Abstract
Aims
To determine whether influenza can trigger heart attacks, we investigated the impact of influenza epidemics on autopsy-proven coronary deaths.
Methods and results
We studied weekly death due to acute myocardial infarction (AMI) and chronic ischaemic heart disease (IHD) in autopsies conducted in 1993 to 2000 in St Petersburg, Russia. We plotted the weekly acute respiratory disease (ARD) counts and influenza epidemics against AMI and chronic IHD deaths. There were 11 892 subjects dying of AMI and 23 000 subjects dying of chronic IHD. Median age was 75 for women and 65 for men. In every year, a peak of AMI and chronic IHD deaths were present and coincided with the influenza epidemic and peak ARD activity. A similar pattern was seen for each subgroup of men, women, subjects 50 years or older, and subjects 70 years or older. When comparing the average influenza epidemic weeks to average off-season weeks, the odds for AMI and chronic IHD death increased by 1.30 (95% confidence interval (CI): 1.08–1.56) and 1.10 (95% CI: 0.97–1.26), respectively.
Conclusion
Influenza epidemics are associated with a rise in autopsy-confirmed coronary deaths. Influenza vaccination should be advocated for patients at high risk of developing cardiovascular events.
Keywords: Influenza, Myocardial infarction, Trigger, Cardiac death, Infection, Epidemiology
Introduction
Influenza epidemics are associated with significant morbidity and mortality in both developed and developing countries.1 Recent reports from the United States have shown that death rate attributed to influenza is increasing and is now the fourth major cause of mortality in USA.2 In fact, the death toll due to influenza may be even higher than this number.3 The reported death rate attributed to influenza is mainly based on the study of death certificates which generally underestimate deaths due to influenza and often misclassify the clinical outcomes as well.4
Influenza and other upper respiratory infections may trigger cardiovascular events by causing an acute and severe inflammatory state in the body which can lead to destabilization and rupture of atherosclerotic plaques.5–8
From our own observational study, and a closer examination of published trials, we estimated that influenza causes up to 92 000 deaths per year in US only through triggering of fatal myocardial infarctions.3 The influenza attack rate in a typical year is 10–20% and, given the high burden of symptomatic and asymptomatic coronary artery disease, many of these cases occur in subjects with coronary heart disease (CHD).9 This attack rate (and in many cases, the severity of the subsequent disease) will be even higher during epidemics and pandemics. Accordingly, it has been noted that during influenza epidemics and pandemics (except for 1918 Spanish flu pandemic), roughly twice as many subjects die of cardiac causes rather than from influenza pneumonia.10
Here, we report our investigation of the impact of influenza epidemics on CHD deaths in St Petersburg, Russia, over a span of 8 years. Unlike the previous studies which relied mainly on death certificates, we selected fatal cases with autopsy-proven CHD as the cause of death. Moreover, by studying a population in which at the time of study only a minority were receiving influenza vaccine and statin drugs, we were able to study the natural history. Determining the effect of influenza epidemics on CHD death is especially important in light of the looming threat of a new global pandemic.10
Study population
We studied weekly mortality due to CHD from 1993 to 2000 in residents of 10 districts of St Petersburg, Russia. Data were provided as number of cases of acute respiratory disease (ARD) reported by the Public Health Service in St Petersburg, and the number of autopsy-confirmed cases of coronary deaths. Influenza epidemics were determined by the Sanitary-Epidemiologic Center based on the data of St Petersburg's Influenza Research Center Epidemics were defined as when the weekly ARD morbidity exceeded the pre-defined epidemic thresholds. Influenza A (H3N2) was the prevailing virus strain in all years except for influenza season 1994–95 when an influenza B strain was the prevailing circulating virus strain. An influenza A (H1N1) virus strain was also circulating in years 1995–96, 1997–98, and 1999–2000 (see Supplementary Data).
The study included a total number of 34 892 subjects aged 30–89 years. The study population covered 10 of 12 central districts of St Petersburg, covering about 2 195 100 persons aged 30 or older (69.6% of the whole city population). The autopsy rate for in-hospital and out-of-hospital fatal cases in St Petersburg exceeds 70%. The overall autopsy rate did not change significantly over the study period.
Causes of death were defined based on the ICD-10 coding criteria. Patients were divided into two groups: the first group included 11 892 subjects who died of acute myocardial infarction (AMI: ICD codes I21, I22, and I23). The second group included 23 000 subjects dying of chronic ischaemic heart disease (chronic IHD: ICD-10 code I25). The study protocol was approved by the institutional review board of the University of Texas Health Science Center at Houston.
Statistical methods
Cases were arrayed according to age group (decade from 30–89 years) and gender, and were totalled by week for each of 417 weeks (from 1993 to 2000). Data for the first week of January 1993 were not available for analysis.
Indicator variables were created to represent epidemic periods when reported weekly influenza cases exceeded an expected threshold value calculated by the St Petersburg Federal Center for Influenza Surveillance, and non-epidemic periods were defined as periods when the number of influenza cases were below the epidemic threshold. We compared the average within-epidemic coronary mortality to the average off-epidemic mortality to quantify the association between influenza epidemic activity and CHD mortality.
The first stage of analysis involved plotting the weekly counts of respiratory morbidity and AMI mortality in both raw and smoothed forms. Smoothed plots of heart disease mortality cycling with influenza case volume across the 8 year study period were produced using cubic spline interpolation. Splines were computed and data plotted using SAS Graph software version 9.13. Analyses were performed separately for myocardial infarction mortality (AMI) and chronic IHD mortality.
Odds ratios were computed within each year's epidemic cycle using stratified analysis, and results were summarized across the 8 years of the study by the Mantel–Haenszel technique using SAS software version 9.13.
The raw data were then subjected to time series cross-correlation analysis to evaluate correlation between AMI mortality and respiratory morbidity associated with time lag over the period of observation. Cross-correlations describing the time-based correlation of influenza activity and heart disease mortality were computed using NCSS 2004 software.
Heart disease mortality was further analysed using time series decomposition forecasting to investigate seasonality as an element of long-range trend. We used the classical time series decomposition forecasting model, which separates time series into five major components: mean, trend, season, cycle, and randomness (error). The model is of the simple multiplicative form: Expected value=mean × trend × seasonality × cycle × error. We estimated expected values of periodic mortality counts for four seasonal periods beyond the data ending in 2000. Time series decomposition models were fitted using NCSS 2004 software.
All tests were conducted two-sided when applicable.
Results
There were 11 892 subjects dying with AMI (47.8% men vs. 52.2% women) and 23 000 subjects dying of chronic IHD (40.1% men vs. 59.9% women). The elderly comprised the majority of subjects; women were older than men (median age: 75 and 65, respectively). The influenza epidemics lasted for an average of 2.9 ± 0.8 months (range: 2 to 4 months).
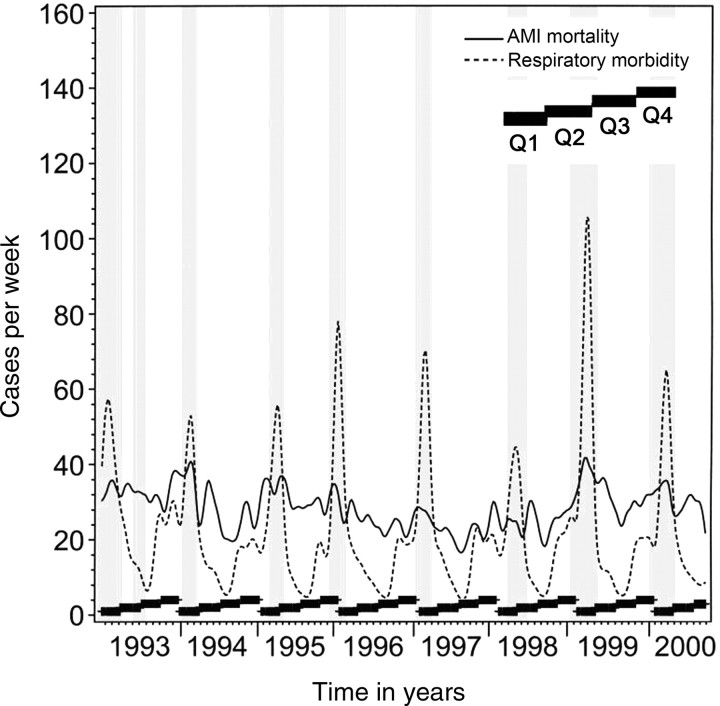
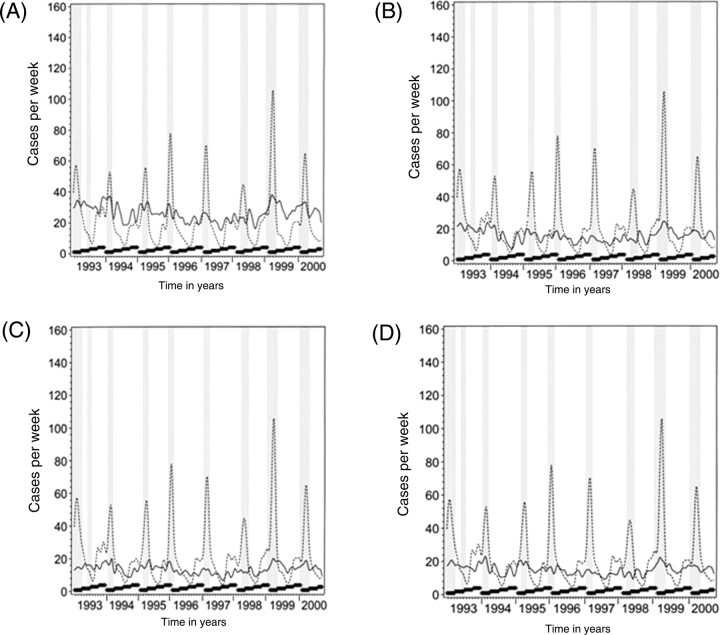
The smoothed data plots of respiratory morbidity vs. AMI mortality for the entire cohort and population subset (i.e. ≥ 50 years, ≥ 70 years, men, and women) are shown in Figures 1 and 2, respectively. Wide fluctuations in respiratory morbidity are apparent and are highly seasonal, as expected. AMI mortality fluctuates less, but appears to track respiratory morbidity closely. Chronic IHD plots followed the same pattern (data not shown). For almost every influenza epidemic, there is a rise in the number of coronary deaths. The peaks in AMI and chronic IHD are coincident with the influenza epidemic and the height of ARD activity in all years (except for 1998 where the ARD activity as depicted by the height of the related curve was milder compared with other years). Very similar patterns could be observed for subgroups of subjects 50 years or older, subjects 70 years or older, men, and women.
Figure 1.
Smoothed data plot of deaths due to acute myocardial infarction and morbidity from acute respiratory disease from 1993 to 2000 in the whole study population. The continuous line depicts acute myocardial infarction mortality and the dashed line indicates acute respiratory disease morbidity and grey columns indicate influenza epidemic periods. The thick black ladders at the bottom of each year's plot indicate the four calendar quarters of Q1, Q2, Q3, and Q4.
Figure 2.
Smoothed data plots of deaths due to acute myocardial infarction and morbidity from acute respiratory disease from 1993 to 2000 in subjects aged 50 or older (A), and aged 70 or older (B), in men (C) and women (D). The continuous line depicts acute myocardial infarction mortality and the dashed line indicates acute respiratory disease morbidity and grey columns indicate influenza epidemic periods. The thick black ladders at the bottom of each year's plot indicate the four seasons in the order of calendar quarters Q1, Q2, Q3, and Q4.
Influenza epidemics were associated with increases in cardiac mortality (see Supplementary Data). We estimated the odds of death comparing the average of the epidemic weeks to the average of non-epidemic weeks. The odds for AMI and chronic IHD were 1.30 (95% CI: 1.08–1.56) and 1.10 (95% CI: 0.97–1.26), respectively (see Supplementary Data).
The cross-correlation plots for acute respiratory morbidity and coronary deaths are shown in Figure 3 for the AMI and chronic IHD. Cross-correlation analysis shows that for any peak ARD activity, peak correlations with CHD are found near time zero, within an approximately 2 week peak window on either side, and the correlation steadily fades away in about 10 weeks. This indicates that as respiratory morbidity begins to rise in the population, so does coronary mortality, and as respiratory morbidity declines, coronary mortality also declines. Of note is that correlations are most negative (i.e. respiratory morbidity and coronary mortality are least correlated) at lags of ±24–26 weeks—about 6 months off from peak. Weaker positive correlations are present at about ±52 weeks, indicating that AMI and chronic IHD mortality at any given peak time echoes ARD activity during peak times across multiple years.
Figure 3.
Time series cross-correlation of acute respiratory disease morbidity and acute myocardial infarction mortality. Peak correlations are near time zero, within an approximately 2 week peak window on either side, and a decline of about 10 weeks duration.
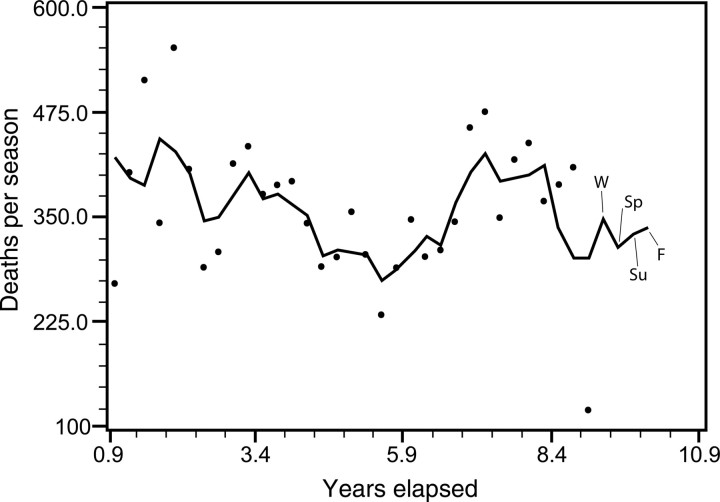
Time series decomposition yields predictable AMI mortality estimates that closely mirror the seasonal cycling of the data (Figure 4). The model can forecast future seasonal mortality 1 year beyond the actual data. Forecasts are 348 AMI deaths for winter, 315 for spring, 329 for summer, and 337 for fall. This indicates that, after removal of the mean and random error, a seasonal periodicity is identified that is predictable from trend, season, and underlying cycle. A similar pattern could be seen for chronic IHD death, except that the peak is at the beginning of the spring rather than the end of the winter. Estimates are 334 deaths for winter, 348 for spring, 314 for summer, 333 for fall.
Figure 4.
Time series decomposition forecast of total seasonal acute myocardial infarction mortality shows that a seasonal periodicity is predictable from trend, season, and underlying cycle. The period beyond the dots shows forecasted future seasonal mortality 1 year beyond the actual data. The predictions are for winter (W), spring (SP), summer (SU), and fall (F) projecting into the hypothetical year 10.
Discussion
We have observed that winter peaks in ARDs and presence of influenza epidemics are associated with an increase in autopsy-confirmed death due to myocardial infarction and chronic IHD. The effect of influenza epidemics on AMI and chronic IHD death was observed during multiple years of study and a rise in AMI and chronic IHD death could be anticipated during influenza epidemics. This effect was observed in both genders at all age groups. Cardiac mortality was associated with epidemic-level respiratory morbidity in the community (in the cross-correlation analysis) and with season (in the time series decomposition). Both of these constitute circumstantial evidence that endemic influenza may play a role in cardiac mortality burden.
A novelty of our current study is the reliance on autopsy results for determination of cause of death. Most of the previous studies have used death certificates as the main source of determining the clinical outcomes. However, the use of death certificate statistics is widely known to be inaccurate for this purpose.4,11 Physicians whose patients have had influenza followed by fatal myocardial infarction generally report the deaths as due to myocardial infarction secondary to traditional risk factors, but neglect to list influenza on the death certificate.3,10 Conversely, in patients with influenza and pneumonia, AMI may be missed as findings of dyspnoea, chest pain, fever, hypoxia, and leucocytosis are attributed to pneumonia alone. By using autopsy reports, we could also differentiate between acute and chronic coronary deaths.
Influenza as a trigger of myocardial infarction
We and others have hypothesized that influenza can trigger AMI.3,5–7,12 We have previously estimated that influenza causes up to 92 000 deaths per year in US by triggering AMI.3 Interestingly, a recent analysis from CDC concluded that influenza is a major cause of death in the elderly population of US, causing up to 51 000 all-cause deaths per year in US,2 a much higher estimate than the previous estimates of 22 000 excess deaths.13 More than 36 000 deaths in this study were due to respiratory and circulatory deaths.2
Epidemics of influenza and cardiovascular death
We described an association of influenza vaccination with reduced risk of non-fatal MI in 2000 and several, except one, subsequent studies have confirmed the finding, but there has been no large randomized trial or autopsy study.14–20 Interestingly, we found data supporting such an association in several old reports. For example, Collins21 reviewed the mortality data from 35 cities from 1917 through 1929 and showed that in almost all epidemics (except for the 1918 pandemic), there was a rise in death due to organic heart disease. Other authors have noticed that death due to cardiovascular causes comprises more than half of the excess deaths during influenza epidemics22 and often exceeds death due to respiratory causes.23,24
Seasonality and the winter excess mortality due to cardiovascular diseases
Multiple studies have shown an excess number of cardiovascular deaths during the winter season.25,26 Although several studies have suggested a role for low temperature in causing the excess winter deaths, other reports have failed to find such relationship, and a recent report which has considered multiple factors has shown that the winter rise in mortality is most likely due to a single cause, namely influenza.27,28 Our data further support the role of influenza in causing the excess winter mortality.
Secular relation between influenza activity and cardiovascular deaths
In clinical studies, the risk for AMI is highest about 3–5 days after influenza or ARD and gradually declines afterwards to vanish within a month.6,7 Collins21 reported a peak in death due to organic heart disease coinciding with practically every epidemic. Other population studies also reported the peak of influenza activity and cardiovascular deaths to be either almost coincident,29 or a week apart.30 A more recent population study found no lag between the peak in respiratory or circulatory deaths and peak incidence of respiratory illness episodes on the community.4 We found a similar pattern of timing between influenza activity and coronary deaths in our population study. In our cross-correlation analysis, for any peak ARD activity, we found peak coronary event correlations at near time zero, within an approximately 2 week peak window on either side. The correlation between ARD and CHD death decreased steadily to zero in about 10 weeks. Therefore, while in clinical studies influenza activity precedes AMI by about a week, in population studies, they may be coincident in time or the relation may even reverse (raising the question of whether AMI patients become more susceptible to influenza, or whether there is a common trigger such as cold, stress, crowding, or pollution); however, these two activities are almost always seen in a slim (1–2 weeks) window of time. The major source for the variation of the relation in large population studies seems to be the variances in reporting methods. These include delays in reporting culture data or death certificates and other variations in the sources of data which are even more different among countries.
Strengths and limitations
Our study, unlike the previous ones, relied on autopsy data; this minimizes bias due to misclassification of the disease. We selected St Petersburg, Russia, because of its very high autopsy rate (close to 70%) and the low rate of vaccine use at the time of study (<3%). Low use of influenza vaccine and statins (<3%) at the time of our study minimizes the potential therapeutic biases and makes our study closer to a natural history. In contrast, these biases would be much more common in studies conducted in the past 5–10 years in the US where a substantial portion of the population at risk would have been receiving influenza vaccine and several cardioprotective drugs; unless one focuses on the less-treated groups (the poor, urban, and minorities). Our study had several limitations. We did not have access to person-specific data on medications, risk factors, or autopsy diagnoses of pneumonia–influenza. We did not have the meteorological data to assess their impact on influenza activity and cardiac mortality and it was out of the scope of this study. However, other groups have shown that even after considering all such parameters, influenza remains a major determinant of winter excess mortality.27
Implications for the emerging influenza pandemic
Our findings have special importance in light of concerns about a pending influenza pandemic. In the majority of influenza epidemics and pandemics (except for the 1918 Spanish flu pandemic), cardiovascular death surpassed other causes of mortality, including superimposed pneumonia.21 During influenza pandemics, patients with CHD may be considered among the priority groups for receipt of vaccine. Meticulous use of plaque-stabilizing agents such as statins, beta-blockers, aspirin, and angiotensin-converting enzyme inhibitors should offer additional protection during an influenza outbreak.21 Also, a substantial number of myocardial infarctions (especially in women) present with atypical symptoms and the diagnosis can easily be missed in the setting of acute influenza and pneumonia. For example, one case series report of SARS patients showed that 40% of victims in that study died of AMI.31
Conclusions
Influenza epidemics are associated with increases in cardiac death due to CHD. Recognition of influenza as a trigger for acute coronary events calls for more intensive efforts to increase the vaccination rate in subjects at risk of CHD. This may be especially important in an influenza pandemic when a high mortality may be observed in the elderly and those affected with CHD or multiple coronary risk factors.10 Vigorous use of cardioprotective medications such as statins during pandemics may prevent many deaths, as CHD is the major cause of death in a substantial proportion of influenza victims. The role of influenza in cardiovascular disease is neglected in the cardiology textbooks and the mainstream literature. A search in PubMed with keywords of influenza and myocardial infarction at the time of preparing this manuscript yielded only a small number of papers while there were thousands of papers on traditional CHD risk factors. The benefit of influenza vaccination for cardiovascular patients was recently recognized by the American Heart Association and American College of Cardiology.32 We suggest that, based on the available data, the European Society of Cardiology adopts a similar policy and recommends influenza vaccine for European patients with CHD. The vaccine may be even more beneficial for the underprivileged subjects who are not taking all the recommended cardioprotective medications due to their high cost (unlike influenza vaccine) or lack of access to proper medical care.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgement
The authors wish to thank Dr Anushayanthan Alfred for his assistance in preparation of this manuscript.
Conflict of interest: none declared.
References
- 1.Couch RB. Prevention and treatment of influenza. N Engl J Med. 2000;343:1778–1787. doi: 10.1056/NEJM200012143432407. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Madjid M, Naghavi M, Litovsky S, Casscells SW. Influenza and cardiovascular disease: a new opportunity for prevention and the need for further studies. Circulation. 2003;108:2730–2736. doi: 10.1161/01.CIR.0000102380.47012.92. [DOI] [PubMed] [Google Scholar]
- 4.Fleming DM, Cross KW, Pannell RS. Influenza and its relationship to circulatory disorders. Epidemiol Infect. 2005;133:255–262. doi: 10.1017/s0950268804003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spodick DH, Flessas AP, Johnson MM. Association of acute respiratory symptoms with onset of acute myocardial infarction: prospective investigation of 150 consecutive patients and matched control patients. Am J Cardiol. 1984;53:481–482. doi: 10.1016/0002-9149(84)90016-x. [DOI] [PubMed] [Google Scholar]
- 6.Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet. 1998;351:1467–1471. doi: 10.1016/s0140-6736(97)11084-4. [DOI] [PubMed] [Google Scholar]
- 7.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 8.Madjid M, Aboshady I, Awan I, Litovsky S, Casscells SW. Influenza and cardiovascular disease: is there a causal relationship? Tex Heart Inst J. 2004;31:4–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Findlay PF, Gibbons YM, Primrose WR, Ellis G, Downie G. Influenza and pneumococcal vaccination: patient perceptions. Postgrad Med J. 2000;76:215–217. doi: 10.1136/pmj.76.894.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madjid M, Casscells SW. Of birds and men: cardiologists' role in influenza pandemics. Lancet. 2004;364:1309. doi: 10.1016/S0140-6736(04)17176-6. [DOI] [PubMed] [Google Scholar]
- 11.Glezen WP, Decker M, Perrotta M. Survey of underlying conditions of persons hospitalized with acute respiratory disease during influenza epidmics in Houston, 1978–1981. Am Rev respir dis. 1987;136:550–555. doi: 10.1164/ajrccm/136.3.550. [DOI] [PubMed] [Google Scholar]
- 12.Bainton D, Jones GR, Hole D. Influenza and ischaemic heart disease—a possible trigger for acute myocardial infarction? Int J Epidemiol. 1978;7:231–239. doi: 10.1093/ije/7.3.231. [DOI] [PubMed] [Google Scholar]
- 13.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87:1944–1950. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naghavi M, Barlas Z, Siadaty S, Naguib S, Madjid M, Casscells W. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation. 2000;102:3039–3045. doi: 10.1161/01.cir.102.25.3039. [DOI] [PubMed] [Google Scholar]
- 15.Siscovick DS, Raghunathan TE, Lin D, Weinmann S, Arbogast P, Lemaitre RN, Psaty BM, Alexander R, Cobb LA. Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol. 2000;152:674–677. doi: 10.1093/aje/152.7.674. [DOI] [PubMed] [Google Scholar]
- 16.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the FLU Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation. 2002;105:2143–2147. doi: 10.1161/01.cir.0000016182.85461.f4. [DOI] [PubMed] [Google Scholar]
- 17.Lavallee P, Perchaud V, Gautier-Bertrand M, Grabli D, Amarenco P. Association between influenza vaccination and reduced risk of brain infarction. Stroke. 2002;33:513–518. doi: 10.1161/hs0202.102328. [DOI] [PubMed] [Google Scholar]
- 18.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 19.Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36:1501–1506. doi: 10.1161/01.STR.0000170674.45136.80. [DOI] [PubMed] [Google Scholar]
- 20.Jackson LA, Yu O, Heckbert SR, Psaty BM, Malais D, Barlow WE, Thompson WW. Influenza vaccination is not associated with a reduction in the risk of recurrent coronary events. Am J Epidemiol. 2002;156:634–640. doi: 10.1093/aje/kwf073. [DOI] [PubMed] [Google Scholar]
- 21.Collins S. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Pub Health Rep. 1932;47:2159–2179. [Google Scholar]
- 22.Eickhoff TC, Sherman IL, Serfling RE. Observations on excess mortality associated with epidemic influenza. JAMA. 1961;176:776–782. doi: 10.1001/jama.1961.03040220024005. [DOI] [PubMed] [Google Scholar]
- 23.Housworth J, Langmuir AD. Excess mortality from epidemic influenza, 1957–1966. Am J Epidemiol. 1974;100:40–48. doi: 10.1093/oxfordjournals.aje.a112007. [DOI] [PubMed] [Google Scholar]
- 24.Scragg R. Effect of influenza epidemics on Australian mortality. Med J Aust. 1985;142:98–102. [PubMed] [Google Scholar]
- 25.Sakamoto-Momiyama M. Changes in the seasonality of human mortality: a medico-geographical study. Soc Sci Med. 1978;12:29–42. [PubMed] [Google Scholar]
- 26.Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol. 1998;31:1226–1233. doi: 10.1016/s0735-1097(98)00098-9. [DOI] [PubMed] [Google Scholar]
- 27.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 28.Laake K, Sverre JM. Winter excess mortality: a comparison between Norway and England plus Wales. Age Ageing. 1996;25:343–348. doi: 10.1093/ageing/25.5.343. [DOI] [PubMed] [Google Scholar]
- 29.Alling DW, Blackwelder WC, Stuart-Harris CH. A study of excess mortality during influenza epidemics in the United States, 1968–1976. Am J Epidemiol. 1981;113:30–43. doi: 10.1093/oxfordjournals.aje.a113063. [DOI] [PubMed] [Google Scholar]
- 30.Perrotta DM, Decker M, Glezen WP. Acute respiratory disease hospitalizations as a measure of impact of epidemic influenza. Am J Epidemiol. 1985;122:468–476. doi: 10.1093/oxfordjournals.aje.a114128. [DOI] [PubMed] [Google Scholar]
- 31.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.