Abstract
Neospora caninum is an intracellular protozoan that infects many domestic and wild animals. Domestic dogs and other canids function as definitive hosts, while other mammals serve as natural intermediate hosts. In the present study, the brain tissues of bats collected in Yunnan Province, Southern China were tested by N. caninum specific-nested PCR, targeting the Nc-5 gene and the internal transcribed spacer 1 (ITS1) region of the ribosomal DNA to determine whether bats could be infected with N. caninum. N. caninum DNA was detected in 1.8% (4/227) of bats, i.e., 1.7% (1/60) in Rousettus leschenaultia, 1.7% (1/58) in Hipposideros pomona, 2.9% (2/69) in Rhinolophus pusillus, and none (0/40) in Myotis daubentoniid. The findings of the present study are only the first indication that bats could serve as an intermediate host, and further studies are necessary to confirm whether bats are involved in the transmission of N. caninum infections.
Keywords: Neospora caninum, Prevalence, Bats, Nested PCR, Southern China
Neospora caninum, an obligate intracellular apicomplexan parasite, is considered one of the most common causes of infectious abortion and reproductive loss in cattle worldwide [1]. The domestic dog has been regarded as a definitive host of N. caninum, but other canids, including coyotes, Australian dingoes and gray wolves, have recently been demonstrated to be definitive natural hosts for the parasite [2]. Many mammals, including cattle, sheep, goat, water buffalo, horse, bison, white-tailed deer, and red fox, can serve as natural intermediate hosts for N. caninum [3]. Recent epidemiological studies have shown that many wild animals are positive for Neospora antibodies or DNA, indicating that these animals may play an important role in the transmission of N. caninum, which makes neosporosis difficult to control [4,5].
Bats have been considered an important natural reservoir for many emerging zoonotic viruses, including the rabies virus, hantavirus, Marburg virus, Nipah virus, Ebola virus [6], and severe acute respiratory syndrome coronaviruses [7]. Bacteria and protozoa have also been detected positive in bats, by which these pathogens may be transmitted to other animals or humans. For example, Toxoplasma gondii DNA has been detected by PCR in bats collected from the Jilin, Liaoning, Jiangxi and Guangdong provinces in China, and they share the same genotype as those found in other wild and domestic animals, and humans [8]. However, there is no evidence of N. caninum infection in bats. Recently, de Jesus examined for N. caninum DNA by PCR in free-living bats in Bahia, Brazil, but the results were negative [9]. The present study aimed to detect N. caninum DNA by PCR in bats from Yunnan Province, Southern China.
The study was approved by the Administrative Committee on Animal Welfare of Military Veterinary Institute, Academy of Military Medical Sciences in China (No. JSY-DW-2010-02). In total, 227 adult bats, belonging to four species that included Rousettus leschenaultia (n = 60), Hipposideros pomona (n = 58), Rhinolophus pusillus (n = 69), and Myotis daubentonii (n = 40), were captured in Yunnan Province, Northern China from March 2010 to March 2015.
The collected bats were euthanized, and the brain tissues were collected from each single animal for genomic DNA extraction using the TIANamp Genomic DNA kit (TianGen, Beijing, China).
The bats were tested for N. caninum infection by two nested PCR assays. The first nested PCR was conducted using N. caninum-specific primers Np21 and Np6 targeting the Nc-5 gene as external primers [10], and Np9 and Np10 as internal primers [11]. The second nested PCR was performed to amplify the internal transcribed spacer 1 (ITS1) region of the ribosomal DNA in N. caninum using the primers NN1 and NN2 as external primers, and NP1 and NP2 as internal primers [12]. Positive and negative controls were included in each test. The genomic DNA of the N. caninum NC-1 strain was used as positive control, and ultrapure water was used as negative control. The PCR products were electrophoresed in a 1.5% agarose gel and stained with an ethidium bromide solution (1 μg/ml). The sample was considered positive if it was tested positive by the two previously mentioned methods.
The PCR products of all positive samples were purified using the TaKaRa MiniBEST Agarose Gel DNA Extraction Kit Ver.4.0 (Takara Biomedical Technology Co., Ltd., Beijing, China) according to the manufacturer's protocols. The PCR product was cloned into vector pMD18-T (Takara) and sequenced using the primers M13-47 and RV-M in an ABI 3730 sequencer.
The sequences were compared with the GenBank entries by Blast N2.2.13. Additionally, the identities of the obtained nucleotide sequences were computed using EMBOOS pairwise alignment after the manual removing of gaps. A phylogenetic tree was constructed using the neighbor-joining method [13]. The evolutionary distances were computed using the maximum composite likelihood method [14]. Phylogenetic analyses were conducted in MEGA 4 [15].
In total, 5 samples (2.2%) were positive for the Nc-5 gene in 227 bat samples, including one R. leschenaultia, two H. pomona, and two R. pusillus. There were 6 samples (2.6%) that tested positive for the ITS1 region, including four R. pusillus, and one each for R. leschenaultia and H. pomona. No positive result was found in M. daubentonii (Table 1 ). The sample was considered positive if both Nc-5 and ITS1 were detected positive in a bat sample. Thus, one sample was positive in 60 R. leschenaultia, one sample positive in 58 H. pomona, and two samples were positive in 69 R. pusillus (Table 1).
Table 1.
Neospora caninum positive PCR findings in brain tissue of bats in Yunnan Province, Southern China.
| Target gene | No. of positive samples/total (%) |
||||
|---|---|---|---|---|---|
| Rousettus leschenaultiaa | Hipposideros pomonab | Rhinolophus pusillusc | Myotis daubentoniid | Total | |
| Nc-5 | 1/60 (1.7) | 2/58 (3.5) | 2/69 (2.9) | 0/40 | 5/227 (2.2) |
| ITS1 | 1/60 (1.7) | 1/58 (1.7) | 4/69 (5.8) | 0/40 | 6/227 (2.6) |
| Nc-5 and ITS1 | 1/60 (1.7) | 1/58 (1.7) | 2/69 (2.9) | 0/40 | 4/227 (1.8) |
Fruit bat in the Pteropodidae family; GenBank accession number for Nc-5 is MF802336, and for ITS1 is MF802339.
Insectivorous bat in the Hipposideridae family; GenBank accession numbers for Nc-5 are MF802334-MF802335, and for ITS1 is MF802344.
Insectivorous bat in the Rhinolophidae family; GenBank accession numbers for Nc-5 are MF802337-MF802338, and for ITS1 are MF802340-MF802343.
Insectivorous bat.
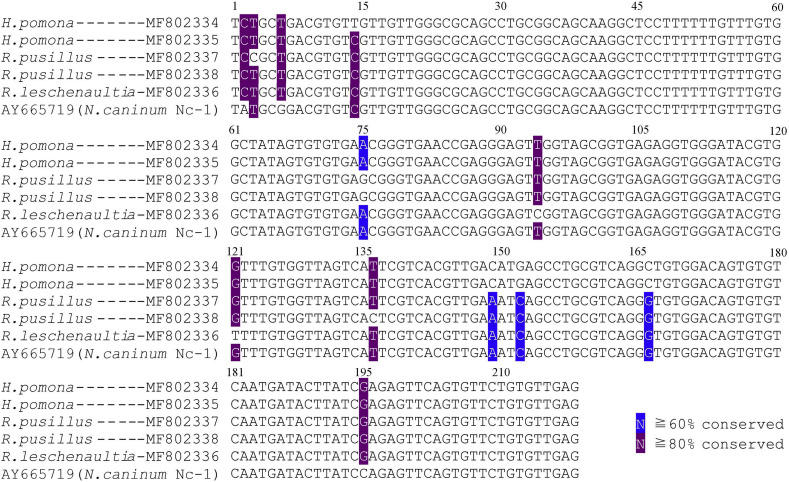
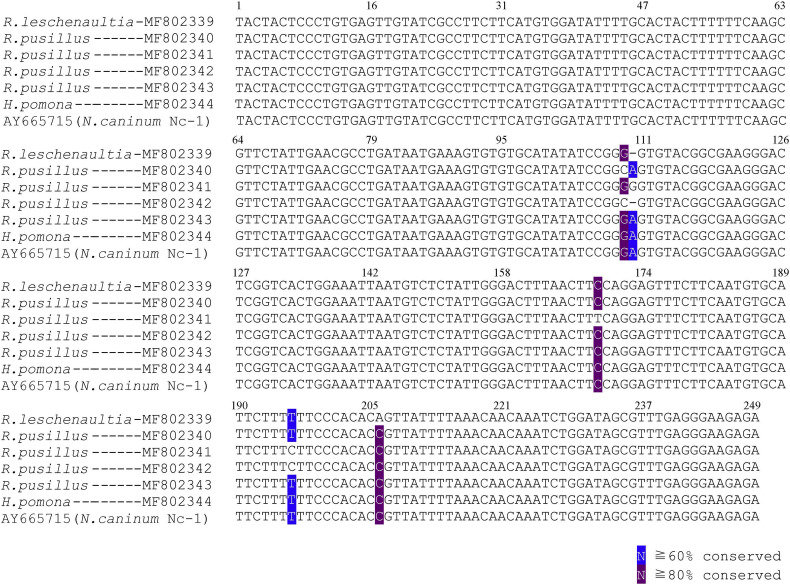
All positive products were sequenced (GenBank Accession numbers MF802334–MF802344), showing that the obtained sequences shared a 98–99% similarity to the reference strain Nc-1. With a difference of 12 nucleotides in the Nc-5 gene (Supplementary Fig. 1), and 5 nucleotides in the ITS1 (Supplementary Fig. 2). This finding indicates that the two genes of N. caninum in bats presented genetic polymorphism, and no association was found between a determined gene sequence of N. caninum and the bat species based on our results. In order to show the phylogenetic relationship between the N. caninum sequence obtained in this study and some other representatives from the family Toxoplasmatidae, we have constructed the phylogenetic tree based on the portion of ITS1 rDNA, showing that N. caninum in bats were grouped into the N. caninum-clade (Supplementary Fig. 3).
Supplementary Fig. 1.
Multiple pair-wise of Nc-5 gene between five positive samples and reference sequences (Accession number: AY665719).
Supplementary Fig. 2.
Multiple pair-wise of ITS1 sequence between positive samples and reference sequences (Accession number: AY665715). The DNA sequences showed 100% homology with the reference (Nc-1 strain) seen in ML14, ML79 and ZY41.
Supplementary Fig. 3.
The evolutionary relationships between members of the family Toxoplasmatidae based on the fragment of ITS1. The un-rooted tree is shown. The evolutionary history was inferred using the neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The evolutionary distances were computed using the maximum composite likelihood method. N. caninum obtained in this study is marked with a black dot. It clusters within the N. caninum-clade.
All positive products were sequenced (GenBank Accession numbers MF802334–MF802344), showing that the obtained sequences shared a 98–99% similarity to the reference strain Nc-1. With a difference of 12 nucleotides in the Nc-5 gene (Supplementary Fig. 1), and 5 nucleotides in the ITS1 (Supplementary Fig. 2). This finding indicates that the two genes of N. caninum in bats presented genetic polymorphism, and no association was found between a determined gene sequence of N. caninum and the bat species based on our results. In order to show the phylogenetic relationship between the N. caninum sequence obtained in this study and some other representatives from the family Toxoplasmatidae, we have constructed the phylogenetic tree based on the portion of ITS1 rDNA, showing that N. caninum in bats were grouped into the N. caninum-clade (Supplementary Fig. 3).
To our knowledge, this is the first molecular evidence of N. caninum DNA in bats that suggest that these animals could serve as naturally infected reservoirs of N. caninum. However, it is unclear how these bats may have acquired the infection. It seems that the bats with positive results could have been infected with oocysts from a contaminated environment where dogs could disseminate the oocysts, or they could have been infected through the transplacental transmission of the parasite. Nonetheless, it is still not clear whether bats are involved in the transmission of N. caninum infections.
Many wild animals are considered intermediate hosts of N. caninum. For example, antibodies to N. caninum have been detected 61.5% in eland, 58.5% in zebra, 19.2% in gazelle, 33.3% in warthog, 50% in African buffalo, 30% in lion, 20% in cheetah, and 33.3% in spotted hyena by an agglutination test in Kenya [16], and 8.4% in white-tailed deer by ELISA in Northern Mexico [17]. N. caninum DNA has been found 6% in magpie in Spain by PCR [18], and 3.7% in house sparrows in Iran [19]. These results show that these wild animals may be infected or contact with the parasite.
We detected PCR positive findings for N. caninum DNA in the brain tissue of one fruit bat and three insectivorous bats in this study. Previous studies have indicated that rodents and insectivores may be a risk factor for domestic animals to get infected with Toxoplasma gondii and N. caninum [20]. Recently, a study conducted in Brazil showed a negative result of N. caninum infection in 97 bats, including 12 fruit bats, 49 insectivorous bats, and 36 vampire bats. These results may be related to the contamination of oocysts in the environment, the susceptibility of bats to N. caninum, and the sensitivity of detection methods. We used nested-PCR methods targeting Nc-5 and ITS1 genes to detect N. caninum DNA in bats, which may be helpful to increase the positive detection rate of the parasite. Unfortunately, the bat tissue had been used for the detection of viruses and parasites and was no longer suitable for histopathological analysis to confirm the molecular detection of N. caninum [21,22].
In conclusion, this is the first report on the molecular detection of N. caninum in bats from Yunnan Province, Southern China. Further studies are needed to confirm these molecular findings and whether bats are involved in the life cycle and transmission of N. caninum.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parint.2018.03.002.
Acknowledgement
This work was financially supported by the National Key R&D Program of China (2016YFC1201602 and 2017YFD0501702), the General Program of the National Natural Science Foundation of China (31572529) and the National Key Basic Research and Development Program (Program 973) of China (2015CB15030 and 2016YFC1200100).
The following are the supplementary data related to this article.
Contributor Information
Quan Liu, Email: liuquan1973@hotmail.com.
Xichen Zhang, Email: xczhang@jlu.edu.cn.
References
- 1.Dubey J.P., Buxton D., Wouda W. Pathogenesis of bovine neosporosis. J. Comp. Pathol. 2006;134:267–289. doi: 10.1016/j.jcpa.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Almeria S. Neospora caninum and wildlife. ISRN Parasitol. 2013;2013 doi: 10.5402/2013/947347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey J.P., Schares G. Neosporosis in animals—the last five years. Vet. Parasitol. 2011;180:90–108. doi: 10.1016/j.vetpar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Rosypal A.C., Lindsay D.S. The sylvatic cycle of Neospora caninum: where do we go from here? Trends Parasitol. 2005;21:439–440. doi: 10.1016/j.pt.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Gondim L.F. Neospora caninum in wildlife. Trends Parasitol. 2006;22:247–252. doi: 10.1016/j.pt.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Holzer M., Krahling V., Amman F., Barth E., Bernhart S.H., Carmelo V.A., Collatz M., Doose G., Eggenhofer F., Ewald J., Fallmann J., Feldhahn L.M., Fricke M., Gebauer J., Gruber A.J., Hufsky F., Indrischek H., Kanton S., Linde J., Mostajo N., Ochsenreiter R., Riege K., Rivarola-Duarte L., Sahyoun A.H., Saunders S.J., Seemann S.E., Tanzer A., Vogel B., Wehner S., Wolfinger M.T., Backofen R., Gorodkin J., Grosse I., Hofacker I., Hoffmann S., Kaleta C., Stadler P.F., Becker S., Marz M. Differential transcriptional responses to Ebola and Marburg virus infection in bat and human cells. Sci. Rep. 2016;6 doi: 10.1038/srep34589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 8.Qin S.Y., Cong W., Liu Y., Li N., Wang Z.D., Zhang F.K., Huang S.Y., Zhu X.Q., Liu Q. Molecular detection and genotypic characterization of Toxoplasma gondii infection in bats in four provinces of China. Parasite Vector. 2014;7:558. doi: 10.1186/s13071-014-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jesus R.F., Rodrigues G.M., Silva E.M., Carneiro A.J., Franke C.R., de Magalhaes Cunha R., Gondim L.F. Toxoplasmatinae parasites in bats from Bahia State, Brazil. J. Wildl. Dis. 2017;53:144–147. doi: 10.7589/2016-03-065. [DOI] [PubMed] [Google Scholar]
- 10.Yamage M., Flechtner O., Gottstein B. Neospora caninum: specific oligonucleotide primers for the detection of brain “cyst” DNA of experimentally infected nude mice by the polymerase chain reaction (PCR) J. Parasitol. 1996;82:272–279. [PubMed] [Google Scholar]
- 11.McInnes L.M., Ryan U.M., O'Handley R., Sager H., Forshaw D., Palmer D.G. Diagnostic significance of Neospora caninum DNA detected by PCR in cattle serum. Vet. Parasitol. 2006;142:207–213. doi: 10.1016/j.vetpar.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Buxton D., Maley S.W., Wright S., Thomson K.M., Rae A.G., Innes E.A. The pathogenesis of experimental neosporosis in pregnant sheep. J. Comp. Pathol. 1998;118:267–279. doi: 10.1016/s0021-9975(07)80003-x. [DOI] [PubMed] [Google Scholar]
- 13.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 14.Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 16.Ferroglio E., Wambwa E., Castiello M., Trisciuoglio A., Prouteau A., Pradere E., Ndungu S., De Meneghi D. Antibodies to Neospora caninum in wild animals from Kenya, East Africa. Vet. Parasitol. 2003;118:43–49. doi: 10.1016/j.vetpar.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Olamendi-Portugal M., Caballero-Ortega H., Correa D., Sanchez-Aleman M.A., Cruz-Vázquez C., Medina-Esparza L., Ortega-S J.A., Cantu A., Garcia-Vazquez Z. Serosurvey of antibodies against Toxoplasma gondii and Neospora caninum in white-tailed deer from Northern Mexico. Vet. Parasitol. 2012;189:369–373. doi: 10.1016/j.vetpar.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Darwich L., Cabezon O., Echeverria I., Pabon M., Marco I., Molina-Lopez R., Alarcia-Alejos O., Lopez-Gatius F., Lavin S., Almeria S. Presence of Toxoplasma gondii and Neospora caninum DNA in the brain of wild birds. Vet. Parasitol. 2012;183:377–381. doi: 10.1016/j.vetpar.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Abdoli A., Arbabi M., Dalimi A., Pirestani M. Molecular detection of Neospora caninum in house sparrows (Passer domesticus) in Iran. Avian Pathol. 2015;44:319–322. doi: 10.1080/03079457.2015.1050583. [DOI] [PubMed] [Google Scholar]
- 20.Meerburg B.G., De Craeye S., Dierick K., Kijlstra A. Neospora caninum and Toxoplasma gondii in brain tissue of feral rodents and insectivores caught on farms in the Netherlands. Vet. Parasitol. 2012;184:317–320. doi: 10.1016/j.vetpar.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Hu T., Qiu W., He B., Zhang Y., Yu J., Liang X., Zhang W., Chen G., Zhang Y., Wang Y., Zheng Y., Feng Z., Hu Y., Zhou W., Tu C., Fan Q., Zhang F. Characterization of a novel orthoreovirus isolated from fruit bat, China. BMC Microbiol. 2014;14:293. doi: 10.1186/s12866-014-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang H.H., Qin S.Y., Wang W., He B., Hu T.S., Wu J.M., Fan Q.S., Tu C.C., Liu Q., Zhu X.Q. Prevalence and genetic characterization of Toxoplasma gondii infection in bats in southern China. Vet. Parasitol. 2014;203:318–321. doi: 10.1016/j.vetpar.2014.04.016. [DOI] [PubMed] [Google Scholar]