Abstract
Background:
The effect of corticosteroid treatment on the viral load of Severe Acute Respiratory Syndrome (SARS) patients is unknown.
Objective:
To compare the plasma SARS-CoV RNA concentrations in ribavirin-treated patients who received early hydrocortisone therapy with those who received placebo.
Study design:
Serial plasma SARS-CoV RNA concentrations measured in the setting of a prospective, randomized double-blinded, placebo-controlled trial designed to assess the efficacy of “early” (<7 days of illness) hydrocortisone use in previously healthy SARS patients were analyzed. SARS-CoV RNA was quantified using a one-step real-time RT-PCR assay targeting the nucleocapsid gene.
Results:
Among 16 non-ICU cases, SARS-CoV RNA was detected in plasma since day 3–4 after fever onset; viral concentration peaked in the first week, which then rapidly declined in the second week of illness. On days 8, 12, 16, and 20, the cumulative proportion of patients with undetectable virus in plasma was 31%, 69%, 92%, and 100%, respectively. Plasma SARS-CoV RNA concentrations in the second and third week of illness were significantly higher in patients who received initial hydrocortisone treatment (n = 9), as compared to those who received placebo (n = 7)(AUC; Mann–Whitney, P = 0.023). The median time for SARS-CoV to become undetectable in plasma was 12 days (11–20 days) versus 8 days (8–15 days), respectively.
Conclusion:
Our findings suggested “early” corticosteroid treatment was associated with a higher subsequent plasma viral load.
Keywords: SARS, Plasma viral load, Corticosteroid
1. Introduction
Severe Acute Respiratory Syndrome (SARS) is a newly emerged infectious disease; little is known about its pathogenesis and its optimal treatment regimen (Peiris et al., 2003a). The use of corticosteroid therapy to reduce possible immune-mediated pulmonary damage remains controversial (Oba, 2003). We previously described a real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR) plasma/serum assay for early diagnosis of SARS (Ng et al., 2003). In this study, we analyzed serial plasma SARS-CoV RNA concentrations measured in the setting of a randomized, placebo-controlled trial initially designed to assess the efficacy of “early” hydrocortisone use (initiated <7 days from fever onset) in previously healthy SARS patients. Effect of corticosteroid treatment on plasma viral load was studied by comparing hydrocortisone-treated patients versus those who received placebo.
2. Methods
A prospective, randomized, double-blinded, placebo-controlled trial to assess for the clinical efficacy of early corticosteroid use on SARS patients was conducted during 20th April 2003 to 30th May 2003 in two regional hospitals in Hong Kong. Ethics approval was obtained from the Instituional Review Board of the Chinese University of Hong Kong. However, the study was terminated because the Hong Kong outbreak ended in June 2003. The study inclusion criteria were: (1) “Probable” cases of SARS with or without laboratory confirmation according to the Center for Disease Control and Prevention (CDC) case definition (CDC, 2003) and (2) age 18–65 years. Exclusion criteria were: (1) late presentation on ≥5 days after symptom onset; (2) presence of co-morbidity (e.g. renal impairment, liver failure, congestive heart failure, major cardiovascular diseases, chronic lung diseases, malignancy, etc.); and (3) evidence of respiratory failure on admission as defined by blood oxygen saturation () ≤ 90% or oxygen tension of < 8 kPa without supplemental oxygen therapy (i.e. in room air). Informed consents from patients were obtained. Routine serial laboratory investigations and plain chest radiographs were performed as described (Lee et al., 2003, Sung et al., 2004). Extent of pulmonary infiltrates on each frontal chest radiograph was scored in percentages by radiologists (Wong et al., 2003a, Wong et al., 2003b). Conventional, qualitative SARS-CoV RT-PCR assays were performed weekly on throat gargles and stool specimens for each patient; and paired SARS-CoV serology (indirect immunofluorescence assay, 2–4 weeks apart) were also obatined (WHO, 2003, Chan et al., 2004). Plasma SARS-CoV RNA concentrations were measured daily from the day of admission until discharge.
For plasma SARS-CoV RNA quantitation, blood samples were collected in EDTA-containing tubes. Plasma was obtained by centrifuging the blood samples at 1600 × g for 10 min, followed by 16,000 × g for 10 min. Viral RNA was extracted from 0.28 mL plasma by a QIAamp viral RNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. RNA was eluted with 50 μL of AVE buffer (included in the kit) and stored at −80 °C. One step quantitative real-time RT-PCR targeting the nucleocapsid gene of the SARS-CoV was performed as previously described to determine the plasma viral RNA concentrations (Ng et al., 2003). Each sample was analyzed in duplicates, and the calibration curve was run in parallel for each analysis. Multiple negative water blanks were also included in every analysis.
The clinical treatment protocol consisted of initial antibacterial therapy for community-acquired pneumonia (Lee et al., 2003, Sung et al., 2004) upon admission. Intravenous ribavirin therapy at a dose of 400 mg eight hourly was given if there was no clinical response after 48 h, for a total of 12 days. Also, cases were randomised in a double-blind fashion to receive either (1) “early” intravenous hydrocortisone 100 mg every eight hourly, or (2) intravenous normal saline 5 mL eight hourly as placebo, for a total of 12 days, or until “pulse” methylprednisolone was given as rescue therapy. “Pulse” of intravenous high-dose methylprednisolone (500 mg/day for three consecutive days) was given for cases having persistent/recurrent fever plus radiographic progression of lung opacities ± hypoxemia as rescue therapy. Further pulses of methylprednisolone were given as required when no clinical or radiological improvement archived, up to a total of 3.0 g (Sung et al., 2004). Detailed clinical, radiological, and laboratory data in patients treated similarly had been described elsewhere (Sung et al., 2004, Hui et al., in press).
2.1. Statistical analysis
Data was analyzed using summary statistics and the area under curve (AUC) method. Mann–Whitney test was used to compare plasma viral concentrations between different arms across time, which were quantified by mean AUC, as calculated by the linear trapezoidal method. Spearman's rank correlation coefficient was used to assess for correlation between plasma viral concentrations and the extent of lung opacities on plain chest radiographs or the nadir lymphocyte counts. SPSS for Windows (Release 11.5; SPSS Inc., Chicago, IL, USA) was used for the analyses, and the level of significance was set at 0.05 for all comparisons.
3. Results
A total of 17 patients were recruited. Their median age was 34 years (range 22–57 years), with a male to female ratio of 1:3. All were previously healthy individuals. Paired serology testing confirmed SARS-CoV infection in 16 cases (IgG titer 40–640); five had bilateral lung infiltrates, with one case requiring supplemental oxygen therapy. All recovered and discharged uneventfully without requiring ICU care. The remaining patient was transferred to ICU and mechanical ventilated because of respiratory failure and died before a convalescence serum could be obtained. His diagnosis of SARS was confirmed by two throat washings tested positive for SARS-CoV by RT-PCR.
The clinical, radiological, and laboratory data in our cohort is summarized in Table 1 . A total of nine patients in the non-ICU group received “early” hydrocortisone treatment, initiated at a mean interval of 4.8 days (95% CI: 4.1–5.5) from fever onset. Normal saline was given in the remaining seven cases. High-dose methylprednisolone was given to six (86%) patients in the placebo group (mean cumulative dose = 1.8 g) and four (44%) patients in the hydrocortisone group (mean cumulative dose = 2.2 g), respectively, initiated at a mean interval of 8.6 days from illness onset. The single ICU case received both “early” hydrocortisone treatment and subsequent high-dose methylprednisolone therapy.
Table 1.
Clinical, radiological, and laboratory data in the “early hydrocortisone” treated group vs. “placebo” group
| Early hydrocortisone | Placebo | |
|---|---|---|
| Number of cases | 9 | 7 |
| Mean age (years) | 35 | 34 |
| Male:female | 1:3.5 | 1:2.5 |
| Cases given methylprednisolone (number) | 4 | 6 |
| Total methylprednisolone dosage (g) | 2.2 | 1.8 |
| Initial extent of pulmonary infiltrate (%) [95% CI] | 1.48 [0.43–2.53] | 1.67 [0.26–3.07] |
| Peak extent of pulmonary infiltrate (%) [95% CI] | 4.54 [2.06–7.02] | 5.00 [2.19–7.81] |
| Initial lymphocyte count (109 L−1) [95% CI] | 0.83 [0.56–1.10] | 0.7 [0.56–0.84] |
| Nadir lymphocyte count (109 L−1) [95% CI] | 0.49 [0.32–0.66] | 0.36 [0.20–0.52] |
| Initial LDH level (IU/L) [95% CI] | 208.33 [181.89–234.77] | 193.43 [125.27–261.59] |
| Peak LDH level (IU/L) [95% CI] | 346.44 [241.23–451.66] | 346.14 [170.10–522.19] |
| Peak CRP level (mg/L) [95% CI] | 34.89 [11.99–57.79] | 35.33 [10.01–69.65] |
| Cases with positive RT-PCR result on respiratory/stool specimens (%) | 5 (55.6) | 3 (42.9) |
| Cases with positive RT-PCR result on respiratory specimens (%) | 4 (44.4) | 2 (28.6) |
LDH, lactate dehydrogenase; CRP, C-reactive protein; RT-PCR, reverse transcriptase polymerase chain reaction.
The detectability (percentage of patients) of plasma SARS-CoV RNA in 16 non-ICU patients (hydrocortisone-treated group versus placebo group) across time is depicted in Fig. 1 . SARS-CoV RNA was detected in plasma since day 3–4 after illness onset. Overall, the mean time for SARS-CoV RNA to become undetectable in plasma was 11.9 days (95% CI: 9.7–14.1) from fever onset; cumulative proportion of patients with undetectable virus was 31%, 69%, 92%, and 100%, on days 8, 12, 16, and 20, respectively. Median time for SARS-CoV RNA to become undetectable in plasma was 12 days (range 11–20 days) versus 8 days (range 8–15 days) in the hydrocortisone and placebo groups, respectively (Mann–Whitney; P = 0.106).
Fig. 1.
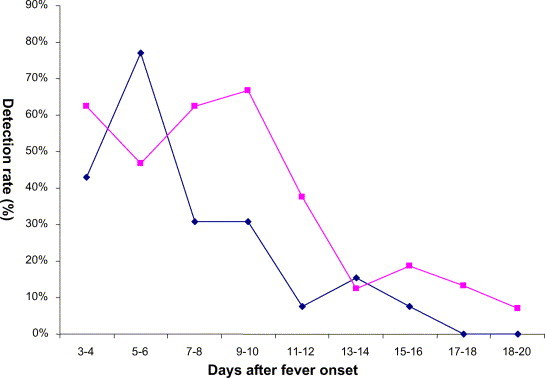
Percentage of patients with detectable plasma SARS-CoV RNA during their first 3 weeks of illness. ( ) “Early hydrocortisone” treated group; (
) “Early hydrocortisone” treated group; ( ) “placebo” group.
) “placebo” group.
Profile of mean plasma SARS-CoV RNA concentrations in 16 non-ICU cases (hydrocortisone-treated group versus placebo group) is depicted in Fig. 2 . In general, peak plasma viral load was reached within the first week of illness and rapidly declined in the second week. The mean peak plasma SARS-CoV RNA concentration was 510 copies/mL (95% CI: 148–1738 copies/mL). The peak value in the single ICU case was 35,086 copies/mL. The plasma viral loads measured in the two treatment arms were compared. Plasma SARS-CoV RNA concentrations in the second and third week of illness were statistical significantly higher in patients who received early hydrocortisone treatment (n = 9), as compared to those who received placebo (n = 7) (AUC; Mann–Whitney, P = 0.023). No difference in plasma viral RNA concentrations was detected between those who ever received subsequent high dose methylprednisolone (n = 10) and those who did not (n = 6) (AUC; Mann–Whitney, P > 0.05).
Fig. 2.
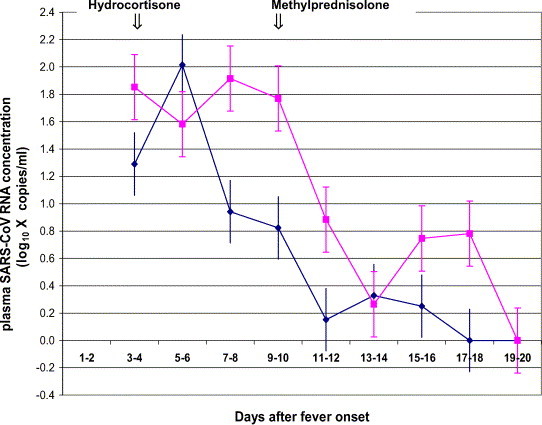
Profile of mean plasma SARS-CoV RNA concentrations in the “early hydrocortisone” treated group vs. “placebo” group. ( ) “Early hydrocortisone” treated group (mean, S.E.); (
) “Early hydrocortisone” treated group (mean, S.E.); ( ) “placebo” group (mean, S.E.).
) “placebo” group (mean, S.E.).
In addition, patients who had upper respiratory specimen(s) tested positive for SARS-CoV by RT-PCR (n = 6) had statistical significantly higher plasma viral RNA concentrations comparing to cases tested negative (n = 11) (AUC; Mann–Whitney test, P = 0.037). No difference in plasma viral RNA concentrations was detected between fecal specimen positive (n = 4) and negative (n = 13) patients (AUC; Mann–Whitney test, P > 0.05). Furthermore, no statistically significant difference in plasma viral RNA concentrations was detected between patients who ever had bilateral lung infiltrates (n = 6) and those who had not (n = 11) (AUC; Mann–Whitney, P > 0.05). No significant correlation was found between plasma SARS-CoV RNA concentration and the extent of lung opacities (%) on plain chest radiographs (Spearman's r = +0.321), or their respective peak values (Spearman's r = +0.144). Also, no significant correlation between peak plasma SARS-CoV RNA concentrations and the nadir lymphocyte counts (Spearman's r = +0.082) was found.
4. Discussion
In young, previously healthy adult SARS patients, SARS-CoV RNA was detected in plasma since day 3–4 after fever onset, reaching its peak concentration in the first week, and declined rapidly in the second week of illness. “Early” hydrocortisone treatment initiated in <7 days of illness was associated with significantly higher subsequent plasma viral concentrations in the second and third week. Duration of viraemia may also be prolonged.
Our findings confirmed previous observations that “viral load” of SARS patients declined rapidly in the second week of illness after an initial peak (day 10 in upper respiratory secretions) (Peiris et al., 2003b, Ng et al., 2003). Since ribavirin has no demonstrable antiviral activity against SARS-CoV (Cyranoski, 2003), host immune defense is likely to be responsible for viral clearance. As shown in our study, “early” initiation of corticosteroid treatment during the viral replication phase (Peiris et al., 2003b, Wong and Hui, 2003) in the first week of illness resulted in delayed viral clearance (thus a higher subsequent plasma viral load), which possibly related to its immunosuppressive effect. Duration of viraemia might also be prolonged (median time to undetectable = 12 days versus 8 days), though the difference did not reach statistical significance.
The use of corticosteroid therapy in SARS patients is controversial (Oba, 2003). Its immunosuppressive effect (SARS is a viral induced pneumonia), potential side effects (e.g. superimposed bacterial/fungal infections, hyperglycemia, electrolyte imbalance, psychosis), and lack of evidence on clinical efficacy from randomized/placebo-controlled studies were the main concerns (Wang et al., 2003, Peiris et al., 2003a). Corticosteroid was given to reduce possible immuno-pathological damage. Preliminary data from retrospective, non-randomized studies did demonstrate some clinical benefits in SARS patients receiving corticosteroid treatment (Sung et al., 2004, Ho et al., 2003). However, the optimal timing and dosage of such therapy is uncertain. Results from our analysis do not support the use of corticosteroid therapy early in the illness because of delayed viral clearance, especially when no concomitant effective antiviral therapy is given. In fact, as shown in a separate study, “early” low-dose corticosteroid/hydrocortisone therapy failed to halt disease progression in >80% of cases (Sung et al., 2004). Although we did not observe abrupt rebound of viraemia in our cohort after “pulse” methylprednisolone therapy given after the first week, further study is needed to clarify the issue. Meanwhile we call for cautious use of such therapy; and if used, preferably given in later course of the disease, when an overexuberant host immune response is causing pathological lung damage (e.g. in the second week following clinical deterioration) (Peiris et al., 2003b, Loutfy et al., 2003, Wong and Hui, 2003), and when SARS-CoV viral load had declined significantly.
In addition to earlier detection of SARS-CoV, plasma viral RNA quantitation provides valuable prognostic information (Ng et al., 2003). In this study, we verify that a significantly higher plasma viral load was present in patients ever had SARS-CoV detected in their upper respiratory specimens by the qualitative RT-PCR method (likely resulting from a higher concomitant viral load in the upper respiratory secretions). In fact, a positive qualitative RT-PCR assay or a high viral load in upper respiratory secretions, and a high plasma viral load on admission were all predictive of adverse clinical outcomes (Tsang et al., 2003, Cheng et al., 2004, Ng et al., 2003). The lack of significant correlation between plasma viral load and fecal specimen RT-PCR positivity, the radiographic extent of lung infiltrate and the degree of lymphopenia may actually reflect the complexity of pathogenic mechanisms of SARS (e.g. immune-mediated pulmonary tissue damage, lymphocyte apoptosis versus direct viral lytic action) and require further study (Cheng et al., 2004, Peiris et al., 2003a, Wong and Hui, 2003, O’Donnell et al., 2003).
Our study was limited by a small sample size. Patients with advanced age, co-morbidity, and those immunocompromised were excluded. Moreover, viral load profiles among more severe SARS cases, and the clinical consequence of a higher plasma viral load in early hydrocortisone treated patients would need further investigation.
5. Conclusion
Our study demonstrated that “early” corticosteroid treatment was associated with a higher subsequent plasma viral load and therefore should be avoided. Judicious use of corticosteroid therapy in SARS is advisable.
Acknowledgements
This work is supported by the Research Fund for the Control of Infectious Diseases (RFCID) from the Health, Welfare and Food Bureau of the Hong Kong SAR Government. Also we thank Miss. P.S. Chan in her technical assistance in preparing the manuscript. Dr. Lee is an infectious disease specialist and honorary clinical assistant professor at the Department of Medicine and Therapeutics, Prince of Wales Hospital, The Chinese University of Hong Kong. His current research interests focus on clinical diagnosis and management of SARS patients.
References
- Centers for Disease Control and Prevention. Severe acute respiratory syndrome (SARS) case definition (http://www.cdc.gov/ncidod/sars/casedefinition.htm).
- Chan P.K.S., Ng K.-C., Chan R.C.W., Lam R.K.Y., Chow V.C.Y., Hui M. Immunofluorescence assay for serological diagnosis of severe acute respiratory syndrome. Emerg. Infect. Dis. 2004;10:530–532. doi: 10.3201/eid1003.030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C., Hung I.F., Tang B.S., Chu C.M., Wong M.M., Chan K.H. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 2004;38:467–475. doi: 10.1086/382681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D. Critics slam treatment for SARS as ineffective and perhaps dangerous. Nature. 2003;423:4. doi: 10.1038/423004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J.C., Ooi G.C., Mok T.Y. High dose pulse versus non-pulse corticosteroid regimens in severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2003;168(12):1449–1456. doi: 10.1164/rccm.200306-766OC. [DOI] [PubMed] [Google Scholar]
- Hui DSC, Wong KT, Antonio GE, Lee N, Wu A, Wong V, et al. Severe Acute Respiratory Syndrome (SARS): correlation of clinical outcome and radiological features. Radiology, in press.
- Lee N., Hui D.S., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. J. Am. Med. Assoc. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- Ng E.K., Hui D.S., Chan K.C., Hung E.C., Chiu R.W., Lee N. Quantitative analysis and prognostic implication of SARS—Coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin. Chem. 2003;49:1976–1980. doi: 10.1373/clinchem.2003.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba Y. The use of corticosteroids in SARS. Correspondence. N. Engl. J. Med. 2003;348:2034–2035. doi: 10.1056/NEJM200305153482017. [DOI] [PubMed] [Google Scholar]
- O’Donnell R., Tasker R.C., Roe M.F. SARS: understanding the Coronavirus: apoptosis may explain lymphopenia of SARS. Br. Med. J. 2003;327:620. doi: 10.1136/bmj.327.7415.620-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of Coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J.J., Wu A., Joynt G.M., Yuen K.Y., Lee N., Chan P.K. Severe Acute Respiratory Syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang O.T.Y., Chau T.-N., Choi K.W. Coronavirus-positive nasopharyngeal aspirate as predictor for Severe Acute Respiratory Syndrome mortality. Emerg. Infect. Dis. 2003;9:1381–1387. doi: 10.3201/eid0911.030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ding Y., Li X. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N. Engl. J. Med. 2003;349:507–508. doi: 10.1056/NEJM200307313490519. [DOI] [PubMed] [Google Scholar]
- Wong G.W., Hui D.S. Severe acute respiratory syndrome (SARS): epidemiology, diagnosis and management. Thorax. 2003;58:558–560. doi: 10.1136/thorax.58.7.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.T., Antonio G.E., Hui D.S.C., Lee N., Yuen E.H.Y., Wu A. Severe Acute Respiratory Syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology. 2003;228(2):401–406. doi: 10.1148/radiol.2282030593. [DOI] [PubMed] [Google Scholar]
- Wong R.S., Wu A., To K.F., Lee N., Lam C.W., Wong C.K. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Br. Med. J. 2003;21(326):1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. PCR primers for SARS developed by WHO Network Laboratories. http://www.who.int/csr/sars/primers/en/.


