19.5 Tg) of fuel in 2002 and estimated that the military contribution is in the range of 10–13% of total aviation emissions (Eyers et al., 2004, Waitz et al., 2005). Table 2 provides estimates of fuel consumption and exhaust emissions from military aviation by the AERO2k model (Eyers et al., 2004). Among the large number of military aircraft, Cheng and Corporan (2010) stated that the three classes of military engines T56, TF33, and T700/T701C fitted in the C130 Hercules, B-52 bomber and Apache/Blackhawk helicopters, respectively, consume 70%–80% of the USA military aviation fuel each year.
4.5. Water vapour
Water is a key product of all hydrocarbon combustion and aircraft engines release H2O as vapour (Lewis et al., 1999). Water vapour is a greenhouse gas and its increase in the stratosphere (Solomon et al., 2010) and the free troposphere (Sherwood et al., 2010) tend to warm the Earth's surface (Prather et al., 1999). Water vapour, via latent heat released or absorbed during condensation and evaporation cycles also play an active role in dynamic processes that shape the global circulation of the atmosphere (Schneider et al., 2010). Moreover its effect on the formation of contrails and on the enhanced cirrus generation in the upper troposphere can be relevant for additional global RF with an indirect consequent potential increase of positive effects on global warming (Lee et al., 2009). The annual and global-mean RF due to present-day aviation water vapour emissions has been found to be 0.9 (range 0.3–1.4) mW m−2 (Wilcox et al., 2012). The increased water vapour in the lower troposphere may have secondary effects on precipitation, fog, visibility and some microphysical processes.
An emission index of 1230 ± 20 g H2O kg Fuel−1 is commonly reported for completely burnt fuel (Lewis et al., 1999, Lee et al., 2010): this represents a little less than 30% of all combustion products in aircraft exhaust (Fig. 3). No differences in emission indices during idle, take-off and cruise power settings are reported (Lewis et al., 1999), as emissions of H2O are a simple function of fuel consumption. The AERO2k inventories (Eyers et al., 2004) estimate a global emission of 217 Tg H2O for 2002, 193 Tg from civil aviation and 24 Tg from military operations. Other more recent estimates report 251 Tg H2O in 2005 (Kim et al., 2007) and 233 Tg H2O in 2006 (Wilkerson et al., 2010). However, the emissions of water by the global aircraft fleet into the troposphere are small if compared with fluxes within the natural hydrological cycle (IPCC, 1999) and thus water vapour from aircraft exhausts is not considered relevant for local air pollution and human health. An estimation of H2O produced by aircraft below 1000 m can be assessed by considering the global use of fuel reported in the literature for LTO cycles: considering the total consumption of 13.9 Tg fuel in 2005 (Kim et al., 2007), a total emission of ∼17 Tg H2O can be estimated (Table 2). Considering the fuel burn breakdown provided by Simone et al. (2013) for the EU (3.1 Tg in 2005), a total of 3.8 Tg y−1 H2O are emitted within European countries.
4.6. Carbon dioxide
Carbon dioxide is recognised as the main greenhouse gas, has a primary role in the Earth's climate warming and its behaviour within the atmosphere is simple and well understood (IPCC, 1999). Its main anthropogenic source is the combustion of fossil fuels: CO2 emissions from fossil fuel combustion, including small contributions from cement production and gas flaring, were estimated to be 8.7 ± 0.5 Pg C yr−1 in 2008 an increase of 2% from 2007, 29% from 2000 and 41% from 1990 (Le Quéré et al., 2009). More recently, Peters et al. (2011) indicated that global CO2 emissions from fossil-fuel combustion and cement production further grew by 5.9% in 2010, surpassing 9 Pg C yr−1 principally due to the strong emissions growth in emerging economies. Once emitted, there are no important processes involving CO2 formation or destruction and sinks occur principally at the Earth surface by exchange with the biosphere and the oceans (Solomon et al., 2007).
Carbon dioxide is the most abundant carbon-based effluent from aircraft engines (e.g., IPCC, 1999, Anderson et al., 2006, Lee et al., 2010) and Lewis et al. (1999) report that it accounts for ∼72% of total combustion products (Fig. 3). Typically, the EI(CO2) from modern aircraft engines is 3160 ± 60 g kg Fuel−1 for complete combustion (Lewis et al., 1999, Lee et al., 2010) and emissions of CO2 are a simple function of fuel consumption (e.g., Owen et al., 2010). However, some studies reported that EI(CO2) decreases slightly at low thrust because incomplete combustion may result in a relative increase of CO and hydrocarbons in the exhaust (e.g., Wey et al., 2006, Stettler et al., 2011). The role of aviation in the rise of CO2 emissions on a global scale may not be neglected and a list of estimates of CO2 emissions is provided in Table 2. In 1992, global aviation emissions of CO2 were about 2% of total anthropogenic sources and equivalent to about 13% of emissions from all transportation sources (IPCC, 1999). The AERO2k inventories (Eyers et al., 2004) estimated a global emission of 553 Tg CO2 for 2002, 492 Tg from civil aviation and 61 Tg from military operations, while a higher global emission of 733 Tg y−1 was reported for 2005 (Lee et al., 2009), accounting for approximately 3% of the total CO2 emissions from the combustion of fossil fuels (Howitt et al., 2011). Other estimates reported are 641 Tg CO2 in 2005 (Kim et al., 2007) and 595 Tg CO2 in 2006 (Wilkerson et al., 2010). As for H2O, an estimate of CO2 produced by aircraft below 1000 m was derived by assuming a constant EI(CO2) of 3160 g kg Fuel−1 and by considering the global use of fuel reported in the literature during LTO cycles in 2005 (Table 2). Results show a global emission of 44 Tg CO2 of which about 9.8 Tg y−1 are emitted within Europe.
4.7. Carbon monoxide
Carbon monoxide (CO) in the atmosphere is mainly generated by photochemical oxidation of methane and nonmethane hydrocarbons as well as direct emissions from anthropogenic combustion processes, such as vehicular exhaust, domestic heating, industrial emissions and biomass burning. In the troposphere, CO has a chemical lifetime varying from 30 to 90 days and its major sink is oxidation by hydroxyl radicals (Novelli et al., 1998, Seinfeld and Pandis, 2006). Its ability to form a strong bond with haemoglobin to form carboxyhaemoglobin can cause adverse effects on human health due to the reduction of blood oxygen-carrying capacity. At high exposure levels, CO can lead to asphyxia, whereas at low doses it may cause impaired neuropsychological performance and risk for myocardial ischaemia and rhythm disturbances in persons with cardiovascular diseases (Samoli et al., 2007, Bell et al., 2009).
Carbon monoxide is generally emitted in aircraft exhaust as result of incomplete combustion of jet fuel. Emissions of CO are regulated by ICAO international standards and engine manufacturers must provide emission indices for this pollutant during an LTO cycle (ICAO, 2008). In the last 40 years, the improvement of engine technology has led to a significant reduction in CO emissions during the LTO cycle. Fig. 6 shows a decrease in CO emissions at the end of the 1970s and nowadays most newly certified engines emit less than 10 kg CO per complete LTO cycle.
Carbon monoxide emissions indices are highest at low power settings where combustor temperatures and pressures are low and combustion is less efficient (Sutkus et al., 2001). Table SI1 summarises values of EI(CO) certified by ICAO for specific in-use aircraft engines and also lists EI(CO) for various military engines. Fig. 7 reports the ICAO data (all in-use engines certified from 1976 to today) as a function of LTO stages and shows that CO emission indices are generally greater at lower thrusts. Generally, average EI(CO) for in-use commercial engines included in the ICAO databank vary from 0.6 g kg Fuel−1 at take-off power to 31 g kg Fuel−1 at idle. Anderson et al. (2006) observed large decreases in CO emissions with increasing engine power for various FSCs (by a factor of ∼8 from idle to 61% F00) and reported that CO was observed to account for ∼1% of the total carbon emissions at engine idle, but emissions drop off at cruise thrust (61% F00) contributing <0.1%. Cain et al. (2013) measured emissions from a turbo-shaft engine burning different types of fuel and observed a decrease of CO with increasing engine power mainly due to improved combustion efficiency at higher power settings. Because of their predominant emission at lower power settings, CO emissions from aircraft are of high relevance to air quality in the vicinity of airports because of idle and taxi phases conducted at low thrust and which take up most of the time aircraft spend at an airport. Fig. 8 reports the total CO emissions for in-use engines during the four LTO phases and shows that CO emissions during idle are generally two orders of magnitude higher than climb and take-off phases.
After emission, CO may undergo to a series of chemical reactions in the troposphere involving hydroxyl radical, O2 and NO to form carbon dioxide, nitrogen dioxide, and ozone.
Some studies have derived EI(CO) directly from measurements during normal operation of idle and taxi at airports and have revealed some considerable differences compared to ICAO data, with results generally higher than those certified. For example, Heland and Schäfer (1998) reported an EI(CO) of 51.8 ± 4.6 g kg Fuel−1 at idle for a CFM56-3 engine, which was about 27–48% higher than the ICAO data. Herndon et al. (2008) reported that EI(CO) observed in ground idle plumes was greater (up to 100%) than predicted by engine certification data for the 7% thrust condition. Since CO emissions increase with decreasing thrust, these studies seem to confirm that normal idle and taxi operations at airports occur at lower thrust than the standardised ICAO LTO cycle, resulting in more CO emitted than certified values (e.g., Schäfer et al., 2003).
Some studies have measured the carbon monoxide in ambient air at airports (e.g., Schürmann et al., 2007, Heland and Schäfer, 1998, Yu et al., 2004, Herndon et al., 2008). In a study carried out at two different airports, Yu et al. (2004) observed that aircraft are an important contributor to CO in Hong Kong airport, whereas emissions from ground vehicles going in and out of the airport dominated emissions at Los Angeles. A study carried out at Zurich airport (Schürmann et al., 2007) demonstrated that CO concentrations in the vicinity of the terminals are highly dependent on aircraft movements.
4.8. Nitrogen oxides and nitrogen acids
Nitrogen oxides (NOx = NO + NO2) in urban environments are principally emitted from fossil fuel combustion as NO, as described by the extended Zeldovich mechanism (Lavoi et al., 1970):
NO plays an important role in atmospheric chemistry by rapidly reacting with ambient ozone or radicals to form NO2 on a timescale of minutes (Finlayson-Pitts and Pitts, 2000, Seinfeld and Pandis, 2006):
Other primary sources of NOx in the troposphere are biomass burning, soil emissions, lightning, transport from the stratosphere and ammonia oxidation (IPCC, 1999). NO2 is a strong respiratory irritant gas and its effects on human health have been extensively reviewed (Samoli et al., 2006, Weinmayr et al., 2010, Chiusolo et al., 2011) indicating a relationship with cardiovascular and respiratory diseases and mortality.
Nitrogen oxides are produced in the high temperature regions of the combustor primarily through the thermal oxidation of atmospheric N2 and therefore NOx formation is sensitive to combustor pressure, temperature, flow rate, and geometry (Sutkus et al., 2001). Additional NOx may derive from the combustion of the fuel-bound nitrogen: nitrogen in the fuel is not controlled or typically measured, but it can range from near zero to perhaps 20 ppm (Chevron Corporation, 2006). Gardner et al. (1997) estimated that 93% of NOx from aircraft is emitted in the Northern Hemisphere and ∼60% at cruise altitudes. More recent estimates indicated that in 2005 the NOx emitted during LTO was 0.23 Tg (Kim et al., 2007), accounting for ∼8% of global emissions from aviation.
NOx is included in the parameters certified by ICAO. There is a difference in the molecular mass of NO and NO2, and in the ICAO methodology data are reported as NO2 equivalent (unless otherwise specified). Being sensitive to combustor pressure, NOx emissions increase monotonically with engine thrust (Table SI1, Fig. 7). Generally, EI(NOx) for in-use engines included in the ICAO databank vary from 4 ± 1 g NOx kg−1 burned Fuel−1 at idle to 29 ± 12 g NOx kg−1 burned Fuel−1 at take-off power. However, despite the strong relationships to power settings, NOx total emissions per each standardised LTO phase are pretty constant during idle, approach and take-off operations (Fig. 8). Carslaw et al. (2008) measured individual plumes from aircraft departing Heathrow Airport and found that engines with higher reported NOx emissions result in proportionately lower concentrations than engines with lower emissions. This result was hypothesised to be linked to aircraft operational factors, such as take-off weight and aircraft thrust setting, which therefore may have an important influence on concentrations of NOx. Furthermore, Carslaw and co-authors reported that NOx concentrations can differ by up to 41% for aircraft using the same airframe and engine type, while those due to the same engine type in different airframes can differ by 28%.
In recent years there has been a growing concern over emissions of primary NO2 as a fraction of NOx from road traffic mainly because of the failure of NOx emission reductions to deliver an improvement in urban NO2 concentrations (e.g., Jenkin, 2004, Carslaw and Beevers, 2004, Carslaw, 2005, Hueglin et al., 2006, Grice et al., 2009, Mavroidis and Chaloulakou, 2011, Cyrys et al., 2012). The ratio of NO2 to NOx in aircraft emissions is diagnostic of combustor efficiency and several studies reported that, unlike many other forms of combustion, the majority of the NOx emitted from modern high bypass TF engines at idle is in the form of NO2. On the contrary, NO is dominant at high power regimes. For example, Wormhoudt et al. (2007) performed ground measurements and observed that emitted NO2 may represent up to 80% of the total NOx emissions for a modern engine at low thrust and 7% at the highest power setting. Other studies (Timko et al., 2010b, Timko et al., 2010c, Wood et al., 2008b) reported that the NO2/NOx ratio may vary between 75% and 98% at low thrust, while for approach, thrust may range from 12% to 20%. Presto et al. (2011) observed that the NO/NOx ratio increases from 0.2–0.3 at 4% F00 to 1 at 30% and 85% F00. Other measurements carried out within 350 m of a taxiway and 550 m of a runway during common airport operations indicated that 28–35% of NOx exists in the form of NO2 (Herndon et al., 2004). However it was reported that the relative abundance of NO and NO2 are subject to large uncertainties due to conversion in the plumes and the contribution of other sources. The results of a study performed by Schäfer et al. (2003) using remote sensing methodologies suggested that NO was rapidly converted to NO2 in the exhaust plume. The NO2 formation and destruction processes of aircraft exhausts were investigated by Wood et al. (2008b), who observed that the NO2/NOx fraction is significantly higher in advected measurements than in engine tests. The results suggested that a significant portion of the NO in the exhaust can be converted into NO2 by mechanisms that do not involve ozone.
Nitrogen oxides may also be oxidised to other reactive nitrogen species and the complete family of reactive nitrogen species is denoted as reactive odd nitrogen (NOy), which includes the sum of NOx and its oxidation products (HNO3, HONO, NO3 •, N2O5, HNO4, peroxyacyl nitrates, alkyl nitrates and others). Nitric acid is the major oxidation product and increasing atmospheric concentrations of NOx favour nitric acid formation as a result of the daytime gas phase recombination reaction of hydroxyl radical with NO2. NOx plays a key role in secondary inorganic aerosol formation (Finlayson-Pitts and Pitts, 2000, Seinfeld and Pandis, 2006).
High levels of NOx, particularly NO2, are a matter of concern for air quality near major airports. For example, current NO2 concentrations breach the UK annual mean air quality objective (40 μg m−3) at some locations around Heathrow, London (UK) (UK Department of Transport, 2006; UK Statutory Instrument, 2007, HAL, 2011), while some exceedences of the Swiss annual mean NO2 limit value (30 μg m−3) have been observed near Zürich airport (Fleuti and Hofmann, 2005). However, as most airports are located in the vicinity of large cities, the contribution of airport-related emissions to those exceedences is hard to quantify due to the major influence of other sources, such as traffic and industry. For example, Yu et al. (2004) observed that ground vehicles were the dominant source of NOx emissions at Los Angeles airport.
Although various studies have attempted to estimate the contribution of airport operations to ambient NOx levels, the results are often conflicting. For example, Carslaw et al. (2006) estimated that Heathrow operations accounted for ∼27% of the annual mean NOx and NO2 at the airfield boundary and less than 15% (<10 μg m−3) at background locations 2–3 km downwind of the airport, while Fleuti and Hofmann (2005) estimated the Zürich airport influence upon NO2 to be below 1 μg m−3 at a distance of three or more kilometres. In both case studies concentrations of NOx close to the airport were dominated by road traffic sources. A detailed emission inventory of UK airports was computed by Stettler et al. (2011), who pointed out that LTO emissions at London Heathrow in 2005 accounted for about 8.19 × 106 kg NOx, of which more than 80% is in the form of NO. An emission inventory study of NOx emissions at Zurich airport in 2003 (Unique, 2004) reported that most nitrogen oxides were released from LTO operations, while minor contributions were calculated for landside traffic, handling/airside traffic and airport infrastructure.
4.8.1. Nitrous oxide
Apart from NOx, other nitrogen species have been detected and analysed in aircraft exhaust plumes and at airports. Few data are available for the emissions of nitrous oxide (N2O) and some are contradictory. Wiesen et al. (1994) examined nitrous oxide emissions from different commercial jet engines using different fuels and reported average EI(N2O) ranging from 97 to 122 mg kg Fuel−1. Heland and Schäfer (1998) further analysed N2O using FTIR techniques and observed that N2O emitted by a CFM56-family engine was under the detection limits at idle thrust and detectable at higher power settings, with a related EI(N2O) of 1300 mg kg Fuel−1. Conversely, Santoni et al. (2011) measured N2O emissions from a CFM56-2C1 engine and concluded that at low thrust EI N2O were 110 ± 50 mg kg Fuel−1 (mean ± standard deviation), while a drop of emissions was observed at higher thrust levels (32 ± 18 mg kg Fuel−1).
4.8.2. Nitrous acid
HONO is generated in the gas turbines via reaction of hydroxyl radical with NO (Wormhoudt et al., 2007, Brundish et al., 2007) and ∼1.1% of the total NOy is in the form of HONO by the engine exit (Lukachko et al., 1998). Anderson et al. (2005) measured nitrous acid (HONO) in the exhaust of a B757 and observed a clear power dependence, increasing with increasing power; at high power, over 2 ppmv of HONO was detected. The same authors (Wormhoudt et al., 2007) further reported an increasing EI(HONO) at increasing thrust, but also reported that the EI(HONO)/EI(NO2) ratio decreases with increasing engine regimes. They found that HONO is a minor constituent (up to 7%) compared with NOx. Herndon et al. (2006) measured NOy at Logan airport in Boston (USA) and reported that the emission index for a B737 increased from idle (2 ± 1.9 g (NOy) kg Fuel−1) to take-off (19.5 ± 3.9 g (NOy) kg Fuel−1). Wood et al. (2008b) reported that HONO accounts for 0.5%–7% of NOy emissions from aircraft exhaust depending on thrust and engine type: 2–7% for low thrust and 0.5–1% for high thrust (65–100% F00). In conclusion, using data available in the literature, Lee et al. (2010) proposed that EI(HONO) should range between 0.08 and 0.8 g kg Fuel−1. More recently, Lee et al. (2011) performed measurements of HONO from a DC-8 aircraft equipped with CFM56-series engines using both traditional and synthetic fuels and observed that the EI(HONO) increases approximately 6-fold from idle to take-off conditions, but plateaus between 65 and 100% of maximum rated engine thrust. This study also discussed the kinetics behind the HONO formation/destruction.
Jurkat et al. (2011) measured the gaseous nitrogen emissions in young aircraft exhaust plumes emitted by 8 different types of modern jet airliners in flight and calculated molar ratios of HONO/NO and HONO/NOy of 0.038 ± 0.010 and 0.027 ± 0.005, respectively. The relative EI(HONO) at cruise thrust was reported to be 0.31 ± 0.12 g NO2 kg Fuel−1.
4.8.3. Nitric acid
Most studies of HNO3 emissions were performed using experimental measurements with chemical ionisation mass spectrometry (CIMS) in both exhaust plumes at cruising altitudes (e.g., Arnold et al., 1992, Arnold et al., 1998a, Tremmel et al., 1998, Miller et al., 2003) and simulated gas turbines (Katragkou et al., 2004) or using plume models (e.g., Garnier et al., 1997, Kraabøl et al., 2002). Generation of HNO3 is generally lower than HONO: Lukachko et al. (1998) reported that only ∼0.07% of the total NOy is oxidised to HNO3 by the engine exit, while Lee et al. (2010, and references therein) reported EI(HNO3) of 0.003–0.3 g kg Fuel−1. Because of the very low levels expected in aircraft exhaust, few studies have been carried out on the ground. There is consequently a lack of data about nitric acid measured in engine exhaust plumes during real working conditions.
4.9. Sulphur oxides and sulphuric acid
4.9.1. Sulphur oxides
Sulphur dioxide (SO2) is emitted into the atmosphere from both natural (volcanic activity, grassland and forest fires) and anthropogenic sources, including crude oil and coal transformation processes, fossil fuel combustion, metal smelting and various industrial processes (e.g., Seinfeld and Pandis, 2006, Smith et al., 2011). Exposure is associated with increased mortality and morbidity (Katsouyanni et al., 1997, Sunyer et al., 2003a) including cardiovascular admissions, particularly for ischaemic heart disease (Sunyer et al., 2003b). Oxidation of SO2 (S(IV)) is recognised as the major channel for the formation of atmospheric sulphuric acid (S(VI)), and sulphur trioxide (SO3) is an important intermediate in the oxidation processes (Vahedpour et al., 2011). Consequently, SO2 has an indirect effect on acid deposition and a key role in the aerosol system by acting as sulphate precursor. Since sulphate aerosol is known to modify the direct and indirect RF, SO2 also has an indirect influence on climate.
Sulphur dioxide is the overwhelmingly predominant S-containing species in aircraft exhaust (Anderson et al., 2005, Lee et al., 2010) and originates mainly from the oxidation of fuel sulphur in the engines (Brown et al., 1996a: Schumann et al., 2002). Therefore, SO2 emissions may vary greatly as a function of FSC. In the past, studies were carried out to analyse and model the sulphur emissions of aircraft and to estimate their role in the formation of visible contrails (e.g., Busen and Schumann, 1995, Schumann et al., 1996, Brown et al., 1996b, Brown et al., 1997, Arnold et al., 1998a). Generally an emission index of 0.8–1.3 g of SOx (as SO2) per kg Fuel was reported for complete combustion (e.g., Lewis et al., 1999, Kim et al., 2007, Lee et al., 2010, Presto et al., 2011), however measurements at flight altitudes have showed that sulphur dioxide varies with the average FSC (e.g., Arnold et al., 1998a, Schumann et al., 1998). For example, Hunton et al. (2000) reported that the EI(SO2) varied from 2.49 g SO2 kg Fuel−1 for a high-sulphur fuel (∼1150 ppmm S) in a test chamber to less than 0.01 g SO2 kg Fuel−1 for a low-sulphur fuel (∼10 ppmm S). They also reported that there is no dependence of emission indices upon engine power.
In this context, it is very important to stress that no S is created or destroyed from the fuel to the exhausts, therefore for every fuel S atom there is a molecule of SO2 or SO3 at the exhaust plane (the SO3 quickly converts to H2SO4). In this way the emission indices of total emitted S may vary according to the FSC, whereas the only uncertainties are in the speciation between S(IV) to S(VI) species, i.e. in the conversion efficiency, which is discussed fully later.
The importance of SO2 emissions at local scale, i.e. near the airports, was highlighted by Yu et al. (2004), who found that sulphur dioxide was a good tracer of aircraft emissions at both Los Angeles and Hong Kong airports. However, on a global scale the aviation source is considered to be secondary with respect to other major sources of SO2: Kjellström et al. (1999) used an atmospheric general circulation model including the atmospheric sulphur cycle to investigate the impact of aircraft sulphur emissions on the global sulphur budget of the atmosphere and concluded that aviation accounted for about 1% of the total sulphate mass north of 40°N, where aircraft emissions are largest. In 2004, about 0.18 Tg of SO2 was estimated to be emitted from aviation (Lee et al., 2010) using an EI(SO2) of 0.8 g Fuel−1. An estimation of SO2 produced by aircraft below 1000 m can be computed by applying a constant EI(SO2) of 0.8 g kg Fuel−1 and by considering the global use of fuel reported by the literature during LTO cycles in 2005 (Table 2). Results show a global emission of 11 Mg SO2 of which about 2.5 Mg y−1 are emitted within Europe.
4.9.2. Conversion of S(IV) to S(VI)
Despite SO2 being the dominant S-species in aircraft exhaust emissions, a fraction can be further oxidised to form S(VI) as SO3 and H2SO4 (Lee et al., 2010). The presence of SO3 has been established in gas turbine engine exhaust and as attributed mainly to the oxidation of SO2 by O atoms (Arnold et al., 1998a) or by hydroxyl radicals in exhaust plumes (Tremmel and Schumann, 1999). The further reaction with water vapour rapidly converts SO3 to sulphuric acid, according to Stockwell and Calvert, 1983, Stockwell, 1994, Brown et al., 1996a and Seinfeld and Pandis (2006):
Starik et al. (2002) computed that ∼1% of the sulphur is converted into SO3 within the combustor and about 10% into SO3 and H2SO4 before the engine exit. Past numerical simulations of H2SO4 formation from atomic oxygen and hydroxyl radical in aircraft engines indicated that between 2% and 10% of the fuel sulphur is emitted as S(VI) (Brown et al., 1996a, Lukachko et al., 1998). However, current understanding indicates a more realistic value of 2% (or possibly less). These studies also indicate that S(VI) conversion in the turbine is kinetically limited by the level of atomic oxygen, resulting in a higher oxidation efficiency at lower FSCs. Katragkou et al. (2004) report that the limiting factor of this series of reactions is the oxidation of SO2 by the hydroxyl radical, which is somewhat uncertain at the high temperatures in gas turbine engines. The knowledge of the mechanisms involving sulphur species and their interactions with H, O atoms and radicals occurring within a combustor is far from complete and are the subject of discussion (e.g., Blitz et al., 2003, Somnitz et al., 2005, DeWitt and Hwang, 2005, Yilmaz et al., 2006, Hindiyarti et al., 2007, Rasmussen et al., 2007, Wheeler and Schaefer, 2009, Hwang et al., 2010).
Once emitted, the gaseous sulphuric acid may act as an important precursor for aerosol because of its low vapour pressure. An understanding of the processes controlling sulphate aerosols is therefore essential to the study of the mechanisms of formation of particles generated by aircraft (e.g., Starik et al., 2004). For example, Arnold et al. (1998a) reported no detectable levels of sulphuric acid in the gas phase behind an in-flight commercial aircraft, leading to the inference that initially formed H2SO4 experiences a rapid gas-to-particle conversion at plume ages <1.6 s. Sulphuric acid was measured in several other studies at cruising altitudes and for different FSCs (e.g., Fahey et al., 1995b, Busen and Schumann, 1995, Schumann et al., 1996, Curtius et al., 1998, Arnold et al., 1998a, Schröder et al., 2000, Schumann et al., 2000, Curtius et al., 2002) as well as in fuel combustion experiments at ground-level (Frenzel and Arnold, 1994, Curtius et al., 1998, Curtius et al., 2002, Kiendler and Arnold, 2002, Sorokin et al., 2004) and during combustor testing (Katragkou et al., 2004). Curtius et al. (2002) reported H2SO4 concentrations measured in the plume were up to 600 pptv for a 56 ppmm FSC, while the average concentration of H2SO4 measured in the ambient atmosphere outside the aircraft plume was 88 pptv and the maximum ambient atmospheric concentration 300 pptv.
The abundance ratio, sometime named conversion factor (ε = (SO3+H2SO4)/total sulphur) has been widely used to assess the ratio of S(VI) to total sulphur at the exit of engines. The literature offers numerous estimates or measures of ε. However, the results are often difficult to compare as they are derived by different methods, ranging from direct measurements, indirect computations and models. In addition, most studies take in account only particulate sulphate, while only a few studies have measured both particulate and gaseous phases. Anyway, Timko et al. (2010b) demonstrated that the conversion of S(IV) to S(VI) is independent of engine technology for most modern in-use engines. Earlier values of ε are well summarised in DeWitt and Hwang (2005), while most recent measurements and modelling studies of aircraft plume chemistry reported other direct, indirect and inferred values of ε. Generally, ε values between 1 and 3% are commonly reported. For example, ε values between 6 and 31% have been calculated for a B757 aircraft (Miake-Lye et al., 1998), while Schumann et al. (2002) observed ε between 0.34 and 4.5% for an old engine (Mk501) and 3.3 ± 1.8% for a modern engine (CFM56-3B1). For low FSC, they also reported that ε was considerably smaller than implied by the volume of volatile particles in the exhaust, while for FSC≥100 ppm, sulphuric acid is the most important precursor of volatile aerosols formed in aircraft exhaust plumes of modern engines. Kiendler and Arnold (2002) inferred an ε value of 2 ± 0.8% for a M45H engine on the ground, while Curtius et al., 1998, Curtius et al., 2002 reported 3.3 ± 1.8% in the plume of a B737-300 aircraft in flight by measuring the total H2SO4 content in both gaseous and aerosol phases. The sulphur conversion fraction of an RB211 engine was computed by Starik et al. (2002) using a model and results showed that increases in FSC cause a minor reduction in ε, reporting values ≈9%, and ≈8.4% for FSC of 0.04% and 0.3%, respectively. Wilson et al. (2004) and Sorokin et al. (2004) observed ε of 2.3 ± 1.2% in an A310 equipped with a CF6-series engine at an exhaust age of about 5 ms from the combustor exit, while Jurkat et al. (2011) derived ε for various in-flight aircraft and reported an average value of 2.2 ± 0.5%, varying from a minimum of 1.2% for a Trent-series and a maximum of 2.8% for a CMF56-series engines. Wong et al. (2008) modelled the microphysical processes involved and suggested conversion efficiency of 1–2%. Timko et al. (2010b) reported ε ranging from 0.08% to 0.01%, while Kinsey et al. (2011) suggest a median value of 2.4%. Petzold et al. (2005b) reported that sulphur partitioning at 150 °C was 97% SO2 ≤ 2.7% gaseous H2SO4 < 0.3% chemisorbed H2SO4 at soot particle surface. Regarding the relative abundance of the two S(VI) species, during the COMS experiments Sorokin et al. (2004) reported that SO3 represented the major fraction of S(VI) in the exhaust behind the combustor and that SO3 conversion to H2SO4 takes place in the sampling line where the exhaust gases spend a sufficiently long time and where the temperature is markedly lower than in the hot exhaust. Other experimental measurements made during the EXCAVATE experiment by Anderson et al. (2005) led to the conclusion that the fraction of total sulphur that existed as SO3 would have to be less than 0.005%.
According to the conversion factors for sulphur species and taking in account the mass conservation of S in the exhaust plumes (no S is created or destroyed from the fuel to the exhausts), the computation of the EIs can be assessed by applying:
and
where M( ) represents the molecular weights of sulphur species, FSC is the fuel sulphur content and ε is the S(IV) to S(VI) conversion efficiency as a fraction, e.g. 0.02 and a unit conversion may be necessary (e.g. if FSC is in expressed ppmm, etc).
Another important consideration concerning the sulphate derived from aircraft engines was pointed out during the APEX-1 project, which was primarily developed to investigate the effects of fuel composition on emissions at various power settings (e.g., Wey et al., 2006, Knighton et al., 2007, Yelvington et al., 2007, Onasch et al., 2009). General results from the testing of a CFM56-series engine showed a strong linear relationship (r 2 = 0.93) between FSC and emission indices for sulphate, which can be approximately described by the linear equation EI (sulphur in mg kg Fuel−1) = 0.0136 FSC (in ppm)+4.4952 (Kinsey, 2009).
4.10. Ozone
Ozone (O3) is a reactive oxidant gas playing a key role in photochemical air pollution and in atmospheric oxidation processes. Ozone is associated with decrements in respiratory function and death from respiratory causes (Jerrett et al., 2009, Yang and Omaye, 2009). Although in the upper atmosphere it acts as a barrier for ultraviolet radiation, in the lower troposphere is a secondary air pollutant generated through a series of complex photochemical reactions involving reactive hydrocarbons, solar radiation and NO2 (Finlayson-Pitts and Pitts, 2000, Seinfeld and Pandis, 2006).
Ozone is not primarily produced by aircraft engines, however some ozone precursor such as CO, NOx and VOCs are emitted from the exhaust and may subsequently increase the boundary layer O3 pollution. Note that, amongst the ozone precursors, both CO and many VOCs are mainly emitted at low power settings during airport taxi and idle operations, while NOx is mainly released during take-off and climb phases, when engines reach higher thrusts. It is reported that NO emissions, which are dominant at highest thrusts, initially cause local ozone reductions in aircraft plumes (Kraabøl et al., 2000a, Kraabøl et al., 2000b) following:
but subsequently the photolysis of NO2 may form atomic oxygen which reacts with molecular O2 to form O3:
where M is N2, O2 or another molecule absorbing the excess energy to stabilise the ozone formed (Seinfeld and Pandis, 2006). A contrary effect, i.e. a decrease in O3 concentrations, may also occur due to the reaction of ozone with other compounds emitted from aircraft. For example, it is recognised that alkenes, which are emitted in the exhaust plumes, are susceptible to reaction with ozone forming primary carbonyls and bi-radicals (e.g., Grosjean et al., 1994, Seinfeld and Pandis, 2006) and consuming O3.
Although the effects of aircraft emissions on ozone depletion in the upper troposphere and stratosphere have been addressed by IPCC (1999) and the European 6th Framework ‘ATTICA’ (Assessment of Transport Impacts on Climate Change and Ozone Depletion) project (Lee et al., 2010), less attention has been given to the effects within the boundary layer due to emissions during LTO operations.
4.11. Hydrocarbons
Unburned hydrocarbons (UHC) are emitted as a result of the inefficiency of jet turbine engines to completely convert fuel to CO2 and H2O (Knighton et al., 2009). Although the levels of UHC emitted by aviation are considered negligible relative to emissions from surface transportation systems such road traffic, they may cause adverse health effects on exposed people, including workers and travellers at airports, and residents who live near large hubs. Therefore, UHC are included as parameter to be monitored during the LTO cycles by ICAO (ICAO, 2008). Analysing the data provided by the ICAO databank (EASA, 2013), a large range in the magnitude of UHC emissions between different engine models can be observed. Moreover, ICAO data clearly show that the emission of UHC during complete LTO cycles have fallen considerably since the 1970s (Fig. 6), mainly due to the development of more efficient technologies.
Unfortunately, the UHC parameter used by ICAO only refers to the lump sum of all hydrocarbons, including contributions from methane, and no corrections are made for background levels within the engine intake air (Anderson et al., 2006, Lee et al., 2010). Consequently, UHC data give no information on the large number of specific non-methane hydrocarbons (NMHCs) nowadays identified, and in some cases quantified, in aircraft exhaust plumes (Wilson et al., 2004, Anderson et al., 2006, Lobo et al., 2007, Agrawal et al., 2008, Herndon et al., 2009). This fact clearly represents a significant gap in the knowledge of impacts of aircraft on both environmental and human health endpoints, because of the very different physicochemical and toxicological properties of each class of organic compounds. Most emitted VOC are known ozone precursors, many are particle precursors and can impact visibility after particle formation. Some compounds are known or are suspected to have adverse effects on human health and the environment. Among the hydrocarbons emitted in aircraft exhaust, 14 species (12 compounds and two groups of complex organic compounds) are present in the Hazardous Air Pollutants (HAP) list compiled by the USEPA (Federal Aviation Administration, 2003). These compounds are 1,3-butadiene, n-hexane, acetaldehyde, xylene, acrolein, propionaldehyde, benzene, styrene, ethylbenzene, toluene, formaldehyde, lead compounds and polycyclic organic matter as 7 and 16 PAH groups.
In the last 20 years, various research programmes and experiments have been carried out to give more detailed data on the speciated hydrocarbon emissions of aircraft engines. Among others, some milestones are listed hereafter. Spicer et al., 1984, Spicer et al., 1994 measured detailed organic emissions for the CFM56-class engines burning various JP-grade fuels; Gerstle et al., 1999, Gerstle et al., 2002 reported UHC emission rates for several military engines not included in the ICAO databank; the EXCAVATE campaign (Anderson et al., 2005, Anderson et al., 2006) investigated the speciated-hydrocarbon emissions from an RB211-535-E4 engine at two different fuel sulphur levels; Herndon et al. (2006) investigated a set of hydrocarbons from in-use aircraft at Boston Logan International Airport; the APEX-1 campaign (Wey et al., 2006) reported the hydrocarbon speciation for a CFM56-2C1 engine using fuels with differing FSC (Knighton et al., 2007, Yelvington et al., 2007); Schürmann et al. (2007) sampled volatile organic compounds in diluted exhausts; the JETS/APEX-2 and APEX-3 campaigns (Lobo et al., 2007, Kinsey, 2009) reported data for speciated hydrocarbons in both a staged aircraft test (Yelvington et al., 2007, Wey et al., 2007, Agrawal et al., 2008, Timko et al., 2010c) and at airports (Wood et al., 2008b, Herndon et al., 2009); Knighton et al. (2009) consolidated earlier data from Spicer et al., 1984, Spicer et al., 1994, EXCAVATE and APEX studies; Cain et al. (2013) measured speciated hydrocarbon emissions from a TS engine burning various (conventional, alternative and surrogate) fuels.
Although those studies have yielded much useful information for characterising the emissions of hydrocarbons, to date there is still a great deal of work to be done, many chemical and physical characteristics remain unclear, and some conflicting results need to the further investigated. Firstly, Spicer et al. (1984) reported that a significant percentage (30%–40%) of the total hydrocarbon emissions at idle are made up of a large number of exhaust compounds with aliphatic, cycloaliphatic and aromatic structures, predominantly ethylene, propylene, acetylene, 1-butene, methane, and formaldehyde. This latter carbonyl was found to be the predominant aldehyde present in the exhaust. In addition to byproducts of combustion, some studies (Spicer et al., 1992, Spicer et al., 1994, Slemr et al., 2001) also observed that unburned/unreacted fuel compounds are emitted in the engine exhaust from fuel cracking and incomplete combustion. Spicer et al. (1984) reported that compounds from unburned fuel may represent a major component of exhausts and that they are mainly composed of normal C10–C16 paraffins with smaller amounts of alkyl substituted aromatics, cycloparaffins, and branched alkanes. The unburned fuel component was also observed to be virtually eliminated at the 30% and 80% F00 conditions, when concentrations of all of the individual hydrocarbons are very low. Similar results were reported by Slemr et al. (2001) in both modern commercial high bypass TF engines (CFM56-2C1) and older technology engines (Rolls Royce M45H Mk501) with emissions dominated by alkenes and alkynes due to fuel cracking and aromatic compounds arising from unburned fuel.
These pioneering results were largely confirmed by more recent studies, which generally reported that emitted hydrocarbons are composed of relatively light weight (C2–C6) species, including alkanes and alkenes, formaldehyde, methanol, ethylene, acetaldehyde, acetic acid, benzene, toluene, phenol, styrene, naphthalene and methylnaphthalenes (Slemr et al., 2001, Anderson et al., 2006, Knighton et al., 2007, Yelvington et al., 2007, Schürmann et al., 2007, Kinsey, 2009). The results of the whole APEX study (Kinsey, 2009) partially confirmed previous data, indicating that generally the gaseous hydrocarbon emissions of various engines primarily consist of formaldehyde (16–28% of total gaseous emissions), ethylene (8–23%), acetaldehyde (5–13%), acetylene (5–15%), propene (2–8%) and glyoxal (3–8%), with significant quantities of acrolein (<4%), benzene (<3%), 1,3-butadiene (<3%), and toluene (<1%), while 16–42% of total non-methane volatile compounds remained unresolved. The sum of HCHO, ethylene, acetaldehyde, and propene may account for roughly 75% of the volatile organic compounds, while benzene, toluene, xylenes, and other substituted benzene compounds, oxygenates (acetone, glyoxal, and propanal), olefins (butene, pentene, hexane), and naphthalenes constitute the remaining 20% (Timko et al., 2010c). In addition to the numerous papers published, US Environmental Protection Agency (US EPA, 2009) also created a companion spreadsheet including data on speciated hydrocarbon from APEX projects. Fig. 9 summarises the data from APEX campaigns in terms of profile (mass fraction) of the emitted hydrocarbons.
Fig. 9.
Results of the APEX campaigns. Profile (mass fractions) of individual hydrocarbon species. The single compounds are ordered to show decreasing fractions.
The total hydrocarbon EIs are highest at low power settings, where combustor temperatures and pressures are low and combustion is less efficient (Sutkus et al., 2001, Yelvington et al., 2007). UHC data provided by ICAO also confirm this behaviour for in-use TF engines (Fig. 7). Similarly, many studies have reported the same behaviour for individual hydrocarbon species. Spicer et al., 1992, Spicer et al., 1994 and Slemr et al. (2001) first reported that the emissions of many hydrocarbon species dropped at higher engine power by a factor of 20–50 and unburned fuel components disappeared. The EXCAVATE campaign (Anderson et al., 2006) also highlighted that most hydrocarbon species are strongly power dependent, with EIs at high thrusts dramatically lower than at idle. During APEX-1,2,3 campaigns, Knighton et al. (2007) observed that at engine power conditions significantly higher than 15% F00, the engine combustion efficiency is close to 100%, resulting in hydrocarbon emissions often below the detection levels for many individual compounds. The inverse dependence of UHC upon thrust has a high relevance for air quality at airports, where idle and taxi phases are conducted at low thrusts and take up most of the time. Fig. 8 shows that the cumulative UHC emission spans over two order of magnitude for in-use engines passing from idle to take-off during standardised LTO cycles.
Despite these interesting studies, the scientific literature still offers poor information on the hydrocarbon speciation and the few available data are often conflicting. For example, the potential changes in the hydrocarbon profiles at varying power are still unclear and deserve further investigation. Despite the large dependence of the magnitude of total UHC emitted from different engines, Knighton et al. (2009) observed that the ratios between the formaldehyde versus other hydrocarbon species were constant and independent of power settings. Although this result indicates constant hydrocarbon profiles with varying thrust, these results are inconsistent with other studies showing clear shifts of the hydrocarbon speciation with power. For example, during the EXCAVATE campaign, Anderson et al. (2006) observed that alkenes (mainly ethene) constituted more than 70% of the observed total NMHC emissions at idle, while at 61% F00 aromatic species (mostly toluene) accounted for over 50% of the total. There is currently a lack of information about the emitted hydrocarbons and this gap is mainly evident for emissions at power settings below the ICAO 7% idle. The behaviour and data for the most important classes of organics are discussed hereafter in separate sub-subsections.
4.11.1. Methane
Methane (CH4) is a radiatively active gas and is estimated to be 25 times more effective on a per-molecule level than CO2 in terms of greenhouse effect at hundred-year time scales (Lelieveld et al., 1998). Moreover, its roles in atmospheric chemistry to produce tropospheric ozone and stratospheric water vapour indirectly enhance its climate forcing effects. Although natural emissions from wetlands are largely recognised as dominant sources of methane at global scales, anthropogenic sources, such as energy, agriculture, waste and biomass burning can further contribute to its load in the atmosphere (Dlugokencky et al., 2011 and references therein). Most studies report that turbine engines are not a significant source of CH4 and have concluded that most engines tend to produce minor amounts of methane at idle and may consume it at higher engine power (Spicer et al., 1992, Spicer et al., 1994, Vay et al., 1998, Slemr et al., 2001, Anderson et al., 2006, Santoni et al., 2011). Wiesen et al. (1994) examined methane emissions from different commercial jet engines (PW 305 and RB211) under various flight conditions using different fuels and concluded that air traffic does not contribute significantly to the global budget of methane. Santoni et al. (2011) measured methane emissions from a CFM56-2C1 engine aboard a NASA DC-8 aircraft and reported that the EI for CH4 was (mean ± standard deviation) 170 ± 160 mg kg Fuel−1 at 4% and 7% F00, while negative values (54 ± 33 mg kg Fuel−1) were reported for higher thrust settings, indicating consumption of methane by the engine.
4.11.2. Alkanes, alkenes and alkynes
During the EXCAVATE campaign, Anderson et al. (2006) reported that the alkene species constituted over 90% of the observed total NMHC at idle but less than 20% at higher engine power settings. They also observed large decreases in alkane and alkene emissions with increasing engine power for various FSCs. In particular, EXCAVATE results showed that propylene underwent the most dramatic decrease, exhibiting a drop of mixing ratios by a factor ∼280 from 7 to 61% F00. In the same manner, isoprene dropped from ∼2.5 ppbv to less than ∼5 pptv (i.e., below the detection limit). On the other hand, these results reported decreases in alkane compounds which were much more modest, typically under a factor of 10. Schürmann et al. (2007) revealed that though isoprene was not directly found in emissions from kerosene refuelling, it was detected in considerable amounts in the aircraft exhaust which indicates that isoprene is most likely formed in the combustion process of a jet engine.
4.11.3. Carbonyls
Due to their known adverse effects on human health, some carbonyls (formaldehyde, acetaldehyde, propionaldehyde and acrolein) have been included in the HAP list (Federal Aviation Administration, 2003). However, nowadays there is a gap in the current state of knowledge regarding the toxicity of many other aldehydes (including glyoxal, methylglyoxal and crotonaldehyde) which are detected in sizeable quantities in aircraft exhaust plumes and have potential toxic effects (Wood et al., 2008). APEX results (Kinsey, 2009) clearly showed that carbonyls generally account for most of the gaseous hydrocarbons emitted by common aircraft engines. Agrawal et al. (2008) reported that the major three contributors to carbonyl emissions are formaldehyde, acetaldehyde and acetone, and showed that carbonyl emissions are significantly higher during the idle mode than at higher thrusts. However, measurements of carbonyl EIs were also found to be very variable since they are sensitive to changes in ambient temperature (Yelvington et al., 2007, Knighton et al., 2007, Agrawal et al., 2008). Similar results were obtained for TS engines: Cain et al. (2013) observed that the EIs for the most prevalent aldehydes emitted at various engine power combinations were formaldehyde, acetaldehyde, and propionaldehyde and also reported a decrease with increasing engine power. The results of such engine tests seem to be confirmed by ambient measurements. For example, Fanning et al. (2007) and Zhu et al. (2011) reported that the time averaged concentrations of formaldehyde and acrolein were elevated at the Los Angeles International airport relative to a background reference site.
4.11.4. Aromatic compounds
Benzene, toluene, ethylbenzene, and ortho-, meta-, and para-xylenes are an important group of VOCs collectively known as BTEX. In urban environments BTEX are principally emitted by vehicle exhaust gases because of their presence in fuels, lubricating and heating oil, while minor sources include gasoline evaporation, use of solvents and paint, leakage from natural gas and liquefied petroleum gas. The adverse health effects of benzene are well known (e.g., WHO, 2000, Saillenfait et al., 2003, Pariselli et al., 2009, and reference therein) and it is included as a known human carcinogen by the IARC classification system. BTEX are highly reactive in the troposphere playing a key role in atmospheric chemistry as important photochemical precursors for tropospheric ozone and secondary organic aerosol generation (Atkinson, 2000, Atkinson and Arey, 2003).
Aromatic compounds are present in jet fuels, and can therefore be emitted as both unburned material and byproducts of incomplete hydrocarbon combustion, but also from fuel evaporation and refuelling (Anderson et al., 2005, Anderson et al., 2006). In this context, the benzene to toluene ratio (B/T) was often proposed to identify the fuel vs combustion origin of hydrocarbon mixtures. For example, Schürmann et al. (2007) observed that the B/T ratio at an airport is well below 1 for refuelling emissions and engine ignition while in the exhaust this value reaches up to 1.7. The US EPA (2009) mass fraction profiles (Fig. 9) clearly show that BTEX account for ∼4% of identified compounds, while other relevant aromatics (in order of decreasing mass fraction) are phenol, 1,2,4-trimethylbenzene, styrene, m-ethyltoluene and 1,2,3-trimethylbenzene. Generally, the literature shows large decreases in benzene and toluene emissions with increasing engine power, both for TF (Anderson et al., 2006) and TS engines (Cain et al., 2013). In particular, by studying the hydrocarbon emissions from a TS engine operating with conventional (JP-8), alternative and surrogate fuels, Cain et al. (2013) hypothesised that fuel composition and structure may play a significant role in the aromatic emissions of aircraft. They speculated that the propensity of the molecular structure of paraffins in fuels to produce benzene or toluene was observed to follow cycloparaffin > iso-paraffin > n-paraffin. This study also attempted to depict the chemical processes at the basis of their observations and hypothesised that iso- and n-paraffins must first undergo either ring closure or decomposition to combustion/pyrolytic intermediates prone to ring formation (e.g., propargyl radicals and propylene) to ultimately form cyclic and aromatic compounds. In addition, Cain et al. (2013) reported that an increased branching ratio of iso-paraffins resulted in higher production rates of the C3-intermediates, which further contribute to ring/aromatic formation and growth.
4.11.5. Polycyclic aromatic hydrocarbons
Among the large number of hydrocarbon species emitted by aircraft engines, the polycyclic aromatic hydrocarbons (PAHs) deserve particular attention because most congeners are known, probable or possible human carcinogens (WHO, 2000, Armstrong et al., 2004, IARC, 2010) and because of their ubiquitous presence in the urban atmosphere (Ravindra et al., 2008, Zhang and Tao, 2009). PAH are semi-volatile and partition between the gaseous and particulate phases; lighter PAHs (2–3 aromatic rings) are present almost exclusively in the vapour-phase, whereas PAHs with higher molecular weights (>4 rings) are almost totally adsorbed on particles. Although PAHs may undergo oxidation by several atmospheric oxidants, their potential for long range transport cannot be disregarded (e.g., Keyte et al., 2013).
Agrawal et al. (2008) showed that lighter congeners such naphthalene and its 1-methyl and 2-methyl derivatives contribute strongly to the total PAH mass in various aircraft (TF) emissions at differing thrust modes. Moreover, they also reported that the EI(naphthalene) increased as power increased from idle mode falling off as the engine operated at the highest power. Chen et al. (2006) characterised the PAH emissions of the TS engine of a helicopter at five power settings and reported a mean total PAH concentration in the exhaust of 843 μg m−3, with a maximum of 1653 μg m−3 emitted during ground idle. The emission level of total PAHs during a complete LTO cycle was estimated to be 1.15 g PAHs LTO−1. Even if the results provide evidence for high mass concentrations of total emitted PAH, the speciation revealed that lighter congeners, which have generally lower carcinogenic potencies, were dominant: 59.7% of total PAHs emissions were made up of naphthalene, 37.8% of three-ring congeners, while the remaining 2.5% of PAHs had four- to seven-rings. The emission factor revealed U-shaped behaviour: maximum at idle (50%), minimum at fly idle (67%) and increasing until max thrust (100% F00).
Although the PAH pollution at airports can be overwhelmed by external sources, such as vehicular traffic and industrial emissions, a number of studies have indicated airport emissions cannot be neglected. Cavallo et al. (2006) measured the concentrations of 23 PAH in three areas (airport apron, building and terminal/office) of a major Italian airport (Fiumicino, Rome). The airport apron was found to be suffering the highest levels of total PAHs (27.7 μg m−3) with a prevalence of 2–3 ring PAH such as methylnaphthalenes and acenaphthene presumably associated with jet fuel combustion. However, they also showed that PAH levels were lower than the threshold limit value proposed for occupational exposure by ACGIH (0.2 mg m−3). Similar results were obtained by Zhu et al. (2011), who observed that the semi-volatile PAHs (from phenanthrene to chrysene) were consistently higher at both blast fence and downwind sites from the take-off runway of Los Angeles airport than at a background site. This study also indicated naphthalene as the most abundant gas-phase PAH (80–85% of the total PAHs).
4.11.6. Organic sulphur, nitrogen and chlorinated species
Since jet fuels contain variable FSC, some organic sulphur species may form during combustion. Anderson et al. (2006) measured the emissions of OCS, CS2 and dimethyl sulphide (DMS) from a RB211-series TF engine at varying engine power and burning two different FSC fuels. Results showed no consistent trends for OCS and CS2 with varying thrust settings and suggested that the sources of those gases are insensitive to the FSC. In contrast, this study revealed that levels of DMS are dramatically reduced from approximately ambient levels at idle to near the instrument detection limit as engine power is increased and speculated that ambient DMS is essentially burned (oxidised) out of the exhaust stream at combustor temperatures associated with high engine power.
The presence of organic nitrogen species in aircraft exhaust may derive from the presence of nitrogen in fuels and from the potential reaction between alkanes and NOx within the exhaust plume. During the EXCAVATE campaign, alkylnitrate species were observed in exhaust plumes with methyl nitrate, iso-propyl nitrate, and 2-butyl nitrate accounting for 80–90% of the total N-containing organic species (Anderson et al., 2006). In particular, methyl nitrate was observed to follow U-shaped curves of EI vs. fuel flow, with minimum emissions at mid-range thrust, slightly increased emissions at low thrust and strongly increased at higher powers.
Chlorinated organic compounds can form in aircraft exhaust as by-products of fossil fuel combustion in the presence of chlorine. Chlorine can be present in fuels because refineries can use salt driers to remove water from fuels (Anderson et al., 2006), and in certain circumstances may be present in ambient air as sea salt, such as in coastal environments. Despite the lack of available data in the literature, there is no evidence to date that chlorinated compounds are produced by aircraft engines. For example, Agrawal et al. (2008) observed that the emissions of dioxins from various aircraft engines are below the detection limit.
4.12. Chemi-ions
Aircraft exhausts also contain gaseous ions, the so called chemi-ions (CIs), have been measured in several studies (e.g., Reiner and Arnold, 1993, Reiner and Arnold, 1994, Arnold et al., 1998b, Yu and Turco, 1997, Kiendler and Arnold, 2002, Eichkorn et al., 2002, Haverkamp et al., 2004, Sorokin et al., 2004, Miller et al., 2005, Anderson et al., 2005). Their formation was also found in various mobile sources (e.g., Seigneur, 2009) and is attributed to the radical–radical reactions during combustion processes. Once emitted, CIs may evolve chemically via ion–ion recombination and ion–molecule reactions involving trace gas molecules present in the exhaust (Kiendler and Arnold, 2002) and may act as aerosol precursors (Sorokin and Mirabel, 2001, Eichkorn et al., 2002). Starik (2008) provides a scheme of ion formation in hydrocarbon flames and inside the combustor.
Relatively high number concentrations of CIs have been measured: in the SULFUR experiments (Schumann et al., 2002 and reference therein) 109 ions cm−3 were reported at ground level, i.e., of the order of 1017 CIs kg Fuel−1, but it was also reported that CIs decrease rapidly with increasing plume age (Arnold et al., 2000, Sorokin and Mirabel, 2001). Haverkamp et al. (2004) measured EI for the total (positive and negative) ions of 1.2 × 1016–2 × 1016 CIs kg Fuel−1 and observed number concentrations of the same order of magnitude for both negative and positive ions: negative CIs varied from 6 × 107 and 2.1 × 108 molecules cm−3, while positive ions ranged from 4 × 107 to 1.7 × 108 molecules cm−3. About 50% of the measured ions have masses heavier than 100 amu and the most massive ions show masses up to 1500–3000 amu, depending on the fuel flow (thrust) and FSC (Haverkamp et al., 2004). Schumann et al. (2002) reported masses also exceeding 8500 amu. Identified negative CIs include many organic ions and cluster ions containing sulphuric acid, e.g., HSO4 ‒(H2SO4)n, HSO4 ‒(H2SO4)n(SO3)m (n < 3, m = 0,1), NO3 ‒ (HNO3)m and HSO4 ‒(HNO3)m (m = 1,2). Kiendler and Arnold (2002) further reported a low stability of HSO4 ‒(H2SO4)n (n ≥ 3) against thermal detachment of H2SO4 at high temperatures, indicating the presence of gaseous H2SO4 in exhaust plumes. Positive CIs are mostly oxygen-containing organic compounds (Schumann et al., 2002) and considering the heavy masses of most CI, Haverkamp et al. (2004) also hypothesised the presence of large organic molecules, such as PAHs.
The generation of CIs in the combustor, their physico-chemical characteristics and the changes occurring along with plume aging are not yet well understood and merit further investigation as these ions may play a key role in the formation of numerous volatile aerosol particles (e.g., Yu and Turco, 1997, Arnold et al., 2000, Sorokin and Mirabel, 2001, Haverkamp et al., 2004, Miller et al., 2005).
4.13. Particulate matter
Particulate matter (PM) is emitted by a great variety of both natural and anthropogenic sources. The latter include a large variety of anthropogenic processes, which emit particles with very different chemical composition and physical properties. Nowadays, PM composition and sources have been extensively investigated in a large number of different environments (e.g., Viana et al., 2008, Harrison et al., 2012, Amato et al., 2013). However, few data on PM emissions are historically available for aircraft engines (Wayson et al., 2009, Kinsey et al., 2011). In addition, ICAO has not yet defined any emission standard for PM to be applied during LTO cycles and is therefore interested in setting a certification limit for this pollutant to address related air quality and climate issues (Kinsey, 2009). In this context, there are some current programmes aiming to describe the PM emissions from aircraft engines, e.g., the Society of Automotive Engineers (SAE) E-31 Committee is developing a standard PM test method for aircraft engine certification (SAE, 2009).
Despite a number of studies which have been published recently on PM emissions from gas turbine engines from both a physical and a chemical point of view (e.g., Corporan et al., 2008, Whitefield et al., 2008, Herndon et al., 2008, Agrawal et al., 2008, Westerdahl et al., 2008, Kinsey et al., 2010, Kinsey et al., 2011), current data on aircraft-generated PM are still wholly inadequate and many open questions wait to be addressed. This gap appears to be a pressing issue because many epidemiological studies have found a strong correlation between the exposure to PM and some significant adverse human health effects (e.g., Pope and Dockery, 2006, Valavanidis et al., 2008, Polichetti et al., 2009, Karakatsani et al., 2012, Anderson et al., 2012, Heal et al., 2012, Martinelli et al., 2013). PM inhalation can affect morbidity and can lead to an increase in hospital admissions, and is significantly associated with mortality and to a substantial reduction in life expectancy (Pope et al., 2009, Hoek et al., 2010, Sapkota et al., 2012, Raaschou-Nielsen et al., 2013).
4.13.1. Volatile and non-volatile PM
PM generated from aircraft engines can be classified into two major fractions: non-volatile and volatile PM (e.g., Kinsey, 2009, Presto et al., 2011), while the combination of both volatile and non-volatile PM is commonly referred as total PM. Non-volatile PM is directly emitted by engines and is mainly composed of graphitic/elemental/black carbon with traces of metals, which are stable at the high temperatures and pressures normally reached in the exhaust plumes. Volatile PM is instead formed through the gas-to-particle partitioning and conversion processes of sulphur and various organic gases (Robinson et al., 2010, Timko et al., 2010b), which occur after the emission in the near-field plume downstream of the engine (Kinsey et al., 2011). Since the most volatile PM components are partitioned into the gas- and particulate-phases, their behaviour is sensitive on the changes in the environmental conditions with respect to the near-plume and in any case many compounds can remain in equilibrium between the two phases. This component is therefore very sensitive to the sampling conditions (Wey et al., 2006, Wong et al., 2011, Presto et al., 2011). In particular, the organic component of the volatile PM undergoing partitioning between the two phases is named organic aerosol (OA) and can be composed of a large number of different hydrocarbon classes. Moreover, as the reactive compounds can be affected by oxidation by a number of atmospheric oxidant species (mainly hydroxyl, nitrate radicals and ozone), it can be expected that the composition and the quantity of volatile PM changes progressively away from the plume, after natural cooling, dilution and chemical processes occur in the atmosphere. Many hydrocarbons of high volatility, such as BTEX, low molecular weight PAHs, alkanes and many others, may be easily oxidised to species with substantially lower volatilities (Kroll and Seinfeld, 2008) and, thus, may act as precursors for the formation of the secondary organic aerosol (SOA). The formation and the properties of the SOA, including their gas/particle partitioning, are an intense area of research (e.g., Pandism et al., 1992, Pankow, 1994, Odum et al., 1996, Kroll and Seinfeld, 2008, Hallquist et al., 2009) and the common way to describe the partitioning of a constituent i between the gas- and the condensed-phases with mass concentration COA can be described by a partitioning coefficient, ξ i:
where is the effective saturation concentration of the compound, i.e. a semi-empirical property describing the partitioning of complex mixtures. Donahue et al. (2009) proposed three different classes of compounds on the basis of their C ∗ values: (i) the low volatility organic compounds, showing C ∗ from 10−2 to 10−1 μg m−3 and mostly remaining in the condensed phase under common atmospheric conditions; (ii) the SVOCs, exhibiting C ∗ between 100 and 102 μg m−3 and undergoing significant partitioning and (iii) the intermediate volatility organic compounds (IVOCs), having C ∗ in the order of magnitude of 103–106 μg m−3, which are almost entirely in the gas-phase. Recently, some studies have pointed out that most hydrocarbons emitted by aircraft engines are thought to be important SOA precursors (Miracolo et al., 2011, Presto et al., 2011), being in the IVOC and SVOC classes. However, the potential of hydrocarbons emitted by aircraft exhaust to form secondary components is currently poorly understood.
4.13.2. Particulate mass
Generally, the emission indices of PM mass range from approximately 10 to 550 mg PM kg Fuel−1 (Kinsey, 2009). U-shaped curves of PM emissions versus thrust are commonly reported in the literature, showing elevated emissions at low power settings, a decrease to a minimum at midrange power, and then an increase at high or full power (Whitefield et al., 2008, Kinsey, 2009, Kinsey et al., 2010, Kinsey et al., 2011). Agrawal et al. (2008) noted a 10–40-fold increase in the EI(PM) as the engine power increased from idle to climb thrust. However, there are deviations from this behaviour: the PM mass emission indices at varying thrusts have been shown to depend on various factors, including engine families, technology, FSC, operating power, cold and warm engine conditions and environmental conditions (e.g., Kinsey, 2009) and real-time emission rates for PM for a typical TF engine have revealed significant PM spikes during changes in power settings (Agrawal et al., 2008).
The measurements of PM from aircraft exhaust are heavily dependent on the adopted methodology (e.g., Presto et al., 2011). Since the volatile PM may undergo rapid changes in time and space, the sampling protocol, such as the distance from the engine exit, and other parameters having implications on the aging of plumes play a key role in the mass of sampled particles. In addition, the environmental conditions (e.g., temperature, humidity, sunlight, wind, etc.) can also affect PM mass, particularly through the potential for particle formation, coagulation, and growth (e.g., Herndon et al., 2005). Timko et al. (2010b) reported that soot is the only type of particle detected at the engine exit plane, while volatile particles are only detected downwind (15–50 m) due to the nucleation of sulphate and organic materials in the cooling exhaust plume. Kinsey et al. (2010) indicated that a variable amount (40%–80%) of the total PM can be composed of volatile matter, mainly in the form of sulphur and organics. Lobo et al. (2012) measured the specific PM emissions during normal LTO operations at a distance of 100–300 m downwind of an active taxi-/runway at the Oakland International Airport and reported EI(PM) between 100 and 700 mg PM kg Fuel−1 under both the idle/taxi and take-off conditions for various aircraft/engine combinations.
4.13.3. Particle number concentration
During the APEX campaigns, the observed EI(#) varied from approximately 1·1015 to 1·1017 particles kg Fuel−1 (Kinsey, 2009, Kinsey et al., 2010) and are therefore comparable on a per unit fuel burn basis to the number of particles generated from other combustion sources, such as ship emissions, biomass burning and forest fires (Kumar et al., 2013). Generally most TF engines tested during APEX projects exhibited EI(#) strongly correlated with fuel flow (Kinsey et al., 2010), with higher EI at low power settings following a logarithmic relationship of EI(#) to thrust:
where m represents the slope of the regression line with values ranging from −2·1015 to −3·1016 and b is the intercept of the regression line varying from 2·1016 to 2·1017 (Kinsey, 2009). Similarly to EI(PM) the particle number indices were however observed to be sensitive to engine technology, FSC, operating power and environmental conditions: Kinsey (2009) also reported a completely different behaviour for a TJ engine (CJ610-8ATJ), with EI(#) lower at idle and relatively constant at higher F00.
It was shown that EI(#) tends to increase moving away from the engine exit plane. EXCAVATE results (Anderson et al., 2005) reported increases by a factor of 10 at 25–35 m than at 1 m downstream of the exhaust plane. Timko et al. (2010b) further observed differences in particle number emissions sampled at engine exit plane and downwind (15–50 m) of the engine. They reported that soot is the main species detected at the engine exit plane, while the nucleation of volatile particles in the cooling exhaust gases measured downwind further led to increases in the particle number of 1–2 orders of magnitude.
Cheng and Corporan (2010) reported particle number emissions from military engines operated with JP-8 fuel in various thrust settings. They observed that a common TF engine emits increasing number of particles at increasing thrust with particle number emission indices of 5.5·1015, 5.3·1015, 9.6·1015, and 8.9·1015 particles kg Fuel−1 for the idle, 80%, 90% and 95% power setting, respectively. An inverse pattern with decreasing emissions at increased power settings was instead reported for a common TP engine equipping the widespread used military cargo C-130 Hercules: averaged EI were 1.8·1016, 1.4·1016, 1.4·1016, 1.0·1016, and 1.2·1016 particles kg-Fuel−1 for 4%, 7%, 20%, 41% and max thrusts, respectively. This study also examined two common TS engines used in most helicopters and aircraft and reported increasing emissions of particles with increasing thrust: 3.1·1015 (idle), 3.3·1015 (75%) and 5.5·1015 (max thrust) particles kg-Fuel−1 and 1.1·1014 (idle) 1.8·1015 (75%) and 3.0·1015 (max thrust), respectively. Similar results were observed by Cain et al. (2013) in a TS engine burning various types of fuel: JP-8 fuel emissions were between 1015 and 1016 particles kg-Fuel−1, while emissions from other alternative and surrogate fuels were 1–2 order of magnitude lower.
Measurements of EI(#) at airports indicated similar results. Lobo et al. (2012) measured the specific PM emissions during normal LTO operations at a distance 100–300 m downwind of an active taxi-/runway at the Oakland International Airport and associated the data with various aircraft/engine combinations. They observed similar EI(#) for both idle/taxi (7·1015–3·1017 particles kg Fuel−1) and take-off (4·1015–2·1017 particles kg Fuel−1) phases. Klapmeyer and Marr (2012) reported that the EI(#) for in-use aircraft at a regional airport varied from 1.4·1016 to 7.1·1016 particles kg Fuel−1 and observed slightly higher concentrations during taxi phases than during take-offs.
The beneficial effects of alternative fuels upon particle emissions are nowadays under discussion. Although this review does not focus on such effects, it is interesting to note that some studies have highlighted potential positive effects on the EI(#) and EI(PM). For example, Lobo et al. (2011) reported reduced emissions of PM number emissions of about one third using 50% FT/50% Jet-A1 blend instead of Jet-A1.
4.13.4. Size distributions
Size distributions of airborne particles influence their residence time and dispersion (Allen et al., 2001). In addition, the dimensions of particles are directly related to their emission sources, as mechanically generated particles (e.g., wind-blown dust, sea spray) are generally largest than 1 μm, while combustion-generated (high-temperature processes, traffic, many industrial activities) are typically smaller than 1 μm (e.g., Lewis and Schwartz, 2004, Seinfeld and Pandis, 2006, Ning and Sioutas, 2010). Ultrafine particles (UFPs, diameter <100 nm) typically constitute ∼90% or more of particle number count in areas influenced by vehicle emissions (Morawska et al., 2008). UFPs have larger surface area per unit mass with respect to larger particles and can potentially contain high proportions of organic material such as polycyclic aromatic hydrocarbons. Moreover, UFPs can penetrate deeper into the respiratory tract and into cells possibly posing an elevated risk for human health (Oberdorster et al., 2004, Delfino et al., 2005, Bräuner et al., 2007, Belleudi et al., 2010, Knibbs et al., 2011).
A large number of studies (e.g., Herndon et al., 2005, Wey et al., 2007, Westerdahl et al., 2008, Cheng et al., 2008, Mazaheri et al., 2009, Dodson et al., 2009, Kinsey, 2009, Kinsey et al., 2011, Zhu et al., 2011, Presto et al., 2011, Hsu et al., 2013) have provided evidence that AEs may lead to increased concentrations of UFPs. However, the nature of semi-volatile compounds emitted by aircraft, the possible mechanisms of secondary aerosol formation and the dilution effect, make it difficult to associate a measured size distribution with a specific source. Studies performed at the exhaust exit-plane or directly downstream of the engine cannot usefully be compared with data obtained in ambient air sampled at airports. However, even if differences and limitations exist, some trends and recurring modes have been identified in most studies.
A study by Shumway (2002) used scanning electron microscopy to analyse individual particles emitted from military engines and reported predominant particles with dimensions ranging from 22 to 120 nm. It was observed that emitted particles were discrete at low thrust (approach and idle), while they tended to agglomerate at higher power (intermediate and military modes). Similar results have recently been reported by Mazaheri et al. (2013), who analysed the aircraft emissions during normal takeoff and landing operations at an international airport by using the transmission electron microscopy technique. They reported particles in the range of 5–100 nm in diameter with a dominant nucleation mode (18−20 nm) and semisolid spherical shapes. Nowadays most studies measure particle size distributions using automatic instruments, such as scanning mobility particle sizers (SMPS), electrical low pressure impactors (ELPI), and differential mobility spectrometers (DMS). A comprehensive review of these devices is provided elsewhere (Kumar et al., 2010). Anderson et al. (2005) reported that exhaust exit-plane measurements on engines mounted in test cells and B757 aircraft in run-up facilities produce of the order of 1015 soot particles per kg of fuel burned with a mean mass diameter of 40–60 nm. Using an improved version of the nanometre aerosol size analyser (nASA), they also reported that the aerosol size distribution at 1 m from a B757 engine is a combination of volatile and non-volatile particles with a bimodal distribution. The first (non-volatile) mode was measured by heating the aerosol to 300 °C before analysis with the nASA and was found to be around 20 nm; this mode was thought to be primarily composed of soot and other components including zinc, aluminium, and titanium which are from the abrasion of engine components or the trace metal impurities in the fuel. The second (volatile) mode was observed at 7 nm and comprised particles that vaporise below 300 °C.
During the APEX campaigns (e.g., Wey et al., 2007, Kinsey, 2009, Kinsey et al., 2010), the particle size distributions of the emissions were generally found to be unimodal and log-normally distributed, with electrical mobility diameters ranging from ∼3 nm to >100 nm and a geometric number mean diameter (GMD) of ∼10–35 nm. A slightly dependence of GMD on thrust was detected, with GMD of 10–20 nm at low fuel flow rates, a decrease at mid-power and then an increase at higher thrust. These studies also reported the presence of a prominent nucleation mode mainly on samples collected farther from the engine exit (30 m) with respect to gases sampled at 1 or 10 m. This second mode was attributed to the secondary aerosol generation caused by the expansion and cooling of the exhaust plume and is composed of sulphuric acid and low-volatility hydrocarbons (Wey et al., 2007). APEX results detected changes in both the GMD and related geometric standard deviation (GSD) of the particle size distributions at varying engine and fuel type, thrust, and environmental conditions.
While APEX reported size distributions for commercial in-use airliner engines, we report data from other studies on differing engine types and technologies. Rogers et al. (2005) showed that the particles measured in the exhaust of two military engines (a FT with afterburner and a TS) were unimodally distributed with peaks at 20–40 nm. Cheng et al. (2008) observed that the particle number size distributions downstream of a C-130 Hercules showed peaks between 50 and 80 nm for engine power settings ranging from idle to maximum thrust. They also observed a clear trend of increasing particle diameter with increasing engine power setting and distance from the engine exit. Cheng et al. (2008) detected the presence of another peak corresponding to the lower instrumental limit, presumed to be an additional mode below 20 nm. Cheng and Corporan (2010) reported unimodal size distributions for military turbofan, turboprop and turboshaft emissions sampled at the engine exhaust plane. They observed that both the total particle number concentration and GMD increased as the engine power increased for all tested engines. In particular, the observed GMD ranged from 55 nm (at idle) to 85 nm (at 95% F00) in turbofan, from 51 nm (at idle) to 67 nm (at max thrust) in turboprop and from 20 nm (at idle) to 42 nm (at max thrust) in a turboshaft engine.
4.13.5. Changes of particle number and size after the dilution of plumes
The effects of the aircraft-related emissions of UFP at airports have received increasing attention in recent years and some studies have demonstrated a clear dependence of UFP concentrations and size distributions upon aircraft operations. In addition, UFP measurements upwind and downwind of airports are of particular importance because they are performed under ambient conditions, i.e. after the plume has been diluted by air and the particle coagulation and gas-to-particle condensation processes have occurred.
Hu et al. (2009) studied the effect of aircraft movements in a neighbourhood adjacent to the regional airport of Santa Monica and observed that spikes in the particle number concentration related to the take-off phase were 440 times elevated above background and reached 2.2 × 106 particles cm−3. At a site located at the blast fence of Los Angeles International Airport, Zhu et al. (2011) reported that total UFPs counts exceeded 107 particles cm−3 during take-offs. This study further investigated temporal profiles in particle concentration of 30 nm mobility diameter (corresponding to the mean geometric mode of emitted particles) due to isolated aircraft take-off events: dramatic increases of particle concentrations (from 1.6·103 to 1.7·104 particles cm−3) were reported when aircraft engines are accelerated to the 100% thrust power for take-off, followed by decreases of number concentrations showing an exponential decay. Similar findings have been reported by Hsu et al. (2012), who observed that departures of jet engine aircraft on a runway may contribute to 1·103 to 7·104 particles cm−3. The same authors further revealed significant higher increases of UFP at Los Angeles International airport (Hsu et al., 2013) due to the LTO activity: 2·106‒7·106 particles cm−3 increase at a monitor at the end of the departure runway, 8·104‒1.4·105 particles cm−3 at a site 250 m downwind from the runway.
Changes in the particle size distributions can also occur after plumes are diluted in ambient air due to coagulation. However, most studies have shown that particle size distributions at airports are comparable with those measured during engine tests. Air monitoring carried out in the surroundings of the Los Angeles International Airport found that the upwind site was dominated by particles of approximately 90 nm diameter whereas downwind sites were dominated by finer particles, peaking at approximately 10–15 nm (Westerdahl et al., 2008), which corresponds to the size reported during APEX campaigns for many in-use engines (Kinsey et al., 2010). Similarly, Fanning et al. (2007) and Zhu et al. (2011) reported very high number concentrations of UFPs collected at the blast fence site, with the highest numbers found at a particle size of approximately 14 nm. The same study further observed that the UFP number concentrations measured in a residential community approximately 2–3 km downwind of the airport were intermediate in concentration between the airport runway and the background reference site. This finding was associated with aircraft take-off activities and the authors noted the significant exposure and possible health implications for people living near the airport. Mazaheri et al. (2009) revealed that size distributions exhibit similar modality during all phases of the LTO cycles with particles predominantly in the range of 4–100 nm in diameter. This latter study also reported two distinct modes: a nucleation mode at diameters <30 nm observed in all LTO modes and an accumulation mode between 40 and 100 nm more pronounced during take-offs. While the nucleation mode exhibited the highest number concentration of all modes, the accumulation mode dominated the particle mass size distributions. Lobo et al. (2012) measured the specific PM emissions during normal LTO operations at a distance of 100–300 m downwind of an active taxi-/runway at the Oakland International Airport and associated the data with various aircraft/engine combinations. The size distributions were typically bimodal with a nucleation mode composed of freshly nucleated PM and an accumulation mode mostly made up of soot with some condensed volatile material. These observations closely parallel the mechanisms and size distribution of particles in diesel exhaust (Harrison et al., 2011).
4.14. Chemical composition of PM
Although the chemical composition of PM may include most of the periodic table of the elements and many thousands of different organic compounds, it is principally composed of few major components, which usually represent several percent of the total mass of particles, and some of those may remain in thermodynamic equilibrium between gaseous and particle phases. The particulate matter emitted directly by aircraft is mostly composed of soot (e.g., Anderson et al., 2005; Timko et al., 2010b), while sulphate and semi-volatile hydrocarbons may further coat the particles after the plume dilution. However, aircraft PM may also contain traces of metals and ions, which are mainly the result of: (i) fuel impurities; (ii) corrosion and wear of mechanical components of engines; (iii) pre-existing PM drawn in the combustor. The following sub-subsections discuss the various components separately.
4.14.1. Carbonaceous PM
Carbonaceous PM consists of a complex mixture of elemental carbon (EC) and organic carbon (OC) (jointly referred to as soot) and commonly accounts for a large fraction of ambient fine particle mass in both rural and urban environments. Soot is primarily generated by incomplete combustion processes through the pyrolysis of organic fuels used in combustion processes. Many studies have discussed the various types of such particles; however there are still controversies and open discussion about the terminology to adopt. The terms used to identify the various fractions of carbonaceous aerosols, such as soot, black carbon (BC), elemental carbon (EC), equivalent black carbon and refractory black carbon are mainly associated with the corresponding measurement methods (e.g., Pöschl, 2003, Andreae and Gelencsér, 2006, Bond and Bergstrom, 2006, Kondo et al., 2011, Buseck et al., 2012, Long et al., 2013, Novakov and Rosen, 2013) and more generally refer to the most refractory and light-absorbing component of carbonaceous combustion particles, even if the underlying definitions and measurement methods are different (Petzold et al., 2013). Without going into the merits of this discussion, this section provides an overview of the data concerning the carbonaceous fraction and the terms used (soot, BC and EC) are the same as reported by the original authors. In any case, Lee et al. (2010) indicated that BC is often used interchangeably with soot in the literature relating to aircraft emissions, although in the strictest sense they are different.
The airliners of 1960s and 1970s emitted visible and dark exhaust plumes, especially during take-off. In recent decades, a great effort has been made by most engine manufacturers to reduce such emissions, which consisted mainly of soot and organics, and nowadays most modern airliners do not emit visible plumes. However, soot is still the primary form of non-volatile PM emitted by jet engines (e.g., Timko et al., 2010b), even if its contribution represents only few percent of the global atmospheric BC emission (Hendricks et al., 2004).
From a morphological point of view, soot particles emitted by aircraft engines have nearly spherical shapes with lognormal size distributions peaking at 30–60 nm (Petzold et al., 2003, Petzold et al., 2005a, Popovicheva et al., 2004). However, once emitted soot particles quickly build complex agglomerates causing a second mode of larger particles between 100 and 500 nm, which are totally amorphous (Petzold et al., 1998, Popovitcheva et al., 2000, Popovicheva et al., 2004, Demirdjian et al., 2007). Despite the structural characteristics of soot being of primary importance in relation to its atmospheric properties, there is a lack of experimental data on microstructure, composition and hygroscopicity of original soot emitted from aircraft engines. Some studies conducted at cruise height (Kärcher et al., 1996, Gleitsmann and Zellner, 1998) have assumed that all the soot particles in exhausts are hydrophobic. Demirdjian et al. (2007) used a combination of several analytical methods to study the microstructure and the composition of soot agglomerates sampled in an aircraft engine combustor and reported that soot was in two main fractions having quite different physicochemical properties. A major fraction of particles was found to be made up of amorphous carbon with small amounts of oxygen, sulphur and iron and was rather hydrophobic, while a second fraction was characterised by various structures and a large amount of impurities and was highly hydrophilic. Vander Wal et al. (2010) compared the physical structure and the chemical composition of soot produced by different sources, including a modern TF engine, using high resolution transmission electron microscopy and X-ray photoelectron spectroscopy. The results showed that some physical characteristics of jet engine soot, such as the lamella length distributions, are intermediate between soot produced by other sources such as wildfires and diesel, while other characteristics are singular. Jet soot was reported to have the highest sp3 carbon content, in fact higher than the sp2 (graphitic) content, the greatest oxygen content in the form of phenolic and carbonyl groups and the widest range of hetero-elements, including S, Na, N, Zn, Ba.
From a chemical point of view, soot is mainly made up of graphitic BC (Petzold et al., 1999, Popovicheva et al., 2004), but some particles can be also coated with organic materials and sulphur species (e.g., Petzold et al., 2003). For example, the hygroscopic properties of jet engine combustion particles have been investigated in several rig-tests and results have confirmed that the water uptake by combustion particles is generally independent of combustor operating conditions, but increases significantly with increasing FSC level, which is attributed to an increasing amount of sulphuric acid adsorbed on the particles (Gysel et al., 2003). The uptake of sulphuric acid and organics seems to be enhanced by the surface irregularities in the soot. The typical fractal agglomerate structure of soot may offer a large specific surface area for adsorption and chemical reactions (Popovitcheva et al., 2000). Recently, Loukhovitskaya et al. (2013) also investigated the uptake of HNO3 on aviation soot.
The EIs of elemental and organic carbon were investigated during APEX campaigns (Kinsey, 2009, Onasch et al., 2009): results showed that EC ranged from 21 to 98 mg kg Fuel−1 and OC between 37 and 83 mg kg Fuel−1. Most studies indicated that BC emissions are a function of engine thrust settings (Anderson et al., 2005, Wey et al., 2007, Kinsey, 2009, Kinsey et al., 2011), but are nearly independent of FSC (e.g., Wilson et al., 2004, Kinsey, 2009). During the EXCAVATE campaign, Anderson et al. (2005) concluded that black carbon emission indices increase significantly from idle to cruise power. These findings are also consistent with the results of the APEX campaigns: Wey et al. (2007) and Kinsey et al. (2011) reported that BC emissions are minimum at low power and increase with thrust settings, reaching values more than 0.3 g kg Fuel−1 at power levels higher than 85% F00 and dominating the total mass emissions. Agrawal et al. (2008) reported that the carbonaceous PM composition (EC + OC mass) significantly increases with power and shifts from OC-rich at idle to EC-rich with rising thrust regimes. Similar findings were observed by Petzold and Schröder (1998), who indicated that the ratio of BC to total carbon ranged from 11% at idle to >80% at take-off thrust. This result is predictable when considering that the highest emissions of hydrocarbons occur at low power. Presto et al. (2011) recently investigated both the elemental carbon and the organic aerosol emitted by a CFM56-series engine at varying thrust settings after the exhaust using a smog chamber. Their findings confirmed the U-shaped curves of PM emissions versus thrust commonly reported in the literature, but also added new important knowledge on the relative contributes of EC and OA. At low power (4%–7% F00), most PM is composed of OA, while at 30% thrust very low emissions of both elemental and organic components were observed. At climb power (85%), an abrupt increase of EI(PM) occurred, mainly driven by EC, which accounted for about two thirds of the total PM.
The chemical characterisation of the organic component of the PM indicated that over 70% of the particle-phase organic compounds are made up of SVOC compounds in the n-alkane (mainly C23 to C33), PAH, and sterane/hopane compound classes (Kinsey et al., 2011). Besides the lighter PAHs, which mainly partition in the gaseous phase, the heavier congeners are principally in the particulate phase and generally also have the highest carcinogenic and mutagenic potencies (Delgado-Saborit et al., 2011). Hu et al. (2009) studied the effect of aircraft movements at a site located 100 m downwind of the regional airport of Santa Monica and reported spikes in concentration of particle-bound PAHs occurring during jet take-offs (440 ng m−3, i.e. 90 times the local background levels), however they did not detect significantly higher average levels of PAHs at airports. It is interesting to note that PAH emissions at airports may also undergo local deposition. In a study carried out at Delhi International Airport, Ray et al. (2008) observed that PAH contamination in the <2 mm surface soil layer reached maximum levels at a site near the landing area. The presence of PM-bound hopanes and steranes is also intriguing because these compounds are present in crude oil and are also largely used as molecular markers of vehicle emissions (e.g., Zielinska et al., 2004, Kam et al., 2012). Additional insights are therefore necessary for the characterisation of these organic compounds, which can derive either from the unburned fuel or from the emission of lubricating oils, which was hypothesised to have an important role in the mass of organic PM (Yu et al., 2010).
The emission of carbonaceous PM was also reported in further studies conducted at airports. For example, Dodson et al. (2009) performed continuous BC measurements at five monitoring sites in close proximity to a small regional airport in Warwick, Rhode Island. By coupling BC data with real-time flight activities (departures and arrivals) and meteorological data, they reported that aircraft departures and arrivals (and other sources coincident in space and time) contribute approximately 24–28% of the total BC concentrations. Further, they also indicated that aircraft take-off makes a greater contribution to BC levels than landing. Hu et al. (2009) studied the effect of aircraft movements in a neighbourhood adjacent to the regional airport of Santa Monica and generally did not observe elevated average levels of BC, although spikes in concentration of this pollutant were observed associated with jet take-offs. At a site located 100 m downwind of the take-off area, jet departures resulted in short time (60 s) peaks with average concentrations of up to 30 μg m−3, i.e. 100 times elevated above the local background.
4.14.2. The smoke number (SN)
Despite soot corresponding to the majority of the non-volatile mass of PM emitted by aircraft, this component is not directly certified by ICAO. However, the ICAO databank requires that an exhaust opacity metric called the smoke number (SN) is measured for TF engines. SN was defined as a “dimensionless term quantifying smoke emission level based upon the staining of a filter by the reference mass of exhaust gas sample and rated on a scale of 0–100” (ICAO, 2008). SN was firstly collected on a filter by flowing a defined volume of the exhaust gas (12–21 kg of exhaust gas per square metre of filter) by a sample probe positioned directly behind the engine nozzle and inside the exhaust jet. The degree of attenuation of the filter before and after the sampling was thus measured using a reflectometer, and the SN was computed as:
where R 0 and R f are the absolute reflectance of the filter before and after the sampling, respectively. Unfortunately, SN gives only a qualitative estimate of particle emission and was recognised to be dependent on sampling conditions, soot characteristics and morphology, and therefore was assumed to have little value for estimating atmospheric impacts (Anderson et al., 2005). Moreover, it was reported that particles with a diameter less than 300 nm passed through the filter and therefore only the larger particles are collected resulting in a relative weak accuracy of measurement (Kugele et al., 2005).
Several studies have attempted to correlate SN to BC mass concentration (e.g., Champagne, 1971, Whyte, 1982, Girling et al., 1990, Petzold and Döpelheuer, 1998, Wayson et al., 2009, Peck et al., 2013, Stettler et al., 2013a, Stettler et al., 2013b) and today an interim methodology named first-order approximation 3.0 (FOA3) was developed and used to estimate BC mass emissions normalised by fuel burn EI(BC) from SN (Wayson et al., 2009). Although this calculation was reported to be dependent upon the mode-specific SN recorded in the engine databank (e.g., Stettler et al., 2011), recently Stettler et al. (2013b) observed that the correlation between BC and SN depends on the particle size distribution and that the methods suggested to convert SN to BC could lead to heavy underestimations of BC concentrations. An alternative method independent of the SN (FOX) was also recently developed and first studies reported an improved estimation of BC (Stettler et al., 2013a), but it needs to be further tested. To fill this gap, recently a group of experts was called to define new standard procedures for BC measurement at ground level for regulatory purposes (SAE, 2009). In the absence of defined standards, the scientific literature offers a number of studies on the emission of soot, BC and EC.
4.14.3. Inorganic ions
The analysis of the major inorganic ions in aircraft exhaust has a clear dependence on the adopted sampling methodology and can be affected by many artefacts. As for most hydrocarbons, ions may undergo gas-to-particle partitioning and some species may further derive from chemical reactions in the atmosphere or on the filter surface. For example, the concentrations of aerosol nitrate can be affected by the adsorption of nitric acid gas on pre-existing particles, while evaporative losses occur at temperatures >20 °C and the exhaust plumes largely exceed this temperature. In addition, sulphate may form quickly due to the oxidation of SO2, coating soot particles. In view of this, Anderson et al. (2005) firstly reported that the concentration of sulphate aerosol rose considerably as sampling was performed progressively downstream of the engine, suggesting that sulphate particles may originate or undergo rapid growth within aircraft exhaust plumes. These findings were further confirmed by APEX campaigns. Agrawal et al. (2008) noted that the mass of the ions collected at 1 m from the engine exit plane were below the detection limit for most ions, while only sulphate was detectable. On the contrary, APEX samplings at 30 m reported EI(ions) in the range of 30–40 mg kg Fuel−1 dominated by sulphate (53%–72% of the total ion EIs) and ammonium (Kinsey et al., 2011). In summary, there is a lack of data on the ionic component of exhaust emissions of aircraft and this merits further investigation.
4.14.4. Elemental composition
There is a severe shortage of data on the elemental composition of PM emitted by aircraft. Kinsey et al. (2011) reported that PM2.5 emissions are composed of various trace elements mainly originating from fuels, lubricating oils, engine wear and corrosion, although release from the sampling line and fugitive dust may contribute to the total load. During the APEX campaigns, the elemental composition of PM emitted from aircraft engines was analysed for a number of different aircraft engines. The total elemental emissions (sum of Mg, Si, P, S, Cl, K, Ca, Ti, Cr, Mn, Fe, Ni, Cu, Zn, Br, Ag, In, Sb, Te, I, Tl) were in the range of 6.3–27.5 mg elements kg Fuel−1, corresponding to 2–7% of the total emitted PM and were dominated by sulphur (54%–80% of total element mass) (Kinsey, 2009, Kinsey et al., 2011). As expected, sulphur was well correlated with sulphate and most of the sulphur on the filter exists as sulphate (Agrawal et al., 2008). Moreover, the variability in the metal emissions was observed to be much greater between different engines than between engine thrust settings (Agrawal et al., 2008).
Recently, Mazaheri et al. (2013) investigated the physical and chemical characteristics of individual particles collected in the exhausts of in-use aircraft during landing and takeoff by using transmission microscopy and energy dispersive X-ray spectroscopy. They reported that most of the measured particles have a spherical shape in the nucleation mode (18−20 nm) and only contain C, O, S, Cl, and in some cases K. They also reported fewer particles having a more irregular shape resulting in a larger average aspect ratio and a much greater and diverse range of elements. While the small spherical particles have been linked to the combustion processes of engines, the latter irregular particles have been linked to a diverse range of sources, including tyre wear, fine dusts, vehicular traffic, and possibly engine wear.
4.14.5. Secondary aerosol
Despite the potential role of aircraft emissions in forming SIA and SOA, there is a lack of information on the chain of processes affecting aircraft emissions once emitted in ambient air. A recent study by Miracolo et al. (2011) used a smog chamber to simulate the aging of the particulate matter emitted from a TF engine under typical (summertime) atmospheric conditions. Their findings pointed out the key role of the photo-oxidation processes in forming both SIA and SOA. They reported that after several hours of photo-oxidation, the ratio of secondary-to primary PM mass was on average 35 ± 4.1, 17 ± 2.5, 60 ± 2.2 and 2.7 ± 1.1 for increasing thrusts settings (4%, 7%, 30% and 85% F00, respectively). Miracolo et al. (2011) also observed that SOA dominates the secondary PM at low thrust, while secondary sulphate becomes the main secondary component at higher power.
It is not clear if aircraft emissions can influence the amount of secondary aerosol on a large scale. In this regard, a recent study by Woody and Arunachalam (2013) used the Community Multiscale Air Quality (CMAQ) model to investigate the impacts of aircraft emissions on SOA at the Hartsfield-Jackson Atlanta International Airport. By applying the model at various spatial resolutions, they reported that aircraft emissions reduced SOA by ∼6% at 36 and 12-km due to the chemistry of the free radicals with aircraft NOx, while at smaller resolution the interaction between the aircraft emissions and external biogenic SOA precursors enhanced SOA (∼12%).
5. Aircraft non-exhaust emissions
Although the vast majority of studies have focussed upon the exhaust emissions from engines, there are other aircraft-related emissions that may influence the air quality within an airport. These include emissions from the power units, i.e. APUs and GPUs, primary particles from tyre erosion and brake wear, oil leaks and corrosion of aluminium alloys, all of which have been recognised to impact air quality near airports but at date have received only limited consideration.
5.1. Tyre, brake and runway surface wear
Tyre and brake wear during landing and runway dust re-suspension have been estimated to be major sources of particulate matter. This is expected as smoke is clearly visible to the naked eye when aircraft wheels contact the ground and spin up to the landing velocity. Despite that, the proportion of the mass lost from aircraft tyres and brakes that becomes suspended as fine PM has not been extensively studied; the few available data indicate that the rubber lost from tyre wear can vary from few grams to ∼0.8 kg per landing (Morris, 2006, Bennett et al., 2011 and references therein). Particulate emissions from tyres have been suggested to be dependent upon the maximum take-off weight, but other factors may have a role in the rubber wear, e.g., number of wheels, weather conditions, engine type, airport runway length and taxiway layout and operating procedures (Morris, 2006). The subsequent activation of brakes to bring the aircraft to a stop may further abrade brake lining material from discs and pads and may release fine particles as for road vehicles (e.g., Pant and Harrison, 2013). From a physicochemical point of view, it is plausible that brake wear includes both the emission of material from the abrasion of discs and the volatilisation and condensation of brake pad materials, while soot may arise from the thermal degradation of tyre polymers. This was confirmed by experimental data collected at a major European airport: Amato et al. (2010) reported unusually high levels of both organic carbon and metals possibly sourced from tyre detritus/smoke in runway dust (Ba, Zn, Mo) and from brake dust in ambient PM10 (Cu, Sb). In addition to tyre and brake wear, landing field wear and re-suspension can also occur, as usually aircraft land on a runway generally constructed of asphalt, concrete, gravel or grass.
For example, studies at Gatwick airport estimated that tyre and brake wear are dominant sources of PM10, accounting about 22 and 4.5 tonnes y−1, respectively, i.e. about 60% and 12% of all aircraft-related emissions, respectively (British Airports Authority, 2006). However, these emissions are subject to large uncertainties as they are dependent on many factors, including speed at landing, some aircraft characteristics (weight, number of wheels, brake material if carbon or steel) and runway characteristics (length, weather conditions) (Underwood et al., 2004).
Bennett et al. (2011) collected landing and braking dust samples from the undercarriage (oleo legs) and wheel hubs of aircraft and reported that they have bimodal distributions, with peaks at aerodynamic diameters of about 10 and 50 μm. A further SEM-EDS analysis has revealed that particles may contain various materials embedded in a carbonaceous substrate: (i) soot arising from the burning of the tyre rubber, from the asphalt tar or from brake abrasion; (ii) runway dust mainly composed of typical crustal materials (quartz and feldspar particles) which are lifted mechanically from the ground surface; (iii) small droplet (35 μm) of Fe, associated with Co and other transition metals (Mn, Ni, V, Zn) which are commonly found in asphalt concrete and (iv) irregular Fe particles (<10 μm). This study also reported that aluminium, which is typically used as tracer for crustal materials from runway wear, can also derive from Al hydroxide included in some tyre formulations.
5.2. Other mechanical components
High-strength aluminium alloys are commonly used as the aircraft fuselage materials in the body and wings, while minor amounts of other elements (Cu, Zn, Mg) may be also present in various airframe components (Wei et al., 1998). Aluminium alloys have a microstructure that can be highly susceptible to intergranular and pitting corrosion, and weathering is recognised as a major cause of structural damage to aircraft structure and coatings (Usmani and Donley, 2002, Russo et al., 2009: Knight et al., 2011), along with long term operations (Ostash et al., 2006), runway de-icing chemicals (Huttunen-Saarivirta et al., 2011) and atmospheric pollution and salts (Cole and Paterson, 2009). The degradation of aircraft mechanical components is also connected with mechanical, and corrosion-mechanical (macrocracks) defects, which lead to a decrease in its load-bearing capacity (Ostash et al., 2006). Corrosion has many forms and affects most structural alloys found in airframes: of particular importance is pitting and intergranular corrosion, which can develop into fatigue cracks, stress corrosion cracks or exfoliation (Liao et al., 2008). In this light, it is plausible that corrosion and mechanical stress of some aircraft components may release metallic particles into the environment. For example, using scanning electron microscopy techniques, Amato et al. (2010) founded the relatively common presence of platy aluminous particles derived from airframe corrosion in the ambient PM10 samples collected near the El Prat airport in Barcelona.
5.3. Oil leaks
In addition to exhaust from jet fuel combustion, oil escaping or burning from lubricated parts may be vented overboard from aircraft engines and therefore may further contribute to the total emissions of aircraft (Onasch et al., 2009, Timko et al., 2010b, Yu et al., 2010, Yu et al., 2012). Aircraft lubricating oils are usually composed of a mixture of synthetic C5–C10 fatty acid esters of pentaerythritol and dipentaerythritol with specialised additives (Yu et al., 2010, Yu et al., 2012). Some of these, such as tricresyl phosphate, are recognised as toxic to humans (Craig and Barth, 1999, Van Netten, 1999, Winder and Balouet, 2002: Marsillach et al., 2011) and have been detected in ambient air and aircraft cabins, posing a risk for aviation technicians, loaders, crew and passengers in case of release into the environment (e.g., Solbu et al., 2010, Liyasova et al., 2011, Denola et al., 2011, Schindler et al., 2013). Yu et al. (2010) reported that the degree of degradation of lubrication oil during aircraft engine operations as a result of friction and/or pyrolysis might be negligible, suggesting that most emitted oil is unburned. Because of its low volatility, unburned lubricating oil may exit from engines as vapour or submicrometre droplets and may further condense and add mass to the organic PM in the wake of the aircraft. Results of exhaust characterisation measurements suggest that the contribution of lubrication system releases to the organic PM may be greater than the engine exhaust (Timko et al., 2010b): they estimated that the contribution of oil leaks to the total mass of organics generally lies within the range 10–20% for low thrust and 50% for high thrust settings. A recent study (Yu et al., 2012) has identified and quantified the lubricating oil in the particulate matter emissions from various engines of in-service commercial aircraft at two airports. This study used the characteristic mass marker of lubricating oil (ion fragment intensity between m/z = 85 and 71) to distinguish lubricating oil from jet engine combustion products. Results revealed that lubricating oil is commonly present in organic PM emissions in association with emitted soot particles, unlike the purely oil droplets observed at the lubrication system vent. The contribution from lubricating oil in aircraft plumes was observed to vary from 5% to 100% in measured aircraft plumes.
Yu et al. (2010) measured the size distributions of submicrometre unburned lubricant oil released from engines with C-TOF-AMS and UHSAS and reported a shift to larger sizes with increasing power. At idle thrust they observed a C-TOF-AMS vacuum aerodynamic diameter (D va) of 260 ± 3 nm, while the UHSAS volume equivalent diameter (Dve) was 281 ± 9 nm. At higher engine power, they observed modes at 272 ± 4 nm and 350 ± 8 nm for C-TOF-AMS and UHSAS, respectively.
6. Other airport-related emissions
Apart from aircraft exhaust and non-exhaust emissions, other sources can be present within an airport and can contribute to the total pollutant load in the atmosphere. Among others, the emissions of the power units providing power to the aircraft (APUs and GPUs), the GSEs, additional sources on the modern terminals, intermodal transportation systems and road traffic are further considered as impacting upon the air quality and must be taken in account in airport emission measurements.
6.1. Auxiliary and ground power units
The APUs are small on-board gas-turbine engines burning jet fuel coupled with an electrical generator capable of supplying electrical power to aircraft systems when required on the ground or providing pneumatic or hydraulic power to start the main engines. Despite APUs being installed in all modern airliners so as to be energetically independent, their use is becoming less significant over time due to the increasing trend toward mains supplied ground power units (GPU) (Mazaheri et al., 2011). This ground equipment is supplied by the airports and includes diesel powered tugs of various types, ground carts, and also APUs installed on ground carts (e.g., Kinsey et al., 2012b). Some airports also provide electrical power to the aircraft by connecting directly to the ground network and by using fixed ground electrical power (FGEP) units. This system avoids the use of fuelled power units, with a subsequent reduction in local emissions and is thus very useful in airports not complying with air quality standards.
The role of the APUs on the air quality at airports is nowadays widely discussed and an increasing number of studies have estimated their contribution. However, the results are often conflicting. Schäfer et al. (2003) indicated that APU emissions at airport service buildings cannot be neglected in comparison to the main engine emissions. The emission inventory of the airport of Zurich in 2004 (Fleuti and Hofmann, 2005) reported that although the aircraft exhaust accounted for most of CO, hydrocarbons and NOx (89%, 45%, 82%, respectively of total emissions), a significant percent was from APUs, GPUs, start-up-idle, handling/GSE, airside traffic and stationary sources, with APUs accounting for about half of the total non-aircraft engine emissions. HAL (2011) reported that 19% of the total NOx emissions of London Heathrow airport are due to the use of APUs. A survey over 325 airports in the USA (Ratliff et al., 2009) estimated the emissions from APUs and LTO cycles and stated that the greatest percentage that APUs contributed to total aircraft emissions was 10–15% for CO and between 15 and 30% for NOx and SOx. However, this study also reported that the airports used by a higher percentage of small and business jets tend to be affected by higher emissions from the APUs. Stettler et al. (2011) estimated that APUs contribute 6% to total PM2.5 emissions at major UK airports. The effect of the APUs upon public health was recently estimated by Yim et al. (2013), who calculated the emissions from aircraft LTO activity, aircraft APUs and GSE at the top 20 UK airports, ranked by passenger numbers. Their findings concluded that the ban on the use of APUs would prevent about 11 averted early deaths per year (90% confidence interval 7–16).
Unlike aircraft engines, APU emissions are not certificated by ICAO, and the manufacturers generally consider information on APU emissions rates as proprietary (ICAO, 2011), therefore there are today few data available on APU emissions. Emissions from APU depend on many factors and are subject to change through provision of GPU facilities from the airport. Some airports have implemented policies to encourage the use of the GPU instead of APUs (Mazaheri et al., 2011 and reference therein), however in the absence of GPU availability, the use of APUs is still the only alternative to provide the energy for aircraft operations with engines off and for the ignition of the engines. The first studies of APU emissions started in the 1970s by the US Army (Kinsey et al., 2012b and references therein) and our literature search has found very few data in comparison to those on the jet engine emissions. However, the main studies reporting (or reprocessing) data on the APU emissions are increasing nowadays (Slogar and Holder, 1976, Williams and Lee, 1985, Gerstle et al., 1999, Gerstle et al., 2002, Wade, 2002, O'Brien and Wade, 2003, Schäfer et al., 2003, Watterson et al., 2004, EASA, 2011, Anderson et al., 2011, Blakey et al., 2011, Kinsey et al., 2012b, Williams et al., 2012).
6.2. Ground service equipment emissions, vehicular traffic and other sources
As they are strictly linked to the airport operations, the amount of GSE vehicles clearly reflects the airport layout and traffic in terms of both cargo and passengers. Moreover, the operation duration is expected to increase with increasing aircraft size. Other factors include the type of engines installed and the quality of fuels used and the status of the vehicle fleet (age, wear and tear). Therefore, it is not possible to identify the unique characteristics common to all the airports and ICAO databanks not include any information about GSE emissions. Similarly, the amount of road traffic in the form of private cars, taxis, shuttle bus and trucks for transporting people and goods in and out to the airport depends on the airport layout, on the quality of the road links and intermodal transport systems and, finally, is directly related to the number of passengers and goods that the airport handles. As both the airport-induced vehicular traffic and most of the GSEs have gasoline or diesel engines, it is reasonable to consider them as common traffic. The traffic source is recognised to be dominant in many urban environments. Its chemical and physical characteristics are reported elsewhere, in a large number of studies and reviews (e.g., Hueglin et al., 2006, Thorpe and Harrison, 2008, Johansson et al., 2009, Gietl et al., 2010, Kumar et al., 2011, Harrison et al., 2012, Pant and Harrison, 2013, Amato et al., 2013).
Some studies have indicated that GSE may contribute a major fraction of the total AEs. For example, a study carried out at the McCarran airport in Las Vegas reported that approximately 60% of the total airport emissions are related to GSE (Nambisan et al., 2000). Schürmann et al. (2007) calculated that NO concentrations at Zurich airport were dominated by emissions from ground support vehicles, while Unal et al. (2005) estimated that the impacts on ozone and PM2.5 of GSE at the Hartsfield–Jackson Atlanta International airport are small compared to the aircraft impacts. In addition, other miscellaneous sources may be also present at airports and may further increase the total pollutant load, including maintenance work, heating facilities, fugitive vapours from refuelling operations, kitchens and restaurants for passengers and operators, etc. Despite being intermittent and depending on the airport layout, these emissions may be dominant in certain circumstances. For example, Amato et al. (2010) reported that the local construction work for a new airport terminal in a major European airport (El Prat, Barcelona) was an important contributor to PM10 crustal dust levels along with road dust and aircraft re-suspension, with a clear drop during the weekends.
7. Airport emissions and public health
While aircraft emissions at cruising altitudes are an air pollution issue at global scale (Barrett et al., 2010, Koo et al., 2013), the emissions within the planetary boundary layer due to the LTO operations are certainly more local and it is plausible to believe they may have a more direct effect on human health. Nevertheless, the potential subsidence of air masses due to the Ferrell and Hadley circulations, which may displace high altitude emissions toward the ground cannot be disregarded (Barrett et al., 2010).
Air quality degradation in the locality of airports is considered by some to pose a real public health hazard (Barrett et al., 2013) and some recent estimates of the aviation contribution to premature mortality have been reported (e.g., Ratliff et al., 2009, Levy et al., 2012, Ashok et al., 2013, Yim et al., 2013). Although at the current time, no specific target toxic compound has been identified to be used as a marker or indicator for human exposure to jet engine fuels and their combustion products (Tesseraux, 2004), it has been estimated that over 2 million civilian and military personnel per year are occupationally exposed to jet fuels and exhaust gases (Pleil et al., 2000, Ritchie, 2003, Cavallo et al., 2006). Kerosene-based fuels have the potential to cause acute or persistent neurotoxic effects from acute, sub-chronic, or chronic exposure of humans or animals (Ritchie et al., 2001), although evidence is lacking that current levels of exposure are harmful. Occupational exposure can occur by dermal, respiratory or oral ingestion routes of raw fuel, vapour, aerosol or exhausts. It has been postulated that chronic exposure to vapours and exhaust fumes could affect the operators inside the airport (Cavallo et al., 2006) and aircraft crew (Denola et al., 2011, Schindler et al., 2013), while occasional exposure can affect all passengers in transit (Liyasova et al., 2011). In addition, also the population living in the vicinity of airports can be exposed (Jung et al., 2011).
However, the impact of LTO emissions on surface air quality and human health is poorly quantified (Barrett et al., 2010) even though most governments have recently focused attention on management and reduction the environmental impacts of aviation. Some studies have attempted to estimate the direct and indirect effects of aviation to support environmental policy assessments and to evaluate many possible future scenarios. A global-scale study by Barrett et al. (2010) estimated that ∼8000 premature deaths per year can be attributed to aircraft emissions at cruising altitudes, representing ∼80% of the total impact of aviation (including LTO emissions) and ∼1% of air quality-related premature mortalities from all sources.
A series of more local studies have been conducted to assess the impact of AEs on human health. Generally the results have highlighted the potential adverse effects of AEs on public health and also revealed the need for more extensive information about this source. Three estimates were given for US airports in 2005: Ratliff et al. (2009) analysed aircraft LTO emissions at 325 US airports with commercial activity and estimated that 160 (90% confidence interval 64–270) premature deaths occurred due to ambient particulate matter exposure attributable to the aircraft emissions; Levy et al. (2012) estimated about 75 early deaths using activity data from 99 US airports; Ashok et al. (2013) estimated that aviation LTO emissions caused about 195 (90% confidence interval 80–340) early deaths, while the same emissions were forecast to cause ∼350 (90% confidence interval 145–610) deaths in 2018. Arunachalam et al. (2011) used the Community Multiscale Air Quality model (CMAQ) to estimate the incremental contribution to PM2.5 due to commercial aviation emissions during LTO cycles in two major and one mid-sized US airport and reported that 8–9, 11–15 and 5 (depending on model resolution) premature deaths per year can be estimated for Atlanta, Chicago and Providence airports, respectively. In Europe, Yim et al. (2013) estimated that 110 (90% CI: 72–160) early deaths occur in the UK each year (based on 2005 data) due to airport emissions. The same study also assessed that up to 65% of the health impacts of UK airports could be mitigated by replacing current fuel with low FSC fuel, by electrifying GSE, avoiding use of APUs and use of a single engine during the taxi phase. Lin et al. (2008) estimated that residents living within five miles of Rochester and La Guardia airports are affected by an increased relative risk of hospital admission of 1.47 and 1.38 respectively compared to resident living >5 miles distant. Jung et al. (2011) characterised the levels of BTEX in the vicinity of the Teterboro airport, New York/New Jersey metropolitan area, by exposing passive samplers for 48 h at the end of airport runways, in households close to the airport and out-of-neighbourhood locations. Results indicated that the average concentrations of benzene, toluene, ethylbenzene, m-/p-xylenes and o-xylene in neighbourhood concentrations (0.8, 3.8, 0.4, 1.2 and 0.4 μg m−3, each BTEX respectively) were not significantly different to those measured at the airport runways (0.8, 3.2, 0.3, 1, and 0.3 μg m−3, respectively) and higher than the out-of-neighbourhood locations (0.5, 1.1, 0.2, 0.8, and 0.4 μg m−3, respectively). Cavallo et al. (2006) characterised the exposure to PAHs in airport personnel and evaluated the genotoxic and oxidative effects in comparison with a selected control group. They analysed 23 PAHs collected from various areas over five working days and urinary 1-hydroxypyrene (1-OHP) following five working days as a biomarker of exposure. They reported an induction of sister chromatid exchange due to PAH exposure, although its health significance was not quantified.
8. Conclusions
The main goal of this review is to give an overview on the current state of knowledge of airport-related emissions and to summarise the key characteristics of pollution and the impacts on local and global air quality. After thoroughly reviewing the latest available scientific literature, it can be concluded that the currently available information on the impact of AEs upon air quality is inadequate and the consequences of future growth in the volume of air traffic are very hard to predict. Most work has focussed upon aircraft engine exhaust during LTO cycles which accounts for a large proportion of the total emitted pollutants. However other sources such as the auxiliary power units, vehicular traffic and ground service equipment are known sources that may seriously affect air quality near to airports. In this way, it is apparent from the literature that while aircraft exhaust may account for most of the pollution at some airports, there are other sources that need to be addressed in more detail in the future, such as:
-
•
tyre, brake, asphalt wear and the re-suspension of particles due to the turbulence created by aircraft movements;
-
•
the emissions from the units providing power to the aircraft when required on the ground (APUs and GPUs);
-
•
the ground support equipment that an airport offers as a service for flights and passengers, including passenger buses, baggage and food carts, container loaders, refilling trucks, cleaning, lavatory servicing and de/anti-icing vehicles, and tugs;
-
•
the effects of the intermodal transportation systems, and road traffic for transporting people and goods in and out to the airport.
Most studies report that airport operations are responsible for significant emissions of a series of non-volatile, gaseous and semi-volatile species. Non-volatile emissions are made up of refractory material such as soot, which is emitted as PM even at high temperatures, but is also comprised of many organics and sulphur compounds, the latter mainly in the form of sulphate. Volatile emissions include compounds that exist as vapour at the engine exit plane and are made up of gaseous and vapour-phase pollutants, such as CO, NOx, SO2 and many organics (i.e. aromatics, alkanes, alkenes and a number of other VOCs). The less volatile fraction is of especial interest as it can react in the atmosphere and undergo gas-to-particle conversion by forming new particles or condensing on pre-existing ones.
The volatile emissions have mostly been fairly well characterised, but a comprehensive chemical speciation of the hydrocarbons and complete knowledge of their chemical processing in the atmosphere is still lacking. Detailed information on the non-volatile and semi-volatile compounds is also scarce. In spite of the increasing attention given to AEs, many issues remain unaddressed and represent a serious gap on which scientific research should focus. A list of the key characteristics of AEs that need to be carefully addressed should include:
-
•
a careful quantification of sulphuric acid, HONO and HNO3 directly emitted by aircraft for a large variety of engines. Currently available data refer only to few engine types and the changes of EI at varying thrusts are not completely clear. This should also include seeking a better knowledge of the characteristics and the evolution of emitted chemi-ions and a better understanding of their role as a source of sulphur and nitrogen species in plumes;
-
•
a more realistic quantification of emission inventories for nitrogen oxides and organic compounds, which includes the variability induced by the common practices of take-off and taxi phases at reduced thrust;
-
•
quantification of the effects of ozone-precursors emitted from aircraft and other AEs on the levels of ground-level ozone at airports, which to date have not been thoroughly investigated. In particular, since well established atmospheric photochemical reactions of many VOCs are known as potential sources of elevated ozone concentrations in the troposphere, improved chemical speciation of organic compounds is much needed. Better apportionment of ozone formation potential from aircraft emissions during LTO cycles and from other AEs should be also estimated;
-
•
standardisation of procedures for measurement of engine exhaust at ground level for regulatory purposes, which appear to be lacking mainly for PM and speciated hydrocarbon emissions. Such methodologies should take into account the semi-volatile components, which have been recognised to make a major contribution to the total mass of emitted PM. Achievement of this objective is vital to be able to obtain data that are comparable across different studies;
-
•
further quantitative knowledge of the chemical and physical modifications affecting many compounds and particulate matter in the atmosphere, including the oxidation of hydrocarbons to less volatile species and the formation of sulphate on the surface of pre-existing particles;
-
•
chemical and physical characterisation of PM. Far fewer data exist for PM than for the main gaseous pollutants. The chemical speciation of PM is not fully understood and the role of plumes aging on PM mass and composition is largely unknown. The role of lubrication oils, fuel type and engine technology, age and maintenance upon aircraft PM emissions also needs to be investigated;
-
•
a more detailed assessment of the health effects of the AEs within and in the surroundings of major airports;
-
•
the identification of particular chemical species to be used as a tracers for most of the AE sources;
-
•
the significance of airport operations for emission reduction and management should be investigated in more depth. There is a lack of information on the effects of time-in-modes, aircraft waiting/idling durations, aircraft weight, and use of APU/GPU/FGEP on the actual emission of pollutants. A more detailed knowledge of such operations will lead to a more reliable assessment of the quantities of exhaust pollutants emitted into the air;
-
•
the relative importance of near-airport, regional, and global scale air quality impacts of airport and aircraft emissions need to be further investigated. Most studies focus on local or global effects of the AEs, but there is no comprehensive view of air pollution over a full range of scales.
Quantification of the impact of airport emissions on local air quality is very difficult due to the complexity of airport emissions and the presence of substantial levels of pollution from other sources, with many airports being located near to urban settlements, major highways and roads or industrial installations. This makes the signal of the AEs and, in particular, of aircraft emissions very hard to distinguish. This is a serious gap because development of cost-effective strategies to improve air quality to meet regulatory requirements demands a clear quantification of the contribution of AEs to the total air pollution.
Acknowledgement
We gratefully acknowledge the European Union for funding the Marie Curie Intra-European Fellowships for career development to M. Masiol through the project entitled ‘Chemical and Physical Properties and Source Apportionment of Airport Emissions in the context of European Air Quality Directives’ (European Commission proposal n° 328542 Project CHEERS, call: FP7-PEOPLE-2012-IEF).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atmosenv.2014.05.070.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- ACARE . vol. 2. Advisory Council for Aeronautics Research in Europe; Brussels: 2002. (Strategic Research Agenda). [Google Scholar]
- ACI . Media Release, Airports Council International; Montreal: 2013. ACI Releases its 2012 World Airport Traffic Report.http://www.aci.aero/media/bc5239b4-07ac-4d1f-8cbb-db8f1b093d80/News/Releases/2013/PR_2013_08_28_WATR_Release_pdf Available at: [Google Scholar]
- ACI . 2008. ACI Global Traffic Forecast Report 2008–2027.http://www.icao.int/Meetings/ceans/Documents/Ceans_Wp_066_en.pdf (ICAO Conference on the Economics of Airports and Air Navigation Service, Montréal, 15–20 September 2008). Working Paper, CEANS-WP/66. Available at: [Google Scholar]
- ACI . 2007. Global Traffic Forecast 2006–2025.http://www.aci.aero/aci/aci/file/Economics/ACI%20Executive%20Summary.pdf Executive Summary. Edition 2007. Available at: [Google Scholar]
- Agrawal H., Sawant A.A., Jansen K., Miller J.W., Cocker D.R., III Characterization of chemical and particulate emissions from aircraft engines. Atmos. Environ. 2008;42:4380–4392. [Google Scholar]
- Airbus . 2012. Global Market Forecast 2012–2031. [Google Scholar]
- Airlines for America . 2013. Annual Results: World Airlines.http://www.airlines.org/Pages/Annual-Results-World-Airlines.aspx Available at: (last accessed August, 2013) [Google Scholar]
- Allen A.G., Nemitz E., Shi J.P., Harrison R.M., Greenwood J.C. Size distributions of trace metals in atmospheric aerosols in the United Kingdom. Atmos. Environ. 2001;35:4581–4591. [Google Scholar]
- Amato F., Schaap M., Reche C., Querol X. Road traffic: a major source of particulate matter in Europe. Urban air quality in Europe. Handb. Environ. Chem. 2013;26:165–193. [Google Scholar]
- Amato F., Moreno T., Pandolfi M., Querol X., Alastuey A., Delgado A., Pedrero M., Cots N. Concentrations, sources and geochemistry of airborne particulate matter at a major European airport. J. Environ. Monit. 2010;12:854–862. doi: 10.1039/b925439k. [DOI] [PubMed] [Google Scholar]
- Amin R.S. Institute of Transportation Studies, University of California; Berkeley: 2001. Airports and the General Conformity Process (No. UCB-ITS-RR-2001-1)http://www.escholarship.org/uc/item/8nw2z88q.pdf Document Number. Available at: (last accessed August, 2013) [Google Scholar]
- Anderson B.E., Cofer W.R., Bagwell D.R., Barrick J.W., Hudgins C.H. Airborne observations of aircraft aerosol emissions I: total nonvolatile particle emission indices. Geophys. Res. Lett. 1998;25:1689–1692. [Google Scholar]
- Anderson B.E., Cofer W.R., Barrick J.D., Bagwell D.R., Hudgins C.H. Airborne observations of aircraft aerosol emissions II: factors controlling volatile particle production. Geophys. Res. Lett. 1998;25:1693–1696. [Google Scholar]
- Anderson B.E., Branham H.-S., Hudgins C.H., Plant J.V., Ballenthin J.O., Miller T.M., Viggiano A.A., Blake D.R., Boudries H., Canagaratna M., Miake-Lye R.C., Onasch T., Wormhoudt J., Worsnop D., Brunke K.E., Culler S., Penko P., Sanders T., Han H.-S., Lee P., Pui D.Y.H., Thornhill K.L., Winstead E.L. National Aeronautics and Space Administration; Hampton, VA: 2005. Experiment to Characterize Aircraft Volatile Aerosol and Trace-Species Emissions (EXCAVATE) NASA/TM-2005-213783. August 2005. [Google Scholar]
- Anderson B.E., Chen G., Blake D.R. Hydrocarbon emissions from a modern commercial airliner. Atmos. Environ. 2006;40:3601–3612. [Google Scholar]
- Anderson B.E., Beyersdorf A.J., Hudgins C.H., Plant J.V., Thornhill K.L., Winstead E.L., Ziemba L.D., Howard R., Corporan E., Miake-Lye R.C., Herndon S.C., Timko M., Woods E., Dodds W., Lee B., Santoni G., Whitefield P., Hagen D., Lobo P., Knighton W.B., Bulzan D., Tacina K., Wey C., Vander Wal R., Bhargava A., Kinsey J., Liscinsky D.S. 2011. Alternative Aviation Fuel Experiment (AAFEX) NASA/TM-;2011-217059. [Google Scholar]
- Anderson J.O., Thundiyil J.G., Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012;8:166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae M.O., Gelencsér A. Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols. Atmos. Chem. Phys. 2006;6:3131–3148. [Google Scholar]
- Armstrong B., Hutchinson E., Unwin J., Fletcher T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: a review and meta-analysis. Environ. Health Perspect. 2004;112:970–978. doi: 10.1289/ehp.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold F., Scheid J., Stilp T., Schlager H., Reinhardt M.E. Measurements of jet aircraft emissions at cruise altitude I: the odd-nitrogen gases NO, NO2, HNO2 and HNO3. Geophys. Res. Lett. 1992;19:2421–2424. [Google Scholar]
- Arnold F., Kiendler A., Wiedemer V., Aberle S., Stilp T., Busen R. Chemiion concentration measurements in jet engine exhaust at the ground: implications for ion chemistry and aerosol formation in the wake of a jet aircraft. Geophys. Res. Lett. 2000;27:1723–1726. [Google Scholar]
- Arnold F., Wohlfrom K.-H., Klemm M.W., Schneider J., Gollinger K., Schumann U., Busen R. First gaseous ion composition measurements in the exhaust plume of a jet aircraft in flight: implications for gaseous sulfuric acid, aerosols, and chemiions. Geophys. Res. Lett. 1998;25:2137–2140. [Google Scholar]
- Arnold F., Stilp T., Busen R., Schumann U. Jet engine exhaust chemiion measurements: implications for gaseous SO2 and H2SO4. Atmos. Environ. 1998;32:3073–3077. [Google Scholar]
- Arunachalam S., Wang B., Davis N., Baek B.H., Levy J.I. Effect of chemistry transport model scale and resolution on population exposure to PM2.5 from aircraft emissions during landing and takeoff. Atmos. Environ. 2011;45:3294–3300. [Google Scholar]
- Ashok A., Lee I.H., Arunachalam S., Waitz I.A., Yim S.H., Barrett S.R. Development of a response surface model of aviation's air quality impacts in the United States. Atmos. Environ. 2013;77:445–452. [Google Scholar]
- Atkinson R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000;34:2063–2101. [Google Scholar]
- Atkinson R., Arey J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003;103:4605–4638. doi: 10.1021/cr0206420. [DOI] [PubMed] [Google Scholar]
- Ayres J.G. Health effects of gaseous air pollutants. In: Hester R.E., Harrison R.M., editors. Issues in Environmental Science and Technology No. 10. Royal Society of Chemistry; Cambridge: 1998. pp. 1–20. [Google Scholar]
- Azar C., Johansson D.J.A. Valuing the non-CO2 climate impacts of aviation. Clim. Change. 2012;111:559–579. [Google Scholar]
- Balkanski Y., Myhre G., Gauss M., Rädel G., Highwood E.J., Shine K.P. Direct radiative effect of aerosols emitted by transport: from road, shipping and aviation. Atmos. Chem. Phys. 2010;10:4477–4489. [Google Scholar]
- Barrett S.R., Britter R.E., Waitz I.A. Global mortality attributable to aircraft cruise emissions. Environ. Sci. Technol. 2010;44:7736–7742. doi: 10.1021/es101325r. [DOI] [PubMed] [Google Scholar]
- Barrett S.R.H., Yim S.H.L., Gilmore C.K., Murray L.T., Tai A.P.K., Kuhn S.R., Jacob D.J., Yantosca R.M., Byun D., Ngan F., Li X.S., Ashok A., Koo J., Levy J., Dessens O., Balasubramanian S., Fleming G.G., Wollersheim C., Malina R., Pearlson M.N., Stratton R.W., Arunachalam S., Binkowski F.S., Hileman J.I., Waitz I.A. Public health, climate and economic impacts of desulfurizing aviation fuel. Environ. Sci. Technol. 2012;46:4275–4282. doi: 10.1021/es203325a. [DOI] [PubMed] [Google Scholar]
- Barrett S.R.H., Britter R.E., Waitz I.A. Impact of aircraft plume dynamics on airport local air quality. Atmos. Environ. 2013;74:247–258. [Google Scholar]
- Baughcum S.L., Begin J.J., Franco F., Greene D.L., Lee D.S. Aircraft emissions: current inventories and future scenarios. In: Penner J.E., Lister D.H., Griggs D.J., Dokken D.J., McFarland M., editors. Aviation and the Global Atmosphere. Cambridge University Press; Cambridge: 1999. (Special Report of the Intergovernmental Panel on Climate Change). (Chapter 9) [Google Scholar]
- Baughcum S.L., Henderson S.C., Tritz T.G. 1996. Scheduled Civil Aircraft Emissions Inventories for 1976 and 1984: Database Development and Analysis. NASA CR 4722. [Google Scholar]
- Baughcum S.L., Henderson S.C., Tritz T.G., Pickett D.C. 1996. Scheduled Civil Aircraft Emission Inventories for 1992: Database Development and Analysis. NASA CR-4700. [Google Scholar]
- Bell M.L., Peng R.D., Dominici F., Samet J.M. Emergency hospital admissions for cardiovascular diseases and ambient levels of carbon monoxide: results for 126 United States urban counties, 1999–2005. Circulation. 2009;120:949–955. doi: 10.1161/CIRCULATIONAHA.109.851113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleudi V., Faustini A., Stafoggia M., Cattani G., Marconi A., Perucci C.A., Forastiere F. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology. 2010;21:414–423. doi: 10.1097/EDE.0b013e3181d5c021. [DOI] [PubMed] [Google Scholar]
- Bennett M., Christie S. An application of backscatter Lidar to model the odour nuisance arising from aircraft tyre smoke. Int. J. Environ. Pollut. 2011;44:316–328. [Google Scholar]
- Bennett M., Christie S.M., Graham A., Thomas B.S., Vishnyakov V., Morris K., Peters D.M., Jones R., Ansell C. Composition of smoke generated by landing aircraft. Environ. Sci. Technol. 2011;45:3533–3538. doi: 10.1021/es1027585. [DOI] [PubMed] [Google Scholar]
- Bennett M., Christie S., Graham A., Raper D.W. Lidar observations of aircraft exhaust plumes. J. Atmos. Ocean Technol. 2010;27:1638–1651. [Google Scholar]
- Beyersdorf A.J., Timko M.T., Ziemba L.D., Bulzan D., Corporan E., Herndon S.C., Howard R., Miake-Lye R., Thornhill K.L., Winstead E., Wey C., Yu Z., Anderson B.E. Reductions in aircraft particulate emissions due to the use of Fischer–Tropsch fuels. Atmos. Chem. Phys. Discuss. 2013;13:15105–15139. [Google Scholar]
- Blakey S., Rye L., Wilson C.W. Aviation gas turbine alternative fuels: a review. P. Combust. Inst. 2011;33:2863–2885. [Google Scholar]
- Blitz M.A., Hughes K.J., Pilling M.J. Determination of the high-pressure limiting rate coefficient and the enthalpy of reaction for OH + SO2. J. Phys. Chem. A. 2003;107:1971–1978. [Google Scholar]
- Boeing . Market Analysis, Boeing Commercial Airplanes; Seattle: 2013. Current Market Outlook 2013–2032.http://www.boeing.com/assets/pdf/commercial/cmo/pdf/Boeing_Current_Market_Outlook_2013.pdf Available at: [Google Scholar]
- Bond T.C., Bergstrom R.W. Light absorption by carbonaceous particles: an investigative review. Aerosol Sci. Technol. 2006;40:27–67. [Google Scholar]
- Brasseur G.P., Müller J.F., Granier C. Atmospheric impact of NOx emissions by subsonic aircraft: a three-dimensional model study. J. Geophys. Res. Atmos. 1996;101(D1):1423–1428. [Google Scholar]
- Bräuner E.V., Forchhammer L., Møller P., Simonsen J., Glasius M., Wåhlin P., Raaschou-Nielsen O., Loft S. Exposure to ultrafine particles from ambient air and oxidative stress-induced DNA damage. Environ. Health Perspect. 2007;115:1177. doi: 10.1289/ehp.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Airports Authority . 2006. Gatwick 2010 Baseline Emission Inventory.http://83.98.24.64/Documents/business_and_community/Publications/2006/2010_basline_emissions_inventory.pdf Available at: (last accessed September, 2013) [Google Scholar]
- Brock C.A., Schröder F., Kärcher B., Petzold A., Busen R., Fiebig M. Ultrafine particle size distributions measured in aircraft exhaust plumes. J. Geophys. Res. Atmos. (1984–2012) 2000;105(D21):26555–26567. [Google Scholar]
- Brown R.C., Miake-Lye R.C., Anderson M.R., Kolb C.E. Aircraft sulfur emissions and the formation of visible contrails. Geophys. Res. Lett. 1997;24:385–388. [Google Scholar]
- Brown R.C., Anderson M.R., Miake-Lye R.C., Kolb C.E., Sorokin A.A., Buriko Y.Y. Aircraft exhaust sulfur emissions. Geophys. Res. Lett. 1996;23:3603–3606. [Google Scholar]
- Brown R.C., Miake-Lye R.C., Anderson M.R., Kolb C.E. Effect of aircraft exhaust sulfur emissions on near field plume aerosols. Geophys. Res. Lett. 1996;23:3607–3610. [Google Scholar]
- Brundish K.D., Clague A.R., Wilson C.W., Miake-Lye R.C., Brown R.C., Wormhoudt J., Lukachko S.P., Chobot A.T., Yam C.K., Waitz I.A., Hagen D.E., Schmid O., Whitefield P.D. Evolution of carbonaceous aerosol and aerosol precursor emissions through a jet engine. J. Propul. Power. 2007;23:959–970. [Google Scholar]
- Brunekreef B., Holgate S.T. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Buseck P.R., Adachi K., Gelencsér A., Tompa É., Pósfai M. Are black carbon and soot the same? Atmos. Chem. Phys. Discuss. 2012;12:24821–24846. [Google Scholar]
- Busen R., Schumann U. Visible contrail formation from fuels with different sulfur contents. Geophys. Res. Lett. 1995;22(11):1357–1360. [Google Scholar]
- Buttress J.C., Morris K.M. British Airways Environmental Affairs, British Airways Technical Documents Relating to the Aircraft Operations Supporting the Project for the Sustainable Development of Heathrow, Document 1, ENV/KMM/1127/14.18, October 2005. British Airways; London: 2005. An estimation of the total NOx emissions resulting from aircraft Engine Ground Running at Heathrow airport.http://www.britishairways.com/cms/global/pdfs/csr/PSDH_Technical_Reports.pdf Available at: (last accessed September 2013) [Google Scholar]
- Cain J., DeWitt M.J., Blunck D., Corporan E., Striebich R., Anneken D., Klingshirn C., Roquemore W.M., Vander Wal R. Characterization of gaseous and particulate emissions from a turboshaft engine burning conventional, alternative, and surrogate fuels. Energ. Fuels. 2013;27:2290–2302. [Google Scholar]
- Carr E., Lee M., Marin K., Holder C., Hoyer M., Pedde M., Cook R., Touma J. Development and evaluation of an air quality modeling approach to assess near-field impacts of lead emissions from piston-engine aircraft operating on leaded aviation gasoline. Atmos. Environ. 2011;45:5795–5804. [Google Scholar]
- Carslaw D.C., Ropkins K., Laxen D., Moorcroft S., Marner B., Williams M.L. Near-field commercial aircraft contribution to nitrogen oxides by engine, aircraft type, and airline by individual plume sampling. Environ. Sci. Technol. 2008;42:1871–1876. doi: 10.1021/es071926a. [DOI] [PubMed] [Google Scholar]
- Carslaw D.C., Beevers S.D., Ropkins K., Bell M.C. Detecting and quantifying aircraft and other on-airport contributions to ambient nitrogen oxides in the vicinity of a large international airport. Atmos. Environ. 2006;40:5424–5434. [Google Scholar]
- Carslaw D.C. Evidence of an increasing NO2/NOx emissions ratio from road traffic emissions. Atmos. Environ. 2005;39(26):4793–4802. [Google Scholar]
- Carslaw D.C., Beevers S.D. New Directions: should road vehicle emissions legislation consider primary NO2? Atmos. Environ. 2004;38:1233–1234. [Google Scholar]
- Cavallo D., Ursini C.L., Carelli G., Iavicoli I., Ciervo A., Perniconi B., Rondinone B., Gismondi M., Iavicoli S. Occupational exposure in airport personnel: characterization and evaluation of genotoxic and oxidative effects. Toxicology. 2006;223:26–35. doi: 10.1016/j.tox.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Champagne D.L. 1971. Standard Measurement of Aircraft Gas Turbine Engine Exhaust Smoke. ASME paper, 71-GT-88. [Google Scholar]
- Chen Y.C., Lee W.-J., Uang S.-N., Lee S.-H., Tsai P.-J. Characteristics of polycyclic aromatic hydrocarbon (PAH) emissions from a UH-1H helicopter engine and its impact on the ambient environment. Atmos. Environ. 2006;40:7589–7597. [Google Scholar]
- Cheng M.-D. 2009. A Comprehensive Program for Measurement of Military Aircraft Emissions. Strategic Environmental Research and Development Program, SERDP Project WP-1401 ORNL/TM-2009/256. [Google Scholar]
- Cheng M.-D., Corporan E. A study of extractive and remote-sensing sampling and measurement of emissions from military aircraft engines. Atmos. Environ. 2010;44:4867–4878. [Google Scholar]
- Cheng M.D., Corporan E., DeWitt M.J., Landgraf B. Emissions of volatile particulate components from turboshaft engines operated with JP-8 and Fischer-Tropsch fuels. Aerosol Air Qual. Res. 2009;9:237–256. [Google Scholar]
- Cheng M.D., Corporan E., DeWitt M.J., Spicer C.W., Holdren M.W., Cowen K.A., Laskin A., Harris D.B., Shores R.C., Kagann R., Hashmonay R. Probing emissions of military cargo aircraft: description of a joint field measurement strategic environmental research and development program. JAWMA. 2008;58:787–796. doi: 10.3155/1047-3289.58.6.787. [DOI] [PubMed] [Google Scholar]
- Chevron Corporation . 2006. Aviation Fuels Technical Review.https://www.cgabusinessdesk.com/document/aviation_tech_review.pdf Available at: (last accessed August 2013) [Google Scholar]
- Chèze B., Gastineau P., Chevallier J. Forecasting world and regional aviation jet fuel demands to the mid-term (2025) Energ. Policy. 2011;39:5147–5158. [Google Scholar]
- Chiusolo M., Cadum E., Stafoggia M., Galassi C., Berti G., Faustini A., Bisanti L., Vigotti M.A., Patrizia Dessì M., Cernigliaro A., Mallone S., Pacelli B., Minerba S., Simonato L., Forastiere F. Short-term effects of nitrogen dioxide on mortality and susceptibility factors in 10 Italian cities: the EpiAir study. Environ. Health Perspect. 2011;119:1233–1238. doi: 10.1289/ehp.1002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole I.S., Paterson D.A. Modelling aerosol deposition rates on aircraft and implications for pollutant accumulation and corrosion. Corros. Eng. Sci. Technol. 2009;44:332–339. [Google Scholar]
- Corporan E., Reich R., Monroig O., DeWitt M.J., Larson V., Aulich T., Mann M., Seames W. Impacts of biodiesel on pollutant emissions of a JP-8-fueled turbine engine. JAWMA. 2005;55:940–949. doi: 10.1080/10473289.2005.10464680. [DOI] [PubMed] [Google Scholar]
- Corporan E., DeWitt M.J., Belovich V., Pawlik R., Lynch A.C., Gord J.R., Meyer T.R. Emissions characteristics of a turbine engine and research combustor burning a fischer-tropsch jet fuel. Energ. Fuels. 2007;21:2615–2626. [Google Scholar]
- Corporan E., Quick A., DeWitt M.J. Characterization of particulate matter and gaseous emissions of a C-130H aircraft. JAWMA. 2008;58:474–483. doi: 10.3155/1047-3289.58.4.474. [DOI] [PubMed] [Google Scholar]
- Corporan E., Edwards T., Shafer L., DeWitt M.J., Klingshirn C., Zabarnick S., West Z., Striebich R., Graham J., Klein J. Chemical, thermal stability, seal swell, and emissions studies of alternative jet fuels. Energ. Fuels. 2011;25:955–966. [Google Scholar]
- Cowen K., Goodwin B., Joseph D., Tefend M., Satola J., Kagann R., Hashmonay R., Spicer C., Holdren M. Extractive sampling and optical remote sensing of F100 aircraft engine emissions. JAWMA. 2009;59:531–539. doi: 10.3155/1047-3289.59.5.531. [DOI] [PubMed] [Google Scholar]
- Craig P.H., Barth M.L. Evaluation of the hazards of industrial exposure to tricresyl phosphate: a review and interpretation of the literature. J. Toxicol. Environ. Health B Crit. Rev. 1999;2:281–300. doi: 10.1080/109374099281142. [DOI] [PubMed] [Google Scholar]
- Curtius J., Sierau B., Arnold F., Baumann R., Busen R., Schulte P., Schumann U. First direct sulfuric acid detection in the exhaust plume of a jet aircraft in flight. Geophys. Res. Lett. 1998;25:923–926. [Google Scholar]
- Curtius J., Arnold F., Schulte P. Sulfuric acid measurements in the exhaust plume of a jet aircraft in flight: implications for the sulfuric acid formation efficiency. Geophys. Res. Lett. 2002;29:1113. doi: 10.1029/2001GL013813. [DOI] [Google Scholar]
- Cyrys J., Eeftens M., Heinrich J., Ampe C., Armengaud A., Beelen R., Bellander T., Beregszaszi T., Birk M., Cesaroni G., Cirach M., de Hoogh K., De Nazelle A., de Vocht F., Declercq C., Dedele A., Dimakopoulou K., Eriksen K., Galassi C., Grauleviciene R., Grivas G., Gruzieva O., Hagenbjork Gustafsson A., Hoffmann B., Iakovides M., Ineichen A., Krämer U., Lanki T., Lozano P., Madsen C., Meliefste K., Modig L., Mölter A., Mosler G., Nieuwenhuijsen M., Nonnemacher M., Oldenwening M., Peters A., Pontet S., Probst-Hensch N., Quass U., Raaschou-Nielsen O., Ranzi A., Sugiri D., Stephanou E.G., Taimisto P., Tsai M.-Y., Vaskovi E., Villani S., Wang M., Brunekreef B., Hoek G. Variation of NO2 and NOx concentrations between and within 36 European study areas: results from the ESCAPE study. Atmos. Environ. 2012;62:374–390. [Google Scholar]
- Dakhel P.M., Lukachko S.P., Waitz I.A., Miake-Lye R.C., Brown R.C. Postcombustion evolution of soot properties in an aircraft engine. J. Propul. Power. 2007;23:942–948. [Google Scholar]
- Daley M.P.S., Naugle M.D.F. Measurement and analysis of airport emissions. J. Air Pollut. Control Assoc. 1979;29:113–116. [Google Scholar]
- Delfino R.J., Sioutas C., Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ. Health Perspect. 2005;113:934. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Saborit J.M., Stark C., Harrison R.M. Carcinogenic potential, levels and sources of polycyclic aromatic hydrocarbon mixtures in indoor and outdoor environments and their implications for air quality standards. Environ. Int. 2011;37:383–392. doi: 10.1016/j.envint.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Demirdjian B., Ferry D., Suzanne J., Popovicheva O.B., Persiantseva N.M., Shonija N.K. Heterogeneities in the microstructure and composition of aircraft engine combustor soot: impact on the water uptake. J. Atmos. Chem. 2007;56:83–103. [Google Scholar]
- Denola G., Hanhela P.J., Mazurek W. Determination of tricresyl phosphate air contamination in aircraft. Ann. Occup. Hyg. 2011;55:710–722. doi: 10.1093/annhyg/mer040. [DOI] [PubMed] [Google Scholar]
- DeWitt K., Hwang S.M. 2005. Sulfur Oxidation and Contrail Precursor Chemistry.http://archive.org/details/nasa_techdoc_20050123900 NASA Grant Number NAG3-2674. Available at: (last accessed August 2013) [Google Scholar]
- DeWitt M.J., Corporan E., Graham J., Minus D. Effects of aromatic type and concentration in Fischer-Tropsch fuel on emissions production and material compatibility. Energ. Fuels. 2008;22:2411–2418. [Google Scholar]
- Dlugokencky E.J., Nisbet E.G., Fisher R., Lowry D. Global atmospheric methane: budget, changes and dangers. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011;369:2058–2072. doi: 10.1098/rsta.2010.0341. [DOI] [PubMed] [Google Scholar]
- Dodson R.E., Houseman E.A., Morin B., Levy J.I. An analysis of continuous black carbon concentrations in proximity to an airport and major roadways. Atmos. Environ. 2009;43:3764–3773. [Google Scholar]
- Donahue N.M., Robinson A.L., Pandis S.N. Atmospheric organic particulate matter: from smoke to secondary organic aerosol. Atmos. Environ. 2009;43:94–106. [Google Scholar]
- EASA (European Aviation Safety Agency) 2011. Studying, sAmpling and Measuring of aircraft ParticuLate Emissions III – Specific Contract 01 (SAMPLE III – SC.01) Research project EASA.2010/FC10 SC.01 23 October 2011.http://www.easa.europa.eu/safety-and-research/research-projects/docs/environment/2011-SAMPLE%20III%20SC.01-Studying,%20sAmpling%20and%20Measuring%20of%20Particulate%20Matter%20III%20Specific%20Contract%2001-Final%20Report%20v2.pdf Available at: (last accessed September 2013) [Google Scholar]
- EASA (European Aviation Safety Agency) 2013. ICAO Aircraft Engine Emissions Databank (updated April 2013)http://easa.europa.eu/environment/edb/aircraft-engine-emissions.php Available at: [Google Scholar]
- Ebbinghaus A., Wiesen P. Aircraft fuels and their effect upon engine emissions. Air Space Eur. 2001;3(1/2):101–103. [Google Scholar]
- Eichkorn S., Wohlfrom K.-H., Arnold F., Busen R. Massive positive and negative chemiions in the exhaust of an aircraft jet engine at ground-level: mass distribution measurements and implications for aerosol formation. Atmos. Environ. 2002;36:1821–1825. [Google Scholar]
- Eyers C.J., Addleton D., Atkinson K., Broomhead M.J., Christou R., Elliff T., Falk R., Gee I., Lee D.S., Marizy C., Michot S., Middel J., Newton P., Norman P., Plohr M., Raper D., Stanciou N. 2004. AERO2k Global Aviation Emissions Inventories for 2002 and 2025 QINETIQ/04/01113, Farnborough, Hants, UK. [Google Scholar]
- Eyring V., Isaksen I.S.A., Berntsen T., Collins W.J., Corbett J.J., Endresen O., Grainger R.G., Moldanova J., Schlager H., Stevenson D.S. Transport impacts on atmosphere and climate: shipping. Atmos. Environ. 2010;44:4735–4771. doi: 10.1016/j.atmosenv.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAA (Federal Aviation Administration) Federal Aviation Administration Office of Environment and Energy; 2005. Aviation & Emissions. A Primer, January 2005.http://www.faa.gov/regulations_policies/policy_guidance/envir_policy/media/aeprimer.pdf Available at: (last accessed September 2013) [Google Scholar]
- Fahey D.W., Schumann U., Ackerman S., Artaxo P., Boucher O., Danilin M.Y., Karcher B., Minnis P., Nakajima T., Toon O.B. Aviation-produced aerosols and cloudiness. In: Penner J.E., Lister D.H., Griggs D.J., Dokken D.J., McFarland M., editors. Aviation and the Global Atmosphere. Cambridge University Press; Cambridge: 1999. (Special Report of the Intergovernmental Panel on Climate Change). (Chapter 3) [Google Scholar]
- Fahey D.W., Keim E.R., Woodbridge E.L., Gao R.S., Boering K.A., Daube B.C., Wofsy S.C., Lohmann R.P., Hintsa E.J., Dessler A.E., Webster C.R., May R.D., Brock C.A., Wilson J.C., Miake-Lye R.C., Brown R.C., Rodriguez J.M., Loewenstein M., Proffitt M.H., Stimpfle R.M., Bowen S.W., Chan K.R. In situ observations in aircraft exhaust plumes in the lower stratosphere at midlatitudes. J. Geophys. Res. 1995;100(D2):3065–3074. [Google Scholar]
- Fahey D.W., Keim E.R., Boering K.A., Brock C.A., Wilson J.C., Jonsson H.H., Anthony S., Hanisco T.F., Wennberg P.O., Miake-Lye R.C., Salawitch R.J., Louisnard N., Woodbridge E.L., Gao R.S., Donnelly S.G., Wamsley R.C., Del Negro L.A., Solomon S., Daube B.C., Wofsy S.C., Webster C.R., May R.D., Kelly K.K., Loewenstein M., Podolske J.R., Chan K.R. Emission measurements of the Concorde supersonic aircraft in the lower stratosphere. Science. 1995;270:70–74. [Google Scholar]
- Farias F., ApSimon H. Relative contributions from traffic and aircraft NOx emissions to exposure in West London. Environ. Modell. Softw. 2006;21:477–485. [Google Scholar]
- Fan W., Sun Y., Zhu T., Wen Y. Emissions of HC, CO, NOx, CO2, and SO2 from civil aviation in China in 2010. Atmos. Environ. 2012;56:52–57. [Google Scholar]
- Fanning E., Yu R.C., Lu R., Froines J. 2007. Monitoring and Modeling of Ultrafine Particles and Black Carbon at the Los Angeles International Airport.http://arb.ca.gov/research/apr/past/04-325.pdf Final Report. California Air Resource Board Contract #04-325. Available at: (last accessed September 2013) [Google Scholar]
- Federal Aviation Administration . 2003. Select Resource Materials and Annotated Bibliography on the Topic of Hazardous Air Pollutants (HAPs) Associated with Aircraft.http://www.faa.gov/regulations_policies/policy_guidance/envir_policy/media/HAPs_rpt.pdf Airports and Aviation Report. Available from: (last accessed September 2013) [Google Scholar]
- Fenger J. Air pollution in the last 50 years – from local to global. Atmos. Environ. 2009;43:13–22. [Google Scholar]
- Finlayson-Pitts B.J., Pitts J.N. Acad. Press; San Diego: 2000. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications; p. 969. [Google Scholar]
- Fleuti E., Hofmann P. 2005. Airport Local Air Quality Studies, ALAQS Case Study: Zürich Airport 2004, a Comparison of Modelled and Measured Air Quality.http://www.eurocontrol.int/eec/gallery/content/public/document/eec/report/2005/037_Comparison_of_Modelled_and_Measured_Air_Quality_Zurich_Airport_2004.pdf Tech. Rep. EEC/SEE/2005/017, EUROCONTROL. Available at: (last accessed August 2013) [Google Scholar]
- Frenzel A., Arnold F. Proceedings of the International Scientific Coll. On Impact of Emissions from Aircraft and Spacecraft upon the Atmosphere, Köln 1994, DLR-Mitt 94-06. 1994. Sulfuric acid cluster ion formation by jet engines: implications for sulfuric acid formation and nucleation; pp. 106–112. [Google Scholar]
- Gardner R.M., Adams K., Cook T., Deidewig F., Ernedal S., Falk R., Fleuti E., Herms E., Johnson C.E., Lecht M., Lee D.S., Leech M., Lister D., Massé B., Metcalfe M., Newton P., Schmitt A., Vandenbergh C., Van Drimmelen R. The ANCAT/EC global inventory of NOx emissions from aircraft. Atmos. Environ. 1997;31:1751–1766. [Google Scholar]
- Garnier F., Baudoin C., Woods P., Louisnard N. Engine emission alteration in the near field of an aircraft. Atmos. Environ. 1997;31:1767–1781. [Google Scholar]
- Gauss M., Isaksen I.S.A., Lee D.S., Søvde O.A. Impact of aircraft NOx emissions on the atmosphere – tradeoffs to reduce the impact. Atmos. Chem. Phys. 2006;6:1529–1548. [Google Scholar]
- Gerstle T., Virag P., Wade M. vol. 3. Institute for Environment, Safety, Occupational Health Risk Analysis (IERA); 1999. Aircraft Engine and Auxiliary Power Unit Emissions Testing.http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA361473 (Participate Matter Results). IERA-RS-BR-TR-1999-0006-Vol. 3. Available at: (last accessed September 2013) [Google Scholar]
- Gerstle T., Virag P., Wade M. United States Air Force: IERA; 2002. Aircraft Engine and Auxiliary Power Unit Emissions Testing: Final Report Addendum F119-PW-100 Engine Emissions Testing Report. [Google Scholar]
- Gettelman A., Chen C. The climate impact of aviation aerosols. Geophys. Res. Lett. 2013;40:2785–2789. [Google Scholar]
- Gietl J.K., Lawrence R., Thorpe A.J., Harrison R.M. Identification of brake wear particles and derivation of a quantitative tracer for brake dust at a major road. Atmos. Environ. 2010;44:141–146. [Google Scholar]
- Girling S.P., Hurley C.D., Mitchell J.P., Nichols A.L. Development and characterization of a smoke generator for the calibration of aerosol emissions from gas turbine engines. Aerosol Sci. Technol. 1990;13:8–19. [Google Scholar]
- Gleitsmann G., Zellner R. A modeling study of the formation of cloud condensation nuclei in the jet regime of aircraft plumes. J. Geophys. Res. 1998;103:19543–19555. [Google Scholar]
- Grice S., Stedman J., Kent A., Hobson M., Norris J., Abbott J., Cooke S. Recent trends and projections of primary NO2 emissions in Europe. Atmos. Environ. 2009;43:2154–2167. [Google Scholar]
- Grosjean D., Grosjean E., Williams E.L. Atmospheric chemistry of olefins: a product study of the ozone-alkene reaction with cyclohexane added to scavenge hydroxyl radical. Environ. Sci. Technol. 1994;28:186–196. doi: 10.1021/es00050a026. [DOI] [PubMed] [Google Scholar]
- Gysel M., Nyeki S., Weingartner E., Baltensperger U., Giebl H., Hitzenberger R., Petzold A., Wilson C.W. Properties of jet engine combustion particles during the PartEmis experiment: hygroscopicity at subsaturated conditions. Geophys. Res. Lett. 2003;30:1566,. doi: 10.1029/2003GL016896. [DOI] [Google Scholar]
- HAL (Heathrow Airport Ltd.) 2011. Heathrow Air Quality Strategy 2011–2020.http://www.heathrowairport.com/static/Heathrow/Downloads/PDF/air-quality-strategy_LHR.pdf Available at: (last accessed August 2013) [Google Scholar]
- Hallquist M., Wenger J.C., Baltensperger U., Rudich Y., Simpson D., Claeys M., Dommen J., Donahue N.M., George C., Goldstein A.H., Hamilton J.F., Herrmann H., Hoffmann T., Iinuma Y., Jang M., Jenkin M., Jimenez J.L., KiendlerScharr A., Maenhaut W., McFiggans G., Mentel T., Monod A., Prevot A.S.H., Seinfeld J.H., Surratt J.D., Szmigielski R., Wildt J. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 2009;9:5155–5235. [Google Scholar]
- Harrison R.M., Jones A.M., Gietl J., Yin J., Green D.C. Estimation of the contributions of brake dust, tire wear, and resuspension to nonexhaust traffic particles derived from atmospheric measurements. Environ. Sci. Technol. 2012;46:6523–6529. doi: 10.1021/es300894r. [DOI] [PubMed] [Google Scholar]
- Harrison R.M., Beddows D.C.S., Dall'Osto M. PMF analysis of wide-range particle size spectra collected on a major highway. Environ. Sci. Technol. 2011;45:5522–5528. doi: 10.1021/es2006622. [DOI] [PubMed] [Google Scholar]
- Haverkamp H., Wilhelm S., Sorokin A., Arnold F. Positive and negative ion measurements in jet aircraft engine exhaust: concentrations, sizes and implications for aerosol formation. Atmos. Environ. 2004;38:2879–2884. [Google Scholar]
- Heal M.R., Kumar P., Harrison R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012;41:6606–6630. doi: 10.1039/c2cs35076a. [DOI] [PubMed] [Google Scholar]
- Heland J., Schäfer K. Determination of major combustion products in aircraft exhausts by FTIR emission spectroscopy. Atmos. Environ. 1998;32:3067–3072. [Google Scholar]
- Heland J., Schäfer K. Analysis of aircraft exhausts with Fourier-transform infrared emission spectroscopy. Appl. Opt. 1997;36:4922–4931. doi: 10.1364/ao.36.004922. [DOI] [PubMed] [Google Scholar]
- Henderson S.C., Wickrama U.K., Baughcum S.L., Begin J.L., Franco F., Greene D.L., Lee D.S., Mclaren M.L., Mortlock A.K., Newton P.J., Schmitt A., Sutkus D.J., Vedantham A., Wuebbles D.J. Aircraft emissions: current inventories and future scenarios. In: Penner J.E., Lister D.H., Griggs D.J., Dokken D.J., McFarland M., editors. Aviation and the Global Atmosphere. Cambridge University Press; Cambridge: 1999. (Special Report of the Intergovernmental Panel on Climate Change). (Chapter 9) [Google Scholar]
- Hendricks J., Kärcher B., Döpelheuer A., Feichter J., Lohmann U., Baumgardner D. Simulating the global atmospheric black carbon cycle: a revisit to the contribution of aircraft emissions. Atmos. Chem. Phys. 2004;4:2521–2541. [Google Scholar]
- Herndon S.C., Wood E.C., Northway M.J., Miake-Lye R., Thornhill L., Beyersdorf A., Anderson B.E., Dowlin R., Dodds W., Knighton W.B. Aircraft hydrocarbon emissions at Oakland international airport. Environ. Sci. Technol. 2009;43:1730–1736. doi: 10.1021/es801307m. [DOI] [PubMed] [Google Scholar]
- Herndon S.C., Jayne J.T., Lobo P., Onasch T.B., Fleming G., Hagen D.E., Whitefield P.D., Miake-Lye R.C. Commercial aircraft engine emissions characterization of in-use aircraft at Hartsfield–Jackson Atlanta International Airport. Environ. Sci. Technol. 2008;42:1877–1883. doi: 10.1021/es072029+. [DOI] [PubMed] [Google Scholar]
- Herndon S.C., Rogers T., Dunlea E.J., Jayne J.T., Miake-Lye R., Knighton B. Hydrocarbon emissions from in-use commercial aircraft during airport operations. Environ. Sci. Technol. 2006;40:4406–4413. doi: 10.1021/es051209l. [DOI] [PubMed] [Google Scholar]
- Herndon S.C., Onasch T.B., Frank B.P., Marr L.C., Jayne J.T., Canagaratna M.R., Grygas J., Lanni T., Anderson B.E., Worsnop D., Miake-Lye R.C. Particulate emissions from in-use commercial aircraft. Aerosol Sci. Technol. 2005;39:799–809. [Google Scholar]
- Herndon S., Shorter J., Zahniser M., Nelson D., Jayne J., Brown R., Miake-Lye R.C., Waitz I., Silva P., Lanni T., Demerjian K., Kolb C. NO and NO2 emission ratios measured from in-use commercial aircraft during taxi and takeoff. Environ. Sci. Technol. 2004;38:6078–6084. doi: 10.1021/es049701c. [DOI] [PubMed] [Google Scholar]
- Hileman J.I., Stratton R.W., Donohoo P.E. Energy content and alternative jet fuel viability. J. Propul. Power. 2010;26:1184–1195. [Google Scholar]
- Hindiyarti L., Glarborg P., Marshall P. Reactions of SO3 with the O/H radical pool under combustion conditions. J. Phys. Chem. A. 2007;111:3984–3991. doi: 10.1021/jp067499p. [DOI] [PubMed] [Google Scholar]
- Hoek G., Boogaard H., Knol A., de Hartog J., Slottje P., Ayres J.G., Borm P., Brunekreef B., Donaldson K., Forastiere F., Holgate S., Kreyling W.G., Nemery B., Pekkanen J., Stone V., Wichmann H.E., van der Sluijs J. Concentration response functions for ultrafine particles and all-cause mortality and hospital admissions: results of a european expert panel elicitation. Environ. Sci. Technol. 2010;44:476–482. doi: 10.1021/es9021393. [DOI] [PubMed] [Google Scholar]
- Howitt O.J.A., Carruthers M.A., Smith I.J., Rodger C.J. Carbon dioxide emissions from international air freight. Atmos. Environ. 2011;45:7036–7045. [Google Scholar]
- Hsu H.H., Adamkiewicz G., Andres Houseman E., Zarubiak D., Spengler J.D., Levy J.I. Contributions of aircraft arrivals and departures to ultrafine particle counts near Los Angeles International Airport. Sci. Tot. Environ. 2013;444:347–355. doi: 10.1016/j.scitotenv.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Hsu H.H., Adamkiewicz G., Andres Houseman E., Vallarino J., Melly S.J., Wayson R.L., Spengler J.D., Levy J.I. The relationship between aviation activities and ultrafine particulate matter concentrations near a mid-sized airport. Atmos. Environ. 2012;50:328–337. [Google Scholar]
- Hu S., Fruin S., Kozawa K., Mara S., Winer A.M., Paulson S.E. Aircraft emission impacts in a neighborhood adjacent to a general aviation airport in Southern California. Environ. Sci. Technol. 2009;43:8039–8045. doi: 10.1021/es900975f. [DOI] [PubMed] [Google Scholar]
- Hueglin C., Buchmann B., Weber R.O. Long-term observation of real-world road traffic emission factors on a motorway in Switzerland. Atmos. Environ. 2006;40:3696–3709. [Google Scholar]
- Hunton D.E., Ballenthin J.O., Borghetti J.F., Federico G.S., Miller T.M., Thorn W.F., Viggiano A.A., Anderson B.E., Coffer W.R., McDougal D.S., Wey C.C. Chemical ionization mass spectrometric measurements of SO2 emissions from jet engines in flight and test chamber operations. J. Geophys. Res. 2000;105(D22):26841–26855. [Google Scholar]
- Huttunen-Saarivirta E., Kuokkala V.T., Kokkonen J., Paajanen H. Corrosion effects of runway de-icing chemicals on aircraft alloys and coatings. Mat. Chem. Phys. 2011;126:138–151. [Google Scholar]
- Hwang S.M., Cooke J.A., De Witt K.J., Rabinowitz M.J. Determination of the rate coefficients of the SO2 + O + M → SO3 + M reaction. Int. J. Chem. Kinet. 2010;42:168–180. [Google Scholar]
- Hyslop N.P. Impaired visibility: the air pollution people see. Atmos. Environ. 2009;43:182–195. [Google Scholar]
- IARC (International Agency for Research on Cancer) 2010. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. (IARC Monogr. Eval. Carcinog. Risk Hum. 92 (IARC, Lyon)). [PMC free article] [PubMed] [Google Scholar]
- ICAO (International Civil Aviation Organization) 2013. Annual Report of the Council 2012. Available at: http://www.icao.int/publications/pages/publication.aspx?docnum=10001. [Google Scholar]
- ICAO (International Civil Aviation Organization) first ed. International Civil Aviation Organization; Montréal: 2011. Airport Air Quality Manual. [Google Scholar]
- ICAO (International Civil Aviation Organization) vol. 2. 2008. Environmental Protection (Annex 16) (Aircraft Engine Emission, International Standards and Recommended Practices). [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) 1999. Aviation and the Global Atmosphere. Summary for Policymakers; p. 23. IPCC Working Groups I and III. [Google Scholar]
- Jenkin M.E. Analysis of sources and partitioning of oxidant in the UK—part 2: contributions of nitrogen dioxide emissions and background ozone at a kerbside location in London. Atmos. Environ. 2004;38:5131–5138. [Google Scholar]
- Jerrett M., Burnett R.T., Pope C.A., Ito K., Thurston G., Krewski D., Shi Y., Calle E., Thun M. Longterm ozone exposure and mortality. N. Engl. J. Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C., Norman M., Burman L. Road traffic emission factors for heavy metals. Atmos. Environ. 2009;43:4681–4688. [Google Scholar]
- Johnson G.R., Mazaheri M., Ristovski Z.D., Morawska L.A. Plume capture technique for the remote characterization of aircraft engine emissions. Environ. Sci. Technol. 2008;42:4850–4856. doi: 10.1021/es702581m. [DOI] [PubMed] [Google Scholar]
- Johnston H. Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust. Science. 1971;173:517–522. doi: 10.1126/science.173.3996.517. [DOI] [PubMed] [Google Scholar]
- Jung K.H., Artigas F., Shin J.Y. Personal, indoor, and outdoor exposure to VOCs in the immediate vicinity of a local airport. Environ. Monit. Assess. 2011;173:555–567. doi: 10.1007/s10661-010-1404-9. [DOI] [PubMed] [Google Scholar]
- Jurkat T., Voigt C., Arnold F., Schlager H., Kleffmann J., Aufmhoff H., Schäuble D., Schaefer M., Schumann U. Measurements of HONO, NO, NOy and SO2 in aircraft exhaust plumes at cruise. Geophys. Res. Lett. 2011;38:L10807. doi: 10.1029/2011GL046884. [DOI] [Google Scholar]
- Kam W., Liacos J.W., Schauer J.J., Delfino R.J., Sioutas C. On-road emission factors of PM pollutants for light-duty vehicles (LDVs) based on urban street driving conditions. Atmos. Environ. 2012;61:378–386. [Google Scholar]
- Kampa M., Castanas E. Human health effects of air pollution. Environ. Pollut. 2008;151:362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Karakatsani A., Analitis A., Perifanou D., Ayres J.G., Harrison R.M., Kotronarou A., Kavouras I.G., Pekkanen J., Hämeri K., Kos G.P.A., de Hartog J.J., Hoek G., Katsouyanni K. Particulate matter air pollution and respiratory symptoms in individuals having either asthma or chronic obstructive pulmonary disease: a European multicentre panel study. Environ. Health. 2012;11:1–16. doi: 10.1186/1476-069X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärcher B., Peter T., Biermann U.M., Schumann U. The initial composition of jet condensation trails. J. Atmos. Sci. 1996;53:3066–3083. [Google Scholar]
- Katragkou E., Wilhelm S., Arnold F. First gaseous sulfur (VI) measurements in the simulated internal flow of an aircraft gas turbine engine during project PartEmis. Geophys. Res. Lett. 2004;31:L02117. doi: 10.1029/2003GL018231. [DOI] [Google Scholar]
- Katsouyanni K., Touloumi G., Spix C., Schwartz J., Balducci F., Medina S., Rossi G., Wojtyniak B., Sunyer J., Bacharova L., Schouten J.P., Ponka A., Anderson H.R. Short-term effects of ambient sulfur dioxide and particulate matter on mortality in 12 European cities: results from time series data from the APHEA project. Air Pollution and Health: a European Approach. Br. Med. J. 1997;314:1658–1663. doi: 10.1136/bmj.314.7095.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyte I.J., Harrison R.M., Lammel G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons – a review. Chem. Soc. Rev. 2013;42:9333–9391. doi: 10.1039/c3cs60147a. [DOI] [PubMed] [Google Scholar]
- Khadilkar H., Balakrishnan H. Estimation of aircraft taxi fuel burn using flight data recorder archives. Transp. Res. Part D. 2012;17:532–537. [Google Scholar]
- Kiendler A., Arnold F. Unambiguous identification and measurement of sulfuric acid cluster chemiions in aircraft jet engine exhaust. Atmos. Environ. 2002;36:1757–1761. [Google Scholar]
- Kim B.Y., Fleming G.G., Lee J.J., Waitz I.A., Clarke J.-P., Balasubramanian S., Malwitz A., Klima K., Locke M., Holsclaw C.A., Maurice L.Q., Gupta M.L. System for assessing Aviation's Global Emissions (SAGE), part 1: model description and inventory results. Transp. Res. Part D. 2007;12:325–346. [Google Scholar]
- Kinsey J.S., Timko M.T., Herndon S.C., Wood E.C., Yu Z., Miake-Lye R.C., Lobo P., Whitefield P., Hagen D., Wey C., Anderson B.E., Beyersdorf A.J., Hudgins C.H., Thornhill K.L., Winstead E., Howard R., Bulzan D.I., Tacina K.B., Knighton W.B. Determination of the emissions from an aircraft auxiliary power unit (APU) during the Alternative Aviation Fuel Experiment (AAFEX) JAWMA. 2012;62:420–430. doi: 10.1080/10473289.2012.655884. [DOI] [PubMed] [Google Scholar]
- Kinsey J.S., Timko M.T., Herndon S.C., Wood E.C., Yu Z., Miake-Lye R.C., Lobo P., Whitefield P., Hagen D., Wey C., Anderson B.E., Beyersdorf A.J., Hudgins C.H., Thornhill K.L., Winstead E., Howard R., Bulzan D.I., Tacina K.B., Knighton W.B. Determination of the emissions from an aircraft auxiliary power unit (APU) during the alternative aviation fuel experiment (AAFEX) JAWMA. 2012;62:420–430. doi: 10.1080/10473289.2012.655884. [DOI] [PubMed] [Google Scholar]
- Kinsey J.S., Hays M.D., Dong Y., Williams D.C., Logan R. Chemical characterization of the fine particle emissions from commercial aircraft engines during the Aircraft Particle Emissions eXperiment (APEX) 1 to 3. Environ. Sci. Technol. 2011;45:3415–3421. doi: 10.1021/es103880d. [DOI] [PubMed] [Google Scholar]
- Kinsey J.S., Dong Y., Williams D.C., Logan R. Physical characterization of the fine particle emissions from commercial aircraft engines during the aircraft particle emissions experiment (APEX) 1 to 3. Atmos. Environ. 2010;44:2147–2156. doi: 10.1021/es103880d. [DOI] [PubMed] [Google Scholar]
- Kinsey J.S. U.S. Environmental Protection Agency; Washington, D.C.: 2009. Characterization of Emissions from Commercial Aircraft Engines during the Aircraft Particle Emissions eXperiment (APEX) 1 to 3. EPA/600/R-09/130. [DOI] [PubMed] [Google Scholar]
- Kjellström E., Feichter J., Sausen R., Hein R. The contribution of aircraft emissions to the atmospheric sulfur budget. Atmos. Environ. 1999;33:3455–3465. [Google Scholar]
- Klapmeyer M.E., Marr L.C. CO2, NOx, and particle emissions from aircraft and support activities at a regional airport. Environ. Sci. Technol. 2012;46:10974–10981. doi: 10.1021/es302346x. [DOI] [PubMed] [Google Scholar]
- Knibbs L.D., Cole-Hunter T., Morawska L. A review of commuter exposure to ultrafine particles and its health effects. Atmos. Environ. 2011;45:2611–2622. [Google Scholar]
- Knight S.P., Salagaras M., Trueman A.R. The study of intergranular corrosion in aircraft aluminium alloys using X-ray tomography. Corros. Sci. 2011;53:727–734. [Google Scholar]
- Knighton W.B., Rogers T.M., Anderson B.E., Herndon S.C., Yelvington P.E., Miake-Lye R.C. Quantification of aircraft engine hydrocarbon emissions using proton transfer reaction mass spectrometry. J. Propul. Power. 2007;23:949–958. [Google Scholar]
- Knighton W.B., Herndon S.C., Miake-Lye R.C. Environmental Protection Agency, Office of Transportation and Air Quality; 2009. Aircraft Engine Speciated Organic Gases: Speciation of Unburned Organic Gases in Aircraft Exhaust.http://origin.www.faa.gov/regulations_policies/policy_guidance/envir_policy/media/FAA-EPA_TSD_Speciated%20OG_Aircraft_052709.pdf Available at: (last accessed August 2013) [Google Scholar]
- Kondo Y., Sahu L., Moteki N., Khan F., Takegawa N., Liu X., Koike M., Miyakawa T. Consistency and traceability of black carbon measurements made by laser-induced incandescence, thermal-optical transmittance, and filter-based photoabsorption techniques. Aerosol Sci. Technol. 2011;45:295–312. [Google Scholar]
- Koo J., Wang Q., Henze D.K., Waitz I.A., Barrett S.R.H. Spatial sensitivities of human health risk to intercontinental and high-altitude pollution. Atmos. Environ. 2013;71:140–147. [Google Scholar]
- Kraabøl A.G., Flatøy F., Stordal F. Impact of NOx emissions from subsonic aircraft: inclusion of plume processes in a three-dimensional model covering Europe, North America, and the North Atlantic. J. Geophys. Res. 2000;105(D3):3573–3581. [Google Scholar]
- Kraabøl A.G., Konopka P., Stordal F., Schlager H. Modelling chemistry in aircraft plumes 1: comparison with observations and evaluation of a layered approach. Atmos. Environ. 2000;34:3939–3950. [Google Scholar]
- Kraabøl A.G., Berntsen T.K., Sundet J.K., Stordal F. Impacts of NOx emissions from subsonic aircraft in a global three-dimensional chemistry transport model including plume processes. J. Geophys. Res. Atmos. (1984–2012) 2002;107(D22):ACH-22. [Google Scholar]
- Kroll J.H., Seinfeld J.H. Chemistry of secondary organic aerosol: formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ. 2008;42:3593–3624. [Google Scholar]
- Kugele A., Jelinek F., Gaffal R. 2005. Aircraft Particulate Matter Emission Estimation Through all Phases of Flight.http://www.eurocontrol.int/eec/gallery/content/public/document/eec/report/2005/034_Aircraft_Particulate_Matter_Emission_Estimation.pdf EUROCONTROL, EEC/SEE/2005/0014. Available at: (last accessed September 2013) [Google Scholar]
- Kuhn P.M. Airborne observations of contrail effects on the thermal radiation budget. J. Atmos. Sci. 1970;27:937–942. [Google Scholar]
- Kumar P., Pirjola L., Ketzel M., Harrison R. Nanoparticle emissions from 11 non-vehicle exhaust sources e a review. Atmos. Environ. 2013;67:252–277. [Google Scholar]
- Kumar P., Ketzel M., Vardoulakis S., Pirjola L., Britter R. Dynamics and dispersion modelling of nanoparticles from road traffic in the urban atmospheric environment—a review. J. Aerosol Sci. 2011;42:580–603. [Google Scholar]
- Kumar P., Robins A., Vardoulakis S., Britter R. A review of the characteristics of nanoparticles in the urban atmosphere and the prospects for developing regulatory controls. Atmos. Environ. 2010;44:5035–5052. [Google Scholar]
- Kurniawan J.S., Khardi S. Comparison of methodologies estimating emissions of aircraft pollutants, environmental impact assessment around airports. Environ. Impact Assess. Rev. 2011;31:240–252. [Google Scholar]
- Lavoi G., Heywood J., Keck J. Experimental and theoretical investigation of nitric oxide formation in internal combustion engines. Combust. Sci. Technol. 1970;1:313–326. [Google Scholar]
- Le Quéré C., Raupach M., Canadell J., Marland G. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009;2:831–836. [Google Scholar]
- Lee B.H., Santoni G.W., Wood E.C., Herndon S.C., Miake-Lye R.C., Zahniser M.S., Wofsy S.C., Lee J.W.M. Measurements of nitrous acid in commercial aircraft exhaust at the Alternative Aviation Fuel Experiment. Environ. Sci. Technol. 2011;45:7648–7654. doi: 10.1021/es200921t. [DOI] [PubMed] [Google Scholar]
- Lee D.S., Pitari G., Grewe V., Gierens K., Penner J.E., Petzold A., Prather M.J., Schumann U., Bais A., Berntsen T., Iachetti D., Lim L.L., Sausen R. Transport impacts on atmosphere and climate: aviation. Atmos. Environ. 2010;44:4678–4734. doi: 10.1016/j.atmosenv.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.S., Fahey D.W., Forster P.M., Newton P.J., Wit R.C.N., Lim L.L., Owen B., Sausen R. Aviation and global climate change in the 21st century. Atmos. Environ. 2009;43:3520–3537. doi: 10.1016/j.atmosenv.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.J., Waitz I.A., Kim B.Y., Fleming G.G., Maurice L., Holsclaw C.A. System for assessing Aviation's Global Emissions (SAGE), part 2: uncertainty assessment. Transp. Res. D Trans. Environ. 2007;12:381–395. [Google Scholar]
- Lelieveld J., Crutzen P.J., Dentener F.J. Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B. 1998;50:128–150. [Google Scholar]
- Levy J.I., Woody M., Baek B.H., Shankar U., Arunachalam S. Current and future particulate-matter-related mortality risks in the United States from aviation emissions during landing and takeoff. Risk Anal. 2012;32:237–249. doi: 10.1111/j.1539-6924.2011.01660.x. [DOI] [PubMed] [Google Scholar]
- Lewis E.R., Schwartz S.E. vol. 152. AGU; Washington DC: 2004. Sea Salt Aerosol Production. Mechanisms, Methods, Measurements, and Models – a Critical Review. (Geophysical Monograph Series). 413 pp. [Google Scholar]
- Lewis J.S., Niedzwiecki R.W., Bahr D.W., Bullock S., Cumpsty N., Dodds W., DuBois D., Epstein A., Freguson W.W., Fiorento A., Gorbatko A.A., Hagen D.E., Hart P.J., Hayashi S., Jamieson J.B., Kerrebrock J., Lecht M., Lowrie B., Miake- Lye R.C., Mortlock A.K., Moses C., Renger K., Sampath S., Sanborn J., Simon B., Sorokin A., Taylor W., Waitz I., Wey C.C., Whitefield P., Wilson C.W., Wu S. Aircraft technology and its relation to emissions. In: Penner J.E., Lister D.H., Griggs D.J., Dokken D.J., McFarland M., editors. Aviation and the Global Atmosphere. Cambridge University Press; Cambridge: 1999. (Special Report of the Intergovernmental Panel on Climate Change). (Chapter 7) [Google Scholar]
- Liao M., Chen W.R., Bellinger N.C. Effects of ultrasonic impact treatment on fatigue behavior of naturally exfoliated aluminum alloys. Int. J. Fatigue. 2008;30:717–726. [Google Scholar]
- Liaquat A.M., Kalam M.A., Masjuki H.H., Jayed M.H. Potential emissions reduction in road transport sector using biofuel in developing countries. Atmos. Environ. 2010;44:3869–3877. [Google Scholar]
- Lin S., Munsie J.P., Herdt-Losavio M., Hwang S.A., Civerolo K., McGarry K., Gentile T. Residential proximity to large airports and potential health impacts in New York State. Int. Arch. Occup. Environ. Health. 2008;81:797–804. doi: 10.1007/s00420-007-0265-1. [DOI] [PubMed] [Google Scholar]
- Lindstedt R.P., Maurice L.Q. Detailed chemical-kinetic model for aviation fuels. J. Propul. Power. 2000;16:187–195. [Google Scholar]
- Liyasova M., Li B., Schopfer L.M., Nachon F., Masson P., Furlong C.E., Lockridge O. Exposure to tri-o-cresyl phosphate detected in jet airplane passengers. Toxicol. Appl. Pharm. 2011;256:337–347. doi: 10.1016/j.taap.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Yan B., Chen G. Technical review on jet fuel production. Renew. Sust. Energ. Rev. 2013;25:59–70. [Google Scholar]
- Lobo P., Hagen D.E., Whitefield P.D. Measurement and analysis of aircraft engine PM emissions downwind of an active runway at the Oakland International Airport. Atmos. Environ. 2012;61:114–123. [Google Scholar]
- Lobo P., Hagen D.E., Whitefield P.D. Comparison of PM emissions from a commercial jet engine burning conventional, biomass, and Fischer–Tropsch fuels. Environ. Sci. Technol. 2011;45:10744–10749. doi: 10.1021/es201902e. [DOI] [PubMed] [Google Scholar]
- Lobo P., Whitefield P.D., Hagen D.E., Herndon S.C., Jayne J.T., Wood E.C., Knighton W.B., Northway M.J., Miake-Lye R.C., Cocker D., Sawant A., Agrawal H., Miller J.W. California Air Resources Board; Sacramento, CA: 2007. The Development of Exhaust Speciation Profiles for Commercial Jet Engines, Final Report.http://www.arb.ca.gov/research/apr/past/04-344.pdf Contract No. 04-344. Oct 31, 2007. Available at: (last accessed September 2013) [Google Scholar]
- Long C.M., Nascarella M.A., Valberg P.A. Carbon black vs. black carbon and other airborne materials containing elemental carbon: physical and chemical distinctions. Environ. Pollut. 2013;181:271–286. doi: 10.1016/j.envpol.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Loukhovitskaya E.E., Talukdar R.K., Ravishankara A.R. Uptake of HNO3 on aviation kerosene and aircraft engine soot: influences of H2O or/and H2SO4. J. Phys. Chem. A. 2013;117:4928–4936. doi: 10.1021/jp401723k. [DOI] [PubMed] [Google Scholar]
- Lukachko S.P., Waitz I.A., Miake-Lye R.C., Brown R.C., Anderson M.R. Production of sulphate aerosol precursors in the turbine and exhaust nozzle of an aircraft engine. J. Geophys. Res. 1998;103(D13):16159–16174. [Google Scholar]
- Mahashabde A., Wolfe P., Ashok A., Dorbian C., He Q., Fan A., Lukachko S., Mozdzanowska A., Wollersheim C., Barrett S.R.H., Locke M., Waitz I.A. Assessing the environmental impacts of aircraft noise and emissions. Prog. Aerosp. Sci. 2011;47:15–52. [Google Scholar]
- Marsillach J., Richter R.J., Kim J.H., Stevens R.C., MacCoss M.J., Tomazela D., Suzuki S.M., Schopfer L.M., Lockridge O., Furlong C.E. Biomarkers of organophosphorus (OP) exposures in humans. Neurotoxicology. 2011;32:656–660. doi: 10.1016/j.neuro.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli N., Olivieri O., Girelli D. Air particulate matter and cardiovascular disease: a narrative review. Eur. J. Intern. Med. 2013;24:295–302. doi: 10.1016/j.ejim.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Mavroidis I., Chaloulakou A. Long-term trends of primary and secondary NO2 production in the Athens area. Variation of the NO2/NOx ratio. Atmos. Environ. 2011;45:6872–6879. [Google Scholar]
- Maynard R.L. Health effects of urban pollution. In: Hester R.E., Harrison R.M., editors. vol. 28. Royal Society of Chemistry; Cambridge: 2009. pp. 108–128. (Issues in Environmental Science and Technology). [Google Scholar]
- Mazaheri M., Johnson G.R., Morawska L. Particle and gaseous emissions from commercial aircraft at each stage of the landing and takeoff cycle. Environ. Sci. Technol. 2009;43:441–446. doi: 10.1021/es8013985. [DOI] [PubMed] [Google Scholar]
- Mazaheri M., Johnson G.R., Morawska L. An inventory of particle and gaseous emissions from large aircraft thrust engine operations at an airport. Atmos. Environ. 2011;45:3500–3507. [Google Scholar]
- Mazaheri M., Bostrom T.E., Johnson G.R., Morawska L. Composition and morphology of particle emissions from in-use aircraft during takeoff and landing. Environ. Sci. Technol. 2013;47(10):5235–5242. doi: 10.1021/es3046058. [DOI] [PubMed] [Google Scholar]
- Mazraati M. World aviation fuel demand outlook. OPEC Energy Rev. 2010;34(1):42–72. [Google Scholar]
- Miake-Lye R.C., Anderson B.E., Cofer W.R., Wallio H.A., Nowicki G.D., Ballenthin J.O., Hunton D.E., Knighton W.B., Miller T.M., Seeley J.V., Viggiano A.A. SOx oxidation and volatile aerosol in aircraft exhaust plumes depend on fuel sulfur content. Geophys. Res. Lett. 1998;25:1677–1680. [Google Scholar]
- Miller T.M., Ballenthin J.O., Hunton D.E., Viggiano A.A., Wey C.C., Anderson B.E. Nitric acid emissions from the F100 jet engine. J. Geophys. Res. 2003;108(D1):4032. doi: 10.1029/2001JD001522. [DOI] [Google Scholar]
- Miller T.M., Ballenthin J.O., Viggiano A.A., Anderson B.E., Wey C.C. Mass distribution and concentrations of negative chemiions in the exhaust of a jet engine: sulfuric acid concentrations and observation of particle growth. Atmos. Environ. 2005;39:3069–3079. [Google Scholar]
- Miracolo M.A., Hennigan C.J., Ranjan M., Nguyen N.T., Gordon T.D., Lipsky E.M., Presto A.A., Donahue N.M., Robinson A.L. Secondary aerosol formation from photochemical aging of aircraft exhaust in a smog chamber. Atmos. Chem. Phys. 2011;11:4135–4147. [Google Scholar]
- Morawska L., Ristovski Z., Jayaratne E.R., Keogh D.U., Ling X. Ambient nano and ultrafine particles from motor vehicle emissions: characteristics, ambient processing and implications on human exposure. Atmos. Environ. 2008;42:8113–8138. [Google Scholar]
- Morris K. British Airways Environmental Affairs, British Airways Technical Documents Relating to the Aircraft Operations Supporting the Project for the Sustainable Development of Heathrow, Document 7, EJT/KMM/1131/14.18. British Airways, London. 2006. An estimation of the tyre material erosion from measurements of aircraft.http://www.britishairways.com/cms/global/pdfs/csr/PSDH_Technical_Reports.pdf Available at: (last accessed September 2013) [Google Scholar]
- Morris K. British Airways; London: 2005. Reverse Thrust Examples from British Airways Operations.http://www.britishairways.com/cms/global/pdfs/csr/PSDH_Technical_Reports.pdf (British Airways Environmental Affairs, British Airways Technical Documents Relating to the Aircraft Operations Supporting the Project for the Sustainable Development of Heathrow, Document 3, WG2/TG4-IP5/7). Available at: (last accessed September 2013) [Google Scholar]
- Morris K. British Airways Environmental Affairs, British Airways Technical Documents Relating to the Aircraft Operations Supporting the Project for the Sustainable Development of Heathrow, Document 5, EJT/KMM/1126/14.8. British Airways; London: 2005. Results from a number of surveys of power settings used during taxi operations.http://www.britishairways.com/cms/global/pdfs/csr/PSDH_Technical_Reports.pdf Available at: (last accessed September 2013) [Google Scholar]
- Morris K., Easey N. British Airways Environmental Affairs, British Airways Technical Documents Relating to the Aircraft Operations Supporting the Project for the Sustainable Development of Heathrow, Document 6, EJT/KMM/1128/14.18. British Airways; London: 2005. Results from two surveys of the use of reverse thrust of aircraft landing at Heathrow airport.http://www.britishairways.com/cms/global/pdfs/csr/PSDH_Technical_Reports.pdf Available at: (last accessed September 2013) [Google Scholar]
- Morris K. British Airways Environmental Affairs, British Airways Technical Documents Relating to the Aircraft Operations Supporting the Project for the Sustainable Development of Heathrow, Document 2, WG 3 AEMTG WP10/10. British Airways; London: 2002. Take-off at less than full power.http://www.britishairways.com/cms/global/pdfs/csr/PSDH_Technical_Reports.pdf Available at: (last accessed September 2013) [Google Scholar]
- Murcray W.B. On the possibility of weather modification by aircraft contrails. Mon. Weather Rev. 1970;98:745–748. [Google Scholar]
- Nambisan S., Kajkowski J., Menon R. A preliminary survey of ground service equipment running times and its implications for air quality estimates at airports. In: Nambisan S., editor. The 2020 Vision of Air Transportation. American Society of Civil Engineers; San Francisco CA: 2000. pp. 144–152. [Google Scholar]
- Naugle F., Fox D.L. Aircraft and air pollution. Environ. Sci. Technol. 1981;1:391–395. doi: 10.1021/es00086a002. [DOI] [PubMed] [Google Scholar]
- Nikoleris T., Gupta G., Kistler M. Detailed estimation of fuel consumption and emissions during aircraft taxi operations at Dallas/Fort Worth International Airport. Transp. Res. D. 2011;16:302–308. [Google Scholar]
- Ning Z., Sioutas C. Atmospheric processes influencing aerosols generated by combustion and the inference of their impact on public exposure: a review. Aerosol Air Qual. Res. 2010;10:43–58. [Google Scholar]
- Novakov T., Rosen H. The black carbon story: early history and new perspectives. AMBIO. 2013;42(7):840–851. doi: 10.1007/s13280-013-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli P.C., Masarie K.A., Lang P.M. Distributions and recent changes of carbon monoxide in the lower troposphere. J. Geophys. Res. 1998;103(D15):19,015–19,033. [Google Scholar]
- Nygren E., Aleklett K., Höök M. Aviation fuel and future oil production scenarios. Energ. Policy. 2009;37:4003–4010. [Google Scholar]
- O'Brien R.J., Wade M.D. 2003. Air Emissions Inventory Guidance Document for Mobile Sources at Air Force Installations (Revised December 2003) IERA-RS-BR-SR-2001-0010. [Google Scholar]
- Oberdorster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W., Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Odum J.R., Hoffmann T., Bowman F., Collins D., Flagan R.C., Seinfeld J.H. Gas/particle partitioning and secondary organic aerosol yields. Environ. Sci. Technol. 1996;30:2580–2585. [Google Scholar]
- Olsen S.C., Wuebbles D.J., Owen B. Comparison of global 3-D aviation emissions datasets. Atmos. Chem. Phys. 2013;13:429–441. [Google Scholar]
- Onasch T.B., Jayne J.T., Herndon S., Worsnop D.R., Miake-Lye R.C., Mortimer I.P., Anderson B.E. Chemical properties of aircraft engine particulate exhaust emissions. J. Propul. Power. 2009;25:1121–1137. [Google Scholar]
- Ostash O.P., Andreiko I.M., Holovatyuk Y.V. Degradation of materials and fatigue durability of aircraft constructions after long-term operation. Mater. Sci. 2006;42:427–439. [Google Scholar]
- Owen B., Lee D.S., Lim L. Flying into the future: aviation emissions scenarios to 2050. Environ. Sci. Technol. 2010;44:2255–2260. doi: 10.1021/es902530z. [DOI] [PubMed] [Google Scholar]
- Paladino J., Whitefield P., Hagen D., Hopkins A.R., Trueblood M. Particle concentration characterization for jet engine emissions under cruise conditions. Geophys. Res. Lett. 1998;25:1697–1700. [Google Scholar]
- Pandism S.N., Harle R.A., Cass G.R., Seinfeld J.H. Secondary organic aerosol formation and transport. Atmos. Environ. A General Top. 1992;26:2269–2282. [Google Scholar]
- Pankow J.F. An absorption model of the gas/aerosol partitioning involved in the formation of secondary organic aerosol. Atmos. Environ. 1994;28:189–193. [Google Scholar]
- Pant P., Harrison R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: a review. Atmos. Environ. 2013;77:78–97. [Google Scholar]
- Pariselli F., Sacco M.G., Ponti J., Rembges D. Effects of toluene and benzene air mixtures on human lung cells (A549) Exp. Toxicol. Pathol. 2009;61:381–386. doi: 10.1016/j.etp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Patterson J., Noel G.J., Senzig D.A., Roof C.J., Fleming G.G. Analysis of departure and arrival profiles using real-time aircraft data. J. Aircr. 2009;46:1094–1103. [Google Scholar]
- Peace H., Maughan J., Owen B., Raper D. Identifying the contribution of different airport related sources to local urban air quality. Environ. Modell. Softw. 2006;21:532–538. [Google Scholar]
- Peck J., Oluwole O.O., Wong H.W., Miake-Lye R.C. An algorithm to estimate aircraft cruise black carbon emissions for use in developing a cruise emissions inventory. JAWMA. 2013;63:367–375. doi: 10.1080/10962247.2012.751467. [DOI] [PubMed] [Google Scholar]
- Peters G.P., Marland G., Le Quéré C., Boden T., Canadell J.G., Raupach M.R. Rapid growth in CO2 emissions after the 2008–2009 global financial crisis. Nat. Clim. Change. 2011;2:2–4. [Google Scholar]
- Petzold A., Ogren J.A., Fiebig M., Laj P., Li S.-M., Baltensperger U., Holzer-Popp T., Kinne S., Pappalardo G., Sugimoto N., Wehrli C., Wiedensohler A., Zhang X.-Y. Recommendations for reporting “black carbon” measurements. Atmos. Chem. Phys. 2013;13:8365–8379. [Google Scholar]
- Petzold A., Gysel M., Vancassel X., Hitzenberger R., Puxbaum H., Vrochticky S., Weingartner E., Baltensperger U., Mirabel P. On the effects of organic matter and sulfur-containing compounds on the CCN activation of combustion particles. Atmos. Chem. Phys. 2005;5:3187–3203. [Google Scholar]
- Petzold A., Fiebig M., Fritzsche L., Stein C., Schumann U., Wilson C.W., Hurley C.D., Arnold F., Katragkou E., Baltensperger U., Gysel M., Nyeki S., Hitzenberger R., Giebl H., Hughes K.J., Kurtenbach R., Wiesen P., Madden P., Puxbaum H., Vrchoticky S., Wahl C. Particle emissions from aircraft engines – a survey of the European project PartEmis. Meteorol. Z. 2005;14:465–476. [Google Scholar]
- Petzold A., Stein C., Nyeki S., Gysel M., Weingartner E., Baltensperger U., Giebl H., Hitzenberger R., Döpelheuer A., Vrchoticky S., Puxbaum H., Johnson M., Hurley C.D., Marsh R., Wilson C.W. Properties of jet engine combustion particles during the PartEmis experiment: microphysics and chemistry. Geophys. Res. Lett. 2003;30:1719. [Google Scholar]
- Petzold A., Strom J., Schroeder F.P., Karcher B. Carbonaceous aerosol in jet engine exhaust: emission characteristics and implications for heterogeneous chemical reactions. Atmos. Environ. 1999;33:2689–2698. [Google Scholar]
- Petzold A., Döpelheuer A. Reexamination of black carbon mass emission indices of a jet engine. Aerosol Sci. Technol. 1998;29:355–356. [Google Scholar]
- Petzold A., Schröder F.P. Jet engine exhaust aerosol characterization. Aerosol Sci. Technol. 1998;28:62–76. [Google Scholar]
- Petzold A., Ström J., Ohlsson S., Schröder F.P. Elemental composition and morphology of ice-crystal residual particles in cirrus clouds and contrails. Atmos. Res. 1998;49:21–34. [Google Scholar]
- Pleil J.D., Smith L.B., Zelnick S.D. Personal exposure to JP-8 jet fuel vapours and exhaust at air force bases. Environ. Health Perspect. 2000;108:183–192. doi: 10.1289/ehp.00108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polichetti G., Cocco S., Spinali A., Trimarco V., Nunziata A. Effects of particulate matter (PM10, PM2.5 and PM1) on the cardiovascular system. Toxicology. 2009;261:1–8. doi: 10.1016/j.tox.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Pope C.A., III, Ezzati M., Dockery D.W. Fine-Particulate air pollution and life expectancy in the United States. N. Engl. J. Med. 2009;360:376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C.A., III, Dockery D.W. Health effects of fine particulate air pollution: lines that connect. JAWMA. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Popovicheva O.B., Persiantseva N.M., Lukhovitskaya E.E., Shonija N.K., Zubareva N.A., Demirdjian B., Ferry D., Suzanne J. Aircraft engine soot as contrail nuclei. Geophys. Res. Lett. 2004;31 doi: 10.1029/2003GL018888. [DOI] [Google Scholar]
- Popovitcheva O.B., Persiantseva N.M., Trukhin M.E., Rulev G.B., Shonija N.K., Buriko Y.Y., Starik A.M., Demirdjian B., Ferry D., Suzanne J. Experimental characterization of aircraft combustor soot: microstructure, surface area, porosity and water adsorption. Phys. Chem. Chem. Phys. 2000;2:4421–4426. [Google Scholar]
- Popp P.J., Bishop G.A., Stedman D.H. Method for commercial aircraft nitric oxide emission measurements. Environ. Sci. Technol. 1999;33:1542–1544. [Google Scholar]
- Pöschl U. Aerosol particle analysis: challenges and progress. Anal. Bioanal. Chem. 2003;375:30–32. doi: 10.1007/s00216-002-1611-5. [DOI] [PubMed] [Google Scholar]
- Prather M., Sausen R., Grossman A.S., Haywood J.M., Rind D., Subbaraya B.H. Potential climate change from aviation. In: Penner J.E., Lister D.H., Griggs D.J., Dokken D.J., McFarland M., editors. Aviation and the Global Atmosphere. Cambridge University Press; Cambridge: 1999. (Special Report of the Intergovernmental Panel on Climate Change). (Chapter 6) [Google Scholar]
- Presto A.A., Nguyen N.T., Ranjan M., Reeder A.J., Lipsky E.M., Hennigan C.J., Miracolo M.A., Riemer D.D., Robinson A.L. Fine particle and organic vapor emissions from staged tests of an in-use aircraft engine. Atmos. Environ. 2011;45(21):3603–3612. [Google Scholar]
- Raaschou-Nielsen O., Andersen Z.J., Beelen R., Samoli E., Stafoggia M., Weinmayr G., Hoffmann B., Fischer P., Nieuwenhuijsen M.J., Brunekreef B., Xun W.W., Katsouyanni K., Dimakopoulou K., Sommar J., Forsberg B., Modig L., Oudin A., Oftedal B., Schwarze P.E., Nafstad P., De Faire U., Pedersen N.L., Östenson C.-G., Fratiglioni L., Penell J., Korek M., Pershagen G., Eriksen K.T., Sørensen M., Tjønneland A., Ellermann T., Eeftens M., Peeters P.H., Meliefste K., Wang M., Bueno-de-Mesquita B., Key T.J., de Hoogh K., Concin H., Nagel G., Vilier A., Grioni S., Krogh V., Tsai M.-Y., Ricceri F., Sacerdote C., Galassi C., Migliore E., Ranzi A., Cesaroni G., Badaloni C., Forastiere F., Tamayo I., Amiano P., Dorronsoro M., Trichopoulou A., Bamia C., Vineis P., Hoek G. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- Ramanathan V., Feng Y. Air pollution, greenhouse gases and climate change: global and regional perspectives. Atmos. Environ. 2009;43:37–50. [Google Scholar]
- Rasmussen C.L., Glarborg P., Marshall P. Mechanisms of radical removal by SO2. Proc. Combust. Inst. 2007;31:339–347. [Google Scholar]
- Ratliff G., Sequeira C., Waitz I., Ohsfeldt M., Thrasher T., Graham M., Thompson T. 2009. Aircraft Impacts on Local and Regional Air Quality in the United States. PARTNER Report 15 Final Report (Report No. PARTNER-COE-2009-002) [Google Scholar]
- Ravindra K., Sokhi R., Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos. Environ. 2008;42:2895–2921. [Google Scholar]
- Ray S., Khillare P.S., Agarwal T., Shridhar V. Assessment of PAHs in soil around the International Airport in Delhi, India. J. Hazard. Mater. 2008;156:9–16. doi: 10.1016/j.jhazmat.2007.11.099. [DOI] [PubMed] [Google Scholar]
- Reiner T., Arnold F. Laboratory flow reactor measurements of the reaction SO3 + H2O + MH2SO4 + M: implications for gaseous H2SO4 and aerosol formation in the plume of jet aircraft. Geophys. Res. Lett. 1993;20:2659–2662. [Google Scholar]
- Reiner T., Arnold F. Laboratory flow reactor measurements of the reaction SO3 + H2O + MH2SO4 + M: implications for gaseous H2SO4 and aerosol formation in aircraft exhaust plumes. J. Chem. Phys. 1994;101:7399–7407. [Google Scholar]
- Reinking R.F. Insolation reduction by contrails. Weather. 1968;23:171–173. [Google Scholar]
- Ritchie G.D. Biological and health effects of exposure to kerosene-based jet fuels and performance additives. J. Toxicol. Environ. Health B. 2003;6:357–451. doi: 10.1080/10937400306473. [DOI] [PubMed] [Google Scholar]
- Ritchie G.D., Still K.R., Alexander W.K., Nordholm A.F., Wilson C.L., Rossi I.J., Mattie D.R. A review of the neurotoxicity risk of selected hydrocarbon fuels. J. Toxicol. Environ. Health B Crit. Rev. 2001;4:223–312. doi: 10.1080/109374001301419728. [DOI] [PubMed] [Google Scholar]
- Robinson A.L., Grieshop A.P., Donahue N.M., Hunt S.W. Updating the conceptual model for fine particle mass emissions from combustion systems. JAWMA. 2010;60:1204–1222. doi: 10.3155/1047-3289.60.10.1204. [DOI] [PubMed] [Google Scholar]
- Rogers F., Arnott P., Zielinska B., Sagebiel J., Kelly K.E., Wagner D., Lighty J.S., Sarofim A.F. Real-time measurements of jet aircraft engine exhaust. JAWMA. 2005;55:583–593. doi: 10.1080/10473289.2005.10464651. [DOI] [PubMed] [Google Scholar]
- Roof C., Hansen A., Fleming G.G., Thrasher T., Nguyen A., Hall C., Dinges E., Bea R., Grandi F., Kim B.Y., Usdrowski S., Hollingsworth P. 2007. Aviation Environmental Design Tool (AEDT) System Architecture AEDT-AD-01.http://www.faa.gov/about/office_org/headquarters_offices/apl/research/models/history/media/AEDT_Architecture.pdf Available at: (last accessed September 2013) [Google Scholar]
- Rückerl R., Schneider A., Breitner S., Cyrys J., Peters A. Health effects of particulate air pollution – a review of epidemiological evidence. Inhal. Toxicol. 2011;23:555–592. doi: 10.3109/08958378.2011.593587. [DOI] [PubMed] [Google Scholar]
- Russo S., Sharp P.K., Dhamari R., Mills T.B., Hinton B.R.W., Clark G., Shankar K. The influence of the environment and corrosion on the structural integrity of aircraft materials. Fatigue Fract. Eng. Mater. 2009;32:464–472. [Google Scholar]
- SAE . vol. 4970. SAE International; Warrendale, PA: 2009. (Aerospace Information Report AIR6037 Aircraft Exhaust Nonvolatile Particle Matter Measurement Method Development). [Google Scholar]
- Saillenfait A.M., Gallissot F., Morel G., Bonnet P. Developmental toxicities of ethylbenzene, ortho-, meta-, para-xylene and technical xylene in rats following inhalation exposure. Food Chem. Toxicol. 2003;41:415–429. doi: 10.1016/s0278-6915(02)00231-4. [DOI] [PubMed] [Google Scholar]
- Samoli E., Aga E., Touloumi G., Nisiotis K., Forsberg B., Lefranc A., Pekkanen J., Wojtyniak B., Schindler C., Niciu E., Brunstein R., Dodič Fikfak M., Schwartz J., Katsouyanni K. Short-term effects of nitrogen dioxide on mortality: an analysis within the APHEA project. Eur. Respir. J. 2006;27:1129–1138. doi: 10.1183/09031936.06.00143905. [DOI] [PubMed] [Google Scholar]
- Samoli E., Touloumi G., Schwartz J., Anderson H.R., Schindler C., Forsberg B., Vigotti M.A., Vonk J., Kosnik M., Skorkovsky J., Katsouyanni K. Short-term effects of carbon monoxide on mortality: an analysis within the APHEA project. Environ. Health Perspect. 2007;115:1578–1583. doi: 10.1289/ehp.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni G.W., Lee B.H., Wood E.C., Herndon S.C., Miake-Lye R.C., Wofsy S.C., McManus J.B., Nelson D.D., Zahniser M.S. Aircraft emissions of methane and nitrous oxide during the alternative aviation fuel experiment. Environ. Sci. Technol. 2011;45:7075–7082. doi: 10.1021/es200897h. [DOI] [PubMed] [Google Scholar]
- Sapkota A., Chelikowsky A.P., Nachman K.E., Cohen A.J., Ritz B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Qual. Atmos. Health. 2012;5:369–381. [Google Scholar]
- Sausen R., Isaksen I., Grewe V., Hauglustaine D., Lee D.S., Myhre G., Köhler M.O., Pitari G., Schumann U., Stordal F., Zerefos C. Aviation radiative forcing in 2000: an update on IPCC (1999) Meteorol. Z. 2005;14:555–561. [Google Scholar]
- Schäfer K., Emeis S., Hoffmann H., Jahn C. Influence of mixing layer height upon air pollution in urban and sub-urban area. Meteorol. Z. 2006;15:647–658. [Google Scholar]
- Schäfer K., Jahn C., Sturm P., Lechner B., Bacher M. Aircraft emission measurements by remote sensing methodologies at airports. Atmos. Environ. 2003;37(37):5261–5271. [Google Scholar]
- Schindler B.K., Weiss T., Schütze A., Koslitz S., Broding H.C., Bünger J., Brüning T. Occupational exposure of air crews to tricresyl phosphate isomers and organophosphate flame retardants after fume events. Arch. Toxicol. 2013;87:645–648. doi: 10.1007/s00204-012-0978-0. [DOI] [PubMed] [Google Scholar]
- Schlager H., Konopka P., Schulte P., Schumann U., Ziereis H., Arnold F., Klemm M., Hagen D.E., Whitefield P.D., Ovarlez J. Situ observation of air traffic emission signatures in the North Atlantic flight corridor. J. Geophys. Res. 1997;102:10739–10750. [Google Scholar]
- Schneider T., O'Gorman P.A., Levine X.J. Water vapor and the dynamics of climate changes. Rev. Geophys. 2010;48:RG3001. [Google Scholar]
- Schröder F., Brock C.A., Baumann R., Petzold A., Busen R., Schulte P., Fiebig M. In situ studies on volatile jet exhaust particle emissions: impact of fuel sulfur content and environmental conditions on nuclei mode aerosols. J. Geophys. Res. 2000;105(D15):19941–19954. [Google Scholar]
- Schumann U. Formation, properties and climatic effects of contrails. C. R. Phys. 2005;6:549–565. [Google Scholar]
- Schumann U., Arnold F., Busen R., Curtius J., Kärcher B., Kiendler A., Petzold A., Schlager H., Schröder F., Wohlfrom K.-H. Influence of fuel sulfur on the composition of aircraft exhaust plumes: the experiments SULFUR 1–7. J. Geophys. Res. Atmos. 2002;107(D15) AAC-2. [Google Scholar]
- Schumann U., Schlager H., Arnold F., Ovarlez J., Kelder H., Hov Ø., Hayman G., Isaksen I.S.A., Staehelin J., Whitefield P.D. Pollution from aircraft emissions in the North Atlantic flight corridor: overview on the POLINAT projects. J. Geophys. Res. Atmos. (1984–2012) 2000;105(D3):3605–3631. [Google Scholar]
- Schumann U., Schlager H., Arnold F., Baumann R., Haschberger P., Klemm O. Dilution of aircraft exhaust plumes at cruise altitudes. Atmos. Environ. 1998;32:3097–3103. [Google Scholar]
- Schumann U. The impact of nitrogen oxides emissions from aircraft upon the atmosphere at flight altitudes 8–15 km – results from the AERONOX Project. Atmos. Environ. 1997;31:1723–1733. [Google Scholar]
- Schumann U. On conditions for contrail formation from air craft exhausts. Meteorol. Z. 1996;5:4–23. [Google Scholar]
- Schumann, Ström J., Busen R., Baumann R., Gierens K., Krautstrunk M., Schröder F.P., Stingl J. In situ observations of particles in jet aircraft exhausts and contrails for different sulfur-containing fuels. J. Geophys. Res. Atmos. (1984–2012) 1996;101(D3):6853–6869. [Google Scholar]
- Shumway L.A. U.S. Navy Aircraft Environmental Support Office by the SSC San Diego Marine Environmental Quality Branch; 2002. Characterization of Jet Engine Exhaust Participates for the F404, F118,T64,andT58 Aircraft Engines. Technical Report 1881, March 2002. [Google Scholar]
- Schürmann G., Schäfer K., Jahn C., Hoffmann H., Bauerfeind M., Fleuti E., Rappenglück B. The impact of NOx, CO and VOC emissions on the air quality of Zurich airport. Atmos. Environ. 2007;41:103–118. [Google Scholar]
- Schwartz J. Air pollution and hospital admissions for cardiovascular disease in Tuscon. Epidemiology. 1997;8:371–377. doi: 10.1097/00001648-199707000-00004. [DOI] [PubMed] [Google Scholar]
- Screpanti A., De Marco A. Corrosion on cultural heritage buildings in Italy: a role for ozone? Environ. Pollut. 2009;157:1513–1520. doi: 10.1016/j.envpol.2008.09.046. [DOI] [PubMed] [Google Scholar]
- Seigneur C. Current understanding of ultrafine particulate matter emitted from mobile sources. JAWMA. 2009;59:3–17. doi: 10.3155/1047-3289.59.1.3. [DOI] [PubMed] [Google Scholar]
- Seinfeld J.H., Pandis S.N. second ed. John Wiley & Sons; NewYork: 2006. Atmospheric Chemistry and Physics. (From Air Pollution to Climate Change). [Google Scholar]
- Shell . 2013. World-wide Civil Jet Fuel Grades.http://www.shell.com/global/products-services/solutions-for-businesses/aviation/shell-aviation-fuels/fuels/types/civil-jet-fuel-grades.html#textwithimage_19 Webpage. Available at: (last accessed: December 2013) [Google Scholar]
- Sherwood S.C., Roca R., Weckwerth T.M., Andronova N.G. Tropospheric water vapor, convection, and climate. Rev. Geophys. 2010;48:RG2001. doi: 10.1029/2009RG000301. [DOI] [Google Scholar]
- Simone N.W., Stettler M.E.J., Barrett S.R.H. Rapid estimation of global civil aviation emissions with uncertainty quantification. Transp. Res. D. 2013;25:33–41. [Google Scholar]
- Slemr F., Giehl H., Habram M., Slemr J., Schlager H., Schulte P., Haschberger P., Lindermeir E., Dopelheuer A., Plohr M. In-flight measurements of aircraft CO and nonmethane hydrocarbon emission indices. J. Geophys. Res. 2001;106:7485–7494. [Google Scholar]
- Slemr F., Giehl H., Slemr J., Busen R., Schulte P., Haschberger P. In-flight measurement of aircraft non-methane hydrocarbon emission indices. Geophys. Res. Lett. 1998;25:321–324. [Google Scholar]
- Slogar G.A., Holder R.C. U.S. Environmental Protection Agency, Office of Mobile Source Air Pollution Control; Ann Arbor: MI: 1976. Determination of Effects of Ambient Conditions on Aircraft Engine Emissions, Engine Testing – GTCP 85 APU, Type 331 Turboprop. EPA-460/3-76-009a and b. [Google Scholar]
- Smith S.J., Aardenne J.V., Klimont Z., Andres R.J., Volke A., Delgado Arias S. Anthropogenic sulfur dioxide emissions: 1850–2005. Atmos. Chem. Phys. 2011;11:1101–1116. [Google Scholar]
- Solbu K., Daae H.L., Thorud S., Ellingsen D.G., Lundanes E., Molander P. Exposure to airborne organophosphates originating from hydraulic and turbine oils among aviation technicians and loaders. J. Environ. Monit. 2010;12:2259–2268. doi: 10.1039/c0em00273a. [DOI] [PubMed] [Google Scholar]
- Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K.B., Tignor M., Miller H.L., editors. Climate Change 2007: the Physical Science Basis. Cambridge University Press; Cambridge, United Kingdom and NewYork, NY, USA: 2007. (Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change). [Google Scholar]
- Solomon S., Rosenlof K.H., Portmann R.W., Daniel J.S., Davis S.M., Sanford T.J., Plattner G.K. Contributions of stratospheric water vapor to decadal changes in the rate of global warming. Science. 2010;327(5970):1219–1223. doi: 10.1126/science.1182488. [DOI] [PubMed] [Google Scholar]
- Somnitz H., Gleitsmann G.G., Zellner R. Novel rates of OH induced sulfur oxidation. Implications to the plume chemistry of jet aircraft. Meteorol. Z. 2005;14:459–464. [Google Scholar]
- Sorokin A., Katragkou E., Arnold F., Busen R., Schumann U. Gaseous SO3 and H2SO4 in the exhaust of an aircraft gas turbine engine: measurements by CIMS and implications for fuel sulfur conversion to sulfur (VI) and conversion of SO3 to H2SO4. Atmos. Environ. 2004;38:449–456. [Google Scholar]
- Sorokin A., Mirabel P. Ion recombination in aircraft exhaust plumes. Geophys. Res. Lett. 2001;28(6):955–958. [Google Scholar]
- Spicer C.W., Holdren M.W., Cowen K.A., Joseph D.W., Satola J., Goodwin B., Mayfield H., Laskin A., Alexander M.L., Ortega J.V., Newburn M., Kagann R., Hashmonay R. Rapid measurement of emissions from military aircraft turbine engines by downstream extractive sampling of aircraft on the ground: results for C-130 and F-15 aircraft. Atmos. Environ. 2009;43:2612–2622. [Google Scholar]
- Spicer C.W., Holdren M.W., Riggin R.M., Lyon T.F. Chemical composition and photochemical reactivity of exhaust from aircraft turbine engines. Ann. Geophys. 1994;12:12,944–12,955. [Google Scholar]
- Spicer C.W., Holdren M.W., Smith D.L., Hughes D.P., Smith M.D. Chemical composition of exhaust from aircraft turbine engines. J. Eng. Gas Turbines Power. 1992;114:111–115. [Google Scholar]
- Spicer C.W., Holdren M.W., Lyon T.F., Riggin R.M. 1984. Composition and Photochemical Reactivity of Turbine Engine Exhaust. Prepared by Battelle Laboratories, Prepared for the Air Force Engineering & Services Center. [Google Scholar]
- Starik A.M. Gaseous and particulate emissions with jet engine exhaust and atmospheric pollution. In: Research and Technology Organisation (RTO) of NATO, editor. Advances on Propulsion Technology for High-Speed Aircraft, Educational Notes RTO-EN-AVT-150, Paper 15. RTO; Neuilly-sur-Seine, France: 2008. http://www.rto.nato.int Available at: (last accessed: September 2013) [Google Scholar]
- Starik A.M., Savel'ev A.M., Titova N.S., Loukhovitskaya E.E., Schumann U. Effect of aerosol precursors from gas turbine engines on the volatile sulfate aerosols and ion clusters formation in aircraft plumes. Phys. Chem. Chem. Phys. 2004;6:3426–3436. [Google Scholar]
- Starik A.M., Savel'ev A.M., Titova N.S., Schumann U. Modeling of sulfur gases and chemiions in aircraft engines. Aer. Sci. Technol. 2002;6:63–81. [Google Scholar]
- Stettler M.E., Boies A.M., Petzold A., Barrett S.R. Global civil aviation black carbon emissions. Environ. Sci. Technol. 2013;47:10397–10404. doi: 10.1021/es401356v. [DOI] [PubMed] [Google Scholar]
- Stettler M.E., Swanson J.J., Barrett S.R., Boies A.M. Updated correlation between aircraft smoke number and black carbon concentration. Aerosol Sci. Technol. 2013;47:1205–1214. [Google Scholar]
- Stettler M.E.J., Eastham S., Barrett S.R.H. Air quality and public health impacts of UK airports. Part I: emissions. Atmos. Environ. 2011;45:5415–5424. [Google Scholar]
- Stockwell W.R. The effect of gas-phase chemistry on aqueous-phase sulfur dioxide oxidation rates. J. Atmos. Chem. 1994;19:317–329. [Google Scholar]
- Stockwell W.R., Calvert J.G. The mechanism of the HO-SO2 reaction. Atmos. Environ. 1983;17:2231–2235. [Google Scholar]
- Sunyer J., Atkinson R., Ballester F., Le Tertre A., Ayres J., Forastiere F., Forsberg B., Vonk J., Bisanti L., Anderson R., Schwartz J., Katsouyanni K. Respiratory effects of sulfur dioxide: a hierarchical multicity analysis in the APHEA 2 study. Occup. Environ. Med. 2003;60:e2. doi: 10.1136/oem.60.8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J., Ballester F., Le Tertre A., Atkinson R., Ayres J.G., Forastiere F., Forsberg B., Vonk J.M., Bisanti L., Tenias J.M., Medina S., Schwartz J., Katsouyanni K. The association of daily sulfur dioxide air pollution levels with hospital admissions for cardiovascular diseases in Europe (The Aphea-II study) Eur. Heart J. 2003;24:752–760. doi: 10.1016/s0195-668x(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Sutkus D.J., Jr., Baughcum S.L., DuBois D.P. 2001. Scheduled Civil Aircraft Emission Inventories for 1999: Database Dev. and Anal. NASA/CRm2001-211216. [Google Scholar]
- Tesseraux I. Risk factors of jet fuel combustion products. Toxicol. Lett. 2004;149:295–300. doi: 10.1016/j.toxlet.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Thorpe A., Harrison R.M. Sources and properties of non-exhaust particulate matter from road traffic: a review. Sci. Tot. Environ. 2008;400:270–282. doi: 10.1016/j.scitotenv.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Timko M.T., Yu Z., Onasch T.B., Wong H.W., Miake-Lye R.C., Beyersdorf A.J., Anderson B.E., Thornhill K.L., Winstead E.L., Corporan E., DeWitt M.J., Klingshirn C.D., Wey C., Tacina K., Liscinsky D.S., Howard R., Bhargava A. Particulate emissions of gas turbine engine combustion of a Fischer–Tropsch synthetic fuel. Energ. Fuels. 2010;24:5883–5896. [Google Scholar]
- Timko M.T., Onasch T.B., Northway M.J., Jayne J.T., Canagaratna M.R., Herndon S.C., Wood E.C., Miake-Lye M.C., Knighton W.B. Gas turbine engine Emissions—part II: chemical properties of particulate matter. J. Eng. Gas Turbines Power. 2010;132:061505. [Google Scholar]
- Timko M.T., Herndon S.C., Wood E.C., Onasch T.B., Northway M.J., Jayne J.T., Canagaratna M.R., Miake-Lye R.C., Knighton W.B. Gas turbine engine emissions – part I: volatile organic compounds and nitrogen oxides. J. Eng. Gas Turbines Power. 2010;132:061504e1. [Google Scholar]
- Tremmel H.G., Schumann U. Model simulations of fuel sulfur conversion efficiencies in an aircraft engine: dependence on reaction rate constants and initial species mixing ratios. Aerosp. Sci. Technol. 1999;3:417–430. [Google Scholar]
- Tremmel H.G., Schalger H., Konopka P., Schulte P., Arnold F., Klemm M., Droste-Franke B. Observations and model calculations of jet aircraft exhaust products at cruise altitude and inferred initial OH emissions. J. Geophys. Res. 1998;103(D9):10803–10816. [Google Scholar]
- Turgut E.T., Usanmaz O., Rosen M.A. Estimation of vertical and horizontal distribution of takeoff and climb NOx emission for commercial aircraft. Energ. Convers. Manage. 2013;76:121–127. [Google Scholar]
- Turgut E.T., Rosen M.A. Assessment of emissions at busy airports. Int. J. Energ. Res. 2010;34:800–814. [Google Scholar]
- Unal A., Hu Y., Chang M.E., Odman M.T., Russell A.G. Airport related emissions and impacts on air quality: application to the Atlanta International Airport. Atmos. Environ. 2005;39:5787–5798. [Google Scholar]
- Underwood B.Y., Walker C.T., Peirce M.J. Netcen; Warrington: 2004. Heathrow Emissions Inventory 2002: Part 1. [Google Scholar]
- Unique . 2004. Aircraft NOx-Emissions within the Operational LTO Cycle.http://www.zurich-airport.com/Portaldata/2/Resources/documents_unternehmen/umwelt_und_laerm/Technical_Report_Operational_Aircraft_Emissions_2004.pdf Unique (Flughafen Zürich AG) in Cooperation with Swiss Flight Data Monitoring. Available at: (last accessed: August 2013) [Google Scholar]
- UK Statutory Instrument . 2007. Air Quality Standards Regulations 2007 SI 2007/64. [Google Scholar]
- US EPA (Environmental Protection Agency) 2009. Recommended Best Practice for Quantifying Speciated Organic Gas Emissions from Aircraft Equipped with Turbofan, Turbojet, and Turboprop Engines, Version 1.0.http://www.epa.gov/nonroad/aviation/420r09901.pdf EPA-420-R-09–901 May 2009. Available at: (last accessed September 2013) [Google Scholar]
- Usmani A.M., Donley M. Aircraft-coating weathering studies by analytical methods. J. Appl. Polym. Sci. 2002;86:294–313. [Google Scholar]
- Vahedpour M., Goodarzi M., Hajari N., Nazari F. Theoretical study on the atmospheric formation of sulfur trioxide as the primary agent for acid rain. J. Struct. Chem. 2011;22:817–822. [Google Scholar]
- Valavanidis A., Fiotakis K., Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- Van Netten C. Multi-elemental analysis of jet engine lubricating oils and hydraulic fluids and their implication in aircraft air quality incidents. Sci. Tot. Environ. 1999;229:125–129. doi: 10.1016/s0048-9697(99)00060-1. [DOI] [PubMed] [Google Scholar]
- Vander Wal R.L., Bryg V.M., Hays M.D. Fingerprinting soot (towards source identification): physical structure and chemical composition. J. Aerosol Sci. 2010;41:108–117. [Google Scholar]
- Vay S.A., Anderson B.E., Sachse G.W., Collins J.E., Jr., Podolske J.R., Twohy C.H., Gandrud B., Chan K.R., Baughcum S.L., Wallio H.A. DC-8-based observations of aircraft CO, CH4, N2O, and H2O (g) emission indices during SUCCESS. Geophys. Res. Lett. 1998;25:1717–1720. [Google Scholar]
- Viana M., Kuhlbusch T.A.J., Querol X., Alastuey A., Harrison R.M., Hopke P.K., Winiwarter W., Vallius M., Szidat S., Prevot A.S.H., Hueglin C., Bloemen H., Wåhlin P., Vecchi R., Miranda A.I., Kasper-Giebl A., Maenhaut W., Hitzenberger R. Source apportionment of particulate matter in Europe: a review of methods and results. J. Aerosol Sci. 2008;39:827–849. [Google Scholar]
- Wade M.D. 2002. Aircraft/Auxiliary Power Units/Aerospace Ground Support Equipment Emission Factors. US Air Force IERA-RS-BR-SR-2003-0002. [Google Scholar]
- Wahner A., Geller M.A., Arnold F., Brune W.H., Cariolle D.A., Douglass A.R., Johnson C., Lister D.H., Pyle J.A., Ramaroson R., Rind D., Rohrer F., Schumann U., Thompson A.M. Subsonic and supersonic aircraft emissions. In: World Meteorological Organization, United Nations Environment Programme, National Oceanic and Atmospheric Administration, National Aeronautics and Space Administration, editors. Scientific Assessment of Ozone Depletion: 1994. 1995. (WMO Global Ozone Research and Monitoring Project – Report No. 37, Geneva). (Chapter 11) [Google Scholar]
- Waitz I.A., Lukachko S.P., Lee J.J. Military aviation and the environment: historical trends and comparison to civil aviation. J. Aircr. 2005;42:329–339. [Google Scholar]
- Watt J., Tidblad J., Kucera V., Hamilton R., editors. The Effects of Air Pollution on Cultural Heritage. Springer; New York: 2009. p. 306. [Google Scholar]
- Watterson J., Walker C., Eggleston S. Netcen; Oxford: 2004. Revisions to the Method of Estimating Emissions from Aircraft in the UK Greenhouse Gas Inventory.http://uk-air.defra.gov.uk/reports/cat07/0504201622_GHG_Tier_3_aviation_method_%5BIssue_1.1%5D.doc Available at: (last accessed September 2013) [Google Scholar]
- Wayson R.L., Fleming G.G., Iovinelli R. Methodology to estimate particulate matter emissions from certified commercial aircraft engines. JAWMA. 2009;59:91–100. doi: 10.3155/1047-3289.59.1.91. [DOI] [PubMed] [Google Scholar]
- Webb S., Whitefield P.D., Miake-Lye R.C., Timko M.T., Thrasher T.G. Transportation Research Board; Washington, D.C.: 2008. Research Needs Associated with Particulate Emissions at Airports. ACRP Report 6. [Google Scholar]
- Wei R.P., Liao C.M., Gao M. A transmission electron microscopy study of constituent-particle-induced corrosion in 7075-T6 and 2024-T3 aluminum alloys. Metall. Mater. Trans. A. 1998;29:1153–1160. [Google Scholar]
- Weinmayr G., Romeo E., De Sario M., Weiland S.K., Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ. Health Perspect. 2010;118:449–457. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerdahl D., Fruin S.A., Fine P.L., Sioutas C. The Los Angeles International Airport as a source of ultrafine particles and other pollutants to nearby communities. Atmos. Environ. 2008;42:3143–3155. [Google Scholar]
- Wey C.C., Anderson B.E., Hudgins C.H., Wey C., Li-Jones X., Winstead E., Thornhill L., Lobo P., Hagen D., Whitefield P.D., Yelvington P.E., Herndon S.C., Onasch T.B., Miake-Lye R.C., Wormhoudt J., Knighton B., Howard R., Bryant D., Corporan E., Moses C., Holve D., Dodds W. 2006. Aircraft Particle Emissions eXperiment (APEX) NASA/TM-2006-214382, 2006, ARL-TR-3903. [Google Scholar]
- Wey C.C., Anderson B.E., Wey C., Miake-Lye R.C., Whitefield P.D., Howard R. Overview of the aircraft particle emissions experiment. J. Propul. Power. 2007;23:898–905. [Google Scholar]
- Wheeler S.E., Schaefer H.F., III Thermochemistry of the HOSO radical, a key intermediate in fossil fuel combustion. J. Phys. Chem. A. 2009;113:6779–6788. doi: 10.1021/jp9029387. [DOI] [PubMed] [Google Scholar]
- Whitefield P.D., Lobo P., Hagen D.E., Timko M.T., Miake-Lye R.C., Taylo C., Ratliff G., Lukachko S., Sequeira C., Hileman J., Waitz I., Webb S., Thrasher T.G., Ohsfeldt M.R., Kaing H.K., Essama S.C. Transportation Research Board; Washington, D.C.: 2008. Summarizing and Interpreting Aircraft Gaseous and Particulate Emissions Data. ACRP Report 9. [Google Scholar]
- Whitt D.B., Jacobson M.Z., Wilkerson J.T., Naiman A.D., Lele S.K. Vertical mixing of commercial aviation emissions from cruise altitude to the surface. J. Geophys. Res. 2011;116:D14109. doi: 10.1029/2010JD015532. [DOI] [Google Scholar]
- WHO (World Health Organization) second ed. WHO, Regional Office for Europe; Copenhagen: 2000. Air Quality Guidelines for Europe. [Google Scholar]
- Whyte R.B. vol. 2. 1982. p. 174. (Alternative Jet Fuels). AGARD 476 Advisory Report No. 181. [Google Scholar]
- Wiesen P.J., Kleffmann J., Kurtenbach R., Becker K. Nitrous oxide and methane emissions from aero engines. Geophys. Res. Lett. 1994;21:2027–2030. [Google Scholar]
- Wilcox L.J., Shine K.P., Hoskins B.J. Radiative forcing due to aviation water vapour emissions. Atmos. Environ. 2012;63:1–13. [Google Scholar]
- Wilkerson J.T., Jacobson M.Z., Malwitz A., Balasubramanian S., Wayson R., Fleming G., Naiman A.D., Lele S.K. Analysis of emission data from global commercial aviation: 2004 and 2006. Atmos. Chem. Phys. 2010;10:6391–6408. [Google Scholar]
- Williams R.C., Lee J.B. Wright-Patterson Air Force Base, OH: U.S. Air Force Wright Aeronautical Laboratories; 1985. Effects of Alternate Fuels on APUs. AFWALTR-85–2083. [Google Scholar]
- Williams P.I., Allan J.D., Lobo P., Coe H., Christie S., Wilson C., Hagen D., Whitefield P., Raper D., Rye L. Impact of alternative fuels on emissions characteristics of a gas turbine engine – part 2: volatile and semivolatile particulate matter emissions. Environ. Sci. Technol. 2012;46:10812–10819. doi: 10.1021/es301899s. [DOI] [PubMed] [Google Scholar]
- Wilson C.W., Petzold A., Nyeki S., Schumann U., Zellner R. Measurement and prediction of emissions of aerosols and gaseous precursors from gas turbine engines (PartEmis): an overview. Aerosol Sci. Technol. 2004;8:131–143. [Google Scholar]
- Winder C., Balouet J.C. The toxicity of commercial jet oils. Environ. Res. 2002;89:146–164. doi: 10.1006/enrs.2002.4346. [DOI] [PubMed] [Google Scholar]
- Wong H.W., Yelvington P.E., Timko M.T., Onasch T.B., Miake-Lye R.C., Zhang J., Waitz I.A. Microphysical modeling of ground-level aircraft-emitted aerosol formation: roles of sulfur-containing species. J. Propul. Power. 2008;24:590–602. [Google Scholar]
- Wong H.-W., Yu Z., Timko M.T., Herndon S.C., de la Rosa Blanco E., Miake-Lye R.C., Howard R.P. Design parameters for an aircraft engine exit plane particle sampling system. J. Eng. Gas Turbines Power. 2011;133:021501. [Google Scholar]
- Wood E., Herndon S., Miake-Lye R.C., Nelson D., Seeley M. Transportation Research Board; Washington, D.C.: 2008. Aircraft and Airport-Related Hazardous Air Pollutants: Research Needs and Analysis. ACRP Report 7. [Google Scholar]
- Wood E.C., Herndon S.C., Timko M.T., Yelvington P.E., Miake-Lye R.C. Speciation and chemical evolution of nitrogen oxides in aircraft exhaust near airports. Environ. Sci. Tecnol. 2008;42:1884–1891. doi: 10.1021/es072050a. [DOI] [PubMed] [Google Scholar]
- Woody M.C., Arunachalam S. Secondary organic aerosol produced from aircraft emissions at the Atlanta Airport: an advanced diagnostic investigation using process analysis. Atmos. Environ. 2013;79:101–109. [Google Scholar]
- Wormhoudt J., Herndon S.C., Yelvington P.E., Lye-Miake R.C., Wey C. Nitrogen oxide (NO/NO2/HONO) emissions measurements in aircraft exhausts. J. Propul. Power. 2007;23:906–911. [Google Scholar]
- Wuebbles D., Gupta M., Ko M. Evaluating the impacts of aviation on climate change. EOS Trans. AGU. 2007;88:157–160. [Google Scholar]
- Yang W., Omaye S.T. Air pollutants, oxidative stress and human health. Mutat. Res. 2009;674:45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Yelvington P.E., Herndon S.C., Wormhoudt J.C., Jayne J.T., Miake-Lye R.C., Knighton W.B., Wey C. Chemical speciation of hydrocarbon emissions from a commercial aircraft engine. J. Propul. Power. 2007;23:912–918. [Google Scholar]
- Yilmaz A., Hindiyarti L., Jensen A.D., Glarborg P., Marshall P. Thermal dissociation of SO3 at 1000–1400 K. J. Phys. Chem. A. 2006;110:6654–6659. doi: 10.1021/jp0557215. [DOI] [PubMed] [Google Scholar]
- Yim S.H.L., Stettler M.E.J., Barrett S.R.H. Air quality and public health impacts of UK airports. Part II: impacts and policy assessment. Atmos. Environ. 2013;67:184–192. [Google Scholar]
- Yu F., Turco R.P. The role of ions in the formation and evolution of particles in aircraft plumes. Geophys. Res. Lett. 1997;24:1927–1930. [Google Scholar]
- Yu Z., Herndon S.C., Ziemba L.D., Timko M.T., Liscinsky D.S., Anderson B.E., Miake-Lye R.C. Identification of lubrication oil in the particulate matter emissions from engine exhaust of in-service commercial aircraft. Environ. Sci. Tecnol. 2012;46:9630–9637. doi: 10.1021/es301692t. [DOI] [PubMed] [Google Scholar]
- Yu Z., Liscinsky D.S., Winstead E.L., True B.S., Timko M.T., Bhargava A., Herdon S.C., Miake-Lye R., Anderson B.E. Characterization of lubrication oil emissions from aircraft engines. Environ. Sci. Technol. 2010;44:9530–9534. doi: 10.1021/es102145z. [DOI] [PubMed] [Google Scholar]
- Yu K.N., Cheung Y.P., Chueng T., Henry R.C. Identifying the impact of large urban airports on local air quality by nonparametric regression. Atmos. Environ. 2004;38:4501–4507. [Google Scholar]
- Zhang Y., Tao S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009;43:812–819. [Google Scholar]
- Zhu Y., Fanning E., Yu R.C., Zhang Q., Froines J.R. Aircraft emissions and local air quality impacts from takeoff activities at a large International Airport. Atmos. Environ. 2011;45:65. [Google Scholar]
- Zielinska B., Sagebiel J., McDonald J.D., Whitney K., Lawson D.R. Emission rates and comparative chemical composition from selected in-use diesel and gasoline-fueled vehicles. JAWMA. 2004;54:1138–1150. doi: 10.1080/10473289.2004.10470973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.