Abstract
This article reviews the virology, immunology and epidemiology of the most common viral causes of acute gastroenteritis (rotaviruses, human caliciviruses, astroviruses and enteric adenoviruses). The clinical symptoms span from mild diarrhoea to life-threatening dehydration, and rotavirus disease is a major cause of childhood mortality, mainly in developing countries. The diagnosis, treatment and preventive measures are reviewed. Uncommon viral causes of acute gastroenteritis and viruses causing gastroenteritis in immunodeficient patients are mentioned. The clinically most important development in this field over the past 3 years has been the wide application of the new live attenuated rotavirus vaccines in universal mass vaccination programmes in many countries.
Keywords: acute viral gastroenteritis, astrovirus, enteric adenovirus, human calicivirus, norovirus, rotavirus, rotavirus vaccine, sapovirus
Acute gastroenteritis with vomiting are easily recognized as a clinical entity, but may be caused by very different agents (viruses, bacteria, parasites), or may have a non-infectious cause.
Table 1 lists viruses found in the human gut that have been recognized as:
-
•
common causes of diarrhoea and vomiting in humans
-
•
uncommon causes or not a cause of diarrhoea and vomiting in humans
-
•
causes of diarrhoea in immunodeficient individuals.
Table 1.
Viruses infecting the human gut
| Common causes of diarrhoea and vomitinga |
|
|
|
|
| Uncommon causes of diarrhoea and vomiting or asymptomatic infection |
|
|
|
|
|
|
|
| Causes of diarrhoea in immunodeficient individualsc |
|
|
|
|
|
Viruses other than those that commonly cause diarrhoea are seen sporadically; on average, viruses represent about one-third of all microbial causes of childhood diarrhoea. SARS CoV, severe acute respiratory syndrome coronavirus.
Figures in parentheses are detection ranges in various surveys.
Most common cause of outbreaks.
In addition to common causes of diarrhoea and vomiting.
This article discusses the major groups of viruses that commonly cause gastroenteritis in humans.
The viruses
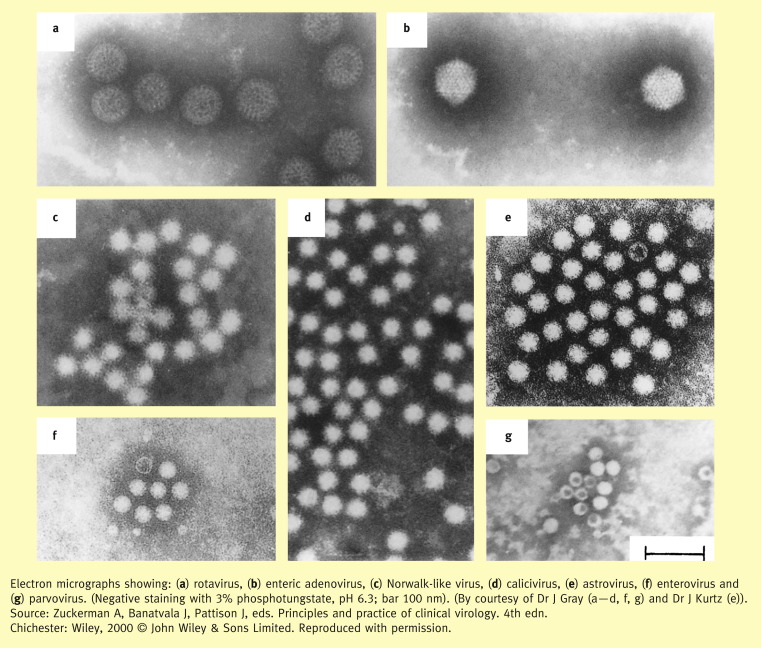
Rotaviruses,1 caliciviruses,2 astroviruses3 and enteric adenoviruses4 are the principal virus families involved. Their size, particle and genome structure, classification and epidemiological significance are summarized in Table 2 . Their appearance by electron microscopy is shown in Figure 1 .
Table 2.
Characteristics of viruses that commonly cause acute gastroenteritis in humans
| Virus (family) | Size and structure | Genome composition | Classification | Epidemiology |
|---|---|---|---|---|
| Rotaviruses (Reoviridae) | 75 nm, triple-layered, wheel-shaped | 11 segments of dsRNA totalling 18.5 kb | Groups A–H Within group A subgroups, G and P types Genotypes of all segments |
Endemic in children worldwide, winter outbreaks in temperate climates, small epidemics in the elderly |
| Caliciviruses (Caliciviridae) | About 30 nm, surface cup-shaped | ssRNA, 7.7 kb | Two genera infecting humans noroviruses sapoviruses | Epidemics in humans of all age groups |
| Enteric adenoviruses (Adenoviridae) | About 70 nm, icosahedral | dsDNA, 36 kb | Group F serotypes 40, 41 | Endemic in children |
| Astroviruses (Astroviridae) | About 30 nm, star-like appearance | ssRNA, 6.8 kb | Eight serotypes/genotypes | Epidemics in children and adults |
Figure 1.
Electron micrographs showing: (a) rotavirus (b) enteric adenovirus (c) Norwalk-like virus (norovirus) (d) calicivirus (e) astrovirus (f) enterovirus and (g) parvovirus (erythrovirus). (Negative staining with 3% phosphotungstate, pH 6.3; bar 100 nm). (By courtesy of Dr J Grey (a–d, f, g) and Dr J Kurtz (e)). (From Zuckerman A, Banatvala J, Pattison J, eds. Principles and practice of clinical virology. 4th edn. Chichester: Wiley, 2000 © John Wiley & Sons Limited. Reproduced with permission.)
Rotaviruses
These are a major cause of infantile gastroenteritis worldwide.
Structure: rotaviruses comprise an inner core containing a genome of 11 segments of double-stranded RNA and the transcription/replication and capping enzyme complex, a middle layer (inner capsid) consisting of viral protein 6 (VP6), and an outer layer made of VP7 and VP4, the latter protruding as spikes.1
Classification: rotaviruses are a genus of the Sedoreovirinae subfamily in the Reoviridae family and are routinely classified according to the immunological reactivities and genomic sequences of three of their structural components. Based on cross-reactivities and sequence diversities of VP6, seven to eight groups/species (A–G/H) are distinguished. The surface proteins VP4 and VP7 elicit type-specific neutralizing antibodies. Accordingly, for group A rotaviruses, which cause most human infections, a dual-type classification system has been established, differentiating G types (VP7-specific, G derived from glycoprotein) and P types (VP4-specific, P derived from protease-sensitive protein). At present, 27 G types and 35 P types have been described, of which at least 11 G types and 11 P types have been found in humans.5 More recently, genotype classification of the other nine RNA segments has been developed,5 permitting the detailed study of the evolution and transmission pathways of these viruses.
Replication and pathogenesis: rotaviruses replicate in mature epithelial cells at the tips of the villi of the small intestine. After virus adsorption to sialic acid, human blood group antigens and various co-receptors, viral replication takes place, first in cytoplasmic inclusion bodies termed ‘viroplasms’ followed by maturation in contact with the endoplasmic reticulum. Mature particles are released from cells by lysis.1 Rotavirus replication in the gut is rapid and reaches high titres (up to 1011 virus particles/ml faeces at the peak of acute diarrhoea) within a short period of time. The diarrhoea arises from epithelial necrosis and atrophy, leading to reduced absorption of carbohydrates and increased osmotic pressure in the gut lumen. There is also a component of hypersecretion which contributes to the diarrhoea. The rotavirus non-structural protein 4 (NSP4) functions as a viral enterotoxin.1
Immune response: primary rotavirus infection leads to a serotype-specific humoral immune response with initially monotypic protection. During the first 2 years of life, children are repeatedly infected with rotaviruses of various types, resulting in a more complex immune response that seems to provide partial heterotypic protection.6 Rotavirus-specific secretory copro-antibodies of the immunoglobulin A (IgA) subclass have been identified as an important correlate of protection.7
Caliciviruses
Noroviruses (previously termed ‘Norwalk-like viruses’) and sapoviruses (previously termed ‘Sapporo-like viruses’) are the two (out of five) genera of the Caliciviridae family that infect humans. The human noro- and sapoviruses are classified into five to six genogroups (I–VI), with each group containing between 1 and 19 different genotypes.2 Noroviruses of different genotypes co-circulate but genotype II-4 noroviruses predominate worldwide. Genetic recombination among norovirus and sapovirus strains is not infrequent.
These viruses were first recognized as a cause of human gastroenteritis outbreaks in the 1960s and are now considered the most important cause of non-bacterial gastroenteritis outbreaks and epidemics worldwide. In the UK, calicivirus outbreaks are common in hospital settings, old people's homes, etc. Human infections with caliciviruses elicit virus-specific immune responses, though these do not seem to provide full protection from subsequent infections.2
Astroviruses
Astroviruses are members of the Astroviridae family and have a characteristic appearance by electron microscopy (Figure 1). Eight different serotypes/genotypes have been distinguished; serotype 1 is most common. Little is known about immunity conveyed after astrovirus infection or the relative cross-protective effect of the immune response on re-infection with heterotypic strains.3
Adenoviruses
Enteric adenoviruses of subgroup F (serotypes 40 and 41) of the Adenoviridae are a less common cause of diarrhoea in infants and small children. They replicate in the cell nucleus and cytoplasm. Some adenovirus proteins inhibit apoptosis and others decrease the expression of host cell proteins, for example, major histocompatibility complex (MHC) class I antigens on the surface of infected cells, thereby reducing susceptibility to adenovirus-specific cytotoxic T cells. A serotype-specific humoral immune response provides homotypic protection.4
Epidemiology
Rotaviruses
Infections occur endemically worldwide, causing over 450,000 deaths each year in children aged under 5 years, mainly in low-income countries of sub-Saharan Africa and South East Asia.8 The epidemiology of these infections is complex. There is a strict winter peak in temperate climates, but in tropical and subtropical regions infections occur throughout the year. Transmission is mainly by the faeco-oral route. Nosocomial infections occur on infant and paediatric wards and are difficult to eradicate.
Group A rotaviruses of different G and P types co-circulate in different populations within a geographical location, varying over time. Types G1, G2, G3, G4 and G9 represent more than 90% of co-circulating strains in temperate climates, but other G types (e.g. G5, G8, G10, G12) are increasing and may even become most prevalent, particularly in tropical and subtropical areas.
The young of many mammalian species harbour rotaviruses of diverse genotypes and have been found to act as reservoirs for human infections.1 Most human infections are caused by group A rotaviruses; however, group B rotaviruses were established as the cause of acute gastroenteritis outbreaks in children and adults in China in the 1980s, and recently in Calcutta, India and in other South East Asian countries. Group C rotavirus infections are associated with isolated cases and small outbreaks of diarrhoea in humans.1
Noroviruses
Norovirus infections exhibit a winter peak, and the associated clinical entity has become known as ‘winter vomiting disease’. Age-related seroprevalence surveys have shown that many infections with noroviruses occur in the young and are often inapparent. About 50% of children have been infected by the age of 2 years. It is now accepted that the incidence of infection with noroviruses and sapoviruses is largely underestimated; with the advent of rotavirus (RV) vaccination (see below) noroviruses are now becoming the main cause of acute gastroenteritis in children.9 Norovirus disease outbreaks result from the ingestion of contaminated food (oysters, green salad) or water, although person-to-person spread is the predominant mode of transmission. Such outbreaks occur in both children and adults in recreational camps, hospitals, nursing homes, schools and on cruise ships. Genetic and antigenic diversity arise through the accumulation of point mutations and the selection of variants through evolutionary pressure likely to be exerted by short-term herd immunity.
Astroviruses
These cause both endemic infections and food-borne outbreaks. Seroprevalence surveys have shown that individuals can become infected by more than one serotype.
Clinical features
The onset of acute viral gastroenteritis follows an incubation period of 1–2 days, with watery diarrhoea lasting 4–7 days, vomiting and varying dehydration. Fever is not common. As a rule, the duration of diarrhoea after infection with norovirus is shorter than after infection with rotaviruses or enteric adenoviruses. Infection may be accompanied by abdominal cramps, headache, myalgia and projectile vomiting, which are regarded as typical of norovirus infection. After rotavirus infection, all degrees of severity of clinical symptoms are seen. The outcome depends on viral pathogenicity factors and on the host immune status. Inapparent infections can occur, particularly in neonates. Although rotavirus infection is often accompanied by respiratory symptoms, there is no strong evidence that rotaviruses replicate in the respiratory tract. Extra-intestinal spread of rotaviruses has been reported and may result in viraemia or, very rarely, central nervous system disease (meningitis).
Chronic gut infections with rotaviruses, adenoviruses, noroviruses, sapoviruses and astroviruses have been seen in immunocompromised children. Chronic gut infections with human cytomegalovirus, adenoviruses of new serotypes (types 42–47) and picobirnaviruses have been reported in HIV-infected patients with AIDS-defining illnesses.
Diagnosis
Diagnosis of rotavirus, astrovirus and enteric adenovirus infections is relatively easy because large numbers of particles are produced and shed during the acute phase of the illness. Noroviruses and sapoviruses are replicated to lower concentrations and for shorter periods. Diagnosis is by electron microscopy of negatively stained specimen suspensions (‘catch-all method’), by passive particle agglutination tests, by virus-specific enzyme-linked immunosorbent assay and, more recently, by viral genome detection using polymerase chain reaction (PCR) analysis for enteric adenoviruses and reverse transcription (RT)-PCR for rotaviruses, caliciviruses and astroviruses.
Management
Treatment is mainly by oral rehydration or, in more severe cases, intravenous rehydration. In tropical areas where rotavirus infections are associated with high mortality, standard formulas of oral rehydration fluid are recommended by the WHO and widely used. Otherwise, treatment is symptomatic. Use of antimotility drugs is not advised in children, although there have been recent promising developments in the use of drugs with antisecretory activity, such as racecadotril.10 There are no specific antiviral chemotherapeutic agents in clinical use.11
Outbreaks of nosocomial rotavirus infections are common in children on hospital wards and in day-care centres. Outbreaks of diarrhoea and vomiting caused by noroviruses occur in children and adults following banquets, on cruise ships, and in cafeterias, schools, hotels and fast-food restaurants. Outbreak control measures focus on interruption of person-to-person transmission and removal of sources of infection (food, water, food-handlers), along with measures to improve environmental hygiene.
Vaccine development
Development of vaccines against viral gastroenteritis has been directed mainly towards rotaviruses, which are a major cause of gastroenteritis and high childhood mortality in developing countries.8, 12
A live attenuated, rhesus rotavirus-based, human reassortant quadrivalent vaccine eliciting immunity to human rotavirus strains G1–G4 was found to protect significantly against severe disease, including dehydration. It received US Food and Drug Administration approval for universal use in the USA in August 1998, and 1.5 million doses were used between September 1998 and July 1999. However, a Vaccine Adverse Events Reporting System found gut intussusception to be a rare complication, epidemiologically correlated with vaccination, particularly on days 3–7 after the first vaccination. The pathogenesis of this association is not clear. Although the vaccine-attributable risk of intussusception was considered very low in recent studies (<1/10,000), the recommendation for use of this vaccine in the USA was withdrawn in October 1999, and it was taken off the market by the manufacturer.1
In the search for alternative vaccines, two further, live attenuated oral rotavirus vaccines have been developed. The underlying concepts of the vaccines are different. The pentavalent vaccine (RotaTeqRTM), containing the human antigens G1–G4 and P[8] in mono-reassortant viruses on a bovine rotavirus (WC3 strain) genetic backbone, is aimed at eliciting type-specific antibodies against all the rotavirus types that are recognized to circulate most frequently. The rationale of the monovalent vaccine (RotarixRTM), an attenuated human G1P[8] strain, is based on two clinical observations. First, cross-protection is accumulated through successive natural infections, and rotavirus disease can be prevented by repeated natural infection.12 Second, vaccination with one rotavirus type can provide protection, even if subsequent infections are caused by rotaviruses of a different type. It should be noted that, despite considerable work, the exact correlates of protection against rotavirus disease are still not determined.7, 13 Both vaccines have been found to be effective and safe.14, 15 They have recently been licensed for sale in numerous countries, and in many of these universal mass vaccination (UMV) against rotavirus disease as part of childhood vaccination schemes has been initiated. In the USA a distinct decline in clinic visits and hospital admissions for rotavirus disease has been ascertained.16 Clinical trials with the new vaccines in developing countries where they are most needed have shown a decreased efficacy in preventing severe RV disease,17, 18 but due to the high RV-associated mortality in these countries, WHO decided in 2009 to recommend the use of RV vaccination worldwide.19 As recorded in the USA,16 in most developed countries where UMV programmes against rotavirus disease have been introduced, a significant decrease of RV-associated diarrhoea requiring medical attention or hospitalization has been observed. In Europe, UMV against rotavirus disease is being carried out in Belgium, Finland, Luxemburg, Austria, and several states of Germany; in the UK, it was announced in November 2012 that rotavirus vaccination would be added to the childhood vaccination schedule planned to begin in September 2013.20, 21 There will be intense post-marketing surveillance in order to determine the impact of the vaccine and also to monitor the emergence of novel rotavirus strains.
Since the licensed RV vaccine contains live, attenuated viruses, attention is focused on the development of virus-like particles (obtained from baculovirus recombinant co-expressed RV proteins), enhancement of rotavirus immunogenicity by micro-encapsidation, DNA-based, and possibly ‘edible’ preparations as candidate vaccines.
So far, no vaccines against other viruses causing gastroenteritis in humans have been licensed. A vaccine candidate specific for norovirus genotype II-4 is under development and seems promising, as its targeted use in healthcare settings could reduce hospital-acquired infection.
References
- 1.Estes M.K., Kapikian A.Z. Rotaviruses. In: Knipe D.M., Howley P.M., editors. Fields virology. 5th edn. Lippincott, Williams & Wilkins; Philadelphia: 2007. pp. 1917–1974. [Google Scholar]
- 2.Green K.Y. Caliciviridae: the noroviruses. In: Knipe D.M., Howley P.M., editors. Fields virology. 5th edn. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 949–979. [Google Scholar]
- 3.Mendez E., Arias C.F. Astroviruses. In: Knipe D.M., Howley P.M., editors. Fields virology. 5th edn. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 981–1000. [Google Scholar]
- 4.Berk A. Adenoviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields virology. 5th edn. Lippincott, Williams & Wilkins; Philadelphia: 2007. pp. 2355–2394. [Google Scholar]
- 5.Matthijnssens J., Ciarlet M., McDonald S.M. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG) Arch Virol. 2011;156:1397–1413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velazquez F.R., Matson D.O., Calva J.J. Rotavirus infection in infants as protection against subsequent infection. N Engl J Med. 1996;355:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 7.Franco M.A., Angel J., Greenberg H.B. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 8.Tate J.E., Burton A.H., Boschi-Pinto C. 2008 Estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 9.Payne D.C., Vinjé J., Szilagyi P.G. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368:1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salazar-Lindo E., Santisteban-Ponce J., Chea-Woo E., Gutierrez M. Racecadotril in the treatment of acute watery diarrhea in children. N Engl J Med. 2000;343:463–467. doi: 10.1056/NEJM200008173430703. [DOI] [PubMed] [Google Scholar]
- 11.Desselberger U. Rotavirus infections: guidelines for treatment and prevention. Drugs. 1999;58:447–452. doi: 10.2165/00003495-199958030-00005. [DOI] [PubMed] [Google Scholar]
- 12.Angel J., Franco M.A., Greenberg H.B. Rotavirus vaccines: recent developments and future considerations. Nat Rev. 2007;5:529–539. doi: 10.1038/nrmicro1692. [DOI] [PubMed] [Google Scholar]
- 13.Desselberger U., Huppertz H.I. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis. 2011;203:188–195. doi: 10.1093/infdis/jiq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz-Palacios G.M., Perez-Schael I., Velazquez F.R. Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 15.Vesikari T., Matson D.O., Dennehy P. Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent humanbovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 16.Payne D.C., Boom J.A., Staat M.A. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US Children <5 years of age, 2009–2011. Clin Infect Dis. 2013;57:13–20. doi: 10.1093/cid/cit164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhi S.A., Cunliffe N.A., Steele D. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 18.Zaman K., Dang D.A., Victor J.C. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 19.SAGE, Meeting of the Immunization Strategic Advisory Group of Experts, April 2009 – conclusions and recommendations. Wkly Epidem Rec. 2009;84:220–235. [PubMed] [Google Scholar]
- 20.Iturriza-Gómara M., Cunliffe N. Rotavirus vaccine: a welcome addition to the immunization schedule in the UK. Br Med J. 2013;346:f2347. doi: 10.1136/bmj.f2347. [DOI] [PubMed] [Google Scholar]
- 21.https://www.gov.uk/government/news/new-vaccine-to-help-protect-babies-against-rotavirus
Further reading
- 22.Grohmann G.S., Glass R.I., Pereira H.G. Enteric viruses and diarrhea in HIV-infected patients. N Engl J Med. 1993;329:14–20. doi: 10.1056/NEJM199307013290103. [DOI] [PubMed] [Google Scholar]
- 23.Matthijnssens J., Otto P.H., Ciarlet M. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch Virol. 2012;157:1177–1182. doi: 10.1007/s00705-012-1273-3. [DOI] [PubMed] [Google Scholar]
- 24.Nakata S., Honma S., Namata K.K. Members of the family Caliciviridae (NV and SV) are the most prevalent causes of gastroenteritis outbreaks among infants in Japan. J Infect Dis. 2000;181:2029–2032. doi: 10.1086/315500. [DOI] [PubMed] [Google Scholar]
- 25.Patel M.M., Glass R., Desai R., Tate J.E., Parashar U.D. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infect Dis. 2012;12:561–570. doi: 10.1016/S1473-3099(12)70029-4. [DOI] [PubMed] [Google Scholar]
- 26.Patel N.C., Hertel P.M., Estes M.K. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N Engl J Med. 2010;362:314–319. doi: 10.1056/NEJMoa0904485. [DOI] [PMC free article] [PubMed] [Google Scholar]