Abstract
The BAFF system plays a key role in the development of autoimmunity, especially in systemic lupus erythematosus (SLE). This often leads to the assumption that BAFF is mostly a B cell factor with a specific role in autoimmunity. Focus on BAFF and autoimmunity, driven by pharmaceutical successes with the recent approval of a novel targeted therapy Belimumab, has relegated other potential roles of BAFF to the background. Far from being SLE-specific, the BAFF system has a much broader relevance in infection, cancer and allergy. In this review, we provide the latest views on additional roles of the BAFF system in health and diseases, as well as an update on BAFF and autoimmunity, with particular focus on current clinical trials.
Abbreviations: ACR, American College of Rheumatology; ALL, acute lymphoblastic leukemia; ANA, anti-nuclear autoantibodies; APRIL, a proliferation inducing ligand; BAFF, B cell activating factor from the TNF family; BAFF-R, BAFF-receptor; BCMA, B cell maturation antigen; BCR, B cell receptor; BOS, bronchiolitis obliterans syndrome; cGVHD, chronic graft versus host disease; CIDP, chronic-inflammatory demyelinating polyneuropathy; CLL, chronic lymphocytic leukemia; CNS, central nervous system; CVID, common variable immunodeficiency; DCs, dentritic cells; dsDNA, double stranded deoxyribonucleic acid; EBV, Epstein–Barr virus; ECP, extracorporeal photopheresis; FDA, Food and Drug Administration; EULAR, European League Against Rheumatism; GC, germinal center; HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplantation; HSPGs, heparan sulfate proteoglycans; ICOS, inducible costimulatory; Ig, immunoglobulin; IFN, interferon; IL, interleukin; LN, lupus nephritis; MMF, mycophenolate mofetil; mRNA, messenger ribonucleic acid; MS, multiple sclerosis; MTX, methotrexate; MVECS, microvacular endothelial cells; MyD88, myeloid differentiation primary response gene 88; MZ, marginal zone; NgR, Nogo-66 receptor; NK, natural killer; NZB, New-Zealand black; NZW, New-Zealand white; PC, plasma cell; PFS, progression-free survival; RF, rheumatoid factor; RSV, respiratory syncytial virus; SAEs, serious adverse events; SCF, stem cell factor; SLE, systemic lupus erythematosus; SLEDAI, SLE Disease Activity Index; SNPs, single nucleotide polymorphisms; SS, Sjögren's syndrome; TACI, transmembrane activator and cyclophilin ligand interactor; Tg, transgenic; TGF-β, transforming growth factor-β; Th, T-helper; TLR, toll-like receptor; TNF, tumor necrosis factor; Treg, regulatory T cells
Keywords: A proliferation inducing ligand (APRIL), Autoimmunity, B cell activating factor from the tumor necrosis factor family (TNF) (BAFF), Cancer, Systemic lupus erythematosus (SLE)
1. Introduction
The B cell activating factor from the tumor necrosis factor (TNF) family (BAFF; also known as BLyS, TNFSF13B) system has been extensively reviewed since its discovery in 1999 [1], [2], particularly in the field of autoimmunity, where BAFF has a crucial role, especially in systemic lupus erythematosus (SLE) (reviewed in Vincent et al. [3]). The role of BAFF on B-lymphocytes and in autoimmunity, and the resulting clinical success in SLE patients, have almost overshadowed the potential role of this system in other immunological fields, such as transplantation/graft versus host disease (GVHD), chronic variable immunodeficiency (CVID), infections and allergy. For instance, BAFF is emerging as a critical factor in various hematological and lymphoid cancers. Indeed, a number of translational studies link progression of various malignancies with BAFF's well-described pro-survival activity, suggesting that the use of BAFF-neutralizing therapies may extend to additional indications, in particular cancer. Furthermore, the BAFF system has recently been described in preeclampsia [4], and the data suggests that both BAFF and APRIL signaling pathways are important for cell viability in the human placenta [5]. This review details the latest results and views on novel functions of the BAFF/APRIL system outside of the autoimmunity arena. However, as the role of BAFF in autoimmunity remains one the most important developments in this decade, this review also provides an update on current clinical trials.
2. BAFF, APRIL and their receptors
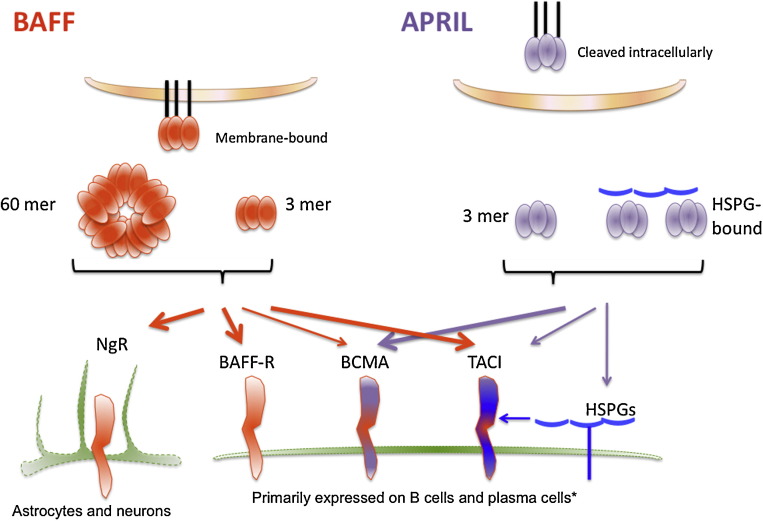
One of the most important paradigm shifts in B-lymphocyte biology in last fifteen years has been the realization that expression of a B cell receptor (BCR) on B cells was important but not enough for survival and maturation. Indeed, BAFF has emerged a critical B cell survival factor without which maturation of B cells does not occur [2]. A proliferation inducting ligand (APRIL; also known as TNFSF13A) was identified as a cell growth stimulator in various cancer cell lines [6]. BAFF and APRIL are produced as type II transmembrane proteins, like many of the TNF family ligands, and are then proteolytically cleaved at a furin protease site and released in a soluble form [7]. BAFF may remain in a membrane-bound form, although processed soluble BAFF is required for B cell homeostasis [8]. In contrast, APRIL is cleaved in the Golgi site prior to release, and normally exists in a soluble form only, once outside of the cell of origin [9]. A recently reported exception to this is APRIL-δ, a malignant isoform of APRIL that lacks a furin cleavage site and remains membrane-bound on leukemia cell precursors [10]; another membrane-bound variation is TWE-PRIL (TNFSF12–TNFSF13), which is a hybrid protein of APRIL and TWEAK (TNF-related weak inducer of apoptosis, TNFSF12) resulting from trans-splicing between their adjacent genes [11]. BAFF is an active ligand as a homotrimer, which is the main form of BAFF found in the circulation [12], however, unlike other TNF family cytokines, a 60-mer form of BAFF has been obtained at physiological pH conditions and this form is also able to bind to its receptors [13], [14]. While this form has been described in mouse models, it remains unclear whether or not it can be detected in humans. Mouse BAFF also has a membrane-bound splice variant, δ-BAFF, which may form trimers with normal BAFF proteins to reduce BAFF availability, although both forms of BAFF modulate B-1 B cell populations [15]. APRIL, but not BAFF can interact with heparin sulfate proteoglycans (HSPGs) [16], which are structurally unrelated to TNF receptors, but may act to increase APRIL signaling at a local site and concentrate APRIL on the cell surface, similar to the 60-mer form of BAFF engaging many receptors at a single site (see Fig. 1 ).
Fig. 1.
BAFF and APRIL signaling. BAFF is expressed as a membrane-bound trimer, which is proteolytically cleaved to form a soluble trimer. BAFF also exists in the circulation as a 60-mer. BAFF binds strongly to BAFF-R and TACI, and weakly to BCMA (as indicated by thick or thin arrows, respectively). BAFF trimers do not activate TACI efficiently and BAFF multimers are required for this. BAFF has also recently been described to bind to a receptor on astrocytes and neurons called Nogo-66 receptor (NgR). APRIL is cleaved intracellularly (with the exception of APRIL-δ), and is found in the circulation as a trimer, and as a multimer in association with heparin sulfate proteoglycans (HSPGs). APRIL binds strongly to BCMA, weakly to TACI, which also binds to HSPGs. BAFF-R is primarily expressed on all B cells, TACI on innate-like B cells, and BCMA on plasmablasts and plasma cells. However, these receptors can be found on other cell types, such as BAFF-R on activated T cells.
BAFF and APRIL share two receptors: transmembrane activator and cyclophilin ligand interactor (TACI; also known as TNFRSF13B) and B cell maturation antigen (BCMA; also known as TNFRSF17). APRIL binds strongly to BCMA and moderately to TACI, whereas BAFF binds weakly to BCMA and strongly to TACI [17]. Additionally, BAFF binds strongly to BAFF receptor (BAFF-R; also known as TNFRSF13C) [17]. These three main receptors, BAFF-R, TACI, and BCMA have distinct expression patterns based on B cell development stages, related to their separate functions (Table 1 ). BAFF-R expression is absent on B cell precursors in the bone marrow but is gained on immature B cells upon acquiring a functional B cell receptor (BCR) [18], and BAFF-R is critical for survival and maturation of immature B cells [19]. In mice, TACI is expressed most highly on mature innate-like B cells such as marginal zone (MZ) B cells and B-1 B cells, and is necessary for T-independent type II and type I responses [20], [21], negative regulation of B cell numbers [22] and T follicular helper (Tfh) cell numbers [23], and promotion of class switch recombination in B cells by signaling directly through myeloid differentiation primary response gene 88 (MyD88) [24]. TACI expression is temporally regulated, such that TACI expression is decreased in newborn humans and mice [25]. In humans, TACI is expressed on memory B cells and TACI mutations are detected in up to 10% of common variable immunodeficiency (CVID) patients making it the most frequently mutated gene for that disease [26], [27]. TACI can interact with APRIL and HSPG in a complex [28]. BCMA expression is restricted to plasmablasts and plasma cells (PC) [29], and BCMA promotes long-lived PC survival [30], [31].
Table 1.
Types of cells expressing BAFF/APRIL system ligands and receptors.
| Cell type | Ligands | Receptors | Function | Ref. |
|---|---|---|---|---|
| Hematopoietic cells | ||||
| Astrocytes | BAFF | Supports B cell survival in inflammatory diseases and B cell lymphoma; major source of BAFF in the CNS. | [33] | |
| Microglia (rat primary cells) | BAFF | BAFF-R, TACI | Ganglioside stimulation induces BAFF expression; BAFF may have a role in CNS inflammation. | [145] |
| Immature B cells (mouse T1, T2) | BAFF-R | BAFF-R supports B cell survival and maturation. | [29] | |
| Mature B cells (mouse Fo, MZ, and B-1) | APRIL, BAFF | BAFF-R, TACI | BAFF-R supports B cell survival (although mouse B-1 B cells are BAFF independent); TACI promotes class switch recombination and negatively regulates survival. | [22], [24], [29], [146] |
| Bone marrow plasma cells | BCMA | BCMA signaling supports PC survival. | [29] | |
| Myeloid precursor cells | APRIL | Provides APRIL in situ for BMPC survival. | [147] | |
| Activated T cells | APRIL, BAFF | BAFF-R | IL-2 activation induces BAFF production. | [148], [149] |
| NK cells (human blood cells) | BAFF | [149] | ||
| pDC | BAFF | TLR9 activation induces BAFF production. | [150] | |
| Neutrophils, Monocytes, Macrophages and Dendritic cells | APRIL, BAFF | Major source of APRIL and BAFF. | [148], [151] | |
| Non-hematopoietic cells | ||||
| Neurites | NgR | BAFF can regulate neurite outgrowth during CNS inflammation. | [32] | |
| Intestinal epithelial cells | BAFF | IFN-γ induces production of BAFF. | [152] | |
| Adipocytes | APRIL, BAFF | BAFF-R, BCMA, TACI | BAFF downregulates adipogenesis mediators (leptin and adipoleptin) and upregulates proinflammatory adipokines (MCP-1, COX-2, IL-6, haptoglobulin); interaction with macrophages upregulates BAFF-R and TACI. | [145], [153], [154], [155] |
| Human skin keratinocytes | APRIL, BAFF | BAFF-R, BCMA, TACI | APRIL/BAFF activate NF-κB and expression of IL-6 and GM-CSF via BCMA. | [156] |
| Human adipose-derived stem cells (hASC) | APRIL, BAFF | BAFF-R, BCMA, TACI | APRIL/BAFF promote proliferation via ERK1/2. | [157] |
| BM CD14+ cells and osteoclasts | APRIL, BAFF | Provides APRIL/BAFF in situ for BMPC and myeloma cell survival. | [158], [159] | |
| Cancer cells | ||||
| Primary CNS lymphoma cells | BAFF-R, BCMA, TACI | BAFF from astrocytes promote CNS lymphoma cell survival. | [33] | |
| Primary leukemia B cell precursor | δ-APRIL | BAFF-R, BCMA, TACI | Abberant isoforms of APRIL promote leukemia cell survival. | [10] |
| Primary multiple myeloma cells and myeloma cell lines | TACI, HSPG | Concentrated signaling from APRIL in complex with HSPG on the cell surface promotes survival and proliferation. | [28] | |
| CLL stromal microvascular endothelial cells (MVECs) | APRIL, BAFF | BAFF and APRIL from MVECs promote CLL survival. | [50] | |
| CLL cells | BAFF-R, BCMA, TACI | |||
| Hepatocellular carcinoma (HCC) cell lines (HepG2, Hep3B) and primary HCC cells | APRIL, BAFF | BAFF-R, BCMA | APRIL:BCMA decreases proliferation and BAFF does not effect cell growth in these cells, in contrast to other cancer settings. | [160] |
| Renal cell carcinoma | APRIL, BAFF | APRIL expression correlated with an aggressive phenotype. | [161] | |
APRIL, a proliferation inducing ligand; BAFF, B cell activating factor of the tumor necrosis factor (TNF) family; BAFF-R, BAFF-receptor; BCMA, B cell maturation antigen; BM, bone marrow; BMPC, bone marrow plasma cell; CLL, chronic lymphocytic leukemia; CNS, central nervous system; Fo, follicular; hASC, human adipose-derived stem cells; HCC, hepatocellular carcinoma; HSPGs, heparan sulfate proteoglycans; IFN, interferon; IL, interleukin; MVECs, microvascular endothelial cells; MZ, marginal zone; NgR, Nogo-66 receptor; NK, natural killer; PC, plasma cell; pDC, plasmacytoid dendritic cell; TACI, transmembrane activator and cyclophilin ligand interactor; TLR, toll-like receptor.
Nogo-66 receptor (NgR, Reticulon 4 Receptor) has recently been identified as an additional high affinity receptor for BAFF [32]. NgR is expressed on neurons, and can inhibit neuron outgrowth in response to BAFF signaling [32]. This interaction has emerging relevance in central nervous system (CNS) injuries and diseases such as multiple sclerosis (MS), all of which are associated with increased local production of BAFF by astrocytes [33].
BAFF and APRIL are predominantly produced by myeloid cells, but recent studies have described regulated expression of BAFF and APRIL, and their receptors in a variety of hematopoietic and non-hematopoietic cell lineages (Table 1), and several recent studies report new mechanisms regulating BAFF expression. BAFF production from monocytes, a major source of BAFF production, can be suppressed by intravenous immunoglobulin (IVIg) treatment in vitro and in chronic-inflammatory demyelinating polyneuropathy (CIDP) patients which have elevated circulating BAFF levels [34]. BAFF expression is upregulated by estrogen [35], which is consistent with the strong gender bias observed in BAFF-driven autoimmune patients and some murine disease models. BAFF expression is increased by interferon-alpha (IFN-α) signaling [35], and B cell-deficient, BAFF-deficient mice were protected from IFN-α-driven systemic lupus erythematosus (SLE) [36], reinforcing the potential for BAFF-overexpression as a driver of autoimmune disease, also supported by a correlation between elevated circulating BAFF levels and SLE in humans (reviewed in Vincent et al. [3]).
3. The role of the BAFF system in transplantation and GVHD
In light of the prominent role of BAFF in autoimmunity, it is not completely surprising that elevated levels of BAFF have also been associated with chronic GVHD (cGVHD) in patients receiving allogeneic hematopoietic stem cell transplantation (HSCT) therapy [37], which mirrors some aspects of immunodeficiency and autoimmunity. Indeed, increased serum BAFF levels and alloantibodies titers strongly suggest a role for B cells in GVHD pathogenesis. The B cell repertoire of the GVHD microenvironment has been defined, identifying increased numbers of CD27+ germinal center (GC) B cells and post-GC plasmablast-like cells [38].
cGVHD in the lung of patients with bronchiolitis obliterans syndrome (BOS) is often fatal and patients have increased numbers of CD19+CD21lo B cells, suggesting a role for transitional type 1 B cells in the disease. Elevated serum BAFF concentrations in combination with CD19+CD21lo cell numbers have been successfully used to assess the risk of BOS in HSCT recipients [39]. BAFF single nucleotide polymorphisms (SNPs) have also been identified and showed usefulness as independent predictors of GVHD phenotype after allogeneic transplant [40]. Serum BAFF levels also show predictive use in monitoring disease activity in the first month following extracorporeal photopheresis (ECP) treatment of refractory cGVHD [41]. Furthermore, BAFF levels have also been used to delineate kidney transplant recipients with normal or abnormal renal function and rejection. Those with abnormal renal function have higher levels of surface BAFF on their CD4 and CD8 T cell populations 5 years after transplantation and moderate IgG deposition suggesting autoantibody production by B cells [42].
A common feature of cGVHD is a delay in naïve B cell reconstitution for up to 12 months after allogeneic transplant. Naïve B cell lymphopenia was also correlated with hypogammaglobulinemia and coincident increases in CD38hi B cells [43]. This is in contrast to autologous HSCT in which there is a drastic increase in the number of naïve and transitional B cells following transplantation, possibly suggesting competitive reconstitution of naïve B cells. Depletion of activated and potentially autoreactive B cells has been suggested, using rituximab [44]. Further studies have shown stably induced changes in B cell distribution 25 months after rituximab therapy, with notable increases in naïve IgD+ B cell numbers and decreased activated BAFF-Rlo CD20lo B cells. Interestingly, however, BAFF levels, often seen as negative prognostic factors, increased after rituximab treatment [45], potentially due to diminished numbers of BAFF receptor-expressing B cells or as a feedback loop response to the loss of B cells [3]. While the sentiment remains that BAFF is a viable therapeutic target, the glaring disconnect between increased serum BAFF and poor reconstitution of naïve B cells, which have higher BAFF-R expression than activated B cells including CD27+ B cells, suggests that other mechanisms are at work. If naïve B cell expansion is necessary for redistribution of B cells, BAFF modulation may not be a simple therapy and inhibition of TACI and/or BCMA may be more appropriate for targeting CD27+ GC and post-GC B cells.
4. The role of the BAFF system in cancer
The classical picture of the BAFF system dynamics is slowly evolving into a more complex picture of interwoven regulatory function and downstream signaling designed to alter cellular survival, growth and migration. While the central role of BAFF in autoimmunity is established, we are still developing our understanding of the contribution of the BAFF system to malignancies. The current conceptual picture posits BAFF and APRIL as primarily pathogenic players in microenvironments of both solid and hematological tumors.
Elevated serum BAFF levels derived from neutrophils have been noted in oral cavity cancers [46], have been associated with promoting invasive migration activity in hypoxic breast cancer cell line studies [47], and correlated with increased serum TNF levels, angiogenesis and poor prognosis in multiple myeloma [48]. Overexpression of BAFF has been shown to enhance c-myc expression and promote chronic lymphocytic leukemia (CLL)-like disease via activation of the canonical IKK in Myc/BAFF Tg mice [49]. BAFF production by stromal support cells such as microvascular endothelial cells (MVECS) has been shown to be triggered by CD40L aberrantly expressed on the surface of CLL B cells, further supporting the importance of support stroma for cancer cell survival and explaining observed ex vivo apoptosis of isolated CLL cells [50].
Given these observations, it might be useful to incorporate measurement of serum BAFF levels into existing scoring systems. Prognostic use of BAFF levels has already shown promise in CLL when combined with CD38, ZAP70 expression and the mutational status [51] and in follicular lymphoma, where expression of both BAFF and BAFF-R are elevated and coincide with inferior progression-free survival (PFS), with some suggestion that overexpression of BAFF is secondary to elevated expression of BAFF-R, and therefore increased sensitivity to BAFF [52].
Prostate cancer appears to be an exceptional case, in which epithelial cell-derived BAFF protects periglandular lymphocyte survival and limits tumor expansion [53]. This latter study provides a rationale for future studies that will include assessment of BAFF receptor phenotype in disease context to more fully understand disease dynamics and how BAFF may polarize cellular behavior. Indeed, investigations of cellular programming of B cell tumors, led to the identification of a novel BAFF splice variant, aptly named Δ4BAFF. The isoform has an exaggerated production in SLE and CLL thereby enhancing autocrine BAFF secretion at the transcriptional level and, as such, acting as a transcription factor. This is the first report of cytokine self-regulation at the transcriptional level and may be the first piece in an unexplored layer of BAFF regulation [54]. This provides some support for the aberrant autocrine feedback seen in these pathologies and suggests that pathogenic BAFF signals may be self-substantiating, with possible implications for future therapeutic strategies. Similarly, APRIL isoforms (β, γ, δ, ɛ) were detected in pre-B acute lymphoblastic leukemia (ALL) cells, potentially contributing to previously BAFF-attributed B cell survival [10].
The above findings emerged against a backdrop of increasing focus on BAFF receptors. Surprisingly, soluble BCMA isoforms have been identified and while their function is not yet elucidated, it suggests the exciting prospect of natural decoys produced to regulate levels of free BAFF or APRIL [10]. Overexpression of TACI has been detected in multiple myeloma and thyroid carcinoma; and correlative analyses suggest that TACI expression is a useful prognostic marker for lymphoma [55]. Most interestingly, overexpression of APRIL was found to coincide with overexpression of HSPGs [55], which are known co-receptors for APRIL when binding TACI. This has renewed speculation that HSPGs may act as primary receptors for APRIL in addition to co-receptor function. BAFF-R has been implicated in nuclear interactions with IKKβ and NF-κB/c-Rel in both normal health and neoplastic B cells, including NHL B cells, which enhance histone H3 phosphorylation and binding of NF-κB on promoters for inducible genes including Bcl-xl, CD40L and most strikingly, BAFF. This complicates further an already complicated picture of BAFF system regulation, in which the receptor itself is a transcriptional regulator and exerts its effects via chromatin remodeling in addition to classical NF-κB activation at the membrane surface [56].
Mutations in BAFF receptors have also emerged as critical factors in disease mechanism and have emerging prognostic power. Substitution mutations in BAFF-R at His159Tyr in non-Hodgkin lymphoma (NHL) patients increased recruitment of TRAF3, TRAF2 and surprisingly, TRAF6, demonstrating for the first time that TRAF6 is an essential component of downstream BAFF-R signaling in NHL and in health [57].
Inhibition of BAFF or its receptors has been a strong focal point for therapeutic development. Merck's Phase Ib trial with atacicept inhibitor of BAFF and APRIL has shown that the agent is well tolerated at moderate dosage but currently no data on the clinical activity in CLL trials is available [58]. Competitive inhibition with a fusion protein comprising BAFF and the toxin gelonin has shown promising efficacy in promoting tumor clearance in childhood ALL when combined with mobilizing agents such as a CXCR4 antagonist [59]. A similar strategy employing mutant BAFF fused with Pin2/TRF1-interacting protein X1 (PinX1), a telomerase reverse transcriptase inhibitor, has also been effective at selectively killing BAFF receptor expressing Burkitt lymphoma lines [60]. The latter example further reinforces growing evidence that BAFF function is not confined to NF-κB activation and pro-survival transcriptional activity but direct alteration at immunosenescence checkpoints of target cells at the chromatin-level to alter proliferative capacity and ultimately survival. These developments will preface development of a new generation of cancer therapeutics.
5. The role of the BAFF system in infections
Many reports over the last five years have implicated BAFF and APRIL in the context of infections. We will review the recent findings with respect to BAFF and APRIL in viral, bacterial and parasitic infections.
5.1. Viral infections
The induction of BAFF as a result of viral infection has been reported in viral infections as divergent as hepatitis C, human immunodeficiency virus (HIV), respiratory syncytial virus (RSV) infection, and H1N1 influenza [61], [62], [63]. In addition, cell lines infected in vitro with viruses including dengue virus, Sendai virus, reovirus-1 and Epstein–Barr virus (EBV) upregulate BAFF expression [64], [65], [66]. There are few examples of APRIL expression being assessed, however one study looking at coronavirus-induced encephalomyelitis described up-regulated APRIL expression that was IFN-γ independent, and there are conflicting results following RSV infection [63], [67]. Many observations have noted that BAFF upregulation observed following viral infection is IFN-dependent, and monocytes have been shown to release BAFF in response to IFN treatment [7]. Indeed both Sendai virus infection of monocytes and RSV infection of bronchial epithelial cells (both ssRNA viruses) have been shown to be abrogated by blocking IFN signaling [63], [66]. The importance of IFN-signaling has been underlined by the Interprim ANRS 112 Study Group. This study shows that patients with acute HIV-1 infection that were treated with pegylated-IFN-alpha2b treatment in combination with antiretroviral therapy produced higher anti-HIV antibody titers with a broader specificity than patients treated with antiretroviral therapy alone [68]. This IFN treatment resulted in higher levels of BAFF, and the success of the treatment was attributed in part to increased BAFF signaling. Whether increasing BAFF alone (without increased IFN treatment) would have proved efficacious in this trial was not tested. Whether the upregulation of BAFF in viral infections is a product of inflammation, or a direct effect of viral infection from BAFF-producing cells, and whether BAFF is a necessary key to producing antiviral antibodies still remains to be determined.
Different viruses will infect different cell types, and the level of BAFF secretion differs from one cell-type to another. Macrophages, neutrophils and dendritic cells are capable of producing high amounts of BAFF, whilst epithelial cells lining mucosal layers have been shown to be able to more modestly upregulate BAFF secretion as a result of infection, none-the-less they are thought to play an important role in local responses [66]. Human and mouse B cells have been shown to upregulate BAFF and APRIL expression as a result of EBV infection, and some human EBV + B cells lines produced comparable levels of BAFF and APRIL to human myeloid cell lines tested (Table 1) [65]. Not only did the EBV-infected B cell lines express BAFF and APRIL, but they expressed BAFF-R, TACI and BCMA transcripts, thus infection by EBV resulted in engagement of BAFF-R/TACI/BCMA by autocrine BAFF and APRIL, which led to class switching, and NF-κB activation. Thus, whilst BAFF and APRIL play an important role in containing the EBV infection, sustained BAFF and APRIL expression lead to inappropriate lymphoproliferation, underscoring the importance of limiting the expression of BAFF and APRIL to appropriate situations and timeframes. HIV-infected patients also suffer from B cell dysregulation that leads to lymphomas, and only 30–40% of these complications are EBV-related. It has been proposed that these lymphomas are a result of polyclonal B-cell activation caused by HIV-infected mDCs secreting BAFF, and other inflammatory cytokines in the vicinity of B cells expressing receptors for BAFF [69].
A study investigating the role of BAFF and APRIL in providing protection in influenza virus-infected mice found that blocking BAFF alone had no effect on antiviral antibody titers systemically, or locally [70], but that blocking both BAFF and APRIL using TACI-Fc resulted in a significant drop in antiviral IgG both systemically and in the BAL. This group showed the importance of the receptor TACI for the maintenance of anti-influenza responses, as TACI−/− mice mounted antiviral responses that were comparable to WT up until 18 days, but by 33 days post-infection the antiviral titers in TACI−/− mice had declined significantly. Indeed TACI has been shown to be important for promoting the sustained expression of Blimp-1 by B cells responding to protein antigens [71]. These studies bring the role of APRIL into focus, and suggest that this underappreciated member of the TNF family deserves more attention.
5.2. Bacterial infections
One example highlighting the importance of the BAFF–APRIL system in defense against bacterial infections is that the subset of CVID patients with a mutation in TACI or BAFF-R often present with recurrent bacterial sinopulmonary infections [72]. Although the fact that there are also patients with TACI and BAFF-R mutations who are asymptomatic tells us that there are other genetic or environmental factors that contribute to this phenotype. However, mirroring the CVID patients with mutant TACI or BAFF-R, mice lacking TACI, or treated with TACI-Ig, and mice deficient in BAFF-R have deficient humoral immunity to T-cell independent antigens, including encapsulated bacterial pathogens such as Streptococcus pneumoniae [73]. BAFF-R-deficient mice have almost no mature B cells, so it is not surprising that these mice have reduced antibody titers, however the TACI−/− mice demonstrate the importance of TACI signaling in generating functional antibody responses, as these animal have B cells and in elevated numbers. The diminished capacity of neonates to mount protective responses to bacterial polysaccharides (and viruses) may be due to reduced expression of TACI, BCMA and BAFF-R on B cells, and thus a decreased ability to generate switched antibody responses [74], although neonates also express low levels of CD40. Similar to the reports from viral infections, patients with active pulmonary tuberculosis, and elevated levels of IFN, showed elevated levels of BAFF and APRIL [75].
5.3. Parasitic infections
The role of BAFF in malarial infection has been the subject of scrutiny over the last two years, and studies have started to assess the role of the BAFF/APRIL system in parasitic infections. Mouse models of malaria infection demonstrated that malarial infection results in a decreased proportion of DCs expressing BAFF, and by overexpressing BAFF, malarial-specific antibody secreting cell numbers were increased, and mice were protected from lethal malaria infections [76]. Whilst the level of BAFF secreted locally by DCs has not been investigated in humans, systemic levels of BAFF increased with acute malarial infection (as did IFN-γ), however, the level of BAFF-R on B cells was reduced during acute infection in humans [77], suggesting that defective BAFF signaling may be the key to the poor humoral responses despite frequent antigen exposure observed in malaria-endemic areas.
Together these studies underline the importance of BAFF and APRIL in developing appropriate responses to infections, and underscores the serious consequences that can arise from dysregulated BAFF production following infection. These studies also highlight the importance of understanding the regulation of BAFF following infection, in the context of the risk of developing autoimmunity. Increased TLR7, TLR9 and IFN-α have been described in SLE patients, suggesting that infection, leading to elevated BAFF, can trigger pathogenesis in individuals who are genetically predisposed to lupus [78].
6. Mutation in the BAFF-R and TACI genes, relationship with CVID
CVID is one of the most common immunodeficiency syndromes and is characterised by a reduction in antibody production in particular IgG, IgA and IgM and a poor response to vaccination or pathogen exposure (reviewed in [79]). As a result CVID patients are vulnerable to infections especially that of the respiratory tract. Other immune disorders such as autoimmune manifestation affect about 20% of CVID patients [79]. The etiology of CVID is still very much debated but appears to be linked to various genetic defects affecting immune functions, although the inheritance pattern of these is very unclear [80]. Mutations or deletion of a number of immune genes important for B cell function have been associated with CVID. This is the case for the inducible costimulator (ICOS), CD19, CD81 and CD20 [80].
More recently, a large body of work has identified a series of mutations in the TACI gene that were found in about 8–10% of CVID patients [81], [72], [82], [83], [84]. Homozygosity for variants C104R, A181E and S144X of the TACI gene has been confirmed in a number of CVID patients [81], [72]. Heterozygosity for variants C104R, A181E, S194X and R202H has also been described in CVID patients [81], [72]. The most common variants are C104R affecting the extracellular domain and A181E located in the transmembrane domain, and for these, heterozygosity is more common than homozygosity. Work dissecting how the C104R mutation leads to impaired TACI function, first showed that this mutated form of TACI interferes with TACI signaling by associating with WT TACI in the absence of ligand, hence forming signaling-defective oligomeric receptor complexes [85]. Later work looking at the equivalent TACI mutation in mice (C76R) demonstrated that B cell function was impaired in mice heterozygous for the mutation suggesting that this mutation impairs TACI function in heterozygotes via haploinsufficiency [86], [87].
Mice lacking TACI develop defects resembling that of CVID patients such as reduced IgA levels and impaired response to T-independent antigens [88], [89], a problem which appears to be linked in part to the role of TACI in maintaining Blimp-1 expression, essential for PC generation and persistence [71]. Yet, relatives of a CVID patient can often carry the same TACI mutations without any immunological abnormalities [80], [26]. Interestingly, both B cells from CVID patients and healthy relatives are defective in vitro, in particular in response to TLR9 activation and TACI stimulation [26]. In contrast, in vitro IgG and IgA production from healthy relative-derived B cells was normal when compared to B cells from CVID relatives [26]. Therefore, mutation in the TACI gene is not the sole contributing factor but may facilitate emergence of CVID in combination with other contributing factors.
BAFF-R is essential for B cell survival and, therefore, a dominant mutation in the BAFF-R gene is likely to lead to immunodeficiency. Indeed, BAFF-R variants, present at the heterozygous state, have been identified in CVID patients with low B cell numbers [90]. These variants appeared to be polymorphic as they do not affect BAFF-R expression, yet no signaling through these variants has been tested [90]. Another report described siblings carrying a homozygous BAFF-R gene deletion preventing BAFF-R protein expression [91]. Yet only one sibling had recurrent infections, the other remained relatively healthy. Both siblings had reduced B cell numbers as well as reduced IgM and IgG levels but IgA levels remained within a normal range [91]. TACI (which was not mutated in these patients) is critical for IgA production in mice [88], [89], which may explain this difference. Therefore, similar to individuals treated with B cell depleting agent belimumab, the incidence of infection in BAFF-R-null patients may vary from one individual to another depending on additional factors and/or exposure to pathogens [92]. For this reason, while defects in BAFF-R or TACI function will certainly increase an individual's vulnerability to immunodeficiency, additional factors are required to precipitate clear CVID clinical manifestations.
7. An emerging role for the BAFF/APRIL system in asthma
Very recently a number of research articles have reported a role for the BAFF/APRIL system in asthma. However, the role of this system in airway inflammation still remains unclear and often controversial. Early work has looked at mouse models of airway inflammation and showed that mice over-expressing BAFF (BAFF Tg mice) were protected against airway inflammation compared to WT animals [93]. Yet, two independent studies looking at the use of a TACI-Ig decoy receptor to neutralize both BAFF and APRIL in the same mouse model, showed that it had a protective effect and protected the animals against airway inflammation [94], [95]. This mouse model of airway inflammation is driven by T-helper 2 (Th2) effector T cells and BAFF Tg produce higher numbers of regulatory T cells (Tregs) [96] which may have inhibited the function of Th2 cells in BAFF Tg mice. Alternatively, TACI-Ig also blocks APRIL, not just BAFF, and APRIL may play a role in this model. It does not seem to be the case as APRIL appears to block Th2 effector responses and APRIL−/− mice are more susceptible to airway inflammation compared to WT animals [97].
In human asthma, early work showed that airway epithelial cells produce BAFF [98]. Moreover, segmental allergen challenge of allergic patients leads to local release of BAFF [99]. The same group also provided evidence that BAFF plays a role in the pathology of chronic rhinosinusitis with nasal polyps [100]. Work on a Korean cohort of children showed that levels of BAFF in the sputum are increased [101]. One study suggested that measuring serum BAFF levels may serve a novel diagnostic parameter for asthma [102], yet another study suggested that other factors such as stem cell factor (SCF) and IL-31 are more reliable indicators [103]. Interestingly, emerging work looking at TACI mutations in Swedish children with asthma described rare mutations in the TACI gene correlating with an increased risk of asthma symptoms [104]. None of these mutations affected IgA production [104]. In conclusion, the role of BAFF in human asthma remains unclear and more work needs to be done to ascertain the role of the BAFF/APRIL system in the function of IgE-producing B cells, mast cells, neutrophils, airway macrophages and DCs as well as the regulation of Th2 effector cells.
8. The role of the BAFF system in autoimmunity
8.1. The BAFF system and autoimmunity in mice
BAFF Tg mice develop a SLE/Sjögren's syndrome (SS)-like syndrome, with an enlarged B cell compartment and lymphoid organs, high titers of anti-double stranded DNA (dsDNA) antibodies and rheumatoid factor (RF), hypergammaglobulinemia, circulating immune complexes and glomerulonephritis with immunoglobulin (Ig) deposits [1]. Moreover, SLE-prone mice New-Zealand Black × New-Zealand White (NZBxNZW)F1, BXSB and MRL-lpr/lpr mice have increased serum BAFF levels, and BAFF blockade led to reduced disease manifestations [105], [106]. Interestingly, BAFF appears to also play a central role in the pathogenesis of autoimmune arthritis. Serum BAFF levels were reportedly elevated in a collagen-induced arthritis (CIA) mouse model, an experimental model of rheumatoid arthritis (RA) [107]. Moreover, BAFF gene silencing in CIA mice abrogated autoimmune arthritis development [108]. Investigation of the role of APRIL in CIA led to conflicting results as CIA incidence was reported lower in both APRIL−/− mice [109] and APRIL Tg mice [110]. In addition to the different genetic modifications in these two mice strains, the genetic background was also different. DBA1 mice [110] are autoimmune prone even without overexpression of APRIL, while the APRIL−/− mice used were on a C57BL6 background. The exact role of APRIL in experimental RA remains to be clarified. Of note, CIA as well as adjuvant arthritis in rats, another experimental model of RA, experienced improvement in arthritis development after TACI-Ig administration which blocks both BAFF and APRIL [111], [112].
8.2. The BAFF system and autoimmunity in humans
Numerous human autoimmune diseases share various pathogenic pathways, including the BAFF/APRIL pathway. In fact, serum BAFF and APRIL levels are reportedly increased in a proportion of patients suffering from SLE, primary Sjögren's syndrome (pSS), RA, immune thrombocytopenia, MS, systemic sclerosis, myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis (only BAFF), myasthenia gravis (BAFF only), graves’ disease (BAFF only), autoimmune pancreatitis (BAFF only) and anti-glomerular basement membrane (GBM) antibody disease (reviewed in Vincent et al. [3]) [113], [114], [115], [116]. As some but not all patients suffering from autoimmune disease have elevated serum BAFF levels, there is the potential to delineate subsets of individuals suffering from a distinct BAFF-driven disease, with shared distinct immunological phenotypes [78].
8.3. Anti-BAFF therapies in autoimmunity
8.3.1. Tabalumab
Tabalumab (LY2127399) is a fully human IgG subclass 4 (IgG4) monoclonal antibody (mAb) targeting and neutralizing both soluble and membrane-bound BAFF currently in ongoing clinical trials for RA, SLE, multiple myeloma, MS and end-stage renal disease (Table 2 ). Tabalumab already showed some early clinical efficiency in RA. However, in a recent randomized placebo-controlled trial (RCT), tabalumab did not meet primary endpoint at week 16 in RA patients with inadequate response to TNF inhibitors [117]. In another RCT in RA patients with inadequate response to MTX and naïve to biologics, tabalumab met the primary endpoint at week 16 [118]. In these two studies, after an initial transient increase, there was a decrease in CD20+ B cells, as well as mature CD27−IgD+ B cells, whereas CD27+IgD− memory B cells increased. Of note, a reduction in IgM and IgA (only in one study [117]) but not IgG levels in patients receiving tabalumab compared to placebo was observed [117], [118]. A recent uncontrolled open-label study showed that RA patients receiving tabalumab had a reduced total number of B cells, and specifically mature naïve B cells, throughout the 52-week study period without reaching total depletion. Memory B cells rapidly increased at week 12 (almost 100%) and remained increased up to week 52 (70%). The median time for B cell recovery since the last subcutaneous injection was 40.6 weeks. Serum IgA, IgM, and IgG decreased at week 52. All B cells subset (and Ig) alterations were not associated with an increased rate of infection, highlighting the satisfactory safety profile of tabalumab [119]. A recent 52-week open-label extension study, following a RCT of 24 weeks, in RA patients receiving a stable dose of methotrexate (MTX) reported sustained long-term efficiency of the drug from week 24 to week 52 according to the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) criteria. A high rate of infections in a dose-dependent manner was reported [120]. A Phase II study involving relapsing-remitting MS patients has recently been completed (clinicaltrials.gov identifier NCT00882999), but no clinical data has been published so far.
Table 2.
Ongoing clinical trials for BAFF and/or APRIL targeted therapies in human autoimmune and cancer diseases (http://www.clinicaltrials.gov, March 2013).
| Disease | Drug | Mechanism of action | Trial | Status | Phase |
|---|---|---|---|---|---|
| SLE | Blisibimod | Anti-BAFF | NCT01305746 | Active | II |
| NCT01395745 | Not yet recruiting | III | |||
| Belimumab | Anti-BAFF | NCT01597622 | Recruiting | III | |
| NCT01484496 | Recruiting | III | |||
| NCT01597492 | Recruiting | IV | |||
| NCT01705977 | Not yet recruiting | IV | |||
| NCT01632241 | Not yet recruiting | IV | |||
| NCT00724867 | Active | III | |||
| NCT01345253 | Recruiting | III | |||
| NCT00712933 | Active | III | |||
| NCT00583362 | Active | II | |||
| NCT01639339 | Recruiting | III | |||
| Tabalumab | Anti-BAFF | NCT01205438 | Recruiting | III | |
| NCT01196091 | Recruiting | III | |||
| NCT01488708 | Recruiting | III | |||
| Pediatric SLE | Belimumab | Anti-BAFF | NCT01649765 | Recruiting | II |
| RA | Tabalumab | Anti-BAFF | NCT01676701 | Active | III |
| NCT01253291 | Active | I | |||
| NCT01215942 | Active | III | |||
| NCT01202773 | Active | III | |||
| NCT01202760 | Active | III | |||
| NCT01198002 | Active | III | |||
| NCT01576549 | Active | II | |||
| MG | Belimumab | Anti-BAFF | NCT01480596 | Not yet recruiting | II |
| Diffuse cutaneous systemic sclerosis | Belimumab | Anti-BAFF | NCT01670565 | Recruiting | II |
| Wegener's granulomatosis or microscopic polyangiitis | Belimumab | Anti-BAFF | NCT01663623 | Not yet recruiting | III |
| Granulomatosis with polyangiitis microscopic polyangiitis | Blisibimod | Anti-BAFF | NCT01598857 | Not yet recruiting | II |
| End-stage renal disease | Tabalumab | Anti-BAFF | NCT01200290 | Active | II |
| Idiopathic membranous glomerulonephropathy | Belimumab | Anti-BAFF | NCT01762852 | Not yet recruiting | II |
| NCT01610492 | Recruiting | II | |||
| Immune thrombocytopenic purpura | Blisibimod | Anti-BAFF | NCT01609452 | Not yet recruiting | II/III |
| Chronic ITP | Belimumab | Anti-BAFF | NCT01440361 | Not yet recruiting | II |
| Symptomatic WM | Belimumab | Anti-BAFF | NCT01142011 | Recruiting | II |
| Relapsed or refractory MM | Tabalumab | Anti-BAFF | NCT00689507 | Active | I |
| NCT01556438 | Recruiting | I | |||
| Previously treated MM | Tabalumab | Anti-BAFF | NCT01602224 | Recruiting | II/III |
| Prevention of kidney transplant rejection | Belimumab | Anti-BAFF | NCT01536379 | Not yet recruiting | II |
APRIL, a proliferation inducing ligand; BAFF, B cell activating factor of the tumor necrosis factor (TNF) family; ITP, immune thrombocytopenia; MG, myasthenia gravis; MM, multiple myeloma; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; WM, Waldenström macroglobulinemia.
8.3.2. Blisibimod
Blisibimod (AMG623, also known as A-623) is a peptibody (fusion polypeptide protein), produced in Escherichia coli, targeting both soluble and membrane BAFF. Blisibimod is composed of a novel BAFF binding domain linked to the N-terminus of human Fc domain [121]. The PEARL-SC RCT Phase IIb clinical trial in SLE has recently been completed in December 2012 (clinicaltrials.gov identifier NCT01162681). However, the only available data comes from the pharmaceutical company website [121]. No published articles, including information pertaining to the Phase IIb trial, are currently available in the public domain.
8.3.3. Belimumab
Belimumab (also known as GSK1550188 or HGS1006) is a recombinant fully human IgG1-λ mAb targeting only soluble BAFF (reviewed in Fairfax et al. [122]). Belimumab efficacy in SLE has been reported in two Phase III trials [123], [124]. Belimumab is the first and sole Food and Drug Administration (FDA)-approved biologic for SLE. Recent data reported an adequate safety profile as well as sustained efficacy of belimumab plus standard therapy in SLE patients over seven years and in excess of 1745 patient-years, particularly those with presence of autoantibodies at baseline in whom decreased autoantibody levels. A steroid sparing effect has been observed [125].
In a 24-week Phase II study with active RA patients, belimumab plus standard of care showed an adequate safety profile, and biological efficacy through both significant decreases in RF and CD20+ B cells, and clinical efficacy according to the ACR20 criteria, but not ACR50 (except with belimumab 10 mg/kg, P = 0.042) and ACR70 (ACR50 and ACR70 results only available on clinicaltrials.gov identifier NCT00071812, not published yet) [126]. In pSS, belimumab is currently in ongoing Phase II clinical trials (clinicaltrials.gov identifier NCT01160666). In the open label Beliss study involving pSS patients receiving belimumab, authors reported a particular improvement in glandular domain with non-malignant parotid swelling, as well as a decrease in disease activity score (ESSDAI, formally EULAR Sjögren's Syndrome Disease Activity Index) at week 28 and 52 [127], [128]. A Phase II trial studying the effect of belimumab in desensitization in sensitized patients awaiting kidney transplant has been terminated due to lack of efficacy in its primary goal (clinicaltrials.gov identifier NCT01025193).
8.3.4. Atacicept
Atacicept is a chimeric recombinant fusion protein comprising the extracellular domain of the TACI receptor linked to the human IgG1 Fc domain. Atacicept inhibits B lymphocyte stimulation by neutralizing BAFF, APRIL and heterotrimer activity and induces significant depletion of PC [129]. Of note, APRIL is implicated in PC survival and antibody production in both mice [31], [130], [30] and humans [131]. In mice and monkeys, atacicept reduces serum IgM levels and inhibits the IgM response to T-dependent antigen [132]. Data in mice showed that TACI-Ig inhibits B cell maturation and survival, T cell activation, and the T cell independent MZ B cell response. In a mouse model, TACI-Ig also significantly decreases PC numbers in the spleen and BM [132], [133], [134]. However, TACI-Ig treatment does not reduce the numbers of mouse memory B cells, which are active in long-term humoral immunity, as their survival is independent of BAFF or APRIL [130]. These biologic changes in response to TACI-Ig are associated with reduced disease scores and prolonged survival in SLE-prone mice [105], [133], [134], [135]. In two Phase Ib studies, atacicept treatment showed a safety profile and reduced serum IgM, IgA and IgG levels, as well as reduced mature and total B cell numbers in a dose-related manner in SLE patients [136], [137]. Of note, promising effects on disease-activity measures were reported [136], [137]. Unfortunately, a RCT Phase II/III trial with concomitant newly initiated corticosteroids and mycophenolate mofetil (MMF) in active lupus nephritis (LN) was stopped due to increased rates of infections and a pronounced decline in serum IgG levels [138]. It is important to note that the reduction in serum IgG levels started after initiation of MMF and corticosteroids, namely two weeks before atacicept induction. Interestingly, while SLE patients with significant kidney disease or severe renal disorder were excluded from the two previous Phase Ib studies [136], [137], the four SLE patients receiving atacicept in the Phase II/III study were characterized by active LN with high levels of proteinuria that may have disrupted IgG excretion as well as drug pharmacokinetics [138]. Another Phase II/III study involving lupus patients, without both active CNS SLE and active moderate to severe glomerulonephritis, has just been completed (clinicaltrials.gov identifier NCT00624338) but no published data is available. Strikingly, disease activity worsened in MS patients receiving atacicept in a Phase II clinical trial (clinicaltrials.gov identifier NCT00642902), leading to termination of the latter as well as two other clinical trials, one in MS (clinicaltrials.gov identifier NCT00853762) and one in optic neuritis (clinicaltrials.gov identifier NCT00624468). In RA, atacicept did not meet primary efficacy end point in two Phase II clinical trials, despite an acceptable safety profile and significant biological activity, which were previously reported in a Phase Ib study in RA patients [139], [140], [141]. A recent Phase II RCT clinical trial studying the effect of atacicept in combination with rituximab (RTX) in RA patients reported more hypersensitivity-related events in atacicept-RTX combination group compared to RTX monotherapy. There were no differences regarding clinical response or infection rates between these two groups [142].
8.3.5. Antibodies toward anti-BAFF therapies: should we pay more attention?
The production of antidrug antibodies (ADAb) in chronic inflammatory disease has been shown to limit both the pharmacodynamics and pharmacokinetics of monoclonal antibodies neutralizing TNF, thereby influencing disease activity. In fact, ADAb formation is associated with lower serum TNF inhibitor drug levels, adverse events and drug failure in these diseases. The use of immunosuppressive agents in combination with TNF inhibitors appears to reduce the incidence of antibody formation against the drug, as prevalence of ADAb is inversely associated with dose and additional use of MTX in chronic inflammatory disease such as RA (reviewed in Vincent et al. [143]). Thus, ADAb could possibly explain in part the failure of anti-BAFF therapy in some patients, especially those not receiving immunosuppressants as demonstrated in studies involving anti-TNF drugs [143], as well as rituximab, where a correlation between the presence of ADAb, weak B cell depletion and clinical outcomes has been noted [144]. Of note, the reported frequency of anti-tabalumab antibodies in RA patients ranged from 0 to 4.4% [117], [118], [120]. In a recent study, in which 3% (3/100) of RA patients had anti-tabalumab ADAb [118], authors reported no association with drug failure or adverse events associated with ADAb, however this is a very small sample size preventing any definitive conclusion on this issue. Whether there are ADAb toward all anti-BAFF drugs is unknown, and whether potential ADAb will limit drug efficiency remains to be determined.
9. Summary
The BAFF system appears to play a major role in immunology, not only in autoimmunity but also in cancer, infection, transplantation, allergy and immunodeficiency. The approved use of belimumab in systemic lupus erythematosus is an important first step in the development of more personalized approaches to targeting the BAFF system and shows promise for use in broader immunological contexts. But like many ligands in the TNF superfamily, BAFF and APRIL most likely have beneficial as well as detrimental effects. The dominant effect will determine how to target this system effectively in pathologies other than autoimmunity and more work is needed to better understand emerging roles of the BAFF system in additional immunological settings. While BAFF inhibitors continue to show some degrees of efficacy in many ongoing clinical trials, the challenge will always be to obtain significantly added benefits of this approach compared to currently used biologics. A better understanding of the therapeutics’ full mechanism of action may help achieve this goal.
Competing interests
The authors have no conflict of interest to declare.
Biographies

Fabien Vincent is a rheumatologist specialized in autoimmunity with broad experience encompassing B cell immunology, molecular immunology and genetic bases of disease. He completed his medical training at the University Hospital Centre of Caen and Paris in France between 1999 and 2010. He has extensive experience with handling clinical data, as well as with ELISA, QPCR, flow cytometry and cell culture and genetic sequence analysis. He is involved in a biomedical research project in Fabienne Mackay's laboratory in order to complete a PhD. His project focuses on the role of B cell activating factor of the tumour necrosis factor (TNF) family (BAFF) and the innate immune system in the pathogenesis of the systemic lupus erythematosus. Another aim of this study is to characterize the role of BAFF and innate immunity markers in the increased frequency of lupus and auto-immune diseases observed in Australian Indigenous population. He also works on the role of antidrug antibodies to TNF-specific neutralizing agents in chronic inflammatory diseases.

Damien Saulep-Easton completed his undergraduate training in Biotechnology, majoring in Biochemistry and Molecular Microbiology. He continued on to complete a Post Graduate Diploma in Medical Microbiology where he mapped and characterized the genetic resistance mechanisms of a lethal multi-drug resistant strain of Pseudomonas aeruginosa, a significant nosocomial pathogen. Damien has since spent half a decade in professional research and project management and is currently in the second year of his PhD. Funded by the federal Department of Innovation Industry, Science and Research (DIISR), Damien's clinical research is focussed on the dissecting the role of BAFF in leukemia and developing novel tools for clinicians to track disease progression.

William A. Figgett obtained his B.Sc. (Molecular Biology & Genetics) (Hons) from the University of Sydney in 2008. He then completed his Ph.D. partly at the Garvan Institute of Medical Research in Sydney and partly at the Monash University department of Immunology in Melbourne. His research focused on dissecting disease mechanisms contributing to BAFF-driven autoimmunity as well as the regulation of innate B cell activation.

Kirsten Fairfax is an NHMRC CJ Martin Fellowship holder. She completed her first post-doc at The Babraham Institute, Cambridge, UK with Martin Turner studying RNA-binding proteins and B cell responses. She is now conducting her second post-doc with Fabienne MacKay and is focusing on B cells and autoimmunity. Kirsten's research interests extend to plasma cells and adaptive immune responses, including the interaction of T cells and DCs with B cells.

Fabienne Mackay is currently the 5th Chair of the Department of Immunology, Monash Alfred Medical Research and Education Precinct (AMREP), Melbourne, Australia, and is an NHMRC Senior Research Fellow. After obtaining her PhD in 1994 at the Louis Pasteur University in Strasbourg, Professor Mackay joined Biogen Idec Inc. in Boston where she dissected the role of TNF-like ligands in autoimmunity and cancer. This work led to many patents and the development of new treatments tested in the clinic. In 2000, Professor Mackay joined the Garvan Institute in Sydney as a Wellcome Trust Senior Research Fellow and was awarded an NHMRC programme grant. Her lab at Garvan discovered the function of BAFF/BLyS as a key B-cell survival factor essential for the maturation of B lymphocytes but also playing a role in autoimmunity, and became one the leading group on BAFF research. In March 2006, Professor Mackay was appointed to Director of the Autoimmunity Research Unit and Adjunct Full Professor at the Faculty of Medicine of the University of New South Wales. In 2008 and 2009, she was a New South Wales Director for the Australian Society of Medical Research (ASMR). Her current group continues to explore new function of BAFF and has a programme studying the role of B-cell subsets and innate immunity in immune tolerance, autoimmunity and cancer. Professor Mackay has authored over 100 articles/reviews/book chapters, many in high-impact-factor journals such as Nature, J Exp Medicine and J Clin Invest. Her h-index is 51, with 9834 citations, an average of 81 citations per article. In 2012, Professor Mackay received the Thomson Reuters Australia citation and innovation award.
Contributor Information
Fabien B. Vincent, Email: fabien.vincent@monash.edu.
Damien Saulep-Easton, Email: damien.easton@monash.edu.
William A. Figgett, Email: william.figgett@monash.edu.
Kirsten A. Fairfax, Email: kirsten.fairfax@monash.edu.
Fabienne Mackay, Email: Fabienne.Mackay@monash.edu.
References
- 1.Mackay F., Woodcock S.A., Lawton P., Ambrose C., Baetscher M., Schneider P. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. Journal of Experimental Medicine. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider P., MacKay F., Steiner V., Hofmann K., Bodmer J.L., Holler N. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. Journal of Experimental Medicine. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent F.B., Morand E.F., Mackay F. BAFF and innate immunity: new therapeutic targets for systemic lupus erythematosus. Immunology and Cell Biology. 2012;90:293–303. doi: 10.1038/icb.2011.111. [DOI] [PubMed] [Google Scholar]
- 4.Fenstad M.H., Johnson M.P., Roten L.T., Aas P.A., Forsmo S., Klepper K. Genetic and molecular functional characterization of variants within TNFSF13B, a positional candidate preeclampsia susceptibility gene on 13q. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0012993. e12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langat D.L., Wheaton D.A., Platt J.S., Sifers T., Hunt J.S. Signaling pathways for B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) in human placenta. American Journal of Pathology. 2008;172:1303–1311. doi: 10.2353/ajpath.2008.071139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahne M., Kataoka T., Schroter M., Hofmann K., Irmler M., Bodmer J.L. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. Journal of Experimental Medicine. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nardelli B., Belvedere O., Roschke V., Moore P.A., Olsen H.S., Migone T.S. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 8.Bossen C., Tardivel A., Willen L., Fletcher C.A., Perroud M., Beermann F. Mutation of the BAFF furin cleavage site impairs B-cell homeostasis and antibody responses. European Journal of Immunology. 2011;41:787–797. doi: 10.1002/eji.201040591. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Fraga M., Fernandez R., Albar J.P., Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Reports. 2001;2:945–951. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maia S., Pelletier M., Ding J., Hsu Y.M., Sallan S.E., Rao S.P. Aberrant expression of functional BAFF-system receptors by malignant B-cell precursors impacts leukemia cell survival. PLoS ONE. 2011;6:e20787. doi: 10.1371/journal.pone.0020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pradet-Balade B., Medema J.P., Lopez-Fraga M., Lozano J.C., Kolfschoten G.M., Picard A. An endogenous hybrid mRNA encodes TWE-PRIL, a functional cell surface TWEAK-APRIL fusion protein. EMBO Journal. 2002;21:5711–5720. doi: 10.1093/emboj/cdf565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossen C., Cachero T.G., Tardivel A., Ingold K., Willen L., Dobles M. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111:1004–1012. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Xu L., Opalka N., Kappler J., Shu H.B., Zhang G. Crystal structure of sTALL-1 reveals a virus-like assembly of TNF family ligands. Cell. 2002;108:383–394. doi: 10.1016/s0092-8674(02)00631-1. [DOI] [PubMed] [Google Scholar]
- 14.Cachero T.G., Schwartz I.M., Qian F., Day E.S., Bossen C., Ingold K. Formation of virus-like clusters is an intrinsic property of the tumor necrosis factor family member BAFF (B cell activating factor) Biochemistry. 2006;45:2006–2013. doi: 10.1021/bi051685o. [DOI] [PubMed] [Google Scholar]
- 15.Gavin A.L., Duong B., Skog P., Ait-Azzouzene D., Greaves D.R., Scott M.L. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. Journal of Immunology. 2005;175:319–328. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 16.Ingold K., Zumsteg A., Tardivel A., Huard B., Steiner Q.-G., Cachero T.G. Identification of proteoglycans as the APRIL-specific binding partners. Journal of Experimental Medicine. 2005;201:1375–1383. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day E.S., Cachero T.G., Qian F., Sun Y., Wen D., Pelletier M. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. 2005;44:1919–1931. doi: 10.1021/bi048227k. [DOI] [PubMed] [Google Scholar]
- 18.Mihalcik S.A., Huddleston P.M., 3rd, Wu X., Jelinek D.F. The structure of the TNFRSF13C promoter enables differential expression of BAFF-R during B cell ontogeny and terminal differentiation. Journal of Immunology. 2010;185:1045–1054. doi: 10.4049/jimmunol.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng L.G., Sutherland A.P., Newton R., Qian F., Cachero T.G., Scott M.L. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. Journal of Immunology. 2004;173:807–817. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 20.von Bulow G.U., Russell H., Copeland N.G., Gilbert D.J., Jenkins N.A., Bram R.J. Molecular cloning and functional characterization of murine transmembrane activator and CAML interactor (TACI) with chromosomal localization in human and mouse. Mammalian Genome. 2000;11:628–632. doi: 10.1007/s003350010125. [DOI] [PubMed] [Google Scholar]
- 21.Ozcan E., Garibyan L., Lee J.J., Bram R.J., Lam K.P., Geha R.S. Transmembrane activator, calcium modulator, and cyclophilin ligand interactor drives plasma cell differentiation in LPS-activated B cells. Journal of Allergy and Clinical Immunology. 2009;123:1277e5–1286e5. doi: 10.1016/j.jaci.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seshasayee D., Valdez P., Yan M., Dixit V.M., Tumas D., Grewal I.S. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–288. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 23.Ou X., Xu S., Lam K.P. Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15401–15406. doi: 10.1073/pnas.1200386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He B., Santamaria R., Xu W., Cols M., Chen K., Puga I. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nature Immunology. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akkoyunlu M. TACI expression is low both in human and mouse newborns. Scandinavian Journal of Immunology. 2012;75:368. doi: 10.1111/j.1365-3083.2011.02651.x. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Gallo M., Radigan L., Almejun M.B., Martinez-Pomar N., Matamoros N., Cunningham-Rundles C. TACI mutations and impaired B-cell function in subjects with CVID and healthy heterozygotes. Journal of Allergy and Clinical Immunology. 2013;131:468–476. doi: 10.1016/j.jaci.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lougaris V., Gallizzi R., Vitali M., Baronio M., Salpietro A., Bergbreiter A. A novel compound heterozygous TACI mutation in an autosomal recessive common variable immunodeficiency (CVID) family. Human Immunology. 2012;73:836–839. doi: 10.1016/j.humimm.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Moreaux J., Sprynski A.C., Dillon S.R., Mahtouk K., Jourdan M., Ythier A. APRIL and TACI interact with syndecan-1 on the surface of multiple myeloma cells to form an essential survival loop. European Journal of Haematology. 2009;83:119–129. doi: 10.1111/j.1600-0609.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 29.Stadanlick J.E., Kaileh M., Karnell F.G., Scholz J.L., Miller J.P., Quinn W.J., 3rd Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nature Immunology. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor B.P., Raman V.S., Erickson L.D., Cook W.J., Weaver L.K., Ahonen C. BCMA is essential for the survival of long-lived bone marrow plasma cells. Journal of Experimental Medicine. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peperzak V., Vikstrom I., Walker J., Glaser S.P., Lepage M., Coquery C.M. Mcl-1 is essential for the survival of plasma cells. Nature Immunology. 2013;14:290–297. doi: 10.1038/ni.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Zheng S., Wu H., Wu Y., Liu S., Fan M. Identification of BLyS (B lymphocyte stimulator), a non-myelin-associated protein, as a functional ligand for Nogo-66 receptor. Journal of Neuroscience. 2009;29:6348–6352. doi: 10.1523/JNEUROSCI.5040-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krumbholz M., Theil D., Derfuss T., Rosenwald A., Schrader F., Monoranu C.M. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. Journal of Experimental Medicine. 2005;201:195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bick S., Tschernatsch M., Karg A., Fuehlhuber V., Trenczek T.E., Faltermeier K. Intravenous immunoglobulin inhibits BAFF production in chronic inflammatory demyelinating polyneuropathy – a new mechanism of action? Journal of Neuroimmunology. 2013;256:84–90. doi: 10.1016/j.jneuroim.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Panchanathan R., Choubey D. Murine BAFF expression is up-regulated by estrogen and interferons: implications for sex bias in the development of autoimmunity. Molecular Immunology. 2013;53:15–23. doi: 10.1016/j.molimm.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob N., Guo S., Mathian A., Koss M.N., Gindea S., Putterman C. B Cell and BAFF dependence of IFN-alpha-exaggerated disease in systemic lupus erythematosus-prone NZM 2328 mice. Journal of Immunology. 2011;186:4984–4993. doi: 10.4049/jimmunol.1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen J.L., Fore M.S., Wooten J., Roehrs P.A., Bhuiya N.S., Hoffert T. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood. 2012;120:2529–2536. doi: 10.1182/blood-2012-06-438911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarantopoulos S., Stevenson K.E., Kim H.T., Cutler C.S., Bhuiya N.S., Schowalter M. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuzmina Z., Krenn K., Petkov V., Kormoczi U., Weigl R., Rottal A. CD19+CD21low B-cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood. 2013;121:1886–1895. doi: 10.1182/blood-2012-06-435008. [DOI] [PubMed] [Google Scholar]
- 40.Clark W.B., Brown-Gentry K.D., Crawford D.C., Fan K.H., Snavely J., Chen H. Genetic variation in recipient B-cell activating factor modulates phenotype of GVHD. Blood. 2011;118:1140–1144. doi: 10.1182/blood-2010-09-310011. [DOI] [PubMed] [Google Scholar]
- 41.Whittle R., Taylor P.C. Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host disease to extracorporeal photopheresis. Blood. 2011;118:6446–6449. doi: 10.1182/blood-2011-05-354019. [DOI] [PubMed] [Google Scholar]
- 42.Xu H., He X., Liu Q., Shi D., Chen Y., Zhu Y. Abnormal high expression of B-cell activating factor belonging to the TNF superfamily (BAFF) associated with long-term outcome in kidney transplant recipients. Transplantation Proceedings. 2009;41:1552–1556. doi: 10.1016/j.transproceed.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 43.Kuzmina Z., Greinix H.T., Weigl R., Kormoczi U., Rottal A., Frantal S. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117:2265–2274. doi: 10.1182/blood-2010-07-295766. [DOI] [PubMed] [Google Scholar]
- 44.Kim S.J., Won J.H. B cell homeostasis and the development of chronic graft-versus-host disease: implications for B cell-depleting therapy. Leukemia and Lymphoma. 2012;53:19–25. doi: 10.3109/10428194.2011.603448. [DOI] [PubMed] [Google Scholar]
- 45.Sarantopoulos S., Stevenson K.E., Kim H.T., Washel W.S., Bhuiya N.S., Cutler C.S. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117:2275–2283. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jablonska E., Slodczyk B., Wawrusiewicz-Kurylonek N., Garley M., Dziemianczyk D., Kretowski A. Overexpression of B cell-activating factor (BAFF) in neutrophils of oral cavity cancer patients – preliminary study. Neoplasma. 2011;58:211–216. doi: 10.4149/neo_2011_03_211. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J., Sun L., Lin S., Zhao R., Zhou L., Fang D. BlyS is up-regulated by hypoxia and promotes migration of human breast cancer cells. Journal of Experimental and Clinical Cancer Research. 2012;31:31. doi: 10.1186/1756-9966-31-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fragioudaki M., Tsirakis G., Pappa C.A., Aristeidou I., Tsioutis C., Alegakis A. Serum BAFF levels are related to angiogenesis and prognosis in patients with multiple myeloma. Leukemia Research. 2012;36:1004–1008. doi: 10.1016/j.leukres.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W., Kater A.P., Widhopf G.F., 2nd, Chuang H.Y., Enzler T., James D.F. B-cell activating factor and v-Myc myelocytomatosis viral oncogene homolog (c-Myc) influence progression of chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18956–18960. doi: 10.1073/pnas.1013420107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cols M., Barra C.M., He B., Puga I., Xu W., Chiu A. Stromal endothelial cells establish a bidirectional crosstalk with chronic lymphocytic leukemia cells through the TNF-related factors BAFF, APRIL, and CD40L. Journal of Immunology. 2012;188:6071–6083. doi: 10.4049/jimmunol.1102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molica S., Digiesi G., Battaglia C., Cutrona G., Antenucci A., Molica M. Baff serum level predicts time to first treatment in early chronic lymphocytic leukemia. European Journal of Haematology. 2010;85:314–320. doi: 10.1111/j.1600-0609.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- 52.Li Y.J., Jiang W.Q., Rao H.L., Huang J.J., Xia Y., Huang H.Q. Expression of BAFF and BAFF-R in follicular lymphoma: correlation with clinicopathologic characteristics and survival outcomes. PLoS ONE. 2012;7:e50936. doi: 10.1371/journal.pone.0050936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Carlo E., D’Antuono T., Pompa P., Giuliani R., Rosini S., Stuppia L. The lack of epithelial interleukin-7 and BAFF/BLyS gene expression in prostate cancer as a possible mechanism of tumor escape from immunosurveillance. Clinical Cancer Research. 2009;15:2979–2987. doi: 10.1158/1078-0432.CCR-08-1951. [DOI] [PubMed] [Google Scholar]
- 54.Le Pottier L., Tobon G.J., Youinou P., Pers J.O. B cell activating factor of the TNF family: Δ4BAFF, an alternate-splice isoform that acts as a transcription factor and exaggerates its production in autoimmunity and cancer. Annals of the Rheumatic Diseases. 2010;69:A14. [Google Scholar]
- 55.Moreaux J., Veyrune J.L., De Vos J., Klein B. APRIL is overexpressed in cancer: link with tumor progression. BMC Cancer. 2009;9:83. doi: 10.1186/1471-2407-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu L., Lin-Lee Y.C., Pham L.V., Tamayo A.T., Yoshimura L.C., Ford R.J. BAFF-R promotes cell proliferation and survival through interaction with IKKbeta and NF-kappaB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells. Blood. 2009;113:4627–4636. doi: 10.1182/blood-2008-10-183467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hildebrand J.M., Luo Z., Manske M.K., Price-Troska T., Ziesmer S.C., Lin W. A BAFF-R mutation associated with non-Hodgkin lymphoma alters TRAF recruitment and reveals new insights into BAFF-R signaling. Journal of Experimental Medicine. 2010;207:2569–2579. doi: 10.1084/jem.20100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kofler D.M., Gawlik B.B., Elter T., Gianella-Borradori A., Wendtner C.M., Hallek M. Phase 1b trial of atacicept, a recombinant protein binding BLyS and APRIL, in patients with chronic lymphocytic leukemia. Leukemia. 2012;26:841–844. doi: 10.1038/leu.2011.286. [DOI] [PubMed] [Google Scholar]
- 59.Parameswaran R., Yu M., Lyu M.A., Lim M., Rosenblum M.G., Groffen J. Treatment of acute lymphoblastic leukemia with an rGel/BLyS fusion toxin. Leukemia. 2012;26:1786–1796. doi: 10.1038/leu.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L., Jiang Y., Zheng Y., Zeng Y., Yang Z., Huang G. Selective killing of Burkitt's lymphoma cells by mBAFF-targeted delivery of PinX1. Leukemia. 2011;25:331–340. doi: 10.1038/leu.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez B., Valdez H., Freimuth W., Butler T., Asaad R., Lederman M.M. Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. AIDS. 2003;17:1983–1985. doi: 10.1097/00002030-200309050-00018. [DOI] [PubMed] [Google Scholar]
- 62.Toubi E., Gordon S., Kessel A., Rosner I., Rozenbaum M., Shoenfeld Y. Elevated serum B-lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. Journal of Autoimmunity. 2006;27:134–139. doi: 10.1016/j.jaut.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 63.McNamara P.S., Fonceca A.M., Howarth D., Correia J.B., Slupsky J.R., Trinick R.E. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax. 2013;68:76–81. doi: 10.1136/thoraxjnl-2012-202288. [DOI] [PubMed] [Google Scholar]
- 64.Dalrymple N.A., Mackow E.R. Endothelial cells elicit immune-enhancing responses to dengue virus infection. Journal of Virology. 2012;86:6408–6415. doi: 10.1128/JVI.00213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He B., Raab-Traub N., Casali P., Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. Journal of Immunology. 2003;171:5215–5224. doi: 10.4049/jimmunol.171.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ittah M., Miceli-Richard C., Lebon P., Pallier C., Lepajolec C., Mariette X. Induction of B cell-activating factor by viral infection is a general phenomenon, but the types of viruses and mechanisms depend on cell type. Journal of Innate Immunity. 2011;3:200–207. doi: 10.1159/000321194. [DOI] [PubMed] [Google Scholar]
- 67.Reed J.L., Welliver T.P., Sims G.P., McKinney L., Velozo L., Avendano L. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. Journal of Infectious Diseases. 2009;199:1128–1138. doi: 10.1086/597386. [DOI] [PubMed] [Google Scholar]
- 68.Adalid-Peralta L., Godot V., Colin C., Krzysiek R., Tran T., Poignard P. Stimulation of the primary anti-HIV antibody response by IFN-alpha in patients with acute HIV-1 infection. Journal of Leukocyte Biology. 2008;83:1060–1067. doi: 10.1189/jlb.1007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poudrier J., Thibodeau V., Roger M. Natural immunity to HIV: a delicate balance between strength and control. Clinical & Developmental Immunology. 2012;2012:875821. doi: 10.1155/2012/875821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf A.I., Mozdzanowska K., Quinn W.J., 3rd, Metzgar M., Williams K.L., Caton A.J. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. Journal of Clinical Investigation. 2011;121:3954–3964. doi: 10.1172/JCI57362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsuji S., Cortesao C., Bram R.J., Platt J.L., Cascalho M. TACI deficiency impairs sustained Blimp-1 expression in B cells decreasing long-lived plasma cells in the bone marrow. Blood. 2011;118:5832–5839. doi: 10.1182/blood-2011-05-353961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castigli E., Wilson S.A., Garibyan L., Rachid R., Bonilla F., Schneider L. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nature Genetics. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 73.von Bulow G.U., van Deursen J.M., Bram R.J. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 74.Kaur K., Chowdhury S., Greenspan N.S., Schreiber J.R. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood. 2007;110:2948–2954. doi: 10.1182/blood-2007-01-069245. [DOI] [PubMed] [Google Scholar]
- 75.Liu K., Zhang Y., Hu S., Yu Y., Yang Q., Jin D. Increased levels of BAFF and APRIL related to human active pulmonary tuberculosis. PLoS ONE. 2012;7:e38429. doi: 10.1371/journal.pone.0038429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu X.Q., Stacey K.J., Horne-Debets J.M., Cridland J.A., Fischer K., Narum D. Malaria infection alters the expression of B-cell activating factor resulting in diminished memory antibody responses and survival. European Journal of Immunology. 2012;42:3291–3301. doi: 10.1002/eji.201242689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nduati E., Gwela A., Karanja H., Mugyenyi C., Langhorne J., Marsh K. The plasma concentration of the B cell activating factor is increased in children with acute malaria. Journal of Infectious Diseases. 2011;204:962–970. doi: 10.1093/infdis/jir438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vincent F.B., Bourke P., Morand E.F., Mackay F., Bossingham D. Focus on systemic lupus erythematosus in Indigenous Australians: towards a better understanding of autoimmune diseases. Internal Medicine Journal. 2013;43:227–234. doi: 10.1111/imj.12039. [DOI] [PubMed] [Google Scholar]
- 79.Scharenberg A.M., Hannibal M.C., Torgerson T., Ochs H.D., Rawlings D.J. Common variable immune deficiency overview. In: Pagon R.A., Bird T.D., Dolan C.R., Stephens K., Adam M.P., editors. GeneReviews. University of Washington, Seattle; Seattle (WA): 2006. [Google Scholar]
- 80.Park J.H., Resnick E.S., Cunningham-Rundles C. Perspectives on common variable immune deficiency. Annals of the New York Academy of Sciences. 2011;1246:41–49. doi: 10.1111/j.1749-6632.2011.06338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salzer U., Chapel H.M., Webster A.D.B., Pan-Hammarstrom Q., Schmitt-Graeff A., Schlesier M. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nature Genetics. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L., Radigan L., Salzer U., Behrens T.W., Grimbacher B., Diaz G. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. Journal of Allergy and Clinical Immunology. 2007;120:1178–1185. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan-Hammarstrom Q., Salzer U., Du L., Bjorkander J., Cunningham-Rundles C., Nelson D.L. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nature Genetics. 2007;39:429–430. doi: 10.1038/ng0407-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinez-Pomar N., Detkova D., Arostegui J.I., Alvarez A., Soler-Palacin P., Vidaller A. Role of TNFRSF13B variants in patients with common variable immunodeficiency. Blood. 2009;114:2846–2848. doi: 10.1182/blood-2009-05-213025. [DOI] [PubMed] [Google Scholar]
- 85.Garibyan L., Lobito A.A., Siegel R.M., Call M.E., Wucherpfennig K.W., Geha R.S. Dominant-negative effect of the heterozygous C104R TACI mutation in common variable immunodeficiency (CVID) Journal of Clinical Investigation. 2007;117:1550–1557. doi: 10.1172/JCI31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bacchelli C., Buckland K.F., Buckridge S., Salzer U., Schneider P., Thrasher A.J. The C76R transmembrane activator and calcium modulator cyclophilin ligand interactor mutation disrupts antibody production and B-cell homeostasis in heterozygous and homozygous mice. Journal of Allergy and Clinical Immunology. 2011;127:1253–1263. doi: 10.1016/j.jaci.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 87.Lee J.J., Jabara H.H., Garibyan L., Rauter I., Sannikova T., Dillon S.R. The C104R mutant impairs the function of transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) through haploinsufficiency. Journal of Allergy and Clinical Immunology. 2010;126:1234–1244. doi: 10.1016/j.jaci.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.von Bulow G.-U., van Deursen J.M., Bram R.J. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 89.Yan M., Wang H., Chan B., Roose-Girma M., Erickson S., Baker T. Activation and accumulation of B cells in TACI-deficient mice. Nature Immunology. 2001;2:638–643. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- 90.Losi C.G., Silini A., Fiorini C., Soresina A., Meini A., Ferrari S. Mutational analysis of human BAFF receptor TNFRSF13C (BAFF-R) in patients with common variable immunodeficiency. Journal of Clinical Immunology. 2005;25:496–502. doi: 10.1007/s10875-005-5637-2. [DOI] [PubMed] [Google Scholar]
- 91.Warnatz K., Salzer U., Rizzi M., Fischer B., Gutenberger S., Bohm J. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13945–13950. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jacobi A.M., Huang W., Wang T., Freimuth W., Sanz I., Furie R. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis and Rheumatism. 2010;62:201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sutherland A.P., Ng L.G., Fletcher C.A., Shum B., Newton R.A., Grey S.T. BAFF augments certain Th1-associated inflammatory responses. Journal of Immunology. 2005;174:5537–5544. doi: 10.4049/jimmunol.174.9.5537. [DOI] [PubMed] [Google Scholar]
- 94.Moon E.Y., Ryu S.K. TACI: Fc scavenging B cell activating factor (BAFF) alleviates ovalbumin-induced bronchial asthma in mice. Experimental and Molecular Medicine. 2007;39:343–352. doi: 10.1038/emm.2007.38. [DOI] [PubMed] [Google Scholar]
- 95.Bilsborough J., Chadwick E., Mudri S., Ye X., Henderson W.R., Jr., Waggie K. TACI-Ig prevents the development of airway hyperresponsiveness in a murine model of asthma. Clinical and Experimental Allergy. 2008;38:1959–1968. doi: 10.1111/j.1365-2222.2008.03099.x. [DOI] [PubMed] [Google Scholar]
- 96.Groom J.R., Fletcher C.A., Walters S.N., Grey S.T., Watt S.V., Sweet M.J. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. Journal of Experimental Medicine. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao Y., Motomura S., Deyev V., Podack E.R. TNF superfamily member 13, APRIL, inhibits allergic lung inflammation. European Journal of Immunology. 2011;41:164–171. doi: 10.1002/eji.201040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kato A., Truong-Tran A.Q., Scott A.L., Matsumoto K., Schleimer R.P. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. Journal of Immunology. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kato A., Xiao H., Chustz R.T., Liu M.C., Schleimer R.P. Local release of B cell-activating factor of the TNF family after segmental allergen challenge of allergic subjects. Journal of Allergy and Clinical Immunology. 2009;123:369–375. doi: 10.1016/j.jaci.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kato A., Peters A., Suh L., Carter R., Harris K.E., Chandra R. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology. 2008;121:1385–1392. doi: 10.1016/j.jaci.2008.03.002. 92.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jee H.M., Choi B.S., Kim K.W., Sohn M.H., Han M.Y., Kim K.E. Increased B cell-activating factor (BAFF) level in the sputum of children with asthma. Korean Journal of Pediatrics. 2010;53:795–800. doi: 10.3345/kjp.2010.53.8.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kang J.-S., Yoon Y.-D., Ahn J.-H., Kim S.-C., Kim K.-H., Kim H.-M. B cell-activating factor is a novel diagnosis parameter for asthma. International Archives of Allergy and Immunology. 2006;141:181–188. doi: 10.1159/000094897. [DOI] [PubMed] [Google Scholar]
- 103.Lei Z., Liu G., Huang Q., Lv M., Zu R., Zhang G.M. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. 2008;63:327–332. doi: 10.1111/j.1398-9995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 104.Janzi M., Melen E., Kull I., Wickman M., Hammarstrom L. Rare mutations in TNFRSF13B increase the risk of asthma symptoms in Swedish children. Genes and Immunity. 2012;13:59–65. doi: 10.1038/gene.2011.55. [DOI] [PubMed] [Google Scholar]
- 105.Gross J.A., Johnston J., Mudri S., Enselman R., Dillon S.R., Madden K. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 106.Ding H., Wang L., Wu X., Yan J., He Y., Ni B. Blockade of B-cell-activating factor suppresses lupus-like syndrome in autoimmune BXSB mice. Journal of Cellular and Molecular Medicine. 2010;14:1717–1725. doi: 10.1111/j.1582-4934.2009.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang M., Ko K.H., Lam Q.L., Lo C.K., Srivastava G., Zheng B. Expression and function of TNF family member B cell-activating factor in the development of autoimmune arthritis. International Immunology. 2005;17:1081–1092. doi: 10.1093/intimm/dxh287. [DOI] [PubMed] [Google Scholar]
- 108.Lai Kwan Lam Q., King Hung Ko O., Zheng B.J., Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14993–14998. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiao Y., Motomura S., Podack E.R. APRIL (TNFSF13) regulates collagen-induced arthritis, IL-17 production and Th2 response. European Journal of Immunology. 2008;38:3450–3458. doi: 10.1002/eji.200838640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandez L., Salinas G.F., Rocha C., Carvalho-Pinto C.E., Yeremenko N., Papon L. The TNF family member APRIL dampens collagen-induced arthritis. Annals of the Rheumatic Diseases. 2012 doi: 10.1136/annrheumdis-2012-202382. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 111.Wang D., Chang Y., Wu Y., Zhang L., Yan S., Xie G. Therapeutic effects of TACI-Ig on rat with adjuvant arthritis. Clinical and Experimental Immunology. 2011;163:225–234. doi: 10.1111/j.1365-2249.2010.04293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y., Zhang L., Wu Y., Tong T., Zhao W., Li P. Therapeutic effects of TACI-Ig on collagen-induced arthritis by regulating T and B lymphocytes function in DBA/1 mice. European Journal of Pharmacology. 2011;654:304–314. doi: 10.1016/j.ejphar.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 113.Xin G., Cui Z., Su Y., Xu L.X., Zhao M.H., Li K.S. Serum BAFF and APRIL might be associated with disease activity and kidney damage in patients with anti-GBM disease. Nephrology (Carlton, Vic.) 2013;18:209–214. doi: 10.1111/nep.12032. [DOI] [PubMed] [Google Scholar]
- 114.Kim J.Y., Yang Y., Moon J.S., Lee E.Y., So S.H., Lee H.S. Serum BAFF expression in patients with myasthenia gravis. Journal of Neuroimmunology. 2008;199:151–154. doi: 10.1016/j.jneuroim.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 115.Vannucchi G., Covelli D., Curro N., Dazzi D., Maffini A., Campi I. Serum BAFF concentrations in patients with Graves’ disease and orbitopathy before and after immunosuppressive therapy. Journal of Clinical Endocrinology and Metabolism. 2012;97:E755–E759. doi: 10.1210/jc.2011-2614. [DOI] [PubMed] [Google Scholar]
- 116.Nagai M., Hirayama K., Ebihara I., Shimohata H., Kobayashi M., Koyama A. Serum levels of BAFF and APRIL in myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis: association with disease activity. Nephron: Clinical Practice. 2011;118:c339–c345. doi: 10.1159/000323393. [DOI] [PubMed] [Google Scholar]
- 117.Genovese M.C., Fleischmann R.M., Greenwald M., Satterwhite J., Veenhuizen M., Xie L. Tabalumab, an anti-BAFF monoclonal antibody, in patients with active rheumatoid arthritis with an inadequate response to TNF inhibitors. Annals of the Rheumatic Diseases. 2012 doi: 10.1136/annrheumdis-2012-202775. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 118.Genovese M.C., Bojin S., Biagini I., Mociran E., Cristei D., Mirea G. Tabalumab in patients with rheumatoid arthritis with an inadequate response to methotrexate and naive to biologic therapy. Arthritis and Rheumatism. 2013;65:880–889. doi: 10.1002/art.37820. [DOI] [PubMed] [Google Scholar]
- 119.Greenwald M.W., Veenhuizen M., Komocsar W., Jones-Taha R., Lee C.H., Berclaz P.Y. Changes in B cell populations and serum immunoglobulins and their relationship to infections in a one year, uncontrolled open label study of tabalumab. ACR meeting. Arthritis and Rheumatism. 2012;64:S545. [Google Scholar]
- 120.Greenwald M.W., Szczepanski L., Kennedy A.C., Lee C.H., Polasek E., Veenhuizen M. Long-term safety and efficacy of tabalumab, an anti-B-cell activating factor monoclonal antibody, in patients with rheumatoid arthritis: a 52-week, open-label extension study. ACR meeting. Arthritis and Rheumatism. 2012;64:S193. doi: 10.1186/s13075-014-0415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blisibimod. A-623. Anthera Pharmaceuticals, Inc. BAFF peptibody for the treatment of lupus. Viewed 19 February 2013 <http://www.anthera.com/products_a623.asp>.
- 122.Fairfax K., Mackay I.R., Mackay F. BAFF/BLyS inhibitors: a new prospect for treatment of systemic lupus erythematosus. IUBMB Life. 2012;64:595–602. doi: 10.1002/iub.1046. [DOI] [PubMed] [Google Scholar]
- 123.Navarra S.V., Guzman R.M., Gallacher A.E., Hall S., Levy R.A., Jimenez R.E. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 124.Furie R., Petri M., Zamani O., Cervera R., Wallace J.D., Tegzova D. A phase 3, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits BLyS, in patients with systemic lupus erythematosus. Arthritis and Rheumatism. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Merrill J.T., Furie R.A., Wallace D.J., Stohl W., Winn Chatham W., Weinstein A. Sustained disease improvement and safety profile over 1745 patient-year experience (7 years) with belimumab in systemic lupus erythematosus patients. Arthritis and Rheumatism. 2012;64:S1110–S1111. [Google Scholar]
- 126.McKay J., Chwalinska-Sadowska H., Boling E., Valente R., Limanni A., Racewicz A. Belimumab (BmAb), a fully human monoclonal antibody to B-lymphocyte stimulator (BLyS), combined with standard of care therapy reduces the signs and symptoms of rheumatoid arthritis in a heterogeneous subject population. Arthritis and Rheumatism. 2005;52:S710–S711. [Google Scholar]
- 127.De Vita S., Seror R., Quartuccio L., Desmoulins F., Salvin S., Baron G. Efficacy of belimumab on non-malignant parotid swelling and systemic manifestations of Sjögren's syndrome: results of the Beliss study. Arthritis and Rheumatism. 2012;64:S926. [Google Scholar]
- 128.Mariette X., Quartuccio L., Seror R., Salvin S., Desmoulins F., Fabris M. Results of the Beliss study, the first open phase 2 study of belimumab in primary Sjogren's syndrome. Arthritis and Rheumatism. 2012;64:S1079. [Google Scholar]
- 129.Munafo A., Priestley A., Nestorov I., Visich J., Rogge M. Safety, pharmacokinetics and pharmacodynamics of atacicept in healthy volunteers. European Journal of Clinical Pharmacology. 2007;63:647–656. doi: 10.1007/s00228-007-0311-7. [DOI] [PubMed] [Google Scholar]
- 130.Benson M.J., Dillon S.R., Castigli E., Geha R.S., Xu S., Lam K.P. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. Journal of Immunology. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 131.Huard B., McKee T., Bosshard C., Durual S., Matthes T., Myit S. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. Journal of Clinical Investigation. 2008;118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carbonatto M., Yu P., Bertolino M., Vigna E., Steidler S., Fava L. Nonclinical safety, pharmacokinetics, and pharmacodynamics of atacicept. Toxicological Sciences. 2008;105:200–210. doi: 10.1093/toxsci/kfn105. [DOI] [PubMed] [Google Scholar]
- 133.Ramanujam M., Wang X., Huang W., Schiffer L., Grimaldi C., Akkerman A. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. Journal of Immunology. 2004;173:3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 134.Ramanujam M., Wang X., Huang W., Liu Z., Schiffer L., Tao H. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. Journal of Clinical Investigation. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gross J.A., Dillon S.R., Mudri S., Johnston J., Littau A., Roque R. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. Impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 136.Dall’Era M., Chakravarty E., Wallace D., Genovese M., Weisman M., Kavanaugh A. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis and Rheumatism. 2007;56:4142–4150. doi: 10.1002/art.23047. [DOI] [PubMed] [Google Scholar]
- 137.Pena-Rossi C., Nasonov E., Stanislav M., Yakusevich V., Ershova O., Lomareva N. An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus. Lupus. 2009;18:547–555. doi: 10.1177/0961203309102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ginzler E.M., Wax S., Rajeswaran A., Copt S., Hillson J., Ramos E. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Research and Therapy. 2012;14:R33. doi: 10.1186/ar3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Vollenhoven R.F., Kinnman N., Vincent E., Wax S., Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis and Rheumatism. 2011;63:1782–1792. doi: 10.1002/art.30372. [DOI] [PubMed] [Google Scholar]
- 140.Genovese M.C., Kinnman N., de La Bourdonnaye G., Pena Rossi C., Tak P.P. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis and Rheumatism. 2011;63:1793–1803. doi: 10.1002/art.30373. [DOI] [PubMed] [Google Scholar]
- 141.Tak P.P., Thurlings R.M., Rossier C., Nestorov I., Dimic A., Mircetic V. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis and Rheumatism. 2008;58:61–72. doi: 10.1002/art.23178. [DOI] [PubMed] [Google Scholar]
- 142.Van Vollenhoven R.F., Wax S., Copt S., Tak P.P. Safety and efficacy of atacicept in combination with rituximab in patients with rheumatoid arthritis: results from the atacicept for reduction of signs and symptoms in rheumatoid arthritis trial (III) Annals of the Rheumatic Diseases. 2012;71(Suppl. 3):183. [Google Scholar]
- 143.Vincent F.B., Morand E.F., Murphy K., Mackay F., Mariette X., Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Annals of the Rheumatic Diseases. 2012;72:165–178. doi: 10.1136/annrheumdis-2012-202545. [DOI] [PubMed] [Google Scholar]
- 144.Albert D., Dunham J., Khan S., Stansberry J., Kolasinski S., Tsai D. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Annals of the Rheumatic Diseases. 2008;67:1724–1731. doi: 10.1136/ard.2007.083162. [DOI] [PubMed] [Google Scholar]
- 145.Kim K.S., Park J.Y., Jou I., Park S.M. Functional implication of BAFF synthesis and release in gangliosides-stimulated microglia. Journal of Leukocyte Biology. 2009;86:349–359. doi: 10.1189/jlb.1008659. [DOI] [PubMed] [Google Scholar]
- 146.Chu V.T., Enghard P., Schurer S., Steinhauser G., Rudolph B., Riemekasten G. Systemic activation of the immune system induces aberrant BAFF and APRIL expression in B cells in patients with systemic lupus erythematosus. Arthritis and Rheumatism. 2009;60:2083–2093. doi: 10.1002/art.24628. [DOI] [PubMed] [Google Scholar]
- 147.Matthes T., Dunand-Sauthier I., Santiago-Raber M.L., Krause K.H., Donze O., Passweg J. Production of the plasma-cell survival factor a proliferation-inducing ligand (APRIL) peaks in myeloid precursor cells from human bone marrow. Blood. 2011;118:1838–1844. doi: 10.1182/blood-2011-01-332940. [DOI] [PubMed] [Google Scholar]
- 148.Lavie F., Miceli-Richard C., Ittah M., Sellam J., Gottenberg J.E., Mariette X. B-cell activating factor of the tumour necrosis factor family expression in blood monocytes and T cells from patients with primary Sjogren's syndrome. Scandinavian Journal of Immunology. 2008;67:185–192. doi: 10.1111/j.1365-3083.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- 149.Suzuki K., Setoyama Y., Yoshimoto K., Tsuzaka K., Abe T., Takeuchi T. Effect of interleukin-2 on synthesis of B cell activating factor belonging to the tumor necrosis factor family (BAFF) in human peripheral blood mononuclear cells. Cytokine. 2008;44:44–48. doi: 10.1016/j.cyto.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 150.Chung N.P., Matthews K., Klasse P.J., Sanders R.W., Moore J.P. HIV-1 gp120 impairs the induction of B cell responses by TLR9-activated plasmacytoid dendritic cells. Journal of Immunology. 2012;189:5257–5265. doi: 10.4049/jimmunol.1201905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moreaux J., Legouffe E., Jourdan E., Quittet P., Reme T., Lugagne C. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Woo S.J., Im J., Jeon J.H., Kang S.S., Lee M.H., Yun C.H. Induction of BAFF expression by IFN-gamma via JAK/STAT signaling pathways in human intestinal epithelial cells. Journal of Leukocyte Biology. 2013;93:363–368. doi: 10.1189/jlb.0412210. [DOI] [PubMed] [Google Scholar]
- 153.Alexaki V.I., Notas G., Pelekanou V., Kampa M., Valkanou M., Theodoropoulos P. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. Journal of Immunology. 2009;183:5948–5956. doi: 10.4049/jimmunol.0901186. [DOI] [PubMed] [Google Scholar]
- 154.Hamada M., Abe M., Miyake T., Kawasaki K., Tada F., Furukawa S. B cell-activating factor controls the production of adipokines and induces insulin resistance. Obesity (Silver Spring, Md.) 2011;19:1915–1922. doi: 10.1038/oby.2011.165. [DOI] [PubMed] [Google Scholar]
- 155.Kim M.Y., Kim D.H., Do M.S. B-cell-activating factor is a regulator of adipokines and a possible mediator between adipocytes and macrophages. Experimental and Molecular Medicine. 2013;45:e4. doi: 10.1038/emm.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alexaki V.I., Pelekanou V., Notas G., Venihaki M., Kampa M., Dessirier V. B-cell maturation antigen (BCMA) activation exerts specific proinflammatory effects in normal human keratinocytes and is preferentially expressed in inflammatory skin pathologies. Endocrinology. 2012;153:739–749. doi: 10.1210/en.2011-1504. [DOI] [PubMed] [Google Scholar]
- 157.Zonca M., Mancheno-Corvo P., DelaRosa O., Manes S., Buscher D., Lombardo E. APRIL and BAFF proteins increase proliferation of human adipose-derived stem cells through activation of Erk1/2 MAP kinase. Tissue Engineering Part A. 2012;18:852–859. doi: 10.1089/ten.TEA.2011.0316. [DOI] [PubMed] [Google Scholar]
- 158.Abe M., Kido S., Hiasa M., Nakano A., Oda A., Amou H. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: a rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia. 2006;20:1313–1315. doi: 10.1038/sj.leu.2404228. [DOI] [PubMed] [Google Scholar]
- 159.Moreaux J., Cremer F.W., Reme T., Raab M., Mahtouk K., Kaukel P. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood. 2005;106:1021–1030. doi: 10.1182/blood-2004-11-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Notas G., Alexaki V.I., Kampa M., Pelekanou V., Charalampopoulos I., Sabour-Alaoui S. APRIL binding to BCMA activates a JNK2-FOXO3-GADD45 pathway and induces a G2/M cell growth arrest in liver cells. Journal of Immunology. 2012;189:4748–4758. doi: 10.4049/jimmunol.1102891. [DOI] [PubMed] [Google Scholar]
- 161.Pelekanou V., Notas G., Theodoropoulou K., Kampa M., Takos D., Alexaki V.I. Detection of the TNFSF members BAFF, APRIL, TWEAK and their receptors in normal kidney and renal cell carcinomas. Analytical Cellular Pathology. 2011;34:49–60. doi: 10.3233/ACP-2011-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]