Abstract
The recent advances in molecular technology have enabled the detection of several new viral agents in specimens collected from the human respiratory tract. Human metapneumovirus was first described in 2001, and is a significant respiratory pathogen, particularly of children. Following the identification of severe acute respiratory syndrome (SARS) associated coronavirus, two other newly detected coronaviruses, NL63 and HKU1, have been linked to respiratory disease in humans. However, identifying a new virus as the causative agent of a specific disease is difficult, and ideally would involve satisfying Koch's postulates. The recently described human bocavirus and polyomaviruses KI and WU have been detected in samples collected from humans with acute respiratory infection, but as yet, have not been conclusively proven to be agents of human disease.
We review the new viral agents that have been detected in respiratory samples since 2001, and examine their contribution as agents of human disease.
Keywords: Emerging virus, Acute respiratory infection, Respiratory viruses, Epidemiology, Molecular, Clinical, Epidemiology
1. Introduction
Acute respiratory tract infections (ARTI) are a major cause of morbidity and mortality worldwide, particularly in children, who may experience multiple infections per year until they are 10 years of age (Arnold et al., 2006). Viruses are responsible for the majority of ARTI, with rhinoviruses (HRV), respiratory syncytial virus (RSV), influenza virus (INF) and parainfluenzaviruses (PIV) considered the major pathogens. Traditionally, the diagnosis of these infections relied on the isolation and identification of the viral agent by cell culture or detection of viral antigens by direct immunofluorescent assays (DFA) (Arnold et al., 2006). However, using these methods a large proportion of respiratory infections remained undiagnosed.
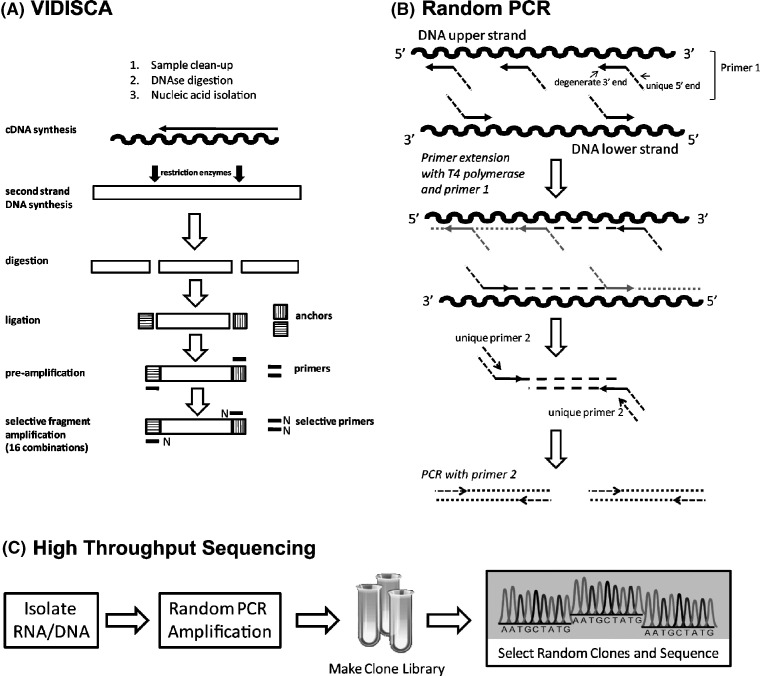
Recent advances in molecular biology, particularly the introduction of the polymerase chain reaction (PCR) assay, have greatly improved the detection of viral respiratory pathogens. Yet, even with the most sensitive molecular techniques, only 40–60% of infections were able to be associated consistently with a causative microorganism (Louie et al., 2005). This suggested that additional respiratory pathogens were likely to exist (van den Hoogen et al., 2001). In fact, since 2001, seven previously undescribed viruses have been identified by analysis of clinical specimens from the human respiratory tract (Table 1 ). These are human metapneumovirus (HMPV) in 2001(van den Hoogen et al., 2001), three new human coronaviruses (HCoV); the severe acute respiratory syndrome (SARS) associated coronavirus in 2003 (Ksiazek et al., 2003), coronavirus NL63 (NL63) in 2004 (van der Hoek et al., 2004), coronavirus HKU1 (HKU1) in 2005 (Woo et al., 2005a), as well as human bocavirus (HBoV) in 2005 (Allander et al., 2005) and the recently described human polyomaviruses KI (KIV) and WU (WUV) in 2007 (Allander et al., 2007a, Gaynor et al., 2007). These new viral agents were detected by novel molecular methods such as VIDISCA (van der Hoek et al., 2004), pan-viral DNA microarrays (Wang et al., 2003), and high throughput sequencing (Allander et al., 2005, Allander et al., 2007a, Gaynor et al., 2007) (Fig. 1 ). These methods were comprehensively reviewed by Ambrose and Clewley (2006). More broadly, the advent of these new technologies has greatly stimulated efforts to identify novel viruses in the respiratory tract and in other human disease states.
Table 1.
Summary of distribution, clinical association and methods of discovery of newly emerging viruses associated with the human respiratory tract
| Virus | Patient group | Prevalence | Clinical signs | Method of discovery | Reference |
|---|---|---|---|---|---|
| Human metapneumovirus | Children and the elderly | 3–25% | Bronchiolitis, pneumonia, bronchitis, rhinorrhoea, cough, sore throat | Virus isolation, electron-microscopy, and random PCR | van den Hoogen et al. (2001) |
| Severe acute respiratory syndrome (SARS) | All ages | Sporadic | Pneumonia | Virus isolation, electron-microscopy, and consensus coronavirus PCR | Ksiazek et al. (2003) |
| Coronavirus NL63 and coronavirus HKU1 | Children and the elderly | 1–10% | Bronchiolitis, pneumonia, rhinorrhoea, fever, cough, wheezing | Virus isolation, VIDISCA consensus coronavirus PCR | van der Hoek et al. (2004), Woo et al. (2005a) |
| Human bocavirus | Children | 1–11% | Bronchiolitis,a pneumonia, acute otitis media, asthma | Random PCR | Allander et al. (2005) |
| Polyomavirus KI and polyomavirus WU | Children | 1–7% | Bronchiolitis,a pneumonia, cough | Random PCR | Allander et al. (2007a), Gaynor et al. (2007) |
Currently there is no definitive evidence that bocavirus and polyomaviruses KIV and WUV are causative agents of respiratory disease.
Fig. 1.
(A) VIDISCA method. Schematic overview of the stages incorporated in the performance of the VIDISCA method (adapted from van der Hoek et al., 2004). (B) Random PCR. Primer 1 with a unique 5′-end and degenerate 3′-end sequence is used in a first round PCR. The degenerate segment binds to template sequences that occur stochastically throughout the viral genome, and primer extension occurs with T4 polymerase. Double-stranded DNA is formed containing unique sequences on each strand. Primer 2 hybridizes to the unique sequence and is used for amplification of fragments of the viral template DNA (adapted from Stang et al., 2005). (C) High throughput sequencing. Randomly amplified PCR products are cloned into a suitable plasmid vector, and subsequently sequenced.
Once new viral agents are identified, it is important to establish their pathogenic potential. Traditionally, Koch's postulates have provided the standard for establishing a causal link between a pathogen and disease, but these have been modified over the years to match new knowledge of viral detection and disease processes (Fredericks and Relman, 1996). These modified postulates now propose that a causal relationship between an organism and disease may be established if (i) the organism is consistently present in patients with the disease at a significantly greater prevalence than in matched control subjects, (ii) the disease may be replicated in an appropriate animal model after challenging with the viral pathogen, and subsequent isolation of the agent from the challenged animal, and (iii) a specific immune response to the virus by the host can be demonstrated.
The process of establishing a causal link between a new virus and disease may be aided by the availability of a well-characterized specimen bank. Such specimens collected from patients with a variety of illnesses, supported with comprehensive clinical and epidemiologic data, are a valuable resource for establishing an association between presence of a virus and disease. However, the conclusion about such an association is only valid if they are tested together with appropriately matched controls.
Of the viruses discovered over the last 7 years, HMPV and the newly emerging HCoV are recognized to have global presence, and are considered causative agents of respiratory disease. However, to date, SARS coronavirus has been restricted geographically and has only been associated with limited and sporadic outbreaks of human disease. Of the other emerging viruses, an association with the respiratory tract, or respiratory infection, has been postulated, but not proven. These are HBoV and the newly described KIV and WUV.
This review examines the state of knowledge of newly emerging viruses that have been detected in the human respiratory tract, and their clinical association with human disease.
2. Human metapneumovirus
HMPV is a new human respiratory tract pathogen first described in 2001. The virus was isolated by cell culture from respiratory secretions collected during a 20-year period from Dutch children with ARTI. HMPV showed slow replication in cell culture with characteristic syncytia formation followed by rapid internal disruption of the cells at 10–14 days after inoculation (van den Hoogen et al., 2001).
It was established that HMPV was a respiratory pathogen of primates, by demonstrating active replication of the virus in four juvenile cynomolgus macaques, resulting in mild upper respiratory tract signs in two of these animals.
The HMPV genome was characterized using random arbitrarily primed-PCR, and the virus was subsequently classified within Paramyxoviridae based on genome structure and organization. HMPV is the first known mammalian metapneumovirus associated with disease.
Many studies have shown a broad spectrum of clinical signs associated with HMPV infection in patients of all age groups. Like other human respiratory viruses, HMPV may cause upper and lower respiratory tract infection in infants and young children (Williams et al., 2004, Williams et al., 2006). It is second only to RSV as a cause of bronchiolitis in early childhood (Boivin et al., 2002, Freymouth et al., 2003, van den Hoogen et al., 2003, Viazov et al., 2003, Mullins et al., 2004, Williams et al., 2004, Chano et al., 2005, Sasaki et al., 2005). Children younger than 5 years of age are most susceptible to HMPV infection (van den Hoogen et al., 2004), and those younger than 2 years of age are most likely to be hospitalized with severe symptoms, including bronchiolitis, pneumonia, and bronchitis (Kahn, 2006, Heikkinen et al., 2008). Recently, Heikkinen et al. (2008) showed that acute otitis media developed in 61% of HMPV-infected children younger than 3 years of age. There are no clinical manifestations of HMPV infection that are unique, and infection in young children cannot be distinguished from infection with RSV or INF (Peret et al., 2002, Bastien et al., 2003, Boivin et al., 2003, Esper et al., 2004, McAdam et al., 2004, van den Hoogen et al., 2004, Williams et al., 2004, Vicente et al., 2007). Several studies have reported the association of HMPV and asthma exacerbations in children (von Linstow et al., 2004, Williams et al., 2004, Williams et al., 2005b, Vicente et al., 2007), and with exacerbations of both asthma and chronic obstructive pulmonary disease (COPD) in adults (Falsey and Walsh, 2006).
HMPV infection in adults usually presents with mild common cold-like respiratory symptoms such as rhinorrhoea, cough and sore throat (Stockton et al., 2002, Falsey et al., 2003). However, disease may be more severe in patients with underlying medical conditions such as cardiopulmonary disease, the elderly and immunocompromized subjects (Boivin et al., 2002, Falsey et al., 2003, Hamelin et al., 2005, Honda et al., 2006). In these subjects the virus can cause prolonged and serious infections, and several case studies have described the detection of HMPV in patients with severe lung disease including several fatalities (Pelletier et al., 2002, Cane et al., 2003, Esper et al., 2004, Larcher et al., 2005, Martino et al., 2005, Williams et al., 2005a). Interestingly, one study reported an immunocompromized child, in whom HMPV infection with two different strains of the virus were detected during a 10-month period (Williams et al., 2005a).
It is now recognized that HMPV has a global distribution (van den Hoogen et al., 2001, Boivin et al., 2002, Boivin et al., 2004, Nissen et al., 2002, Cuevas et al., 2003, Esper et al., 2003, Freymouth et al., 2003, Maggi et al., 2003b, Peiris et al., 2003, Dollner et al., 2004, Ebihara et al., 2004, Al-Sonboli et al., 2005, Kim and Lee, 2005, Ludewick et al., 2005, Noyola et al., 2005, Robinson et al., 2005, Garcia-Garcia et al., 2006, Gray et al., 2006, Samransamruajkit et al., 2006) circulating predominantly in late winter and spring. Usually the peak of infection is coincident with or follows the peak of RSV infection (Boivin et al., 2003, Karron et al., 2003, Osterhaus and Fouchier, 2003, Peiris et al., 2003, Esper et al., 2004, Konig et al., 2004, Mullins et al., 2004, Bouscambert-Duchamp et al., 2005, Chano et al., 2005, Agapov et al., 2006, Kaida et al., 2006, Nicholson et al., 2006, Sarasini et al., 2006). Many respiratory viruses share seasonality and susceptible populations. As a result, co-detection of HMPV with other respiratory viruses has been reported at rates ranging from 1% to greater than 10% (Viazov et al., 2003, Esper et al., 2004, Williams et al., 2004, Xepapadaki et al., 2004). One study reported that dual infection with HMPV and RSV is associated with severe bronchiolitis and significantly increased the risk of admission to a paediatric intensive-care unit (Semple et al., 2005). This is at odds with previous findings which failed to detect a predisposition to more severe disease in patients with multiple respiratory virus detections (Lina et al., 1996).
Two major genotypes of HMPV, designated A and B are recognized, each with two subtypes (A1 and A2; B1 and B2) (Peret et al., 2002, Biacchesi et al., 2003, Esper et al., 2004, Mackay et al., 2004, Williams et al., 2004, Chano et al., 2005, Vicente et al., 2007). Circulating strains of HMPV differ in different populations at any single time, and the same strain may be present in geographically distinct locations in different years. In an Australian study, the predominant genotype of HMPV switched over 4 consecutive years from genotype A1 to A2 to B1 (Sloots et al., 2006a). Similar observations have been reported elsewhere (Gerna et al., 2005). So far there are no data to suggest that severity of illness may be associated with a specific genotype (Kahn, 2006).
Seroprevalence studies have shown that the presence of HMPV-specific antibodies was greater than 90% by age 5 years, and nearly 100% in adults (van den Hoogen et al., 2001, Leung et al., 2005). This suggests that primary infection with HMPV occurs early in childhood, typically between the ages of 1–5 years (van den Hoogen et al., 2001, Ebihara et al., 2003, Wolf et al., 2003, Leung et al., 2005). The reported seroprevalence in infants younger than 3 months of age was greater than 90%, decreasing to a minimum by age 13 months and increasing to 52% by age 2 years (Wolf et al., 2003). Although this appears to be indicative of maternally derived antibodies (Leung et al., 2005), the role of such antibodies in the protection or moderation of HMPV infection remains to be determined.
3. Emerging human coronaviruses
Coronaviruses (CoV) infect many species of animals, including humans. The first HCoV, of which 229E and OC43 are the representatives, were identified in the 1960s (Tyrrell and Bynoe, 1965, Hamre and Procknow, 1966, McIntosh et al., 1967). Coronaviruses are classified into three distinct groups, with 229E and NL63 included in Group 1 coronaviruses and OC43 and HKU1 in Group 2 (Lai and Cavanagh, 1997). SARS–CoV, represents an early split from Group 2 coronaviruses (Eickmann et al., 2003, Marra et al., 2003, Rota et al., 2003, Snijder et al., 2003) and is believed to have originated from wild animals (Guan et al., 2003, Lau et al., 2005).
3.1. SARS coronavirus
First reports of an outbreak of a new respiratory illness involving “atypical pneumonia” were published in 2003 (Center for Disease Control and Prevention (CDC), 2003; Tsang et al., 2003). The novel virus present in these cases was isolated from patients’ lungs and sputa on Vero E6 cells (Drosten et al., 2003a, Drosten et al., 2003b, Peiris et al., 2003, Ksiazek et al., 2003). Sequencing of the viral isolate showed the presence of a new human coronavirus (Rota et al., 2003, Snijder et al., 2003). It was demonstrated that this virus was the aetiologic agent for SARS by transmission of respiratory disease to nonhuman primates (Fouchier et al., 2003). The SARS epidemic was halted by a highly effective global public health response co-ordinated by the World Health Organization, and there is no evidence that SARS–CoV is currently circulating in humans. However, the SARS outbreak focussed renewed attention on coronaviruses generally, resulting in the identification of two more human coronaviruses, NL63 and HKU1.
3.2. Human coronaviruses NL63 and HKU1
HCoV–NL63 was first detected in 2004 in a child with bronchiolitis in The Netherlands (van der Hoek et al., 2004), whilst HCoV–HKU1 was detected in 2005 in an adult with chronic pulmonary disease in Hong Kong (Woo et al., 2005a). After the initial discovery, several groups have reported presence of these viruses in their populations illustrating global presence (Fouchier et al., 2004, Arden et al., 2005, Bastien et al., 2005, Chiu et al., 2005, Ebihara et al., 2005a, Kaiser et al., 2005, Moes et al., 2005, Choi et al., 2006, Esper et al., 2006, Gerna et al., 2006, Sloots et al., 2006b, Vabret et al., 2005, Vabret et al., 2006). These reports showed that NL63 and HKU1 may be detected in 1 to 10% of patients with acute respiratory tract infections. Co-detection of these viruses with other respiratory viruses was commonly reported (van der Hoek et al., 2006a). Like SARS–CoV, NL63 has been successfully cultured in monkey epithelial cell lines (Fouchier et al., 2004, van der Hoek et al., 2004, Schildgen et al., 2006), but to date, it has not been possible to culture HCoV–HKU1, despite attempts to do so in many cell lines. This difficulty with isolating some newly identified viruses particularly explains why they have not previously been detected.
Initial studies described the presence of NL63 in young children admitted to hospital with severe lower respiratory tract infections (LRTIs) (Fouchier et al., 2004, van der Hoek et al., 2004), but the virus has also been detected in elderly patients with a fatal outcome, showing that the severity of the respiratory disease can be substantial (Bastien et al., 2005).
HCoV–NL63 was proven to be a respiratory pathogen and has been identified as an agent of laryngotracheitis (croup) (Forster et al., 2004, Konig et al., 2004, van der Hoek et al., 2006b). In a case-controlled study of young German children it was reported that the chance of croup is 6.6 times higher in NL63-positive children than in NL63-negative children (Forster et al., 2004, Konig et al., 2004). Also, a link between NL63 and Kawasaki disease has been suggested (Esper et al., 2005), but other groups recently questioned this association (Belay et al., 2005, Ebihara et al., 2005b, Shimizu et al., 2005, Baker et al., 2006, Chang et al., 2006).
The first described HKU1 cases were amongst elderly patients and children with major underlying disease (Woo et al., 2005b). Similar findings in the USA (Esper et al., 2006) and France (Vabret et al., 2006) suggested that HKU1 infection may aggravate the conditions of persons with an underlying disease, thereby resulting in hospitalization.
Common respiratory symptoms accompanying HKU1 infection are rhinorrhoea, fever, coughing, wheezing, and disease manifestations include bronchiolitis and pneumonia (Lau et al., 2006, Sloots et al., 2006b). It has been suggested that HKU1 might also be involved in gastrointestinal disease (Vabret et al., 2006).
HKU1 and NL63 infections are generally not life threatening, certainly not in otherwise healthy persons. This suggests that these coronaviruses, like 229E and OC43, are predominantly common cold viruses that can cause more severe clinical symptoms in young children, elderly persons, and the immunocompromized (McIntosh et al., 1974, Holmes, 2001, van Elden et al., 2004).
Phylogenetic analysis has shown that NL63 may be classified into two genotypes (van der Hoek et al., 2004, Arden et al., 2005, Chiu et al., 2005, Vabret et al., 2005), and full-genome sequencing of HKU1 isolates has also revealed the presence of two genotypes (A and B) with the possibility of a third genotype (C) that is the resultant of recombination between genotypes A and B (Woo et al., 2006).
4. New viruses identified in the respiratory tract of humans
4.1. Human bocavirus
Human bocavirus was first described in 2005 (Allander et al., 2005). This virus was identified by a procedure based on DNase treatment of pooled respiratory samples, random amplification and cloning, followed by large-scale sequencing and bioinformatic analyses. In their original study, Allander et al. (2005) showed HBoV had a prevalence of 3.1% in Swedish children with LRTI. Soon thereafter, a second study from Australia reported a 5.2% prevalence of HBoV in respiratory samples collected from children with ARTI during winter (Sloots et al., 2006b). In a study of hospitalized patients, Arnold et al. (2006) detected HBoV in 5.6% of children, showing a peak prevalence of infection in spring. Subsequently, presence of the virus has been reported by investigators in Europe, the United Kingdom, the United States, Canada, Asia, and Australia, with prevalence rates ranging from 1.5% to 11.3% in these populations (Allander et al., 2005, Arnold et al., 2006, Bastien et al., 2006, Bastien et al., 2007, Choi et al., 2006, Foulongne et al., 2006, Kesebir et al., 2006, Ma et al., 2006, Manning et al., 2006, Sloots et al., 2006b, Fry et al., 2007, Maggi et al., 2007, Naghipour et al., 2007, Neske et al., 2007). However, the results from these studies, regarding seasonality, indicate that there is no obvious regular seasonal occurrence of HBoV. A common feature of these studies was that HBoV was most frequently detected in infants younger than 3 years of age.
Arnold et al. (2006) proposed an association between detection of HBoV in the upper respiratory tracts of children and LRTI. They demonstrated that presence of HBoV in the upper respiratory tract was positively associated with asthma, acute otitis media, and pneumonia. The most common signs observed in HBoV-infected children were respiratory distress and hypoxia associated with a diagnosis of bronchiolitis, supporting the concept that HBoV may directly infect the lower respiratory tract.
Recent studies reporting an association of HBoV with ARTI, differed from other similar studies by including control populations (Kesebir et al., 2006, Manning et al., 2006, Fry et al., 2007, Maggi et al., 2007). HBoV was detected at high frequency in subjects with overt disease, whereas the virus was either not found or was found very infrequently in control subjects. Although a positive association with ARTI was suggested, the results remain inconclusive, given the high rate at which other viruses were detected in HBoV-positive specimens. These findings add important corroborative support, but still do not prove causality of disease.
In a similar study using control subjects, Allander et al. (2007b) proposed HBoV as a cause of acute wheezing in children. However, differences in specimen type, age groups, and time of year tested for controls and test subjects were considerable and may have confounded detection rates in this study.
HBoV has only rarely been detected in immunocompetent adult subjects (Bastien et al., 2006) yet was frequently detected in immunosuppressed adults (Kupfer et al., 2006, Manning et al., 2006, Maggi et al., 2007). However, the presence of HBoV in these subjects may be the result of re-infection, viral persistence or reactivation (Allander, 2008). To answer these questions, prospective longitudinal examination of HBoV in immunosuppressed patients will be an important area for future studies.
The idea that HBoV infection may play a possible role in gastroenteritis has been suggested because of the frequent manifestation of gastrointestinal symptoms in HBoV-positive subjects. Several studies have examined an association of HBoV with gastrointestinal disease, and have reported HBoV presence in faeces with a prevalence rate ranging from 0.8% to 9.1% in these patients (Lau et al., 2007, Lee et al., 2007, Neske et al., 2007, Vicente et al., 2007). Also, Albuquerque et al. (2008) reported the detection of HBoV in Brazilian children with gastroenteritis in the absence of respiratory symptoms. Although these findings suggest that HBoV may play a role as an agent of acute gastroenteritis, it is possible that this is a clinical presentation of a systemic response to HBoV infection by the host. This data however, does add new knowledge about potential mechanisms of transmission for this virus.
So far, two slightly different genetic lineages of HBoV have been reported globally that share greater than 98% amino acid identity (Allander et al., 2005, Kesebir et al., 2006, Bastien et al., 2007, Chieochansin et al., 2007, Neske et al., 2007). Also, Lau et al. (2007) reported a high level of genomic conservation of HBoV detected in samples from different anatomical sites, showing that a single lineage of HBoV was found in both respiratory tract and enteric specimens. Similarly, HBoV detected in patients over a 21-month period showed little genetic variation within the NP-1 and NS1 genes (Arnold et al., 2006).
4.2. Novel human polyomaviruses KIV and WUV
Recently, two new human polyomaviruses, KIV and WUV were detected in respiratory tract specimens (Allander et al., 2007a, Gaynor et al., 2007). The viruses were identified following large-scale molecular screening using high throughput DNA sequencing of random clones.
KIV and WUV are related members of a new group (or possibly sub-family) within the Polyomaviridae, small DNA viruses with circular, covalently closed double-stranded genomes infecting a wide range of mammalian and avian species. Polyomaviruses frequently establish persistent, lifelong infections characterized by highly restricted or latent infection.
In their initial reports Allander et al. (2007a) detected KIV in 1% of nasopharyngeal aspirates and 0.5% of stool samples collected from a Swedish population and Gaynor et al. (2007) reported a prevalence of 3% and 0.6% for WUV in respiratory samples from Australia and the USA, respectively. Neither group was able to detect these viruses in urine samples.
Since these first reports, KIV and WUV DNA have been detected in respiratory specimens from a number of geographic locations, suggesting a global presence for these viruses. Studies from Australia and Scotland reported a prevalence for KIV of 2.5% and 1.4%, respectively (Bialasiewicz et al., 2008, Norja et al., 2007), and for WUV prevalence rates ranged from 1% in a Scottish population to 7% in Australian and South Korean populations (Bialasiewicz et al., 2008, Han et al., 2007, Norja et al., 2007).
One striking feature of early findings concerning KIV and WUV is their high rate of co-detection with other respiratory viruses. A co-detection rate of 74% has been observed for KIV (Bialasiewicz et al., 2008) and rates ranging from 68% to 79% for WUV (Bialasiewicz et al., 2008, Han et al., 2007, Le et al., 2007). A level of 10% co-detection of KIV with WUV in the absence of any other respiratory virus was reported by Bialasiewicz et al. (2008).
Although an aetiologic role in childhood respiratory disease has been proposed for KIV and WUV (Allander et al., 2007a, Bialasiewicz et al., 2008, Gaynor et al., 2007), initial investigations have been based on large-scale screening of respiratory samples referred for general diagnostic testing from patients with moderate to severe respiratory disease. It is however difficult to assess the pathogenic role of these viruses without examining samples collected from matched control populations. Three recent studies have sought to do this for WUV, reporting prevalence rates in symptomatic versus asymptomatic subjects of 7.0% versus 4.2% (Han et al., 2007), 2.5% versus 6.4% (Abed et al., 2007) and 1.0% versus 5.4% (Norja et al., 2007). However, these data must be interpreted with caution as each of these studies examined only small numbers of controls and some of these were not well matched to the symptomatic population tested. Interestingly, although Norja et al. (2007) reported a high prevalence of KIV (5.4%) and WUV (5.4%) in their control subjects, 5 of the 6 (83%) positive specimens came from subjects who were classified as immunosuppressed. If consistent, the presence of KIV and WUV in control populations may represent a latent or persistent infection with subsequent asymptomatic reactivation or prolonged shedding from a prior respiratory tract infection.
Persistent shedding of WUV over a 6–8 week period was amongst a number of interesting observations reported in two children (1.5 and 4 years old) from St. Louis, USA, and WUV was also the only virus detected in a 1-day-old infant with respiratory symptoms raising interesting questions about possible transmission mechanisms of this virus (Le et al., 2007).
To date, little information is available regarding the molecular diversity of KIV and WUV strains present in different populations. Early data from Australian, USA and Canadian WUV-positive samples showed limited or no genetic variations based on sequencing from the large TAg region and the VP2 region (Abed et al., 2007, Gaynor et al., 2007). This was substantiated by whole genome analysis of Australian and USA samples which confirmed limited sequence variation amongst KIV and WUV strains from those patient groups (Bialasiewicz et al., 2008).
Further studies will need to be completed before the role of KIV and WUV as respiratory pathogens can be confirmed. It remains possible that these viruses are not involved in respiratory disease and their presence in the respiratory tract simply reflects their mode of transmission. However, considering the oncogenic potential of polyomaviruses in mammals, linking KIV and WUV to a particular disease will be important and may have significant medical implications.
5. Conclusion
With the development of new molecular technology, our ability to detect and characterize new viral agents has greatly improved. As a result, genome sequences have been described for new viruses that are associated with the human respiratory tract, gastrointestinal tract as well as new blood-borne viruses. Some of these are recognized as significant human pathogens causing disease in certain population groups. Others can be found in clinical specimens without definitive evidence for their role as the causative agent of disease, and yet others, like TT-(torqueteno) virus (Maggi et al., 2003a, Pifferi et al., 2006) and mimivirus (Khan et al., 2007, Raoult et al., 2007) have been loosely associated with respiratory disorders in humans.
Many putative viral respiratory pathogens could not be isolated from patients with overt disease, and researchers have as yet been unable to satisfy the modified Koch's postulates that will prove a causal relationship. It is important therefore, that further extensive clinical studies be continued in an attempt to define the role of these agents as human pathogens.
Still, for a significant proportion of clinical infectious disease of suspected viral origin, a pathogen cannot be identified. Although new molecular methods are increasingly used to investigate these unknown causes of disease, they remain technically challenging and prone to the amplification of non-viral related sequence artefacts. However, with continuing advances in molecular technology and the development of more reliable, robust and reproducible molecular techniques, it seems certain that new potential viral pathogens of humans will be discovered.
References
- Abed Y., Wang D., Boivin G. WU polyomavirus in children, Canada. Emerg Infect Dis. 2007;13:1936–1938. doi: 10.3201/eid1312.070909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agapov E., Sumino K.C., Gaudreault-Keener M., Storch G.A., Holtzman M.J. Genetic variability of human metapneumovirus infection: evidence of a shift in viral genotype without a change in illness. J Infect Dis. 2006;193:396–403. doi: 10.1086/499310. [DOI] [PubMed] [Google Scholar]
- Albuquerque M.C.M., Rocha L.N., Benati F.J., Soares C.C., Maranhao A.G., Ramírez M.L. Human bocavirus infection in children with gastroenteritis, Brazil. Emerg Infect Dis. 2008;13:1756–1758. doi: 10.3201/eid1311.060671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sonboli N., Hart C.A., Al-Aeryani A., Banajeh S.M., Al-Aghbari N., Dove W. Respiratory syncytial virus and human metapneumovirus in children with acute respiratory infections in Yemen. Pediatr Infect Dis J. 2005;24:734–736. doi: 10.1097/01.inf.0000172937.80719.7f. [DOI] [PubMed] [Google Scholar]
- Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T., Andreasson K., Gupta S., Bjerkner A., Bogdanovic G., Persson M.A. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T., Jartti T., Gupta S., Niesters H.G., Lehtinen P., Osterback R. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T. Human bocavirus. J Clin Virol. 2008;41:29–33. doi: 10.1016/j.jcv.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Ambrose H.E., Clewley J.P. Virus discovery by sequence-independent genome amplification. Rev Med Virol. 2006;16:365–383. doi: 10.1002/rmv.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden K.E., Nissen M.D., Sloots T.P., Mackay I.M. New human coronavirus, HCoV–NL63, associated with severe lower respiratory tract disease in Australia. J Med Virol. 2005;75:455–462. doi: 10.1002/jmv.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J.C., Singh K.K., Spector S.A., Sawyer M.H. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43:283–288. doi: 10.1086/505399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.C., Shimizu C., Shike H., Garcia F., van der H.L., Kuijper T.W. Human coronavirus-NL63 infection is not associated with acute Kawasaki disease. Adv Exp Med Biol. 2006;581:523–526. doi: 10.1007/978-0-387-33012-9_94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N., Ward D., Van C.P., Brandt K., Lee S.H., McNabb G. Human metapneumovirus infection in the Canadian population. J Clin Microbiol. 2003;41:4642–4646. doi: 10.1128/JCM.41.10.4642-4646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N., Anderson K., Hart L., Van C.P., Brandt K., Milley D. Human coronavirus NL63 infection in Canada. J Infect Dis. 2005;191:503–506. doi: 10.1086/426869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N., Brandt K., Dust K., Ward D., Li Y. Human Bocavirus infection, Canada. Emerg Infect Dis. 2006;12:848–850. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N., Chui N., Robinson J.L., Lee B.E., Dust K., Hart L. Detection of human bocavirus in Canadian children in a 1-year study. J Clin Microbiol. 2007;45:610–613. doi: 10.1128/JCM.01044-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay E.D., Erdman D.D., Anderson L.J., Peret T.C., Schrag S.J., Fields B.S. Kawasaki disease and human coronavirus. J Infect Dis. 2005;192:352–353. doi: 10.1086/431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biacchesi S., Skiadopoulos M.H., Boivin G., Hanson C.T., Murphy B.R., Collins P.L. Genetic diversity between human metapneumovirus subgroups. Virology. 2003;315:1–9. doi: 10.1016/s0042-6822(03)00528-2. [DOI] [PubMed] [Google Scholar]
- Bialasiewicz S., Whiley D.M., Lambert S.B., Jacob K., Bletchly C., Wang D. Presence of the newly discovered human polyomaviruses KI and WU in Australian patients with acute respiratory tract infection. J Clin Virol. 2008;41:63–68. doi: 10.1016/j.jcv.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G., Abed Y., Pelletier G., Ruel L., Moisan D., Cote S. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- Boivin G., De S.G., Cote S., Gilca R., Abed Y., Rochette L. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G., Mackay I., Sloots T.P., Madhi S., Freymuth F., Wolf D. Global genetic diversity of human metapneumovirus fusion gene. Emerg Infect Dis. 2004;10:1154–1157. doi: 10.3201/eid1006.031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouscambert-Duchamp M., Lina B., Trompette A., Moret H., Motte J., Andreoletti L. Detection of human metapneumovirus RNA sequences in nasopharyngeal aspirates of young French children with acute bronchiolitis by real-time reverse transcriptase PCR and phylogenetic analysis. J Clin Microbiol. 2005;43:1411–1414. doi: 10.1128/JCM.43.3.1411-1414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane P.A., van den Hoogen B.G., Chakrabarti S., Fegan C.D., Osterhaus A.D. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 2003;31:309–310. doi: 10.1038/sj.bmt.1703849. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control Prevention (CDC) Outbreak of severe acute respiratory syndrome—worldwide. Morb Mortal Wkly Rep. 2003;52(11):226–228. [PubMed] [Google Scholar]
- Chang L.Y., Chiang B.L., Kao C.L., Wu M.H., Chen P.J., Berkhout B. Lack of association between infection with a novel human coronavirus (HCoV), HCoV–NH, and Kawasaki disease in Taiwan. J Infect Dis. 2006;193:283–286. doi: 10.1086/498875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chano F., Rousseau C., Laferriere C., Couillard M., Charest H. Epidemiological survey of human metapneumovirus infection in a large pediatric tertiary care center. J Clin Microbiol. 2005;43:5520–5525. doi: 10.1128/JCM.43.11.5520-5525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieochansin T., Chutinimitkul S., Payungporn S., Hiranras T., Samransamruajkit R., Theamboolers A. Complete coding sequences and phylogenetic analysis of Human Bocavirus (HBoV) Virus Res. 2007;129:54–57. doi: 10.1016/j.virusres.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Chiu S.S., Chan K.H., Chu K.W., Kwan S.W., Guan Y., Poon L.L. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.H., Lee H.J., Kim S.J., Eun B.W., Kim N.H., Lee J.A. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43:585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas L.E., Nasser A.M., Dove W., Gurgel R.Q., Greensill J., Hart C.A. Human metapneumovirus and respiratory syncytial virus, Brazil. Emerg Infect Dis. 2003;9:1626–1628. doi: 10.3201/eid0912.030522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollner H., Risnes K., Radtke A., Nordbo S.A. Outbreak of human metapneumovirus infection in norwegian children. Pediatr Infect Dis J. 2004;23:436–440. doi: 10.1097/01.inf.0000126401.21779.74. [DOI] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der W.S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Drosten C., Preiser W., Gunther S., Schmitz H., Doerr H.W. Severe acute respiratory syndrome: identification of the etiological agent. Trends Mol Med. 2003;9:325–327. doi: 10.1016/S1471-4914(03)00133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T., Endo R., Kikuta H., Ishiguro N., Yoshioka M., Ma X. Seroprevalence of human metapneumovirus in Japan. J Med Virol. 2003;70:281–283. doi: 10.1002/jmv.10391. [DOI] [PubMed] [Google Scholar]
- Ebihara T., Endo R., Kikuta H., Ishiguro N., Ishiko H., Hara M. Human metapneumovirus infection in Japanese children. J Clin Microbiol. 2004;42:126–132. doi: 10.1128/JCM.42.1.126-132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T., Endo R., Ma X., Ishiguro N., Kikuta H. Detection of human coronavirus NL63 in young children with bronchiolitis. J Med Virol. 2005;75:463–465. doi: 10.1002/jmv.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T., Endo R., Ma X., Ishiguro N., Kikuta H. Lack of association between New Haven coronavirus and Kawasaki disease. J Infect Dis. 2005;192:351–352. doi: 10.1086/430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickmann M., Becker S., Klenk H.D., Doerr H.W., Stadler K., Censini S. Phylogeny of the SARS coronavirus. Science. 2003;302:1504–1505. doi: 10.1126/science.302.5650.1504b. [DOI] [PubMed] [Google Scholar]
- Esper F., Boucher D., Weibel C., Martinello R.A., Kahn J.S. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111:1407–1410. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- Esper F., Martinello R.A., Boucher D., Weibel C., Ferguson D., Landry M.L. A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189:1388–1396. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F., Shapiro E.D., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Coronavirus HKU1 infection in the United States. Emerg Infect Dis. 2006;12:775–779. doi: 10.3201/eid1205.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R., Erdman D., Anderson L.J., Walsh E.E. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Walsh E.E. Viral pneumonia in older adults. Clin Infect Dis. 2006;42:518–524. doi: 10.1086/499955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster J., Ihorst G., Rieger C.H., Stephan V., Frank H.D., Gurth H. Prospective population-based study of viral lower respiratory tract infections in children under 3 years of age (the PRI.DE study) Eur J Pediatr. 2004;163:709–716. doi: 10.1007/s00431-004-1523-9. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A., Kuiken T., Schutten M., van A.G., van Doornum G.J., van den Hoogen B.G. Aetiology: Koch's postulates fulfilled for SARS virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R.A., Hartwig N.G., Bestebroer T.M., Niemeyer B., de Jong J.C., Simon J.H. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci USA. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulongne V., Rodiere M., Segondy M. Human Bocavirus in children. Emerg Infect Dis. 2006;12:862–863. doi: 10.3201/eid1205.051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks D.N., Relman D.A. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev. 1996;9:18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freymouth F., Vabret A., Legrand L., Eterradossi N., Lafay-Delaire F., Brouard J. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr Infect Dis J. 2003;22:92–94. doi: 10.1097/00006454-200301000-00024. [DOI] [PubMed] [Google Scholar]
- Fry A.M., Lu X., Chittaganpitch M., Peret T., Fischer J., Dowell S.F. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia M.L., Calvo C., Martin F., Perez-Brena P., Acosta B., Casas I. Human metapneumovirus infections in hospitalised infants in Spain. Arch Dis Child. 2006;91:290–295. doi: 10.1136/adc.2005.082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor A.M., Nissen M.D., Whiley D.M., Mackay I.M., Lambert S.B., Wu G. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:595–604. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Campanini G., Rovida F., Sarasini A., Lilleri D., Paolucci S. Changing circulation rate of human metapneumovirus strains and types among hospitalized pediatric patients during three consecutive winter-spring seasons. Brief report. Arch Virol. 2005;150:2365–2375. doi: 10.1007/s00705-005-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Campanini G., Rovida F., Percivalle E., Sarasini A., Marchi A. Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J Med Virol. 2006;78:938–949. doi: 10.1002/jmv.20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G.C., Capuano A.W., Setterquist S.F., Sanchez J.L., Neville J.S., Olson J. Human metapneumovirus Peru. Emerg Infect Dis. 2006;12:347–350. doi: 10.3201/eid1202.051133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Hamelin M.E., Cote S., Laforge J., Lampron N., Bourbeau J., Weiss K. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2005;41:498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Han T.H., Chung J.-Y., Koo J.W., Kim S.W., Hwang E.-S. WU polyomavirus in children with acute lower respiratory tract infections, South Korea. Emerg Infect Dis. 2007;13:1766–1768. doi: 10.3201/eid1311.070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T., Osterback R., Peltola V., Jartti T., Vainionpaa R. Human metapneumovirus infections in children. Emerg Infect Dis. 2008;14:101–106. doi: 10.3201/eid1401.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. Coronaviruses. In: Knipe D., Howley P., editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1187–1203. [Google Scholar]
- Honda H., Iwahashi J., Kashiwagi T., Imamura Y., Hamada N., Anraku T. Outbreak of human metapneumovirus infection in elderly inpatients in Japan. J Am Geriatr Soc. 2006;54:177–180. doi: 10.1111/j.1532-5415.2005.00575_10.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J.S. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida A., Iritani N., Kubo H., Shiomi M., Kohdera U., Murakami T. Seasonal distribution and phylogenetic analysis of human metapneumovirus among children in Osaka City, Japan. J Clin Virol. 2006;35:394–399. doi: 10.1016/j.jcv.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Kaiser L., Regamey N., Roiha H., Deffernez C., Frey U. Human coronavirus NL63 associated with lower respiratory tract symptoms in early life. Pediatr Infect Dis J. 2005;24:1015–1017. doi: 10.1097/01.inf.0000183773.80217.12. [DOI] [PubMed] [Google Scholar]
- Karron R.A., Belshe R.B., Wright P.F., Thumar B., Burns B., Newman F. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr Infect Dis J. 2003;22:394–405. doi: 10.1097/01.inf.0000066244.31769.83. [DOI] [PubMed] [Google Scholar]
- Kesebir D., Vazquez M., Weibel C., Shapiro E.D., Ferguson D., Landry M.L. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194:1276–1282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., La S.B., Lepidi H., Raoult D. Pneumonia in mice inoculated experimentally with Acanthamoeba polyphaga mimivirus. Microb Pathog. 2007;42:56–61. doi: 10.1016/j.micpath.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Kim Y.K., Lee H.J. Human metapneumovirus-associated lower respiratory tract infections in korean infants and young children. Pediatr Infect Dis J. 2005;24:1111–1112. doi: 10.1097/01.inf.0000190042.65120.23. [DOI] [PubMed] [Google Scholar]
- Konig B., Konig W., Arnold R., Werchau H., Ihorst G., Forster J. Prospective study of human metapneumovirus infection in children less than 3 years of age. J Clin Microbiol. 2004;42:4632–4635. doi: 10.1128/JCM.42.10.4632-4635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kupfer B., Vehreschild J., Cornely O., Kaiser R., Plum G., Viazov S. Severe pneumonia and human bocavirus in adult. Emerg Infect Dis. 2006;12:1614–1616. doi: 10.3201/eid1210.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M., Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher C., Geltner C., Fischer H., Nachbaur D., Muller L.C., Huemer H.P. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant. 2005;24:1891–1901. doi: 10.1016/j.healun.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Li K.S., Huang Y., Tsoi H.W., Wong B.H. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Yip C.C., Tse H., Tsoi H.W., Cheng V.C. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Yip C.C., Que T.L., Lee R.A., Au-Yeung R.K., Zhou B. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis. 2007;196:986–993. doi: 10.1086/521310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le B.-M., Demertzis L.M., Wu G., Tibbetts R.J., Buller R., Arens M.Q. Clinical and epidemiologic characterization of WU polyomavirus infection, St. Louis, Missouri. Emerg Infect Dis. 2007;13:1936–1938. doi: 10.3201/eid1312.070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.I., Chung J.Y., Han T.H., Song M.O., Hwang E.S. Detection of human bocavirus in children hospitalized because of acute gastroenteritis. J Infect Dis. 2007;196:994–997. doi: 10.1086/521366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J., Esper F., Weibel C., Kahn J.S. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J Clin Microbiol. 2005;43:1213–1219. doi: 10.1128/JCM.43.3.1213-1219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lina B., Valette M., Foray S., Luciani J., Stagnara J., See D.M. Surveillance of community-acquired viral infections due to respiratory viruses in Rhone-Alpes (France) during winter 1994 to 1995. J Clin Microbiol. 1996;34:3007–3011. doi: 10.1128/jcm.34.12.3007-3011.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie J.K., Hacker J.K., Gonzales R., Mark J., Maselli J.H., Yagi S. Characterization of viral agents causing acute respiratory infection in a San Francisco University Medical Center Clinic during the influenza season. Clin Infect Dis. 2005;41:822–828. doi: 10.1086/432800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewick H.P., Abed Y., van N.N., Boivin G., Klugman K.P., Madhi S.A. Human metapneumovirus genetic variability, South Africa. Emerg Infect Dis. 2005;11:1074–1078. doi: 10.3201/eid1107.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Endo R., Ishiguro N., Ebihara T., Ishiko H., Ariga T. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol. 2006;44:1132–1134. doi: 10.1128/JCM.44.3.1132-1134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay I.M., Bialasiewicz S., Waliuzzaman Z., Chidlow G.R., Fegredo D.C., Laingam S. Use of the P gene to genotype human metapneumovirus identifies 4 viral subtypes. J Infect Dis. 2004;190:1913–1918. doi: 10.1086/425013. [DOI] [PubMed] [Google Scholar]
- Maggi F., Pifferi M., Fornai C., Andreoli E., Tempestini E., Vatteroni M. TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity. J Virol. 2003;77:2418–2425. doi: 10.1128/JVI.77.4.2418-2425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi F., Pifferi M., Vatteroni M., Fornai C., Tempestini E., Anzilotti S. Human metapneumovirus associated with respiratory tract infections in a 3-year study of nasal swabs from infants in Italy. J Clin Microbiol. 2003;41:2987–2991. doi: 10.1128/JCM.41.7.2987-2991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi F., Andreoli E., Pifferi M., Meschi S., Rocchi J., Bendinelli M. Human bocavirus in Italian patients with respiratory diseases. J Clin Virol. 2007;38:321–325. doi: 10.1016/j.jcv.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Manning A., Russell V., Eastick K., Leadbetter G.H., Hallam N., Templeton K. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis. 2006;194:1283–1290. doi: 10.1086/508219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Martino R., Porras R.P., Rabella N., Williams J.V., Ramila E., Margall N. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam A.J., Hasenbein M.E., Feldman H.A., Cole S.E., Offermann J.T., Riley A.M. Human metapneumovirus in children tested at a tertiary-care hospital. J Infect Dis. 2004;190:20–26. doi: 10.1086/421120. [DOI] [PubMed] [Google Scholar]
- McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. 1967;57:933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Chao R.K., Krause H.E., Wasil R., Mocega H.E., Mufson M.A. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis. 1974;130:502–507. doi: 10.1093/infdis/130.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moes E., Vijgen L., Keyaerts E., Zlateva K., Li S., Maes P. A novel pancoronavirus RT-PCR assay: frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium. BMC Infect Dis. 2005;5:6. doi: 10.1186/1471-2334-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J.A., Erdman D.D., Weinberg G.A., Edwards K., Hall C.B., Walker F.J. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghipour M., Cuevas L.E., Bakhshinejad T., Dove W., Hart C.A. Human bocavirus in Iranian children with acute respiratory infections. J Med Virol. 2007;79:539–543. doi: 10.1002/jmv.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neske F., Blessing K., Tollmann F., Schubert J., Rethwilm A., Kreth H.W. Real-time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. J Clin Microbiol. 2007;45:2116–2122. doi: 10.1128/JCM.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K.G., McNally T., Silverman M., Simons P., Stockton J.D., Zambon M.C. Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine. 2006;24:102–108. doi: 10.1016/j.vaccine.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Nissen M.D., Siebert D.J., Mackay I.M., Sloots T.P., Withers S.J. Evidence of human metapneumovirus in Australian children. Med J Aust. 2002;176:188. doi: 10.5694/j.1326-5377.2002.tb04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norja P., Ubillos I., Templeton K., Simmonds P. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J Clin Virol. 2007;40:307–311. doi: 10.1016/j.jcv.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyola D.E., puche-Solis A.G., Herrera-Diaz A., Soria-Guerra R.E., Sanchez-Alvarado J., Lopez-Revilla R. Human metapneumovirus infections in Mexico: epidemiological and clinical characteristics. J Med Microbiol. 2005;54:969–974. doi: 10.1099/jmm.0.46052-0. [DOI] [PubMed] [Google Scholar]
- Osterhaus A., Fouchier R. Human metapneumovirus in the community. Lancet. 2003;361:890–891. doi: 10.1016/S0140-6736(03)12785-7. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G., Dery P., Abed Y., Boivin G. Respiratory tract reinfections by the new human Metapneumovirus in an immunocompromised child. Emerg Infect Dis. 2002;8:976–978. doi: 10.3201/eid0809.020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peret T.C., Boivin G., Li Y., Couillard M., Humphrey C., Osterhaus A.D. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–1663. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifferi M., Maggi F., Caramella D., De M.E., Andreoli E., Meschi S. High torquetenovirus loads are correlated with bronchiectasis and peripheral airflow limitation in children. Pediatr Infect Dis J. 2006;25:804–808. doi: 10.1097/01.inf.0000232723.58355.f4. [DOI] [PubMed] [Google Scholar]
- Raoult D., La S.B., Birtles R. The discovery and characterization of Mimivirus, the largest known virus and putative pneumonia agent. Clin Infect Dis. 2007;45:95–102. doi: 10.1086/518608. [DOI] [PubMed] [Google Scholar]
- Robinson J.L., Lee B.E., Bastien N., Li Y. Seasonality and clinical features of human metapneumovirus infection in children in Northern Alberta. J Med Virol. 2005;76:98–105. doi: 10.1002/jmv.20329. [DOI] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Samransamruajkit R., Thanasugarn W., Prapphal N., Theamboonlers A., Poovorawan Y. Human metapneumovirus in infants and young children in Thailand with lower respiratory tract infections; molecular characteristics and clinical presentations. J Infect. 2006;52:254–263. doi: 10.1016/j.jinf.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Sarasini A., Percivalle E., Rovida F., Campanini G., Genini E., Torsellini M. Detection and pathogenicity of human metapneumovirus respiratory infection in pediatric Italian patients during a winter–spring season. J Clin Virol. 2006;35:59–68. doi: 10.1016/j.jcv.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A., Suzuki H., Saito R., Sato M., Sato I., Sano Y. Prevalence of human metapneumovirus and influenza virus infections among Japanese children during two successive winters. Pediatr Infect Dis J. 2005;24:905–908. doi: 10.1097/01.inf.0000180984.61778.1e. [DOI] [PubMed] [Google Scholar]
- Schildgen O., Jebbink M.F., de V.M., Pyrc K., Dijkman R., Simon A. Identification of cell lines permissive for human coronavirus NL63. J Virol Methods. 2006;138:207–210. doi: 10.1016/j.jviromet.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple M.G., Cowell A., Dove W., Greensill J., McNamara P.S., Halfhide C. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu C., Shike H., Baker S.C., Garcia F., van der H.L., Kuijpers T.W. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J Infect Dis. 2005;192:1767–1771. doi: 10.1086/497170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloots T.P., Mackay I.M., Bialasiewicz S., Jacob K.C., McQueen E., Harnett G.B. Human metapneumovirus, Australia, 2001–2004. Emerg Infect Dis. 2006;12:1263–1266. doi: 10.3201/eid1208.051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloots T.P., McErlean P., Speicher D.J., Arden K.E., Nissen M.D., Mackay I.M. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol. 2006;35:99–102. doi: 10.1016/j.jcv.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol. 2003;331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A., Korn K., Wildner O., Uberla K. Characterization of virus isolates by particle-associated nucleic acid PCR. J Clin Microbiol. 2005;43:716–720. doi: 10.1128/JCM.43.2.716-720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton J., Stephenson I., Fleming D., Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis. 2002;8:897–901. doi: 10.3201/eid0809.020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Tyrrell D.A., Bynoe M.L. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. 1965;1:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret A., Mourez T., Dina J., van der H.L., Gouarin S., Petitjean J. Human coronavirus NL63, France. Emerg Infect Dis. 2005;11:1225–1229. doi: 10.3201/eid1108.050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret A., Dina J., Gouarin S., Petitjean J., Corbet S., Freymuth F. Detection of the new human coronavirus HKU1: a report of 6 cases. Clin Infect Dis. 2006;42:634–639. doi: 10.1086/500136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de G.R., Fouchier R.A. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen B.G., van Doornum G.J., Fockens J.C., Cornelissen J.J., Beyer W.E., de G.R. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- van den Hoogen B.G., Osterhaus D.M., Fouchier R.A. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23:S25–S32. doi: 10.1097/01.inf.0000108190.09824.e8. [DOI] [PubMed] [Google Scholar]
- van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Pyrc K., Berkhout B. Human coronavirus NL63, a new respiratory virus. FEMS Microbiol Rev. 2006;30:760–773. doi: 10.1111/j.1574-6976.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Sure K., Ihorst G., Stang A., Pyrc K., Jebbink M.F. Human coronavirus NL63 infection is associated with croup. Adv Exp Med Biol. 2006;581:485–491. doi: 10.1007/978-0-387-33012-9_86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elden L.J., van Loon A.M., van A.F., Hendriksen K.A., Hoepelman A.I., van Kraaij M.G. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J Infect Dis. 2004;189:652–657. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viazov S., Ratjen F., Scheidhauer R., Fiedler M., Roggendorf M. High prevalence of human metapneumovirus infection in young children and genetic heterogeneity of the viral isolates. J Clin Microbiol. 2003;41:3043–3045. doi: 10.1128/JCM.41.7.3043-3045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente D., Cilla G., Montes M., Perez-Yarza E.G., Perez-Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis. 2007;13:636–637. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Linstow M.L., Larsen H.H., Eugen-Olsen J., Koch A., Nordmann W.T., Meyer A.M. Human metapneumovirus and respiratory syncytial virus in hospitalized Danish children with acute respiratory tract infection. Scand J Infect Dis. 2004;36:578–584. doi: 10.1080/00365540410018166. [DOI] [PubMed] [Google Scholar]
- Wang D., Urisman A., Liu Y.T., Springer M., Ksiazek T.G., Erdman D.D. Viral discovery and sequence recovery using DNA microarrays. PLoS Biol. 2003;1:257–260. doi: 10.1371/journal.pbio.0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.V., Harris P.A., Tollefson S.J., Halburnt-Rush L.L., Pingsterhaus J.M., Edwards K.M. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.V., Martino R., Rabella N., Otegui M., Parody R., Heck J.M. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192:1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.V., Tollefson S.J., Heymann P.W., Carper H.T., Patrie J., Crowe J.E. Human metapneumovirus infection in children hospitalized for wheezing. J Allergy Clin Immunol. 2005;115:1311–1312. doi: 10.1016/j.jaci.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.V., Wang C.K., Yang C.F., Tollefson S.J., House F.S., Heck J.M. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D.G., Zakay-Rones Z., Fadeela A., Greenberg D., Dagan R. High seroprevalence of human metapneumovirus among young children in Israel. J Infect Dis. 2003;188:1865–1867. doi: 10.1086/380100. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Tsoi H.W., Huang Y., Poon R.W., Chu C.M. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005;192:1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Yip C.C., Huang Y., Tsoi H.W., Chan K.H. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J Virol. 2006;80:7136–7145. doi: 10.1128/JVI.00509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xepapadaki P., Psarras S., Bossios A., Tsolia M., Gourgiotis D., Liapi-Adamidou G. Human Metapneumovirus as a causative agent of acute bronchiolitis in infants. J Clin Virol. 2004;30:267–270. doi: 10.1016/j.jcv.2003.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]