Abstract
Enterovirus 71 (EV71) has emerged as a clinically important neurotropic virus that can cause acute flaccid paralysis and encephalitis, leading to cardiopulmonary failure and death. Recurring outbreaks of EV71 have been reported in several countries. The current lack of approved anti-EV71 therapy has prompted intense research into antiviral development. Several strategies – ranging from target-based chemical design to compound library screenings – have been employed, while others revisited compound series generated from antiviral developments against poliovirus and human rhinoviruses. These efforts have given rise to a diversity of antiviral candidates that include small molecules and non-conventional nucleic-acid-based strategies. This review aims to highlight candidates with potential for further clinical development based on their putative modes of action.
Enterovirus 71 (EV71) is described as one of the human enterovirus A species under the genus Enterovirus in the Picornaviridae family of viruses, which includes poliovirus [1]. While efforts to eradicate poliovirus through vaccination programs have limited the number of polio-endemic countries to just four (Afghanistan, India, Nigeria and Pakistan) [2], EV71 has emerged as an important non-polio neurotropic enterovirus.
EV71 was first isolated from patients with central nervous system diseases in California between 1969 and 1974 [3]. The same authors also described the EV71 prototype strain, BrCr, isolated from a two-month-old patient who presented with aseptic meningitis. Since then, EV71 outbreaks have been reported in several countries beyond North America, including Taiwan, Australia, Malaysia and Singapore [4]. These outbreaks have mainly involved young children, with most cases displaying mild, self-limiting hand, foot and mouth disease; however, EV71 outbreaks have also been associated with a variety of severe neurological complications that can deteriorate rapidly to involve cardiopulmonary failure with high mortality rates. Transmission of EV71 can occur rapidly via the fecal–oral and droplet and/or aerosol routes. During the largest EV71 outbreak to date, in Taiwan in 1998, more than 100,000 children were affected, with 405 severe cases involving neurological or cardiopulmonary complications, of which 78 were fatal [5].
The spectrum of EV71-associated neurological diseases includes aseptic meningitis, brain stem encephalitis and poliomyelitis-like acute flaccid paralysis. Like poliomyelitis, severe EV71 infections can result in permanent neurological damage. In a long-term study of children who survived neurologically involved EV71 infections during the 1998 outbreak in Taiwan, patients who had a more severe infection (involving both neurological and cardiopulmonary complications) displayed signs of neurologic sequelae, impaired neurodevelopment and impaired cognitive functions [6].
There is currently no effective vaccine or antiviral against EV71. Treatments for acute EV71 infections with neurological manifestations mainly aim to alleviate symptoms. Mechanical cardiopulmonary support systems and the administration of milrinone, a positive inotropic agent, have been used to prevent cardiopulmonary failure and thus improve the clinical outcome of patients [7]. The lack of antiviral treatment options against EV71 remains a worrying situation, however, because EV71 has been found to circulate endemically with peak activity in warmer seasons (e.g. summer to fall) [8]. Considering the propensity of EV71 to cause severe neurological diseases in children, there is a need to develop effective antiviral treatment options to prevent or reduce EV71-related deaths and long-term neuropathy in the next EV71 global outbreak.
In this review, we focus on documenting recent developments towards an antiviral for EV71 infections. Previous reviews have covered the development of antivirals targeting picornaviruses or enteroviruses in general 9, 10, 11, 12, 13, 14, 15; however, with the exception of poliovirus, EV71 has distinguished itself from other enteroviruses in terms of circulation and severity of associated diseases. In contrast to poliovirus, against which highly effective vaccines are available, the global population remains largely unprotected against EV71. Our review, therefore, highlights promising anti-EV71 strategies and their putative modes of inhibition.
General virology of picornaviruses
An understanding of the molecular mechanisms of viral infection and replication can enable the identification of potential antiviral targets. Although the exact mechanisms for EV71 replication have not been elucidated, picornaviruses generally share a highly similar virus architecture and mode of replication. Picornaviruses are non-enveloped viruses of ∼30 nm in diameter with an icosahedral capsid made up of 60 protomers, each of which is, in turn, made up of four structural proteins: VP1, VP2, VP3 and VP4. The picornavirus genome is encoded as a single-stranded, positive-sense RNA of 7500–8000 bases that consists of an open reading frame flanked by 5′ and 3′ untranslated regions (UTRs).
Upon virus attachment and entry into the host cell, an uncapping event occurs to release the RNA genome into the cell. Covalently attached to the 5′ end is a viral protein, VPg (3B), which is cleaved by a cellular enzyme during early infection, whereas the 3′ end has a poly(A) tract. The 5′ and 3′ UTRs are highly structured regions that interact with a variety of viral and host proteins in translation and RNA synthesis events.
Cap-independent translation of the viral RNA takes place through the recruitment of host replication machinery at the internal ribosome entry site located in the 5′ UTR of the viral RNA. The viral polyprotein can be divided into three precursor molecules (P1, P2 and P3) (Fig. 1 ). P1 contains all the viral capsid proteins, VP1–4, and P2 and P3 carry the viral non-structural proteins. Functional viral proteins and precursor proteins are produced upon maturation cleavage of the polyprotein by the virus-encoded proteases 2Apro and 3Cpro. Negative RNA intermediates of the viral genome are also generated to serve as templates for the replication of positive-sense RNA viral genomes. These events typically take place in virus-induced membrane complexes within host cells. Progeny virions are then self-assembled from the synthesized viral proteins and RNA genomes before their subsequent release from the host cell [16] (Fig. 1).
Figure 1.
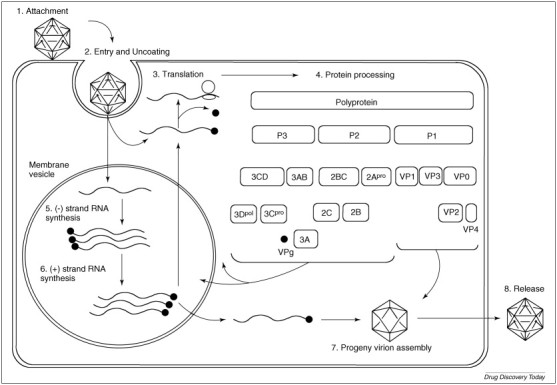
Graphical overview of picornavirus replication. Virus particle first attaches to host cell surface via a cellular receptor before entering and uncoating to unveil the viral RNA genome. Viral RNA is translated by cellular translational machinery to give a polyprotein that is then cleaved by the virus-encoded proteases 2Apro and 3Cpro to give functional precursor proteins (e.g. 2BC and 3CD) and individual proteins. Within virus-induced membrane vesicles, viral RNA (+) is copied by the viral RNA polymerase, 3Dpol, to give (−) strand RNA intermediates, which in turn provide the template for the synthesis of (+) strand viral RNA. The (+) strand viral RNAs are used to generate more (−) strand viral RNAs, translated into viral proteins or packaged into progeny virions. Lysis of host cells will result in the release of progeny virions. Adapted and modified from Figure 2 in Ref. [10] and Figure 4 In Ref. [16].
Inhibitors of virus attachment, entry and uncoating
EV71 receptors
Receptor binding is an essential event in virus infection. The ability to recognize and bind specific receptors typically determines the host range and tissue tropism of viruses. The recent characterizations of EV71 receptors have opened the door to the development of antiviral strategies targeting EV71 entry into host cells.
At least three cellular receptors for EV71 have been reported to date. Human scavenger receptor class B, member 2 (SCARB2) was described as a receptor for EV71 strains from all three genogroups (A, B and C) [17]. SCARB2 is a type III double-transmembrane protein primarily located in endosomes, although surface expression of SCARB2 has also been demonstrated [17]. The second EV71 receptor, human P-selectin glycoprotein ligand-1 (PSGL-1/CD162), is a sialomucin membrane protein expressed mainly in leukocytes, including dendritic cells and macrophages [18]. PSGL-1 was postulated to facilitate the viremic phase of EV71 by enabling replication in circulating leukocytes [19]. Using monoclonal antibodies targeting different parts of PSGL-1, the authors identified the N-terminal region as a crucial interaction site for EV71. The sialic acid residue of PSGL-1 might also be important for EV71 interaction. In the study by Yang et al. [20], sialidase removal of sialic acid residues from plasma membrane proteins was able to protect EV71-susceptible DLD-1 intestinal cells from infection. In another study, EV71 was found to infect immature dendritic cells via DC-SIGN. Infection was reduced by up to 50% when anti-DC-SIGN antibody was used to block DC-SIGN [21].
The potential for the development of antiviral strategies targeting EV71 receptor binding was demonstrated in all these studies. Anti-SCARB2 antibodies and soluble SCARB2–Fc conjugates were able to inhibit EV71 infection in a dose-dependent manner [17]. Likewise, soluble PSGL-1, monoclonal antibodies targeting the N-terminal of PSGL-1 [19] and sialylated glycans purified from human milk [20] displayed dose-dependent inhibition of EV71 in co-infection and pretreatment experiments. Unfortunately, these were unable to inhibit EV71 infection completely at the highest concentrations tested, suggesting the involvement of multiple receptors during EV71 infection. This can be addressed in the future by the combined administration of inhibitors targeting different EV71 receptors. The potential discovery of more EV71 receptors in the future (in particular, neural-specific receptors) might eventually lead to the development of effective receptor inhibitors that can prevent EV71-induced neuropathology.
Viral capsid-binding molecules
The hydrophobic pocket within the viral protein, VP1, is a well-known target for antiviral design because its occupancy by suitable compounds will stabilize the virus capsid and prevent uncoating of virus for RNA release [22]. Some of the more prominent series of capsid-binding compounds include the WIN series from Sterling-Winthrop (e.g. Pleconaril) [23], the SCH series from Schering Plough (e.g. SCH 48973) [24] and R 77975-related compounds from the Janssen Research Foundation (e.g. Pirodavir) [25]. Although antiviral activity was claimed for a broad spectrum of picornaviruses, EV71 was notably absent from the list of picornaviruses tested in these studies. The characteristics and clinical developments of picornavirus capsid-binding molecules are detailed in several comprehensive reviews 9, 13, 15. After the 1998 Taiwan outbreak of EV71, Pleconaril was tested against EV71 but failed to prevent virus-induced cytopathic effects in cell cultures [26]. In view of the emergence of EV71 as a clinically important picornavirus, there is a need to re-evaluate more of these compounds against EV71.
Pyridyl imidazolidones
Using WIN compounds as templates, a series of imidazolidone derivatives were designed through computer modeling, synthesized and evaluated for their ability to inhibit EV71-induced cytopathic effects in rhabdomyosarcoma (RD) cells. Several lead molecules with strong inhibition against all three genogroups of EV71 (EC50 = 2.13–4.67 μm) and low cytotoxicity (CC50 > 25 μm) were discovered. A series of publications by the same group later documented the various chemical modifications that improved antiviral activity, including substitutions on the phenoxyl group (BPROZ-194, EC50 = 1.55 μm), oxime ether group addition (BPROZ-101, EC50 = 0.0012 μm) and methyl group addition (BPROZ-033, EC50 = 0.009 μm) [27]. These compounds were evaluated for anti-EV71 activity in a murine model with promising results, although a current lack of funding is impeding progress to clinical trials (S.R. Shih, pers. commun.).
Pyridanzinyl oxime ethers
Oxime-ether derivatives of Pirodavir displayed improved metabolic stability and antiviral activity against a greater spectrum of picornaviruses relative to Pirodavir [28]. Most notably, one of these compounds, BTA39, inhibited EV71 with an EC50 of 0.001 μm and a reported CC50 ≥ 4.588 μm [29].
Lactoferrin
Bovine lactoferrin and human lactoferrin were found to inhibit EV71 infection in RD cells during early stages of infection at a mean EC50 of 10.5–24.5 μg/ml and 103.3–185.0 μg/ml, respectively [30]. Bovine lactoferrin delayed EV71-induced paralysis and death for up to two weeks post-infection in 17-day-old mice when co-injected with EV71, and interaction assays using conjugated antibodies demonstrated direct binding of lactoferrin with VP1 and cell surfaces [31]. These suggested lactoferrin's role in preventing virus attachment by blocking cellular receptors and/or receptor-interaction sites on EV71. The exact antiviral mechanism of lactoferrin remains to be determined; however, its potency as an early inhibitor of EV71 has led to the authors of these studies to propose investigating the ingestion of milk as a prophylaxis.
NF449
Suramin, a polysulfonate, was shown to be an effective inhibitor of HIV replication in infected patients. Polysulfonates are known to be multi-targeting inhibitors of HIV, with viral targets including reverse transcriptase and the viral envelope gp120 glycoprotein [32]. A suramin analog, NF449, 4,4′,4′′,4′′′-(carbonylbis(imino-5,1,3-benzenetriylbis(carbonylimino)))tetrakis-benzene-1,3-disulfonic acid, was identified as an early inhibitor of EV71 infection (virus uncoating or host receptor binding) with an EC50 of 6.7 μm and CC50 of >1000 μm in a screen for anti-EV71 compounds in the LOPAC1280 drug library (Sigma–Aldrich) [33]. NF449-resistant EV71 strains isolated in the same study displayed mutations in the viral capsid protein VP1, suggesting VP1 as a putative target of NF449.
Inhibitors of protein synthesis
Translation of the viral RNA is the next key step of virus replication. Because the virus essentially uses cellular machinery for protein translation, it is important to develop antiviral strategies that inhibit viral protein synthesis without affecting host cell translation events. Currently, there are no reports of viral-specific small-molecule inhibitors of protein synthesis with activity against EV71. Amantadine was found to inhibit cap-independent translation initiated by the EV71 internal ribosome entry site in a bicistronic reporter system, although direct antiviral activity was not verified [34]. A more promising but non-conventional antiviral approach targeting viral RNA translation might be RNA interference (RNAi).
RNA interference
The potential of nucleic-acid-based therapy can be seen from the FDA approval of fomivirsen (Vitravene) for use against cytomegalovirus retinitis [35]. Many RNAi-based antiviral therapeutics are currently undergoing various phases of clinical trials against viruses such as HIV-1, respiratory syncytial virus and hepatitis C virus [36].
Several highly conserved sequences have been identified as targets for RNAi in the EV71 genome. Small interfering RNA (siRNA) and plasmid-encoded short hairpin RNA (shRNA) were found to effectively block replication of EV71 when targeted against 3′ UTR [37] or regions encoding the structural proteins (e.g. VP1 and VP2) [38] and non-structural proteins (e.g. 2C, 3C and 3D) 37, 39. Two of these studies reported the greatest inhibitory effects in targeting the viral RNA polymerase 3D 39, 40. siRNA and plasmid-encoded shRNA targeting 3D were able to prevent EV71-induced paralysis, weight loss and death in suckling mice when delivered via the oral or intraperitoneal route [40]. The authors also observed similar antiviral effects for nucleotides delivered with or without a lipid carrier. These promising preclinical results should be followed up with clinical trials for RNA-based anti-EV71 therapeutics.
Inhibitor of 3C protease
Aside from their roles in the maturation cleavages of the viral polyprotein, the EV71-encoded proteases 2A and 3C also target several host proteins, such as eukaryotic translation initiation factor 4G [41] and cleavage stimulation factor 64 [42], to halt host protein synthesis and induce apoptosis [43]. The essential roles of these proteases in virus replication make them attractive targets for antiviral therapeutics.
Rupintrivir and its structural and functional analogs
Several peptide aldehydes were designed to irreversibly inhibit human rhinovirus (HRV) 3Cpro by forming covalent adducts (reviewed in Ref. [9]). One of these compounds, rupintrivir (AG-7088, Pfizer) reached phase II clinical trials but further studies were ceased after it displayed poor efficacy in natural infection cases of HRV [44].
Kuo et al. [45] developed a series of compounds based on rupintrivir and tested against EV71 3Cpro in vitro. A compound, 10b, was identified as a potent inhibitor with EC50 of 0.018 μm while showing no toxicity (CC50 > 25 μm). Further studies are needed to evaluate the compound's efficacy in vivo. More recently, rupintrivir was shown to inhibit EV71 with an EC50 of 0.8 μm using a real-time, cell-based fluorescence resonance energy transfer assay and plaque reduction assay [46]. Compound 1, an orally bioavailable 3Cpro inhibitor developed in parallel with rupintrivir, also showed in vitro antiviral properties against several other human enteroviruses and should also be evaluated for anti-EV71 properties [47].
Inhibitors of protein 2C
The highly conserved picornavirus protein 2C is a multifunctional protein that possesses nucleoside triphosphatase activity [48] and was shown to be involved in the synthesis of the viral negative-strand RNA [49] and encapsidation of progeny virions in poliovirus [50]. EV71 2C protein was reported to recruit host-encoded reticulon 3 in forming replication complexes [51].
Metrifudil and N6-benzyladenosine
Metrifudil, N-(2-methylphenyl)methyl-adenosine and N6-benzyladenosine were identified as inhibitors of EV71 in the same screen as NF449 (inhibitors of virus attachment, entry and uncoating). Reported EC50 of 1.3 μm (metrifudil) and 0.10 μm (N6-benzyladenosine) were derived against EV71 pseudovirus (structural genes replaced by firefly luciferase gene). Metrifudil and N6-benzyladenosine, both adenosine receptor agonists, also showed low cytotoxicity with CC50 of >50 μm and 3300 μm, respectively [33]. The authors managed to isolate drug-resistant EV71 with mutations in 2C proteins after three passages, thus suggesting non-conserved regions in 2C as probable targets of the compounds.
Inhibitors of protein 3A
Picornavirus 3A protein is an essential, multifunctional protein that has been shown to modulate the host cell's intracellular membrane transport [52]. Protein 3A functions primarily in its precursor form, 3AB, which has RNA-binding properties and is known to stimulate the cleavage of 3CDpro and the activity of 3D RNA polymerase 3Dpol 53, 54.
Enviroxime and its structural analogs
Enviroxime is a benzimidazole derivative that inhibits rhinoviruses and poliovirus in vitro by targeting protein 3A 55, 56. Recently, enviroxime was reported to have strong antiviral effects against EV71 with an EC50 of 0.15 μm [57]. Enviroxime, however, was unable to show significant clinical effect (p > 0.05) against HRV9, with poor bioavailability and gastrointestinal side-effects observed in trial subjects [58]. Vinlyacetylene analogs of enviroxime were reported to display better oral bioavailability while retaining protein 3A targeting antiviral activity against poliovirus [59] and, therefore, should be evaluated for anti-EV71 activity (Table 1 ).
Table 1.
Anti-EV71 activity of select compounds
| Inhibitor | Structure | EC50 (μm) | CC50 (μm) | Current status (reference) |
|---|---|---|---|---|
| Capsid-binding | ||||
| BPROZ-194 | 1.552 | >50 | In vitro[27] | |
| BPROZ-101 | 0.0012 | >50 | In vitro[27] | |
| BPROZ-033 | 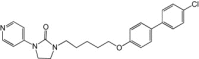 |
0.0088 | >50 | In vitro[27] |
| BTA39 | 0.001 | ≥4.588 | In vitro[29] | |
| Bovine lactoferrin | N/A | 10.5* | N/R | In vivo; mice [31] |
| NF449 |
 |
6.7 |
>1000 |
In vitro[33] |
| Translation-inhibition | ||||
| siRNA (3D) | N/A | < 5nmola | N/R | In vivo; mice [40] |
| shRNA (psi-3D) | N/A | <25 μga | N/R | In vivo; mice [40] |
| 3Cpro inhibitor | ||||
| Compound 10b | 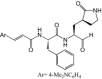 |
0.018 | >25 | In vitro[45] |
| Rupintrivir |
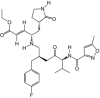 |
0.8 |
N/R |
In vitro[46] |
| 2C inhibitors | ||||
| Metrifudil | 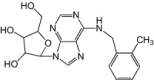 |
1.3 | >50 | In vitro[33] |
| N6-benzyladenosine |
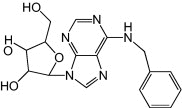 |
0.10 |
3300 |
In vitro[33] |
| 3A inhibitors | ||||
| Enviroxime | 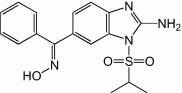 |
0.15 | N/R | In vitro[57] |
| GW5074 |
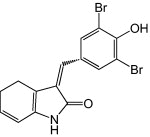 |
2.0 |
170 |
In vitro[33] |
| 3Dpolinhibitor | ||||
| DTriP22 | 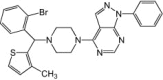 |
0.15 | >100 | In vitro[64] |
| Aurintricarboxylic acid |
 |
2.9 |
211 |
In vitro[65] |
| Nucleoside analog | ||||
| Ribavirin | 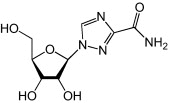 |
65 μg/ml | >200 μg/ml | In vivo; mice [69] |
| Antioxidant | ||||
| Epigallocatechin gallate |
 |
<10 |
N/R |
In vitro[70] |
| Interferon inducer | ||||
| Aloe-emodin |
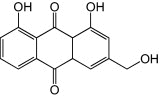 |
0.14 μg/ml |
2632 μg/ml |
In vitro[73] |
| Unknown mechanism | ||||
| Chloroquine | 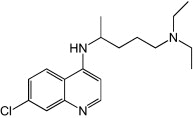 |
<1.2 mmb | N/R | In vitro[74] |
| Allophycocyanin | N/A | 0.101 | 1.521 | In vitro[77] |
| Raoulic acid | 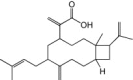 |
0.1 μg/ml | 65.86 μg/ml | In vitro[78] |
| Ursolic acid |  |
0.5 μg/ml | 100.5 μg/ml | In vitro[79] |
EC50, 50% effective concentration; CC50, 50% cytotoxic concentration; N/A, not applicable; N/R, not reported.
50% reduction of EV71 RNA from extracted intestinal cells (mice).
104-fold inhibition of EV71 RNA synthesis.
GW5074
Other scholars have reported several functional analogs of enviroxime that also target protein 3A. GW5074, 3-(3,5-dibromo-4-hydroxybenzylidine-5-iodo-1,3-dihydro-indol-2-one), is a Raf-1 inhibitor identified as an inhibitor of EV71 in the same drug library screen described previously for metrifudil (inhibitors of 2C protein). GW5074 was found to have a CC50 of 170 μm and inhibited EV71 pseudovirus at an EC50 of 2.0 μm. Post-infection addition of 50 μm of GW5074 also showed 102 to 103-fold of inhibition [33]. The target of GW5074, identified in a later study, was found to be the same region targeted by enviroxime in the protein 3A [60]. GW5074 is currently being evaluated for in vivo anti-EV71 activity in a murine model (M. Arita, pers. commun.).
Inhibitor of 3D RNA polymerase
The picornavirus 3D RNA polymerase is involved in several crucial replication events besides the incorporation of nucleotides during the synthesis of negative and positive viral RNA strands. Studies have shown that 3Dpol is responsible for the uridylation of VPg, which confers the priming function of VPg during RNA replication [61]. Viral protein 3CD, the precursor form of 3Dpol, is also involved in polyprotein processing [62] and promotes RNA synthesis by binding to the 5′ cloverleaf structure on the viral RNA [63].
DTriP-22
DTriP-22 is a synthetic compound from a series of piperazine-containing, pyrazolo[3,4-d]pyrimidine derivatives that was identified as a novel class of compounds with anti-EV71 activities. This compound was evaluated to have an EC50 of 0.15–0.98 μm against strains from all genotypes of EV71 and a CC50 greater than 100 μm. It was found to suppress the synthesis of both positive and negative strands of viral RNA during EV71 infection, and subsequent analyses of resistant viruses identified DTriP-22's target as the RNA polymerase of EV71, 3Dpol [64].
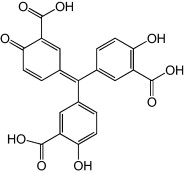
Aurintricarboxylic acid
Aurintricarboxylic acid (ATA) is a polyanionic compound with broad-spectrum antiviral activity reported against a variety of viruses (e.g. HIV and severe acute respiratory syndrome coronavirus). It was recently reported to be a potent EV71 inhibitor with a plaque reduction assay derived EC50 of 2.9 μm, while displaying low cytotoxicity in African green monkey kidney (Vero) cells with a CC50 of 211 μm [65]. The authors managed to rule out ATA targeting of viral adsorption, viral RNA translation and the viral proteases (2Apro and 3Cpro) while observing ATA inhibition of RNA elongation by EV71 3Dpol.
Nucleoside analog
Ribavirin
Ribavirin is a broad-spectrum antiviral that has been used in treating hepatitis C virus infections (in combination with interferon-α) [66] and severe respiratory syncytial virus infections [67]. Studies have shown that the main antiviral mechanism of ribavirin is through lethal mutagenesis of viruses during RNA replication events [68]. Ribavirin was found to inhibit EV71 in RD cells with EC50 of 65 μg/ml (266 μm) while preventing EV71-induced paralysis and death in mice [69]. With continued progress in antiviral development for EV71, ribavirin might prove to be clinically useful for EV71 infections in the future, when used at lower concentrations in combination with other antivirals.
Modulators of host cell environment
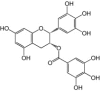
Antioxidant
Epigallocatechin gallate. Polyphenolic compounds isolated from green tea leaves (Camellia sinensis) were tested for their antiviral properties against EV71. Epigallocatechin gallate (EGCG) was found to be the most potent of these compounds with post-infection addition of 10 μm of EGCG resulting in 54% reduction in plaque formation, while EV71-induced cytopathic effects were reduced by two-fold and viral RNA levels were significantly decreased (p < 0.05, treated vs control). EGCG's potency as an anti-EV71 agent among the polyphenols tested correlated with its high antioxidative capacity [70]. EV71 infection was observed to result in increased oxidative stress, while cells deficient in glucose-6-phosphate dehydrogenase supported more efficient EV71 replication [71]. The association between cellular redox status and EV71 replication led the authors to suggest EGCG inhibits EV71 replication by modulating oxidative stress. However, it remains unclear whether this association represents a direct causal relationship between EGCG and EV71 inhibition.
Type I interferons
The induction of Type I interferons (IFNs; e.g. interferon-α/β) is an early, non-specific host immune response to viral infections that can lead to the induction of antiviral mechanisms. Injection of 10 μg of polyriboinosinic:polyribocytidylic acid [poly(I:C)], a potent IFN inducer, into mice 12 hours before EV71 inoculation resulted in increased serum levels of IFN-α, improved survival rate and significantly decreased tissue viral load (p < 0.05, treated vs control) and mortality [72].
Aloe-emodin. Aloe-emodin, a plant-derived anthraquinone derivative, was able to induce a 2.5-fold increase in IFN-α expression in human medullablastoma (TE-671) cells while showing low toxicity to both human promonocyte (HL-CZ) and TE-671 cell lines (CC50 of 2632 μg/ml and 2881 μg/ml, respectively). Pretreatment of cells from both cell lines with aloe-emodin resulted in lowered plaque formation when infected with EV71 (EC50 of 0.14 μg/ml and 0.52 μg/ml, respectively) [73]. The resulting high therapeutic index (CC50/EC50) values for aloe-emodin suggest its therapeutic potential as an anti-EV71 compound.
Compounds with unverified targets and modes of action
Synthetic compound
Chloroquine
Chloroquine was used as an inhibitor of virus uncoating in a study of EV71-induced apoptosis whereby the experiments were not designed to investigate chloroquine's anti-EV71 characteristics. Nonetheless, treatment with 1.2 mm of chloroquine resulted in a 104-fold reduction of EV71 RNA synthesis [74].
The antimalarial drug chloroquine has gained interest as a potent antiviral drug, with studies showing its antiviral activities against diverse viruses such as severe acute respiratory syndrome coronavirus [75] and HIV [76]. Investigations into the inhibitory mechanisms of chloroquine on these viruses showed a variety of targets and processes involved. Given the varied antiviral pathways of chloroquine, the authors’ claim of uncoating inhibition has to be verified with further experiments. However, the experience from the use of chloroquine in malaria treatment and its wide availability add to the appeal of investigating its anti-EV71 potential.
Natural compounds
Allophycocyanin. Allophycocyanin is a red fluorescent protein purified from the marine algae Spirulina platensis that has been found to prevent EV71-induced apoptosis, delay viral RNA synthesis and reduce plaque formation at an EC50 of 0.1 μm and CC50 of 1.52 μm in post-infection experiments on Vero cells [77]. The specific targets for inhibition by allophycocyanin are currently unknown.
Raoulic acid. Raoulic acid was purified from whole-plant extract of a New Zealand plant, Raoulia australis, and tested for antiviral activity against a wide range of viruses. It has a CC50 of 65.86 μg/ml in Vero cells and was able to inhibit EV71 with an EC50 of less than 0.1 μg/ml, giving it a therapeutic index of greater than 656.8 [78]. Drug treatment was performed post-infection, and the targets for inhibition were not investigated in the study.
Ursolic acid. Ursolic acid is a triterpenoid purified from the water extract of Ocimum basilicum, a herb commonly used in traditional Chinese medicine. Ursolic acid showed low cytotoxicity against Hep G2 (hepatoblastoma-derived) cells (CC50 = 100.5 μg/ml) and inhibited EV71-induced cytopathic effects (EC50 = 0.5 μg/ml) [79]. Time-of-addition studies revealed only post-infection inhibition of EV71 for the lower doses of ursolic acid tested (0.125 and 0.25 μg/ml), although the exact mechanisms of inhibition remain unclear.
Concluding remarks and future prospects
Because most of the antiviral agents of EV71 in the literature are only in the preliminary stages of development (i.e. in vitro studies), promising candidates were selected based on their antiviral potencies and cytotoxicity profiles. Among the compounds mentioned in this review, those that are approved for clinical use in other diseases (e.g. chloroquine) or are generally non-toxic (e.g. lactoferrin) are attractive candidates for evaluation in vivo and in clinical trials.
Earlier efforts in the development of antiviral agents against HRV and poliovirus have yielded compound series with anti-picornaviral activities. Research into these series, however, seems to have ceased with the highly successful implementation of oral polio vaccine. These compounds might still prove useful as anti-EV71 agents or lead molecules for designing effective anti-EV71 agents, as exemplified by the development of anti-EV71 capsid binders, and should be evaluated against current EV71 strains. In fact, the extensive pharmacokinetic characterizations in various stages of clinical trials for compounds such as Pleconaril (capsid binder), rupintrivir (3Cpro inhibitor) and enviroxime (3A inhibitor) can serve to inform future developments of anti-EV71 agents.
The mechanisms behind the neuropathogenesis of EV71 infection are currently unknown. Several theories have been proposed, including host immune-mediated inflammation of the central nervous system (CNS) and neural apoptotic cell death as EV71 infection spreads to the CNS [80]. A murine study reported retrograde axonal transport to be the major transmission route for EV71 neuroinvasion, in contrast to a hematogenous transmission with virus crossing the blood–brain barrier. Skeletal muscle was found to serve as an important site for viral replication and entry into the CNS via peripheral nerves innervating the infected site [81]. In fatal EV71 infection cases, patients usually present with three to five days of fever, headache, oral ulcers and vesicular rashes on hand, foot or buttocks that test positive for EV71 before rapid deterioration to severe disease [82]. The lag time between primary infection to severe neural disease might prove to be an important window of opportunity for antiviral intervention. Although the neuropathogenesis of EV71 would argue for a systemic antiviral that can cross the blood–brain barrier, other routes of administration might also prove to be useful in preventing EV71 neuroinvasion and transmission by inhibiting EV71 replication at other sites of infection (e.g. skeletal muscle, gastrointestinal lining and vesicular rashes on the skin).
With no antiviral or prophylactic available for severe EV71 infections, all available antiviral options should be considered pending future progress in our understanding of disease progression in EV71 infection and future reports on the in vivo efficacies and pharmacokinetics of antiviral candidates. An effective antiviral is an essential tool in nullifying the growing threat of EV71 as a neurotopic virus. Even as the search for an EV71 vaccine continues, development of antiviral agents remain pertinent, as seen in the case of poliovirus, in which the lack of antiviral options is preventing a complete eradication of polio [56].
References
- 1.Oberste M.S. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 1999;73:1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC Progress toward interruption of wild poliovirus transmission – worldwide, 2008. Morb. Mortal. Wkly. Rep. 2009;58:308–312. [PubMed] [Google Scholar]
- 3.Schmidt N.J. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 1974;129:304–309. doi: 10.1093/infdis/129.3.304. [DOI] [PubMed] [Google Scholar]
- 4.McMinn P.C. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol. Rev. 2002;26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin T.Y. The 1998 enterovirus 71 outbreak in Taiwan: pathogenesis and management. Clin. Infect. Dis. 2002;34(Suppl. 2):S52–S57. doi: 10.1086/338819. [DOI] [PubMed] [Google Scholar]
- 6.Chang L.Y. Neurodevelopment and cognition in children after enterovirus 71 infection. N. Engl. J. Med. 2007;356:1226–1234. doi: 10.1056/NEJMoa065954. [DOI] [PubMed] [Google Scholar]
- 7.Wang J.N. Critical management in patients with severe enterovirus 71 infection. Pediatr. Int. 2006;48:250–256. doi: 10.1111/j.1442-200X.2006.02198.x. [DOI] [PubMed] [Google Scholar]
- 8.Hsiung G.D., Wang J.R. Enterovirus infections with special reference to enterovirus 71. J. Microbiol. Immunol. Infect. 2000;33:1–8. [PubMed] [Google Scholar]
- 9.Shih S.R. Selective human enterovirus and rhinovirus inhibitors: an overview of capsid-binding and protease-inhibiting molecules. Med. Res. Rev. 2004;24:449–474. doi: 10.1002/med.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Palma A.M. Selective inhibitors of picornavirus replication. Med. Res. Rev. 2008;28:823–884. doi: 10.1002/med.20125. [DOI] [PubMed] [Google Scholar]
- 11.Chen T.C. Development of antiviral agents for enteroviruses. J. Antimicrob. Chemother. 2008;62:1169–1173. doi: 10.1093/jac/dkn424. [DOI] [PubMed] [Google Scholar]
- 12.Patick A.K., Potts K.E. Protease inhibitors as antiviral agents. Clin. Microbiol. Rev. 1998;11:614–627. doi: 10.1128/cmr.11.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diana G.D. Inhibitors of picornavirus uncoating as antiviral agents. Pharmacol. Ther. 1985;29:287–297. doi: 10.1016/0163-7258(85)90005-1. [DOI] [PubMed] [Google Scholar]
- 14.Barnard D.L. Current status of anti-picornavirus therapies. Curr. Pharm. Des. 2006;12:1379–1390. doi: 10.2174/138161206776361129. [DOI] [PubMed] [Google Scholar]
- 15.Li C. The efficacy of viral capsid inhibitors in human enterovirus infection and associated diseases. Curr. Med. Chem. 2007;14:847–856. doi: 10.2174/092986707780363032. [DOI] [PubMed] [Google Scholar]
- 16.Bedard K.M., Semler B.L. Regulation of picornavirus gene expression. Microbes Infect. 2004;6:702–713. doi: 10.1016/j.micinf.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Yamayoshi S. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat. Med. 2009;15:798–801. doi: 10.1038/nm.1992. [DOI] [PubMed] [Google Scholar]
- 18.Laszik Z. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood. 1996;88:3010–3021. [PubMed] [Google Scholar]
- 19.Nishimura Y. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat. Med. 2009;15:794–797. doi: 10.1038/nm.1961. [DOI] [PubMed] [Google Scholar]
- 20.Yang B. Sialylated glycans as receptor and inhibitor of enterovirus 71 infection to DLD-1 intestinal cells. Virol. J. 2009;6:141. doi: 10.1186/1743-422X-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y.W. Enterovirus 71 infection of human dendritic cells. Exp. Biol. Med. (Maywood) 2009;234:1166–1173. doi: 10.3181/0903-RM-116. [DOI] [PubMed] [Google Scholar]
- 22.Smyth M.S., Martin J.H. Picornavirus uncoating. Mol. Pathol. 2002;55:214–219. doi: 10.1136/mp.55.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pevear D.C. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 1999;43:2109–2115. doi: 10.1128/aac.43.9.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buontempo P.J. SCH 48973: a potent, broad-spectrum, antienterovirus compound. Antimicrob. Agents Chemother. 1997;41:1220–1225. doi: 10.1128/aac.41.6.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andries K. In vitro activity of pirodavir (R 77975), a substituted phenoxy-pyridazinamine with broad-spectrum antipicornaviral activity. Antimicrob. Agents Chemother. 1992;36:100–107. doi: 10.1128/aac.36.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shia K.S. Design, synthesis, and structure–activity relationship of pyridyl imidazolidinones: a novel class of potent and selective human enterovirus 71 inhibitors. J. Med. Chem. 2002;45:1644–1655. doi: 10.1021/jm010536a. [DOI] [PubMed] [Google Scholar]
- 27.Chen T.C. Antiviral activity of pyridyl imidazolidinones against enterovirus 71 variants. J. Biomed. Sci. 2008;15:291–300. doi: 10.1007/s11373-007-9228-5. [DOI] [PubMed] [Google Scholar]
- 28.Watson K.G. An orally bioavailable oxime ether capsid binder with potent activity against human rhinovirus. J. Med. Chem. 2003;46:3181–3184. doi: 10.1021/jm0202876. [DOI] [PubMed] [Google Scholar]
- 29.Barnard D.L. In vitro activity of expanded-spectrum pyridazinyl oxime ethers related to pirodavir: novel capsid-binding inhibitors with potent antipicornavirus activity. Antimicrob. Agents Chemother. 2004;48:1766–1772. doi: 10.1128/AAC.48.5.1766-1772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin T.Y. Lactoferrin inhibits enterovirus 71 infection of human embryonal rhabdomyosarcoma cells in vitro. J. Infect. Dis. 2002;186:1161–1164. doi: 10.1086/343809. [DOI] [PubMed] [Google Scholar]
- 31.Weng T.Y. Lactoferrin inhibits enterovirus 71 infection by binding to VP1 protein and host cells. Antiviral Res. 2005;67:31–37. doi: 10.1016/j.antiviral.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Bugatti A. Heparin-mimicking sulfonic acid polymers as multitarget inhibitors of human immunodeficiency virus type 1 Tat and gp120 proteins. Antimicrob. Agents Chemother. 2007;51:2337–2345. doi: 10.1128/AAC.01362-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arita M. Characterization of pharmacologically active compounds that inhibit poliovirus and enterovirus 71 infectivity. J. Gen. Virol. 2008;89:2518–2530. doi: 10.1099/vir.0.2008/002915-0. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y.J. Amantadine as a regulator of internal ribosome entry site. Acta Pharmacol. Sin. 2008;29:1327–1333. doi: 10.1111/j.1745-7254.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 35.Roehr B. Fomivirsen approved for CMV retinitis. J. Int. Assoc. Phys. AIDS Care. 1998;4:14–16. [PubMed] [Google Scholar]
- 36.Haasnoot J. RNA interference against viruses: strike and counterstrike. Nat. Biotechnol. 2007;25:1435–1443. doi: 10.1038/nbt1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sim A.C. RNA interference against enterovirus 71 infection. Virology. 2005;341:72–79. doi: 10.1016/j.virol.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z. Identification of small interfering RNAs which inhibit the replication of several enterovirus 71 strains in China. J. Virol. Methods. 2009;159:233–238. doi: 10.1016/j.jviromet.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Lu W.W. Selective inhibition of enterovirus 71 replication by short hairpin RNAs. Biochem. Biophys. Res. Commun. 2004;325:494–499. doi: 10.1016/j.bbrc.2004.10.062. [DOI] [PubMed] [Google Scholar]
- 40.Tan E.L. Inhibition of enterovirus 71 in virus-infected mice by RNA interference. Mol. Ther. 2007;15:1931–1938. doi: 10.1038/sj.mt.6300287. [DOI] [PubMed] [Google Scholar]
- 41.Kuo R.L. Infection with enterovirus 71 or expression of its 2A protease induces apoptotic cell death. J. Gen. Virol. 2002;83:1367–1376. doi: 10.1099/0022-1317-83-6-1367. [DOI] [PubMed] [Google Scholar]
- 42.Weng K.F. Enterovirus 71 3C protease cleaves a novel target CstF-64 and inhibits cellular polyadenylation. PLoS Pathog. 2009;5:e1000593. doi: 10.1371/journal.ppat.1000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li M.L. The 3C protease activity of enterovirus 71 induces human neural cell apoptosis. Virology. 2002;293:386–395. doi: 10.1006/viro.2001.1310. [DOI] [PubMed] [Google Scholar]
- 44.Patick A.K. Rhinovirus chemotherapy. Antiviral Res. 2006;71:391–396. doi: 10.1016/j.antiviral.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo C.J. Design, synthesis, and evaluation of 3C protease inhibitors as anti-enterovirus 71 agents. Bioorg. Med. Chem. 2008;16:7388–7398. doi: 10.1016/j.bmc.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai M.T. Real-time monitoring of human enterovirus (HEV)-infected cells and anti-HEV 3C protease potency by fluorescence resonance energy transfer. Antimicrob. Agents Chemother. 2009;53:748–755. doi: 10.1128/AAC.00841-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patick A.K. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 2005;49:2267–2275. doi: 10.1128/AAC.49.6.2267-2275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez P.L., Carrasco L. Poliovirus protein 2C has ATPase and GTPase activities. J. Biol. Chem. 1993;268:8105–8110. [PubMed] [Google Scholar]
- 49.Banerjee R. Poliovirus-encoded 2C polypeptide specifically binds to the 3′-terminal sequences of viral negative-strand RNA. J. Virol. 1997;71:9570–9578. doi: 10.1128/jvi.71.12.9570-9578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vance L.M. Poliovirus 2C region functions during encapsidation of viral RNA. J. Virol. 1997;71:8759–8765. doi: 10.1128/jvi.71.11.8759-8765.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang W.F. Reticulon 3 binds the 2C protein of enterovirus 71 and is required for viral replication. J. Biol. Chem. 2007;282:5888–5898. doi: 10.1074/jbc.M611145200. [DOI] [PubMed] [Google Scholar]
- 52.Belov G.A., Ehrenfeld E. Involvement of cellular membrane traffic proteins in poliovirus replication. Cell Cycle. 2007;6:36–38. doi: 10.4161/cc.6.1.3683. [DOI] [PubMed] [Google Scholar]
- 53.Paul A.V. Studies with poliovirus polymerase 3Dpol. Stimulation of poly(U) synthesis in vitro by purified poliovirus protein 3AB. J. Biol. Chem. 1994;269:29173–29181. [PubMed] [Google Scholar]
- 54.Xiang W. Molecular dissection of the multifunctional poliovirus RNA-binding protein 3AB. RNA. 1995;1:892–904. [PMC free article] [PubMed] [Google Scholar]
- 55.Heinz B.A., Vance L.M. Sequence determinants of 3A-mediated resistance to enviroxime in rhinoviruses and enteroviruses. J. Virol. 1996;70:4854–4857. doi: 10.1128/jvi.70.7.4854-4857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Palma A.M. Potential use of antiviral agents in polio eradication. Emerg. Infect. Dis. 2008;14:545–551. doi: 10.3201/eid1404.070439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Palma A.M. Mutations in the nonstructural protein 3A confer resistance to the novel enterovirus replication inhibitor TTP-8307. Antimicrob. Agents Chemother. 2009;53:1850–1857. doi: 10.1128/AAC.00934-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillpotts R.J. Therapeutic activity of enviroxime against rhinovirus infection in volunteers. Antimicrob. Agents Chemother. 1983;23:671–675. doi: 10.1128/aac.23.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Victor F. Synthesis, antiviral activity, and biological properties of vinylacetylene analogs of enviroxime. J. Med. Chem. 1997;40:1511–1518. doi: 10.1021/jm960718i. [DOI] [PubMed] [Google Scholar]
- 60.Arita M. Cellular kinase inhibitors that suppress enterovirus replication have a conserved target in viral protein 3A similar to that of enviroxime. J. Gen. Virol. 2009;90:1869–1879. doi: 10.1099/vir.0.012096-0. [DOI] [PubMed] [Google Scholar]
- 61.Paul A.V. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature. 1998;393:280–284. doi: 10.1038/30529. [DOI] [PubMed] [Google Scholar]
- 62.Ypma-Wong M.F. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988;166:265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]
- 63.Xiang W. Interaction between the 5′-terminal cloverleaf and 3AB/3CDpro of poliovirus is essential for RNA replication. J. Virol. 1995;69:3658–3667. doi: 10.1128/jvi.69.6.3658-3667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen T.C. Novel antiviral agent DTriP-22 targets RNA-dependent RNA polymerase of enterovirus 71. Antimicrob. Agents Chemother. 2009;53:2740–2747. doi: 10.1128/AAC.00101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hung H.C. Inhibition of enterovirus 71 replication and the viral 3D polymerase by aurintricarboxylic acid. J. Antimicrob. Chemother. 2010;65:676–683. doi: 10.1093/jac/dkp502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Ledinghen V. Daily or three times a week interferon alfa-2b in combination with ribavirin or interferon alone for the treatment of patients with chronic hepatitis C. J. Hepatol. 2002;36:672–680. doi: 10.1016/s0168-8278(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 67.Wyde P.R. Respiratory syncytial virus (RSV) disease and prospects for its control. Antiviral Res. 1998;39:63–79. doi: 10.1016/s0166-3542(98)00029-1. [DOI] [PubMed] [Google Scholar]
- 68.Crotty S., Andino R. Implications of high RNA virus mutation rates: lethal mutagenesis and the antiviral drug ribavirin. Microbes Infect. 2002;4:1301–1307. doi: 10.1016/s1286-4579(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 69.Li Z.H. Ribavirin reduces mortality in enterovirus 71-infected mice by decreasing viral replication. J. Infect. Dis. 2008;197:854–857. doi: 10.1086/527326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho H.Y. Antiviral effect of epigallocatechin gallate on enterovirus 71. J. Agric. Food Chem. 2009;57:6140–6147. doi: 10.1021/jf901128u. [DOI] [PubMed] [Google Scholar]
- 71.Ho H.Y. Glucose-6-phosphate dehydrogenase deficiency enhances enterovirus 71 infection. J. Gen. Virol. 2008;89:2080–2089. doi: 10.1099/vir.0.2008/001404-0. [DOI] [PubMed] [Google Scholar]
- 72.Liu M.L. Type I interferons protect mice against enterovirus 71 infection. J. Gen. Virol. 2005;86:3263–3269. doi: 10.1099/vir.0.81195-0. [DOI] [PubMed] [Google Scholar]
- 73.Lin C.W. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int. J. Antimicrob. Agents. 2008;32:355–359. doi: 10.1016/j.ijantimicag.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shih S.R. Viral protein synthesis is required for enterovirus 71 to induce apoptosis in human glioblastoma cells. J. Neurovirol. 2008;14:53–61. doi: 10.1080/13550280701798980. [DOI] [PubMed] [Google Scholar]
- 75.Vincent M.J. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savarino A. Anti-HIV effects of chloroquine: inhibition of viral particle glycosylation and synergism with protease inhibitors. J. Acquir. Immune Defic. Syndr. 2004;35:223–232. doi: 10.1097/00126334-200403010-00002. [DOI] [PubMed] [Google Scholar]
- 77.Shih S.R. Inhibition of enterovirus 71-induced apoptosis by allophycocyanin isolated from a blue-green alga Spirulina platensis. J. Med. Virol. 2003;70:119–125. doi: 10.1002/jmv.10363. [DOI] [PubMed] [Google Scholar]
- 78.Choi H.J. Antiviral activity of raoulic acid from Raoulia australis against picornaviruses. Phytomedicine. 2009;16:35–39. doi: 10.1016/j.phymed.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 79.Chiang L.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005;32:811–816. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 80.Weng K.F. Neural pathogenesis of enterovirus 71 infection. Microbes Infect. 2010;12:505–510. doi: 10.1016/j.micinf.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Chen C.S. Retrograde axonal transport: a major transmission route of enterovirus 71 in mice. J. Virol. 2007;81:8996–9003. doi: 10.1128/JVI.00236-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan K.P. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg. Infect. Dis. 2003;9:78–85. doi: 10.3201/eid1301.020112. [DOI] [PMC free article] [PubMed] [Google Scholar]


