Abstract
Background
Multipathogen reverse transcription real-time PCR (RT-qPCR) platforms have proven useful in surveillance for acute respiratory illness (ARI) and study of respiratory outbreaks of unknown etiology. The TaqMan® Array Card (TAC, Life Technologies™), can simultaneously test 7 clinical specimens for up to 21 individual pathogens (depending on arrangement of controls and use of duplicate wells) by arrayed singleplex RT-qPCR on a single assay card, using minimal amounts of clinical specimens. A previous study described the development of TAC for the detection of respiratory viral and bacterial pathogens; the assay was evaluated against well-characterized analytical materials and a limited collection of human clinical specimens.
Objectives
We wished to compare TAC assay performance against standard individual RT-qPCR assays for respiratory viral detection, focusing on 10 viruses (adenovirus, human metapneumovirus, human parainfluenza viruses 1–4, influenza viruses A and B, respiratory syncytial virus, and rhinovirus) from a larger collection of human specimens.
Study design
We used specimens from 942 children with ARI enrolled systematically in a population-based, ARI surveillance study (New Vaccine Surveillance Network, NVSN).
Results
Compared with standard individual RT-qPCR assays, the sensitivity of TAC for the targeted viruses ranged from 54% to 95% (54%, 56%, and 75% for adenovirus, human parainfluenza viruses-1 and -2, respectively, and 82%–95% for the other viruses). Assay specificity was 99%, and coefficients of variation for virus controls ranged from 1.5% to 4.5%.
Conclusion
The TAC assay should prove useful for multipathogen studies and rapid outbreak response.
Keywords: Viral diagnostics, Respiratory viruses, Acute respiratory infection
1. Background
A number of multipathogen molecular platforms for the simultaneous detection of a wide array of pathogens are being developed to rapidly determine the cause of respiratory disease outbreaks of unknown etiology, and to facilitate surveillance for expanded etiologies of acute respiratory infection (ARI).1, 2, 3, 4, 5, 6, 7, 8 One such system, the TaqMan® Array Card (TAC), (Life Technologies™, Carlsbad CA), allows simultaneous, singleplex, reverse transcription real-time PCR (RT-qPCR) to be performed in a 384-well microfluidic card format. This card-based system has been used successfully to study brain endothelial cell gene expression, miRNA expression profiling, and biologic agent detection.9, 10, 11, 12 Flexible card formatting allows simultaneous testing of 6–7 clinical specimens for the presence of 21 or more targeted pathogens along with appropriate positive and negative assay controls.
Kodani et al.,2 reported the first application of TAC (formerly TLDA, TaqMan® Low Density Array) for infectious diseases diagnostics using a card designed for simultaneous detection of 21 different respiratory viral and bacterial pathogens. Advantages of the TAC system included simple and easy operation; reproducible and simultaneous analyses of multiple pathogens using small sample volumes; a level of detection generally within one ten-fold dilution of individual RT-qPCR assays; a lower risk of amplicon contamination as the system is entirely closed; and unlike other multiplex platforms, the ability to modify or replace individual pathogen assay primer/probes without requiring the re-optimization and validation of the entire panel.2 However, several gaps in knowledge remained after completion of that study.2 First, the frozen, archived clinical specimens utilized were collected from various studies and locations over a 10 year period, preventing evaluation of standardized methods or establishing epidemiologic associations. Second, the total nucleic acid (TNA) extracts were diluted in order to provide sufficient volume to perform comparisons of TAC with individual RT-qPCR assays; this factor, along with the frozen storage, may have contributed to the decreased sensitivity of the TAC assay relative to the individual RT-qPCR assays. Third, in order to achieve a consensus in the amplification conditions for all deployed individual RT-qPCR and TAC assays, a higher probe-annealing temperature of 60 °C was chosen as required for optimal bacterial assay specificity, but with the potential loss in sensitivity of some viral assays designed to run at 55 °C. Loss of viral sensitivity might have biased the results toward increased concordance among falsely negative individual RT-qPCR assays and TAC assays, resulting in an overestimate of TAC sensitivity for viruses.
2. Objectives
We wished to extend the work of Kodani et al.,2 by applying TAC to a larger field study of systematically collected specimens from children with well-characterized recent fever or acute respiratory infection (ARI). We used specimens from 942 children enrolled in the New Vaccine Surveillance Network (NVSN), which has conducted systematic, population-based surveillance for ARI under standardized protocols for the collection of epidemiologic and microbiologic data.13, 14, 15, 16, 17, 18 The sensitivity and specificity of viral detection by the TAC assay format was determined for 10 target respiratory viruses for which we had standard individual RT-qPCR assays in place, using the identical primer and probe sets for TAC and individual RT-qPCR (Table 1 ).13, 18, 19, 20, 21, 22, 23 The individual RT-qPCR assays were optimized for viral detection, using an annealing temperature of 55 °C, to maximize the sensitivity of the “gold standard”.
Table 1.
Primers and probes used in this study for both individual RT-qPCR and TAC assays.
| Assay | Primer/probe sequence (5′–3′)a | Source or reference |
|---|---|---|
| Adenovirus | F, GCC CCA GTG GTC TTA CAT GCA CAT C | 2 |
| R, GCC ACG GTG GGG TTT CTA AAC TT | ||
| P, TGC ACC AGA CCC GGG CTC AGG TAC TCC GA | ||
| HMPV | F, CAA GTG TGA CAT TGC TGA YCT RAA | 2 |
| R, ACT GCC GCA CAA CAT TTA GRA A | ||
| P, TGG CYG TYA GCT TCA GTC AAT TCA ACA GA | ||
| HPIV-1b | F, AGT TGT CAA TGT CTT AAT TCG TAT CAA T | 2 |
| R, TCG GCA CCT AAG TAA TTT TGA GTT | ||
| P, ATA GGC CAA AGA “T”TG TTG TCG AGA CTA TTC CAA | ||
| HPIV-2 | F, GCA TTT CCA ATC TAC AGG ACT ATG A | 2 |
| R, ACC TCC TGG TAT AGC AGT GAC TGA AC | ||
| P, CCA TTT ACC “T”AA GTG ATG GAA TCA ATC GCA AA | ||
| HPIV-3 | F, TGG YTC AAT CTC AAC AAC AAG ATT TAA G | 2 |
| R, TAC CCG AGA AAT ATT ATT TTG CC | ||
| P, CCC RTC TG“T” TGG ACC AGG GAT ATA CTA CAA A | ||
| HPIV-4 | F, CTG CCA AAT CGG CAA TTA AAC | 20 |
| R, CTG GCA GCA ATC ATA AGR TGA TTC | ||
| P, CA TTA TTA TCT CTG C“T”T TCC TTA CAG GCC ACA TCA | ||
| Influenza A | F, GAC CRA TCC TGT CAC CTC TGA C | 2, 23 |
| R, AGG GCA TTY TGG ACA AAK CGT CTA | ||
| P, TGC AGT CCT CGC TCA CTG GGC ACG | ||
| Influenza B | F, TCC TCA ACT CAC TCT TCG AGC G | 2, 22 |
| R, CGG TGC TCT TGA CCA AAT TGG | ||
| P, CCA ATT CGA GCA GCT GAA ACT GCG GTG | ||
| Rhinovirusc | F1, C(P)[A] GCC [T]GC GTG GC | 2, 27 |
| F2, C(P)[A] GCC [T]GC GTG GT | ||
| R, GAA ACA CGG ACA CCC AAA GTA | ||
| P, TCC TCC GGC CCC TGA ATG YGG C | ||
| RSV | F, GGC AAA TAT GGA AAC ATA CGT GAA | 2 |
| R, TCT TTT TCT AGG ACA TTG TAY TGA ACA G | ||
| P, CTG TGT ATG TGG AGC CTT CGT GAA GCT | ||
| RNP3 control | F, CCA AGT GTG AGG GCT GAA AAG | This work |
| R, TGT TGT GGC TGA TGA ACT ATA AAA GG | ||
| P, CC CCA GTC TCT GTC AGC ACT CCC TTC |
F, forward primer; R, reverse primer; P, probe; (P) = dP-CE (pyrimidine derivative); [A] = LNA-dA, [T] = LNA-dT [Locked Nucleic Acid (LNA) primers, Exiqon, Woburn, MA]. TaqMan® probes labeled at the 5′-end with the reporter molecule 6-carboxyfluorescein (FAM) and at the 3′-end with Black Hole Quencher® 1 (BHQ1; Biosearch Technologies Inc., Novato, CA) with the exception of probes that are internally quenched with BHQ1 located at a modified “T” residue and a 3′ terminal Spacer 3 (Biosearch Technologies, Inc.) to prevent probe extension by Taq polymerase.
Subsequent to this study, the HPIV-1 primers were redesigned to improve assay performance at 60 °C annealing temperature: F, ACA AGT TGT CAA YGT CTT AAT TCR TAT; R, TCG GCA CCT AAG TAR TTY TGA GTT.
The rhinovirus RT-qPCR assay may cross-react with some enterovirus strains if present at high viral load.
3. Study design
3.1. Overall study design
3.1.1. Population-based surveillance and study cohort
The NVSN conducted surveillance for ARI among children during 2000–2009 in 3 US counties that include the cities of Rochester, New York, Nashville, Tennessee, and Cincinnati, Ohio. The overall surveillance design and laboratory methodology have been described previously.13, 14, 15, 16, 17, 18, 19 Since the distribution of viruses has been relatively similar across the 3 counties and resources were limited, we utilized only specimens from the Rochester (Monroe County) New York NVSN site for the study cohort. TAC and individual RT-qPCR assay results were compared for in the cohort, which was comprised of 942 children with fever or acute respiratory infection: 281 hospitalized children <5 years of age enrolled from October, 2008 through September, 2009, and 661 children <13 years of age seen in the Emergency Department or Ambulatory Clinic enrolled from October, 2008 through June, 2009.
3.1.2. Specimen and data collection
As part of standard NVSN protocols, informed consent from parents/guardians, detailed epidemiologic data from parent interviews, provider immunization data, and both mid-turbinate nasal and throat swab specimens (sterile non-flocked polyester-tipped applicators, Puritan Medical, Guilford, ME) were collected from all enrolled children. The swabs were combined in Universal Transport Medium (Diagnostic Hybrids, Athens, OH), refrigerated and added to lysis buffer (Qiagen Lysis Buffer AVL, QIAGEN Inc., Valencia, CA) within 24 h; aliquots of original sample and sample in lysis buffer were stored at −80 °C. The lysis buffer served to prevent viral particle degradation during sample freezing, to inactivate RNases present in samples, and to stabilize intact viral RNA for future analysis.
3.2. Molecular virology
All laboratory testing for this work was performed at the University of Rochester Medical Center.
3.2.1. Total nucleic acid extraction (TNA)
TNA was extracted from 140 μL of each specimen aliquot in lysis buffer, to a final eluate volume of 100 μL, using the QIAamp Viral RNA Mini Kit (QIAGEN) on a QIAcube robot (QIAGEN). To minimize freeze-thawing, three TNA aliquots for each extracted clinical specimen were prepared and stored at −80 °C until use.
3.2.2. Individual RT-qPCR assays
Individual RT-qPCR assays for 10 viruses (adenovirus, human metapneumovirus [HMPV], human parainfluenza viruses 1–4 [HPIV-1-4], influenza viruses A and B, respiratory syncytial virus [RSV], and rhinovirus) and the human RNase P (RNP) reference gene control were used for comparison with TAC (Table 1). All individual RT-qPCR assays, except those for HPIV-4 and influenza viruses A and B, were performed on an Applied Biosystems® 7500 Real-Time PCR system (Life Technologies™), using the AgPath-ID™ One-Step RT-PCR (Life Technologies™) kit previously described by Kodani et al.,2 but modified to use standard optimal thermocycling conditions for viral assays: 45 °C for 10 min, 95 °C for 10 min, and 45 cycles of 95 °C for 15 s and 55 °C for 45 s. The individual RT-qPCR for HPIV4 was performed on a Bio-Rad MyiQ™ RT-qPCR thermal cycler (Bio-Rad Laboratories, Hercules, CA), using the conditions noted above. Influenza viruses A and B assays were performed on the Bio-Rad MyiQ™ RT-qPCR thermal cycler, using SuperScript® III Platinum® One-Step RT-PCR chemistry (Life Technologies™) with the following thermocycling conditions: 50 °C for 30 min, 95 °C for 2 min, and 45 cycles of 95 °C for 15 s and 55 °C for 30 s.23 Cycle threshold (CT) values were assigned to specimens exhibiting exponential fluorescence curves and acceptable control results (i.e., negative results for no-template controls and positive results for RNP reference gene control). For this study, a CT value of ≤40 was considered a positive test result.
3.2.3. TAC assay
The panel of TAC assays used in this study was modified from Kodani et al.,2 and included 16 viral and 7 bacterial respiratory pathogens (adenovirus, HMPV, HPIV 1-4, influenza viruses A, B, and C, RSV, rhinovirus, human coronaviruses 229E, NL63, OC43, HKU1, enterovirus, Bordetella pertussis, Chlamydophila pneumoniae, Haemophilus influenzae, Legionella pneumophila, Mycoplasma pneumoniae, Streptococcus pneumoniae, and S. pyogenes). Because of resource limitations, individual RT-qPCR assays could be performed for only 10 viruses; TAC assay results presented herein therefore are limited to those for the 10 viral pathogens (Table 1). The TAC cards were created and handled in the same fashion as in the Kodani et al. study,2 with identical primers and probes used (Table 1) except that in this work HPIV-4 was added to our new TAC cards and to our panel of individual RT-qPCR assays. In addition, the rhinovirus primers and probes were slightly modified for both our TAC and RT-qPCR (Table 1). Our TAC cards also contained primers & probes for influenza C and 4 coronavirus types, but as noted, these are not relevant for the current report.
All synthetic oligonucleotide primer/probes used for individual RT-qPCR assays and TAC were designed by the Centers for Disease Control and Prevention (CDC) and synthesized by BioSearch Technologies Inc., Novato, CA. Primer/probes for the 10 selected virus assays were spotted in duplicate on each card by Life Tchnologies™; quality control assays were performed by both the company and CDC. All viral assays on the two lots of TAC cards used in this study were qualified for use by the using standard reference control reagents. In addition to the viral primers/probes, a proprietary internal positive control (IPC) assay was spotted in duplicate with its oligonucleotide template (Life Technologies™) to monitor for specimen inhibitors of RT-qPCR, and the RNP assay noted above was spotted singly to monitor specimen quality. An assay for human glyceraldehyde 3-phosphate dehydrogenase also was included on each card by the manufacturer for quality control of card production lots; results from this assay were not included in our analysis. Each card tested included 7 clinical specimens and 1 no-template negative control (reaction mix plus water) to monitor for assay contamination. Positive control pools (viral genome or synthetic templates) were periodically run at pre-specified intervals (including at the beginning, mid-point, and end of the study) on cards to monitor pathogen- and RNP-specific assay performance. All controls were monitored throughout the study to ensure test validity.
For each specimen tested, 20 μL of TNA extract and 80 μL of AgPath-ID™ One-Step RT-PCR master mix were loaded into one of the 8 TAC ports. The cards were centrifuged twice to distribute the TNA/master mix throughout the 48 inter-connected wells (per specimen) containing the pre-spotted primer/probes. Each card was then sealed and run on a ViiA™-7 Real-time PCR System (Life Technologies™) using the following thermocycling conditions: 45 °C for 10 min, 94 °C for 10 min, and 45 cycles of 94 °C for 30 s followed by 60 °C for 1 min as described by Kodani et al.2 Assignment of CT values and positive test results were as indicated above for individual RT-qPCR assays.
3.3. Statistical analyses
CT values are presented as the mean of two duplicate wells for specimens yielding positive assays. For specimens with discordant positive and negative duplicate well CT values (<8% of positive viral specimens by TAC), only the positive value was assigned. Sensitivity and specificity were calculated using the individual RT-qPCR results as the “gold standard”. The 95% confidence intervals (95% CI) around the point estimates for sensitivity were calculated according to the efficient-scores method (corrected for continuity)24, 25, 26 using the VassarStats: Website for Statistical Computation (http://vassarstats.net/). Grouped data are presented as means ± SD or as medians with range or interquartile range (IQR) as noted.
4. Results
4.1. TAC sensitivity
Of the 942 clinical specimens tested by individual RT-qPCR assays, 713 (76%) were positive for one or more of the 10 respiratory viruses evaluated; 109 (12%) specimens were positive for more than one virus for a total of 837 viruses detected. The comparative sensitivities of the TAC assay for each of the 10 individual target virus RT-qPCR assays are shown in Table 2 . The overall sensitivity for all viruses of TAC was 83%, with individual virus assay sensitivities ranging from 54 to 95%. The TAC assays for influenza A and B, HMPV, and RSV exhibited the highest sensitivities, ranging from 86% to 95%, whereas the assays for adenovirus and HPIV-1 had the lowest sensitivities (54% and 56%, respectively).
Table 2.
Comparison of individual RT-qPCR and TAC assay results.
| Virus | Characteristic | Individual RT-qPCR Assay | TAC assay | % Sensitivity (95% CI)a |
|---|---|---|---|---|
| Adenovirus | No. positive | 81 | 44 | 54 (43–65) |
| Mean CT ± SD | 30.74 ± 6.31 | 30.60 ± 5.17 | ||
| Median CT (Range) | 33.27 (15.03–38.27) | 31.10 (16.31–39.58) | ||
| HMPV | No. positive | 73 | 63 | 86 (76–93) |
| Mean CT ± SD | 28.03 ± 6.11 | 29.34 ± 5.09 | ||
| Median CT (Range) | 28.32 (13.04–39.44) | 29.03 (18.02–38.63) | ||
| HPIV-1 | No. positive | 16 | 9 | 56 (31–79) |
| Mean CT ± SD | 30.68 ± 4.84 | 35.88 ± 3.74 | ||
| Median CT (Range) | 30.00 (22.30–39.40) | 36.01 (29.63–40.00) | ||
| HPIV-2 | No. positive | 4 | 3 | 75 (22–99) |
| Mean CT ± SD | 32.14 ± 6.24 | 33.31 ± 5.36 | ||
| Median CT (Range) | 33.97 (23.15–37.48) | 36.10 (27.13–36.69) | ||
| HPIV-3 | No. positive | 44 | 36 | 82 (67–91) |
| Mean CT ± SD | 24.88 ± 7.34 | 25.30 ± 4.50 | ||
| Median CT (Range) | 24.07 (13.28–38.01) | 25.31 (18.24–34.82) | ||
| HPIV-4 | No. positive | 12 | 11 | 92 (60–99) |
| Mean CT ± SD | 32.57 ± 4.44 | 30.74 ± 4.34 | ||
| Median CT (Range) | 33.15 (24.93–39.47) | 32.04 (23.15–36.44) | ||
| Influenza A | No. positive | 115 | 109 | 95 (89–98) |
| Mean CT ± SD | 29.62 ± 5.22 | 27.77 ± 4.67 | ||
| Median CT (Range) | 29.28 (18.40–39.62) | 27.63 (18.23–36.47) | ||
| Influenza B | No. positive | 25 | 23 | 92 (72–99) |
| Mean CT ± SD | 30.38 ± 5.53 | 26.74 ± 4.43 | ||
| Median CT (Range) | 29.61 (22.09–39.64) | 27.22 (20.05–34.19) | ||
| Rhinovirus | No. positive | 271 | 218 | 80 (75–84) |
| Mean CT ± SD | 28.58 ± 5.40 | 30.23 ± 4.61 | ||
| Median CT (Range) | 28.60 (12.72–39.69) | 30.81 (17.47–40.00) | ||
| RSV | No. positive | 201 | 189 | 94 (90–97) |
| Mean CT ± SD | 23.65 ± 4.94 | 24.78 ± 4.09 | ||
| Median CT (Range) | 22.46 (14.75–39.36) | 24.09 (16.20–35.35) |
Sensitivity of TAC assay calculated assuming individual RT-qPCR test results as “gold standard”.
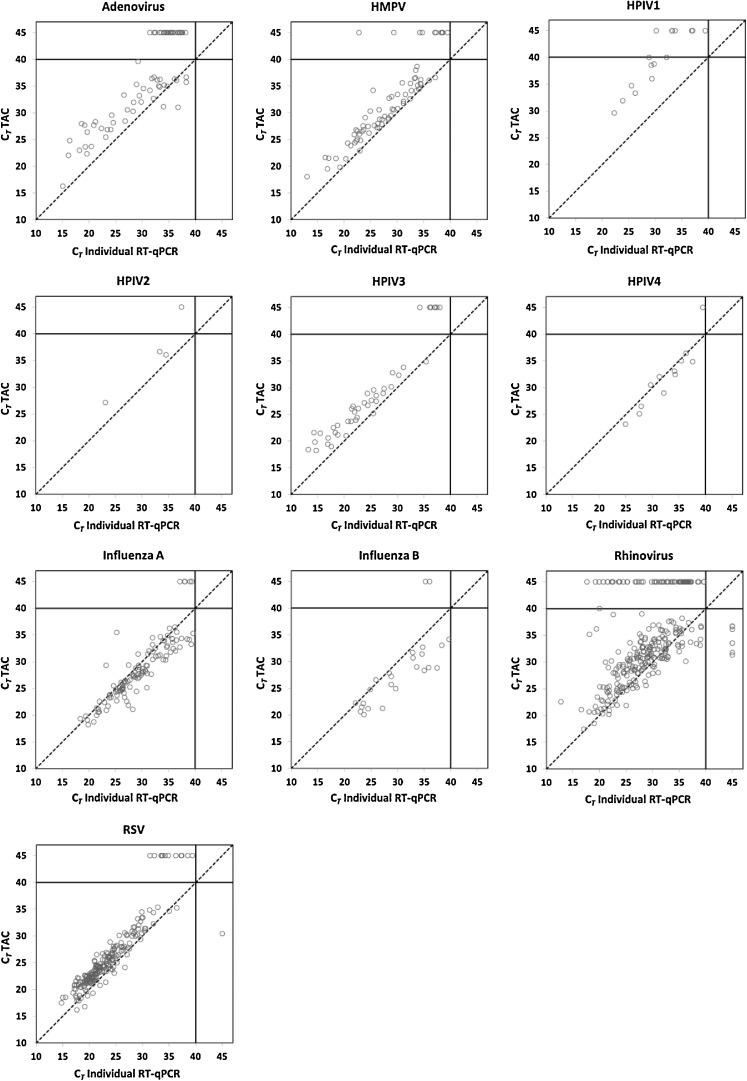
In general, CT values for TAC were similar to those of the individual RT-qPCR assays (Table 2), and the linear concordance between the two systems was high (Fig. 1 ). TAC CT values tended to be slightly higher than individual RT-qPCR values, as denoted in Fig. 1 by values above the reference line, except for influenza viruses A and B. When TAC results were most discordant with individual RT-qPCR assay results, it nearly always was at the higher range of the individual RT-qPCR CT values (30–40 CT); TAC was almost always positive when the individual RT-qPCR CT values were <30, with the notable exception of the rhinovirus assay (Fig. 1).
Fig. 1.
TAC CT values plotted against those of individual RT-qPCR assays by target virus. For better visualization of the results, all negative test results for either RT-qPCR or TAC were assigned a CT value of 45. The dotted line represents the line of identity between the individual RT-qPCR and TAC results.
During the study it was noted that the HPIV-1 TAC performance lagged behind that of the other TAC assays. Thirteen samples positive for HPIV-1 by individual RT-qPCR were available for further testing, and retested by TAC with annealing temperatures of 55 °C. An improvement in TAC assay HPIV-1 performance was obtained at the lower annealing temperature, with assay sensitivity increasing from 69% (9/13) to 100% (13/13).
A comparison of the point estimates of sensitivity, as well as the number of true positive viruses tested (as defined by positive individual RT-qPCR results), from both the work of Kodani et al.2 and the present work is presented in Table 3 (95% CI shown in Table 3 for the results of Kodani et al.2 were recalculated by the same methods as those for the present results).
Table 3.
Comparison of TAC sensitivity in this and previous worka
| Virus | Characteristic | TAC assay—This Work | TAC assay—Kodani et alb |
|---|---|---|---|
| Adenovirus | No. true positives | 81 | 28 |
| % Sensitivity (95% CI) | 54 (43–65) | 97 (80–100) | |
| HMPV | No. true positives | 73 | 19 |
| % Sensitivity (95% CI) | 86 (76–93) | 95 (73–100) | |
| HPIV-1 | No. true positives | 16 | 7 |
| % Sensitivity (95% CI) | 56 (31–79)c | 100 (56–100) | |
| HPIV-2 | No. true positives | 4 | 11 |
| % Sensitivity (95% CI) | 75 (22–99) | 79 (49–94) | |
| HPIV-3 | No. true positives | 44 | 21 |
| % Sensitivity (95% CI) | 82 (67–91) | 95 (75–100) | |
| HPIV-4 | No. true positives | 12 | Not tested |
| % Sensitivity (95% CI) | 92 (60–99) | ||
| Influenza A | No. true positives | 115 | 22 |
| % Sensitivity (95% CI) | 95 (89–98) | 92 (72–99) | |
| Influenza B | No. true positives | 25 | 14 |
| % Sensitivity (95% CI) | 92 (72–99) | 100 (73–100) | |
| Rhinovirus | No. positives | 271 | 41 |
| % Sensitivity (95% CI) | 80 (75–84) | 98 (86–100) | |
| RSV | No. true positives | 201 | 30 |
| % Sensitivity (95% CI) | 94 (90–97) | 100 (86–100) |
Sensitivity of TAC assay for each work calculated assuming individual RT-qPCRtest results as “gold standard”.
FromKodani et al.2, with recalculated 95% CIs calculated by efficient-score method, corrected for continuity (see Methods).
Rose to 100% (95% CI 76–100) when new primer/probe set used—see text.
4.2. TAC specificity
There were 8 instances of apparent false positive TAC assay results (1 RSV and 7 rhinovirus positives) among samples negative by RT-qPCR, for an overall TAC specificity of 99%.
4.3. TAC precision and reproducibility
Throughout the study, CT values of the positive control pools were tightly distributed, with coefficients of variation (CV) ranging from 1.5 to 4.5% for each target virus and for the RNP gene control (Table 4 ). CT values for the IPC were also tightly distributed (CV ≤3%), and had similar mean and median values whether respiratory specimen TNA extracts or water (no-template control) were present, indicating the absence of RT-qPCR inhibitors in the study specimens (Table 4).
Table 4.
Reproducibility of TAC assay controls throughout the study.
| CT |
||||
|---|---|---|---|---|
| Control | No. tested | Mean ± SD | Median (IQR) | CV (%) |
| Virus positive control | ||||
| Adenovirus | 24 | 30.08 ± 0.71 | 30.21 (29.57–30.61) | 2.36 |
| HMPV | 24 | 30.33 ± 1.14 | 29.96 (29.60–30.43) | 3.76 |
| HPIV-1 | 24 | 29.18 ± 0.56 | 29.15 (28.25–29.44) | 1.92 |
| HPIV-2 | 24 | 30.16 ± 0.47 | 30.10 (29.87–30.47) | 1.56 |
| HPIV-3 | 24 | 31.24 ± 0.97 | 31.18 (30.72–31.54) | 3.10 |
| HPIV-4 | 24 | 30.56 ± 1.00 | 30.57 (29.62–31.22) | 3.27 |
| Influenza A | 8 | 26.57 ± 1.17 | 26.24 (25.74–26.92) | 4.42 |
| Influenza B | 8 | 31.67 ± 1.44 | 29.97 (30.69–32.29) | 4.55 |
| Rhinovirus | 24 | 29.82 ± 1.11 | 29.95 (29.15–30.26) | 3.72 |
| RSV | 24 | 29.89 ± 0.69 | 29.87 (29.51–30.26) | 2.31 |
| RNP reference gene controla | 24 | 29.09 ± 0.88 | 29.06 (28.68–29.73) | 3.03 |
| Internal positive control | ||||
| Clinical specimens | 942 | 25.19 ± 0.67 | 25.28 (24.78–25.63) | 2.68 |
| No-template control (water) | 517 | 25.71 ± 0.78 | 25.68 (25.20–26.22) | 3.03 |
Virus positive control pools contained human nucleic acid for RNP control.
5. Discussion
We found that the TAC assay offers a simple, sensitive, reproducible and moderate throughput system for simultaneous detection of multiple respiratory pathogens. TAC assays for all pathogens were accomplished with only 20 μL of nucleic acid extract and 80 μL of reaction mix per patient sample, with card setup and run times of ~3 h, excluding sample extraction; 3 cards (up to 21 samples) could be feasibly run on a single machine in a normal workday. In contrast, completing up to 21 individual RT-qPCR reactions for each clinical sample would require 125 μL of nucleic acid extract and 420 μL of reaction mix, as well as a much greater amount of technician time, precluding throughput of even 1 clinical specimen tested for all pathogens per day. Additional advantages of the TAC assay were a combined datafile output of all pathogens tested per specimen, such that merging of results of separate RT-qPCR assays was not required; and the avoidance of pipetting errors in the multi-well plate RT-qPCR assays by the automated setup of the TAC array assay. Indeed, excellent reproducibility of all controls was maintained throughout the duration of the study.
Among the 942 clinical specimens tested by both individual RT-qPCR and TAC assays for the 10 target viruses, the overall TAC sensitivity and specificity were 83% and 99%, respectively. Kodani et al.,2 found TAC sensitivity and specificity to be 95% and 98% in a smaller test set of 192 virus-positive specimens for 9 of the same target viruses (HPIV-4 not included). Our analysis yielded similar estimates of sensitivity and specificity for each virus assay, except for lower sensitivity for adenovirus, rhinovirus, and HPIV-1 (Table 3). Part of this discrepancy might be explained by the fact that Kodani et al.,2 used an annealing temperature of 60 °C for their individual RT-qPCR assays, which may have decreased the RT-qPCR sensitivity, thus making it more concordant with any negative TAC assay results (i.e., more false negative RT-qPCR results would have increased the concordance with false negative TAC results, raising the estimates of TAC sensitivity for adenoviruses and rhinoviruses). An additional factor may be greater strain variation in adenoviruses and rhinoviruses, reducing the performance of the TAC primers/probes for those viruses when analyzed at 60 °C rather than at 55 °C. We were not able to pursue studies of the effects of adenovirus and rhinovirus strain variation and annealing temperature on the TAC assay due to limited resources.
We also noted decreased TAC assay sensitivity for HPIV-1. Since one of the advantages of the closed TAC system is the ability to modify or replace primer/probes for individual pathogens without requiring the re-optimization and validation of the entire panel,2 we have updated the HPIV-1 primers for future TAC cards to improve assay sensitivity at 60 °C (Table 1).
One major limitation of this study is that we did not perform individual RT-qPCR analyses for several of the viral and bacterial pathogens on the TAC array, and were therefore unable to study these assays in this work; however, Kodani et al. found good performance for detection of bacterial pathogens in their study.2 Also, low numbers of positive clinical specimens for HPIV1, 2, and 4 and influenza B virus led to wide 95% confidence intervals around the point estimates for TAC sensitivity. However, it should be noted that the 95% confidence intervals around the estimates of viral sensitivity overlapped for our results and those of Kodani et al2 for these and nearly all other viruses tested (Table 3).
We were not able to compare the TAC performance directly with other array technologies such as the FilmArray® Respiratory Panel (BioFire Diagnostics, Inc., Salt Lake City, UT) or the Luminex xTAG® Respiratory Viral Panel (Luminex Corporation, Austin, TX). The daily throughput of patient samples may be several-fold greater with the TAC assay, but the instrumentation costs may be greater as well. As clinical laboratories move toward implementation of array technology, such inter-assay comparative data would be informative.
The major strength of this study was the use of a large collection of field specimens from a well-characterized group of children enrolled in the NVSN to more fully examine the clinical performance of the TAC assay in a more “real-world” clinical diagnostic laboratory setting, with the individual RT-qPCR optimized for maximum sensitivity of the “gold standard”. The rich NVSN epidemiologic and clinical database, combined with TAC assay results, should permit an evaluation of the clinical relevance of co-detection of two or more pediatric respiratory pathogens,1, 2, 3, 5, 6, 7, 21 including those not commonly studied (e.g., influenza C, HPIV-4, human coronaviruses). As new pathogens become recognized and RT-qPCR assays become available, they can be quickly and easily introduced into the TAC array, and used to investigate outbreaks of respiratory illness of unknown etiology.21, 27, 28
Funding
Supported in part through cooperative agreement U01-IP-000017 from the Centers for Disease Control and Prevention (CDC); and by the U.S. Department of Health and Human Services (DHHS), Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (BARDA) for advanced product development through Pandemic Influenza Diagnostic funding.
Competing interests
The authors declare no financial conflicts of interest with this work.
Ethical approval
This work was approved by the Institutional Review Boards of the University of Rochester and the Centers for Disease Control and Prevention.
Acknowledgments
We thank the following members of the New Vaccine Surveillance Network for their technical assistance with this work: Jennifer Carnahan, Laura Cole, Aaron Curns, Lisa Denmark, Geraldine Lofthus, Andrea S. Marino, and Jessica Moore. We thank NVSN Co-Investigators Kathryn Edwards, John Williams, and Mary Staat for helpful discussions. We thank Barry Fields, Maja Kodani, Stephen Lindstrom, and M. Steven Oberste from the CDC for providing reagents. The authors greatly benefitted from the research studies, mentorship, and friendship of the late Dr. Caroline Breese Hall.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or views of the CDC or the DHHS. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the CDC or the DHHS.
References
- 1.Brunstein J.D., Cline C.L., McKinney S., Thomas E. Evidence from multiplex molecular assays for complex multipathogen interactions in acute respiratory infections. J Clin Microbiol. 2008;46:97–102. doi: 10.1128/JCM.01117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kodani M., Yang G., Conklin L.M., Travis T.C., Whitney C.G., Anderson L.J. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49:2175–2182. doi: 10.1128/JCM.02270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeffelholz M.J., Pong D.L., Pyles R.B., Xiong Y., Miller A.L., Bufton K.K. Comparison of the FilmArray respiratory panel and Prodesse real-time PCR assays for detection of respiratory pathogens. J Clin Microbiol. 2011;49:4083–4088. doi: 10.1128/JCM.05010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohrmalm C., Eriksson R., Jobs M., Simonson M., Stromme M., Bondeson K. Variation-tolerant capture and multiplex detection of nucleic acids: application to detection of microbes. J Clin Microbiol. 2012;50:3208–3215. doi: 10.1128/JCM.06382-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen R.R., Schinkel J., Koekkoek S., Pajkrt D., Beld M., de Jong M.D. Development and evaluation of a four-tube real time multiplex PCR assay covering fourteen respiratory viruses, and comparison to its corresponding single target counterparts. J Clin Virol. 2011;51:179–185. doi: 10.1016/j.jcv.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen R.R., Wieringa J., Koekkoek S.M., Visser C.E., Pajkrt D., Molenkamp R. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pabbaraju K., Wong S., Tokaryk K.L., Fonseca K., Drews S.J. Comparison of the Luminex xTAG Respiratory Viral Panel with xTAG Respiratory Viral Panel Fast for diagnosis of respiratory virus infections. J Clin Microbiol. 2011;49:1738–1744. doi: 10.1128/JCM.02090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rand K.H., Rampersaud H., Houck H.J. Comparison of two multiplex methods for detection of respiratory viruses: FilmArray RP and xTAG RVP. J Clin Microbiol. 2011;49:2449–2453. doi: 10.1128/JCM.02582-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demarest T.G., Murugesan N., Shrestha B., Pachter J.S. Rapid expression profiling of brain microvascular endothelial cells by immuno-laser capture microdissection coupled to TaqMan((R)) low density array. J Neurosci Methods. 2012;206:200–204. doi: 10.1016/j.jneumeth.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurley J., Roberts D., Bond A., Keys D., Chen C. Stem-loop RT-qPCR for microRNA expression profiling. Methods Mol Biol. 2012;822:33–52. doi: 10.1007/978-1-61779-427-8_3. [DOI] [PubMed] [Google Scholar]
- 11.Rachwal P.A., Rose H.L., Cox V., Lukaszewski R.A., Murch A.L., Weller S.A. The potential of TaqMan Array Cards for detection of multiple biological agents by real-time PCR. PloS ONE. 2012;7:e35971. doi: 10.1371/journal.pone.0035971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weller S.A., Cox V., Essex-Lopresti A., Hartley M.G., Parsons T.M., Rachwal P.A. Evaluation of two multiplex real-time PCR screening capabilities for the detection of Bacillus anthracis, Francisella tularensis and Yersinia pestis in blood samples generated from murine infection models. J Med Microbiol. 2012;61:1546–1555. doi: 10.1099/jmm.0.049007-0. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg G.A., Hall C.B., Iwane M.K., Poehling K.A., Edwards K.M., Griffin M.R. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154:694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Poehling K.A., Edwards K.M., Weinberg G.A., Szilagyi P., Staat M.A., Iwane M.K. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 15.Iwane M.K., Edwards K.M., Szilagyi P.G., Walker F.J., Griffin M.R., Weinberg G.A. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 16.Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin M.R., Walker F.J., Iwane M.K., Weinberg G.A., Staat M.A., Erdman D.D. Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. Pediatr Infect Dis J. 2004;23:S188–S192. doi: 10.1097/01.inf.0000144660.53024.64. [DOI] [PubMed] [Google Scholar]
- 18.Williams J.V., Edwards K.M., Weinberg G.A., Griffin M.R., Hall C.B., Zhu Y. Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201:1890–1898. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg G.A., Erdman D.D., Edwards K.M., Hall C.B., Walker F.J., Griffin M.R. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189:706–710. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
- 20.Sakthivel S.K., Whitaker B., Lu X., Oliveira D.B., Stockman L.J., Kamili S. Comparison of fast-track diagnostics respiratory pathogens multiplex real-time RT-PCR assay with in-house singleplex assays for comprehensive detection of human respiratory viruses. J Virol Methods. 2012;185:259–266. doi: 10.1016/j.jviromet.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers For Disease Control And Prevention Unexplained Respiratory Disease Outbreak working group activities - worldwide, March 2007-September 2011. MMWR Morb Mortal Wkly Rep. 2012;61:480–483. [PubMed] [Google Scholar]
- 22.Kim C., Ahmed J.A., Eidex R.B., Nyoka R., Waiboci L.W., Erdman D. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PloS ONE. 2011;6:e21610. doi: 10.1371/journal.pone.0021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shu B., Wu K.H., Emery S., Villanueva J., Johnson R., Guthrie E. Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 A (H1N1) pandemic influenza virus. J Clin Microbiol. 2011;49:2614–2619. doi: 10.1128/JCM.02636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newcombe R.G. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Newcombe R.G. Know your limitations: not just for clinicians. Estimation of confidence intervals is not straightforward. J Public Health Med. 1999;21:481–482. doi: 10.1093/pubmed/21.4.481-a. [DOI] [PubMed] [Google Scholar]
- 26.Newcombe R.G. Confidence intervals for proportions – the Cinderella of statistical analyses. Clin Oncol. 2008;20:92–93. doi: 10.1016/j.clon.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Centers For Disease Control And Preventio Clusters of acute respiratory illness associated with human enterovirus 68 – Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1301–1304. [PubMed] [Google Scholar]
- 28.Jacobson L.M., Redd J.T., Schneider E., Lu X., Chern S.W., Oberste M.S. Outbreak of lower respiratory tract illness associated with human enterovirus 68 among American Indian children. Pediatr Infect Dis J. 2012;31:309–312. doi: 10.1097/INF.0b013e3182443eaf. [DOI] [PubMed] [Google Scholar]