Abstract
Genetic variants have been reported to cause several genetic diseases. Various genotyping assays have been developed for diagnostic and screening purposes but with certain limitations in sensitivity, specificity, cost effectiveness and/or time savings. Since the discovery of ligase chain reaction (LCR) in the late nineties, it became one of the most favored platforms for detecting these variants and also for genotyping low abundant contaminants. Recent and powerful modifications with the integration of various detection strategies such as electrochemical and magnetic biosensors, nanoparticles (NPs), quantum dots, quartz crystal and leaky surface acoustic surface biosensors, DNAzyme, rolling circle amplification (RCA), strand displacement amplification (SDA), surface enhanced raman scattering (SERS), chemiluminescence and fluorescence resonance energy transfer have been introduced to both LCR and ligation based amplifications to enable high-throughput and inexpensive multiplex genotyping with improved robustness, simplicity, sensitivity and specificity. In this article, classical and up to date modifications in LCR and ligation based amplifications are critically evaluated and compared with emphasis on points of strength and weakness, sensitivity, cost, running time, equipment needed, applications and multiplexing potential. Versatile genotyping applications such as genetic diseases detection, bacterial and viral pathogens detection are also detailed. Ligation based gold NPs biosensor, ligation based RCA and ligation mediated SDA assays enhanced detection limit tremendously with a discrimination power approaching 1.5 aM, 2 aM and 0.1 fM respectively. MLPA (multiplexed ligation dependent probe amplification) and SNPlex assays have been commercialized for multiplex detection of at least 48 SNPs at a time. MOL-PCR (multiplex oligonucleotide ligation) has high-throughput capability with multiplex detection of 50 SNPs/well in a 96 well plate. Ligase detection reaction (LDR) is one of the most widely used LCR versions that have been successfully integrated with several detection strategies with improved sensitivity down to 0.4 fM.
Abbreviation: ACV, Altering Current Voltammetry; AG-LCR, Asymmetric Gap-Ligase Chain Reaction; ALP-SA, Alkaline Phosphatase-Streptavidin; ASO, Allele Specific Oligonucleotide; AuNP, Gold Nanoparticles; BRCA1, Breast Cancer1; CCP, Cationic Conjugated Polymer; cDNA, Complementary DNA; CE, Capillary Electrophoresis; CE-fSSCP, Capillary Electrophoresis- fluorescent Single Stranded Conformational Polymorphism; CFTR, Cystic Fibrosis Transmembrane Conductance Regulator; CGE, Capillary Gel Electrophoresis; CP, Capture Probes; cPLP, circular Padlock Probe; CRET, Chemiluminescence Resonance Energy Transfer; CTV, Citrus tristeza virus; DOL, Dye-labeled Oligonucleotide Ligation; eLCR, electrochemical Ligase Chain Reaction; FO-SPR, Fiber Optic Surface Plasmon Resonance; FRET, Fluorescence Resonance Energy Transfer; G-LCR, Gap-Ligase Chain Reaction; HBV, Hepatitis B Virus; HCC, Hepatocellular Carinoma; HFE, Human Hemochromatosis Protein; HRCA, Hyberbranching Rolling Circle Amplification; HRP, Horse-Radish Peroxidase; HRP-SA, Horse-Radish Peroxidase-Streptavidin; ICP-MS, Inductively Coupled Plasma-Mass Spectrometry; LCR, Ligase Chain Reaction; LDR, Ligase Detection Reaction; LHON, Leber’s Hereditary Optic Neuropathy; LIF, Laser Induced Fluorescence; LSAW, Leaky Surface Acoustic Wave; LSO, Locus Specific Oligonucleotide; MEIA, Microparticle Capture Enzyme Immunoassay; MLPA, Multiplex Ligation Dependent Probe Amplification; MOL-PCR, Multiplexed Oligonucleotide Ligation PCR; MS-MLPA, Methylation-Specific Multiplex Ligation Dependent Probe Amplification; MTBC, Mycobacterium tuberculosis complex; NAT2, N-acetyltransferase-2 gene; NP, Nanoparticles; ONS, Oligonucleotide incorporated Nonfouling Surface; PCR, Polymerase Chain Reaction; PLP, Padlock Probe; PRNP, PRioN Protein; QCM, Quartz Crystal Microbalance; QD, Quantum Dots; QG-LCR, Quantitative Gap-Ligase Chain Reaction; qPCR, quantitative Polymerase Chain Reaction; RCA, Rolling Circle Amplification; RSV, Respiratory Cyntical Virus; RT-MLPA, Reverse Transcriptase-Multiplex Ligation Dependent Probe Amplification; SAPE, Streptavidin-R-phycoerythrin; SARS, Severe Acute Respiratory Syndrome; SDA, Strand Displacement Amplification; SERS, Surface Enhanced Raman Scattering; SNP, Single Nucleotide Polymorphism; SSCP, Single Stranded Conformational Polymorphism; WNV, West Nile virus; μ-eLCR, Microdevice based electrochemical LCR
Keywords: LCR, Nanoparticles, SERS, Biosensors, Chemiluminescence, FRET, SNP genotyping
1. Introduction
The human genome contains around 3 billion nucleotides that are tightly packed into 23 chromosomes in each haploid cell. These nucleotides regulate various cellular processes according to cell needs. Single nucleotide polymorphisms (SNPs) are genetic variations that are present in more than 1% of the population while genetic mutations are present in less than 1% of the population [1], [2], [3]. These genetic variations could occur in coding or non-coding regions of the DNA. The latter are important for disease association studies and genetic mapping. They also could still have an effect on transcription, splicing, regulatory non coding RNA or even mRNA degradation. Although some of these variants in coding regions are silent with no effect on proteins formed, the majority of other variants in the same coding regions could silence gene expression or produce a malfunctioning protein leading to genetic disorders. Several genetic variants, whether classified as mutations or polymorphisms, have been reported to be causative factors for various diseases such as cancer [4], [5], retinitis pigmentosa [6], [7], [8], sickle cell anemia [9], [10], and hearing impairment [11], [12], [13]. Early detection of these variants is inevitable to enable physicians to take all possible parameters that could ameliorate severity of various diseases, prevention of progression of associated complications and, if possible, starting early medication to save lives. This necessitates the development of innovative biosensing platforms that are inexpensive, specific, robust, time saving and affordable with multiplexing and high-throughput capabilities. These platforms should also be sensitive enough to detect minimal copy number that carries these genetic variations especially in very limited or precious samples. They also should precisely screen and genotype biological samples for low abundant contaminants such as microbes and viruses. Although next generation and the gold standard Sanger sequencing approaches have been used successfully for this purpose, various limitations have been reported in terms of being unaffordable, very expensive, challenging and time consuming [14], [15]. Several other affordable screening assays have been developed and used routinely for genotyping these variants. These assays include restriction fragment length polymorphism [9], [16], [17], real-time PCR [18], [19], TaqMan allelic discrimination assay [20], [21], single stranded conformational polymorphism (SSCP) [22], [23], [24], [25], heteroduplex analysis [6], [26], [27], dynamic allele-specific hybridization [28], [29], [30], single base extension [31], [32], [33], denaturing high performance liquid chromatography [34], [35], temperature gradient gel electrophoresis [36], [37] and Microarray platform [38], [39], [40], [41]. Most of those screening assays have drawbacks in specificity, sensitivity, cost effectiveness, multiplexing capability and/or time savings.
Ligase chain reaction (LCR) and ligation based amplifications have drawn attention as promising and powerful genotyping assays. They were proven to be cost effective, simple, and robust with high degree of sensitivity and specificity in addition to multiplexing ability [42], [43], [44]. Here in this article, traditional and recent modifications in LCR and ligation based amplifications are highlighted. Particular emphases on the recently integrated detection strategies such as nanoparticles (NP), quantum dots (QD), electrochemical and magnetic biosensors, DNAzyme, rolling circle amplification, strand displacement amplification, quartz crystal and leaky surface acoustic surface biosensors, surface enhanced raman scattering (SERS) and fluorescence resonance energy transfer (FRET) are also detailed. Some of those integrated technologies have already been commercialized for real applications in various fields. Various genotyping assays for diverse genetic diseases, bacterial and viral pathogen detections are also discussed in this article.
2. Principle of LCR
LCR is a DNA dependent amplification that requires four oligonucleotides as primers [45]. Two oligonucleotides pair in close proximity with their complementary sequences on one template strand, whereas the other two oligonucleotides anneal with the second template strand in a similar way (Fig. 1 ). The resulting nick between adjacent oligonucleotides is covalently sealed by DNA ligase through a phosphodiester bond provided that the primer bases at the junction are perfectly matched with the target and hence one fragment will be generated for each template strand [45]. The formed ligated products serve as templates in subsequent cycles as in PCR. This leads to exponential amplification for the desired fragments [46]. In the presence of a single base change at the junction site, primers will not hybridize perfectly and hence no further amplification will occur. It is crucial to use DNA ligase that lacks blunt-end ligation activity, to prevent target independent ligation [47]. Ligation occurs through three main steps: (i) enzyme activation with either NADH or ATP [47], (ii) substrate adenylation, and (iii) nick-closure following nucleophilic attack. LCR is more sensitive and specific than gold standard PCR technique. It has been reported that the specificity and sensitivity for LCR, in presence of template DNA, were 100% and 97.6% respectively while for PCR they were 90% and 99.5% respectively [48]. Despite maximal specificity reported for LCR in comparison with PCR in the presence of template DNA, target independent ligation especially in the absence of template DNA remains one of the most limiting drawbacks that could occur at low frequency resulting in an increase in background signal with false positive results in some instances.
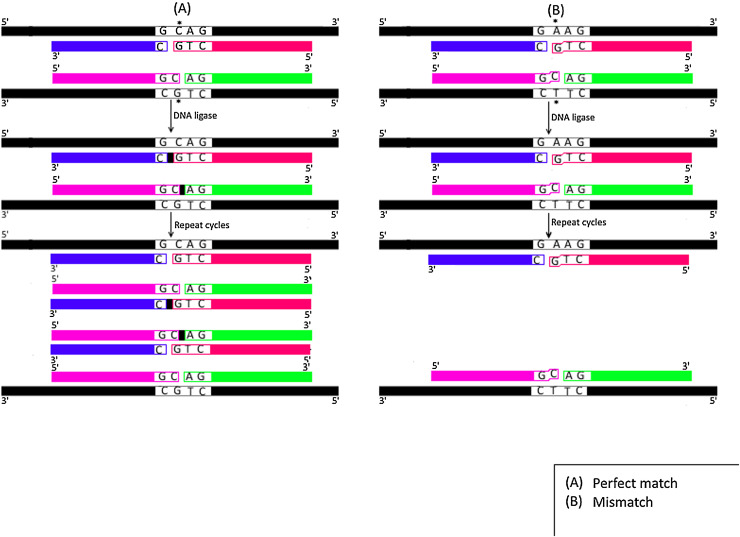
Fig. 1.
Schematic representation for Ligase Chain Reaction (LCR) technique.
A) Perfect Match; Four oligonucleotide primers (each is colored/shaded differently) anneal perfectly with their complementary sequences of perfect match wild type sample (colored in black). DNA ligase enzyme then seal the nick (indicated by small black box) between two adjacent primers hybridizing to the same template strand yielding one ligated fragment for each template strand. Following repeated cycles, exponential ligation of products is achieved. B) Mis-Match; The four oligonucleotide primers (each is colored/shaded differently) do not anneal perfectly with the mis-matched sample that contains only one bp change from wild type sample (colored in black). No ligation is achieved by DNA ligase and hence ligated products are not produced following repeated cycles. Black spot (•) refers to either perfect match (A) or mismatch base (B).
3. Modified LCR techniques
3.1. Ligase Detection Reaction (LDR)
LDR is a modified LCR technique where only two oligonucleotides, instead of 4, bind adjacently on one target strand. LDR achieves linear amplification rather than exponential amplification as in LCR [49]. The effect of several additives on specificity and efficiency of LDR was studied. Unlike formamide, glycerol and DMSO, 5% PEG-6000 was reported to improve specificity without compromising efficiency [50]. LDR was used to screen for a low abundant single base mutation at codon 12 in the Kras oncogene using high resolving capillary gel electrophoresis (CGE)-Laser induced fluorescence (LIF) [51], [52]. Thomas et al. used three primers that have the same fluorophore at the 5′ end but with varying length each corresponding to a particular genotype along with a common primer phosphorylated at the 5′ end [51]. Hamada et al. used similar integrated technique but with a slight modification through the use of three fluorescently labeled discriminating primers with different nucleotides at 3′end along with a conservative primer phosphorylated at 5’end [52]. McNamara et al. developed PCR-LDR based assay for multiplex detection of human malaria infection caused by four parasite species [53]. In this study, five specific primers that vary in length by 3 nucleotides along with two fluorescently labeled conserved-sequence primers were used. Qi et al. used PCR-LDR assay for genotyping the MIR196A2 polymorphism in HBV and HCC Chinese patients [54]. Gerry et al. developed a universal DNA array platform combining PCR and LDR techniques with zip code hybridization for multiplex detection of low abundant mutations in Kras gene [55]. In this study, each allele specific primer was modified to contain a zip code sequence on its 5′ end whereas the common primer was phosphorylated at 5′ end with a fluorophore on its 3′ end. This universal chip platform has also been microfabricated with polycarbonate and poly(methyl methacrylate) for the detection of K-ras oncogene SNPs [56]. Choi et al. reported a multiplex SNP genotyping assay consisting of multiplex PCR pre-amplification, ligation with DNA ligase and CE-fSSCP to increase resolving power for similar sized probes [57]. This assay was able to accurately discriminate SNPs in the tp53 gene.
3.2. Nested LCR
Nested LCR is a modified dual LCR technique where two separate tubes are used for the primary amplification. Each tube contains four primers (∼15-mer each) that are flanking either the left or the right side for the allele-specific base pair. In each tube, two primers bind adjacently on the sense strand while the other two anneal with the antisense strand in a similar manner. Following multiple cycles of LCR, each adjacent pair in each tube will be sealed to form ∼30-mer product. The two tubes are then mixed using fresh ligase and a new round of amplification is performed. After LCR amplification, full length product (∼ 60-mer) will be formed only in presence of correct DNA target allele [58]. Nested LCR technique was reported to be more superior to LCR in terms of improved signal to noise ratio and also nonspecific binding reduction as the generated products, through primary LCR amplification, serve as primers in the secondary allele amplification.
3.3. Gap-Ligase Chain Reaction (G-LCR)
G-LCR, formerly known as PLCR, has been developed to overcome drawbacks reported for standard LCR technique in terms of sensitivity and target independent ligation that increases background signal and reduces specificity. In G-LCR, four primers are designed to create a short gap of one to several bases between two adjacent probes (Fig. 2 a) hybridizing to each target strand [58], [59]. Thermostable DNA polymerase, lacking 3′-5′ proofreading and 5′-3′ nick translation exonuclease activities, and DNA ligase are then used to fill and seal this gap respectively. To improve specificity by minimizing target independent ligation process, an extension to the 3′ end of the primer is added. It was reported that Gap-LCR can detect down to 10 target molecules in a reaction [59]. G-LCR was reported to be very specific and sensitive in detecting the G145R mutation in HBsAg [60]. Wei et al. demonstrated that GLCR was highly sensitive in detecting Chlamydia trachomatis in neonates with pneumonia [61]. Zhou et al. developed a DNAzyme based Gap-LCR assay that can detect SNPs through either colorimetric or fluorometric methods [62]. This assay was designed to amplify mutant allele of sickle cell anemia but not the wild type allele. A short functional G-quadruplex DNAzyme sequence was introduced into one of the LCR primers to be captured by anti-G sequence. Following normal steps in GLCR, lambda exonuclease and exonuclease III enzymes were used for digestion of unreacted probes and for the release of the embedded DNAzyme which forms G-quadruplex structure that ultimately binds hemin to confer a peroxidase like activity. Jenner et al. reported that G-LCR technique was efficiently able to detect the presence of as low as 1 mutant allele in one million wild type alleles for Kras gene codons 12 and 13 [63].
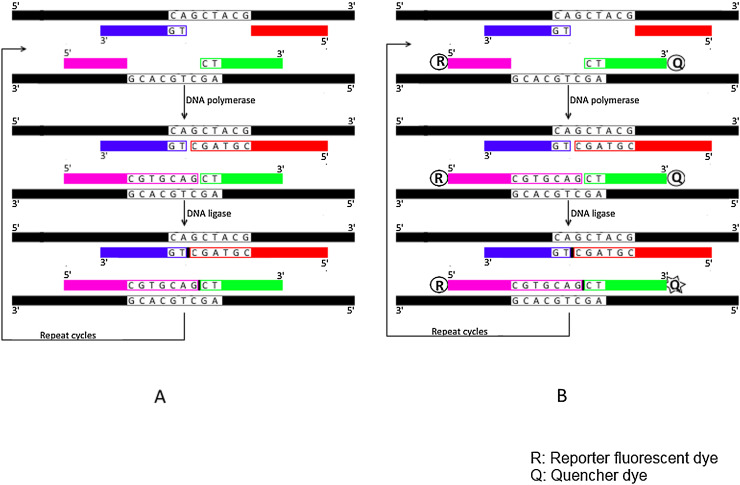
Fig. 2.
Schematic representation for Gap-LCR (GLCR) and Quantitative GLCR (QGLCR) techniques.
A) Gap-LCR (GLCR); Four oligonucleotide primers (each is colored/shaded differently) anneal perfectly with their complementary sequences of perfect match sample (colored in black) leaving a gap that is then filled by DNA polymerase and ultimately ligated (indicated by small black box) with DNA ligase enzyme to produce one ligated fragment for each template strand. Following repeated cycles, exponential ligation of products is achieved. B) Quantitative GLCR (QGLCR); Same as for GLCR except that the 5′ end of one primer is labeled with a reporter dye (R) while the 3′ end of the other primer that anneals adjacently on the same template strand is labeled with a quencher dye (Q). Following the two steps of polymerization and ligation, the ligated product (with small black box) brings reported dye (R) in close proximity with quencher dye (Q) resulting in fluorescence resonance energy transfer (FRET) from reporter dye into quencher dye upon excitation. Repeated cycles result in subsequent fluorescence of quencher dye.
3.4. Real −time (Quantitative) G-LCR (QGLCR)
Real-time G-LCR, also named as quantitative G-LCR, is a modified G-LCR with real-time quantification that is enabled through the use of fluorescently-labeled oligonucleotides. This technique was developed by Harden et al. In this technique, the first primer is labeled with a fluorescent dye at the 5′ end while the second primer that binds adjacently on the same target strand is labeled at the 3′ end with a quencher dye (Fig. 2b). After excitation of the reporter dye, the emitted fluorescence is transferred to the quencher dye resulting in subsequent fluorescence for the quencher dye. The rise in quencher fluorescence by increased cycle numbers is proportional to the starting DNA amount. This assay was initially developed for K-ras and p-53 mutations utilizing radio labeled primes. This assay significantly reduces time as compared to plaque hybridization assay [64]. Psifidi et al. used real time LCR technique for the accurate and sensitive detection of PRNP gene mutation (p.A136 V) [65]. Yi et al. combined GLCR and qPCR methods to detect the low abundant CD17 (A > T) mutation on β-globin gene [66]. One pair of the GLCR primers was modified through the addition of unique sequences to the 5′ end and the 3′ end of both the upstream and the downstream primers respectively for tagging in the following qPCR steps.
3.5. Asymmetric gap-ligase chain reaction (AGLCR)
The efficiency of DNA ligase to join closely adjacent primers on RNA template is very limited and hence LCR technique is not suitable for RNA genotyping. Asymmetric G-LCR (AGLCR) is a modification of gap-LCR to amplify and detect nucleotide changes in RNA molecule. This technique was developed by Marshal et al. (1994). Asymmetric GLCR involves the use of 3 or less nucleotides, instead of 4, in both cDNA and the gap-LCR steps (Fig. 3 ). Firstly, cDNA is synthesized from RNA by reverse transcriptase using only one primer and three dNTPs. Reverse transcription is terminated upon reaching the base that is not supplied in the reaction. Secondly, cDNA is denatured and then mixed with three probes, DNA polymerase and DNA ligase. The 3′ end of the first probe is complementary to the 3′ end of the synthesized cDNA strand and therefore hybridizes spontaneously. The second probe anneals to the 5′ stretch of the first probe leaving no gap but a nick with the 3′ end of the synthesized cDNA strand. The third probe is complementary to the 5′ stretch of the synthesized DNA strand and therefore hybridizes spontaneously leaving a gap with the first probe. DNA polymerase starts filling the gap between probe one and three and the process terminates when the enzyme reaches the addition of the unsupplied nucleotide. The two nicks formed are then sealed by DNA ligase to form DNA hybridized complex that serve as a template for further GLCR amplification. Less than 50 copies of RNA transcripts could be detected using micro-particle enzyme immunoassay (MEIA) [45] and this method was reported to correlate with RNA-PCR and HCV antibody tests.
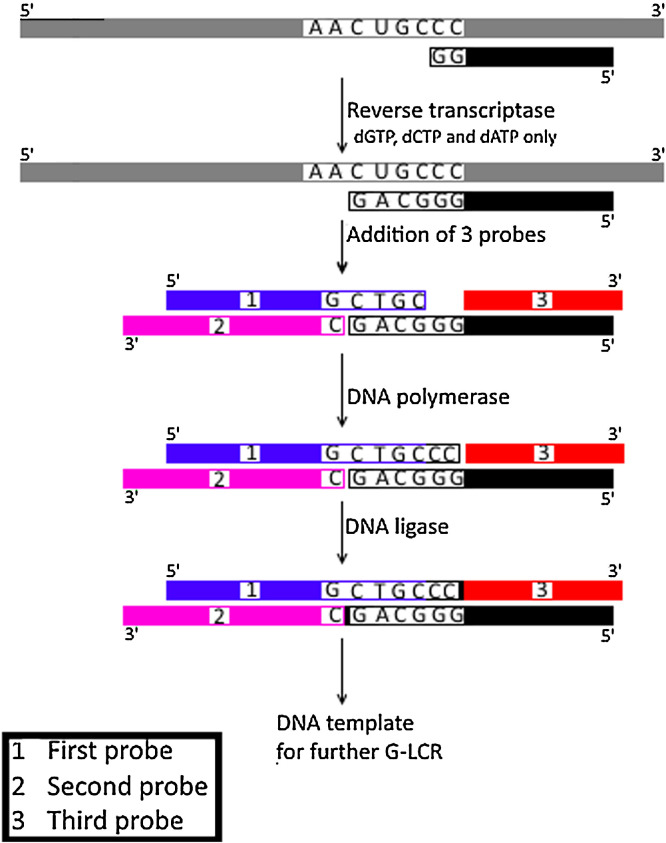
Fig. 3.
Schematic representation for Asymmetric GLCR (AGLCR) technique.
RNA template (colored in grey) is reverse transcribed using one oligonucleotide DNA primer (colored in black) and only 3 deoxynucleotides (dGTP, dCTP and dATP). This process is terminated upon reaching the unsupplied nucleotide in this reaction. Following denaturation, the produced cDNA (colored in black) is then mixed with three probes (each is numbered and colored/shaded differently) generating double stranded DNA with a gap between probe 1 and probe 3 and a nick between probe 2 and synthesized cDNA. The gap is then filled with DNA polymerase while the nick is sealed (indicated by small black box) with DNA ligase enzyme generating a double stranded cDNA that acts as a template for subsequent cycles using GLCR.
3.6. LCR with cleavase-mediated correction
This system was proposed as no DNA ligase possesses absolute accuracy and therefore could potentially introduce target independent ligation. This technique was developed by Demchinskaya et al. This approach involves careful design of two primers that harbor extra penta-nucleotide sequence on their 5’end not to be complementary with the temple. Cleavase, an endonuclease enzyme, is then used to precisely cut the non-complementary 5′ extension on the two primers. The other two primers bear additional nucleotides at their 3’end to replace the cleavage removed nucleotides allowing perfect sealing with DNA ligase. The two primers harboring non complementary extra stretch on their 5′ end also contain biotin and UP region at their 3 ‘ends to bind streptavidin coated on a microwell plate and to enable colorimetric detection respectively. Although this approach prevents target independent ligation observed in conventional LCR, the produced signal is slightly decreased and the minimal limit for target DNA is 30 amol [67].
3.7. Multiplex ligation dependent probe amplification (MLPA)
This technique was developed by Schoutedn et al. and is currently a registered trademark of MRC-Holland [68]. This technique is extensively used as it is very simple, robust and can amplify multiple targets in one reaction using one primer pair following ligation step. Briefly, two oligonucleotide probes that bind adjacently on a target sequence are ligated only in presence of their complementary target DNA. One oligonucleotide probe is designed to contain a recognition sequence for forward universal primer on its 5′ end while the other probe contains the reverse universal primer recognition sequence on its 3′end (Fig. 4 ). In addition one of those probes contains a stuffer sequence that can be tailored with variable lengths to be assigned for each target for multiplexing purposes using one reaction. A fluorescently labeled universal forward primer is then used to generate up to 50 amplicons that can be visualized by capillary electrophoresis. This assay has been used routinely for the detection and quantitation of DNA copy number variation, relative ploidy, mRNA profiling, SNP detection, chromosomal aberration, methylation changes and transgene genotyping [68], [69], [70], [71]. Wang et al. developed a similar approach with replacement of stuffer sequences with unique zip codes. PCR amplification is performed with unlabelled universal primers and Cy5-labeled dCTP nucleotides. Cy5-labelled amplicons are then captured on microarray chip through hybridization with their complementary sequences of chip immobilized probes [72]. Sanchez et al. utilized the same method for profiling tumour markers using unique barcode sequences that are then captured by their complementary sequences that are immobilized on inexpensive electrode microarray. Detection is enabled through the use of horse-radish peroxidase (HRP) labelled secondary probes that are complementary to universal primer sequence. Besides cost reduction and high specificity reported for this assay, sensitivity limit was as low as 25pM [73]. The high success rate for MLPA in multiplex SNP genotyping led to the development of several other MLPA versions for other applications. Methylation specific-MLPA (MS-MLPA) and reverse transcriptase-MLPA (RT-MLPA) were developed for DNA Methylation profiling, gene copy number determination and genetic expression analysis.
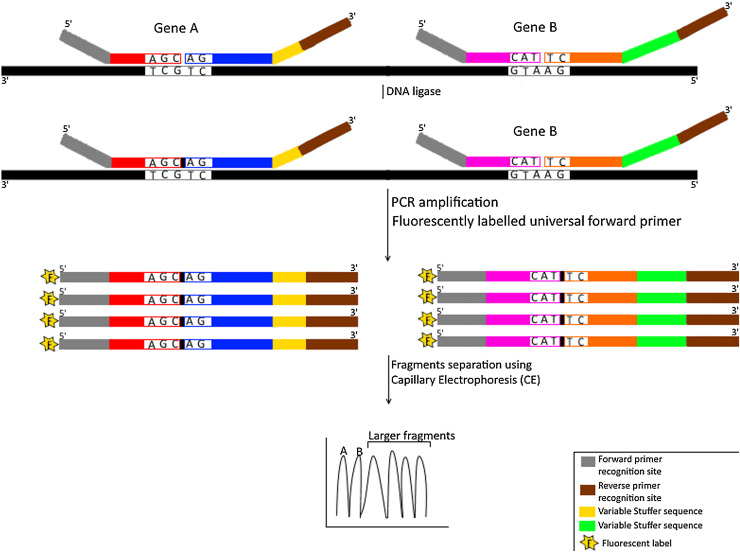
Fig. 4.
Schematic representation for Multiplex Ligation Dependent Probe Amplification (MLPA) technique.
The diagram shows simultaneous detection of 2 different genes (A & B) using four probes (two probes for each gene) that are colored/shaded differently. The first probe contains a recognition sequence for forward universal primer on its 5′ end and a specific sequence on its 3′ end that is complementary to template strand (colored in black). The second probe contains an allele specific sequence on its 5′ end to bind adjacently to the first probe on the same template strand and a reverse universal primer recognition sequence on its 3’end. A tailored stuffer sequence that is assigned for each target gene (for multiplexing purposes) is located between the reverse universal primer recognition sequence and the allele specific sequence of the second probe. DNA ligase seals the nick (indicated as small black box) between the two probes hybridizing to the same target gene. Fluorescently labeled universal forward primers are then used to generate 5′-fluorescently (indicated by letter F) labeled amplicons from ligated products. These products, with variable lengths attributed to the variable stuffer sequences used for each target gene, are separated using capillary electrophoresis.
3.8. Multiplexed oligonucleotide ligation PCR (MOL-PCR)
This assay is similar to MLPA technique except that stuffer sequence is replaced by unique tag sequence to enable fluorescently labelled amplicons to be captured by its complementary sequence that is covalently linked to microsphere bead array. A flow cytometric device is used to identify each bead to measure fluorescence intensity for each bead-probe complex. Also the reverse primer, instead of the forward primer, is fluorescently labelled. If the target is not present, no fluorescence will be generated from the microsphere as no ligation would occur [74], [75]. The MOL-PCR assay was designed for multiplex detection of various targets and for SNP genotyping [74]. This technology has been applied for the multiplex detection of 15 genetically distinct plant pathogens including Citrus tristeza virus (CTV), Xanthomonas genus and Xylella fastidiosa. Mol-PCR has also been used in the detection of pathogens along with antibiotic (doxycycline and ciprofloxacin) resistance in Yersinia pestis, F. tularensis and Bacillus anthracis [74], [75]. The detection limit for this flow cytometry approach is estimated to be less than 103 molecules [74]. Mol-PCR has also been useful in identification and differentiation of the six main human-associated lineages of Mycobacterium tuberculosis complex (MTBC) with sensitivity approaching 99.2% [76]. In 2008, Bruse et al. made few improvements to MOL-PCR assays by using fewer beads and universal biotinylated oligonucleotides to reduce costs significantly but also maintaining accuracy [77]. The assay enables the detection of 50 SNPs per well in a 96 well plate. In addition to a common probe, two allele specific probes that are labelled at their 5′ ends with specific tag each are used for each SNP. Following successful ligation, the 5′-tag hybridizes with its complementary sequence that is linked to a unique FlexMAP microsphere whereas the common probe 3′ end anneals with its complementary universal biotinylated oligonucleotides. Following hybridization with streptavidin-R-phycoerythrin (SAPE), generated fluorescence is developed and detected using flow cytometric analysis.
3.9. Dye labeled oligonucleotide ligation (DOL)
Chen et al. developed a diagnostic assay named as dye-labeled oligonucleotide ligation (DOL) combining oligonucleotide ligation reaction and PCR in one reaction tube along with fluorescence resonance energy transfer (FRET) detection for real time monitoring [78]. This test has been implemented successfully for the detection of 10 SNPs in CFTR, BRCA1, HFE, β-globin and NAT2 genes. Three dye labeled probes that have low Tm are used for each biallelic marker. The common probe is labeled with a donor dye at the 5′ end whereas the remaining two discriminating probes are labeled differently with acceptor dyes at their 3′ end. PCR is firstly performed at high annealing temperature (65 °C) that only allows PCR primers but not the dye labeled probes to bind and to be extended with a thermostable DNA polymerase lacking 5′ exonuclease activity. After generation of sufficient PCR amplicons, ligation step is performed at low annealing temperature (45 °C). The genotype of a DNA target can be determined by monitoring the ligation of dye labeled probes through FRET.
3.10. The SNPlex genotyping system
SNPlex genotyping system was primarily designed by Applied Biosystems in 2005 for genotyping 48 SNPs simultaneously using oligonucleotide ligation [79]. It is reported that less than 1 ng target DNA can be used for each genotype. Four probes are used in this genotyping system named as; allele specific oligonucleotide (ASO), locus specific oligonucleotide (LSO), universal ASO linker and universal LSO linker. ASO and LSO hybridize adjacently with complementary sequences of the target DNA at the SNP site. The 5′ end of the ASO contains a specific zip code sequence while the 3′ end of the LSO contains the reverse primer site for multiplexing PCR. The universal ASO and LSO linkers contain complementary sequences to ASO and LSO probes respectively. Universal ASO linker also contains a forward priming site for multiplex PCR purposes. Following ligation step, exonucleases are used to remove target DNA and unligated oligonucleotides and linkers. Following purification, multiplex PCR is performed using universal forward and biotinylated reverse primer. Biotinylated amplicons are then captured by streptavidin-coated microtiter plates. After washing step, fluorescently labelled universal Zip-Chute oligonucleotides hybridize to the biotinylated single-stranded products. Each Zip-Chute oligonucleotide contains complementary sequence to zipcode sequence on each ASO probe and also contains a mobility modifier for analytical tracking during CE. ZipChute oligonucleotides are then eluted and analysed using Applied Biosystems analyzer. This assay has been used extensively for SNP genotyping in various fields. It has been applied successfully for the detection of SNPs in grapevine (Vitis vinifera L.) [80]. Also 109 unrelated Chinese Han samples were successfully genotyped for 29 Insertion/deletion markers with the SNPlex genotyping system [81].
3.11. Ligation based quartz crystal biosensor (QCM) & leaky surface acoustic wave (LSAW) biosensor
A novel gold electrode biosensor for β-thalassemia SNP genotyping was introduced in 2006 [82]. In this assay DNA target anneal with complementary sequences of gold electrode immobilized probes. A biotinylated discriminating probe was then ligated to immobilized probe using DNA ligase. Following denaturation, HRP conjugated streptavidin (HRP-SA) bind biotinylated ligated product leading to precipitation of 3,3 diaminobenzidine in the presence of H2O2. The latter can then be detected through quartz crystal biosensor (QCM) as a cost effective mean with a detection limit of 0.1 nM. Zhang et al. used a similar approach, for the detection of K-ras SNP in codon 12, except for the replacement of HRP-SA with alkaline phosphatase streptavidin (ALP-SA). ALP converted ascorbic acid 2-phosphate to ascorbic acid which in turn reduced silver ions causing precipitation for silver metal on electrode surface and this was quantified using linear sweep voltammetry. The reported sensitivity for this assay was 80fM [83]. In 2011, Xu et al. reported a similar biosensor for the one developed by Feng et al. but with leaky surface acoustic wave (LSAW) biosensor instead of quartz crystal. This biosensor was used for SNP genotyping in Japanese encephalitis virus with a reported sensitivity of 1 × 10(−12) mol/L [84].
3.12. Integrating ligation based rolling circle amplification (RCA) with biosensors
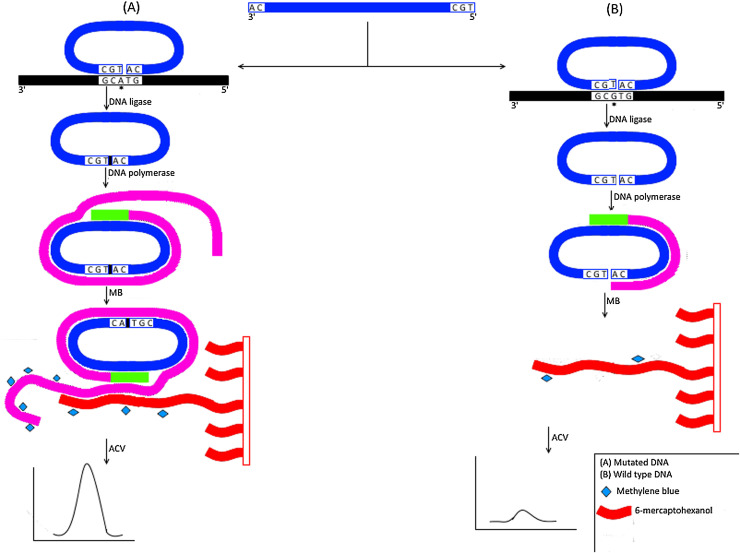
Zhang et al. implemented a label free ligation based rolling circle amplification (RCA) for the detection of as low as 40 amol of mutant strands (Fig. 5 ). In this method, a padlock probe was first circularized upon hybridization with its complementary sequences on mutant strand through DNA ligase. DNA polymerase was then used to amplify the circularized padlock probe using primers complementary to the middle region of the padlock probe. Elongated products annealed with their complementary sequences on capture probes tethered to gold electrode. Detection of hybridized products was performed using methylene blue which produced current measured through altering current voltammetry (ACV) [85]. Su et al. utilized a similar technique except for the use of biotinylated primer to allow elongated products to be captured with streptavidin-coated paramagnetic beads [86]. This method was reported to detect down to 2 amol mutated strands. Long et al. integrated hyperbranching RCA (HRCA) technology with electrochemiluminescence using paramagnetic beads for specific and sensitive identification of hly genes of Listeria monocytogenes. In this assay biotin as well as ruthenium labeled primers were used for generation of elongated products with a reported sensitivity as low as 10 aM [87]. Dong et al. developed a novel ligation based RCA technique with chemiluminescence imaging for SNP detection. In this assay, biotin labeled probes were immobilized on streptavidin coated 96 well plate and then allowed to anneal with target DNA. A second discriminating probe was ligated to immobilized probe through DNA ligase. The 3′ end of the discriminating probe acted as a primer for circularized probe DNA to initiate RCA using Klenow exo- DNA polymerase. HRP-mimicking DNAzyme generated through the binding of hemin to elongated RCA products catalyzed the chemiluminescence reaction between H2O2 and luminal. This assay was reported to detect as low as 0.26 fM target DNA [88]. Cheng et al. integrated LCR with RCA for SNP detection. In this assay, one pair of primers along with a padlock probe (PLP) is used. Following ligation and repetitive cycles, exponential amplification of circular PLP (cPLP) and ligated products is achieved. RCA is then initiated with cPLP as a template and ligated product as a primer to produce several copies of long dsDNA products that are detected through binding with Syber Green I dye [89]. This assay is reported to be specific with a detection limit of 1fM of target DNA. He et al. integrated magnetic beads based RCA and gold nanoparticles tags for specific and sensitive SNP detection using inductively coupled plasma mass spectrometry (ICP-MS) detection strategy [90]. This method was reported to detect as low as 0.1fM. Colorimetric detection of the HRCA using metal indicator was employed to detect H5N1 virus [91].
Fig. 5.
Schematic representation for Ligation Based Rolling Circle Amplification (RCA) technique.
A) Mutated DNA; A padlock probe (PLP) that is complementary to mutant DNA is circularized with DNA ligase (indicated by small black box) and is then amplified using DNA polymerase and primers that are complementary to the middle region of the PLP. Amplified products are then tethered to their complementary sequences of capture probes attached to gold electrode. Using methylene blue (indicated by a diamond shape), hybridized products can be measured by altering current voltammetry (ACV). B) Wild type; The PLP is not complementary with wild type and therefore no ligation occurs and hence neither complete amplification nor hybridization with capture probes would occur. Addition of methylene blue (indicated by a diamond shape) would not result in current change on ACV.
3.13. Multiplexed ligation based electrochemical biosensor with nonfoulingsurface
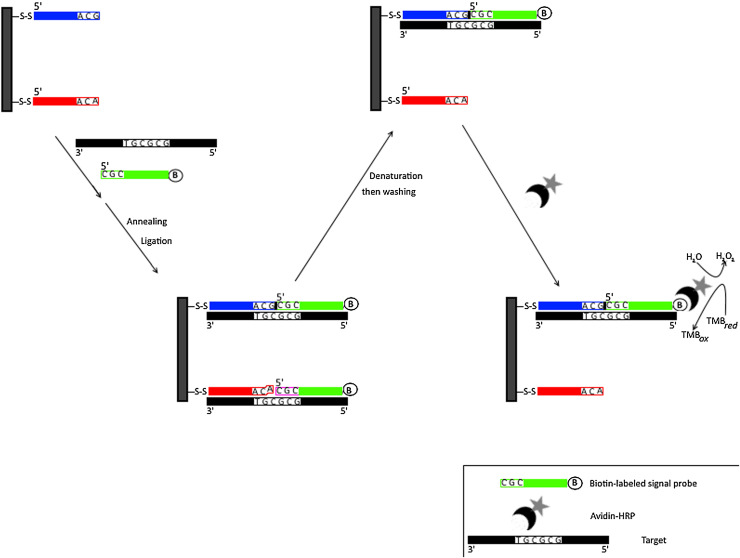
Liu et al. reported a novel ligation dependent SNP genotyping assay using oligonucleotide incorporated nonfouling surface (ONS) as electrochemical DNA biosensor [92]. This multiplex assay has been successfully implemented in the detection of the pre-core G1896A mutation in HBV and also two SNPs of the human CYP2C19 genome C680T and G681A. Thiolated capture probes and thiolated ethylene glycol were co-assembled on gold electrode to obtain mixed self assembled monolayer of ONS. The 3′-end of the capture probe contains nucleotides that are complementary to the corresponding SNP target sequences (Fig. 6 ). The assay also used a signal probe containing sequences adjacent to the SNP nucleotide on its 5 end and a biotin at its 3‘end to enable detection. Following hybridization between target DNA and capture DNA based on full complementarity, the signal probe was then ligated, through DNA ligase, to the capture probe. Stringent denaturation and washing steps were carried out to remove target DNA and unligated signal probe. Covalently ligated signal probes that were not washed, were bound to avidin–horseradish peroxidase conjugate to sequentially develop an amperometric signal.
Fig. 6.
Schematic representation for Multiplexed Ligation Based Electrochemical Biosensor with Nonfouling Surface.
The 3′ end of the gold electrode tethered thiolated capture probes hybridize with their complementary sequences of target DNA. A biotinylated (indicated by letter B) signal probe that is also complementary to other part of the target DNA binds adjacently to thiolated capture probe leaving a nick. This nick is then sealed (indicated by small black box) with DNA ligase. Following denaturation and washing, only ligated products would be tethered to the gold electrode. These ligated products are biotinylated at their 3′ end and therefore can bind to avidin-horseradish peroxidase conjugate (indicated by crescent and star shapes respectively) for generation of amperometric signal
3.14. Gold nanoparticle enabled ligase chain reaction
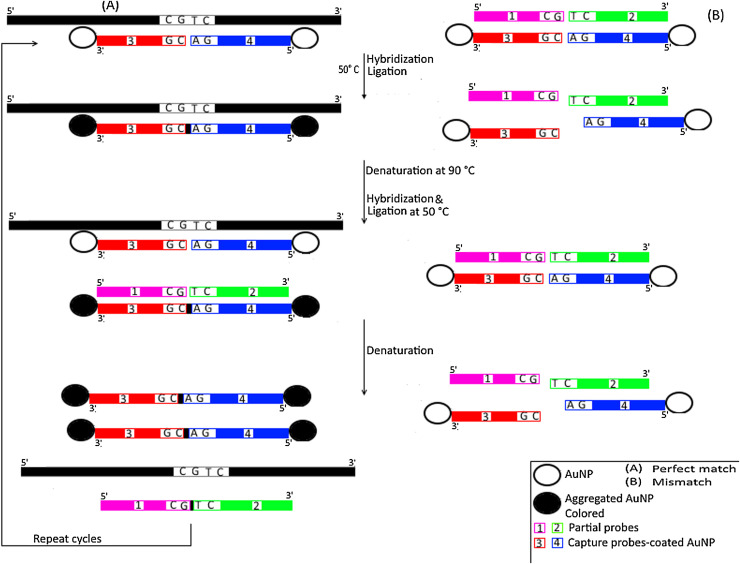
Gold nanoparticles enabled ligase chain reaction was introduced in 2012 as a simple SNP genotyping assay with high sensitivity limits [42]. Briefly, two capture probes (CPs) coated AuNPs were prepared to hybridize adjacently on both target DNA and also on complementary partial probes at 50 °C (Fig. 7 ). Using ampligase, the nick site at the junction between target DNA and the CP coated AuNPs was ligated whereas the duplex formed between CP coated AuNPs and partial probes was not sealed. Temperature was then increased to 90 °C to denature all formed duplex structures. During subsequent hybridizations and ligations, exponential amplification of ligated CP coated AuNPs occured leading to formation of AuNP aggregates that changes colour from red to purple/gray. As low as 20 aM can be detected with 103 times enhanced specificity was reported in this assay. This assay has been implemented successfully for the detection of the oncogene Kras associated mutation. Two years later, Knez et al. replaced the colorimetric detection method with fiber optic surface plasmon resonance (FO-SPR) platform to improve sensitivity with real-time monitoring of the assay in a simplified way without the need for any further analysis following the reaction [93]. Yin et al. developed an ultrasensitive LCR based AuNPs biosensor for DNA detection [94]. This assay was similar to the one developed by Shen et al. except for the probe overloading process on AuNPs through employment of salt aging steps. Following subsequent cycles, exponential amplification of target DNA and increasing assembly of aggregated AuNPs were achieved. This method had much more improved sensitivity of 1.5 aM and could be measured either colorimetrically or by dynamic light scattering.
Fig. 7.
Schematic representation for Gold Nanoparticles Enabled Ligase Chain Reaction.
A) Perfect match; At 50 °C, the two capture probes (CPs) (numbered as 3 & 4 and are colored/shaded differently) that are coated on non-aggregated gold nanoparticles (AuNPs) (indicated by unfilled circle) hybridize adjacently on target DNA (colored in black) leaving a nick that is sealed (indicated by small black box) with ampligase enzyme producing aggregated and coloured NPs (Black filled circle). Following denaturation at 90 °C and second hybridization, more aggregated NPs are produced from sealing the nick at the junction between target DNA and the CP coated AuNPs and also the nick at the junction between ligated CPs (with a small black box) coated AuNPs and partial probes (numbered as 1 & 2 and colored/shaded differently). Denaturation is then repeated with subsequent hybridization and ligation leading to exponential amplification of ligated CP coated AuNPs with a colour change from red to purple/grey. B) Mismatch; The two CPs (numbered as 3 & 4 and colored/shaded differently) coated AuNPs do not hybridize with target DNA (colored in black) and hence neither ligation nor aggregation of CPs coated AuNPs occur with no colour change.
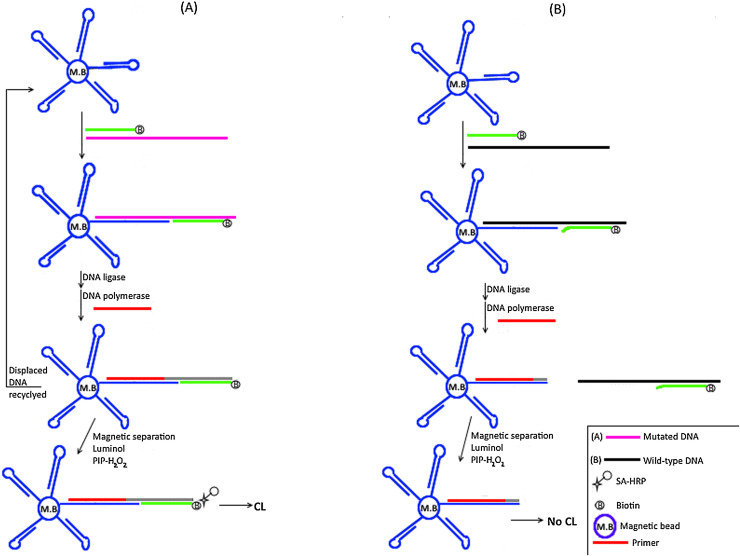
3.15. Integrating LCR based DNAzyme–fluorescein chemiluminescence resonance energy transfer imaging with magnetic biosensors
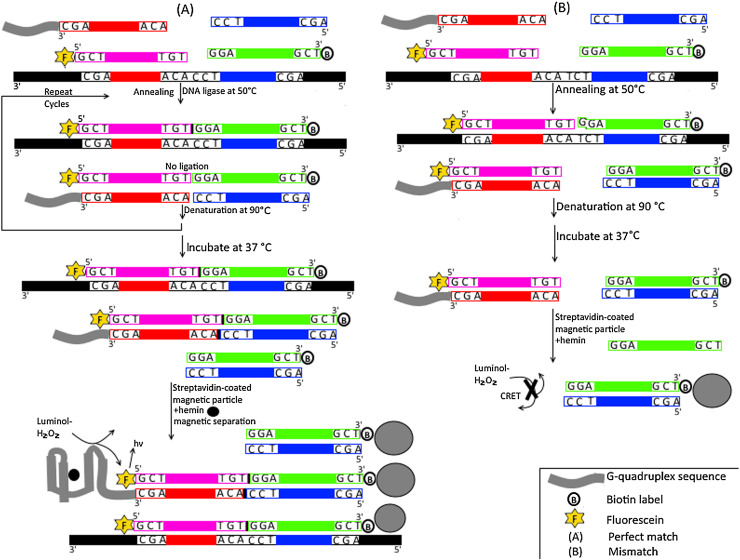
Chemiluminiscence resonance energy transfer from peroxidase-mimicking DNAzyme catalyzed luminal-H2O2 to fluorescein with real-time imaging on magnetic particles was reported as a novel LCR methodology in 2015 [95]. Two pairs of complementary probes are used in this assay (Fig. 8 ). The first pair had similar sequences to the upstream sequence of the SNP site while the second pair had similar sequences to the downstream sequence of the SNP. For the first pair, only one probe had biotin on its 3’end. For the second pair, the 3′ end of one probe had G-quadruplex sequence while the 5′-end of the other probe had fluorescein. At 50 °C, the biotin and the fluorescein labeled probes hybridize adjacently on target DNA and are then sealed by a thermostable ligase whereas the duplex formed for each pair of complementary probes cannot be ligated together. All formed duplexes are then denatured at 90 °C to release ligated product, target DNA and the 4 complementary probes. The ligated product is then used as a new template for the ligation of both the unlabelled probe and the G-quadruple containing probe. Exponential amplification of ligated products is achieved through subsequent cycles. The resultant products are then allowed to form duplexes through incubation at 37 °C. All biotinylated products are then immobilized on streptavidin-coated magnetic particles (SA-MPs) while all non-biotinylated products are removed during magnetic separation process. G-quadruplex containing biotinylated duplex intercalates hemin forming peroxidase-mimicking DNAzyme that catalyzes energy transfer from luminal-H2O2 to fluorescein. This assay has been implemented in the SNP genotyping of the K-ras (G12C) mutation. Exponential amplification along with the use of SA-MPs has significantly improved detection limit down to 0.86fM DNA without compromising specificity.
Fig. 8.
Schematic representation for LCR Based DNAzyme–Fluorescein Chemiluminescence Resonance Energy Transfer technique.
A) Perfect match; Four probes are used in this assay (colored/shaded differently). Two probes are identical to the upstream and the downstream sequences for the SNP site while the other two probes are complementary to the upstream and the downstream sequences for the SNP site. The 3′ end for one of the complementary probes is biotinylated (indicated by letter B) while the 5′ end for the other complementary probe is labeled with fluorescein (indicated by letter F). The 3′ end for one of the identical probes has G-quadruplex sequence (colored in grey) while the 5′ end for the other identical probe is unlabeled. At 50 °C, the biotin and the fluorescein labeled complementary probes hybridize adjacently on target DNA and are then sealed (indicated by small black box) by ligase enzyme whereas the duplex formed between complementary and identical probes (four probes) is not sealed. The mixture is then allowed to hybridize following denaturation at 90 °C. The ligated product (with a small black box) is then used as a new template for the ligation of both the unlabelled probe and the G-quadruple containing probe. Exponential amplification of ligated products is achieved through subsequent cycles. All biotinylated products (with letter B) are then immobilized on streptavidin-coated magnetic particles (SA-MPs) (indicated by large grey circle) while all non-biotinylated products are removed during magnetic separation process. G-quadruplex containing biotinylated duplex intercalates hemin (indicated by small black circle) forming peroxidase-mimicking DNAzyme that catalyzes chemiluminiscence energy transfer from luminal-H2O2 to fluorescein (indicated by letter F). B) Mismatch; Since there is no hybridization with target DNA, no ligation occurs and only the biotinylated (indicated by letter B) complementary probe binds SA-MPs (indicated by large grey circle). The latter does not contain the G-quadruplex which is essential to generate the CRET signal.
3.16. Ligation-mediated strand displacement amplification based chemiluminescence biosensor
Shi et al. developed a ligation mediated strand displacement amplification (SDA) based chemiluminescence biosensor for SNP detection with minimal noisy background [96]. In this assay, the hairpin probes immobilized on magnetic beads were opened and hybridized with target DNA through their 5’end. A reporter probe, with a biotin label on its 5’end, was used in this assay to be complementary to 5’end of target DNA. The 3′ end of the biotinylated probe is sealed with the 5’end of immobilized probe through DNA ligase (Fig. 9 ). In the next step, Klenow fragment (exo−) polymerase and short primer were added to synthesize a new strand that displaced target DNA. The latter initiated another cycle of hybridization with hairpin and biotinylated probes for signal amplification. Magnetic separation was then carried out followed by addition of HRP conjugated streptavidin to bind biotinylated products and to develop chemiluminescence signal through catalytic reaction of luminol- paraiodopenol-H2O2. If there is a mismatch, no ligation would take place and hence neither displacement nor a signal would be generated. Wang et al. developed a simple but sensitive and specific label free SNP genotyping assay based on ligase reaction. In this approach, allele discrimination is achieved through DNA ligase and then nickase based strand displacement amplification is utilized to generate huge amount of peroxidase-mimicking DNAzyme sequences against hemin to enable label-free chemiluminescence detection. This model has been tested successfully for SNP detection in cytochrome P450 monooxygenase CYP2C19*2 [97]. Xu et al. developed a novel ligation dependent SDA using tailored hairpin probes and an initiation primer. The first hairpin probe was labelled with fluorophore and quencher at both ends while the second hairpin probe had an extension that was complementary to initiation primer sequence. The two hairpin molecular beacons contained recognition sites for nickase cleavage. Following hybridization of target DNA and initiation primer with the two hairpin probes, DNA ligase seal the nick between the two opened hairpin probes. The 3′ end of the initiation primer was then extended through polymerization to fill the gap with nick site formation and target recycling through displacement step. Detection was based on measuring fluorescence intensity generated through the opening of the labelled hairpin probe with resultant nicked trigger portion [98].
Fig. 9.
Schematic representation for Ligation-Mediated Strand Displacement Amplification Based Chemiluminescence Biosensor.
A) Mutated DNA; The hairpin probes that are immobilized on magnetic beads (indicated by letters M.B) hybridize with their complementary sequences on target DNA. A 5′ biotinylated (indicated by letter B) reporter probe anneals adjacently to the opened hairpin probe on the target DNA leaving a nick that is sealed with DNA ligase. Klenow fragment (exo−) polymerase and short primer are then used to synthesize a new strand that displaces target DNA. The latter initiates another cycle of hybridization with hairpin and biotinylated probes (indicated by letter B) for signal amplification. Following magnetic separation, horseradish peroxidase (HRP) conjugated streptavidin (indicated by star and small unfilled circle shapes) is added to bind biotinylated products for development of chemiluminescence signal through catalytic reaction of luminol- paraiodopenol-H2O2. B) Wild type DNA; Since there is no hybridization between target DNA and the hairpin probe, neither ligation with biotinylated reporter probe (indicated by letter B) nor displacement of the target DNA would occur and hence no development of chemiluminiscence signal.
4. Detection of LCR products
4.1. Autoradiography
Autoradiography was the first LCR detection method that has been implemented using 3′-radiolabelled 32P oligonucleotides. Radiolabelled LCR products were electrophoresed on denaturing polyacrylamide gels and then visualized by autoradiography [99]. This was a very tedious process that requires at least 12 h and all chemicals to be freshly prepared. This method could detect down to 200 target molecules.
4.2. Nonradioactive detection assay
Several nonradioactive detection methods were developed for detection of ligated LCR products as follows;
4.2.1. Fluorescent method
Winn-Deen and Iovannisc utilized fluorescently labelled oligonucleotides to generate fluorescently labelled LCR products that were then detected on a fluorescent sequencer [100]. As little as 0.5 fmol of fluorescently labelled products could be detected. The main drawback for fluorescent detection is photo-bleaching. Fluorescent detection has been successfully applied in multiplex detection of various SNPs and mutations using various fluorescent probes or the same fluorescent label but with different sized LCR amplicons [53], [57].
Meng et al. developed a fluorescent detection of ligated products on microbeads [101]. In this method, three primers were used. The common downstream primer was phosphorylated and biotinylated at the 5′-end and the 3′-end respectively. Two allele-specific primers were labeled with two different fluorescent dyes at their 5’end. The ligation products were then captured by streptavidin-coated microbeads. A fluorescent microscope was then used for genotyping based on detected fluorescence signals.
4.2.2. Microparticle capture enzyme immunoassay (MEIA)
Microparticle Capture Enzyme Immunoassay (MEIA) which is a sandwich immunoassay approach was introduced in 1994 for detecting LCR products. The termini, distal from the ligation site, of the primers that hybridize with sense strand were labeled with fluorescein at the 5′ end and biotin at the 3′end. The other two ends of the primers annealing to antisense strand were labeled with biotin and fluorescein at the 5′end and the 3’end respectively. After ligation, the LCR products would carry a different binding ligand with either biotin or fluorescein at each end. LCR products were then captured by anti-fluorescein antibody coated microparticles. A washing step was performed to remove unbound biotinylated primers. Anti-biotin antibody conjugated to ALP was then added to bind only the ligated LCR products containing biotin at the other end. After another washing step, the retained ALP removed phosphate group from the substrate methyl umbelliferyl phosphate to form a fluorescent signal that was read by an enzyme immunoassay analyzer. This method was reported to detect less than 100 molecules [102].
Abravaya et al. developed another sandwich assay approach for the detection of GLCR products but with different ligands namely carbazole and adamantine [59]. Anti-carbazole coated microparticles were used to capture ligated GLCR products. Anti-adamantane antibody conjugated ALP generates the fluorescent signal from the same substrate [59].
4.2.3. Luminogenic
A luminogenic assay that is dependent on MEIA strategy was reported by Deen et al. [103]. Primers were labelled with biotin and digoxigenin at their 5 ends and 3′-ends respectively. Ligated products were then captured on streptavidin coated microplate. ALP conjugated Anti-digoxigenin antibody was then bound to the digoxigenin end of captured LCR product. Lumi-phos 530 was then added as a luminogenic substrate for luminogenic detection. This method was reported to be very sensitive with a detection limit 10-fold less than using either radioisotopes or fluorescent sequencer.
4.2.4. Colorimetric assay
Zebala and Barany reported a colorimetric assay that utilized one primer labeled with poly (dA) tail at the 5′ end and another primer labeled with biotin at the 3′ end. Ligated products were then captured on poly (dT)-coated paramagnetic iron beads. After bead separation using magnetic field, the biotin moiety on the 5′ end was bound to streptavidin-ALP conjugate for colorimetric detection [104]. Toubanaki et al. developed colorimetric biosensor assay for SNP genotyping based on ligation reaction [105]. In this assay one common upstream primer was labeled with biotin at the 5′ end and another two allele specific primers were having poly (dA) tail at their 3′ ends. The DNA biosensor was assembled on a plastic adhesive backing with dried oligo(dT)-conjugated gold nanoparticles. The poly dA tail of ligated product was bound to dT-conjugated gold nanoparticles while the other biotin end was captured by streptavidin. Accumulation of nanoparticles resulted in development of a red line after 30 min. Several other colorimetric assays have been developed using other strategies such as DNAzyme [62], [106], Cleavase enzyme [67], gold nanoparticles [42] and magnetic beads [107].
4.2.5. Chemiluminescence and chemiluminescence resonance energy transfer (CRET)
Chemiluminescence and CRET have been widely used in the detection of LCR products as reported previously [95], [97]. In chemiluminescence, light is emitted in response to a chemical reaction while in CRET, the produced chemiluminescence excites the acceptor dye once gets in close proximity.
4.2.6. Fluorescence resonance energy transfer (FRET)
FRET has been implemented for the detection of LCR products. The acceptor dye fluoresces only once gets in close proximity with the donor dye [78], [108]. Peng et al. combined LDR with FRET for the detection of bacterial pathogens. In this assay, the two LDR primers were designed to form a reverse molecular beacon following ligation process due to their high Tm values. Primers termini also contained donor and acceptor dyes to enable FRET once brought into close proximity through the formation of a molecular beacon [108]. Yuan et al. developed an integrated LCR-lambda exonuclease-assisted cationic conjugated polymer (CCP) biosensor for miRNA genotyping [109]. The ability of CCPs to harvest and amplify signal was used as a donor in the FRET transfer to fluorophore. In this assay, one primer was designed to have a phosphorothioate modification at its 3′ end while the other primer was labeled with fluorescein at its 5′ end. Following LCR, one ligated product was formed with fluorescein label on its 5′ end and phosphorothioate on its 3′ end. The latter was resistant to exonuclease I and exonuclease III degradation. Sun et al. developed a robust real-time based LCR for p53 SNP (Arg282Trp) genotyping [110]. In this assay, two probes were used. The first probe was labeled with a fluorophore while the second one was labeled with a quencher. In the presence of the target DNA, the two probes were ligated and the close proximity of the fluorophore and the quencher caused FRET with a decrease in the fluoresecent signal generated. This decrease in fluorescence is directly proportional to DNA target amount. The reported sensitivity for this assay was estimated to be as low as 10 aM.
4.2.7. Electrochemiluminescense (ECL) detection
Electrochemilumeniscence method for GLCR detection of HBsAg was reported in 2002 (60). In this assay, primers complementary to sense strand of HBV DNA were labeled as follows: downstream primer was labeled at the 3′ end with biotin, while upstream primer had a 5′ ruthenium label. Primers hybridizing with antisense strand were not labeled. Streptavidin coated paramagnetic beads were then used to capture ligated products with biotin label. Electrical excitation of ruthenium label on captured products produced a light signal [60].
4.2.8. LCR In passivated silicon-glass microchips
Silicon-glass microchips enabled LCR was developed as a highly sensitive and specific detection strategy for various SNPs [111]. Amplification efficiency was increased significantly through dynamic polymer-based surface passivation method [112], [113], [114].
4.2.9. Electrochemical LCR (eLCR)
Wee et al. developed a simple and inexpensive eLCR for SNP detection based on LCR. This methods depends on the electrical conductivity of dsDNA which results from the pi-stacking of aromatic base pairs. In this method, active redox compounds which are attached to double stranded DNA through covalent attachment or intercalation were used to transfer charge from the base-pair stacking of double stranded DNA to electrodes [115]. Wee et al. developed a microdevice based electrochemical LCR (μ-eLCR) assay for detection of minor nucleotide base changes in breast cancer with up to 10 fold improvement in sensitivity [116]. Koo et al. used eLCR for the detection of methylated CpG islands. After bisulfite treatment of DNA sample, A duplexed LCR was then performed and the generated LCR products were captured by immobilized probes on gold electrodes. Methylation pattern was then detected through electrocatalytic reduction of H2O2 mediated by peroxidase mimicking-DNAzyme approach on electrodes surfaces [117].
4.2.10. Single quantum dots (QD)
Single quantum dot (QD) strategy was integrated with GLCR technique for multiplex SNP detection in 2013 [118]. In this assay, one primer was biotinylated while the other primer was labeled with a fluorophore. Following GLCR, ligated product was bound to streptavidin coated QDs to form DNA-QD complexes that were subsequently analyzed with the use of coincidence laser spectroscopy [118].
4.2.11. Surface enhanced Raman scattering (SERS)
Huh et al. combined LDR and SERS for the detection of k-ras oncogene SNP [119]. In this assay, the upstream LDR primer contained the discriminating base and SERS active dye whereas the downstream primer harbored a modified amine to bind the SERS enhancer of silver nanoparticle. Following ligation, the SERS dye and the enhancer were brought into close vicinity and hence Raman signature was detected. The assay has been implemented in a microfluidic device that was electrokinetically active. Detection limit was reported to be 20 pM of target DNA.
5. Applications
5.1. Detection of pathogens
The following are examples of pathogens that have been detected using Ligation based techniques;
-
•
Detection of Neisseria gonorrhoeae [120], [121], [122], [123], [124], [125]. Traditional detection methods were time consuming with a minimum of 24 h. LCR provided a rapid and effective diagnostic approach which is essential for disease control [120].
-
•
LCR has been used for diagnosis of Chlamydia trachomatis infection [123], [124], [125], [126], [127].
-
•
Pingle et al. developed a multiplex system for detection of 20 bacterial pathogens in blood (Bacillus anthracis, Streptococcus pyogenes, Bacillus cereus, Enterococcus faecalis, Streptococcus agalactiae, Neisseria meningitides, Enterococcus faecium, Staphylococcus epidermidis, Listeria monocytogenes, Staphylococcus aureus, Klebsiella pneumonia, Streptococcus pneumonia, Yersinia pestis, Francisella tularensis, Pseudomonas aeruginosa, Brucella abortus, Haemophilus influenza, Acinetobacter baumannii and Escherichia coli) [128]. Liu et al. analyzed the mutant site (T93G) in the E. coli uidA gene [129].
-
•
MLPA Multiplex identification of food pathogens such as; Bacillus cereus, Campylobacter coli, Shigella spp., Cronobacter sakazakii, Enterococcus spp., Staphylococcus aureus, Salmonella spp., Yersinia enterocolitica and Vibrio vulnificus [130].
-
•
Mycobacterium tuberculosis complex was detected by LCR directly in clinical specimens [131]. MOL-PCR technique has been used for the identification and differentiation of the 6 main Mycobacterium tuberculosis complex (MTBC) lineages associated with human with sensitivity approaching 99.2% (76). MLPA was used to evaluate drug resistance in TB infections [132].
-
•
Detection of HCV RNA using blood samples [45], [133] or from formalin-fixed paraffin-embedded tissues [134].
-
•
LCR technique has been used to detect mutations in the precore (preC), the presurface (PreS) regions in HBV genome with high detection rate [135], [136]. The rtA181 V/T and rtN236T mutations that cause Adefovir dipivoxil (ADV) drug resistance and hence treatment failure were also assessed [137], [138]. The rtM204 V and rtM204I mutations that cause resistance to lamivudine drug were detected by QDs-DNA nanosesnors [139].
-
•
It is also used in Respiratory Cyntical Virus (RSV) DNA detection even in complicated biological samples [140].
-
•
LCR is utilized in the assessment of the virulence of Newcastle disease virus isolates [141].
-
•
West Nile virus (WNV) detection in both clinical and mosquito pool samples [142], [143].
-
•
LCR was used for detecting cowpox virus in the presence of other orthopoxviruses species [144] as well as typing of dengue virus with 98.7% sensitivity, and 98.4% specificity [145]. Another study was performed by Das et al. for simultaneous identification of 32 different isolates from viral strains of Ebola, Crimean Congo fever, Marburg, Yellow fever virus, Rift Valley fever, Dengue and Lassa [146]. Bourgeois et al. used LCR to detect the 594 mutation in UL97 gene of resistant strains of human cytomegalovirus [147].
-
•
Mendoza et al. used a commercially available assay to detect human immunodeficiency virus [148].
-
•
Severe acute respiratory syndrome (SARS) epidemic was attributed to high mutation rate. The 6 found mutations (nt27827, nt22222, nt9404, nt19838, nt21721 and nt9479) were assessed by universal microarray using RT-PCR/LDR technique [149].
-
•
Detection of human papillomavirus [150].
-
•
Detection of distinct plant pathogens including Xanthomonas species, Citrus tristeza virus and Xylella fastidiosa [74].
-
•
Detection of antibiotic (ciprofloxacin and doxycycline) resistance in Bacillus anthracis, F. tularensis and Yersinia pestis [74], [75].
-
•
Detection of NIAID category B bacterial water and food-borne pathogens. A multiplex detection for 26 bacterial species have been designed across 7 different genera, including Salmonella spp., Listeria monocytogenes., Vibrio spp., Shigella spp. Yersinia enterocolitica., Campylobacter spp. and diarrheagenic Escherichia coli [151].
-
•
Ligation based leaky surface acoustic wave (LSAW) biosensor was used in the A2293G SNP genotyping of the Japanese encephalitis virus [84].
5.2. Detection of genetic diseases
-
•
LCR has been used for screening human sickle cell mutations [62], [99], [152].
-
•
LCR was used in multiplex SNP genotyping of nine SNP in p53 gene. It has advantages over conventional PCR in lack of constraints over primer size as well as the use of primers of similar sizes to facilitate optimization of ligation conditions [57].
-
•
It is used to screen for the K-ras oncogene mutation at codon 12 [51], [52], [55], [63].
-
•
Genotyping the MIR196A2 polymorphism In HBV and HCC patients [54].
-
•
Multiplex detection of mutations across CFTR gene using PCR-Oligonucleotide ligation assay. Nine mutations clustered in 2 exons [153] 15 mutations [154] and 31 mutations in Italian patients [155]. The ΔF508 deletion in the CFTR gene was detected using DOL technique [78] as well as the W1282X and G551D mutations using LCR technique [156].
-
•
Detection of the p.A136 V mutation in PRNP gene [65].
-
•
Detection of low abundant CD17 (A > T) mutation on β-globin gene [66].
-
•
Detection of cytochrome P450 monooxygenase CYP2C19*2 mutation [97].
-
•
Detection of familial breast cancer mutations namely 5382insC and 185delAG in the BRCA1 gene [78].
-
•
Detection of the H63D mutation in the HFE gene [78].
-
•
Detection of the 39C/T mutation in the β −globin gene responsible for β −thalassemia in Sardinia [78], [157].
-
•
Detection of the T341C and C282T variants in the N-acetyl-transferase (NAT2) gene [78].
-
•
LDR was implemented to detect and quantify the A3243G mutation that is associated with mitochondrial encephalopathy, myopathy, stroke and lactic acidosis [158].
-
•
LCR was used to detect three Leber’s hereditary optic neuropathy (LHON) associated mitochondrial DNA mutations, G3460A, G11778A, and G14459A [159].
-
•
MLPA was implemented for the determination of variation in copy number that is associated with limb girdle muscular dystrophy [160].
6. Comparative evaluation for various versions of LCR and ligation based amplifications
Conventional LCR technique has been used routinely as an inexpensive and specific technique for detection of wide range of samples (Table 1 ) with 97.6% sensitivity [48], [49]. Samples are amplified exponentially and therefore this technique was suitable for detection of limited samples. Limitations in multiplexing potential and high-throughput along with high background signal due to target independent ligation, especially in absence of target DNA, were the main drawbacks for conventional LCR [48], [49], [154]. The conventional method requires a conventional thermal cycler with either an autoradiography, spectrophotometric or fluorometric detection method [99], [100], [101], [102], [103], [104], [110], [111]. Electrochemical detection was recently enabled with conventional LCR through the use of microdevices and a potentiostat with 3 electrode system [115], [116]. Nested LCR was developed to overcome the high background signal obtained in conventional LCR due to target independent ligation especially in the absence of target DNA [58]. This was achieved through amplification of each template strand separately prior to ligation reaction. Nested LCR is not common at all and is considered to be more expensive than conventional LCR as it requires more reagents and reactions. LDR is one of the most widely used versions of LCR that has overcome several drawbacks reported earlier on for conventional LCR [51], [52], [53], [54], [55], [56], [57], [149], [150], [151], [152], [153], [154], [155]. In this technique, only two oligonucleotides are used for linear amplification of samples and this in turn preserves the ratio for the two alleles and also reduces background signal for target independent ligation. Various detection methods such as microarray, SERS, FRET, capillary electrophoresis and magnetic beads have been integrated with LDR for improved sensitivity, multiplexing potential and high-throughput applications [51], [52], [53], [54], [55], [56], [57], [101], [105], [106], [107], [108], [119]. LDR can detect as low as 1 mutant allele in 1000 copies of wild type sample with a detection limit of 0.4-1fM [101], [106], [107].
Table 1.
Comparative Evaluation For Various Versions Of Ligase Chain Reaction (LCR) And Ligation-Based Amplifications .
| Assay | Points of Strength | Points of Weakness | Discrimination power/ Sensitivity |
Cost | Time to run | Multiplexing potential/ some measure of throughput |
Special Equipment needed | Commonly used? |
Detection Method | Class of Allele Discrimination that method has been used for | Are quantitative data produced? | Are commercial versions available? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LCR | - Exponential amplification and is therefore useful for limited samples (48–49) - More Specific than conventional PCR (48–49) - Relatively inexpensive (49) - Various detection methods (49, 99, 100, 102, 103 110, 111, 115, 116) - Commonly used with wide applications (49, 58, 116, 136, 141, 144, 147, 152) |
- High background signal due to target independent ligation (49) - Moderate sensitivity for older versions, advanced detection assays have been integrated with recent versions for greater sensitivity and automation - Limited multiplexing potential (49) - Limited high-throughput |
- 97.6% in comparison to PCR (48) | Variable cost based on readout equipment (vary from inexpensive to expensive) | Variable based on detection method used | Has limited potential for multiplexing assays (154) | - Thermal cycler and either - Spectrophotometer (104) - Or fluorometer (100, 102, 103, 110) - Or silicon glass microchip and a microchip capillary electrophoresis (111) - Or microdevice and a potentiostat with 3 electrode system (116) |
Common (49, 58, 99, 100, 102, 103, 104, 110, 111, 115, 116, 136, 141, 144, 147, 152) | Variable - Autoradiography (49, 99) - Colorimetric (104) - Fluorescence (100) - MEIA and fluorescence (102) - MEIA and luminescence (103) - FRET (110) - Microchip capillary electrophoresis (111) - Electrochemical detection (115,116) |
- β-sickle cell hemoglobinemia (58, 49, 100,152) - Leber's hereditary optic neuropathy (104) - Cystic fibrosis (49, 156) - Hyperkalemic periodic paralysis (49) - Bovine leukocyte adhesion deficiency (49) - Mycobacterium tuberculosis (49, 103) - Listeria monocytogenes (49) - Ha-ras protooncogene (49) - Human papillomavirus (49) - Herpes simplex virus (49) - HIV (49) - Breast cancer in cell lines (116) - Precore mutation of HBV (136) - Virulence of Newcastle disease virus (141) - Cowpox virus in the presence of other orthopoxviruses species (144) - 594 mutation in UL97 gene of resistant strains of human cytomegalovirus (147) - G3460A, G11778A, and G14459A in Leber’s hereditary optic neuropathy (LHON) associated mitochondrial DNA mutations (159) |
- Yes | - No |
| 1- LDR | - Low background since only one pair of oligonucleotides is used and therefore no chances for target independent blunt ligation (49) - Accurately preserving the ratio of the two alleles (49) - Higher multiplexing ability (146) - Can be scaled for high-throughput (51–52, 55–56) - High sensitivity (101, 106, 107, 145) - Commonly used with wider applications (49, 51–57, 101, 105, 107, 119, 108, 128, 137, 138, 140, 142, 145, 146, 149–155, 158) - Various detection methods (49, 51–57, 101, 105–108, 119) |
- Linear and limited amplification especially for very limited mutant allele/samples (49) - Amplified products reflect target DNA in the original specimen (49) |
- Ability to detect 1 mutant in 1000 copies of wild type with a detection limit as low as 0.4-1fM (101, 106, 107) - The detection threshold ranged from 0.004 to 0.7 plaque forming unit (PFU)/reaction. (145) |
- Variable cost based on readout equipment (Vary from inexpensive to very expensive) | - Variable based on detection method used | - Yes - Used for detecting 4 malaria species using different sizes of primers (53) - Also used in Kras genotyping (55) - The PCR/LDR has a significant advantage in testing 9 agents simultaneously and is amenable to automation. (146) |
- Thermal cycler and either - Spectrophotometer (105–107) - Or fluorometer (49, 51–53, 57, 101) - Or Genetic analyzer (49, 53, 57) - Or microarray chip and a scanner (55–56) |
Very Common (49, 51–57, 101, 105–108, 119, 108, 128, 137, 138, 140, 142, 145, 146,149, 150–155, 158) | - Detection of fluorescently labeled primers (49, 53, 57) - Capillary electrophoresis (51,52) - MEIA and fluorescence (101) - Colorimetric using gold nanoparticles (105) - Colorimetric using magnetic beads (106, 107) - FRET (108) - SERS (119) - DNA microarray platform with zip codes and fluorescent labels (55, 56) |
- Cystic Fibrosis (49, 153–155) - CYP2D6 and CYP2C19 SNPs (105) - β-globin thalassemia (101) - Malaria causing parasite(53) - tp53 SNP (57) - K-ras (51–56, 107, 119) - MIR196A2 polymorphism in HBV and HCC (54) - Staphylococcus. aureus (108) - Staphylococcus. epidermidis (108) - E. coli K-12 (108) - 20 bacterial pathogens in blood (Bacillus anthracis, Streptococcus pyogenes, Bacillus cereus, Enterococcus faecalis, Streptococcus agalactiae, Neisseria meningitides, Enterococcus faecium, Staphylococcus epidermidis, Listeria monocytogenes, Staphylococcus aureus, Klebsiella pneumonia, Streptococcus pneumonia, Yersinia pestis, Francisella tularensis, Pseudomonas aeruginosa, Brucella abortus, Haemophilus influenza, Acinetobacter baumannii and Escherichia coli) (128) - HBV genotyping and also rtA181V/T and rtN236T mutations that cause HBV drug resistance (137,138) - Respiratory Cyntical Virus (RSV) detection (140) - West Nile virus (WNV) (142) - Dengue virus genotyping (145) - 32 different isolates from viral strains of Ebola, Crimean Congo fever, Marburg, Yellow fever virus, Rift Valley fever, Dengue and Lassa (146) - nt27827, nt22222, nt9404, nt19838, nt21721 and nt9479 in severe acute respiratory syndrome SARS (149) - human papillomavirus (150) - NIAID category B bacterial food and water-borne pathogens (151) - A3243G mutation that is associated with mitochondrial encephalopathy, myopathy, stroke and lactic acidosis (158) |
- Yes | - No |
| 2- Nested LCR | - Diminished target independent ligation with improved signal to noise ratio as primers generated from primary amplification step are used in secondary amplification step (58) | - Organic solvents such as ethylene glycol, DMSO, formamide or glycerol should be added to decrease Tm value for ligated primers (58) - More expensive than conventional LCR - No multiplexing ability - No high-throughput - Requires more time since 2 reactions are used prior to ligation (58) - Not common (58) |
- Not reported | - Slightly more expensive than traditional LCR due to the use of two separate reactions for each amplification step with more reagents but is considered cheaper than recently developed LCR (58) | - 30–45 min for LCR in each reaction tube (60–90 min for 2 tubes), then another 30–45 for LCR in combined tubes (58) | - No - As each SNP needs two separate tube reactions before ligation |
- Thermal cycler and either - A flurometer - Or scintillation counter |
- Not Common | - Autoradiography (58) - Or Fluorescence (58) |
- Not reported | - No | - No |
| 3- GLCR | - Suitable for limited mutant alleles/samples (59) - Greater specificity and less false positive than LCR 60) - Improved sensitivity compared to conventional LCR (46) - No target independent ligation (46, 59, 60) - Suitable for high-throughput applications - Specificity is improved with a mismatch at penultimate 3′end (59) - Not tedious (62, 63) - Commonly used in wide applications (49, 60–63,118-127, 131, 135, 148, 156) |
- The use of multiplex G-LCR gave a lot of false positive results, while monoplex G-LCR was false-positive free. (63) | - 1 mutant allele in million wild type alleles (63). - Can detect down to 10 target molecules in a reaction (59) - Can detect 10 copies in presence of wild type (1 to 5% of total DNA) (60) |
- Variable based on readout equipment but in general it is relatively inexpensive | - 1.5-2 h for GLCR (62,63) - 1.5 h for colorimetric detection - Or 5 h for fluorometric detection (62) |
- No - As it gives false positive results (63) |
- Thermal cycler and either - Spectrophotometer (62) - Or fluorometer (62) - Or Abbott LCx analyzer (121, 148,168) |
- Common | - Colorimetric (62) - Or Flurometric (62) - Or MEIA and fluorescence (59) - Or autoradiography (49) - Or electrochemiluminiscence and magnetic beads (60) - Or Quantum dot (118) - Or genetic analyzer (63) |
- Cystic Fibrosis (49, 156) - Neisseria gonorrhea (49, 120–125) - Chlamydia trachomatis (49, 61, 120–121, 123–127) - HIV (59, 148) - GJB2 235delC (62) - β-globin thalassemia (62) - G145R mutation in HBsAg (60) - K-ras (63, 118) - Sickle cell anemia (62) - Mycobacterium tuberculosis (131) - Mutations in the precore (preC), the presurface (PreS) regions in HBV genome (135) |
- Yes | - LCx Abbott Diagnostics was approved by FDA in 1994 and was taken off the market in 2002 |
| 4- QGLCR | - Quantitative results obtained in real time (64) - Robust and can be monitored accurately after each cycle (64) - Sensitive (64–66) - Specific (64–66) - High-throughput applications (64–66) |
- Relatively Expensive - Limited multiplex potential - Requires real-time PCR machine (64–66) |
Detection level of 1:10,000 or greater. (64) | Relatively Expensive | 1.5 h (64) | - Although this technique has the potential for limited multiplexing reactions through the use of available fluorescent dyes and their corresponding quenchers, no reported data yet in this regards | - Real time PCR | - Common | - FRET detection using real time PCR | - K-ras mutations (64) - p-53 (64) - PRNP mutation (p.A136V)(65) - CD17 (A>T) mutation on β-globin (66) |
- Yes | - No |
| 5- AGLCR | - The first developed technique to detect SNPs in RNA molecules through reverse transcription into DNA molecules which are detected 10 fold better than for RNA molecules (45) -Sensitive - Can be automated - Not time consuming - Relatively inexpensive |
- Requires special design of probes, since the first amplification depend on using 3 primers (45) - No multiplexing ability - Not common with very limited applications - Limited high-throughput |
- Can detect less than 50 RNA transcripts (45) - Can detect up to 20 copies of recombinant RNA templates (45) |
- Relatively inexpensive | - 35 min for RT - 45 min for GLCR |
- No | - Thermal cycler and MEIA reader Abbott IMX | - Not Common | - MEIA | - HCV RNA detection (45) | - Yes | - No |
| 6- LCR with Cleavase Mediated Correction | - Excludes possibility of target independent ligation through the use of cleavase enzyme (67) - Relatively robust - Does not require sophisticated equipment neither advanced detection assay |
- Decrease in LCR signal with cleavase-mediated correction when compared to conventional LCR (67) - No multiplexing ability (67) - No high-throughput (67) - Not common |
- 30 aM of DNA (67) | - Relatively inexpensive | - 3 h for LCR - 2–10 minutes for cleavase mediated correction alone (67) |
- No (67) | - Thermal cycler and Titer plate reader | - Not common | - Colorimetric detection (67) | - Not reported | - Yes | - No |
| 7- MLPA | - The most commonly used technique with wide applications - Commercially available and trusted - Robust (68, 72–73) - Simple (68, 72–73) - Stuffer sequence could be easily tailored for multiplexing purposes (68, 72–73) - Improved sensitivity (68, 72–73) - Improved specificity (68, 72–73) - Can also be used for SNP detection in RNA following reverse transcription (68–71) - High-throughput and fully automated (68, 72–73) |
- Expensive if genetic analyzer or a microarray scanner to be used - Low cost could be significantly reduced through the use of fabricated electrode array (73) |
- 25 pM (73) - Detect gain or loss of one copy of a single exon in human DNA samples as small as 20 ng. - Can detect up to 50 different SNPs/ reaction (68) |
- Expensive if genetic analyzer or a microarray scanner to be used - Low cost could be significantly reduced through the use of fabricated electrode array (73) |
- Variable based on detection method used | - Yes - Has the ability to detect 50 SNPs simultaneously (68) - Was applied for detection of 7 circulating markers for breast cancer (73) |
- Thermal cycler and - Genetic analyzer (68) - Or Microarray chip and a scanner (72) - Or electrode array (73) |
- Very common | - Capillary electrophoresis Genetic Analyzer (68) - Or Microarray chip (72) - Or Electrochemical detection through hybridization (73) |
- Transgenic mouse alleles (71) - Breast cancer (73) - Methylation in Prader-Willy syndrome (70) - Methylation in Angelman syndrome (70) - Methylation in acute myeloid leukemia (70) - Potato virus (72) - Bacillus cereus (130) - Campylobacter coli (130) - Shigella spp.(130) - Cronobacter sakazakii (130) - Enterococcus spp.(130) - Staphylococcus aureus (130) - Salmonella spp. (130) - Yersinia enterocolitica (130) - Vibrio vulnificu (130) - Mycobacterium tuberculosis (132) - β-globin thalassemia (157) - limb girdle muscular dystrophy Genotyping (160) |
- Yes | - Yes MRC-Holland® |
| 8- MOL-PCR | - Ease of high level of multiplexing (74) - Improved sensitivity (76) - Enhanced specificity - Reduced cost for multiplexing reactions (76) - Robustness and reduced analysis time (74) - High-throughput with full automation - Commonly used with wide applications |
- Requires expensive luminex flow cytometry analyzer and the use of xMAP microsphere | - less than 1000 molecules (74) - Detection limit of as low as 0.1 ng of input DNA (76) |
- Reagent costs/SNP is estimated to be 0.8 euro in a singleplex reaction and drops down to 0.15 for 8-plex reaction (76). Although reagents are not expensive, high costs are needed once for the Luminex flow cytometry analyzer - Costs could also be reduced significantly by using less beads and universal biotinylated oligonucleotide |
- Reading time for a 96 well plate is less than 45 min with quick analysis time in 30 s for multiplexed samples through the use of flow cytometry (74) | - Yes - Detection of 50 SNPs/well in a 96 well plate (77) |
- Thermal cycler and Flow cytometry based analyzer (Luminex) and xMAP microsphere beads (74–76) | - Very common | - Flow cytometric detection using xMAP microsphere and analyzer (74–76) | - Detection of plant pathogens such as Citrus tristeza virus (CTV), Xanthomonas genus and Xylella fastidiosa (74,75) - Antibiotic resistant bacteria such as Yersinia pestis, F. tularensis and Bacillus anthracis (74,75) - Mycobacterium tuberculosis complex (MTBC) (76) |
- Yes | - No |
| 9- DOL | - Fully automated (78) - Simple (78) - Robust in limited time (78) - Multiplex potential (78) - Specific (78) - DOL assay has built-in internal controls (78) - Scalable for high-throughput applications |
- Although real-time PCR is usually used to monitor changes in fluorescence intensity (78) which adds to the total cost, fluorescence plate reader could be used instead to reduce costs - Requires also dye-labeled ligation probes (78) |
- Not reported | - Relatively expensive | - The entire assay takes <3 h (78) | - Yes - Multiplex assays can be run under the same standard condition of the reaction by designing PCR primers and ligation probe (78) |
- Real time PCR | - Common | - FRET in real time (78) | - CFTR (78) - BRCA1 (78) - HFE (78) - β-globin (78) - NAT2 (78) |
- Yes | - No |
| 10- SNPlex Genotyping System | - High multiplexing potential with high throughput capability as it can detect several hundred SNPs in a hundred or more samples at the same time. (80) - Highly sensitive - Highly specific - High throughput - Commonly used and commercially available |
- Requires special and expensive equipment (80) - Requires 2 days for running the full assay |
- Less than 1 ng | - Expensive | - 2 days for the whole assay (80) | - Yes - 48 SNPs at a time |
- 3730xl DNA Analyzer (Applied Biosystems) (80) | - Very common | - Capillary electrophoresis - Genetic Analyzer 3730xl DNA Analyzer (Applied Biosystems) (80) |
- 521 SNPs in Human (79) - SNPs in grapevine (Vitis vinifera L.) (80) - 29 insertion/deletion SNPs in Chinese Han samples (81) |
- Yes | - Yes SNPlex™ Genotyping System 48-plex |
| 11- QCM and LSAW | - Highly sensitive (82–83) - Can be used directly for unamplified DNA samples at microgram level (83) - Robust and requires less experimental time - Inexpensive - Portable (83) |
- No multiplexing | - 0.1 nM for Beta-thalassemia detection (82) - 80fM with silver deposition (83) 1 × 10(-12) mol/L for LSAW |
- Inexpensive | - For optimal set up, hybridization time was allowed for 1 h (82) - Color development/silver deposition for 30 min |
- No | - Quartz crystal - Microbalance and either - Microgravimetric analysis - Or Linear sweep voltammetry |
- Common | - Quartz crystal - Microbalance and microgravimetric analysis (83) - Or Linear sweep voltammetry for measurement of silver deposition (84) |
- β-thalassemia SNP (82) - k-ras (83) - Japanese encephalitis virus (84) |
- Yes | - No |
| 12- RCA based ligation | - Highest sensitivity approaching 2 amol (86) - Highly specific - Limited operational time - Multiplexing potential - Scalable for high-throughput analysis - Commonly used with wide applications (86, 87, 91) |
- Very expensive if using ICP-MS or chemiluminescence imager (88, 90). - Costs could be reduced significantly with the use of electrochemical workstation or colorimetric detection (91) |
- Down to 2 amol for mutant alleles (86) | - Very expensive if using ICP-MS or chemiluminescence imager. - Costs could be reduced significantly with the use of electrochemical workstation or colorimetric detection |
- Assay can be accomplished in 70 min (85) | - Yes - Using QDs barcodes (85) |
- Electrochemical workstation (85–87) - Or Chemiluminescence imager (88) - Or Mass spectrometry (90) - Or colorimetry (91) |
- Common | - Altering current voltammetry (85–87) - Inductively coupled plasma mass spectrometry (ICP-MS)(90) - Chemiluminescence (88) - Colorimetric detection (91) |
- p53 SNPs (86) - hly genes of Listeria monocytogenes (87) - H5N1 influenzavirus (91) |
- Yes | - No |
| 13- ONS electrochemical biosensor enabled ligation | - Specific - Sensitive - 16 electrode array with high-throughput analysis - High signal/noise ratio - The array can be stored for 5 days in a fridge with high stability - Cost effective |
- Limited multiplexing potential | - 10% of mutant gene could be detected with 16 fold signal distinction | - Cost efficient | - 3 h for ONS preparation on gold electrode - 1hour for annealing, ligation, denaturation and signal development |
- Yes | - Thermal cycler and - 16 electrode sensor array and 16-channel PM3000 workstation |
- Common | - Cyclic voltammetric and amperometric measurement | - Detection of the pre-core G1896A mutation in HBV (92) - Detection of two SNPs of the human CYP2C19 genome C680T and G681A. (92) - (T93G) mutation in the E. coli uidA gene (129) |
- Yes | - No |
| 14- AuNP enabled LCR | - Extremely sensitive - Very simple - Specific - portable - Real time monitoring - Eliminates time required for post reaction analysis (93) - Inexpensive |
- No multiplexing - No high-throughput |
- As low as 1.5 aMcan be detected (94) - 1000 times enhanced specificity (42) |
- Inexpensive | - Preparation of capture probe coated NPs requires 1 day - Ligation and detection approximately 30–60 min (93) |
- No | - Thermal cycler and either - a spectrophotometer - Or SPR platform |
- Common (42, 93, 94) | - Colorimetric detection (42) - Fiber optic surface plasmon resonance (93) - Dynamic light scattering (94) |
- K-ras mutations (42) | - Yes (42, 93, 94) | - No |
| 15- LCR based DNAzyme-CRET magnetic biosensor | - Sensitive (95) - Highly specific - Exponential amplification (95) - Rapid (95) - Operational easiness through magnetic separation. (95) |
- No multiplexing potential - Designing 4 probes for each SNP - Limited high-throughput |
- Detection limit down to 0.86fM DNA (95) - Detection achieved with a wild-type to mutant ratio of 10,000:1. (95) |
- Relatively inexpensive | - LCR (1.5 h) (95) - CRET imaging (1 h) (95) |
- No | - Thermal cycler and - CRET imaging system with a cooled low-light CCD and a magnet (95) |
- Common | - CRET (95) | - Not reported | - Yes | - No |
| 16- Ligation mediated SDA | - Sensitive (96–97) - Specific - Robust (97) - Use of magnetic separation decreased background signals. (96) - High signal to noise ratio (96,97) - Can be automated - Can be scaled for high-throughput - Multiplex potential - Commonly used (96–98) |
- Limited multiplexing potential | - Mutant DNA can be noticeable that as low as 1:400 (96) - Detection limit of 0.1 fM (97) |
- Cost effective | - Ligation (30 min) - SDA (2 h) (97) Chemiluminiscence (30 min) |
- Yes - Can be used for simultaneous detection of various SNPs. (96) |
- Spectrofluorometer (97) | - Very Common | - Chemiluminiscence (97) - Fluorescence intensity (98) |
- K-ras SNPs (96) - CYP2C19*2 (97) - p-53 SNPs (98) |
- Yes | - No |
GLCR utilizes four oligonucleotides that hybridize with template strands leaving a gap to be filled first with DNA polymerase prior to ligation and exponential amplification [59], [60]. This version has diminished background signal attributed to target independent ligation with increased sensitivity (detection of one mutant allele in million copy of a wild type allele) and specificity compared to conventional LCR [46], [59], [60]. It is robust with high capability for high-throughput applications and has been commercialized by Abbott Diagnostics company as Abbott LCx® which was FDA approved since 1994 [62], [63]. It has been widely applied for detection of various microorganisms such as Mycobacterium tuberculosis, Chlamydia trachomatis and Neisseria gonorrhea [49], [120], [121], [122], [123], [124], [125], [126], [127], [131]. The main disadvantage for this version is that it is not suitable for multiplexing applications due to false positive results obtained and therefore it was taken off the market in 2002 [63]. QGLCR is a real time quantitative version of GLCR. It is very robust and data is obtained after each amplification cycle with enhanced sensitivity, specificity and high-throughput applications [64], [65], [66]. It is relatively expensive as it requires a real time PCR machine to detect FRET from a fluorescent dye into a quencher and has limited applications. The reported multiplex potential for this technique is very limited.
AGLCR was the first developed LCR version for detection of RNA molecules through reverse transcription into cDNA that were detected 10 times better than RNA copies [45]. It is robust and relatively inexpensive with a detection limit down to 20 copies of RNA molecules [45]. This technique is not common as it has several drawbacks such as lack of multiplexing potential, requirement for special design of probes and limited high-throughput applications. LCR with cleavase mediated correction eliminates signal background associated with target independent ligation. It does not require sophisticated equipment and detection is enabled through colorimetric analysis with relative robustness [67]. Detection limit was reported to be as low as 30 aM. It does not have multiplex potential nor high-troughput applications with very limited applications and therefore it is not considered to be a common technique.
MLPA is the most widely used LCR based technique for SNP genotyping [68], [69], [70], [71], [72], [73], [130], [132], [157], [160]. It is very simple, robust, highly sensitive, specific, fully automated with multiplexing potential and high-throughput applications. It can detect up to 50 different SNPs at a time with a detection limit of as low as 25pM [68], [73]. It has been widely used for various genotyping platforms. It has been developed by MRC Holland since 2002. Unlike the GLCR based LCx Abbott Diagnostic technique, the MLPA technique is considered to be the most effective and the most favored LCR technique for SNP genotyping. The only drawback for this powerful technique is high incurred cost with the use of either a genetic analyzer or a microarray scanner. This could be overcome through the use of fabricated electrode array [73]. MOL-PCR is another widely used LCR technique that is similar to MLPA but with flow cytometric detection that enabled better sensitivity and faster analysis [74], [75], [76]. It is widely used with multiplexing potential and high-throughput application as it can detect 50 SNPs/well in a 96 well plate. It requires very expensive flow cytometry based analyzer (Luminex) and xMAP microsphere beads. DOL utilizes dye labeled ligation probes and FRET detection with real-time monitoring for presence of alleles. This assay is very simple, sensitive, specific and amenable for multiplexing and high-throughput analysis. The entire assay takes less than 3 h [78].
The SNPlex genotyping system is the second most widely used LCR technique, following the MLPA technique that was developed by Applied Biosystems [80]. It is very sensitive as it can detect less than 1 ng of template. It has high multiplexing potential with high-throughput capability as it can detect several hundred SNPs in a hundred or more samples at the same time [79], [80], [81]. It has been used successfully for the detection of 521 SNPs in Human [79], SNPs in grapevine (Vitis vinifera L.) [80] and 29 insertion/deletion SNPs in Chinese Han samples [81]. The main drawback for this assay is that is takes 2 days for the whole assay and is very expensive as it requires the 3730xl DNA Analyzer.
QCM and LSAW are used as inexpensive, portable, robust and sensitive LCR techniques. They can detect as low as 80 fM with silver deposition within 90 min only [82], [83]. These assays do not support multiplexing applications. ONS electrochemical biosensor is another version for modified LCR that is used for detection of SNPs via cyclic voltammetric and amperometric measurement but with limited multiplexing potential [92], [129]. 10% of mutant gene could be detected with 16 fold signal distinction. It is also inexpensive and stable with high signal/noise ratio. The whole assay takes around 4 h. AuNP enabled LCR is a portable, inexpensive technique that is extremely sensitive with real-time monitoring capability [42], [93], [94], It can detect as low as 1.5aM [94]. Preparation of probe coated gold nanoparticles takes 1 day and ligation assay takes less than an hour. Colorimetric methods, surface plasmon resonance or dynamic light scattering could be used for detection [42], [93], [94]. The main drawbacks for this assay are lack of multiplexing potential and high-throughput applications.
RCA based ligation is a commonly used LCR technique with detection limit approaching 2 amol for mutant alleles. It has multiplexing and high-throughput capabilities [85], [86], [87], [88], [90], [91]. It takes 70 min for the whole assay. Detection methods using either ICP-MS or chemiluminescence make this assay very expensive. Costs could be reduced significantly with the use of electrochemical workstation or colorimetric detection. LCR based DNAzyme-CRET magnetic biosensor is another modified LCR version that enables detection down to 0.86fM [85]. It is robust as it takes 3 h for the whole assay. It has no multiplexing potential with limited high-throughput applications. Ligation mediated SDA is a cost effective and sensitive version of LCR with detection limit down to 0.1 fM [97] with high signal/noise ratio due to the use of magnetic separation. It is a robust assay that takes 3 h for the whole assay with the possibility for high-throughput applications but with limited multiplexing potential. It is commonly used with wide application in various fields [96], [97], [98].
7. Concluding remarks
Several genetic variants have been reported to be causing factors for susceptibility to many genetic diseases such as cancer, sickle cell anemia and retinitis pigmentosa. Early screening for these genetic variants is of extreme importance as it could be considered as a warning alarm to take all possible parameters that could help in alleviating disease severity, progression and, if possible, launching of early medication to save lives. This necessitates the use of advanced bio-sensing platforms that are sensitive, specific, robust and affordable with multiplexing and high-throughput capabilities without being expensive and time consuming. Next generation and Sanger sequencing have been used successfully for this purpose for a while but there are still many limitations with this approach as sequencing is very expensive, unaffordable by many researchers, time consuming and also challenging. Several other affordable screening assays have been developed over the past few decades with certain limitations in specificity, sensitivity, cost effectiveness and/or time savings.
LCR was reported in 1991 and was proven to be more specific and sensitive than gold standard PCR method due to the use of DNA ligase enzyme that seals only adjacent target complementary probes prior to amplification. Since that time, LCR was considered the most favored platform for SNP genotyping as it was inexpensive, affordable and time saving. The main limitations for LCR were lack of multiplexing capabilities and high-throughput applications. Over the past few decades, several modifications and detection strategies have been introduced to both LCR and ligation based amplifications to overcome those limitations. This review outlines and evaluates recent advances and modifications introduced to LCR and ligation based amplifications such as electrochemical and magnetic biosensors, nanoparticles, quantum dots, DNAzyme, quartz crystal and leaky surface acoustic wave biosensors, rolling circle amplification, strand displacement amplification, surface enhanced raman scattering, chemiluminescence and fluorescence resonance energy transfer. Particular emphasis on sensitivity and limitation parameters for each platform is also detailed. Some of those integrated technologies have been commercialized for real applications in various fields. SNP genotyping assays for diverse genetic diseases, bacterial and viral pathogen detections are also discussed in this article. The versatility of these integrated technologies could pave the way towards achieving ideal multiplex SNP genotyping assays that are affordable with enhanced sensitivity, specificity, simplicity, robustness and high-throughput analyses in genome wide screening. Currently, the only limitation left for these advanced technologies is our imagination for such powerful innovations.
Key points
-
(1)
MLPA multiplex technique can detect up to 50 SNPs in one reaction with enhanced sensitivity down to 25 pM when integrated with microarray biosensor.
-
(2)
LCR based DNAzyme-fluorescein CRET enabled the detection of as low as 0.86 fM.
-
(3)
The reported sensitivity for FRET assisted LCR is estimated to be as low as 10 aM.
-
(4)
Introducing SA coated magnetic beads to ligation based RCA significantly improved sensitivity down to 2 amol.
-
(5)
LCR based AuNP biosensor dramatically enhanced sensitivity to as low as 1.5 aM with the ability for either colorimetric or dynamic light scattering detection.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
Dr. Gibriel was supported by the YIRG Research fund from the British University in Egypt (YIRG2015.7)
References
- 1.Brookes A.J. The essence of SNPs. Gene. 1999;2:177–186. doi: 10.1016/s0378-1119(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 2.Wang D.G., Fan J.B., Siao C.J. Large-scale identification, mapping, and genotyping of single-nucleotide polymorphisms in the human genome. Science. 1998;280:1077–1082. doi: 10.1126/science.280.5366.1077. [DOI] [PubMed] [Google Scholar]
- 3.Kruglyak L., Nickerson D.A. Variation is the spice of life. Nat. Genet. 2001;3:234–236. doi: 10.1038/85776. [DOI] [PubMed] [Google Scholar]
- 4.Kawahara M., Sakayori M., Shiraishi K. Identification and evaluation of 55 genetic variations in the BRCA1 and the BRCA2 genes of patients from 50 Japanese breast cancer families. J. Hum. Genet. 2004;7:391–395. doi: 10.1007/s10038-004-0160-5. [DOI] [PubMed] [Google Scholar]
- 5.Maniwa Y., Yoshimura M., Bermudez V.P. His239Arg SNP. of HRAD9 is associated with lung adenocarcinoma. Cancer. 2006;5:1117–1122. doi: 10.1002/cncr.21705. [DOI] [PubMed] [Google Scholar]
- 6.Keen T.J., Inglehearn C.F., Lester D.H. Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site. Genomics. 1991;1:199–205. doi: 10.1016/0888-7543(91)90119-y. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez J.A., Herrera C.A., Birch D.G. A leucine to arginine amino acid substitution at codon 46 of rhodopsin is responsible for a severe form of autosomal dominant retinitis pigmentosa. Hum. Mutat. 1993;3:205–213. doi: 10.1002/humu.1380020309. [DOI] [PubMed] [Google Scholar]
- 8.Saga M., Mashima Y., Akeo K. A novel homozygous Ile535Asn mutation in the rod cGMP phosphodiesterase beta-subunit gene in two brothers of a Japanese family with autosomal recessive retinitis pigmentosa. Curr. Eye Res. 1998;3:332–335. doi: 10.1076/ceyr.17.3.332.5214. [DOI] [PubMed] [Google Scholar]
- 9.Saiki R.K., Scharf S., Faloona F. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 10.Bae H.T., Baldwin C.T., Sebastiani P. Meta-analysis of 2040 sickle cell anemia patients: BCL11A and HBS1L-MYB are the major modifiers of HbF in African Americans. Blood. 2012;9:1961–1962. doi: 10.1182/blood-2012-06-432849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabriel H., Kupsch P., Sudendey J. Mutations in the connexin26/GJB2 gene are the most common event in non-syndromic hearing loss among the German population. Hum. Mutat. 2001;6:521–522. doi: 10.1002/humu.1138. [DOI] [PubMed] [Google Scholar]
- 12.Yan D., Tekin D., Bademci G. Spectrum of DNA variants for non-syndromic deafness in a large cohort from multiple continents. Hum. Genet. 2016;8:953–961. doi: 10.1007/s00439-016-1697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakchouk I., Ben Said M., Jbeli F. NADf chip, a two-color microarray for simultaneous screening of multigene mutations associated with hearing impairment in North African Mediterranean countries. J. Mol. Diagn. 2015;2:155–161. doi: 10.1016/j.jmoldx.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Shendure J., Ji H. Next-generation DNA sequencing. Nat. Biotechnol. 2008;10:1135–1145. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- 15.Travers K.J., Chin C.S., Rank D.R. A flexible and efficient template format for circular consensus sequencing and SNP detection. Nucleic Acids Res. 2010;15:e159. doi: 10.1093/nar/gkq543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamoto S., Kanda T., Imazeki F. Simple assay based on restriction fragment length polymorphism associated with IL28 B in chronic hepatitis C patients. Scand. J. Gastroenterol. 2011;7–8:955–961. doi: 10.3109/00365521.2011.574731. [DOI] [PubMed] [Google Scholar]
- 17.Yoon Y.J., Chang H.Y., Ahn S.H. MDM2 and p53 polymorphisms are associated with the development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Carcinogenesis. 2008;6:1192–1196. doi: 10.1093/carcin/bgn090. [DOI] [PubMed] [Google Scholar]
- 18.Livak K.J. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet. Anal. 2016;999(5–6):143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 19.Tyagi S., Bratu D.P., Kramer F.R. Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 1998;1:49–53. doi: 10.1038/nbt0198-49. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L., Cui G., Li Z. Comparison of high-resolution melting analysis, TaqMan Allelic discrimination assay, and sanger sequencing for Clopidogrel efficacy genotyping in routine molecular diagnostics. J. Mol. Diagn. 2013;5:600–606. doi: 10.1016/j.jmoldx.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Kamau E., Alemayehu S., Feghali K.C. Development of a TaqMan Allelic Discrimination assay for detection of single nucleotides polymorphisms associated with anti-malarial drug resistance. Malar. J. 2012:1123. doi: 10.1186/1475-2875-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozlowski P., Krzyzosiak W.J. Combined SSCP/duplex analysis by capillary electrophoresis for more efficient mutation detection. Nucleic Acids Res. 2001;14:E71. doi: 10.1093/nar/29.14.e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozlowski P., Krzyzosiak W.J. Optimum sample medium for single-nucleotide polymorphism and mutation detection by capillary electrophoresis. Electrophoresis. 2004;7–8:990–998. doi: 10.1002/elps.200305782. [DOI] [PubMed] [Google Scholar]
- 24.Gibriel A.A., Tate R.J., Yu Y. The p.Arg86Gln change in GARP2 (glutamic acid-rich protein-2) is a common West African-related polymorphism. Gene. 2013;1:155–158. doi: 10.1016/j.gene.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Fulton R.E., Salasek M.L., DuTeau N.M. SSCP analysis of cDNA markers provides a dense linkage map of the Aedes aegypti genome. Genetics. 2016;200(2):715–726. doi: 10.1093/genetics/158.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keen J., Lester D., Inglehearn C. Rapid detection of single base mismatches as heteroduplexes on Hydrolink gels. Trends Genet. 1991;1:5. doi: 10.1016/0168-9525(91)90004-a. [DOI] [PubMed] [Google Scholar]
- 27.Nagamine C.M., Chan K., Lau Y.F. A PCR artifact: generation of heteroduplexes. Am. J. Hum. Genet. 1989;2:337–339. [PMC free article] [PubMed] [Google Scholar]
- 28.Howell W.M., Jobs M., Gyllensten U. Dynamic allele-specific hybridization: a new method for scoring single nucleotide polymorphisms. Nat. Biotechnol. 1999;1:87–88. doi: 10.1038/5270. [DOI] [PubMed] [Google Scholar]
- 29.Prince J.A., Brookes A.J. Towards high-throughput genotyping of SNPs by dynamic allele-specific hybridization. Exp. Rev. Mol. Diagn. 2001;3:352–358. doi: 10.1586/14737159.1.3.352. [DOI] [PubMed] [Google Scholar]
- 30.Jobs M., Howell W.M., Stromqvist L. DASH-2: flexible, low-cost, and high-throughput SNP genotyping by dynamic allele-specific hybridization on membrane arrays. Genome Res. 2003;5:916–924. doi: 10.1101/gr.801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouakaze C., Keyser C., Gonzalez A. Matrix-assisted laser desorption ionization-time of flight mass spectrometry-based single nucleotide polymorphism genotyping assay using iPLEX gold technology for identification of Mycobacterium tuberculosis complex species and lineages. J. Clin. Microbiol. 2011;9:3292–3299. doi: 10.1128/JCM.00744-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurst C.D., Zuiverloon T.C., Hafner C. A SNaPshot assay for the rapid and simple detection of four common hotspot codon mutations in the PIK3CA gene. BMC Res. Notes. 2009:266. doi: 10.1186/1756-0500-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borsting C., Tomas C., Morling N. Typing of 49 autosomal SNPs by single base extension and capillary electrophoresis for forensic genetic testing. Methods Mol. Biol. 2012:83087–83107. doi: 10.1007/978-1-61779-461-2_7. [DOI] [PubMed] [Google Scholar]
- 34.Yu B., Sawyer N.A., Chiu C. DNA mutation detection using denaturing high-performance liquid chromatography (DHPLC) Curr. Protoc. Hum. Genet. 2006 doi: 10.1002/0471142905.hg0710s48. Chapter 7Unit7 10. [DOI] [PubMed] [Google Scholar]
- 35.Hansen T.V., Bisgaard M.L., Jonson L. Novel de novo BRCA2 mutation in a patient with a family history of breast cancer. BMC Med. Genet. 2008:958. doi: 10.1186/1471-2350-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones B.M., Knapp L.A. Temporal temperature gradient electrophoresis for detection of single nucleotide polymorphisms. Methods Mol. Biol. 2009:578153–578165. doi: 10.1007/978-1-60327-411-1_9. [DOI] [PubMed] [Google Scholar]
- 37.Hsia AP Wen TJ, Liu Chen HD. Temperature gradient capillary electrophoresis (TGCE) – a tool for the high-throughput discovery and mapping of SNPs and IDPs. Theor. Appl. Genet. 2005;2:218–225. doi: 10.1007/s00122-005-1997-5. [DOI] [PubMed] [Google Scholar]
- 38.Lindroos K., Sigurdsson S., Johansson K. Multiplex SNP genotyping in pooled DNA samples by a four-colour microarray system. Nucleic Acids Res. 2002;30(14):e70. doi: 10.1093/nar/gnf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunderson K.L., Steemers F.J., Lee G. A genome-wide scalable SNP genotyping assay using microarray technology. Nat. Genet. 2005;5:549–554. doi: 10.1038/ng1547. [DOI] [PubMed] [Google Scholar]
- 40.Gibriel A.A. Options available for labelling nucleic acid samples in DNA microarray-based detection methods. Brief Funct. Genom. 2012;4:311–318. doi: 10.1093/bfgp/els015. [DOI] [PubMed] [Google Scholar]
- 41.Podder M., Ruan J., Tripp B.W. Robust SNP genotyping by multiplex PCR and arrayed primer extension. BMC Med. Genom. 2008:15. doi: 10.1186/1755-8794-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen W., Deng H., Gao Z. Gold nanoparticle-enabled real-time ligation chain reaction for ultrasensitive detection of DNA. J. Am. Chem. Soc. 2012;36:14678–14681. doi: 10.1021/ja306265n. [DOI] [PubMed] [Google Scholar]
- 43.Monis P.T., Giglio S. Nucleic acid amplification-based techniques for pathogen detection and identification. Infect. Genet. Evol. 2006;1:2–12. doi: 10.1016/j.meegid.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin G.W., Chung B., Jung G.Y. Multiplex ligase-based genotyping methods combined with CE. Electrophoresis. 2014;7:1004–1016. doi: 10.1002/elps.201300361. [DOI] [PubMed] [Google Scholar]
- 45.Marshall R.L., Laffler T.G., Cerney M.B. Detection of HCV RNA by the asymmetric gap ligase chain reaction. PCR Methods Appl. 1994;2:80–84. doi: 10.1101/gr.4.2.80. [DOI] [PubMed] [Google Scholar]
- 46.Andras S.C., Power J.B., Cocking E.C. Strategies for signal amplification in nucleic acid detection. Mol. Biotechnol. 2001;1:29–44. doi: 10.1385/MB:19:1:029. [DOI] [PubMed] [Google Scholar]
- 47.Cao W. Recent developments in ligase-mediated amplification and detection. Trends Biotechnol. 2004;1:38–44. doi: 10.1016/j.tibtech.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Davis J.D., Riley P.K., Peters C.W. A comparison of ligase chain reaction to polymerase chain reaction in the detection of Chlamydia trachomatis endocervical infections. Infect. Dis. Obstet. Gynecol. 1998;2:57–60. doi: 10.1002/(SICI)1098-0997(1998)6:2<57::AID-IDOG5>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiedmann M., Wilson W.J., Czajka J. Ligase chain reaction (LCR) – overview and applications. PCR Methods Appl. 1994;4:S51–S64. doi: 10.1101/gr.3.4.s51. [DOI] [PubMed] [Google Scholar]
- 50.Xiao Z.X., Cao H.M., Luan X.H. Effects of additives on efficiency and specificity of ligase detection reaction. Mol. Biotechnol. 2007;2:129–133. doi: 10.1007/BF02686107. [DOI] [PubMed] [Google Scholar]
- 51.Thomas G., Sinville R., Sutton S. Capillary and microelectrophoretic separations of ligase detection reaction products produced from low-abundant point mutations in genomic DNA. Electrophoresis. 2004;10–11:1668–1677. doi: 10.1002/elps.200405886. [DOI] [PubMed] [Google Scholar]
- 52.Hamada M., Shimase K., Tsukagoshi K. Discriminative detection of low-abundance point mutations using a PCR/ligase detection reaction/capillary gel electrophoresis method and fluorescence dual-channel monitoring. Electrophoresis. 2014;8:1204–1210. doi: 10.1002/elps.201300584. [DOI] [PubMed] [Google Scholar]
- 53.McNamara D.T., Thomson J.M., Kasehagen L.J. Development of a multiplex PCR-ligase detection reaction assay for diagnosis of infection by the four parasite species causing malaria in humans. J. Clin. Microbiol. 2004;6:2403–2410. doi: 10.1128/JCM.42.6.2403-2410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qi P., Dou T.H., Geng L. Association of a variant in MIR 196A2 with susceptibility to hepatocellular carcinoma in male Chinese patients with chronic hepatitis B virus infection. Hum. Immunol. 2010;6:621–626. doi: 10.1016/j.humimm.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 55.Gerry N.P., Witowski N.E., Day J. Universal DNA microarray method for multiplex detection of low abundance point mutations. J. Mol. Biol. 1999;2:251–262. doi: 10.1006/jmbi.1999.3063. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto M., Barany F., Soper S.A. Polymerase chain reaction/ligase detection reaction/hybridization assays using flow-through microfluidic devices for the detection of low-abundant DNA point mutations. Biosens. Bioelectron. 2006;10:1915–1923. doi: 10.1016/j.bios.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Choi W., Shin G.W., Hwang H. A multiplex single nucleotide polymorphism genotyping method using ligase-based mismatch discrimination and CE-SSCP. Electrophoresis. 2014;8:1196–1203. doi: 10.1002/elps.201300486. [DOI] [PubMed] [Google Scholar]
- 58.Barany F. The ligase chain reaction in a PCR world. PCR Methods Appl. 1991;1:5–16. doi: 10.1101/gr.1.1.5. [DOI] [PubMed] [Google Scholar]
- 59.Abravaya K., Carrino J.J., Muldoon S. Detection of point mutations with a modified ligase chain reaction (Gap-LCR) Nucleic Acids Res. 1995;4:675–682. doi: 10.1093/nar/23.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osiowy C. Sensitive detection of HBsAg mutants by a gap ligase chain reaction assay. J. Clin. Microbiol. 2002;7:2566–2571. doi: 10.1128/JCM.40.7.2566-2571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei H., Wu S.X., Yu J.L. Development of a gap ligase chain reaction for detection of Chlamydia trachomatis in newborn infants. Zhonghua Er Ke Za Zhi. 2003;8:578–581. [PubMed] [Google Scholar]
- 62.Zhou L., Du F., Zhao Y. DNAzyme based gap-LCR detection of single-nucleotide polymorphism. Biosens. Bioelectron. 2013:45141–45147. doi: 10.1016/j.bios.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 63.Jenner S., Techel D. Development of a gLCR-based KRAS mutation detection approach and its comparison with other screening methods. Tumour Biol. 2015;8:6361–6368. doi: 10.1007/s13277-015-3323-4. [DOI] [PubMed] [Google Scholar]
- 64.Harden S.V., Thomas D.C., Benoit N. Real-time gap ligase chain reaction: a rapid semiquantitative assay for detecting p53 mutation at low levels in surgical margins and lymph nodes from resected lung and head and neck tumors. Clin. Cancer Res. 2004;7:2379–2385. doi: 10.1158/1078-0432.ccr-03-0405. [DOI] [PubMed] [Google Scholar]
- 65.Psifidi A., Dovas C., Banos G. Novel quantitative real-time LCR for the sensitive detection of SNP frequencies in pooled DNA: method development, evaluation and application. PLoS One. 2011;1:e14560. doi: 10.1371/journal.pone.0014560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yi P., Jiang H., Li L. A new genotyping method for detecting low abundance single nucleotide mutations based on gap ligase chain reaction and quantitative PCR assay. Cell Biochem. Biophys. 2012;1:161–167. doi: 10.1007/s12013-011-9277-2. [DOI] [PubMed] [Google Scholar]
- 67.Demchinskaya A.V., Shilov I.A., Karyagina A.S. A new approach for point mutation detection based on a ligase chain reaction. J. Biochem. Biophys. Methods. 2001;1:79–89. doi: 10.1016/s0165-022x(01)00178-6. [DOI] [PubMed] [Google Scholar]
- 68.Schouten J.P., McElgunn C.J., Waaijer R. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;12:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eldering E., Spek C.A., Aberson H.L. Expression profiling via novel multiplex assay allows rapid assessment of gene regulation in defined signalling pathways. Nucleic Acids Res. 2003;23:e153. doi: 10.1093/nar/gng153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nygren A.O., Ameziane N., Duarte H.M. Methylation-specific MLPA (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res. 2005;14:e128. doi: 10.1093/nar/gni127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kozlowski P., Lin M., Meikle L. Robust method for distinguishing heterozygous from homozygous transgenic alleles by multiplex ligation-dependent probe assay. Biotechniques. 2007;5:584–588. doi: 10.2144/000112473. [DOI] [PubMed] [Google Scholar]
- 72.Wang P., Guo Y., Cheng J. Development of multiplex reverse transcription-ligase detection reaction-polymerase chain reaction (MRLP) mediated universal DNA microarray for diagnostic platform. Biosens. Bioelectron. 2011;8:3719–3724. doi: 10.1016/j.bios.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez J.L., Henry O.Y., Joda H. Multiplex PCB-based electrochemical detection of cancer biomarkers using MLPA-barcode approach. Biosens. Bioelectron. 2016:82224–82232. doi: 10.1016/j.bios.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 74.Mark J.A., Green L.D., Deshpande A. System integration and development for biological warfare agent surveillance. Proceedings of SPIE, Optics and Photonics in Global Homeland Security III. 2007 (65401D) [Google Scholar]
- 75.Song J., Li P.E., Gans J. Simultaneous pathogen detection and antibiotic resistance characterization using SNP-based multiplexed oligonucleotide ligation-PCR (MOL-PCR) Adv. Exp. Med. Bio. 2010:680455–680464. doi: 10.1007/978-1-4419-5913-3_51. [DOI] [PubMed] [Google Scholar]
- 76.Stucki D., Malla B., Hostettler S., Huna T. Two new rapid SNP-typing methods for classifying Mycobacterium tuberculosis complex into the main phylogenetic lineages. PloS One. 2012;7:e41253. doi: 10.1371/journal.pone.0041253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bruse S., Moreau M., Azaro M. Improvements to bead-based oligonucleotide ligation SNP genotyping assays. Biotechniques. 2008;5:559–571. doi: 10.2144/000112960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X., Livak K.J., Kwok P.Y. A homogeneous, ligase-mediated DNA diagnostic test. Genome Res. 1998;5:549–556. doi: 10.1101/gr.8.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tobler A.R., Short S., Andersen M.R. The SNPlex genotyping system: a flexible and scalable platform for SNP genotyping. J. Biomol. Tech. 2005;4:398–406. [PMC free article] [PubMed] [Google Scholar]
- 80.Pindo M., Vezzulli S., Coppola G. SNP high-throughput screening in grapevine using the SNPlex genotyping system. BMC Plant Biol. 2008:812. doi: 10.1186/1471-2229-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li C., Zhang S., Li L. Selection of 29 highly informative InDel markers for human identification and paternity analysis in Chinese Han population by the SNPlex genotyping system. Mol. Biol. Rep. 2012;3:3143–3152. doi: 10.1007/s11033-011-1080-z. [DOI] [PubMed] [Google Scholar]
- 82.Feng K., Li J., Jiang J.H. QCM detection of DNA targets with single-base mutation based on DNA ligase reaction and biocatalyzed deposition amplification. Biosens. Bioelectron. 2007;8:1651–1657. doi: 10.1016/j.bios.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 83.Zhang P., Chu X., Xu X. Electrochemical detection of point mutation based on surface ligation reaction and biometallization. Biosens. Bioelectron. 2008;10:1435–1441. doi: 10.1016/j.bios.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 84.Xu Q., Chang K., Lu W. Detection of single-nucleotide polymorphisms with novel leaky surface acoustic wave biosensors, DNA ligation and enzymatic signal amplification. Biosens. Bioelectron. 2011;1:274–278. doi: 10.1016/j.bios.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 85.Zhu W., Su X., Gao X. A label-free and PCR-free electrochemical assay for multiplexed microRNA profiles by ligase chain reaction coupling with quantum dots barcodes. Biosens. Bioelectron. 2014:53414–53419. doi: 10.1016/j.bios.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 86.Su Q., Xing D., Zhou X. Magnetic beads based rolling circle amplification-electrochemiluminescence assay for highly sensitive detection of point mutation. Biosens. Bioelectron. 2009;7:1615–1621. doi: 10.1016/j.bios.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 87.Long Y., Zhou X., Xing D. Sensitive and isothermal electrochemiluminescence gene-sensing of Listeria monocytogenes with hyperbranching rolling circle amplification technology. Biosens. Bioelectron. 2010;6:2897–2904. doi: 10.1016/j.bios.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 88.Dong H., Wang C., Xiong Y. Highly sensitive and selective chemiluminescent imaging for DNA detection by ligation-mediated rolling circle amplified synthesis of DNAzyme. Biosens. Bioelectron. 2012:41348–41353. doi: 10.1016/j.bios.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 89.Cheng Y., Zhao J., Jia H., Yuan Z. Ligase chain reaction coupled with rolling circle amplification for high sensitivity detection of single nucleotide polymorphisms. Analyst. 2013;10:2958–2963. doi: 10.1039/c3an36920j. [DOI] [PubMed] [Google Scholar]
- 90.He Y., Chen D., Li M. Rolling circle amplification combined with gold nanoparticles-tag for ultra sensitive and specific quantification of DNA by inductively coupled plasma mass spectrometry. Biosens. Bioelectron. 2014:58209–58213. doi: 10.1016/j.bios.2014.02.072. [DOI] [PubMed] [Google Scholar]
- 91.Hamidi S.V., Ghourchian H. Colorimetric monitoring of rolling circle amplification for detection of H5N1 influenza virus using metal indicator. Biosens. Bioelectron. 2015:72121–72126. doi: 10.1016/j.bios.2015.04.078. [DOI] [PubMed] [Google Scholar]
- 92.Liu G., Lao R., Xu L. Single-nucleotide polymorphism genotyping using a novel multiplexed electrochemical biosensor with nonfouling surface. Biosens. Bioelectron. 2013:42516–42521. doi: 10.1016/j.bios.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Knez K., Spasic D., Delport F. Real-time ligation chain reaction for DNA quantification and identification on the FO-SPR. Biosens. Bioelectron. 2014:67394–67399. doi: 10.1016/j.bios.2014.08.067. [DOI] [PubMed] [Google Scholar]
- 94.Yin H., Huang X., Ma W. Ligation Chain Reaction based gold nanoparticle assembly for ultrasensitive DNA detection. Biosens. Bioelectron. 2014:12–528. doi: 10.1016/j.bios.2013.07.064. [DOI] [PubMed] [Google Scholar]
- 95.Bi S., Zhang Z., Dong Y. Chemiluminescence resonance energy transfer imaging on magnetic particles for single-nucleotide polymorphism detection based on ligation chain reaction. Biosens. Bioelectron. 2015:65139–65144. doi: 10.1016/j.bios.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 96.Shi C., Ge Y., Gu H. Highly sensitive chemiluminescent point mutation detection by circular strand-displacement amplification reaction. Biosens. Bioelectron. 2011;12:4697–4701. doi: 10.1016/j.bios.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 97.Wang H.Q., Liu W.Y., Wu Z. Homogeneous label-free genotyping of single nucleotide polymorphism using ligation-mediated strand displacement amplification with DNAzyme-based chemiluminescence detection. Anal. Chem. 2011;6:1883–1889. doi: 10.1021/ac200138v. [DOI] [PubMed] [Google Scholar]
- 98.Xu J., Wu Z.S., Li H. Dual-cyclical nucleic acid strand-displacement polymerization based signal amplification system for highly sensitive determination of p53 gene. Biosens. Bioelectron. 2016:861024–861030. doi: 10.1016/j.bios.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 99.Barany F. Genetic disease detection and DNA amplification using cloned thermostable ligase. Proc. Natl. Acad. Sci. U. S. A. 1991;1:189–193. doi: 10.1073/pnas.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winn-Deen E.S., Iovannisci D.M. Sensitive fluorescence method for detecting DNA-ligation amplification products. Clin. Chem. 1991;9:1522–1523. [PubMed] [Google Scholar]
- 101.Meng X., Yang X., Wang K. Direct fluorescence detection of point mutations in human genomic DNA using microbead-based ligase chain reaction. Talanta. 2010;5:1725–1729. doi: 10.1016/j.talanta.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 102.Kratochvil J., Laffler T.G. Nonradioactive oligonucleotide probes for detecting products of the ligase chain reaction. Methods Mol. Biol. 1994:28243–28252. doi: 10.1385/0-89603-254-x:243. [DOI] [PubMed] [Google Scholar]
- 103.Winn-Deen E.S., Batt C.A., Wiedmann M. Non-radioactive detection of Mycobacterium tuberculosis LCR products in a microtitre plate format. Mol. Cell. Probes. 2016;199(3):179–186. doi: 10.1006/mcpr.1993.1027. [DOI] [PubMed] [Google Scholar]
- 104.Zebala J.A., Barany F. Detection of Leber's hereditary optic neuropathy by non radioactive-LCR. In: Gelfand D.H., Sninsky J.J., Innis M.A., editors. PCR Strategies. Academic Press; San Diego, CA: 1993. [Google Scholar]
- 105.Toubanaki D.K., Christopoulos T.K., Ioannou P.C. Dry-reagent disposable biosensor for visual genotyping of single nucleotide polymorphisms by oligonucleotide ligation reaction: application to pharmacogenetic analysis. Hum. Mutat. 2008;8:1071–1078. doi: 10.1002/humu.20774. [DOI] [PubMed] [Google Scholar]
- 106.Deng H., Shen W., Gao Z. Colorimetric detection of single nucleotide polymorphisms in the presence of 10(3)-fold excess of a wild-type gene. Biosens. Bioelectron. 2015:68310–683150. doi: 10.1016/j.bios.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 107.Chen X., Ying A., Gao Z. Highly sensitive and selective colorimetric genotyping of single-nucleotide polymorphisms based on enzyme-amplified ligation on magnetic beads. Biosens. Bioelectron. 2012;1:89–94. doi: 10.1016/j.bios.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 108.Peng Z., Soper S.A., Pingle M.R. Ligase detection reaction generation of reverse molecular beacons for near real-time analysis of bacterial pathogens using single-pair fluorescence resonance energy transfer and a cyclic olefin copolymer microfluidic chip. Anal. Chem. 2010;23:9727–9735. doi: 10.1021/ac101843n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan Z., Zhou Y., Gao S. Homogeneous and sensitive detection of microRNA with ligase chain reaction and lambda exonuclease-assisted cationic conjugated polymer biosensing. ACS Appl. Mater. Interfaces. 2014;9:6181–6185. doi: 10.1021/am500883q. [DOI] [PubMed] [Google Scholar]
- 110.Sun Y., Lu X., Su F. Real-time fluorescence ligase chain reaction for sensitive detection of single nucleotide polymorphism based on fluorescence resonance energy transfer. Biosens. Bioelectron. 2015:74705–74710. doi: 10.1016/j.bios.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 111.Lou X.J., Panaro N.J., Wilding P. Mutation detection using ligase chain reaction in passivated silicon-glass microchips and microchip capillary electrophoresis. Biotechniques. 2004;3:392–398. doi: 10.2144/04373ST03. [DOI] [PubMed] [Google Scholar]
- 112.Giordano B.C., Ferrance J., Swedberg S. Polymerase chain reaction in polymeric microchips: DNA amplification in less than 240 seconds. Anal. Biochem. 2001;1:124–132. doi: 10.1006/abio.2000.4974. [DOI] [PubMed] [Google Scholar]
- 113.Giordano B.C., Copeland E.R., Landers J.P. Towards dynamic coating of glass microchip chambers for amplifying DNA via the polymerase chain reaction. Electrophoresis. 2001;2:334–340. doi: 10.1002/1522-2683(200101)22:2<334::AID-ELPS334>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 114.Lou X.J., Panaro N.J., Wilding P. Increased amplification efficiency of microchip-based PCR by dynamic surface passivation. Biotechniques. 2004;2:248–252. doi: 10.2144/04362ST01. [DOI] [PubMed] [Google Scholar]
- 115.Wee E.J., Shiddiky M.J., Brown M.A. eLCR: electrochemical detection of single DNA base changes via ligase chain reaction. Chem. Commun. (Camb) 2012;98:12014–12016. doi: 10.1039/c2cc35841g. [DOI] [PubMed] [Google Scholar]
- 116.Wee E.J., Rauf S., Koo K.M. μ –eLCR: a microfabricated device for electrochemical detection of DNA base changes in breast cancer cell lines. Lab Chip. 2013;22:4385–4391. doi: 10.1039/c3lc50528f. [DOI] [PubMed] [Google Scholar]
- 117.Koo K.M., Wee E.J., Rauf S. Microdevices for detecting locus-specific DNA methylation at CpG resolution. Biosens. Bioelectron. 2014:56278–56285. doi: 10.1016/j.bios.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 118.Song Y., Zhang Y., Wang T.H. Single quantum dot analysis enables multiplexed point mutation detection by gap ligase chain reaction. Small. 2013;7:1096–1105. doi: 10.1002/smll.201202242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huh Y.S., Lowe A.J., Strickland A.D. Surface-enhanced Raman scattering based ligase detection reaction. J. Am. Chem. Soc. 2009;6:2208–2213. doi: 10.1021/ja807526v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Birkenmeyer L., Armstrong A.S. Preliminary evaluation of the ligase chain reaction for specific detection of Neisseria gonorrhoeae. J. Clin. Microbiol. 1992;12:3089–3094. doi: 10.1128/jcm.30.12.3089-3094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boyadzhyan B., Yashina T., Yatabe J.H. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol. 2004;7:3089–3093. doi: 10.1128/JCM.42.7.3089-3093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ching S., Lee H., Hook E.W. Ligase chain reaction for detection of Neisseria gonorrhoeae in urogenital swabs. J. Clin. Microbiol. 1995;12:3111–3114. doi: 10.1128/jcm.33.12.3111-3114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Buimer M., van Doornum G.J., Ching S. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by ligase chain reaction-based assays with clinical specimens from various sites: implications for diagnostic testing and screening. J. Clin. Microbiol. 1996;10:2395–2400. doi: 10.1128/jcm.34.10.2395-2400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kacena K.A., Quinn S.B., Hartman S.C. Pooling of urine samples for screening for Neisseria gonorrhoeae by ligase chain reaction: accuracy and application. J. Clin. Microbiol. 1998;12:3624–3628. doi: 10.1128/jcm.36.12.3624-3628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kissin D.M., Holman S., Minkoff H.L. Epidemiology and natural history of ligase chain reaction detected chlamydial and gonococcal infections. Sex. Transm. Infect. 2002;3:208–209. doi: 10.1136/sti.78.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schachter J. DFA, EIA, PCR, LCR and other technologies: what tests should be used for diagnosis of chlamydia infections? Immunol. Invest. 1997;1–2:157–161. doi: 10.3109/08820139709048923. [DOI] [PubMed] [Google Scholar]
- 127.Castriciano S., Luinstra K., Jang D. Accuracy of results obtained by performing a second ligase chain reaction assay and PCR analysis on urine samples with positive or near-cutoff results in the LCx test for Chlamydia trachomatis. J. Clin. Microbiol. 2002;7:2632–2634. doi: 10.1128/JCM.40.7.2632-2634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pingle M.R., Granger K., Feinberg P. Multiplexed identification of blood-borne bacterial pathogens by use of a novel 16S rRNA gene PCR-ligase detection reaction-capillary electrophoresis assay. J. Clin. Microbiol. 2007;6:1927–1935. doi: 10.1128/JCM.00226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu G., Lao R., Xu L. Detection of single-nucleotide polymorphism on uidA gene of Escherichia coli by a multiplexed electrochemical DNA biosensor with oligonucleotide-incorporated nonfouling surface. Sensors (Basel) 2011;8:8018–8027. doi: 10.3390/s110808018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim S.Y., Chung B., Chang J.H. Simultaneous identification of 13 foodborne pathogens by using capillary electrophoresis-single strand conformation polymorphism coupled with multiplex ligation-dependent probe amplification and its application in foods. Foodborne Pathog. Dis. 2016;10:566–574. doi: 10.1089/fpd.2016.2143. [DOI] [PubMed] [Google Scholar]
- 131.Lindbrathen A., Gaustad P., Hovig B. Direct detection of Mycobacterium tuberculosis complex in clinical samples from patients in Norway by ligase chain reaction. J. Clin. Microbiol. 1997;12:3248–3253. doi: 10.1128/jcm.35.12.3248-3253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bergval I.L., Vijzelaar R.N., Dalla Costa E.R. Development of multiplex assay for rapid characterization of Mycobacterium tuberculosis. J. Clin. Microbiol. 2008;2:689–699. doi: 10.1128/JCM.01821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hsuih T.C., Park Y.N., Zaretsky C. Novel, ligation-dependent PCR assay for detection of hepatitis C in serum. J. Clin. Microbiol. 1996;3:501–507. doi: 10.1128/jcm.34.3.501-507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park Y.N., Abe K., Li H. Detection of hepatitis C virus RNA using ligation-dependent polymerase chain reaction in formalin-fixed, paraffin-embedded liver tissues. Am. J. Pathol. 1996;5:1485–1491. [PMC free article] [PubMed] [Google Scholar]
- 135.Datta S., Chatterjee S., Veer V. Recent advances in molecular diagnostics of hepatitis B virus. World J. Gastroenterol. 2014;40:14615–14625. doi: 10.3748/wjg.v20.i40.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Minamitani S., Nishiguchi S., Kuroki T. Detection by ligase chain reaction of precore mutant of hepatitis B virus. Hepatology. 1997;1:216–222. doi: 10.1053/jhep.1997.v25.pm0008985293. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y.Z., Xiao J.H., Liu L.G. Simultaneous detection of hepatitis B virus genotypes and mutations associated with resistance to lamivudine, adefovir, and telbivudine by the polymerase chain reaction-ligase detection reaction. Braz. J. Infect. Dis. 2011;6:560–566. doi: 10.1016/s1413-8670(11)70251-3. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y.Z., Xiao J.H., Ruan L.H. Detection of the rtA181 V/T and rtN236T mutations associated with resistance to adefovir dipivoxil using a ligase detection reaction assay. Clin. Chim. Acta. 2009;1–2:70–74. doi: 10.1016/j.cca.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 139.Wang X., Lou X., Wang Y. QDs-DNA nanosensor for the detection of hepatitis B virus DNA and the single-base mutants. Biosens. Bioelectron. 2010;8:1934–1940. doi: 10.1016/j.bios.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 140.Du Y.Q., Gao P.F., Wang W. A simple rapid detection method of DNA based on ligation-mediated real-time fluorescence PCR. Analyst. 2013;19:5745–5750. doi: 10.1039/c3an00763d. [DOI] [PubMed] [Google Scholar]
- 141.Collins M.S., Govey S.J., Alexander D.J. Rapid in vitro assessment of the virulence of Newcastle disease virus isolates using the ligase chain reaction. Arch. Virol. 2003;9:1851–1862. doi: 10.1007/s00705-003-0131-8. [DOI] [PubMed] [Google Scholar]
- 142.Rondini S., Pingle M.R., Das S. Development of multiplex PCR-ligase detection reaction assay for detection of West Nile virus. J. Clin. Microbiol. 2008;7:2269–2279. doi: 10.1128/JCM.02335-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang L., Veselinovic M., Yang L. A sensitive DNA capacitive biosensor using interdigitated electrodes. Biosens. Bioelectron. 2017:87646–87653. doi: 10.1016/j.bios.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pfeffer M., Meyer H., Wiedmann M. A ligase chain reaction targeting two adjacent nucleotides allows the differentiation of cowpox virus from other Orthopoxvirus species. J. Virol. Methods. 1994;3:353–360. doi: 10.1016/0166-0934(94)90150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Das S., Pingle M.R., Munoz-Jordan J. Detection and serotyping of dengue virus in serum samples by multiplex reverse transcriptase PCR-ligase detection reaction assay. J. Clin. Microbiol. 2008;10:3276–3284. doi: 10.1128/JCM.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Das S., Rundell M.S., Mirza A.H. A multiplex PCR/LDR assay for the simultaneous identification of category an infectious pathogens: agents of viral hemorrhagic fever and variola virus. PloS One. 2015;9:e0138484. doi: 10.1371/journal.pone.0138484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bourgeois C., Sixt N., Bour J.B. Value of a ligase chain reaction assay for detection of ganciclovir resistance-related mutation 594 in UL97 gene of human cytomegalovirus. J. Virol. Methods. 1997;2:167–175. doi: 10.1016/s0166-0934(97)00093-1. [DOI] [PubMed] [Google Scholar]
- 148.de Mendoza C., Alcami J., Sainz M. Evaluation of the Abbott LCx quantitative assay for measurement of human immunodeficiency virus RNA in plasma. J. Clin. Microbiol. 2002;4:1518–1521. doi: 10.1128/JCM.40.4.1518-1521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Long W.H., Xiao H.S., Gu X.M. A universal microarray for detection of SARS coronavirus. J. Virol. Methods. 2004;1:57–63. doi: 10.1016/j.jviromet.2004.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Delrio-Lafreniere S.A., Browning M.K., McGlennen R.C. Low-density addressable array for the detection and typing of the human papillomavirus. Diagn. Microbiol. Infect. Dis. 2004;1:23–31. doi: 10.1016/j.diagmicrobio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 151.Rundell M.S., Pingle M., Das S., Hussain A. A multiplex PCR/LDR assay for simultaneous detection and identification of the NIAID category B bacterial food and water-borne pathogens. Diagn. Microbiol. Infect. Dis. 2014;2:135–140. doi: 10.1016/j.diagmicrobio.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reyes A.A., Carrera P., Cardillo E. Ligase chain reaction assay for human mutations: the Sickle Cell by LCR assay. Clin. Chem. 1997;1:40–44. [PubMed] [Google Scholar]
- 153.Grossman P.D., Bloch W., Brinson E., Chang C.C. High-density multiplex detection of nucleic acid sequences: oligonucleotide ligation assay and sequence-coded separation. Nucleic Acids Res. 1994;21:4527–4534. doi: 10.1093/nar/22.21.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eggerding F.A. A one-step coupled amplification and oligonucleotide ligation procedure for multiplex genetic typing. PCR Methods Appl. 1995;6:337–345. doi: 10.1101/gr.4.6.337. [DOI] [PubMed] [Google Scholar]
- 155.Gasparini P., Arbustini E., Restagno G. Analysis of 31 CFTR mutations by polymerase chain reaction/oligonucleotide ligation assay in a pilot screening of 4476 newborns for cystic fibrosis. J. Med. Screen. 1999;2:67–69. doi: 10.1136/jms.6.2.67. [DOI] [PubMed] [Google Scholar]
- 156.Fang P., Bouma S., Jou C. Simultaneous analysis of mutant and normal alleles for multiple cystic fibrosis mutations by the ligase chain reaction. Hum. Mutat. 1995;2:144–151. doi: 10.1002/humu.1380060207. [DOI] [PubMed] [Google Scholar]
- 157.Cui J., Azimi M., Baysdorfer C. Application of multiplex ligation-dependent probe amplification to screen for beta-globin cluster deletions: detection of two novel deletions in a multi ethnic population. Hemoglobin. 2013;3:241–256. doi: 10.3109/03630269.2013.782461. [DOI] [PubMed] [Google Scholar]
- 158.Nigou M., Parfait B., Clauser E. Detection and quantification of the A3243G mutation of mitochondrial DNA by ligation detection reaction. Mol. Cell. Probes. 1998;5:273–282. doi: 10.1006/mcpr.1998.0191. [DOI] [PubMed] [Google Scholar]
- 159.Muth J., Williams P.M., Williams S.J. Fast capillary electrophoresis-laser induced fluorescence analysis of ligase chain reaction products: human mitochondrial DNA point mutations causing Leber's hereditary optic neuropathy. Electrophoresis. 1996;12:1875–1883. doi: 10.1002/elps.1150171212. [DOI] [PubMed] [Google Scholar]
- 160.Wildforster V., Dekomien G. Detecting copy number variations in autosomal recessive limb-girdle muscular dystrophies using a multiplex ligation-dependent probe amplification (MLPA) assay. Mol. Cell. Probes. 2009;1:55–59. doi: 10.1016/j.mcp.2008.11.002. [DOI] [PubMed] [Google Scholar]