Abstract
Background and objectives
Conventional cell culture (CC) has limited clinical utility as a result of the extended incubation period often required for virus isolation. Alternative methodologies have been introduced in an effort to improve turnaround times. One such system, the R-mix™ shell vial is discussed herein. The study objectives were: (a) to establish R-mix™ testing parameters as compared to direct antigen testing (DAT) and CC, and (b) to assess technical aspects and cost of R-mix™ in a high volume clinical virology laboratory.
Study design
A prospective analysis of respiratory samples submitted to the clinical virology laboratory between November 2004 and April 2005 was performed. All specimens were inoculated onto R-mix™ shell vials (SV) and CC tubes; and a subset also underwent DAT for influenza A and B and/or RSV. A retrospective estimated cost analysis was made.
Results
A total of 563 samples were included in the study, which collectively revealed a total of 207 viruses. Sensitivity of R-mix™ for seven major respiratory viruses ranged from 45% to 83% compared to CC and DAT, while mean time to detection (TTD) varied from 1.1 to 1.4 days. In addition to these viruses, 23 picornaviruses, 11 CMV isolates and 5 HSV isolates were detected by CC alone.
Conclusions
The R-mix™ system has similar sensitivity as CC for the detection of parainfluenza 1–3 and influenza A/B while dramatically reducing the TTD. Furthermore, it is significantly more sensitive and produces more timely results for RSV than CC; yet, neither method offers a diagnostic benefit over rapid DAT for RSV detection. The sensitivity of R-mix™ for adenovirus appears to be significantly lower than that of CC. Lastly, methodologies other than R-mix™ must remain in place under circumstances where identification of other potential viral respiratory pathogens, including herpesviruses and picornaviruses, is desired.
Abbreviations: CC, cell culture; CI, confidence interval; CMV, cytomegalovirus; DAT, direct antigen testing; EIA, enzyme immunoassay; HAD, hemadsorption; HSV, herpes simplex virus; IF, immunofluorescence; NPV, negative predictive value; PCR, polymerase chain reaction; PPV, positive predictive value; RSV, respiratory syncytial virus; SV, shell vial; TN, true negatives; TP, true positives; TTD, time to detection
Keywords: Respiratory virus, Cell culture, Direct antigen test, Shell vial assay
1. Introduction
During recent years clinical virology laboratories have witnessed an increasing number of commercially available virus detection systems that vary considerably in terms of methodology, efficiency, accuracy and cost. While a desire for faster, better and cheaper results continually provides an impetus for new test development, an improvement in one arena often yields shortcomings in another. Thus, laboratory directors face difficult decisions regarding assay implementation for which numerous factors need be considered, not the least of which is the overall impact on patient care.
With respect to diagnostic testing for pathogenic respiratory viruses, cell culture (CC) has traditionally been considered the gold standard reference method (Yolken et al., 2003). One major disadvantage to this approach, however, is the extended incubation period often required for virus isolation, such that results are unavailable to clinicians during the initial stages of patient management (Shetty et al., 2003). Additional tests including enzyme immunoassays (EIAs), immunofluorescence (IF) assays and other direct antigen tests (DAT) (Johnston and Siegel, 1990, Reina et al., 2004, Shetty et al., 2003, Weinberg and Walker, 2005), shell vial techniques (Engler and Preuss, 1997, Schirm et al., 1992) and nucleic acid amplification tests (Kehl et al., 2001, Templeton et al., 2004, Weinberg et al., 2004b) have all been introduced into the viral diagnostic armamentarium with the logical rationale that more rapid detection may favorably influence patient management, leading to reductions in antibiotic usage, shorter and less costly hospitalizations with fewer extraneous laboratory tests ordered, better cohorting capabilities and infection control measures, and better utilization of anti-viral therapies. A number of investigations have addressed one or more of these issues directly, each having employed some DAT as the methodological basis by which patient outcome comparisons were made (Adcock et al., 1997, Barenfanger et al., 2000, Byington et al., 2002, Noyola and Demmler, 2000, Woo et al., 1997).
Considering all parameters, molecular tests utilizing the polymerase chain reaction (PCR) have performed exceedingly well in detecting respiratory viruses and will likely supersede cell culture as the new gold standard (Kehl et al., 2001, Templeton et al., 2004, Weinberg et al., 2004b). Yet many clinical laboratories are not equipped with this capability at present. Direct antigen tests are inexpensive relative to PCR and offer the benefit of simplicity and very short turnaround times. These assays often have considerably lower sensitivities than cell culture, however. The shell vial technique, which incorporates the speed and specificity of virus detection by immunofluorescent antibodies with the sensitivity of virus propagation on cell monolayers, provides an attractive option in terms of performance, rapidity and cost.
One commercially available system, the R-mix™ shell vial system (Diagnostic Hybrids Inc. [DHI], Athens, OH), combines epithelial cells derived from mink lung (Mv1Lu) with a human adenocarcinoma cell line (A549) in order to support the replication of several respiratory viruses, including influenza A and B, RSV, adenovirus and serotypes 1–3 of parainfluenza virus. While analytical sensitivity (St. George et al., 2002), cost analysis (Barenfanger et al., 2001), and performance characteristics (Dunn et al., 2004, Fong et al., 2000, Weinberg et al., 2004a) of the R-mix™ shell vial system have been addressed in the medical literature, the present study was conducted in an effort to (a) substantiate these data at a large, tertiary-care hospital laboratory over an entire respiratory virus season using traditional CC and DAT as methods of comparison, and (b) assess the technical aspects and cost of R-mix™ in order to determine the feasibility of its replacing CC.
2. Materials and methods
2.1. Design
Appropriately labeled and adequately transported nasal washes, nasopharyngeal aspirates/swabs and throat swabs submitted for DAT and/or respiratory viral culture to the University of Texas Medical Branch clinical microbiology laboratory between 8 November 2004 and 1 April 2005 were included in the analysis. All specimens were cultured using both a conventional tube monolayer method and the R-mix™ shell vial system, whereas DAT was performed only upon request. Specimens were accepted from outpatient clinics, urgent care facilities and UTMB emergency department and inpatient wards.
2.2. Specimen processing
Acceptable specimens included swabs received in viral transport medium (M4, Remel, Lenexa, KS) and fluid samples (i.e. washings and aspirates) received directly, in transport medium or in sterile PBS. Aliquots for EIA testing, when ordered, were made prior to a centrifugation step (1290 × g, 10 min at 4 °C). Following centrifugation, a portion of the supernatant was frozen at −70 °C for possible future use and the remainder was utilized for cultures and shell vials as detailed below. Direct IF tests, when ordered, were performed following resuspension of the cell pellet in 0.4–1.0 mL sterile PBS. Choice of DAT methodology was dependant upon time of specimen arrival in laboratory. If no rapid DAT was requested, samples were stored at 4 °C until processing. Time to detection (TTD) of all viruses was generated using specimen processing as start point.
2.3. Direct antigen testing
For IF, resuspended cell pellets were spotted onto 4-well glass slides and fixed in acetone. Flourophore-conjugated monoclonal antibody was used for RSV, influenza A and influenza B (Imagen, DAKO, Carpinteria, CA) detection. Evans blue counterstain provided nuclear detail for determination of specimen adequacy—negative spots containing fewer than 20 epithelial cells were reported as insufficient. For EIA testing, immunochromatographic kits for RSV and influenza A and B (NOW, Binax, Portland, ME) were used according to manufacturer's instructions.
2.4. Conventional tube culture
Standard tube monolayers of primary rhesus monkey kidney cells (RhMK) (two tubes), human lung carcinoma cells (A-549) (one tube) and diploid human embryonic lung cells (MRC-5) (two tubes) (DHI) were used for all conventional respiratory virus cultures. A 0.2–0.3 mL volume of sample was inoculated into each tube and adsorbed for 2 h. Cells were incubated on a roller drum at 37 °C or 33 °C (replicate MRC-5 and RhMK). Tubes were screened for cytopathic effects (CPE) daily for 5 days, then every other day for 9 additional days. Hemadsorption (HAD) using 0.2 mL of a 0.4% solution of guinea pig erythrocytes was performed at least twice on an RhMK replicate. Cell spots from positive HAD tubes were stained with unconjugated monoclonal antibody for influenza A and B and parainfluenza serotypes 1–3 (Bartels, Trinity Biotech, Wicklow, Ireland). For HAD-negative specimens, spots were stained with individual monoclonal antibody for RSV, adenovirus, HSV 1 and 2, CMV (Bartels, Trinity Biotech) or polyclonal antibody for pan-enterovirus, coxsackie B and echovirus (Chemicon International, Temecula, CA) based on CPE and/or cell tropism. Slides stained with conjugated primary antibodies (CMV, HSV and enterovirus) were read directly. For indirect assays, spots were stained with a flourophore-conjugated secondary anti-murine antibody (Bartels, Trinity Biotech).
2.5. R-mix™ shell vial culture
All shell vial cultures were set-up as recommended by the manufacturer. Briefly, R-mix™ shell vials were inoculated with 0.2–0.3 mL of specimen. As with CC, the final volume of inoculum varied depending upon the total amount of specimen received; however, equal volumes of each sample were added to CC and R-mix™ cultures. Assay-specific refeed medium was utilized (DHI). Shell vials were centrifuged at 700 × g for 60 min and incubated at 37 °C on a stationary rack. A flourophore-conjugated anti-respiratory virus antibody cocktail (D3, DHI) was used for broad-spectrum screening stain at 24 and 48 h. When positive, a replicate shell vial was prepared for confirmation stain using individual monoclonal antibodies for RSV, adenovirus, influenza A or B or parainfluenza serotypes 1–3 (D3, DHI). If both 24- and 48-h broad-spectrum stains were negative, the third replicate vial was discarded.
2.6. Calculations and statistics
Given the high analytical specificity rates of all assays included in our study, we chose to define a true positive as any positive value, regardless of methodology. Based on this definition and using individual positivity rates to determine overall prevalence, sensitivity and negative predictive value (NPV) were determined for each virus/methodology combination. Comparison of proportions was performed using chi square analyses with generated two-tailed p values considered significant if ≤0.05.
3. Results
During the nearly 21-week period of study, a total of 563 samples were processed for CC and R-mix™ shell vial assay. Rapid DAT was performed on a subset of 329 of these samples (58%). Patient age ranged from 2 weeks to 79 years (mean 4.4 years, median 1.0 years); and 95% of specimens received were collected from patients <18 years of age. From the 563 specimens included in the analysis, a total of 207 viruses were detected by one or more method, resulting in an overall positivity rate of 36.8%. These figures include eight samples from which dual virus infection was detected (Table 1 ).
Table 1.
Results from 8 samples in which >1 virus was detected
| No. of samples | Results by method |
||
|---|---|---|---|
| CC | R-mix™ | DAT | |
| 2 | Adenovirus | RSV | RSV |
| 1 | Adenovirus | RSV | Not performed |
| 3 | Adenovirus + RSV | RSV | RSV |
| 1 | Rhinovirus | Negative | Influenza A |
| 1 | Parainfluenza 3 | Parainfluenza 3 | RSV |
The most frequently detected virus during the study period was RSV (n = 78), followed by influenza A/B (n = 36), adenovirus (n = 31), parainfluenza 1–3 and picornaviruses (n = 23 each) (Table II). Sensitivity/NPV for the detection of RSV by R-mix™ was significantly higher than by CC (73%/95.8% and 42%/91.5%, respectively; p = 0.0001); and mean TTD of all RSV positive specimens by R-mix™ shell vial assay was 1.2 days compared to 8.0 days required by CC. On the other hand, DAT identified 72/72 RSV-positive samples (20 by EIA, 52 by DFA), resulting in a significantly greater sensitivity/NPV for RSV (100%/100%) than for either CC or R-mix™ (p < 0.0001). In addition, all results were reported within 24 h of specimen processing. Sensitivity/NPV for the detection of adenovirus was significantly higher by CC than by R-mix™ (100%/100% and 45%/96.9%, respectively; p < 0.0001), while mean TTD by CC was 4.8 days versus 1.4 days by R-mix™. Interestingly, CC required an average of 2.6 days for isolation of the 14 adenovirus strains detected by the R-mix™ system, whereas mean TTD for the other 17 isolates (identified by CC only) was 6.7 days. Sensitivity/NPV for the detection of all parainfluenza isolates was 87%/99.4% by CC and 83%/99.2% by R-mix™. These differences (as well as differences between detection of individual serotypes) were not significant. Additionally, 23 picornavirus isolates and 16 herpesvirus isolates were recovered by CC only (Table 2 ).
Table 2.
Performance characteristics of all three assays by virus type
| Virus | CC |
R-mix™ |
DAT |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of detected/TPa | Sensitivity (95% CI) (%) | NPVb (%) | Mean TTDc | No. of detected/TP | Sensitivity (95% CI) (%) | NPV (%) | Mean TTD | No. of detected/TP | Sensitivity (95% CI) (%) | NPV (%) | Mean TTD | |
| RSV | 33/78 | 42 (32–53%) | 91.5 | 8.0 | 57/78 | 73 (62–82%) | 95.8 | 1.2 | 72/72d | 100 (94–100%) | 100 | <1.0 |
| Influenza A and B | 27/36 | 75 (59–86%) | 98.3 | 3.4 | 28/36 | 78 (62–89%) | 98.5 | 1.1 | 15/23d | 65 (45–81%) | 98.5 | <1.0 |
| Adenovirus | 31/31 | 100 (87–100%) | 100 | 4.8 | 14/31 | 45 (29–62%) | 96.9 | 1.4 | – | – | – | – |
| Parainfluenza 1–3 | 20/23 | 87 (67–96%) | 99.4 | 6.0 | 19/23 | 83 (62–94%) | 99.2 | 1.2 | – | – | – | – |
| Enterovirus/rhinovirus | 23/23 | 100 (83–100%) | n.d.e | n.d. | – | – | – | – | – | – | – | – |
| CMV | 11/11 | 100 (70–100%) | n.d. | n.d. | – | – | – | – | – | – | – | – |
| HSV | 5/5 | 100 (51–100%) | n.d. | n.d. | – | – | – | – | – | – | – | – |
True positives defined as positive results by any methodology (specificity and PPV = 100%).
True positives.
Negative predictive value.
Time to detection (days) = duration between completion of sample processing and issuance of test result.
Lower denominators for DAT compared to cultures reflect fewer samples tested by this methodology.
Not determined.
A total of 36 specimens containing either influenza A (n = 31) or B (n = 5) was identified in the study, 27 by CC, 28 by R-mix™, and 15 by DAT (11 by EIA and 4 by DFA) (Table 2). Sensitivities and 95% confidence intervals for combined influenza A/B detection were 75% (59–86%), 78% (62–89%) and 65% (45–81%) for CC, R-mix™ and DAT, respectively (Table 2). No statistical difference in sensitivity between the two culture methods was identified, yet mean TTD by R-mix™ was 2.3 days shorter than by CC. Sensitivity for influenza A/B detection by DAT did not differ significantly when compared to either culture methodology. Further analysis of influenza A and B data subsets individually yielded similar results, as did sub-analysis of individual DAT methodologies (data is not shown).
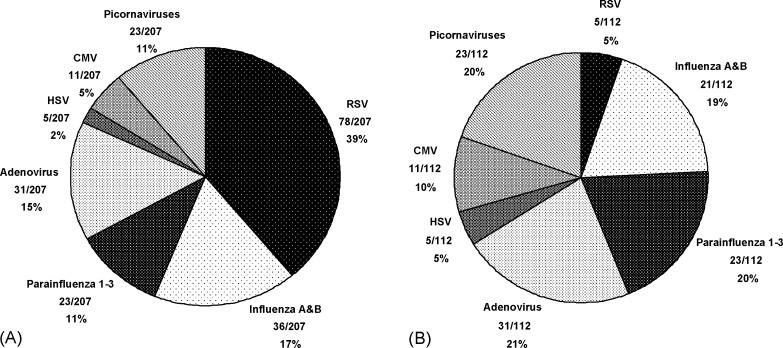
Because our routine laboratory protocol does not incorporate culture for DAT-positive samples throughout the epidemic season, we chose to analyze separately culture results from the subset of specimens that were either DAT-negative or on which DAT was not performed (due to lack of request). Of 468 such cultures, 6 (1.3%) and 21 (4.5%) were positive for RSV and influenza A/B, respectively, by either R-mix™ or CC (Table 3 ). When analyzed in this fashion, no statistical differences in sensitivity between R-mix™ and CC for the detection of RSV or influenza A/B were noted. Mean TTD by R-mix™ remained shorter than by CC, averaging 3.2 and 8.6 days faster for influenza A/B and RSV, respectively. Had this culture protocol been in place during the study period, however, the overall detection rate by either CC or R-mix™ would have been effectively reduced. As illustrated in Fig. 1 , the relative proportions of viruses detected by culture alone would have differed in comparison with the proportions detected by all three methodologies. As a result, while RSV, influenza A/B and parainfluenza 1–3 comprise 67% of the total number of viruses detected by all methods (Fig. 1A), they represent only 44% of viruses detected by culture alone (Fig. 1B). Put differently, specimens for which culture would have been the sole manner of virus detection (e.g. DAT negative or DAT not requested) gave similar overall positivity rates for RSV (5/468; 1.1%), HSV (5/468; 1.1%) and CMV (11/468; 2.4%) as well as for influenza A/B (21/468; 4.5%), picornaviruses (23/468; 4.9%), adenovirus (31/468; 6.6%) and parainfluenza 1-3 (23/468; 4.9%) (Fig. 1B).
Table 3.
Comparison of sensitivity and mean time to detection between CC and R-mix™ after exclusion of 95 DAT (+) results
| Virus | CC |
R-mix™ |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of positive/TPa | Sensitivity (95% CI) (%) | Mean TTDb | NPVc (%) | No. of positive/TP | Sensitivity (95% CI) (%) | Mean TTDb | NPV (%) | |
| Influenza A and B | 18/21 | 86 (65–96) | 4.3 | 99.3 | 19/21 | 90 (70–99) | 1.1 | 99.6 |
| RSV | 5/64 | 83 (42–99) | 10.2 | 99.8 | 5/6d | 83 (42–99) | 1.6 | 99.8 |
True positives.
Time to detection (days) = duration between completion of sample processing and issuance of test result.
Negative predictive value.
Includes one dual infection, RSV(+) by R-mix™ and EIA, adenovirus(+) by CC only.
Fig. 1.
Relative proportion of viruses detected (A) by CC, R-mix™ and/or DAT (n = 207), and (B) by CC and/or R-mix™ alone (i.e. after exclusion of 95 DAT-positive samples) (n = 112).
Equivocal results were occasionally obtained by R-mix™ when screening with the pooled antibody was positive but confirmation staining of a replicate SV with individual antibodies was negative. A total of 33 specimens gave such initial equivocal results (17 at 24 h and 16 at 48 h). Of these, 11 (33%) were identified as virus-positive by CC and/or DAT but were negative by R-mix™ upon repeat staining (five adenovirus, four influenza A, and two parainfluenza 3). Thirteen samples (39%) were negative by CC, DAT and R-mix™. The remaining nine samples were initially equivocal at 24 h but became positive at 48 h upon repeat staining with individual, virus-specific antibodies (one adenovirus, two influenza A, four RSV and two parainfluenza 3 isolates). Seven of these nine isolates were concomitantly identified by CC and/or DAT.
Overall, the R-mix™ system was well accepted among laboratory personnel due to its performance ease and time-savings. Given the two culture protocols used in this study, we found negligible differences in set-up times (e.g. specimen processing, inoculations, centrifugation steps, etc.) and baseline material costs (i.e. cell monolayers/shell vials) between the two culture methodologies. The primary differences in cost were noted in immunofluorescent reagents and overall technologist time. We calculated having spent approximately $12,000 for R-mix™ ($21 per specimen) and approximately $3000 for CC ($5 per specimen) on immune reagents over the entire study period. This was partially compensated for by savings in technologist time—∼0.37 h per specimen for R-mix™ versus ∼1.02 h per specimen for CC, representing a difference of $13 per specimen based upon a $20 h−1 salary. Other items either represented nominal costs or were difficult to calculate due to their use in other assays.
4. Discussion
Our results indicate that the R-mix™ system outperformed CC with respect both to sensitivity and mean TTD for RSV (Table 2). When compared with DAT, however, neither R-mix™ nor CC provided as sensitive or as timely results, suggesting that DAT should remain the test of choice for RSV at our institution. Given the liability of RSV and prior reports demonstrating that DAT frequently detects uncultivable RSV (Johnston and Siegel, 1990, Kellogg, 1991), we have no reason to doubt that most RSV-positive samples detected by DAT alone were actually true positives. Indeed, other investigations of the R-mix™ system have illustrated the benefits of DAT for RSV detection (Dunn et al., 2004, Fong et al., 2000). Yet given the possibility that a small number of RSV-positive samples detected only by DAT may have been falsely positive, we cannot rule out having slightly underestimated the sensitivity for RSV detection by CC and SV. Additionally, because pediatric-aged patients (a) were overrepresented in our data, and (b) have been shown to harbor higher RSV titers and, hence, test positive by DAT more frequently than adults (Kellogg, 1991), we cannot draw conclusions from the present study regarding appropriate testing for RSV in the adult population.
We found that the R-mix™ shell vial system performed quite favorably in comparison with CC for the detection of influenza A/B and parainfluenza 1–3. Potentially significant reductions in mean TTD were achieved without any appreciable compromise in sensitivity (Table 2). Surprisingly, comparison of sensitivity for influenza A/B by DAT and either culture methodology revealed no statistical differences. Because influenza virus is not generally considered to be as labile as RSV, a scenario wherein DAT is the sole positive assay for this virus seems somewhat less likely. Review of our data indicated that 5 of 15 DATs positive for influenza A/B (all of which were performed by EIA) were unconfirmed by either culture method. While we speculate that some of these results may have been falsely positive (and therefore decreased the calculated sensitivity of both culture systems), supplemental testing (e.g. RT-PCR) and/or detailed information (e.g. duration between DAT and culture set-up), which might have corroborated this suspicion, were not readily available.
One limitation of the R-mix™ shell vial system appears to be a lower sensitivity for the detection of adenovirus from clinical samples. Reports by Huang and Turchek (2000) and St. George et al. (2002) demonstrated similar detection rates and analytical sensitivity for adenovirus isolates between R-mix™ shell vials harvested 24 h post-inoculation as compared to other 24-h shell vial or conventional CC methods. However, the two studies utilized (a) stored patient samples that had previously tested positive by IFA or (b) dilutions of passaged virus. By contrast, two prospective studies using fresh, non-passaged patient samples reported low sensitivity rates for adenovirus similar to the present study—namely 69% and 25% based on 51 and 16 isolates, respectively (Dunn et al., 2004, Weinberg et al., 2004a). These authors concluded that abbreviated incubation periods (24 h) or differing inoculum volumes (0.2 mL versus 0.3 mL) may have accounted for their observed low sensitivity values. Yet these variables cannot account for our observed adenovirus sensitivity, as we (a) extended the incubation period of R-mix™ shell vials to 48 h and (b) used identical inoculum volumes of each specimen for CC and R-mix™. Still, the lower sensitivity observed in R-mix™ could have been due to low viral titers in some samples, particularly given the mean TTD by CC for the 17 samples not detected by R-mix™ (6.7 days). In that case, extended incubation of shell vials beyond 48 h may have resulted in an increased recovery rate, although this seems to us to defeat the overall purpose of shell vial techniques. Lastly, because different antibody reagents were utilized for R-mix™ screen/confirmation (D3, DHI) and CC confirmation (Bartels, Trinity Biotech), we cannot exclude the unlikely possibility that differences in observed adenovirus sensitivity between the two culture systems were partly attributable to inherent antigen specificities between the virus-specific monoclonal antibodies.
As others have previously described (Barenfanger et al., 2001, Dunn et al., 2004), we also encountered a number of clinical samples that appeared to be virus-positive by initial R-mix™ screen, but that were negative by individual IF antibody staining performed on the same day. Of 33 such specimens, 11 (33%) were virus-positive by CC and/or DAT but not R-mix™, 13 (39%) were negative by all three methodologies, and nine (27%) were ultimately identified as virus positive by repeat R-mix™ confirmation staining. As the virus season progressed, staff became more attuned to the fact that weak positivity on screening often did not confirm. A minor modification of the SV protocol was instituted so that confirmation of weakly positive screens from day 1 was postponed until day 2. Indeed, the number of equivocal samples at 24-h decreased as the study progressed, and we were able to confirm six virus-positive samples on day 2 that may have otherwise gone undetected by R-mix™. Had we chosen to extend incubation of equivocal samples from day 2 for an additional 24 h (i.e. perform confirmation staining at 36 h post-inoculation), we may have recovered some or all of the 11 viruses that were detected only by other methods.
Based upon the relative proportions of all viruses identified in this study (Fig. 1A), replacement of CC by R-mix™ might have seemed justified given that for 67% of isolates (i.e. RSV, influenza A/B, parainfluenza 1–3) R-mix™ clearly outperformed CC in terms of sensitivity and/or mean TTD. However, we found that the majority of viruses detected after elimination of the 95 DAT-positive specimens (i.e. samples on which culture would not have been performed under our usual laboratory protocol) were more readily identified by CC (Fig. 1B). In fact, use of the R-mix™ system in place of CC as the exclusive follow-up method to DAT in this study would have resulted in a reduction in overall cultivation rate from 23.5% (112/476) to 12.8% (61/476). While the clinical relevance of the herpesvirus and picornavirus isolates from our study is unknown, these viruses have been implicated in respiratory disease among certain populations (de la Hoz et al., 2002, Ghosh et al., 1999, Greenberg, 2003). Furthermore, as a number of less common respiratory viruses, including metapneumovirus, some strains of coronavirus, measles and mumps are more likely to be recognized in CC than shell vials, it would seem reasonable for laboratories to retain some form of conventional CC if possible.
From a technical and logistical standpoint, implementation of R-mix™ in the setting of a large clinical virology laboratory is appealing and potentially cost-effective. We found that the overall cost of the R-mix™ system using the described protocol was only slightly higher (∼$4 per sample) than our current CC protocol after taking into consideration the marked reduction in labor required by the former (∼300% average reduction/specimen). As with other shell vial systems, additional advantages included simplicity of the methodology and less dependence on experience in discerning CPE. Importantly, our cost estimates were based upon an “all or nothing” scenario (i.e. CC alone versus R-mix™ alone). As a result, implementation of R-mix™ with retention of some CC capabilities would have served to increase the overall cost of the “R-mix™ approach”.
In summary, we conclude that the R-mix™ system has a comparable sensitivity as CC for the detection of parainfluenza serotypes 1–3 and influenza A/B, but dramatically reduces the TTD. For RSV, the R-mix™ system is significantly more sensitive and more timely than CC, but neither appears to offers diagnostic benefit over DAT. The sensitivity of R-mix™ for adenovirus detection in clinical material is significantly lower than that of CC. Finally, while technical and logistical advantages of the R-mix™ system exist, alternative methodologies must remain in place if identification of other recognized viral respiratory pathogens, including CMV, HSV and picornaviruses is desired.
Acknowledgements
The authors are indebted to Deborah Briggs, Raylene Flores, Elizabeth Hinson, Candace Sanders and Theresa Tompkins and for their tremendous assistance in the laboratory.
References
- Adcock P.M., Stout G.G., Hauck M.A., Marshall G.S. Effect of rapid viral diagnosis on the management of children hospitalized with lower respiratory tract infection. Pediatr Infect Dis J. 1997;16(9):842–846. doi: 10.1097/00006454-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Barenfanger J., Drake C., Mueller T., Troutt T., O’Brien J., Guttman K. R-mix cells are faster, at least as sensitive and marginally more costly than conventional cell lines for the detection of respiratory viruses. J Clin Virol. 2001;22(1):101–110. doi: 10.1016/s1386-6532(01)00171-8. [DOI] [PubMed] [Google Scholar]
- Barenfanger J., Drake C., Leon N., Mueller T., Troutt T. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J Clin Microbiol. 2000;38(8):2824–2828. doi: 10.1128/jcm.38.8.2824-2828.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byington C.L., Castillo H., Gerber K., Daly J.A., Brimley L.A., Adams S. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children's hospital. Arch Pediatr Adolesc Med. 2002;156(12):1230–1234. doi: 10.1001/archpedi.156.12.1230. [DOI] [PubMed] [Google Scholar]
- de la Hoz R., Stephens G., Sherlock C. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25(Suppl 2):S1–S12. doi: 10.1016/s1386-6532(02)00091-4. [DOI] [PubMed] [Google Scholar]
- Dunn J.J., Woolstenhulme R.D., Langer J., Carroll K.C. Sensitivity of respiratory virus culture when screening with R-mix fresh cells. J Clin Microbiol. 2004;42(1):79–82. doi: 10.1128/JCM.42.1.79-82.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H.D., Preuss J. Laboratory diagnosis of respiratory virus infections in 24 h by utilizing shell vial cultures. J Clin Microbiol. 1997;35(8):2165–2167. doi: 10.1128/jcm.35.8.2165-2167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C.K.Y., Lee M.K., Griffith B.P. Evaluation of R-mix fresh cells in shell vials for detection of respiratory viruses. J Clin Microbiol. 2000;38(12):4660–4662. doi: 10.1128/jcm.38.12.4660-4662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Champlin R., Couch R., Englund J., Raad I., Malik S. Rhinovirus infections in myelosuppresed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29:528–532. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]
- Greenberg S.B. Respiratory consequences of rhinovirus infection. Arch Intern Med. 2003;163:278–284. doi: 10.1001/archinte.163.3.278. [DOI] [PubMed] [Google Scholar]
- Huang Y.T., Turchek B.M. Mink lung cells and mixed mink lung cells and A549 cells for rapid detection of influenza virus and other respiratory viruses. J Clin Microbiol. 2000;38(1):422–423. doi: 10.1128/jcm.38.1.422-423.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S.L., Siegel C.S. Evaluation of direct immunofluorescence, enzyme immunoassay, centrifugation culture, and conventional culture for the detection of respiratory syncytial virus. J Clin Microbiol. 1990;28(11):2394–2397. doi: 10.1128/jcm.28.11.2394-2397.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl S.C., Henrickson K.J., Hua W., Fan J. Evaluation of the hexaplex assay for detection of respiratory viruses in children. J Clin Microbiol. 2001;39(5):1696–1701. doi: 10.1128/JCM.39.5.1696-1701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg J.A. Culture vs direct antigen assays for detection of microbial pathogens from lower respiratory tract specimens suspected of containing the respiratory syncytial virus. Arch Pathol Lab Med. 1991;115:451–458. [PubMed] [Google Scholar]
- Noyola D.E., Demmler G.J. Effect of rapid diagnosis on management of influenza A infections. Pediatr Infect Dis J. 2000;19(4):303–307. doi: 10.1097/00006454-200004000-00008. [DOI] [PubMed] [Google Scholar]
- Reina J., Gonzalez Gardenas M., Ruiz de Gopegui E., Padilla E., Ballesteros F., Mari M. Prospective evaluation of a dot-blot enzyme immunoassay (Directigen RSV) for the antigenic detection of respiratory syncytial virus from nasopharyngeal aspirates of pediatric patients. Clin Microbiol Infect. 2004;10(11):967–971. doi: 10.1111/j.1469-0691.2004.00986.x. [DOI] [PubMed] [Google Scholar]
- Schirm J., Luijt D.S., Pastoor G.W., Mandema J.M., Schroder F.P. Rapid detection of respiratory viruses using mixtures of monoclonal antibodies on shell vial cultures. J Med Virol. 1992;38(2):147–151. doi: 10.1002/jmv.1890380214. [DOI] [PubMed] [Google Scholar]
- Shetty A.K., Treynor E., Hill D.W., Gutierrez K.M., Warford A., Baron E.J. Comparison of conventional viral cultures with direct fluorescent antibody stains for diagnosis of community-acquired respiratory virus infections in hospitalized children. Pediatr Infect Dis J. 2003;22(9):789–794. doi: 10.1097/01.inf.0000083823.43526.97. [DOI] [PubMed] [Google Scholar]
- St. George K., Patel N.M., Hartwig R.A., Scholl D.R., Jollick J.A., Jr., Kauffmann L.M. Rapid and sensitive detection of respiratory virus infections for directed antiviral treatment using R-mix cultures. J Clin Virol. 2002;24(1/2):107–115. doi: 10.1016/s1386-6532(01)00239-6. [DOI] [PubMed] [Google Scholar]
- Templeton K.E., Scheltinga S.A., Beersma M.F.C., Kroes A.C.M., Claas E.C.J. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42(4):1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Brewster L., Clark J., Simoes E ARIVAC consortium. Evaluation of R-mix shell vials for the diagnosis of viral respiratory tract infections. J Clin Virol. 2004;30(1):100–105. doi: 10.1016/j.jcv.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Walker M.L. Evaluation of three immunoassay kits for rapid detection of influenza virus A and B. Clin Diagn Lab Immunol. 2005;12(3):367–370. doi: 10.1128/CDLI.12.3.367-370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg G.A., Erdman D.D., Edwards K.M., Hall C.B., Walker F.J., Griffin M.R. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189:706–710. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
- Woo P.C.Y., Chiu S.S., Seto W.H., Peiris M. Cost-effectiveness of rapid diagnosis of viral respiratory tract infections in pediatric patients. J Clin Microbiol. 1997;35(6):1579–1581. doi: 10.1128/jcm.35.6.1579-1581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R.H., Smith T.F., Waner J.L., Landry M.L. Algorithms for the detection and identification of viruses. In: Murray P.R., Baron E.J., Jorgensen J.H., Pfaller M.A., Yolken R.H., editors. 8th ed. vol. 2. ASM Press; Washington: 2003. pp. 1242–1252. (Manual of clinical microbiology). [Google Scholar]