Abstract
Carbohydrates and their conjugates are involved in various biological events, including viral and bacterial infection, the immune response, differentiation and development, and the progression of tumor cell metastasis. Glycan arrays are a new technology that has enabled the high-sensitivity and rapid analysis carbohydrate–protein interaction and contribute to significant advances in glycomics. Glycan arrays use a minute amount of materials and can be used for high-throughput profiling and quantitative analysis and provide information for the development of carbohydrate-based vaccines and new drug discovery.
Introduction
Recent advances in the study of functional glycomics in living organisms have received a great deal of attention [1••]. Carbohydrates, presented in the form of glycoproteins, glycolipids, glycosaminoglycans, or other glycoconjugates, have long been known to play important roles in a variety of biological processes. They participate in molecular recognition events such as cell adhesion, migration, and metastasis; host–pathogen interactions such as bacterial and viral infections; and initiation of the immune response [2]. Despite the increased awareness of the important function of carbohydrates, the study of carbohydrate–protein interactions is difficult. This is largely because of the structure complexity of carbohydrates, and the low affinity of their interactions with glycan-binding proteins (GBPs) — typically the monomeric K D values are in the micromolar to millimolar range, thus biological responses are often because of multivalent display and interaction of these glycans [3]. Carbohydrates are generated cotranslationally/post-translationally through a nontemplated synthetic process, which involves over 200 glycosyltransferase genes, whose differential expression and combined specificities contribute to the complexity and diversity of carbohydrate structure.
Glycan arrays have become a powerful platform to map out interactions involving carbohydrates in a high-throughput manner because they were introduced in 2002 [4•, 5•]. In general, the chip-based format unites a diverse set of novel technologies: generation of carbohydrate libraries (from natural sources or chemical and enzymatic synthesis); attachment of the saccharides to a surface (covalent or noncovalent bindings); high-throughput expression of carbohydrate-binding proteins; analysis of carbohydrate–protein binding by fluorescence measurements or mass spectrometry (Figure 1 ).
Figure 1.

General scheme for the glycan array fabrication and detection.
Till date, most published glycan array reviews have emphasized the methods of fabrication [1••, 5•, 6, 7]. These reviews focus on new applications of glycan arrays in glycomics and drug discovery over the past few years.
Generation of a glycan library
Based on a limited pool of about nine monosaccharides found in mammalian glycans, it has been calculated that ∼1012 different hexasaccharides are possible [8]. Not all of these possibilities exist in nature, but the current list of known N-linked and O-linked glycans found in proteins contains more than 2000 structures [9]. Therefore, the construction of the complete array of an entire glycome on a single chip is a major challenge. The recent development of new methods for chemical and enzymatic synthesis of oligosaccharides will increase the complexity and utility of carbohydrate arrays [1••, 5•]. The discovery of uncharacterized natural glycans is another challenge, typically achieved by the extraction from natural sources of glycoproteins or glycolipids. A novel technology has recently been developed and used to modify sugar for detecting specifically glycosylated structures on cells. Alkynyl sugar analogs were attached to the cellular glycans through biosynthesis, and the modified glycans were detected by click chemistry with an azido fluorgenic probe or biotin handles [10]. Application of proteomic methods to the metabolically engineered cells has allowed for the identification of new glycoproteins and glycans (GIDmap) [11•]. This glycoproteomic method will allow for a complete analysis of a subset of the glycoproteome, and for the differential comparison between cancer and normal cells for differences in glycosylation pattern.
Profiling carbohydrate–lectin interactions
Lectins are carbohydrate-binding proteins, which are highly specific for their sugar ligands, and are the most common detection tool for the characterization of glycan arrays [12, 13, 14]. In the landmark studies by researchers at the Consortium for Functional Glycomics (www.functionalglycomics.org), a comprehensive array of more than 200 glycans on a glass slide was used to analyze the specific binding of mammalian, plant, viral, and bacterial lectins [15••]. Recently, Gildersleeve and coworkers used a glycan array that contained 73 different glycans to do the high-throughput analysis of 24 lectins [16]. Carbohydrate specificity profiling of the C-type lectin macrophage galactose-type lectin (MGL) revealed that the lectin specifically recognized terminal α-linked and β-linked GalNAc moieties. This result led to the postulation that the expression of Tn antigen (GalNAc-α-Thr) by tumors might modulate the host immune response via the interaction of Tn with C-type lectin, such as MGL [17].
Glycosaminoglycans — growth factor and cytokine interactions
Glycosaminoglycans are complex carbohydrates known to play a key role in regulating growth factors, virus entry, and angiogenesis, but their structure–activity relationship is poorly understood. The first microarrays of heparin-like glycosaminoglycans have been constructed by Seeberger and coworkers to tackle this problem [18•]. Heparin oligosaccharides on the arrays were incubated with the acidic and basic fibroblast growth factors (FGF-1 and FGF-2) and it was found that FGF-1 not only interacted with the hexamer and tetramer of heparin oligosaccharides but also with the unusual 2,4-O-sulfated monomer, which may become important for inhibitor design. The Hsieh-Wilson group reported the use of a chondroitin sulfate microarray to probe the specificity of TNF-α [19], as well as midkine-derived and brain-derived neurotrophic factor [20•]. The tetrasulfated tetrasaccharide CS-E was found to react strongly with these growth factors within the physiological concentration range. A brain neuron growth experiment confirmed that the CS-E motif stimulated neurite outgrowth by about 50% [20•]. It is anticipated that these microarrays will accelerate the understanding of glycosaminoglycan–protein interactions and pinpoint the sulfation patterns responsible for modulating physiological and disease states. These microarrays also provide valuable structure information for the design of inhibitors or antagonists of these therapeutically important cytokines and growth factors.
Carbohydrate–antibody interactions
Pathogen-induced antibody recognition
Most pathogens contain specific polysaccharides on their cell surfaces, which can elicit antibody responses in infected humans. Microbial polysaccharide microarrays can be used for the diagnosis of pathogen infection by analyzing patient serum samples. Several examples of this application are described below (Figure 2 ). In an application with relevance to AIDS vaccine development, glycan arrays were used to dissect the glycan-binding specificity of the HIV-1 broadly neutralizing antibody 2G12 [21•, 22•]. An array of oligomannose structures was analyzed against 2G12, and a minimal binding element was identified. Smaller oligomannose structures (Man4) were found to be as effective as the Man9GlcNAc2-high mannose core at binding to 2G12 and inhibiting complexation with HIV gp120. These results may lead to the development of potential HIV vaccines by the synthesis of polyvalent derivatives of these oligomannose core structures. Wang et al. discovered that rabbit IgG antibodies elicited by Bacillus anthracis spores specifically recognize a rhamnose tetrasaccharide chain that decorates the outermost surface of the B. anthracis exosporium [23]. This tetrasaccharides appear to be a key biomarker for the detection of B. anthracis spores and may guide the development of novel anthrax vaccines. The same group used the glycan arrays to characterize the carbohydrate-binding activity of SARS-CoV neutralizing antibodies induced by an inactivated SARS-CoV vaccine and found potential crossreactivity between the immune response to an inactivated SARS-CoV vaccine and a host carbohydrate [24]. Blixt et al. reported an array containing oligosaccharide antigens specifically expressed by serogroups Salmonella enterica sv. Paratyphi, Typhimurium, and Enteritidis [25]. This microarray was used to detect the sera from patients with salmonellosis. Disaccharides (Tyvα1-3Man, Abeα1-3Man) and trisaccharide (Manα1-2Rhaα1-2Gal) were found to have high-specificity serological recognition. By using the same strategy, a polysaccharide microarray was prepared by immobilizing bacterial polysaccharides to detect bacterial infection by using human or animal serum sample [26, 27]. It is obvious that glycan array applications in this field may facilitate the identification of key immunogenic carbohydrates expressed by microbial pathogens.
Figure 2.
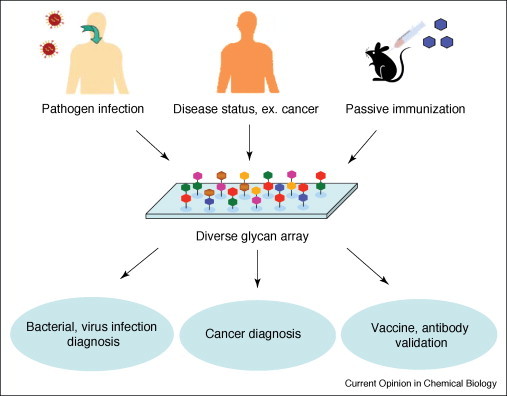
Glycan-binding specificity profiling for the diagnosis of disease state or antibody validation.
Cancer-induced antibody recognition
Aberrant glycosylation is one of the hallmarks of cancer; tracking differences in cell surface glycan expression may therefore be useful for diagnosing cancer, and provide a solution for specifically targeting drugs to cancerous cells (Figure 2). The Globo H hexasaccharide cancer marker and nine structural analogs were arrayed and used to test monoclonal antibodies raised against Globo H (MBR-1 and VK-9), as well as patient sera [28••]. A commercially available array of 37 different carbohydrates microarray was used to profiling of Hodgkin's lymphoma sera and showed marked deviation in glycan-binding specificity compared to normal samples [29]. Another strategy that used lectin-affinity purification and natural glycoprotein microarrays to screen different glycosylation patterns between healthy and different disease stages of the pancreas was developed [30]. Glycan array profiling is expected to facilitate the identification of more specific biomarkers, adding to currently used DNA and protein biomarker for improved cancer diagnosis and early detection.
Carbohydrates for passive immunization
The unique glycan structures from pathogens and aberrantly glycosylated antigens of cancer cells have guided the development of carbohydrate-based vaccines. Specific carbohydrates were conjugated to carrier proteins or virus particles for passive immunization in animals to induce antibodies against these carbohydrates. The glycan array serves as a rapid and convenient method to validate the specificity of antibodies generated by these potential vaccines. Anticarbohydrate antibodies elicited by the polyvalent display of glycans on a virus scaffold were detected by glycan array to validate the immunogenic scaffold design [31]. Using a glycoprotein array to assay the anti-Tn antibodies, Gildersleeve and coworkers evaluated the potential of Tn antigen as a cancer biomarker [32•].
Carbohydrate–virus and carbohydrate–bacterial interactions
Carbohydrates on the cell surface of human cells are used by viruses and bacteria as initial recognition and attachment sites [33]. The specificity of hemagglutinin (HA) from avian and human influenza sources, including those reconstructed from past pandemic strains, was examined [34••, 35, 36]. Virus entry into host cells is initialed by HA binding to cell surface sialic acid-containing glycans, which vary in structure based on the host species and anatomical location. Binding of HA variants recovered from pandemic and circulating strains on a 260-member glycan array demonstrated differences in the recognition of carbohydrate linkages (α2,3 or α2,6 sialic acid, characteristic of avian or human virus, respectively), sulfation and fucosylation. Remarkably, pandemic 1918 HA switched specificity to human epithelial cells, a change from α-2,3 to α-2,6 NeuAc-Gal-binding preference with only two amino acid substitutions. These findings provide information to assess the host–virus interactions associated with different influenza strains and to understand their evolution. Binding of intact influenza virus to a glycan array surface is also possible [15••].
A microarray displaying monosaccharides was also explored for binding to Escherichia coli ORN178. It was found that E. coli adhere specifically to mannose-containing slides [37]. By using glycoconjugate arrays, the Ruhl group has demonstrated for the characterization of unknown adhesion specificities of Helicobacter pylori and other bacteria [38]. These findings introduce the possibility of using carbohydrate microarrays as a detection system for pathogens.
Glycan microarray in drug discovery
High-throughput screening of new inhibitors of carbohydrate-processing enzymes has been performed with glycan arrays. For example, α-(1,3)-fucosyltransferase (FucT) is an enzyme which mediates the final reaction in the biosynthesis of SLex by transferring the fucose from GDP-Fucose (GDP-Fuc) to sialyl lactosamine to form SLex and is therefore a target for the identification of inhibitors to block the inflammation cascade. Eighty-five synthetic compounds were incubated with FucT and GDP-Fuc on a LacNAc-coated microtiter-type microarray and then detected by peroxidase-coupled T. purpureas lectin. Four inhibitors with nanomolar K i were discovered [39]. The technique was further improved by using a glass slide glycan array to test several monosaccharides to inhibit the binding of surface-coated mannose with Con A [40••]. Recently, an aminoglycoside microarray platform has been developed for directly monitoring and studying antibiotic resistance [41, 42]. This type of array will be a useful tool for the discovery of new antibiotics that evade resistance. Inhibition or disruption of the biosynthesis of disease-related glycans may be an effective approach for the treatment of various diseases.
Quantitative carbohydrate–protein interaction using glycan array
Most reported glycan arrays binding experiments have been performed at one or two concentrations of proteins to globally profile carbohydrate–protein interactions. Although qualitative assessment of binding by this method is useful, using fluorescence intensities to quantify binding affinities might be misleading. Values are highly variable from batch to batch because the spot intensities depend on immobilization efficiency. Recently, the Wong lab has developed a quantitative method to assess the binding affinities between carbohydrates and proteins (Figure 3 ) [40••]. A series of protein concentrations were incubated with repeated subarrays which contained several carbohydrates to get surface dissociations (K D,surf). A similar strategy was also reported to determine the dissociation constant of α-LacNAc and β-LacNAc for RCA120 [43]. By varying the printing concentration of glycans, multivalent interactions were probed. Higher density printing generally led to lower observed K D,surf, indicating a multivalency effect which mimics the multivalent display of glycans on cell surfaces. Furthermore, solution dissociation constants (K D) were determined by the competition between carbohydrates or inhibitors in solution-bound and surface-bound glycans. It is envisioned that this method will be useful for the quantitative characterization of sugar-binding specificities of proteins and for the high-throughput discovery of inhibitors of carbohydrate-binding proteins of therapeutic interest.
Figure 3.

Quantitative analysis of protein–carbohydrate interactions to obtain surface and solution dissociation constants from glycan arrays.
Glycan array for measuring enzyme specificities
Glycan microarrays can be utilized to rapidly determine the substrate specificity or activity of carbohydrate-processing enzymes. In a model study reported by Shin and coworkers, a microarray containing GlcNAc and fucose was treated with β-1,4-galactosyltransferase (GalT) and UDP-Gal and then probed with fluorophore-labeled lectins [44•]. Enzymatic conversion of GlcNAc to LacNAc by GalT was detected by fluorescence lectin. Recently, the method was extended to profile 20 carbohydrates as GalT acceptors [43]. Maltose derivatives were covalently conjugated on the microtiter-type glycan array and glycosylation products were detected by FITC-labeled Con A. New acceptor substrate specificities of glycosyltransferase were observed [45]. Owing to the limitation of lectins as detection tool (low-binding specificities and weak affinities), a broad spectrum of detection method was developed to study various recombinant sialyltransferase specificities. The donor substrate cytidine-5′-monophospho-N-acetylneuraminic acid (CMP-Neu5Ac) was biotinylated at the 9-position of Neu5Ac. After screening of a series of sialyltransferase against a +200 member glycans microarray, the sialylation products were observed with fluorescein-streptavidin to determine acceptor substrate specificities [46]. Similarly, GDP-[14C]Fuc was transferred to surface carbohydrates on an array using plant cell wall glycosyltransferase [47]. Arrays have proven to be powerful tools for high-throughput profiling of the substrate specificities of carbohydrate-processing enzymes.
Conclusions
Glycan arrays are powerful tools for screening the specificity of GBPs, including profiling of lectins, growth factors, cytokines, antibodies, and microbial toxins, and have demonstrated their utility in the identification and the characterization of glycan–protein interactions. More detailed quantitative analysis and enzyme characterization have also shown that arrays can be used in inhibitor and drug discovery. Glycan arrays continue to be an important tool in the evolving field of glycomics, and new applications will be developed in the future.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by TSRI and Academia Sinica.
References
- 1••.Paulson J.C., Blixt O., Collins B.E. Sweet spots in functional glycomics. Nat Chem Biol. 2007;2:238–248. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]; An excellent review that provides a brief overview of the major functions ascribed to GBPs and of recent advances in glycan array technology.
- 2.Varki A., Cummings R., Esko J.D., Freeze H., Hart G.W., Marth J. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 1999. Essentials of Glycobiology. 1–635. [PubMed] [Google Scholar]
- 3.Weis W.I., Drickamer K. Structural basis of lectin–carbohydrate recognition. Annu Rev Biochem. 1996;65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 4•.Mellet C.O., Garcia Fernandez J.M. Carbohydrate microarrays. Chembiochem. 2002;3:819–822. doi: 10.1002/1439-7633(20020902)3:9<819::AID-CBIC819>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]; A highlight that provides an overview of glycan arrays published in 2002.
- 5•.Feizi T., Fazio F., Chai W., Wong C.H. Carbohydrate microarrays — a new set of technologies at the frontiers of glycomics. Curr Opin Struct Biol. 2003;13:637–645. doi: 10.1016/j.sbi.2003.09.002. [DOI] [PubMed] [Google Scholar]; A comprehensive review of the glycan array fabrication methods and sources for carbohydrates.
- 6.Shin I., Park S., Lee M. Carbohydrate microarrays: an advanced technology for functional studies of glycans. Chem Eur J. 2005;11:2894–2901. doi: 10.1002/chem.200401030. [DOI] [PubMed] [Google Scholar]
- 7.Culf A.S., Cuperlovic-Culf M., Ouellette R.J. Carbohydrate microarrays: survey of fabrication techniques. Omics. 2006;10:289–310. doi: 10.1089/omi.2006.10.289. [DOI] [PubMed] [Google Scholar]
- 8.Laine R.A. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 1012 structures for a reducing hexasaccharide: the isomer barrier to development of single-method saccharide sequencing or synthesis system. Glycobiology. 1994;4:759–767. doi: 10.1093/glycob/4.6.759. [DOI] [PubMed] [Google Scholar]
- 9.Pilobello K.T., Mahal L. Deciphering the glycocode: the complexity and analytical challenge of glycomics. Curr Opin Chem Biol. 2007;11:300–305. doi: 10.1016/j.cbpa.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Hsu T.L., Hanson S.R., Kishikawa K., Wang S.K., Sawa M., Wong C.H. Alkynyl sugar analogs for labeling and visualization of glycoconjugates in cells. Proc Natl Acad Sci U S A. 2007;104:2614–2619. doi: 10.1073/pnas.0611307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Hanson S.R., Hsu T.L., Weerapana E., Kishikawa K., Simon G.M., Cravatt B.F., Wong C.H. Tailored glycoproteomics and glycan site mapping using saccharide-selective bioorthogonal probes. J Am Chem Soc. 2007;129:7266–7267. doi: 10.1021/ja0724083. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use metabolic oligosaccharide engineering and glycoproteomic methods to identify inventory cellular glycoproteins.
- 12.Bochner B.S., Alvarez R.A., Mehta P., Bovin N.V., Blixt O., White J.R., Schnaar R.L. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y., Feinberg H., Conroy E., Mitchell D.A., Alvarez R., Blixt O., Taylor M.E., Weis W.I., Drickamer K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 14.Coombs P.J., Taylor M.E., Drickamer K. Two categories of mammalian galactose-binding receptors distinguished by glycan array profiling. Glycobiology. 2006;16:1C–7C. doi: 10.1093/glycob/cwj126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Blixt O., Head S., Mondala T., Scanlan C., Huflejt M.E., Alvarez R., Bryan M.C., Fazio F., Calarese D., Stevens J. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]; An array of more than 200 carbohydrates on a slide used to analyze the specific binding of mammalian, plant, viral and bacterial lectins, antibodies, and intact viruses.
- 16.Manimala J.C., Roach T.A., Li Z., Gildersleeve J.C. High-throughput carbohydrate microarray analysis of 24 lectins. Angew Chem Int Ed. 2006;45:3607–3610. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- 17.van Vliet S.J., van Liempt E., Saeland E., Aarnoudse C.A., Appelmelk B., Irimura T., Geijtenbeek T.B., Blixt O., Alvarez R., van Die I., van Kooyk Y. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. 2005;17:661–669. doi: 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- 18•.de Paz J.L., Noti C., Seeberger P.H. Microarray of synthetic heparin oligosaccharides. J Am Chem Soc. 2006;128:2766–2767. doi: 10.1021/ja057584v. [DOI] [PubMed] [Google Scholar]; The first report of heparin arrays will be useful to assay the specificity of heparin–protein interactions.
- 19.Tully S.E., Rawat M., Hsieh-Wilson L. Discovery of a TNF-α antagonist using chondroitin sulfate microarrays. J Am Chem Soc. 2006;128:7740–7741. doi: 10.1021/ja061906t. [DOI] [PubMed] [Google Scholar]
- 20•.Gama C.I., Tully S.E., Sotogaku N., Clark P.M., Rawat M., Vaidehi N., Goddard W.A., III, Nishi A., Hsieh-Wilson L.C. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]; Comprehensive study using a chrondroitin array to assay binding specificity of different growth factors and sulfation patterns important for neurite outgrowth.
- 21•.Calarese D.A., Lee H.K., Huang C.Y., Best M.D., Astronomo R.D., Stanfield R.L., Katinger H., Burton D.R., Wong C.H., Wilson I.A. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci U S A. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use a specially designed oligomannose array to detail the carbohydrate-binding specificity of a broadly neutralizing anti-HIV antibody.
- 22•.Ratner D.M., Seeberger P.H. Carbohydrate microarrays as tools in HIV. Curr Pharm Des. 2007;13:173–183. doi: 10.2174/138161207779313650. [DOI] [PubMed] [Google Scholar]; An excellent review that illustrates carbohydrate arrays as useful tools for HIV study.
- 23.Wang D., Carroll G.T., Turro N.J., Koberstein J.T., Kovac P., Saksena R., Adamo R., Herzenberg L.A., Herzenberg L.A., Steinman L. Photogenerated glycan arrays identify immunogenic sugar moieties of Bacillus anthracis exosporium. Proteomics. 2007;7:180–184. doi: 10.1002/pmic.200600478. [DOI] [PubMed] [Google Scholar]
- 24.Wang D., Lu J. Glycan arrays lead to the discovery of autoimmunogenic activity of SARS-CoV. Physiol Genomics. 2004;18:245–248. doi: 10.1152/physiolgenomics.00102.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blixt O., Hoffmann J., Svenson S., Norberg T. Pathogen specific carbohydrate antigen microarrays: a chip for detection of Salmonella O-antigen specific antibodies. Glycoconj J. 2008;25:27–36. doi: 10.1007/s10719-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 26.Parthasarathy N., DeShazer D., England M., Waag D.M. Polysaccharide microarray technology for the detection of Burkholderia preudomallei and Burkholderia mallei antibodies. Diagn Microbiol Infect Dis. 2006;56:329–332. doi: 10.1016/j.diagmicrobio.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thirumalapura N.R., Morton R.J., Ramachandran A., Malayer J.R. Lipopolysaccharide microarrays for the detection of antibodies. J Immunol Methods. 2005;298:73–81. doi: 10.1016/j.jim.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 28••.Huang C.Y., Thayer D.A., Chang A.Y., Best M.D., Hoffmann J., Head S., Wong C.H. Carbohydrate microarray for profiling the antibodies interacting with Globo H tumor antigen. Proc Natl Acad Sci U S A. 2006;103:15–20. doi: 10.1073/pnas.0509693102. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate the potential of carbohydrate arrays as a diagnostic tool for cancer-related glycans.
- 29.Lawrie C.H., Marafioti T., Hatton C.S.R., Dirnhofer S., Roncador G., Went P., Tzankov A., Pileri S.A., Pulford K., Banham A.H. Cancer-associated carbohydrate identification in Hodgkin's lymphoma by carbohydrate array profiling. Int J Cancer. 2006;118:3161–3166. doi: 10.1002/ijc.21762. [DOI] [PubMed] [Google Scholar]
- 30.Patwa T.H., Zhao J., Anderson M.A., Simeone D.M., Lubman D.M. Screening of glycosylation patterns in serum using natural glycoprotein microarrays and multi-lectin fluorescence detection. Anal Chem. 2006;78:6411–6421. doi: 10.1021/ac060726z. [DOI] [PubMed] [Google Scholar]
- 31.Kaltgrad E., Sen Gupta S., Punna S., Huang C.Y., Chang A., Wong C.H., Finn M.G., Blixt O. Anti-carbohydrate antibodies elicited by polyvalent display on a virus scaffold. Chembiochem. 2007;8:1455–1462. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- 32•.Manimala J.C., Li Z., Jain A., VedBrat S., Gildersleeve J.C. Carbohydrate array analysis of anti-Tn antibodies and lectins reveals unexpected specificities: implications for diagnostic and vaccine development. Chembiochem. 2005;6:2229–2241. doi: 10.1002/cbic.200500165. [DOI] [PubMed] [Google Scholar]; Explores the potential for using the Tn carbohydrate antigen as a biomarker by analyzing the binding specificity with a glycan array.
- 33.Hooper L.V., Gordon J.I. Glycans as legislators of host–microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiology. 2001;11:1R–10R. doi: 10.1093/glycob/11.2.1r. [DOI] [PubMed] [Google Scholar]
- 34••.Stevens J., Blixt O., Glaser L., Taubenberger J.K., Palese P., Paulson J.C., Wilson I.A. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J Mol Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]; Together with [35], uses a +200-member glycan array to probe binding specificity of hemagglutinin variants and site-directed mutants to detail the mutations that result in changes in specificity allowing for avian flu strains to cross species.
- 35.Stevens J., Blixt O., Tumpey T.M., Taubenberger J.K., Paulson J.C., Wilson I.A. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–410. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 36.Kumari K., Gulati S., Smith D.F., Gulati U., Cummings R.D., Air G.M. Receptor binding specificity of recent human H3N2 influenza viruses. Virol J. 2007;4:42. doi: 10.1186/1743-422X-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Disney M.D., Seeberger P.H. The use of carbohydrate microarrays to study carbohydrate–cell interactions and to detect pathogens. Chem Biol. 2004;11:1701–1707. doi: 10.1016/j.chembiol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Walz A., Odenbreit S., Mahdavi J., Boren T., Ruhl S. Identification and characterization of binding properties of Helicobacter pylori by glycoconjugate arrays. Glycobiology. 2005;15:700–708. doi: 10.1093/glycob/cwi049. [DOI] [PubMed] [Google Scholar]
- 39.Bryan M.C., Lee L.V., Wong C.H. High-throughput identification of fucosyltransferase inhibitors using carbohydrate microarrays. Bioorg Med Chem Lett. 2004;14:3185–3188. doi: 10.1016/j.bmcl.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 40••.Liang P.H., Wang S.K., Wong C.H. Quantitative analysis of carbohydrate–protein interactions using glycan microarrays: determination of surface and solution dissociation constants. J Am Chem Soc. 2007;129:11177–11184. doi: 10.1021/ja072931h. [DOI] [PubMed] [Google Scholar]; The authors demonstrate that glycan arrays can be used for high-throughput quantitative analysis of carbohydrate–protein interactions.
- 41.Disney M.D., Barrett O.J. An aminoglycoside microarray platform for directly monitoring and studying antibiotic resistance. Biochemistry. 2007;46:11223–11230. doi: 10.1021/bi701071h. [DOI] [PubMed] [Google Scholar]
- 42.Bryan M.C., Wong C.H. Aminoglycoside array for the high-throughput analysis of small molecule–RNA interactions. Tetrahedron Lett. 2004;45:3639–3642. [Google Scholar]
- 43.Park S., Shin I. Carbohydrate microarray for assaying galactosyltransferase activity. Org Lett. 2007;9:1675–1678. doi: 10.1021/ol070250l. [DOI] [PubMed] [Google Scholar]
- 44•.Park K., Lee M.R., Pyo S.J., Shin I. Carbohydrate chips for studying high-throughput carbohydrate–protein interactions. J Am Chem Soc. 2004;126:4812–4819. doi: 10.1021/ja0391661. [DOI] [PubMed] [Google Scholar]; A model study that uses glycan microarrays for the evaluation of glycan-processing enzyme specificities.
- 45.Seibel J., Hellmuth H., Hofer B., Kicinska A.M., Schmalbruch B. Identification of new acceptor specificities of glycosyltransferase R with the aid of substrate microarrays. Chembiochem. 2006;7:310–320. doi: 10.1002/cbic.200500350. [DOI] [PubMed] [Google Scholar]
- 46.Blixt O., Allin K., Bohorov O., Liu X., Andersson-Sand H., Hoffmann J., Razi N. Glycan microarrays for screening sialyltransferase specificities. Glycoconj J. 2008;25:59–68. doi: 10.1007/s10719-007-9062-z. [DOI] [PubMed] [Google Scholar]
- 47.Shipp M., Nadella R., Gao H., Farkas V., Sigrist H., Faik A. Glyco-array technology for efficient monitoring of plant cell wall glycosyltransferase activities. Glycoconj J. 2008;25:49–58. doi: 10.1007/s10719-007-9060-1. [DOI] [PubMed] [Google Scholar]


