Abstract
Background
The disease burden caused by recently identified respiratory viruses like HCoV-NL63 is unknown.
Objectives
We determined the burden of disease due to HCoV-NL63 infections using the population-based PRI.DE cohort of children under the age of 3 with lower respiratory tract infections (LRTIs).
Study design
In total 1756 respiratory samples, from hospitalized children or children who visited the outpatient clinic, were tested for HCoV-NL63. Sampling covered a period of 2 years and the frequency of infection in different years was compared to other Western European studies that tested for this virus in 2 or more consecutive years.
Results
Sixty-nine samples were HCoV-NL63 positive, 35 were with high loads, and of these 25 were single HCoV-NL63 infections. Based on the number of children with high HCoV-NL63 infection and no additional infection, the overall annual incidence in outpatients was 7 per 1000 children per year (95% confidence interval (CI) 3–13 per 1000 children per year), which can be extrapolated to an absolute number of 16,929 visits to the physician due to an HCoV-NL63 infection in Germany per year. The estimated hospitalization rate is 22 per 100,000 children (95% CI: 7–49 per 100,000 children per year). This number reflects 522 HCoV-NL63 children in Germany per year. A large year-to-year difference in HCoV-NL63 infection frequency was observed. Combining these data with those of other studies in Western Europe revealed that HCoV-NL63 infections follow a 2-year inter-epidemic period with peaks of infection in the winters of 2000/2001, 2002/2003 and 2004/2005 (p < 0.0001).
Conclusions
HCoV-NL63 infection in children below 3 years of age often requires a visit to the physician in an outpatient clinic, especially during peak-years, but hospitalizations are relatively infrequent.
Abbreviations: LRTI, lower respiratory tract infection; HCoV, human coronavirus; CI, confidence intervals; NPS, nasopharyngeal secretions; RSV, respiratory syncytial virus; PIV, human parainfluenza virus
Keywords: Human coronavirus NL63, Croup, Burden of disease
1. Background
Coronavirus infections of humans are mostly restricted to the respiratory tract. The human coronaviruses were first identified in the mid 1960s (HCoV-229E and HCoV-OC431, 2, 3), but in 2003, 2004 and 2005, three new human coronavirus species have been identified (SARS-CoV, HCoV-NL63 and HCoV-HKU1, respectively4, 5, 6, 7). HCoV-NL63 was first described in a child with bronchiolitis in Amsterdam, the Netherlands,6 but it soon turned out that HCoV-NL63 infection occurs frequently in children,8, 9, 10 is observed around the globe (reviewed in Ref. 11), and infection is associated with croup and acute otitis media.10, 12
2. Objectives
The disease burden caused by respiratory viruses is huge in children, which led to the development of vaccines that can be used in young children. For the new respiratory viruses the disease burden is unknown, therefore we determined the burden of disease due to HCoV-NL63 infections in the population-based study (PRI.DE study) on children under the age of 3 with lower respiratory tract infections (LRTI). The PRI.DE study covers 2 years, and children were recruited in the north, east, south and west of Germany at pediatric practices and referral children's hospitals.13 This study design ascertained representation of the German children as 95% of them have a pediatrician, and – in case of hospitalization – over 95% of them are referred to a children's hospital.
3. Study design
3.1. Participants, materials and laboratory testing
Paediatric practices (outpatients) and hospitals (inpatients) from Hamburg, Bochum, Freiburg and Dresden participated in the study.13 Children with clinical signs of apnoea (under the age of 6 months), laryngotracheitis (croup), bronchitis, bronchiolitis, or pneumonia were included in the study. Signs and symptoms were defined according to Denny and Clyde.14 Recruitment covered the period from November 1999 to October 2001. Nasopharyngeal secretions (NPS) were collected in a standardized manner as described.13 Of the 3.677 NPS that were collected within the PRI.DE study, half were randomly assigned to this study. The randomization was performed via a program generating random numbers in order to distribute samples equally between 2 different laboratories. The ethics committee of each participating center approved the study protocol and written informed consent was obtained from parents of all children. RNA was extracted and tested for RSV, PIV-1, PIV-2, PIV-3, influenza viruses and HCoV-NL63 by hexaplex PCR and real-time RT-PCR as described.10 Samples collected in France (winter 2003/2004) and the Netherlands (2002 till 2005) that were screened for HCoV-NL63 were analyzed as described.6, 15 Furthermore, data from literature were included only for those studies that screened consecutive years in Western Europe and in which each year the sample selection criteria did not change. Thus a year-to-year difference is a reflection of periodicity of infection.
3.2. Incidence calculations
Incidence rates were estimated based on the PRI.DE results,13 i.e. an incidence of lower respiratory tract infections of 28.7 per 100 children per year seen by a doctor, and using HCoV-NL63 infection rates obtained from our analysis. The PRI.DE population at risk was determined using numbers of well-baby visits which are attended regularly.16 These figures were used to estimate total numbers of LRTI cases and HCoV-NL63-LRTI cases in the German pediatric population, based on German census data (population size on December 31st, 1999; data from Statistisches Bundesamt, Statistisches Jahrbuch 2001).
3.3. Hospitalization rates
Figures for the total number of hospitalizations in the German pediatric population were determined by applying the observed proportion of HCoV-NL63-related LRTI cases in the PRI.DE study hospitals to the total number of hospitalizations in 1999 in Germany (data from Statistisches Bundesamt, Statistisches Jahrbuch 2002; interpolation of age groups 0–1 year and 1–5 years).
3.4. Confidence intervals
95% confidence intervals (CIs) for incidence rates and hospitalization rates were obtained by calculating exact 97.5% CIs from the binomial distribution for infection rates and combining these with 97.5% CIs for the hospitalization rate due to LRTI or annual LRTI incidence rate.16
4. Results
4.1. Burden of disease
The PRI.DE study is a prospective population-based study. One can thus calculate the annual incidence rate and the total national disease burden attributable to HCoV-NL63-related LRTIs in children under the age of 3 years. This calculation uses national census data (outpatients) and national hospital statistics (inpatients). In total 1756 PRI.DE samples are included in the present study, representing 48% of the total number of samples that were collected. To confirm whether the HCoV-NL63-tested samples were representative for the entire PRI.DE study (which comprised 3677 children), we checked collection dates, study region, age, male/female distribution, diagnosis, virus detection and determined that in none of these categories there was any bias in sample selection (shown in Table 1, Table 2 ). Of the 1756 children, 685 visited the outpatient clinic, and 1071 were hospitalized.
Table 1.
Comparison of demographic, clinical and virological characteristics of PRI.DE study population and the randomly selected subgroup of the present HCoV-NL63 study.
| Category | PRI.DE study patients | HCoV-NL63-tested patients (%) | HCoV-NL63-positive patients (%) |
|---|---|---|---|
| Study region | |||
| Freiburg | 1350 | 657 (49%) | 24 (3.6%) |
| Dresden | 995 | 490 (49%) | 24 (4.9%) |
| Bochum | 467 | 208 (44%) | 6 (2.9%) |
| Hamburg | 865 | 401 (46%) | 15 (3.7%) |
| Age | |||
| 0 year | 1989 | 936 (47%) | 31 (3.3%) |
| 1 year | 1115 | 535 (48%) | 25 (4.7%) |
| 2 years | 569 | 284 (50%) | 13 (4.6%) |
| M/F | |||
| Male | 2133 | 1015 (48%) | 43 (4.2%) |
| Female | 1533 | 734 (48%) | 26 (3.5%) |
| Diagnosis | |||
| Croup | 290 | 134 (46%) | 18 (13.4%)* |
| Bronchitis | 851 | 423 (50%) | 18 (4.3%) |
| Bronchiolitis or wheezing bronchitis | 2054 | 965 (47%) | 31 (3.2%) |
| Pneumonia | 853 | 398 (47%) | 8 (2.0%) |
| Apnoea | 80 | 37 (46%) | 0 (0.0%) |
| Virus detected | |||
| RSV A/B | 1247 | 545 (43%) | 28 (5.1%) |
| PIV 1/2/3 | 377 | 194 (51%) | 6 (3.1%) |
| INF A/B | 141 | 63 (45%) | 0 (0.0%) |
p < 0.000 two-sided Fisher's exact test.
Table 2.
HCoV-NL63 analysis on PRI.DE samples.
| Month of sampling | PRI.DE samples collected | HCoV-NL63 study patients (% of PRI.DE study) | Nr (%) of samples positive for HCoV-NL63 |
|---|---|---|---|
| November 1999 | 162 | 69 (43%) | 1 (1.4%) |
| December 1999 | 213 | 90 (42%) | 1 (1.1%) |
| January 2000 | 307 | 130 (42%) | 3 (2.3%) |
| February 2000 | 292 | 139 (47%) | 3 (2.2%) |
| March 2000 | 347 | 156 (45%) | 0 (0%) |
| April 2000 | 151 | 73 (48%) | 2 (2.7%) |
| May 2000 | 83 | 41 (49%) | 0 (0%) |
| June 2000 | 36 | 15 (42%) | 0 (0%) |
| July 2000 | 28 | 13 (46%) | 0 (0%) |
| August 2000 | 44 | 20 (45%) | 0 (0%) |
| September 2000 | 82 | 40 (49%) | 0 (0%) |
| October 2000 | 103 | 51 (50%) | 0 (0%) |
| November 2000 | 215 | 108 (50%) | 9 (8.3%) |
| December 2000 | 279 | 134 (48%) | 18 (13.4%) |
| January 2001 | 344 | 171 (49%) | 12 (7.0%) |
| February 2001 | 227 | 114 (50%) | 13 (11.4%) |
| March 2001 | 205 | 107 (52%) | 4 (3.7%) |
| April 2001 | 148 | 75 (51%) | 0 (0%) |
| May 2001 | 95 | 41 (43%) | 1 (2.4%) |
| June 2001 | 65 | 31 (48%) | 0 (0%) |
| July 2001 | 45 | 24 (53%) | 0 (0%) |
| August 2001 | 22 | 11 (50%) | 0 (0%) |
| September 2001 | 73 | 35 (48%) | 1 (2.9%) |
| October 2001 | 111 | 68 (61%) | 1 (1.5%) |
In total we detected 69 HCoV-NL63 infections (3.9%). Of these, 38 were children visiting the outpatient clinic (5.5% of outpatients) and 31 children were hospitalized (2.9% of all hospitalized patients). Similar to what was published previously10 a high frequency of croup was noted in the HCoV-NL63 infected children (26.1% of all HCoV-NL63 positives, p < 0.0001 two-sided Fisher's exact test).
The peak of infection was centered around the winter months of each year (Fig. 1 ). When we include all HCoV-NL63 infections (n = 69) to calculate the burden of disease, a total of 16 visits to the physician per 1000 children per year were calculated, corresponding to 37,841 visits to a physician per year in Germany. The hospitalization rate is 85 per 100,000 children per year, corresponding to 2022 hospitalizations per year in Germany. However, during double infections it is debatable whether one or the combination of the two viruses causes the disease encountered. Double infections with HCoV-NL63 and a second respiratory virus were frequently found (49.3%). Furthermore we observed a very strong association between a high HCoV-NL63 viral load and single infections, and in case of double infections the viral load was low (p < 0.0001, Wilcoxon's two sample test).10 To ascertain that the disease burden is calculated for HCoV-NL63 infections only and not influenced by infections with another respiratory virus we included only those infections which were not accompanied by a second respiratory virus (n = 35). Furthermore we included merely HCoV-NL63 infections with high viral load, which was arbitrarily defined as samples with more than 10,000 copies per ml (n = 25). Using these restrictions we calculated that the annual incidence of HCoV-NL63 infection was 7 per 1000 children per year (95% confidence interval (CI) 3–13 per 1000 children per year), reflecting an absolute number of 16,929 visits to the physician in Germany per year (95% CI: 7255–31,440). The estimated hospitalization rate due to HCoV-NL63 alone is 22 per 100,000 children (95% CI: 7–49 per 100,000 children per year). This number reflects 522 children in Germany per year (95% CI: 166–1163), which is a conservative estimate.
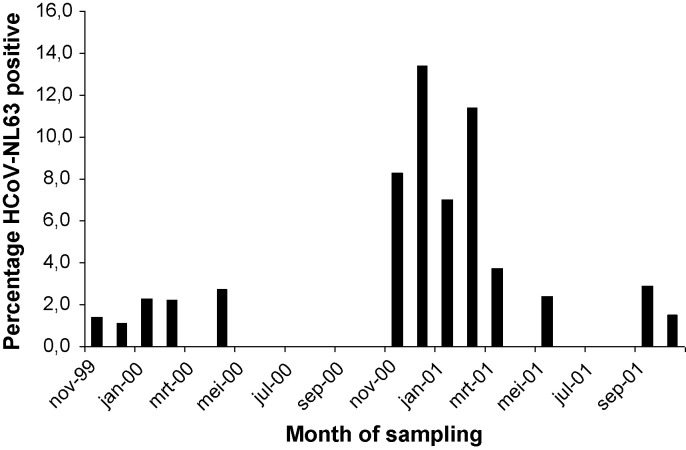
Fig. 1.
Percentage HCoV-NL63 positive samples in the PRI.DE study.
4.2. Periodicity of infection
The winter season is the preferred season for infection by HCoV-NL63, yet large year-to-year differences in frequency is apparent. As shown in Fig. 1, infection by HCoV-NL63 is limited to a small percentage in the first year of the study, whereas in the winter of 2000/01 the frequency reaches levels above 10%. This indicates that there is an inter-epidemic period,17 but it cannot be determined whether HCoV-NL63 peaks every 2, 3 or more years. To investigate this periodicity and the inter-epidemic period we combined literature data and performed additional screening for HCoV-NL63 in hospitalized patients and outpatients with acute respiratory infections in the Netherlands and France during the period 2001 till 2005. A higher frequency of infection in the winters of 2000/01, 2002/03 and 2004/05 and less infections in the 2001/02 and 2003/04 winter was observed (Table 3 and 15, 18, 19), indicating that the frequency is high every other year. To verify whether the inter-epidemic period is 2 years we combined all our data and the published data on HCoV-NL63 screenings during 2 or more consecutive years in Western Europe6, 19, 20, 21, 22, 23 and confirmed that in 2000/2001, 2002/2003 and 2004/2005 significantly higher infection rates are observed compared to 1999/2000, 2001/2002 and 2003/2004 (combined rates 4.5% vs 1.5%, p < 0.0001, Chi-square test, Fig. 2 ).
Table 3.
Overview of HCoV-NL63 screening in Western European studies covering 2 or more consecutive years.
| Country | Years testeda | Samples tested | Samples positive | Percentage positive | Reference |
|---|---|---|---|---|---|
| The Netherlands | 2001 | 85 | 4 | 4.71% | 19 |
| 2002 | 54 | 0 | 0.00% | 19 | |
| 2003 | 578 | 9 | 1.56% | 6, this study | |
| 2004 | 40 | 0 | 0.00% | 6, this study | |
| 2005 | 258 | 6 | 2.33% | 6, this study | |
| Belgium | 2003 | 233 | 6 | 2.58% | 22 |
| 2004 | 70 | 1 | 1.43% | 22 | |
| Switzerland | 2000 | 22 | 1 | 4.55% | 21 |
| 2001 | 28 | 5 | 17.86% | 21 | |
| 2002 | 10 | 0 | 0.00% | 21 | |
| 2003 | 16 | 0 | 0.00% | 21 | |
| 2004 | 22 | 1 | 4.55% | 21 | |
| 2005 | 22 | 2 | 9.09% | 21 | |
| 2006 | 2 | 0 | 0.00% | 21 | |
| Germany | 2000 | 726 | 10 | 1.38% | This study |
| 2001 | 916 | 57 | 6.22% | This study | |
| 2002 | 114 | 2 | 1.75% | This study | |
| Sweden | 2004 | 116 | 1 | 0.86% | 23 |
| 2005 | 106 | 11 | 10.38% | 23 | |
| France | 2003 | 300 | 28 | 9.33% | 18 |
| 2004 | 200 | 4 | 2.00% | This study | |
| 2005 | 1002 | 33 | 3.29% | 15 | |
A year is defined from August to July, e.g. year 2000 is the period between August 1999 till July 2000.
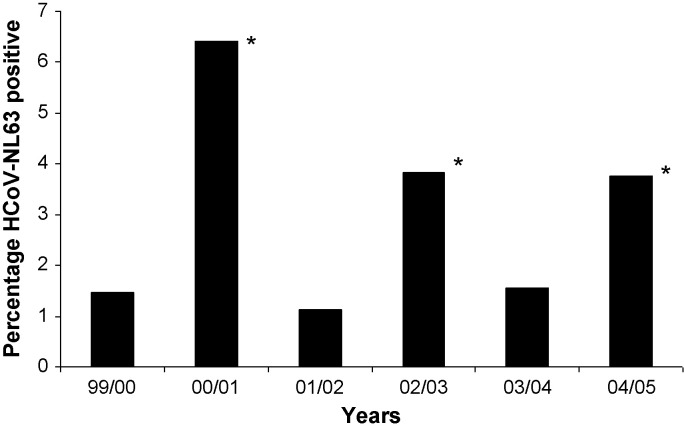
Fig. 2.
Percentage HCoV-NL63 positive samples in Western Europe, the included studies are described in Table 3. A year is defined from August to July. *: significantly higher percentage compared to years 99/00, 01/02 and 03/04, p < 0.0001, Chi-square test.
We found no significant differences when comparing 1999/2000, 2001/2002 and 2003/2004 (rates 1.5%/1.1%/1.6%, p = 0.92, Chi-square test with 2df), but modest differences were noticeable when comparing 2000/2001, 2002/2003 and 2004/2005 (rates 6.4%/3.8%/3.8%, p = 0.003, Chi-square test with 2df).
5. Discussion
In Germany paediatricians are available for all children and they are consulted instead of general practitioners for primary care. Therefore, sampling in paediatric practices provides the opportunity to collect a representative patient set that reflects the population of German children that are ill but not hospitalized. Most studies that have looked at the frequency of HCoV-NL63 infection in patients with respiratory infections have focused exclusively at hospitalized patients. In the PRI.DE study it is possible to investigate the frequency of infection in both groups: those hospitalized and those visiting the paediatric clinic (outpatients). In a previous study we noticed that HCoV-NL63 infection is observed more frequently in outpatients,10 and here we calculated that it reflects almost 17,000 visits to the paediatrician each year in Germany, whereas less than 600 children under the age of 3 years need to be hospitalized.
There are only 2 previous studies that calculated the incidence of HCoV-NL63 infection, one from Hong Kong and one from Nashville, USA.12, 24 The Hong Kong study noticed high numbers of hospitalizations due to HCoV-NL63 infection (210 hospital admissions per 100,000 children ≤6 years of age) whereas we estimate 22 hospitalizations per 100,000 children below the age of 3. The difference in age of the test populations is unlikely to explain the difference. We think that other reasons are more significant. In the Hong Kong study all HCoV-NL63-children were included regardless of the viral load in the NPA, or the presence of a second infection. Furthermore, only a single year was monitored in Hong Kong, and we noticed that the incidence of HCoV-NL63 displays year-to-year differences with a 3–4 times higher rate in uneven winters in Western Europe. We did not include any double infections and HCoV-NL63 infections with low viral load, to diminish the chance that children who are ill due to another respiratory virus infection are counted. In addition, it cannot be ruled out that we even missed some double infections since our samples were not tested for all respiratory pathogens (e.g. hMPV, human bocavirus, and rhinoviruses).
The Nashville study included children with LRTI from several years and a high incidence of HCoV-NL63 in the 6–23 months age group was observed: 12.3 HCoV-NL63 associated LRTIs per 1000 children, although the number of samples that were tested was small (only 5 positives of the 119 samples analyzed).12 These data are in concordance with our findings as most of the children in their study were not hospitalized, therefore the incidence numbers are comparable to what we noticed for outpatients (7 per 1000 children).
The periodicity of infection that we observed is not unexpected. Already in the mid-1970s Monto & Lim described high infection rates with HCoV-OC43 in the years 1966, 1968 and 1969, but low numbers in 1967.25 Actually many childhood diseases tend to vary in frequency between years. These oscillations are often of a regular nature, tending to rise as a function of susceptible persons.17 We noticed that the inter-epidemic period is 2 years with 2000/2001, 2002/2003 and 2004/2005 being strong years.
In our study we tested for HCoV-NL63. Taking into account that infection by HCoV-OC43, HCoV-229E, and HCoV-HKU1 can also result in LRTI,26, 27 the incidence numbers for coronavirus infection are probably several folds higher than the numbers we present for just HCoV-NL63 infections. It would be of great interest to determine the incidence of the other coronaviruses in the sample set tested for HCoV-NL63.
6. Conflict of interest and acknowledgements
LvdH, MdV and RD are supported by VIDI Grant 016.066.318 from the Netherlands Organization for Scientific Research (NWO) and by the sixth framework grant LSHM-CT-2006-037276 from the European Union. Financial support for PRI.DE was received from Wyeth Pharma, Münster, Germany. The Center for Clinical Trials receives funding from the German Federal Ministry of Education and Research (BMBF).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, all authors declare that they have no conflict of interest. We would like to thank all patients, their parents and the staff at all participating centers.
References
- 1.Tyrrell D.A.J., Bynoe M.L. Cultivation of novel type of common-cold virus in organ cultures. Br Med J. 1965;1:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 3.McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. 1967;57:933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 5.Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 6.van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C. Identification of a new human coronavirus. Nat Med. 2004;10(4):368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkman R., Jebbink M.F., El Idrissi N.B., Pyrc K., Müller M.A., Kuijpers T.W. Human coronavirus NL63 and 229E seroconversion in children. J Clin Microbiol. 2008;46(7):2368–2373. doi: 10.1128/JCM.00533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao X., Guo X., Esper F., Weibel C., Kahn J.S. Seroepidemiology of group I human coronaviruses in children. J Clin Virol. 2007;40(3):207–213. doi: 10.1016/j.jcv.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Hoek L., Sure K., Ihorst G., Stang A., Pyrc K., Jebbink M.F. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005;2(8):e240. doi: 10.1371/journal.pmed.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Hoek L. Human coronaviruses: what do they cause? Antivir Ther. 2007;12(4 Pt B):651–658. [PubMed] [Google Scholar]
- 12.Talbot H.K., Shepherd B.E., Crowe J.E., Jr., Griffin M.R., Edwards K.M., Podsiad A.B. The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr Infect Dis J. 2009;28(8):682–687. doi: 10.1097/INF.0b013e31819d0d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster J., Ihorst G., Rieger C.H., Stephan V., Frank H.D., Gurth H. Prospective population-based study of viral lower respiratory tract infections in children under 3 years of age (the PRI.DE study) Eur J Pediatr. 2004;163(12):709–716. doi: 10.1007/s00431-004-1523-9. [DOI] [PubMed] [Google Scholar]
- 14.Denny F.W., Clyde W.A., Jr. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986;108(5 Pt 1):635–646. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- 15.Vabret A., Dina J., Gouarin S., Petitjean J., Tripey V., Brouard J. Human (non-severe acute respiratory syndrome) coronavirus infections in hospitalised children in France. J Paediatr Child Health. 2008;44(4):176–181. doi: 10.1111/j.1440-1754.2007.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ihorst G., Forster J., Petersen G., Schumacher M. Determination of the population size in an epidemiological study with children. Methods Inf Med. 2004;43(5):479–482. [PubMed] [Google Scholar]
- 17.Anderson R.M., May R.M. Directly transmitted infections diseases: control by vaccination. Science. 1982;215(4536):1053–1060. doi: 10.1126/science.7063839. [DOI] [PubMed] [Google Scholar]
- 18.Vabret A., Mourez T., Dina J., van der Hoek L., Gouarin S., Petitjean J. Human coronavirus NL63, France. Emerg Infect Dis. 2005;11(8):1225–1229. doi: 10.3201/eid1108.050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouchier R.A., Hartwig N.G., Bestebroer T.M., Niemeyer B., de Jong J.C., Simon J.H. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci USA. 2004;101(16):6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser L., Regamey N., Roiha H., Deffernez C., Frey U. Human coronavirus NL63 associated with lower respiratory tract symptoms in early life. Pediatr Infect Dis J. 2005;24(11):1015–1017. doi: 10.1097/01.inf.0000183773.80217.12. [DOI] [PubMed] [Google Scholar]
- 21.Regamey N., Kaiser L., Roiha H.L., Deffernez C., Kuehni C.E., Latzin P. Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J. 2008;27(2):100–105. doi: 10.1097/INF.0b013e31815922c8. [DOI] [PubMed] [Google Scholar]
- 22.Moës E., Vijgen L., Keyaerts E., Zlateva K., Li S., Maes P. A novel pancoronavirus RT-PCR assay: frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium. BMC Infect Dis. 2005;5(1):6. doi: 10.1186/1471-2334-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koetz A., Nilsson P., Linden M., van der Hoek L., Ripa T. Detection of human coronavirus NL63, human metapneumovirus and respiratory syncytial virus in children with respiratory tract infections in south-west Sweden. Clin Microbiol Infect. 2006;12(11):1089–1096. doi: 10.1111/j.1469-0691.2006.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu S.S., Chan K.H., Chu K.W., Kwan S.W., Guan Y., Poon L.L. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40(12):1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monto A.S., Lim S.K. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J Infect Dis. 1974;129(3):271–276. doi: 10.1093/infdis/129.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Elden L.J., van Loon A.M., van Alphen F., Hendriksen K.A., Hoepelman A.I., van Kraaij M.G. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J Infect Dis. 2004;189(4):652–657. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo P.C., Lau S.K., Tsoi H.W., Huang Y., Poon R.W., Chu C.M. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005;192(11):1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]