Abstract
Dipeptidyl peptidase-4 (DPP4) is a widely expressed enzyme transducing actions through an anchored transmembrane molecule and a soluble circulating protein. Both membrane-associated and soluble DPP4 exert catalytic activity, cleaving proteins containing a position 2 alanine or proline. DPP4-mediated enzymatic cleavage alternatively inactivates peptides or generates new bioactive moieties that may exert competing or novel activities. The widespread use of selective DPP4 inhibitors for the treatment of type 2 diabetes has heightened interest in the molecular mechanisms through which DPP4 inhibitors exert their pleiotropic actions. Here we review the biology of DPP4 with a focus on: 1) identification of pharmacological vs physiological DPP4 substrates; and 2) elucidation of mechanisms of actions of DPP4 in studies employing genetic elimination or chemical reduction of DPP4 activity. We review data identifying the roles of key DPP4 substrates in transducing the glucoregulatory, anti-inflammatory, and cardiometabolic actions of DPP4 inhibitors in both preclinical and clinical studies. Finally, we highlight experimental pitfalls and technical challenges encountered in studies designed to understand the mechanisms of action and downstream targets activated by inhibition of DPP4.
Introduction
-
Molecular Biology of DPP4
Discovery, genomic organization, and superfamily of related enzymes
Molecular function
Regulation of DPP4 expression
Post-translational modifications of DPP4
-
DPP4 Substrates
Brain natriuretic peptide (BNP)
Erythropoietin
Eotaxin
Gastrin-releasing peptide (GRP)
Glucagon
Glucagon-like peptide-1 (GLP-1)
Glucagon-like peptide-2 (GLP-2)
Glucose-dependent insulinotropic polypeptide (GIP)
Granulocyte colony-stimulating factor (G-CSF)
Granulocyte-macrophage CSF (GM-CSF)
GHRH and IGF-1
High-mobility group box 1 (HMGB1)
Macrophage-derived chemokine (MDC)
Macrophage inflammatory protein-1 α (MIP-1 α), chemokine (C-C motif) ligand 3-like 1 (CCL3L1), or LD78β
Oxyntomodulin
Pituitary adenylate cyclase-activating polypeptide (PACAP)
Neuropeptide Y (NPY)
Peptide tyrosine tyrosine (PYY)
Regulated on activation, normal T cell expressed and secreted (Rantes)
Stromal cell-derived factor-1 (SDF-1)
Substance P (SP)
-
Discovery of DPP4 as a Drug Target
Selective DPP4 inhibitors, glucoregulatory substrates, and the treatment of type 2 diabetes
Metabolic phenotypes in animal models of DPP4 deficiency
-
Mechanism(s) of Action of DPP4 Inhibitors
Selectivity of DPP4 inhibitors
Mechanisms through which DPP4 inhibitors lower glucose
Proof of concept and mechanisms for DPP4-dependent glucose control in humans
-
Role of DPP4 in Endocrine Pathophysiology
Adipose tissue
Cardiovascular system
Autoimmune disorders and inflammation
Miscellaneous metabolic actions of DPP4 inhibitors
Summary and Future Directions
I. Introduction
Dipeptidyl peptidase-4 (DPP4) is a multifunctional protein that exerts biological activity through pleiotropic actions including: protease activity (1), association with adenosine deaminase (ADA) (2), interaction with the extracellular matrix (3), cell surface coreceptor activity mediating viral entry (4), and regulation of intracellular signal transduction coupled to control of cell migration and proliferation (5). The complexity of DPP4 action is amplified by the panoply of bioactive DPP4 substrates, which in turn act as elegant biochemical messengers in multiple tissues, including the immune and neuroendocrine systems. Biological interest in the DPP4 enzyme has heightened after the approval of highly selective DPP4 inhibitors for the treatment of type 2 diabetes. Several excellent reviews have highlighted results of clinical trials using DPP4 inhibitors to treat type 2 diabetes (6); others have compared the structures, pharmacokinetic differences, and comparative efficacy of unique DPP4 inhibitors (7) or summarized cardiovascular (8–10), renal (11), or safety data (12) surrounding the use of DPP4 inhibitors. In this review, we approach the literature from a different perspective, highlighting the biology of the DPP4 enzyme and critically assessing experiments reporting the identification and role(s) of DPP4 substrates. We emphasize studies identifying DPP4 substrates and in turn assess our knowledge of how DPP4 inhibitors, acting through these substrates, transduce their pleiotropic actions in both preclinical and clinical studies. Given the clinical relevance of DPP4 biology for the treatment of diabetes, we focus most of our attention on mechanisms of action most relevant to the pathophysiology and treatment of diabetes and its complications. Readers interested in reviews summarizing clinical trial data are encouraged to consult several excellent recent summaries (6, 7).
II. Molecular Biology of DPP4
A. Discovery, genomic organization, and superfamily of related enzymes
DPP4 was discovered in 1966 as a new aminopeptidase with unique substrate characteristics (13). It was later determined to be identical to the T-cell activation antigen cluster of differentiation (CD)-26, rat liver membrane glycoprotein gp110, and the mouse thymocyte-activating molecule (14–16). The 70-kb human gene identified in 1992 (17–19) is located on the long arm of chromosome 2 (2q24.3) and comprises 26 exons that encode a 766-amino acid protein; the classic serine protease catalytic site is encoded by two exons, exons 21 and 22, respectively. In the mouse, Dpp4 is found on chromosome 2 (2C2–2D), and interestingly, exon 21 and exon 22 are present as a single 156-bp exon (19, 20). DPP4 is widely expressed in numerous tissues including endothelial cells in multiple vascular beds (21), rendering the enzyme highly accessible to peptide substrates circulating through the gut, liver, lung, and kidney (22). The human gene encodes two predominant mRNA transcripts, a larger 4.2-kb mRNA whose distribution is widespread, and a 2.8-kb transcript restricted to the placenta, kidney, lung, and liver. Furthermore, multiple minor splice variants of the DPP4 gene have been described; however, the functional significance, if any, of the various mRNA transcripts, which may contain different 3′-untranslated sequences or poly A tails, for translation of the classical bioactive DPP4 protein remains unclear (19, 20).
DPP4 is a member of the serine peptidase/prolyl oligopeptidase gene family, often subclassified partly by structure and function, which includes: the membrane-bound peptidases, fibroblast activation protein (FAP)/seprase; the resident cytoplasmic enzymes, DPP8 and DPP9; and the nonenzymatic members, DPP6 and DPP10, which are present in neuronal membranes, and prolyl endopeptidase. The position and identity of the residues essential for catalytic activity within the C-terminal region of these related enzymes are highly conserved in prokaryotes and eukaryotes despite other significant differences in sequence (23). Further enzymatic complexity is engendered by the functionally related DASH (dipeptidyl peptidase 4 activity and/or structure homologs) enzymes that replicate the enzymatic activity of DPP4 despite differences in structure and localization, thus explaining DPP4-like activity that persists after genetic deletion or pharmacological inhibition of DPP4 (24).
B. Molecular function
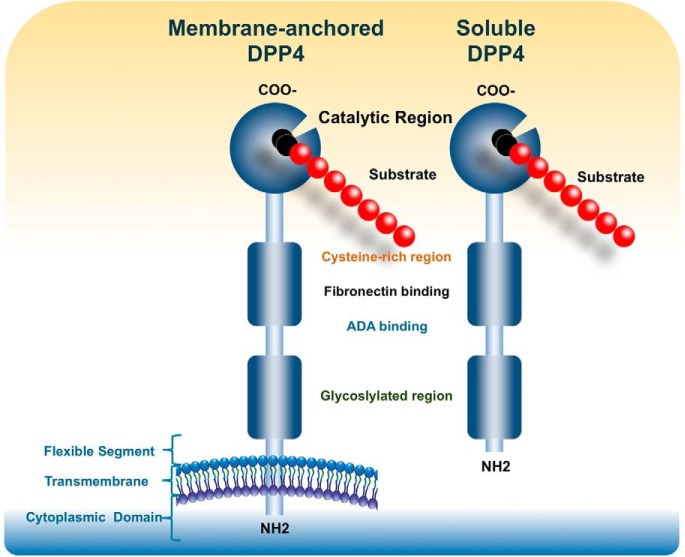
DPP4 transmits signals across cell membranes and interacts with other membrane proteins (Figure 1). Remarkably, most of the protein is extracellular, including the C-terminal catalytic domain, a cysteine-rich area, and a large glycosylated region linked by a flexible stalk to the transmembrane segment. Only six N-terminal amino acids are predicted to extend into the cytoplasm. The active site, Ser 630, is flanked by the classic serine peptidase motif Gly-Trp-Ser630-Tyr-Gly-Gly-Tyr-Val (23, 25, 26). Chemical cross-linking experiments performed in Caco-2 cells and native gel analysis of DPP4 isolated from seminal fluid demonstrated that dimers are the predominant species (27). Further studies analyzing recombinant DPP4 produced from baculoviral-infected Sf9 insect cells demonstrated that DPP4 exhibits a much smaller amount of enzymatic activity in the monomeric form, with activity significantly increasing upon homodimerization (28). Mutation studies demonstrated that the C-terminal loop of DPP4 is essential for both dimer formation and optimal catalytic efficacy (27).
Figure 1.
Membrane-bound DPP4 contains residues 1–766, whereas sDPP4 contains residues 39–766. sDPP4 is lacking the cytoplasmic domain [residues 1–6], transmembrane domain [residues 7–28], and the flexible stalk [residues 29–39]. Both membrane-bound and circulating sDPP4 share many domains including the glycosylated region [residues 101–535, specific residues 85, 92, 150], ADA binding domain [340–343], fibronectin binding domain [468–479], cysteine-rich domain [351–506, disulfide bonds are formed from 385–394, 444–472, and 649–762], and the catalytic domain [507–766 including residues composing the catalytic active site 630, 708, and 740].
Analysis of the protein crystal structure demonstrated that DPP4 can also form tetramers between two soluble proteins or two membrane-bound proteins, which may affect the efficiency of entry and cleavage of substrates by the catalytic active site or allow cell-cell communication (23). Studies in COS-1 cells coexpressing DPP4 and FAPα have demonstrated formation of heteromeric complexes (29), with both enzymes retaining their protease activity (30).
Membrane-bound DPP4 initiates intracellular signaling through interactions with ADA (19), caveolin-1 (31), caspase recruitment domain containing protein 11 (32), and the T-cell antigen CD45 (33). Other prospective binding partners include the CXCR4 receptor, the Na+/H+ exchanger, and the thromboxane A2 receptor, although the specific interaction sites have not been identified. DPP4 also binds to the extracellular matrix components collagen and fibronectin; binding to these proteins and to ADA is mediated by amino acid residues independent of the substrate binding site (34) (Figure 1).
Catalytically active DPP4 is liberated from the plasma membrane, producing a soluble circulating form, sDPP4 (727 aa), which lacks the intracellular tail and transmembrane regions (35) and accounts for a substantial proportion of DPP4 activity in human serum (36, 37) (Figure 1). The soluble form was first identified in serum and saliva (36) and has been detected in cerebrospinal and seminal fluid and bile. sDPP4 can also activate intracellular signaling pathways and increases the proliferation of human lymphocytes, independent of either its catalytic activity (38) or the binding of ADA (38). sDPP4 impairs insulin-mediated activation of Akt in human adipocyte, skeletal muscle, and smooth muscle cells in vitro (39). Remarkably, some of the actions attributed to sDPP4, such as regulation of T-cell migration or up-regulation of costimulatory molecules such as CD86, require functional catalytic sDPP4 catalytic activity (40, 41). The mechanisms through which sDPP4 activates signal transduction, possibly through interaction with the mannose 6-phosphate/IGF-2 receptor (41) or via other molecular interactions (42), are poorly understood.
C. Regulation of DPP4 expression
DPP4 activity is subject to regulation at many levels, including control of gene and protein expression, interaction with binding partners, and modulation of enzyme activity. The DPP4 gene does not contain conventional TATAA or CCAAT promoter sequences but is characterized by a cytosine/guanine-rich promoter region (43, 44). The human DPP4 gene promoter contains consensus sites for transcription factors with key roles in metabolism including specificity protein 1, activator protein 1, egfr-specific transcription factor, hepatocyte nuclear factor-1, and nuclear factor-κB (45). The importance of cytokines for DPP4 activity was demonstrated in chronic B lymphocytic leukemia cells, where interferons α, β, and γ stimulated STAT1α to bind the GAS (interferon γ-activated sequence) consensus sequences in the promoter of the DPP4 gene, leading to up-regulation of both intracellular and cell-surface DPP4 expression as well as DPP4 activity (46).
Cell-surface and intracellular DPP4 expression is also highly regulated, often low under basal conditions, then induced markedly, for example, upon T-cell activation with mitogenic or antigenic stimuli (47). In phytohaemagglutinin-activated cultured human peripheral blood mononuclear cells, immunofluorescence analysis determined that DPP4 is significantly up-regulated in response to IL-12, a key factor in the differentiation of naive T cells into Th1 cells (48). These data, together with evidence from other studies (1), suggest an important role for DPP4 in the activation of immune cells.
The control of DPP4 shedding, which increases levels of the circulating or sDPP4 form (Figure 1), is poorly understood. In human adipocytes isolated from visceral depots, both inflammatory stimuli (TNF-α) and insulin increased the release of sDPP4 despite a lack of change in DPP4 mRNA expression (39). Analysis of DPP4 mRNA expression in visceral adipose from obese human subjects demonstrated relative increases in DPP4 mRNA transcripts and enhanced release of sDPP4 from cultured adipocytes ex vivo (49). Nevertheless, whereas many studies have measured sDPP4 and associated its levels with different disease states, very little insight has been provided into the mechanisms regulating the shedding and biological activity of sDPP4 under these conditions. Studies in Dark Agouti rats demonstrated that kidney extracts exhibited the highest DPP4 activity (kinetic assay Gly-Pro-pNA substrate, presented per gram of wet weight) of any organ tested. High activity was also found in spleen, lung, thymus, and liver, whereas medium levels of activity were found in the intestine, aorta, and bone marrow. Interestingly, plasma DPP4 activity was not reduced after kidney transplantation from a Dpp4-deficient congenic Dark Agouti donor rat into a wild-type recipient rat, suggesting that the kidney is not an important source of sDPP4. Further studies in which Dpp4-deficient congenic Dark Agouti donor rats were lethally irradiated and received transfer of wild-type bone marrow demonstrated that 10 weeks after surgery, bone marrow was the source of 47% of sDPP4 activity in plasma; however, the marrow-derived cell type responsible was not determined (50). These studies highlight our limited understanding of the cell types and tissues that contribute to generation of sDPP4 plasma activity in vivo.
D. Post-translational modifications of DPP4
DPP4 contains eight to 11 potential N-glycosylation sites, which can contribute to its folding and stability (51). Although glycosylation may contribute approximately 18–25% of the total molecular weight (17, 52), mutational analysis has determined that the glycosylation sites are not required for dimerization, catalytic activity, or ADA binding (53). However, N-terminal sialylation facilitates trafficking of DPP4 to the apical membrane (54). Interestingly, molecular analysis of DPP4 isoforms isolated from the rat kidney brush border membrane reveals extensive heterogeneity in the oligosaccharides of DPP4 (55). A single study of sialylated DPP4 isoforms from peripheral blood mononuclear cells of HIV-infected subjects correlated the extent of sialylation with increasing patient age (56). This interaction has been proposed to alter the ionic charge of DPP4 antigens because highly sialylated DPP4 is more readily inhibited by cationic peptides. Desialylation of DPP4 with Vibrio cholera neuraminidase increased DPP4 activity; hence, the extent of sialylation may represent another mechanism for control of DPP4 activity (37, 57).
III. DPP4 Substrates
DPP4 was first investigated for its role in hydrolysis of dietary prolyl peptides (58); subsequent studies using DPP4 isolated using immunoaffinity chromatography and ADA binding identified DPP4 as the primary enzyme responsible for the generation of Gly-Prop-nitroanilide substrates in human serum. It is now known that DPP4 can cleave dozens of peptides, including chemokines, neuropeptides, and regulatory peptides, most containing a proline or alanine residue at position 2 of the amino-terminal region. Despite the preference for a position 2 proline, alternate residues (hydroxyproline, dehydroproline > alanine >, glycine, threonine, valine, or leucine) at the penultimate position are also cleaved by DPP4, suggesting a required stereochemistry. The DPP4 cleavage at postproline peptide bonds inactivates peptides and/or generates new bioactive peptides (see Figure 3), thereby regulating diverse biological processes.
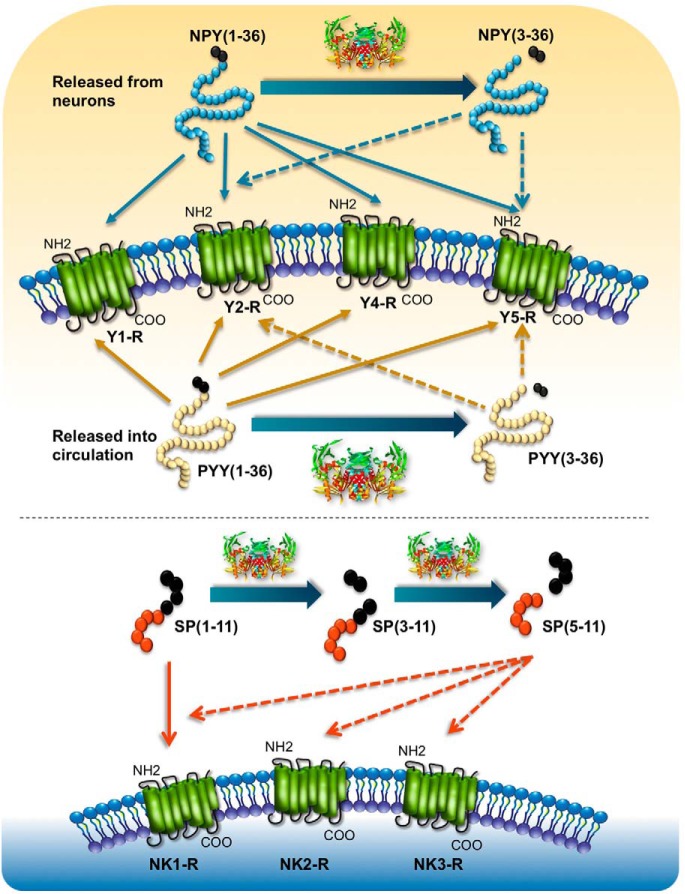
Figure 3.
DPP4 cleavage regulates substrate/receptor interactions. A, DPP4 cleaves NPY [1–36] and PYY [1–36]. The intact forms of these peptides signal through Y1R-Y5R. After DPP4 cleavage, NPY [3–36] and PYY [3–36] are generated and preferentially signal through the Y2R and Y5R. B, DPP4 cleaves SP [1–11], which signals through the NK1R receptor to generate SP [5–11], which can signal through (NK1R, -2R, -3R).
Multiple experimental approaches have been employed for identification and characterization of DPP4 substrates (Figure 2). Most commonly, putative DPP4 substrates are incubated with plasma containing DPP4, cells expressing endogenous or transfected DPP4, or purified sDPP4, after which peptide cleavage can be determined using one or more analytical techniques. The kinetic parameters for many substrates have been determined using in vitro enzyme assays (1). These approaches allow for use of sufficient quantities of peptide substrate to enable detection of intact vs cleaved substrate in vitro. Extension of DPP4 substrate identification to animals or humans in vivo is much more challenging because most DPP4 substrates are neuropeptides, chemokines, or regulatory peptides that circulate at very low concentrations.
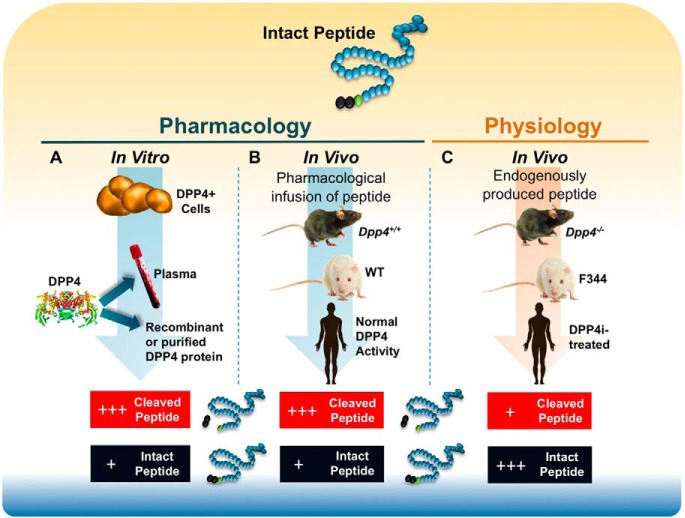
Figure 2.
Experimental paradigms for identification of DPP4 substrates. A, In vitro pharmacology. Cells expressing DPP4, serum, or recombinant/isolated DPP4 are utilized as an enzyme source and incubated with putative DPP4 substrates in vitro, after which the extent of truncation of the peptide at the penultimate residue can be analyzed. B, In vivo pharmacology. Pharmacological concentrations of peptides of interest are infused into wild-type mice or rats or Dpp4−/− mice, Fischer 344 rats, or animals or humans treated with a selective DPP4 inhibitor, and quantification of relative levels of intact vs cleaved peptide are monitored. C, In vivo physiology. Identification of endogenous physiological peptide substrates. In this paradigm, intact peptides and DPP4-cleaved peptides are detected at different levels in wild-type vs Dpp4 knockout animals or in animals or humans treated with a selective DPP4 inhibitor.
Experimental strategies for inferring the biological importance of DPP4 for peptide cleavage include infusion of a putative substrate into a mouse or rat with an inactivating mutation in the Dpp4 gene or coadministration of a peptide substrate with a DPP4 inhibitor in normal animals or humans (Figure 2). These approaches, while yielding interesting data, reveal the importance of DPP4 for potentiating the pharmacological actions of exogenous substrates. Whether DPP4 also controls circulating intact vs cleaved physiological levels of the same endogenous substrate in vivo is less clear and requires analysis of levels of intact vs cleaved peptide in 1) animals with inactivating mutations of the Dpp4 gene; or 2) animals or humans treated with a DPP4 inhibitor (Figure 2). The more challenging identification of endogenous physiological substrates cleaved by DPP4 (Table 1), those peptides whose relative levels of intact vs cleaved isoforms are significantly different after DPP4 inhibition in vivo, generally requires highly sensitive region-specific immunoassays and/or analytic techniques combining immunopurification and peptide identification by mass spectrometry.
Table 1.
Experimental Substrates of DPP4
| Physiological Substrates | N-Terminal Sequence | Pharmacological Substrates | N-Terminal Sequence |
|---|---|---|---|
| GIP | YAEGTF… | Aprotinin | RPD |
| GLP-1 | HAEGTF… | β-Casomorphin | YPFVEPI |
| GLP-2 | HADGSF… | BNP | SPKMVQ… |
| PYY | YPIKPE… | Chorionic gonadotropin | APD… |
| SDF-1 | KPVSLS… | Endomorphin-1 | YPFF-NH2 |
| SP | RPKPQQFFGLM… | Endomorphin-2 | YPWF-NH2 |
| Enterostatin | VPDPR… | ||
| Eotaxin | GPASVP… | ||
| Erythropoietin | APPRL… | ||
| GCSF | EATPL… | ||
| GCP2 | GPVS… | ||
| GHRH44 | YADAIF… | ||
| GHRH29 | YADAIF… | ||
| GMCSF | APAR… | ||
| GRP (1–27) | VPLPA… | ||
| GRP (3–27) | LPA… | ||
| Glucagon | HSQGTF… | ||
| Hemomorphin-7 | YPWTQRF… | ||
| HMGB1 | GKGD… | ||
| IGF-1 | GPETLCGA… | ||
| IP-10 | VPLSRT… | ||
| Kentsin | TPRK… | ||
| LD78β (CCL3L1) | APLAAD… | ||
| MDC | GPYGAN… | ||
| Mig | TPVVRK… | ||
| Morphiceptin | YPFP-NH2 | ||
| NPY | YPSKPD… | ||
| Oxyntomodulin | HSQGTF… | ||
| PACAP (1–38) | HSEGIF… | ||
| PACAP (1–27) | HSEGIF… | ||
| Promelittin (1–50) | APEPEP… | ||
| Promelittin (5–50) | EPEP… | ||
| Promelittin (3–50) | EP… | ||
| Procalcitonin | APFRSA | ||
| PHM | HADGVF… | ||
| Procolipase | VPDPR… | ||
| Prolactin | FPT… | ||
| Rantes | SPYSSD… | ||
| Secretin | HSDGTF… | ||
| SR-17 | SAEFPDFY… | ||
| SP (3–11) | KPQQFFGLM… | ||
| Trypsinogen propeptide | FPT… | ||
| Tyr-MIF-1 | YPLG-NH2 | ||
| Vasostatin-1 | SAEFPDFY… | ||
| VIP (1–59) | HSDAVFTDNY… |
Challenges in understanding the biology of DPP4 may include the inability to demonstrate changes in levels of intact vs cleaved peptide in vivo due to technical limitations of assay sensitivity. Furthermore, it may be similarly difficult to ascertain the physiological or pharmacological relevance of changes in peptide biology after genetic elimination or pharmacological reduction of DPP4 activity, due to the absence of selective peptide antagonists or mouse knockout models for the peptide or receptor of interest. Alternative experimental approaches for defining the importance of a DPP4 substrate include precise replacement of circulating levels of a (cleaved) substrate while maintaining DPP4 inhibition or use of a DPP4-resistant peptide to reproduce physiological levels of the native peptide. We designate substrates cleaved exclusively by recombinant or cell-associated or plasma DPP4 in vitro, or those whose biology is altered only after pharmacological infusion in vivo as “pharmacological” DPP4 substrates (Table 1 and Figure 2). Given the technical challenges in quantification and characterization of very low levels of intact vs cleaved DPP4 substrates, it seems likely that some of the pharmacological substrates shown in Table 1 may be ultimately reclassified as physiological substrates, pending further advances in the sensitivity of analytical techniques. Wherever possible, we distinguish between pharmacological vs physiological DPP4 substrates, providing an overview of commonly studied DPP4 substrates with potential significance in the pathophysiology of endocrine and metabolic disorders.
Although DPP4 activity may broadly regulate the physiology of numerous signaling systems impacting numerous organs and diseases, we focus our discussion on DPP4 substrates relevant to metabolism and related neuroendocrine systems (Table 1). Because DPP4 activity controls immune cell activity and both subclinical and overt inflammation can accelerate the progression of diabetes and obesity, we also discuss select data linking regulation of chemokine biology to DPP4 activity.
Below we highlight a subset of DPP4 substrates, evaluating evidence for identification of: 1) physiological peptide substrates (ie, intact peptides and DPP4 cleavage products are detected at different levels in wild-type vs Dpp4 knockout animals or in animals or human subjects treated with a selective DPP4 inhibitor); and 2) pharmacological substrates (ie, in vitro truncation has been observed at the penultimate residue, predominantly in vitro).
A. Brain natriuretic peptide (BNP)
BNP is synthesized as a 134-amino acid precursor (PreproBNP) that is processed to a 108-residue proBNP [1–108] and subsequently cleaved by furin or corin to BNP [1–32], which promotes natriuresis through engagement of natriuretic peptide receptor type A. BNP [1–32] is cleaved by DPP4 to BNP [3–32] in vitro (59), and BNP [1–32], BNP [3–32], and BNP [5–32] have been detected in plasma from patients with heart failure (60). Both BNP [1–32] and BNP [3–32] stimulate production of cGMP; however, the natriuretic, diuretic, and vasodilatory activity of BNP [3–32] is reduced, relative to that of BNP [1–32] in vivo (61). Sitagliptin potentiated the recovery of cardiac function after infusion of BNP [1–32] in pigs with pacing-induced heart failure (62); however, there is little information on changes of BNP [1–32] vs BNP [3–32] in animals or humans treated with DPP4 inhibitors.
B. Erythropoietin
Erythropoietin is a 166-amino acid protein with a position 2 proline that is cleaved by DPP4 in vitro and in vivo. Subcutaneous administration of erythropoietin in Dpp4 null mice augmented reticulocyte release into blood, whereas injection of the DPP4-truncated protein led to diminished reticulate responses and inhibited responses to full-length erythropoietin, suggesting that cleaved erythropoietin may act as a competitive inhibitor (63). Hematopoiesis in response to radiation or chemotherapy was augmented by genetic or pharmacological reduction of DPP4 activity in mice (63); however, it is less clear whether DPP4 inhibition augments erythropoietin-dependent hematopoiesis in humans. The available data suggest that erythropoietin may be a physiological DPP4 substrate; however, more rigorous biochemical quantification of intact vs cleaved erythropoietin in animals or humans with reduced DPP4 activity is required.
C. Eotaxin
Chemokines such as eotaxin are small peptides with chemoattractant properties that contain one or more cysteine residues at the N terminus. CC chemokines contain four consecutive conserved cysteine residues, whereas CXL chemokines display four cysteines, with two tandem cysteine residues separated by a single amino acid. Eotaxin, or CC motif chemokine (CCL)11 is a 74-amino acid chemokine secreted by Th2 cells to attract eosinophils. It is cleaved by DPP4 to eotaxin [3–74], reducing the potency for eosinophil chemotaxis (64). However, eotaxin [3–74] retains the anti-HIV activity in CC chemokine receptor 3-transfected cells exhibited by eotaxin [1–74]; hence, DPP4 cleavage selectively dampens eotaxin-mediated Th2-like immune responses, without modulating eotaxin-dependent viral entry (64).
D. Gastrin-releasing peptide (GRP)
GRP is a 27-amino acid peptide member of the bombesin family widely expressed in the neuroendocrine system, including the parasympathetic neurons of the pancreas, gut, and brain (65). Cleavage of GRP [1–27] by DPP4 produces GRP [3–27] and GRP [5–27] (66). Administration of a DPP4 inhibitor (valine-pyrrolidide) by gavage before infusion of GRP led to a 25% increase in the acute insulin response to iv glucose in anesthetized mice (67). However, GRP also stimulates GLP-1 secretion, which may contribute to indirect stimulation of insulin secretion (68) that would be further augmented in the presence of a DPP4 inhibitor. Although GRP [5–27] has been isolated from the canine intestine and brain, its biological importance is unknown (69). Furthermore, whether modulation of DPP4 activity controls the biology of endogenous GRP in vivo is unclear.
E. Glucagon
Glucagon is a 29-amino acid hormone secreted from pancreatic α-cells that stimulates hepatic glucose production. Diabetes is characterized by a lack of suppression of glucagon in the postprandial state that contributes to aberrant metabolism of glucose and lipids. Mass spectrometry analysis demonstrated that incubation of purified porcine DPP4 with glucagon in vitro resulted in formation of glucagon [3–29] and glucagon [5–29]. In human serum, glucagon can be metabolized to glucagon [3–29] ex vivo, and a DPP4 inhibitor (Ile-thiazolidide) prevented formation of this cleavage product in vitro (70). Nevertheless, the degradation of glucagon in porcine plasma is not affected by DPP4 inhibition with valine-pyrrolidide, and glucagon degradation was not modulated in anesthetized pigs treated with DPP4 inhibitors (71). Furthermore, the physiological or therapeutic significance of this enzymatic cleavage is unclear because DPP4 inhibitors universally decrease levels of intact glucagon in rodents and humans via potentiation of GLP-1 action, and circulating levels of glucagon [3–29] and glucagon [5–29] have not been reported in animals or humans treated with a DPP4 inhibitor. Collectively, the available data are consistent with the possibility that glucagon is a pharmacological but not a physiological substrate.
F. Glucagon-like peptide-1 (GLP-1)
GLP-1 is secreted from L cells found mainly in the distal portion of the intestinal tract after post-translational cleavage of proglucagon by prohormone convertase (PC) 1/3. Active GLP-1 circulates as GLP-1 [7–37] and GLP-1[7–36]NH2; these peptides are cleaved by DPP4 to generate GLP-1 [9–37] and GLP-1[9–36]NH2, respectively (72). Intact GLP-1 enhances glucose-stimulated insulin secretion and suppresses glucagon secretion, appetite, and gastric emptying (73), actions mediated by a single GLP-1 receptor (GLP-1R). DPP4 cleavage eliminates the classical glucoregulatory actions of GLP-1 and generates peptide(s) with 100-fold less receptor affinity (74), demonstrating that the N-terminal residues are required for engagement of the GLP-1R.
Plasma levels of intact active GLP-1 are increased in Dpp4−/− mice (52), in Fischer344/DuCrj rats with reduced DPP4 activity (75), and in animals and humans treated with DPP4 inhibitors (73). Hence, GLP-1 is a physiological DPP4 substrate. Although GLP-1 [9–36] amide is a weak antagonist of the GLP-1R (74), the physiological relevance of GLP-1 [9–36] remains unclear. Pharmacological administration of GLP-1 [9–36] to achieve levels higher than those circulating physiologically results in enhanced glucose clearance after meal ingestion, independent of changes in insulin, glucagon, or gastric emptying (76). Pharmacological infusion of GLP-1 [9–36] also reduced hepatic glucose production in obese (but not normal-weight) human subjects through incompletely defined mechanisms (77). Nevertheless, DPP4 inhibition results in marked reduction of circulating GLP-1 [9–36], and little data exist surrounding the biological implications of reducing GLP-1 [9–36] to very low or undetectable levels in vivo. Hence, although pharmacological administration of GLP-1 [9–36] enhances glucose clearance, reduces hepatic glucose production, and decreases oxidative stress in blood vessels and cardiomyocytes (78), the biological relevance of lowering levels of GLP-1 [9–36] in the context of DPP4 inhibition has not been demonstrated.
G. Glucagon-like peptide-2 (GLP-2)
GLP-2 is a 33-amino acid peptide with intestinotrophic activity (79) cosecreted with GLP-1 from enteroendocrine L cells in the distal bowel. Both GLP-2 [1–33] and its DPP4 cleavage product GLP-2 [3–33] have been detected by HPLC in rat ileal extracts and human plasma (80), and a DPP4 inhibitor (Val-Pyr) completely abolished formation of GLP-2 [3–33] in human plasma in vitro (81). GLP-2 [3–33] exerts both weak agonism and antagonism at the rodent and human GLP-2 receptors in vitro (82, 83) and in rodents in vivo (82). Nevertheless, whether DPP4 inhibitor-mediated reduction of GLP-2 [3–33] in vivo has any physiological consequence remains unclear. Native GLP-2 potently stimulates small bowel (SB) growth in mice; however, only crypt plus villus height, but not SB mass, was increased in rats treated with native GLP-2 [1–33]. In contrast, equimolar doses of native GLP-2 potently increased SB mass in F344 mutant DPP4 rats (84), illustrating the importance of endogenous DPP4 for inactivating GLP-2 in rodents. Furthermore, DPP4-resistant analogs of GLP-2 exert potent intestinotrophic effects in rodents and humans (84, 85), and plasma levels of intact bioactive GLP-2 [1–33] are increased after treatment of animals (86, 87) with a DPP4 inhibitor. Hence, GLP-2 is a physiological substrate of DPP4. Although DPP4 inhibitors potentiate the intestinotrophic actions of coadministered native GLP-2, administration of DPP4 inhibitors alone has little or no consequence for the intestinal actions of native endogenous GLP-2 in vivo (88).
H. Glucose-dependent insulinotropic polypeptide (GIP)
GIP is a 42-amino acid peptide derived from preproGIP via post-translational processing by PC1/3, mainly within K cells in the duodenum and proximal jejunum (73). GIP is also expressed in α-cells of the islet where PC2 processing liberates GIP [1–31], which, like GIP [1–42], stimulates insulin secretion (89). DPP4 cleaves GIP to release the dipeptide (Tyr-Ala) (90–92). GIP [3–42] is unable to activate the GIP receptor and, at pharmacological levels, functions as a weak antagonist in vitro. Nevertheless, whether GIP [3–42] functions as a physiologically relevant antagonist in vivo is uncertain (93, 94). GIP is a physiological substrate for DPP4 because plasma levels of intact GIP [1–42] are increased in animals (95) and humans (96) after administration of DPP4 inhibitors, and levels of intact GIP are increased in Dpp4−/− mice (52).
I. Granulocyte colony-stimulating factor (G-CSF)
G-CSF is a 174-amino acid chemokine that stimulates the bone marrow to produce hematopoietic stem cells. It was identified as a DPP4 target substrate, and cleavage by DPP4 reduces its chemotaxic potency in vitro and in mice (63). Whether intact vs cleaved forms of G-CSF are significantly different after administration of a DPP4 inhibitor is unclear.
J. Granulocyte-macrophage CSF (GM-CSF)
GM-CSF is a 127-amino acid chemokine secreted by immune cells to induce hematopoiesis. DPP4 inhibition enhances the colony-stimulating activity of GM-CSF ex vivo and in vivo (63). Interestingly, the DPP4 cleavage product of GM-CSF binds to the GM-CSF receptor with a greater affinity than native GM-CSF, which despite lower concentrations allows truncated GM-CSF to competitively inhibit signaling by full-length G-CSF (97).
K. GHRH and IGF-1
GHRH [1–44] and [1–40] are produced in the arcuate nucleus of the hypothalamus and bind its receptor on the anterior pituitary to stimulate the release of GH, and in turn, GH stimulates hepatic IGF-1 release. GHRH was among the first peptides to be identified as a DPP4 substrate; it is rapidly degraded in rodent and human plasma to GHRH [3–44]/GHRH [3–40] (98), and this cleavage was blocked upon incubation of human plasma with the DPP4 inhibitor, diprotin A (99). GHRH [1–44] or [1–40] exhibits a very short half-life (6 min) (98), and DPP4 cleavage was initially perceived to be a critical regulator of GHRH bioactivity and, in turn, the GH-IGF-1 axis.
IGF-1, the downstream effector of GHRH and GH, is a 105-amino acid protein produced mainly by the liver. IGF-1 was identified as a pharmacological DPP4 substrate by matrix-assisted laser desorption/ionization-time of flight analysis of molecular forms of IGF-1 generated after incubation with DPP4 purified from baculovirus-infected insect cells (100). However, studies in pigs treated with sitagliptin at doses inhibiting 90% of DPP4 activity failed to demonstrate an increase in active intact IGF-1 (101). Clinically, treatment of healthy human male subjects with sitagliptin (25–600 mg) for 10 days did not produce increased concentrations of serum IGF-1 or IGF-binding protein 3 as measured by ELISA (102). Furthermore, Dpp4−/− mice or rats do not exhibit increased organ growth or body size. Hence, the available data suggest that although DPP4 cleaves and inactivates both GHRH and IGF-1, enzymatic inactivation by DPP4 is not the major mechanism regulating the bioactivity of the GHRH-IGF-1 axis.
L. High-mobility group box 1 (HMGB1)
HMGB1 is a 215-amino acid nuclear protein that regulates gene transcription. HMGB1 may be released from cells during necrosis or in response to tissue damage, or it can be actively secreted during inflammation. Pharmacologically, HMGB1 stimulates the mobilization of endothelial cell progenitors, enhancing angiogenesis and wound healing (103). Immunoblotting of HMGB1 protein from plasma of two diabetic patients treated with DPP4 inhibitors using N-terminal-specific HMGB1 antisera suggests that levels of intact HMGB1 may be increased in the presence of DPP4 inhibitors (103). However, it remains unclear whether DPP4 cleavage regulates the biological activity of HMGB1 in vivo.
M. Macrophage-derived chemokine (MDC)
MDC is a 69-amino acid protein produced by macrophages and dendritic cells during a Th2-mediated immune response. Cleavage of recombinant MDC in vitro by DPP4 results in a small amount of MDC [3–69] but largely MDC [5–69] (104). Interestingly, whereas cleaved forms of MDC exhibited reduced chemotactic ability, isolated human peripheral blood monocytes remained responsive to the cleaved peptide, and truncated MDC retains its ability to resist HIV infection of peripheral blood mononuclear cells (105). Whether modulation of DPP4 activity similarly regulates the biology of MDC isoforms in vivo requires further analysis.
N. Macrophage inflammatory protein-1 α (MIP-1 α), chemokine (C-C motif) ligand 3-like 1 (CCL3L1), or LD78β
MIP-1 α/CCL3L1/LD78β is a 70-amino acid protein with chemoattractant properties for mononuclear cells (66). sDPP4 cleaves LD78β [1–70] in vitro to generate LD78β [3–70] and LD78β [5–70]. Human peripheral blood mononuclear cells treated with LD78β [3–70] exhibited greater chemotaxis and increased Ca2+ signaling compared with responses to LD78β [1–70] (106). Although DPP4 cleavage to generate LD78β [3–70] enhances chemotaxis in vitro, whether the reduction of DPP4 modulates the proportion of intact vs cleaved LD78β in vivo is less clear (106).
O. Oxyntomodulin
Oxyntomodulin is a 37-amino acid proglucagon-derived peptide secreted from L cells (107) that contains the 29-amino acid glucagon sequence with an additional eight amino acid extension at the C terminus. A unique receptor for oxyntomodulin has not been identified; however, oxyntomodulin activates both the GLP-1R and the glucagon receptor (108, 109). Soluble recombinant human DPP4 cleaves oxyntomodulin in vitro (110). The first 29 amino acids of the N-terminal sequence of oxyntomodulin are identical to glucagon, which, as noted above, is a pharmacological DPP4 substrate that does not appear to undergo meaningful DPP4 cleavage in vivo. Furthermore, the lack of an oxyntomodulin-specific antagonist or receptor challenges elucidation of the importance of oxyntomodulin as a physiological target for DPP4, and most commercially available assays do not reliably distinguish between intact and DPP4-cleaved forms of oxyntomodulin (111).
P. Pituitary adenylate cyclase-activating polypeptide (PACAP)
PACAP is a 38-amino acid neuropeptide that exhibits numerous actions in the endocrine system (112) and is released locally in islets upon stimulation of parasympathetic nerves in the pancreas. PACAP [1–38] is cleaved by DPP4 in vitro, and a C-terminal variant, PACAP [1–27], is also weakly cleaved by DPP4 (66, 110). PACAP signals through a family of structurally related PAC-1R, vasoactive intestinal peptide pituitary adenylate cyclase activating pepdite (VPAC) 1R, and VPAC-2R receptors (113), and both PAC-1R and VPAC-2R have been localized by in situ hybridization to islets. PACAP is a direct regulator of insulin secretion at concentrations as low as 10−14 mol/L, placing it among the most potent insulinotropic peptides described (114). The presence of multiple PACAP isoforms, as well as a family of related receptors that also recognize vasoactive intestinal peptide (VIP), renders generation of a PACAP-specific antagonist highly challenging (113). Although Pac1r−/− mice exhibit a complex metabolic phenotype, they display significant glucose intolerance and defective insulin secretion after both oral and iv glucose and impaired insulinotropic responses to PACAP, but not VIP, consistent with the importance of endogenous PACAP for control of insulin secretion (115). Circulating levels of PACAP [1–38] remain elevated after iv administration in Dpp4−/− mice, with markedly reduced generation of PACAP [3–38] (110). Similarly, the DPP4 inhibitor valine-pyrrolidide potentiated the insulinotropic actions of coadministered synthetic ovine PACAP [1–38] in mice (67). However, whether circulating or local concentrations of endogenous PACAP [1–38] are increased in the presence of a DPP4 inhibitor or altered in Dpp4−/− mice remains unclear, and it is difficult to ascertain due to the low concentrations of PACAP isoforms in vivo.
Q. Neuropeptide Y (NPY)
NPY is a 36-amino acid peptide produced in the hypothalamus and secreted into cerebral spinal fluid. NPY has also been localized to human adipose tissue, the sympathetic nerve termini associated with pancreatic blood vessels, and a subset of β-cells in the developing pancreas or in cells adapting to metabolic stress. NPY and related peptides (peptide YY and pancreatic polypeptide) exert their biological actions through a family of structurally related receptors (Y1R-Y5R) (116). Cleavage of native NPY [1–36] by DPP4 generates NPY [3–36], which has a strong affinity for the YR2/Y5R receptors, and considerable data demonstrate that cleavage of NPY [1–36] in vitro modulates NPY receptor selectivity (Figure 3). Nevertheless, enzymatic cleavage of NPY is complex, with at least five peptidases identified that cleave NPY in vitro (117). Although plasma immunoreactive NPY levels were elevated in Dpp4−/− mice with experimental colitis, these analyses did not distinguish between intact and cleaved forms of NPY (118). Furthermore, there are little data assessing whether envisioned changes in levels of endogenous NPY [1–36] vs NPY [3–36] in circulation and/or tissues after DPP4 inhibition accounts for a component of the relevant biological effects observed after DPP4 inhibition in vivo.
R. Peptide tyrosine tyrosine (PYY)
PYY is a 36-amino acid peptide secreted from intestinal L cells in response to nutrient ingestion. Cleavage of PYY [1–36] by DPP4 in vitro yields PYY [3–36] (119), a selective Y2R agonist (Figure 3). Both intact and cleaved PYY peptides have been detected in human plasma. Peripheral infusion of PYY [3–36], but not PYY [1–36], in healthy human subjects inhibits energy intake (120); conversely, infusion of PYY [1–36] inhibits food intake in wild-type but not in Fischer 344 rats with an inactivating point mutation in the Dpp4 gene (121). Furthermore, circulating levels of PYY [3–36] were significantly reduced, whereas levels of PYY [1–36] were increased in diabetic human subjects treated with sitagliptin (122). Hence, PYY [1–36] is a physiological substrate for DPP4 in vivo.
S. Regulated on activation, normal T cell expressed and secreted (Rantes)
Rantes is a 68-amino acid chemokine secreted by T cells and fibroblasts that interacts with the CCR1, CCR3, and CCR5 receptors. N-Terminal truncation produces Rantes [3–68], which retains chemotactic properties for lymphocytes but no longer stimulates migration of monocytes or eosinophils. After Rantes [1–68] is cleaved by DPP4, Rantes [3–68] continues to signal through the CCR5 receptor in vitro (123). Rantes [3–68] is more effective than Rantes [1–68] at inhibiting viral infection of mononuclear cells in vitro (124). The complexity of the DPP4-dependent potentiation of Rantes activity is underscored by observations that sDPP4 still potentiated the ability of Rantes [3–68] to activate T cells in vitro, suggesting enzyme-independent cooperatively between sDPP4 and chemokine activity (125). Although DPP4-mediated cleavage of Rantes produces potent biological actions in vitro, much less is known about the DPP4 dependence of Rantes biology in vivo.
T. Stromal cell-derived factor-1 (SDF-1)
Two different isoforms of SDF-1 (also known as C-X-C motif chemokine 12) have been described: SDF-1α, a mature protein of 68 amino acids; and SDF-1β, with four additional C-terminal residues, hence 72 amino acids, which are derived from a single SDF-1 gene through alternative RNA splicing (126). SDF-1 is widely expressed in numerous cell types, and SDF-1 expression and secretion are often induced concomitant with cellular injury. Both SDF-1 isoforms are cleaved by soluble or cellular DPP4, which inactivates their antiviral and chemotactic properties in cell-based assays in vitro (127). Because SDF-1 activity enhances migration of hematopoietic and endothelial progenitor cells to sites of ischemic injury, DPP4 inhibitors have been employed to enhance tissue healing, most commonly in the setting of myocardial or vascular ischemia. Importantly, DPP4 inhibition reduces N-terminal degradation of SDF-1 and potentiates SDF-1 action in vivo by enabling enhanced activation of its receptor, CXCR4, with greater stem cell mobilization to sites of injury (128). As is the case with many low abundance DPP4 substrates, quantification of levels of intact vs DPP4-cleaved SDF-1α in vivo is challenging, due to the lack of sensitive assays for discriminating SDF-1α [1–67] from SDF-1α [3–67].
Using an assay that employs a first step of antibody-based immunoenrichment followed by quantification of peptide isoforms using mass spectrometry, Wang et al (129) detected increased plasma levels of SDF-1α [1–67] and decreased levels of SDF-1α [3–67] in C57BL/6 mice acutely treated with the DPP4 inhibitor MK-0626. Furthermore, relative increases in circulating levels of intact SDF-1α [1–67] were detected in plasma from rhesus monkeys acutely treated with MK-0626, with more prolonged (15 d of dosing) DPP4 inhibition markedly reducing circulating levels of SDF-1α [3–67] (129). Similarly, an assay employing antibody capture followed by mass spectrometry detected marked reduction of SDF-1α [3–67] in plasma from Dpp4−/− mice (130). Taken together, the available data demonstrate that SDF-1α [1–67] is a physiological DPP4 substrate.
U. Substance P (SP)
SP, or tachykinin, is an 11-amino acid neurotransmitter that signals predominantly through the neurokinin-1 receptor (NK1R). Plasma from wild-type (but not Fischer 344) rats cleaves recombinant SP [1–11] to generate SP [3–11] and SP [5–11], via mechanisms sensitive to the DPP4 inhibitor diprotin A (131). Dpp4−/− mice exhibit approximately 2-fold higher levels of circulating SP, implicating an essential biological role for DPP4 in control of SP biology (132). Although potentiation of SP activity has been postulated to underlie the pathophysiology of nasopharyngitis or neurogenic inflammation occasionally reported with use of DPP4 inhibitors, SP is also rapidly cleaved by angiotensin-converting enzyme and neutral endopeptidase, and levels of intact vs cleaved SP are very low and difficult to quantify in vivo. Furthermore, the extent of cutaneous inflammation induced by exogenous intradermal administration of SP in human subjects was not modified by coadministration of sitagliptin (133). Although pharmacological infusion of SP potentiates vascular release of norepinephrine and increases heart rate in human studies, there is currently no firm clinical evidence that DPP4 inhibition potentiates the cardiovascular activity of endogenous SP in human subjects. Hence, the biological importance of SP as a putative endogenous physiological DPP4 substrate requires further scrutiny.
IV. Discovery of DPP4 as a Drug Target
DPP4 was initially characterized as a modulator of T-cell activation and proliferation. Observations that DPP4 levels and activity were elevated in T cells of patients with autoimmune disorders and inflammatory conditions including rheumatoid arthritis (134) led to evaluation of DPP4 inhibitors for treatment of immune disorders involving aberrant T-cell function. However, interpretation of these studies was complicated by observations that catalytic activity was not required for DPP4 to mediate its effects on T-cell function (135).
Because DPP4 expression is up-regulated in many cancers and associates with extracellular matrix proteins, the consequences of DPP4 inhibition were evaluated in T-cell malignancies and solid tumor metastases. A nonselective inhibitor for DPP4, FAP, DPP8, and DPP9 (Val-Boro-Pro, PT-100, talabostat) was studied in clinical trials for the treatment of solid tumor malignancy and advanced stage nonsmall cell lung cancer; the available evidence did not reveal a sufficiently robust therapeutic response to merit further clinical development (136, 137). Furthermore, treatment of DPP4 null (Dpp4−/− or Cd26−/−) mice with PT-100 demonstrated stimulation of cytokine and chemokine production and a reduction in tumor incidence, suggesting that inhibition of DPP4 is not the main molecular target for these actions of PT-100 (138). Many of the first-generation DPP4 inhibitors exhibited potent actions in the immune system and modified neoplastic cell growth, yet were subsequently found to exhibit nonselective activity independent of their actions to inhibit DPP4.
A. Selective DPP4 inhibitors, glucoregulatory substrates, and the treatment of type 2 diabetes
Interest in the therapeutic potential of inhibiting DPP4 for the treatment of metabolic disorders followed observations that GLP-1 [7–37]/GLP-1 [7–36] amide, and not GLP-1 [1–37], were potent glucose-dependent stimulators of insulin secretion (73), yet native GLP-1 [7–37]/[7–36] amide exhibited a very short circulating half-life, and continuous infusion was required to optimally control glycemia (139). In 1993, Mentlein et al (91) identified DPP4 as the enzyme responsible for hydrolysis of the incretin hormones GLP-1 [7–36] and GIP [1–42] in vitro, findings subsequently extended to rats by Kieffer et al (90). Furthermore, the rapid N-terminal cleavage of GLP-1 and GIP by purified DPP4 or rat serum was substantially reduced by coincubation with the DPP4 inhibitor diprotin A and diminished in the presence of serum from DPP4-deficient Fisher 344 rats. Infusion of [125 I]GIP [1–42] or [125 I]GLP-1 [7–36]NH2 into DPP4 rats resulted in substantially less N-terminal cleavage of these peptides (90), illustrating the critical role of DPP4 for incretin inactivation in vivo. Simultaneously, Deacon et al (72) demonstrated cleavage of GLP-1 [7–36] amide to GLP-1 [9–36] amide in human plasma in vitro, with these actions sensitive to the DPP4 inhibitor diprotin A, findings consistent with those reported by Pauly et al (140) analyzing the degradation of GIP and GLP-1 by human serum in vitro. Furthermore, the circulating levels of both intact and cleaved GLP-1 isoforms were lower in the fasting state and rose after meal ingestion in human subjects (72). Subcutaneous or iv administration of native GLP-1 in normal and diabetic human subjects led to rapid degradation of the intact GLP-1 [7–36] amide and accumulation of the DPP4-generated metabolite GLP-1 [9–36] amide (141). Collectively, these findings highlighted the role of DPP4 as a key determinant of the enzymatic cleavage and inactivation of GLP-1 both in vitro and in vivo; hence, GLP-1 is a major physiological substrate for DPP4.
Complementary evidence supporting an essential role of DPP4 in the control of incretin degradation and glucose homeostasis was derived from studies of Dpp4-deficient rodents and administration of DPP4 inhibitors to animals and humans. The DPP4 inhibitor valine pyrrolidide prevented GLP-1 degradation and potentiated the insulinotropic and glucoregulatory actions of exogenously infused GLP-1 in anesthetized pigs (142). Treatment of rats with Ile-thiazolidide inhibited DPP4 activity, improved oral glucose tolerance, and enhanced the insulin response to glucose, findings consistent with the potentiation of endogenous GLP-1 and GIP activity in vivo (143). Notably, DPP4 inhibition improved glycemia not only in lean insulin-sensitive rats, but also in obese Zucker rats with marked glucose intolerance, demonstrating that inhibition of DPP4 activity produced glucoregulatory actions in experimental models of dysglycemia (143). Similarly, acute administration of NVP-DPP728, a selective inhibitor of DPP4, to lean and obese insulin-resistant Zucker rats improved oral glucose tolerance and augmented the increases in plasma insulin levels, in association with increased plasma levels of intact GLP-1 and no change in gastric emptying (144). Collectively, these and related studies provided proof of concept for the glucoregulatory actions of DPP4 inhibitors.
B. Metabolic phenotypes in animal models of DPP4 deficiency
Insight into the metabolic consequences of reduced or absent DPP4 activity came from animal models with inactivating mutations in DPP4. The Fischer rat (F344/DuCrj and F344/CRG; Charles River Japan and Germany, respectively) exhibits loss of DPP4 activity due to a G633R substitution near the DPP4 active site (145), which results in degradation of the immature DPP4 protein in the endoplasmic reticulum (146). The Japanese rat exhibits enhanced glucose tolerance, increased levels of circulating GLP-1, and enhanced insulin secretion under basal conditions (75, 147). Both the F344/DuCrj and F344/CRG rats demonstrate a reduction in body weight, associated with decreased food and water intake (148). The F344/DuCrj exhibits favorable metabolic phenotypes including improved oral glucose tolerance, increased insulin secretion, and higher levels of intact plasma GLP-1. Notably, the DPP4 inhibitor valine-pyrrolidide did not modify glucose tolerance of F344/DuCrj rats but improved glucose tolerance in control F344/Jcl rats (75). DPP4-deficient rats also exhibit resistance to high-fat diet-induced obesity, improved insulin sensitivity (149), and resistance to streptozotocin-induced hyperglycemia (150).
The generation and phenotypic characterization of Dpp4−/− mice provided complementary evidence for the physiological importance of DPP4 in glucose homeostasis. Dpp4−/− mice displayed increased circulating levels of GLP-1 and GIP in response to an oral glucose challenge, enhanced glucose-stimulated insulin secretion, and improved oral glucose tolerance. Consistent with data from F344/DuCrj rats, Dpp4−/− mice also exhibited resistance to diet-induced obesity and reduced STZ-induced β-cell destruction (151).
Studies using DPP4 inhibitors in wild-type animals and characterization of rats and mice with DPP4 mutations provided important validation of DPP4 as a glucoregulatory target; however, several caveats qualify interpretation of data from these experiments. First, the initial generation of DPP4 inhibitors was not completely selective for DPP4, raising the possibility that some of the actions ascribed to use of these agents in vivo may reflect off-target activity (152). Second, DPP4-deficient rats and Dpp4−/− mice do not represent models of selective inactivation or deficiency of the catalytic subunit; rather, these animals exhibit reduced expression (F344 rats) or complete genetic elimination (Dpp4−/− mice) of the entire DPP4 protein. Hence, attribution of mechanisms responsible for favorable metabolic phenotypes in these animals is complicated by potential actions ensuing from loss of DPP4 actions arising independent of its catalytic activity.
V. Mechanism(s) of Action of DPP4 Inhibitors
Understanding the mechanisms through which DPP4 inhibitors exert diverse metabolic actions requires assessment of the selectivity of these agents and rigorous evaluation of evidence linking changes in levels or molecular forms of a candidate DPP4 substrate to actions emanating from administration of a DPP4 inhibitor in vivo. As outlined in Table 1 and discussed herein, a large number of peptides are cleaved by DPP4 in vitro, and many of these peptides are also cleaved by DPP4 in vivo. Furthermore, DPP4 inhibitors modify the relative levels of intact vs cleaved substrates (Figure 2), which may exhibit varying affinities for structurally related receptors (Figure 3), further complicating assignment of mechanistic roles for key peptide substrates in transducing actions arising from DPP4 inhibition. In the following sections, we describe experiments supporting the importance of DPP4 inhibitor selectivity and critically evaluate evidence linking changes in levels of key substrates and metabolites to metabolic and glucoregulatory activities ascribed to DPP4 inhibition. Wherever possible we highlight data generated using selective antagonists or mouse genetics to link the presence or absence of peptide action to therapeutic actions ascribed to DPP4 inhibitors.
A. Selectivity of DPP4 inhibitors
Discovery that DPP4 is a member of a family of proteases that exhibit postproline cleavages, including DPP4-like 1, DPP4-like 2, DPP7, DPP8, DPP9, and FAP, prompted scientists to re-evaluate the selectivity of DPP4 inhibitors (152). The critical importance of DPP4 as the glucoregulatory target for DPP4 inhibitors is exemplified by studies in F344/DuCrj rats treated with valine pyrrolidide, which demonstrated no improvement in glucose tolerance compared with reduction in glycemic excursion in control rats (75). Similarly, valine pyrrolidide improved glucose tolerance in wild-type mice but failed to lower glucose in Dpp4−/− mice (52). Importantly, both mice and rats with inactivating DPP4 mutations remain relatively healthy while exhibiting favorable metabolic profiles. Hence, these rodent models provide key genetic evidence demonstrating that DPP4 is the glucoregulatory target for DPP4 inhibitors.
The selectivity of DPP4 inhibitors and the importance of DPP4 for enzymatic inactivation of key substrates is of great interest because DPP7, DPP8, DPP9, and FAP also display DPP4-like activity (DPP4L1 and DPP4L2 do not because they have a mutation at the active site serine) (152, 153). Many DPP4 substrates, including GLP-1, GLP-2, NPY, PYY, SDF-1β, interferon γ-induced protein-10 (IP-10), and interferon-inducible T-cell α chemoattractant, are also target substrates for DPP8 and/or DPP9, albeit with much lower efficiencies compared to DPP4. NPY, BNP, SP, and PYY as well as GLP-1 and GIP may all be hydrolyzed by FAP; however, no overlap in the enzymatic specificity of chemokine truncation was observed (154). The relative importance of ubiquitously expressed DPP8/DPP9 as potential mediators of toxicity emanating from the use of nonselective DPP4 inhibitors remains a subject of some uncertainty (152). Lankas et al (155) assessed the consequences of administering selective inhibitors of DPP4, DPP8/9, or quiescent cell proline peptidase in rats, dogs, and wild-type and Dpp4−/− mice. Selectivity was assessed in vitro against the respective recombinant human enzymes. After 4 weeks of treatment with L-threo-isoleucyl thiazolidine 1, rats demonstrated lung histiocytosis and thrombocytopenia. Dogs given the same treatment exhibited acute central nervous toxicities after one or two doses including seizure, tremor, and ataxia were observed at lower doses (155) with bloody diarrhea in dogs observed after more prolonged therapy. After 5–6 weeks of treatment in dogs, mortality was significantly increased, and additional toxicities including anemia, thrombocytopenia, splenomegaly, and multiorgan pathology were observed. The allo isomer (allo-isoleucyl thiazolidine) was shown to exhibit 10-fold greater potency for inhibition of DPP8/9, potentially accounting for some of the observed preclinical toxicities (155). Subsequent studies employing DPP8/9 selective inhibitors produced thrombocytopenia, splenomegaly, lymphadenopathy, lymphocyte necrosis, and mortality in rats and bloody diarrhea, emesis, and tenesmus in dogs (155). Notably, splenomegaly, bone marrow myeloid hyperplasia, and excess mortality were observed in both wild-type and Dpp4−/− mice treated with high doses of DPP8/9 selective inhibitors (155). Furthermore, Val-boro-pro and DPP8/9 selective inhibitors reduced cytokine release and T-cell proliferation, whereas DPP4-selective inhibitors had no effect in these assays in vitro.
Contrasting results were obtained in studies examining the putative importance of DPP8/9 as potential targets for vildagliptin in some cell types (156). Vildagliptin was a much more potent inhibitor of DPP4, relative to inhibition of DPP8 or DPP9 as assessed in studies employing recombinant human enzyme in vitro, with Ki values of 3, 810, and 95 nm, respectively. Vildagliptin was administered to rodents at concentrations up to 1500 mg/kg/d, doses that produced plasma concentrations well beyond the Ki values determined using in vitro enzyme assays to inhibit DPP4, DPP8, and DPP9. Importantly, tissue concentrations of vildagliptin exceeded plasma levels except for muscle, adipose tissue, and brain; however, specific details of drug levels achieved in different tissues were not provided. No deaths were observed in the vildagliptin-treated rats or mice, and very few adverse effects were noted, except for modest increases in red blood cell counts at the two highest doses (156).
Even less well understood are observations that some DPP4 inhibitors (vildagliptin and saxagliptin), but not others, produce cutaneous necrosis and vasculitis in nonhuman primates (157). It seems likely that these findings reflect differences in selectivity, intracellular penetration, or metabolites with unique activities, rather than mechanism-based DPP4-mediated toxicities; however, the molecular pathophysiology underlying these preclinical findings remains poorly understood.
How can we reconcile differences in preclinical toxicity and contrasting conclusions arising from the studies of Lankas et al (155) and Burkey et al (156) regarding the potential importance of DPP8/9 as mediators of off-target (non-DPP4-mediated) toxicity with specific enzyme inhibitors? First, these studies assessed structurally distinct compounds profiled in different enzymatic assays in vitro and separate groups of animals in vivo, and we do not have external validation of the relative toxicities of the various compounds studied in independent laboratories. Importantly, measuring plasma and even tissue levels of various inhibitors may not reveal whether various inhibitors differentially pass across cell membranes and penetrate intracellular compartments. Because many of the enzymes such as DPP8/9 may not be similarly accessible to all compounds, some of the experimental differences observed may reflect differences with which various compounds access and interact with the enzymes residing in key cellular subcompartments (158). None of the studies routinely assess the extent to which enzyme activities are selectively reduced in tissues and within cells in vivo, a challenging task given the large number of cellular enzymes with related enzymatic profiles and activities. Intriguingly, Dpp9−/− mutant mice with a Ser-Ala point mutation at the catalytic site (S729A) exhibit normal enzyme expression, reduced DPP9 enzymatic activity, and normal embryonic development (159); although Dpp9−/− mice are born with the expected Mendelian frequency, they exhibit neonatal mortality and die shortly after birth. Hence, selective genetic ablation of murine DPP9 catalytic activity, a scenario distinct from partial inhibition of DPP9 activity, produces major organ toxicities in vivo (159). Even less is known about the consequences of selective genetic or chemical reduction of DPP8 enzyme activity in vivo, and it seems clear that toxicology data generated in rodents may not always be readily extrapolated to primates.
Furthermore, it is important to consider whether selectivity of DPP4 inhibitors and their interaction with the catalytic site is relevant for understanding nonenzymatic actions of DPP4. For example, binding to extracellular matrix proteins including fibronectin (160), collagen I and III (161) is mediated through residues independent of the active site. Although these actions would not be predicted to be impaired by pharmacological inhibition targeting the protease activity, genetic evidence demonstrates that some actions of DPP4 not involving substrate cleavage do require catalytic DPP4 activity (40, 41). Nevertheless, there are little data demonstrating that highly selective DPP4 inhibitors meaningfully perturb actions of DPP4 independent of its ability to cleave peptide substrates.
B. Mechanisms through which DPP4 inhibitors lower glucose
1. Preclinical studies
Mice with genetic inactivation of one or both incretin receptors have yielded key insights into glucoregulatory mechanisms of DPP4 inhibitors. Consistent with the cytoprotective actions of both GLP-1 and GIP, sitagliptin reduces β-cell apoptosis in wild-type mice treated with streptozotocin but fails to modify β-cell survival in Glp1r−/−:Gipr−/− mice (162). Acute administration of four structurally distinct DPP4 inhibitors (valine pyrrolidide, SYR-106124, LAF237, and TP8211) improved oral glucose tolerance and increased levels of plasma insulin in wild-type mice and in both Glp1r−/− and Gipr−/− mice. In contrast, none of the DPP4 inhibitors lowered glycemia or increased plasma levels in acute studies with double incretin receptor knockout (DIRKO; Glp1r−/−:Gipr−/−) mice (163). To assess whether the development of insulin resistance and glucose intolerance unmasked the importance of additional DPP4-sensitive glucoregulatory substrates beyond GLP-1 and GIP, Flock et al (164) administered vildagliptin in the drinking water continuously for 8 weeks to high-fat-fed C57BL/6 wild-type and DIRKO mice. Vildagliptin improved glucose tolerance and increased levels of plasma insulin in wild-type mice, but no improvement in glycemia or changes in plasma insulin levels were detected in vildagliptin-treated DIRKO mice, demonstrating the requirement for GIP and GLP-1R signaling for transduction of the chronic glucose-lowering actions of DPP4 inhibitors (164). In contrast, selective changes in expression of genes regulating hepatic lipid metabolism were seen in both wild-type and vildagliptin-treated DIRKO mice.
The exact cellular site(s) that transduce the glucoregulatory actions of DPP4 inhibitors remain uncertain. Although GLP-1 and GIP receptors on islet β-cells are logical candidates, levels of intact circulating GLP-1 and GIP are low, and a considerable proportion of intact GLP-1 and GIP is degraded shortly after secretion from gut endocrine cells (165). Studies in rodents using doses of sitagliptin sufficient to inhibit intestinal but not systemic DPP4 activity implicate local intestinal-neural GLP-1R-dependent mechanisms for potentiation of glucoregulatory actions of DPP4 inhibitors (166); however, the existence and potential importance of similar circuits in humans is difficult to determine. Furthermore, selective genetic restoration of GLP-1R signaling only in Glp1r−/− β-cells, but not in the brain or most neurons, is sufficient to normalize glucose intolerance in Glp1r−/− mice (167). Hence, elucidation of potential sites, including peripheral and central neuronal circuits and islet cells contributing to DPP4-dependent glucoregulation, is challenging. Collectively, the available data demonstrate that GLP-1 and GIP are necessary and sufficient to explain the glucoregulatory actions of DPP4 inhibitors in mice; however, additional DPP4 substrates may be involved in mediating other metabolic nonglycemic actions ascribed to DPP4 inhibition.
C. Proof of concept and mechanisms for DPP4-dependent glucose control in humans
A crossover, placebo-controlled, clinical trial was undertaken in 93 patients with type 2 diabetes previously controlled with diet and exercise, baseline glycated hemoglobin (HbA1c) 7.4%, treated with 100 mg three times daily or 150 mg twice daily with the DPP4 inhibitor, 1-[[[2-[(5-cyanopyridin-2-yl)amino]eth-yl]amino]acetyl]-2-cyano-(S)-pyrrolidine (NVP DPP728) for 4 weeks. Administration of NVP DPP728 reduced 24-hour glucose profiles and decreased levels of HbA1c (to 6.9%) and fasting and postprandial glucose (168). Unexpectedly, these improvements in glycemic parameters were associated with reductions in mean 24-hour plasma insulin levels; however, an increase in the postprandial insulin:glucose ratio was noted, consistent with an improvement in β-cell function (168). Subsequent studies using LAF237 (vildagliptin, 100 mg/d) for 4 weeks in overweight human subjects with diet-controlled type 2 diabetes revealed that DPP4 inhibition increased GLP-1 and decreased plasma glucagon levels, whereas plasma insulin concentrations remained unchanged (169). Consistent with earlier studies using NVP DPP728, insulin:glucose ratios were improved in DPP4 inhibitor-treated subjects. These studies were the first to highlight the correlation between improvements in glycemia and reductions in plasma glucagon in diabetic subjects treated with a DPP4 inhibitor (169).
Further studies demonstrated sustained glucose control in diabetic subjects treated with metformin and either placebo or vildagliptin 50 mg twice daily, initially for 12 weeks and followed by an additional 40-week open-label extension (170). Treatment with vildagliptin reduced HbA1c over the initial 12-week treatment period, then prevented deterioration in glycemic control over 52 weeks; sustained improvements in postprandial glucose control and fasting glucose were evident in subjects treated for 52 weeks, with no significant differences in insulin levels (170).
Balas et al (171) assessed the mechanisms through which vildagliptin improved glycemia in obese human subjects with type 2 diabetes (body mass index [BMI], 34 kg/m2; baseline HbA1c, 9%). Patients were given a single 100-mg dose of vildagliptin or placebo at 5:30 pm, followed 30 minutes later by a meal tolerance test. Vildagliptin markedly suppressed plasma glucagon levels, decreased endogenous glucose production, and enhanced insulin secretion rates, despite a fall in glucose (171). The mechanisms of sitagliptin action were studied in overweight human subjects with type 2 diabetes treated with sitagliptin or placebo for 6 weeks. Study protocols included a meal tolerance test and an iv glucose infusion for assessment of β-cell function. Key findings included suppression of endogenous glucose production and reduced levels of glucagon levels during a meal in sitagliptin-treated subjects (172).
The pharmacodynamic effects of chronic sitagliptin therapy, administered alone or in combination with metformin, were assessed in 16 subjects with type 2 diabetes treated with metformin or sitagliptin, alone or in combination, for 6 weeks (173). Sitagliptin, either alone or in combination with metformin, significantly reduced plasma glucagon levels during a meal tolerance test, associated with a drop in endogenous glucose production.
Most studies using DPP4 inhibitors to treat human subjects with type 2 diabetes demonstrate increased levels of intact GLP-1 and GIP, and often a reduction of total circulating GLP-1 and GIP, consistent with feedback inhibition of L and K cell secretion, respectively (174). Aulinger et al (175) assessed the importance of endogenous GLP-1 for the glucose-lowering effects of sitagliptin in human diabetic subjects (mean HbA1c, 6.2%; BMI, 27.7 kg/m2). Subjects were studied using oral and iv glucose tolerance tests and acute administration of sitagliptin or placebo, in the presence or absence of iv exendin [9–39] (175). Sitagliptin increased fasting levels of intact GLP-1 and GIP, and consistent with tonic feedback inhibition of GLP-1 secretion by active GLP-1, exendin [9–39] further increased fasting levels of active GLP-1 in the presence of sitagliptin. Exendin [9–39] partially reversed (by about 50%) the glucose reduction observed with sitagliptin treatment during the oral glucose tolerance test (175). Sitagliptin alone suppressed levels of plasma glucagon during the oral glucose tolerance test, whereas coadministration of exendin [9–39] led to significant increases in plasma glucagon levels in sitagliptin-treated subjects. These findings are consistent with an important role for augmentation of GLP-1R signaling in the DPP4 inhibitor-mediated suppression of glucagon secretion and oral glucose tolerance (175). The partial, but not complete, reversal of sitagliptin-mediated improvements in glycemic control with exendin [9–39] is entirely consistent with mouse studies implicating a key role for both GLP-1 and GIP in the glucose-lowering actions of DPP4 inhibitors (52, 163).
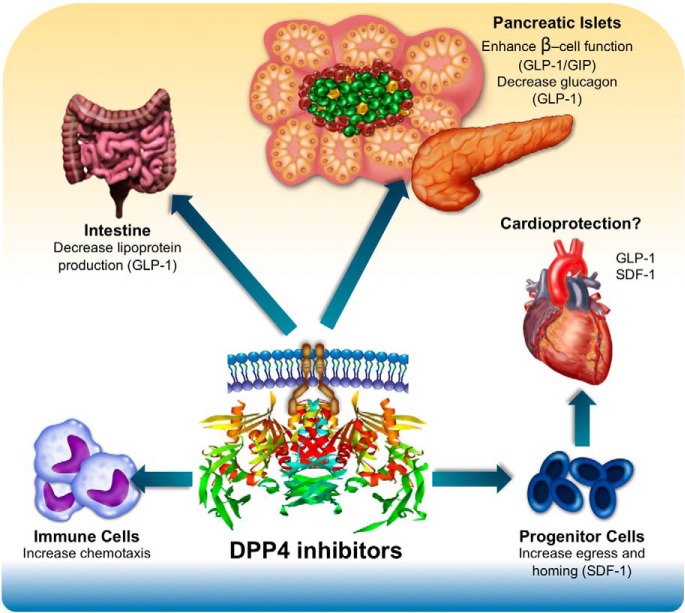
VI. Role of DPP4 in Endocrine Pathophysiology
A. Adipose tissue
1. DPP4 expression in adipose tissue
DPP4 is expressed in both sc and visceral adipose depots isolated from human subjects (39). Increased DPP4 expression is associated with impaired glucose tolerance, increased BMI, and increased levels of HbA1c. Positive correlations of circulating DPP4 with the extent of adipocyte hypertrophy, macrophage infiltrations, and insulin resistance were observed in insulin-resistant morbidly obese patients (49). Dendritic cells and macrophages resident in visceral adipose depots also express DPP4, and relative DPP4 expression in these cell types is increased in obese states and is further induced by experimental inflammation (176). Although DPP4 is well positioned to control local concentrations of key bioactive substrates within adipose tissue, it remains unclear whether adipose DPP4 mechanistically contributes to expansion and dysfunction of different adipose depots.
2. DPP4, appetite, energy expenditure, and weight gain
Dpp4 −/− mice exhibit resistance to high-fat feeding-induced obesity, associated with increased expression of uncoupling protein-1 and the β3 adrenergic receptor in brown adipose tissue and increased whole-body energy expenditure (151). Several DPP4 substrates including BNP [1–32], GLP-1, and oxyntomodulin have been shown to stimulate VO2 consumption and/or thermogenesis in brown adipose tissue (177, 178). However, the mechanism(s) enabling Dpp4 null mice to exhibit resistance to diet-induced obesity have not been identified.
Multiple DPP4 substrates are pharmacological regulators of food intake, including NPY, PYY, enterostatin, GLP-1, and GLP-2. Unlike GLP-1R agonists, DPP4 inhibitors do not suppress food intake or gastric emptying (179). The weight-neutral effects of DPP4 inhibitors may simply reflect the modest elevations in intact GLP-1 to levels insufficient to control satiety and/or changes in levels and activity of multiple anorectic and orexigenic peptides, with the integrated result reflecting the absence of a dominant anorectic signal.
B. Cardiovascular system
1. DPP4 in the cardiovascular system
DPP4 is expressed in vascular endothelial cells (180, 181) and venous capillary beds, and DPP4 enzyme activity has been detected in human fibroblasts. Because the reduction of cardiovascular complications is a major goal for the treatment of type 2 diabetes, we briefly review data linking DPP4 inhibition to mechanisms of action in the cardiovascular system. Readers are directed to more focused comprehensive cardiovascular reviews of DPP4 action for more detail (8, 182).
2. Myocardial infarction and heart failure: preclinical data
Dpp4 null mice demonstrate a cardioprotective gene/protein profile in the heart, smaller remodeled infarct scars, and reduced mortality after left anterior descending artery ligation (183). A similar cardioprotective phenotype was observed in wild-type diabetic mice treated with sitagliptin. Direct intracoronary administration of sitagliptin ex vivo did not improve left ventricular developed pressure in ischemia reperfusion studies of the isolated heart. In contrast, hearts from Dpp4−/− mice or wild-type mice treated with two doses of sitagliptin over 24 hours exhibited improved recovery of left ventricular developed pressure (183). Hence, DPP4 inhibition likely mediates cardioprotection indirectly through one or more substrates in vivo. Despite abundant preclinical evidence linking DPP4 inhibition to cardioprotection in mice or rats with cardiac ischemia (8), only GLP-1 and SDF-1 have been clearly identified as likely candidates transducing DPP4-dependent cardioprotection.
Several studies have employed GLP-1R blockade with exendin [9–39] to identify a role for GLP-1R signaling in DPP4-dependent cardioprotection. DPP4-deficient rats subjected to 45 minutes of ischemia with 2 hours of reperfusion exhibited cardioprotection characterized by reduced infarct size, better cardiac performance, and reduced levels of BNP compared to control rats, findings partially reversed by coadministration of exendin [9–39] (184). Similarly, administration of exendin [9–39] to sitagliptin-treated Sprague Dawley rats with transient cardiac ischemia increased cardiomyocyte apoptosis and reversed the sitagliptin-induced improvement in ventricular function (185).
Male C57Bl6 mice subjected to experimental myocardial infarction and treated with the CXCR4 inhibitor AMD3100 (1.25 mg/kg/d), in combination with diprotin A (70 mg/kg twice daily) and G-CSF (100 μg/kg/d), demonstrated an increase in infarct size 30 days after myocardial infarction, suggesting that the beneficial effects observed were mediated directly through a SDF-1α/CXCR4 signaling axis (186).
Interestingly, regulation of SDF-1α is maintained by signaling through another DPP4 substrate, G-CSF. Normally, within the microenvironment of the bone marrow, G-CSF induces DPP4 and suppresses intramarrow concentrations of SDF-1α. These conditions favor the egress of cells and increase homing to potential sites of injury. The importance of DPP4 in maintaining the signals for egress is demonstrated in Dpp4−/− mice that exhibited selectively defective responses to G-CSF with impaired mobilization of hematopoietic progenitor cells into peripheral blood (187). Consistent with these findings, diabetic DPP4 mutant F344 rats exhibited defective mobilization of Sca1+c-kit+ or Sca1+CD31+ progenitor cells in response to a 5-day treatment with G-CSF (188).
3. Myocardial infarction and heart failure: clinical data
The DPP4-regulated induction of circulating stem cells extends to diabetic human subjects because plasma SDF-1α levels and levels of CD34+KDR+ endothelial progenitor cells isolated by flow cytometry were increased in diabetic subjects after 4 weeks of daily sitagliptin administration (189). Although multiple preclinical studies implicate SDF-1 as a key cardioprotective substrate for DPP4 inhibitors (128, 186, 190), data affirming the importance of SDF-1 for cardioprotection in humans is lacking. The SITAGRAMI trial randomized patients with acute myocardial infarction to placebo or lenograstim (G-CSF, 10 μg/kg/d for 5 d) and sitagliptin (100 mg for 28 d) (191). No difference in ventricular function was observed, despite a 7-fold increase in circulating stem cell progenitors (CD34+, CD45−) at day 5.
Assessment of the cardiovascular safety of DPP4 inhibitors in two outcome studies revealed no differences in the rates of myocardial infarction or death from cardiovascular causes. Subjects randomized to receive either saxagliptin in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) or alogliptin in the Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care (EXAMINE) cardiovascular outcome trials did not experience a reduction in cardiovascular events (192, 193). Unexpectedly, the SAVOR-TIMI53 trial reported a small but significant increase in the rate of hospitalization for heart failure in patients treated with saxagliptin. Although the EXAMINE trial reported no statistically significant increase in hospitalization for heart failure in patients randomized to alogliptin, a small numeric excess of heart failure events was noted in alogliptin-treated subjects. The Vildagliptin in Ventricular Dysfunction Diabetes (VIVIDD) trial (194) was conducted in patients with type 2 diabetes and functional heart failure (Class I-III, New York Heart Association). There were no differences in left ventricular function reported after 1 year in patients randomized to vildagliptin or placebo, but an increase in left ventricular end-diastolic volume was reported for patients randomized to vildagliptin (195). Therefore, the current clinical trial data in patients with established cardiovascular disease does not support the data from preclinical studies suggesting that DPP4 inhibitors are cardioprotective.
4. Blood pressure and endothelial function: preclinical and clinical studies
DPP4 inhibitors reduce blood pressure in some preclinical studies, although potential mechanisms are poorly understood. DPP4 inhibitors produce direct vasodilatory effects in isolated preconstricted aortic rings, actions not requiring circulating DPP4 substrates, which may be transduced through nitric oxide and the cGMP signaling pathway (196). In prospective clinical trials, where flow-mediated dilatation (FMD) of the brachial artery was measured in patients with type 2 diabetes treated with sitagliptin (50 mg/d) or the α glucosidase inhibitor, voglibose (0.6 mg/d), endothelial function was significantly improved after 12 weeks of treatment (197). In contrast, data from other clinical trials assessing changes in vascular function after treatment with DPP4 inhibitors reveal different outcomes. Men with type 2 diabetes that received sitagliptin (50 mg/d) for 6 weeks exhibited significantly attenuated FMD, whereas treatment with voglibose (0.9 mg/d) had no effect on FMD. Furthermore, both sitagliptin (50 mg/d) and alogliptin (25 mg/d) significantly reduced FMD in subjects with type 2 diabetes (198). Hence, it is not possible to make clear conclusions about the effects of DPP4 inhibitors on endothelial function in diabetic subjects.
DPP4 inhibition also promotes natriuresis. Whether enhanced sodium excretion occurs through modulation of interactions with the renal sodium hydrogen exchange protein 3 is unclear; however, preclinical studies demonstrate that alogliptin acutely enhances the urinary flow rate and sodium secretion in both wild-type and Glp1r−/− mice (199). Hence, although GLP-1R agonists potently induce natriuresis through mechanisms requiring a functional GLP-1R, the mechanism(s) for increased natriuresis induced by DPP4 inhibition are not known. Similarly, although DPP4 inhibitors may produce a modest 1- to 2-mm reduction of systolic blood pressure in some clinical trials, the underlying mechanisms linking DPP4 inhibition to reduction in blood pressure have not been identified.
5. DPP4 and control of intestinal lipoprotein secretion: preclinical and clinical studies
DPP4 is expressed in high levels in the brush border membranes (enterocytes) and vascular and lymphatic endothelial cells of the small intestine; however, levels of DPP4 are much lower in the colon. Treatment of mice and hamsters with sitagliptin, either acutely or for several weeks, reduced production of triglyceride rich lipoproteins actions mimicked by the GLP-1R agonist exendin-4. Conversely, the lipid-lowering actions of sitagliptin were abrogated in wild-type mice treated with exendin [9–39] or in sitagliptin-treated Glp1r−/− mice (200).
Multiple studies in normoglycemic, glucose-intolerant, and diabetic human subjects have demonstrated that DPP4 inhibitors reduce postprandial levels of apoB48-containing lipoproteins, plasma triglyceride, and circulating free fatty acids (201, 202). Furthermore, kinetic studies in healthy nondiabetic human subjects studied under pancreatic clamp conditions demonstrated that sitagliptin reduced the production rate of apoB48 lipoproteins, with no effect noted on the production or clearance of ApoB100 (203). The mechanisms underlying the effects of DPP4 inhibitors, acting through GLP-1R signaling, to inhibit intestinal lipoprotein secretion, remain to be determined.
C. Autoimmune disorders and inflammation
Dpp4 −/− mice exhibit subtle but significant differences in proportions of peripheral lymphocyte subpopulations in spleen and peripheral blood, and activated Dpp4−/− splenocytes exhibit dysregulated stimulation of cytokine production ex vivo with significant decreases in IL-4 production and increases in IL-10 and interferon-γ. Furthermore, Ig responses to immunization with pokeweed mitogen were lower in Dpp4−/− mice; together, these results suggest a requirement for Dpp4 in the growth and maturation of CD4+, NK, and NKT cells (204). However, whether these defects reflect the selective absence of catalytic DPP4 activity or enzyme-independent requirements for DPP4 signaling was not discerned. Vora et al (205) compared immune responses in Dpp4−/− mice with responses obtained after identical immune challenges in wild-type mice treated with a highly selective DPP4 inhibitor. Antibody responses to T-cell antigen were comparable, and T-cell responses to multiple antigenic stimuli were identical in Dpp4−/− mice vs Dpp4+/+ mice vs wild-type mice treated with a selective DPP inhibitor (205). Enhanced airway inflammation characterized by increased cellular infiltration and enhanced cytokine production was observed in Dpp4−/− mice challenged with systemic and aerosolized ovalbumin (206). Whether these findings reflected global loss of DPP4 or reduction of DPP4 catalytic activity was not determined.
Chemical inhibition of DPP4 activity attenuates features of encephalomyelitis; however, most of these studies use nonselective DPP4 inhibitors, and mechanisms linking selective DPP4 inhibition to immunomodulation remain incompletely understood. In contrast, several studies administering highly selective DPP4 inhibitors demonstrate delay of diabetes onset, decreased insulitis, increased numbers of regulatory T cells, and improved survival of transplanted islets in nonobese diabetic mice, a widely studied model of type 1 diabetes (207). Remarkably, ex vivo treatment with sDPP4 increased migration of CD4+ T cells; hence, the authors envisioned a direct role for DPP4 inhibitors in the control of T-cell migration by modulating DPP4 catalytic activity in vivo (208, 209).
Immunomodulatory actions of sitagliptin have also been described in human subjects with type 2 diabetes. A single administration of sitagliptin 100 mg reduced expression of Toll-like receptor 2, I-kappa-B kinaseβ, C-C chemokine receptor type 2, and CD26 and decreased nuclear factor-κB binding in isolated peripheral blood mononuclear cells (210). However, van Poppel et al (211) found no effect of 4-week therapy with vildagliptin vs acarbose on plasma cytokine levels or cytokine production ex vivo in mononuclear cells from subjects with type 2 diabetes. Moreover, no significant differences in plasma levels of TGF-β, plasma cytokine or chemokine levels, or proportions of major B- and T-cell lymphocyte subsets, including numbers of regulatory T cells, were observed in healthy human subjects treated with placebo or sitagliptin 100 mg daily for 28 days (212). Despite considerable evidence linking DPP4 to control of retroviral entry, selective inhibition of DPP4 activity in HIV-positive human subjects treated with sitagliptin, 100 mg daily, for up to 26 weeks had no effect on CD4+ T cells, levels of plasma HIV RNA, or plasma chemokine levels (213). Hence, although multiple studies report modulation of inflammation and immune parameters after reduction of DPP4 activity, most studies examining anti-inflammatory actions of DPP4 inhibitors do not control for the indirect beneficial consequences of improving metabolic control. Furthermore, some of the effects of DPP4 in mediating viral entry or immune function are independent of its catalytic activity. Hence, it remains unclear whether partial selective reduction of DPP4 enzymatic activity directly regulates inflammation or the cellular immune response in preclinical and clinical settings.
D. Miscellaneous metabolic actions of DPP4 inhibitors
1. Liver
Treatment of diabetic rodents with DPP4 inhibitors reduces hepatic steatosis and fibrosis and decreases hepatic inflammation; however, the underlying mechanisms have not been identified (214). Similarly, high-fat-fed F344/DuCrj DPP4 rats exhibited a reduction in both plasma and hepatic triglyceride and lower levels of molecular markers of proinflammatory and profibrotic cytokines including IL-6, TNFα, plasminogen activator inhibitor-1, connective tissue growth factor and TGFβ (215). Although the authors postulate that these actions were mediated through potentiation of direct GLP-1 actions on hepatocytes, it seems unlikely that hepatocytes express the canonical GLP-1R (216). DPP4 inhibitors also reduce liver enzymes in clinical studies; however, whether these salutary actions are secondary to or independent of improvements in glycemic control is unknown.
2. Kidney
DPP4 has been localized to the brush border membrane of the proximal tubules (217), the capillary endothelium of glomeruli, and the epithelium of Bowman's capsule. Multiple studies in rats and mice demonstrate renoprotective actions of DPP4 inhibitors in experimental kidney injury, often associated with a reduction in albumin excretion, reduced expression of inflammatory markers, and decreased oxidative stress. Liu et al (218) demonstrated that sitagliptin improved renal blood flow and endothelium-dependent relaxation in renal arteries and decreased blood pressure in rats with spontaneous hypertension, findings attenuated by inhibition of cAMP or GLP-1R signaling. However, in most studies, the molecular mechanisms through which DPP4 inhibition attenuates renal injury have not been identified. Similarly, several clinical trials demonstrate reduction of microalbuminuria in subjects with type 2 diabetes treated with DPP4 inhibitors (219); however, there are little mechanistic data linking reduction of DPP4 activity to improvements in kidney function independent of changes in glucose, inflammation, or blood pressure.
3. Bone
Several DPP4 substrates including GIP, GLP-1, PYY, NPY, and GLP-2 regulate bone formation or resorption in rodents (220). Dpp4−/− mice exhibited normal bone formation (221), and treatment of high-fat-fed mice with sitagliptin had no effect on bone quality (221); however, sitagliptin increased trabecular bone mineralization and architecture. Nevertheless, the mechanisms and key substrates through which DPP4 regulates bone turnover in preclinical studies have not been addressed. Although diabetic subjects treated with DPP4 inhibitors exhibited modest reductions in markers of bone turnover (222), the available data examining putative changes in bone density or fracture rates are not sufficient for firm conclusions to be drawn.
VII. Summary and Future Directions
Since the regulatory approval of the first DPP4 inhibitor on October 17, 2006, millions of patients with type 2 diabetes have been treated with DPP4 inhibitors. Surprising to some, despite the pleiotropic actions of DPP4 and the large number of potential DPP4 substrates, these drugs have been very well tolerated, and few major adverse events have emerged (12). The recent description of increased rates of hospitalization for heart failure in a small subset of saxagliptin-treated patients mandates additional investigation to identify potential mechanisms linking DPP4 inhibition to potential impairment of ventricular function in some individuals (192).
Despite hundreds of preclinical studies examining DPP4 action in experimental models of disease, our fundamental understanding of key substrates transducing important actions of DPP4 inhibitors remains limited (Figure 4). Furthermore, despite the potential importance of DPP4 for cleavage of target substrates, many putative DPP4 substrates are cleaved by multiple enzymes, and their disappearance from plasma is largely regulated by renal and, to a lesser extent, hepatic clearance. Hence, identification of a dominant role for DPP4 in controlling peptide bioactivity is often challenging. New methodologies using liquid chromatography-mass spectrometry-based technology to perform global peptide profiling are discovering novel DPP4 substrates by directly measuring changes in levels of intact vs cleaved peptides in response to genetic deletion or catalytic inhibition of DPP4 (223–226). These types of analyses should expedite identification of multiple new physiological DPP4 substrates and will generate new hypotheses surrounding the mechanisms of action of DPP4 inhibitors. The combination of modern peptide profiling technology with state of the art biochemistry represents a powerful promising approach for deciphering the mechanisms and key substrates transducing classical and novel actions of DPP4 inhibitors in animals and humans.
Figure 4.
Endocrine pathways altered during DPP4 inhibition. Inhibition of DPP4 protease activity with selective DPP inhibitors produces multiple biological actions in peripheral tissues. The peptide substrates that have been identified as downstream targets mediating key cardiometabolic actions of DPP4 are indicated.
Acknowledgments
We thank Apothecom for their expert assistance in preparing the figures.
D.J.D. is supported by a Canada Research Chair in Regulatory Peptides and the Banting and Best Diabetes Center-Novo Nordisk Chair in Incretin Biology and Heart and Stroke Foundation of Canada Grant in Aid G-14–0005953 1417. E.E.M. has received postdoctoral fellowship funding from the Canadian Diabetes Association and Canadian Institute of Health Research.
Disclosure Summary: D.J.D. has served as an advisor or consultant within the past 12 months to Arisaph Pharmaceuticals Inc, Intarcia, Merck Research Laboratories, Novo Nordisk Inc, Sanofi, Takeda, Teva, and Transition Pharmaceuticals Inc. Neither D.J.D. nor his family members hold stock directly or indirectly in any of these companies. D.J.D. is one of several parties to a master patent licensing agreement for DPP4 inhibitor patents, together with University Health Network, University of Toronto, Tufts University, Arisaph, and Prosidion.
Abbreviations
- ADA
adenosine deaminase
- BMI
body mass index
- BNP
brain natriuretic peptide
- CCL3L1
chemokine (C-C motif) ligand 3-like 1
- CD
cluster of differentiation
- DIRKO
double incretin receptor knockout
- DPP4
dipeptidyl peptidase-4
- FAP
fibroblast activation protein
- FMD
flow-mediated dilatation
- G-CSF
granulocyte colony-stimulating factor
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- GLP-1R
GLP-1 receptor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GRP
gastrin-releasing peptide
- HbA1c
glycated hemoglobin
- HMGB1
high-mobility group box 1
- MDC
macrophage-derived chemokine
- MIP-1 α
macrophage inflammatory protein-1 α
- NK1R
neurokinin-1 receptor
- NPY
neuropeptide Y
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PC
prohormone convertase
- PYY
peptide tyrosine tyrosine
- Rantes
regulated on activation, normal T cell expressed and secreted
- SB
small bowel
- SDF-1
stromal cell-derived factor-1
- sDPP4
soluble DPP4
- SP
substance P
- VIP
vasoactive intestinal peptide
- VPAC
vasoactive intestinal peptide pituitary adenylate cyclase activation peptide.
References
- 1. Lambeir AM, Durinx C, Scharpé S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. [DOI] [PubMed] [Google Scholar]
- 2. Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. [DOI] [PubMed] [Google Scholar]
- 3. López-Otín C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. [DOI] [PubMed] [Google Scholar]
- 4. Lu G, Hu Y, Wang Q, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kahne T, Lendeckel U, Wrenger S, Neubert K, Ansorge S, Reinhold D. Dipeptidyl peptidase IV: a cell surface peptidase involved in regulating T cell growth (review). Int J Mol Med. 1999;4:3–15. [DOI] [PubMed] [Google Scholar]
- 6. Davidson JA. The placement of DPP-4 inhibitors in clinical practice recommendations for the treatment of type 2 diabetes. Endocr Pract. 2013;19:1050–1061. [DOI] [PubMed] [Google Scholar]
- 7. Deacon CF, Holst JJ. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety. Expert Opin Pharmacother. 2013;14:2047–2058. [DOI] [PubMed] [Google Scholar]
- 8. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solun B, Marcoviciu D, Dicker D. Dipeptidyl peptidase-4 inhibitors and their effects on the cardiovascular system. Curr Cardiol Rep. 2013;15:382. [DOI] [PubMed] [Google Scholar]
- 10. Scheen AJ. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors: from risk factors to clinical outcomes. Postgrad Med. 2013;125:7–20. [DOI] [PubMed] [Google Scholar]
- 11. Ramirez G, Morrison AD, Bittle PA. Clinical practice considerations and review of the literature for the use of DPP-4 inhibitors in patients with type 2 diabetes and chronic kidney disease. Endocr Pract. 2013;19:1025–1034. [DOI] [PubMed] [Google Scholar]
- 12. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs–FDA and EMA assessment. N Engl J Med. 2014;370:794–797. [DOI] [PubMed] [Google Scholar]
- 13. Hopsu-Havu VK, Glenner GG. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-β-naphthylamide. Histochemie. 1966;7:197–201. [DOI] [PubMed] [Google Scholar]
- 14. McCaughan GW, Wickson JE, Creswick PF, Gorrell MD. Identification of the bile canalicular cell surface molecule GP110 as the ectopeptidase dipeptidyl peptidase IV: an analysis by tissue distribution, purification and N-terminal amino acid sequence. Hepatology. 1990;11:534–544. [DOI] [PubMed] [Google Scholar]
- 15. Ulmer AJ, Mattern T, Feller AC, Heymann E, Flad HD. CD26 antigen is a surface dipeptidyl peptidase IV (DPPIV) as characterized by monoclonal antibodies clone TII-19–4–7 and 4EL1C7. Scand J Immunol. 1990;31:429–435. [DOI] [PubMed] [Google Scholar]
- 16. Vivier I, Marguet D, Naquet P, et al. Evidence that thymocyte-activating molecule is mouse CD26 (dipeptidyl peptidase IV). J Immunol. 1991;147:447–454. [PubMed] [Google Scholar]
- 17. Misumi Y, Hayashi Y, Arakawa F, Ikehara Y. Molecular cloning and sequence analysis of human dipeptidyl peptidase IV, a serine proteinase on the cell surface. Biochim Biophys Acta. 1992;1131:333–336. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka T, Camerini D, Seed B, et al. Cloning and functional expression of the T cell activation antigen CD26. J Immunol. 1992;149:481–486. [PubMed] [Google Scholar]
- 19. Abbott CA, Baker E, Sutherland GR, McCaughan GW. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics. 1994;40:331–338. [DOI] [PubMed] [Google Scholar]
- 20. Bernard AM, Mattei MG, Pierres M, Marguet D. Structure of the mouse dipeptidyl peptidase IV (CD26) gene. Biochemistry. 1994;33:15204–15214. [DOI] [PubMed] [Google Scholar]
- 21. Fukasawa KM, Fukasawa K, Sahara N, Harada M, Kondo Y, Nagatsu I. Immunohistochemical localization of dipeptidyl aminopeptidase IV in rat kidney, liver, and salivary glands. J Histochem Cytochem. 1981;29:337–343. [DOI] [PubMed] [Google Scholar]
- 22. Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept. 2005;128:117–124. [DOI] [PubMed] [Google Scholar]
- 23. Engel M, Hoffmann T, Wagner L, et al. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc Natl Acad Sci USA. 2003;100:5063–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Busek P, Malík R, Sedo A. Dipeptidyl peptidase IV activity and/or structure homologues (DASH) and their substrates in cancer. Int J Biochem Cell Biol. 2004;36:408–421. [DOI] [PubMed] [Google Scholar]
- 25. Hiramatsu H, Kyono K, Shima H, et al. Crystallization and preliminary x-ray study of human dipeptidyl peptidase IV (DPPIV). Acta Crystallogr D Biol Crystallogr. 2003;59:595–596. [DOI] [PubMed] [Google Scholar]
- 26. Rasmussen HB, Branner S, Wiberg FC, Wagtmann N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat Struct Biol. 2003;10:19–25. [DOI] [PubMed] [Google Scholar]
- 27. Chien CH, Huang LH, Chou CY, et al. One site mutation disrupts dimer formation in human DPP-IV proteins. J Biol Chem. 2004;279:52338–52345. [DOI] [PubMed] [Google Scholar]
- 28. Chien CH, Tsai CH, Lin CH, Chou CY, Chen X. Identification of hydrophobic residues critical for DPP-IV dimerization. Biochemistry. 2006;45:7006–7012. [DOI] [PubMed] [Google Scholar]
- 29. Scanlan MJ, Raj BK, Calvo B, et al. Molecular cloning of fibroblast activation protein α, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA. 1994;91:5657–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghersi G, Dong H, Goldstein LA, et al. Seprase-dPPIV association and prolyl peptidase and gelatinase activities of the protease complex. Adv Exp Med Biol. 2003;524:87–94. [DOI] [PubMed] [Google Scholar]
- 31. Ohnuma K, Yamochi T, Uchiyama M, et al. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc Natl Acad Sci USA. 2004;101:14186–14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohnuma K, Uchiyama M, Yamochi T, et al. Caveolin-1 triggers T-cell activation via CD26 in association with CARMA1. J Biol Chem. 2007;282:10117–10131. [DOI] [PubMed] [Google Scholar]
- 33. Torimoto Y, Dang NH, Vivier E, Tanaka T, Schlossman SF, Morimoto C. Coassociation of CD26 (dipeptidyl peptidase IV) with CD45 on the surface of human T lymphocytes. J Immunol. 1991;147:2514–2517. [PubMed] [Google Scholar]
- 34. De Meester I, Korom S, Van Damme J, Scharpé S. CD26, let it cut or cut it down. Immunol Today. 1999;20:367–375. [DOI] [PubMed] [Google Scholar]
- 35. Durinx C, Lambeir AM, Bosmans E, et al. Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is responsible for the release of X-Pro dipeptides. Eur J Biochem. 2000;267:5608–5613. [DOI] [PubMed] [Google Scholar]
- 36. Nagatsu I, Nagatsu T, Yamamoto T. Hydrolysis of amino acid β-naphthylamides by aminopeptidases in human parotid salva and human serum. Experientia. 1968;24:347–348. [DOI] [PubMed] [Google Scholar]
- 37. Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother. 2009;58:1723–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu DM, Slaitini L, Gysbers V, et al. Soluble CD26/dipeptidyl peptidase IV enhances human lymphocyte proliferation in vitro independent of dipeptidyl peptidase enzyme activity and adenosine deaminase binding. Scand J Immunol. 2011;73:102–111. [DOI] [PubMed] [Google Scholar]
- 39. Lamers D, Famulla S, Wronkowitz N, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohnuma K, Munakata Y, Ishii T, et al. Soluble CD26/dipeptidyl peptidase IV induces T cell proliferation through CD86 up-regulation on APCs. J Immunol. 2001;167:6745–6755. [DOI] [PubMed] [Google Scholar]
- 41. Ikushima H, Munakata Y, Iwata S, et al. Soluble CD26/dipeptidyl peptidase IV enhances transendothelial migration via its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cell Immunol. 2002;215:106–110. [DOI] [PubMed] [Google Scholar]
- 42. Zhong J, Rao X, Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis. 2013;226:305–314. [DOI] [PubMed] [Google Scholar]
- 43. Erickson RH, Gum JR, Lotterman CD, Hicks JW, Lai RS, Kim YS. Regulation of the gene for human dipeptidyl peptidase IV by hepatocyte nuclear factor 1 α. Biochem J. 1999;338:91–97. [PMC free article] [PubMed] [Google Scholar]
- 44. Qvist H, Sjöström H, Norén O. The TATA-less, GC-rich porcine dipeptidylpeptidase IV (DPPIV) promoter shows bidirectional activity. Biol Chem. 1998;379:75–81. [PubMed] [Google Scholar]
- 45. Böhm SK, Gum JR Jr, Erickson RH, Hicks JW, Kim YS. Human dipeptidyl peptidase IV gene promoter: tissue-specific regulation from a TATA-less GC-rich sequence characteristic of a housekeeping gene promoter. Biochem J. 1995;311:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bauvois B, Djavaheri-Mergny M, Rouillard D, Dumont J, Wietzerbin J. Regulation of CD26/DPPIV gene expression by interferons and retinoic acid in tumor B cells. Oncogene. 2000;19:265–272. [DOI] [PubMed] [Google Scholar]
- 47. Mattern T, Reich C, Duchrow M, Ansorge S, Ulmer AJ, Flad HD. Antibody-induced modulation of CD26 surface expression. Immunology. 1995;84:595–600. [PMC free article] [PubMed] [Google Scholar]
- 48. Cordero OJ, Salgado FJ, Viñuela JE, Nogueira M. Interleukin-12 enhances CD26 expression and dipeptidyl peptidase IV function on human activated lymphocytes. Immunobiology. 1997;197:522–533. [DOI] [PubMed] [Google Scholar]
- 49. Sell H, Blüher M, Klöting N, et al. Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care. 2013;36:4083–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Z, Grigo C, Steinbeck J, von Hörsten S, Amann K, Daniel C. Soluble DPP4 originates in part from bone marrow cells and not from the kidney. Peptides. 2014;57:109–117. [DOI] [PubMed] [Google Scholar]
- 51. Fan H, Meng W, Kilian C, Grams S, Reutter W. Domain-specific N-glycosylation of the membrane glycoprotein dipeptidylpeptidase IV (CD26) influences its subcellular trafficking, biological stability, enzyme activity and protein folding. Eur J Biochem. 1997;246:243–251. [DOI] [PubMed] [Google Scholar]
- 52. Marguet D, Baggio L, Kobayashi T, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA. 2000;97:6874–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aertgeerts K, Ye S, Shi L, et al. N-linked glycosylation of dipeptidyl peptidase IV (CD26): effects on enzyme activity, homodimer formation, and adenosine deaminase binding. Protein Sci. 2004;13:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Delacour D, Gouyer V, Leteurtre E, et al. 1-Benzyl-2-acetamido-2-deoxy-α-D-galactopyranoside blocks the apical biosynthetic pathway in polarized HT-29 cells. J Biol Chem. 2003;278:37799–37809. [DOI] [PubMed] [Google Scholar]
- 55. Yamashita K, Tachibana Y, Matsuda Y, Katunuma N, Kochibe N, Kobata A. Comparative studies of the sugar chains of aminopeptidase N and dipeptidylpeptidase IV purified from rat kidney brush-border membrane. Biochemistry. 1988;27:5565–5573. [DOI] [PubMed] [Google Scholar]
- 56. Smith RE, Talhouk JW, Brown EE, Edgar SE. The significance of hypersialylation of dipeptidyl peptidase IV (CD26) in the inhibition of its activity by Tat and other cationic peptides. CD26: a subverted adhesion molecule for HIV peptide binding. AIDS Res Hum Retroviruses. 1998;14:851–868. [DOI] [PubMed] [Google Scholar]
- 57. Cuchacovich M, Gatica H, Pizzo SV, Gonzalez-Gronow M. Characterization of human serum dipeptidyl peptidase IV (CD26) and analysis of its autoantibodies in patients with rheumatoid arthritis and other autoimmune diseases. Clin Exp Rheumatol. 2001;19:673–680. [PubMed] [Google Scholar]
- 58. Tiruppathi C, Miyamoto Y, Ganapathy V, Leibach FH. Genetic evidence for role of DPP IV in intestinal hydrolysis and assimilation of prolyl peptides. Am J Physiol. 1993;265:G81–G89. [DOI] [PubMed] [Google Scholar]
- 59. Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpé S, De Meester I. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem. 2006;52:82–87. [DOI] [PubMed] [Google Scholar]
- 60. Niederkofler EE, Kiernan UA, O'Rear J, et al. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail. 2008;1:258–264. [DOI] [PubMed] [Google Scholar]
- 61. Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC Jr. Des-serine-proline brain natriuretic peptide 3–32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–R901. [DOI] [PubMed] [Google Scholar]
- 62. Gomez N, Touihri K, Matheeussen V, et al. Dipeptidyl peptidase IV inhibition improves cardiorenal function in overpacing-induced heart failure. Eur J Heart Fail. 2012;14:14–21. [DOI] [PubMed] [Google Scholar]
- 63. Broxmeyer HE, Hoggatt J, O'Leary HA, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18:1786–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Struyf S, Proost P, Schols D, et al. CD26/dipeptidyl-peptidase IV down-regulates the eosinophil chemotactic potency, but not the anti-HIV activity of human eotaxin by affecting its interaction with CC chemokine receptor 3. J Immunol. 1999;162:4903–4909. [PubMed] [Google Scholar]
- 65. Spindel ER, Giladi E, Segerson TP, Nagalla S. Bombesin-like peptides: of ligands and receptors. Recent Prog Horm Res. 1993;48:365–391. [DOI] [PubMed] [Google Scholar]
- 66. Lambeir AM, Durinx C, Proost P, Van Damme J, Scharpé S, De Meester I. Kinetic study of the processing by dipeptidyl-peptidase IV/CD26 of neuropeptides involved in pancreatic insulin secretion. FEBS Lett. 2001;507:327–330. [DOI] [PubMed] [Google Scholar]
- 67. Ahrén B, Hughes TE. Inhibition of dipeptidyl peptidase-4 augments insulin secretion in response to exogenously administered glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pituitary adenylate cyclase-activating polypeptide, and gastrin-releasing peptide in mice. Endocrinology. 2005;146:2055–2059. [DOI] [PubMed] [Google Scholar]
- 68. Roberge JN, Gronau KA, Brubaker PL. Gastrin-releasing peptide is a novel mediator of proximal nutrient-induced proglucagon-derived peptide secretion from the distal gut. Endocrinology. 1996;137:2383–2388. [DOI] [PubMed] [Google Scholar]
- 69. Reeve JR Jr, Walsh JH, Chew P, Clark B, Hawke D, Shively JE. Amino acid sequences of three bombesin-like peptides from canine intestine extracts. J Biol Chem. 1983;258:5582–5588. [PubMed] [Google Scholar]
- 70. Pospisilik JA, Hinke SA, Pederson RA, et al. Metabolism of glucagon by dipeptidyl peptidase IV (CD26). Regul Pept. 2001;96:133–141. [DOI] [PubMed] [Google Scholar]
- 71. Deacon CF, Kelstrup M, Trebbien R, Klarskov L, Olesen M, Holst JJ. Differential regional metabolism of glucagon in anesthetized pigs. Am J Physiol Endocrinol Metab. 2003;285:E552–E560. [DOI] [PubMed] [Google Scholar]
- 72. Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–957. [DOI] [PubMed] [Google Scholar]
- 73. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. [DOI] [PubMed] [Google Scholar]
- 74. Knudsen LB, Pridal L. Glucagon-like peptide-1-(9–36) amide is a major metabolite of glucagon-like peptide-1-(7–36) amide after in vivo administration to dogs, and it acts as an antagonist on the pancreatic receptor. Eur J Pharmacol. 1996;318:429–435. [DOI] [PubMed] [Google Scholar]
- 75. Nagakura T, Yasuda N, Yamazaki K, et al. Improved glucose tolerance via enhanced glucose-dependent insulin secretion in dipeptidyl peptidase IV-deficient Fischer rats. Biochem Biophys Res Commun. 2001;284:501–506. [DOI] [PubMed] [Google Scholar]
- 76. Meier JJ, Gethmann A, Nauck MA, et al. The glucagon-like peptide-1 metabolite GLP-1-(9–36) amide reduces postprandial glycemia independently of gastric emptying and insulin secretion in humans. Am J Physiol Endocrinol Metab. 2006;290:E1118–E1123. [DOI] [PubMed] [Google Scholar]
- 77. Elahi D, Egan JM, Shannon RP, et al. GLP-1 (9–36) amide, cleavage product of GLP-1 (7–36) amide, is a glucoregulatory peptide. Obesity (Silver Spring). 2008;16:1501–1509. [DOI] [PubMed] [Google Scholar]
- 78. Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: the extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94:1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brubaker PL, Crivici A, Izzo A, Ehrlich P, Tsai CH, Drucker DJ. Circulating and tissue forms of the intestinal growth factor, glucagon-like peptide-2. Endocrinology. 1997;138:4837–4843. [DOI] [PubMed] [Google Scholar]
- 81. Hartmann B, Harr MB, Jeppesen PB, et al. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab. 2000;85:2884–2888. [DOI] [PubMed] [Google Scholar]
- 82. Shin ED, Estall JL, Izzo A, Drucker DJ, Brubaker PL. Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide-2 in mice. Gastroenterology. 2005;128:1340–1353. [DOI] [PubMed] [Google Scholar]
- 83. Thulesen J, Knudsen LB, Hartmann B, et al. The truncated metabolite GLP-2 (3–33) interacts with the GLP-2 receptor as a partial agonist. Regul Pept. 2002;103:9–15. [DOI] [PubMed] [Google Scholar]
- 84. Drucker DJ, Shi Q, Crivici A, et al. Regulation of the biological activity of glucagon-like peptide 2 in vivo by dipeptidyl peptidase IV. Nat Biotechnol. 1997;15:673–677. [DOI] [PubMed] [Google Scholar]
- 85. Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561–583. [DOI] [PubMed] [Google Scholar]
- 86. Okawada M, Holst JJ, Teitelbaum DH. Administration of a dipeptidyl peptidase IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome. Surgery. 2011;150:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hansen L, Hare KJ, Hartmann B, et al. Metabolism of glucagon-like peptide-2 in pigs: role of dipeptidyl peptidase IV. Regul Pept. 2007;138:126–132. [DOI] [PubMed] [Google Scholar]
- 88. Hartmann B, Thulesen J, Kissow H, et al. Dipeptidyl peptidase IV inhibition enhances the intestinotrophic effect of glucagon-like peptide-2 in rats and mice. Endocrinology. 2000;141:4013–4020. [DOI] [PubMed] [Google Scholar]
- 89. Fujita Y, Wideman RD, Asadi A, et al. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet α-cells and promotes insulin secretion. Gastroenterology. 2010;138:1966–1975. [DOI] [PubMed] [Google Scholar]
- 90. Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. [DOI] [PubMed] [Google Scholar]
- 91. Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. [DOI] [PubMed] [Google Scholar]
- 92. Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab. 2000;85:3575–3581. [DOI] [PubMed] [Google Scholar]
- 93. Deacon CF, Plamboeck A, Rosenkilde MM, de Heer J, Holst JJ. GIP-(3–42) does not antagonize insulinotropic effects of GIP at physiological concentrations. Am J Physiol Endocrinol Metab. 2006;291:E468–E475. [DOI] [PubMed] [Google Scholar]
- 94. Gault VA, Parker JC, Harriott P, Flatt PR, O'Harte FP. Evidence that the major degradation product of glucose-dependent insulinotropic polypeptide, GIP(3–42), is a GIP receptor antagonist in vivo. J Endocrinol. 2002;175:525–533. [DOI] [PubMed] [Google Scholar]
- 95. Deacon CF, Wamberg S, Bie P, Hughes TE, Holst JJ. Preservation of active incretin hormones by inhibition of dipeptidyl peptidase IV suppresses meal-induced incretin secretion in dogs. J Endocrinol. 2002;172:355–362. [DOI] [PubMed] [Google Scholar]
- 96. Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612–4619. [DOI] [PubMed] [Google Scholar]
- 97. Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34:81–89. [DOI] [PubMed] [Google Scholar]
- 98. Frohman LA, Downs TR, Williams TC, Heimer EP, Pan YC, Felix AM. Rapid enzymatic degradation of growth hormone-releasing hormone by plasma in vitro and in vivo to a biologically inactive product cleaved at the NH2 terminus. J Clin Invest. 1986;78:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Frohman LA, Downs TR, Heimer EP, Felix AM. Dipeptidylpeptidase IV and trypsin-like enzymatic degradation of human growth hormone-releasing hormone in plasma. J Clin Invest. 1989;83:1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lin CT, Tang HY, Han YS, et al. Downregulation of signaling-active IGF-1 by dipeptidyl peptidase IV (DPP-IV). Int J Biomed Sci. 2010;6:301–309. [PMC free article] [PubMed] [Google Scholar]
- 101. Faidley TD, Leiting B, Pryor KD, Lyons K, Hickey GJ, Thompson DR. Inhibition of dipeptidyl-peptidase IV does not increase circulating IGF-1 concentrations in growing pigs. Exp Biol Med (Maywood). 2006;231:1373–1378. [DOI] [PubMed] [Google Scholar]
- 102. Bergman AJ, Stevens C, Zhou Y, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72. [DOI] [PubMed] [Google Scholar]
- 103. Marchetti C, Di Carlo A, Facchiano F, et al. High mobility group box 1 is a novel substrate of dipeptidyl peptidase-IV. Diabetologia. 2012;55:236–244. [DOI] [PubMed] [Google Scholar]
- 104. Proost P, Struyf S, Schols D, et al. Truncation of macrophage-derived chemokine by CD26/dipeptidyl-peptidase IV beyond its predicted cleavage site affects chemotactic activity and CC chemokine receptor 4 interaction. J Biol Chem. 1999;274:3988–3993. [DOI] [PubMed] [Google Scholar]
- 105. Struyf S, Proost P, Sozzani S, et al. Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemokine (MDC) imply an additional MDC receptor. J Immunol. 1998;161:2672–2675. [PubMed] [Google Scholar]
- 106. Proost P, Menten P, Struyf S, Schutyser E, De Meester I, Van Damme J. Cleavage by CD26/dipeptidyl peptidase IV converts the chemokine LD78β into a most efficient monocyte attractant and CCR1 agonist. Blood. 2000;96:1674–1680. [PubMed] [Google Scholar]
- 107. Pocai A. Unraveling oxyntomodulin, GLP1's enigmatic brother. J Endocrinol. 2012;215:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127:546–558. [DOI] [PubMed] [Google Scholar]
- 109. Pocai A. Action and therapeutic potential of oxyntomodulin. Mol Metab. 2014;3:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhu L, Tamvakopoulos C, Xie D, et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38). J Biol Chem. 2003;278:22418–22423. [DOI] [PubMed] [Google Scholar]
- 111. Bak MJ, Albrechtsen NW, Pedersen J, et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol. 2014;170:529–538. [DOI] [PubMed] [Google Scholar]
- 112. Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. [DOI] [PubMed] [Google Scholar]
- 113. Harmar AJ, Fahrenkrug J, Gozes I, et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 2012;166:4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yada T, Sakurada M, Ihida K, et al. Pituitary adenylate cyclase activating polypeptide is an extraordinarily potent intra-pancreatic regulator of insulin secretion from islet β-cells. J Biol Chem. 1994;269:1290–1293. [PubMed] [Google Scholar]
- 115. Jamen F, Persson K, Bertrand G, et al. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest. 2000;105:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Brothers SP, Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol Med. 2010;2:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Frerker N, Wagner L, Wolf R, et al. Neuropeptide Y (NPY) cleaving enzymes: structural and functional homologues of dipeptidyl peptidase 4. Peptides. 2007;28:257–268. [DOI] [PubMed] [Google Scholar]
- 118. Baticic L, Detel D, Kucic N, Buljevic S, Pugel EP, Varljen J. Neuroimmunomodulative properties of dipeptidyl peptidase IV/CD26 in a TNBS-induced model of colitis in mice. J Cell Biochem. 2011;112:3322–3333. [DOI] [PubMed] [Google Scholar]
- 119. Mentlein R, Dahms P, Grandt D, Krüger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept. 1993;49:133–144. [DOI] [PubMed] [Google Scholar]
- 120. Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. [DOI] [PubMed] [Google Scholar]
- 121. Unniappan S, McIntosh CH, Demuth HU, Heiser U, Wolf R, Kieffer TJ. Effects of dipeptidyl peptidase IV on the satiety actions of peptide YY. Diabetologia. 2006;49:1915–1923. [DOI] [PubMed] [Google Scholar]
- 122. Aaboe K, Knop FK, Vilsbøll T, et al. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12:323–333. [DOI] [PubMed] [Google Scholar]
- 123. Oravecz T, Pall M, Roderiquez G, et al. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med. 1997;186:1865–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Proost P, Schutyser E, Menten P, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98:3554–3561. [DOI] [PubMed] [Google Scholar]
- 125. Iwata S, Yamaguchi N, Munakata Y, et al. CD26/dipeptidyl peptidase IV differentially regulates the chemotaxis of T cells and monocytes toward RANTES: possible mechanism for the switch from innate to acquired immune response. Int Immunol. 1999;11:417–426. [DOI] [PubMed] [Google Scholar]
- 126. Shirozu M, Nakano T, Inazawa J, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. [DOI] [PubMed] [Google Scholar]
- 127. Shioda T, Kato H, Ohnishi Y, et al. Anti-HIV-1 and chemotactic activities of human stromal cell-derived factor 1α (SDF-1α) and SDF-1β are abolished by CD26/dipeptidyl peptidase IV-mediated cleavage. Proc Natl Acad Sci USA. 1998;95:6331–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zaruba MM, Theiss HD, Vallaster M, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. [DOI] [PubMed] [Google Scholar]
- 129. Wang W, Choi BK, Li W, et al. Quantification of intact and truncated stromal cell-derived factor-1α in circulation by immunoaffinity enrichment and tandem mass spectrometry. J Am Soc Mass Spectrom. 2014;25:614–625. [DOI] [PubMed] [Google Scholar]
- 130. Busso N, Wagtmann N, Herling C, et al. Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol. 2005;166:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ahmad S, Wang L, Ward PE. Dipeptidyl(amino)peptidase IV and aminopeptidase M metabolize circulating substance P in vivo. J Pharmacol Exp Ther. 1992;260:1257–1261. [PubMed] [Google Scholar]
- 132. Guieu R, Fenouillet E, Devaux C, et al. CD26 modulates nociception in mice via its dipeptidyl-peptidase IV activity. Behav Brain Res. 2006;166:230–235. [DOI] [PubMed] [Google Scholar]
- 133. Grouzmann E, Bigliardi P, Appenzeller M, Pannatier A, Buclin T. Substance P-induced skin inflammation is not modulated by a single dose of sitagliptin in human volunteers. Biol Chem. 2011;392:217–221. [DOI] [PubMed] [Google Scholar]
- 134. Cordero OJ, Salgado FJ, Mera-Varela A, Nogueira M. Serum interleukin-12, interleukin-15, soluble CD26, and adenosine deaminase in patients with rheumatoid arthritis. Rheumatol Int. 2001;21:69–74. [DOI] [PubMed] [Google Scholar]
- 135. Hegen M, Mittrücker HW, Hug R, et al. Enzymatic activity of CD26 (dipeptidylpeptidase IV) is not required for its signalling function in T cells. Immunobiology. 1993;189:483–493. [DOI] [PubMed] [Google Scholar]
- 136. Nemunaitis J, Vukelja SJ, Richards D, et al. Phase I trial of PT-100 (PT-100), a cytokine-inducing small molecule, following chemotherapy for solid tumor malignancy. Cancer Invest. 2006;24:553–561. [DOI] [PubMed] [Google Scholar]
- 137. Eager RM, Cunningham CC, Senzer N, et al. Phase II trial of talabostat and docetaxel in advanced non-small cell lung cancer. Clin Oncol (R Coll Radiol). 2009;21:464–472. [DOI] [PubMed] [Google Scholar]
- 138. Adams S, Miller GT, Jesson MI, Watanabe T, Jones B, Wallner BP. PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res. 2004;64:5471–5480. [DOI] [PubMed] [Google Scholar]
- 139. Nauck MA, Wollschläger D, Werner J, et al. Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7–36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–1553. [DOI] [PubMed] [Google Scholar]
- 140. Pauly RP, Rosche F, Wermann M, McIntosh CH, Pederson RA, Demuth HU. Investigation of glucose-dependent insulinotropic polypeptide-(1–42) and glucagon-like peptide-1-(7–36) degradation in vitro by dipeptidyl peptidase IV using matrix-assisted laser desorption/ionization-time of flight mass spectrometry. A novel kinetic approach. J Biol Chem. 1996;271:23222–23229. [DOI] [PubMed] [Google Scholar]
- 141. Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. [DOI] [PubMed] [Google Scholar]
- 142. Deacon CF, Hughes TE, Holst JJ. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764–769. [DOI] [PubMed] [Google Scholar]
- 143. Pederson RA, White HA, Schlenzig D, Pauly RP, McIntosh CH, Demuth HU. Improved glucose tolerance in Zucker fatty rats by oral administration of the dipeptidyl peptidase IV inhibitor isoleucine thiazolidide. Diabetes. 1998;47:1253–1258. [DOI] [PubMed] [Google Scholar]
- 144. Balkan B, Kwasnik L, Miserendino R, Holst JJ, Li X. Inhibition of dipeptidyl peptidase IV with NVP-DPP728 increases plasma GLP-1 (7–36 amide) concentrations and improves oral glucose tolerance in obese Zucker rats. Diabetologia. 1999;42:1324–1331. [DOI] [PubMed] [Google Scholar]
- 145. Karl T, Chwalisz WT, Wedekind D, et al. Localization, transmission, spontaneous mutations, and variation of function of the Dpp4 (dipeptidyl-peptidase IV; CD26) gene in rats. Regul Pept. 2003;115:81–90. [DOI] [PubMed] [Google Scholar]
- 146. Tsuji E, Misumi Y, Fujiwara T, Takami N, Ogata S, Ikehara Y. An active-site mutation (Gly633–>Arg) of dipeptidyl peptidase IV causes its retention and rapid degradation in the endoplasmic reticulum. Biochemistry. 1992;31:11921–11927. [DOI] [PubMed] [Google Scholar]
- 147. Pederson RA, Kieffer TJ, Pauly R, Kofod H, Kwong J, McIntosh CH. The enteroinsular axis in dipeptidyl peptidase IV-negative rats. Metabolism. 1996;45:1335–1341. [DOI] [PubMed] [Google Scholar]
- 148. Karl T, Hoffmann T, Pabst R, von Hörsten S. Extreme reduction of dipeptidyl peptidase IV activity in F344 rat substrains is associated with various behavioral differences. Physiol Behav. 2003;80:123–134. [DOI] [PubMed] [Google Scholar]
- 149. Yasuda N, Nagakura T, Yamazaki K, Inoue T, Tanaka I. Improvement of high fat-diet-induced insulin resistance in dipeptidyl peptidase IV-deficient Fischer rats. Life Sci. 2002;71:227–238. [DOI] [PubMed] [Google Scholar]
- 150. Kirino Y, Sato Y, Kamimoto T, Kawazoe K, Minakuchi K, Nakahori Y. Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: a streptozotocin-induced model using wild-type and DPP4-deficient rats. J Endocrinol. 2009;200:53–61. [DOI] [PubMed] [Google Scholar]
- 151. Conarello SL, Li Z, Ronan J, et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:6825–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kirby M, Yu DM, O'Connor S, Gorrell MD. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin Sci (Lond). 2010;118:31–41. [DOI] [PubMed] [Google Scholar]
- 153. Thornberry NA, Weber AE. Discovery of JANUVIA (Sitagliptin), a selective dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Curr Top Med Chem. 2007;7:557–568. [DOI] [PubMed] [Google Scholar]
- 154. Keane FM, Nadvi NA, Yao TW, Gorrell MD. Neuropeptide Y, B-type natriuretic peptide, substance P and peptide YY are novel substrates of fibroblast activation protein-α. FEBS J. 2011;278:1316–1332. [DOI] [PubMed] [Google Scholar]
- 155. Lankas GR, Leiting B, Roy RS, et al. Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes. 2005;54:2988–2994. [DOI] [PubMed] [Google Scholar]
- 156. Burkey BF, Hoffmann PK, Hassiepen U, Trappe J, Juedes M, Foley JE. Adverse effects of dipeptidyl peptidases 8 and 9 inhibition in rodents revisited. Diabetes Obes Metab. 2008;10:1057–1061. [DOI] [PubMed] [Google Scholar]
- 157. Hoffmann P, Bentley P, Sahota P, et al. Vascular origin of vildagliptin-induced skin effects in cynomolgus monkeys: pathomechanistic role of peripheral sympathetic system and neuropeptide Y. Toxicol Pathol. 2014;42:684–695. [DOI] [PubMed] [Google Scholar]
- 158. Bank U, Heimburg A, Wohlfarth A, et al. Outside or inside: role of the subcellular localization of DP4-like enzymes for substrate conversion and inhibitor effects. Biol Chem. 2011;392:169–187. [DOI] [PubMed] [Google Scholar]
- 159. Gall MG, Chen Y, Vieira de Ribeiro AJ, et al. Targeted inactivation of dipeptidyl peptidase 9 enzymatic activity causes mouse neonate lethality. PLoS One. 2013;8:e78378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hung TT, Wu JY, Liu JF, Cheng HC. Epitope analysis of the rat dipeptidyl peptidase IV monoclonal antibody 6A3 that blocks pericellular fibronectin-mediated cancer cell adhesion. FEBS J. 2009;276:6548–6559. [DOI] [PubMed] [Google Scholar]
- 161. Hanski C, Huhle T, Gossrau R, Reutter W. Direct evidence for the binding of rat liver DPP IV to collagen in vitro. Exp Cell Res. 1988;178:64–72. [DOI] [PubMed] [Google Scholar]
- 162. Maida A, Hansotia T, Longuet C, Seino Y, Drucker DJ. Differential importance of glucose-dependent insulinotropic polypeptide vs glucagon-like peptide 1 receptor signaling for β cell survival in mice. Gastroenterology. 2009;137:2146–2157. [DOI] [PubMed] [Google Scholar]
- 163. Hansotia T, Baggio LL, Delmeire D, et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53:1326–1335. [DOI] [PubMed] [Google Scholar]
- 164. Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007;56:3006–3013. [DOI] [PubMed] [Google Scholar]
- 165. Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–5363. [DOI] [PubMed] [Google Scholar]
- 166. Waget A, Cabou C, Masseboeuf M, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology. 2011;152:3018–3029. [DOI] [PubMed] [Google Scholar]
- 167. Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest. 2012;122:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ahrén B, Simonsson E, Larsson H, et al. Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes. Diabetes Care. 2002;25:869–875. [DOI] [PubMed] [Google Scholar]
- 169. Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–2084. [DOI] [PubMed] [Google Scholar]
- 170. Ahrén B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874–2880. [DOI] [PubMed] [Google Scholar]
- 171. Balas B, Baig MR, Watson C, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92:1249–1255. [DOI] [PubMed] [Google Scholar]
- 172. Muscelli E, Casolaro A, Gastaldelli A, et al. Mechanisms for the antihyperglycemic effect of sitagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:2818–2826. [DOI] [PubMed] [Google Scholar]
- 173. Solis-Herrera C, Triplitt C, Garduno-Garcia Jde J, Adams J, DeFronzo RA, Cersosimo E. Mechanisms of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin when used alone or with metformin in type 2 diabetes: a double-tracer study. Diabetes Care. 2013;36:2756–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. El-Ouaghlidi A, Rehring E, Holst JJ, et al. The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide-induced hypoglycemia but reduces glucose-induced glucagon-like peptide 1 and gastric inhibitory polypeptide secretion. J Clin Endocrinol Metab. 2007;92:4165–4171. [DOI] [PubMed] [Google Scholar]
- 175. Aulinger BA, Bedorf A, Kutscherauer G, et al. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes. 2014;63:1079–1092. [DOI] [PubMed] [Google Scholar]
- 176. Zhong J, Rao X, Deiuliis J, et al. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013;62:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Lockie SH, Heppner KM, Chaudhary N, et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes. 2012;61:2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. [DOI] [PubMed] [Google Scholar]
- 180. Matheeussen V, Waumans Y, Martinet W, et al. Dipeptidyl peptidases in atherosclerosis: expression and role in macrophage differentiation, activation and apoptosis. Basic Res Cardiol. 2013;108:350. [DOI] [PubMed] [Google Scholar]
- 181. Shigeta T, Aoyama M, Bando YK, et al. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation. 2012;126:1838–1851. [DOI] [PubMed] [Google Scholar]
- 182. Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114:1788–1803. [DOI] [PubMed] [Google Scholar]
- 183. Sauvé M, Ban K, Momen MA, et al. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59:1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Ku HC, Chen WP, Su MJ. DPP4 deficiency preserves cardiac function via GLP-1 signaling in rats subjected to myocardial ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:197–207. [DOI] [PubMed] [Google Scholar]
- 185. Chang G, Zhang P, Ye L, et al. Protective effects of sitagliptin on myocardial injury and cardiac function in an ischemia/reperfusion rat model. Eur J Pharmacol. 2013;718:105–113. [DOI] [PubMed] [Google Scholar]
- 186. Theiss HD, Vallaster M, Rischpler C, et al. Dual stem cell therapy after myocardial infarction acts specifically by enhanced homing via the SDF-1/CXCR4 axis. Stem Cell Res. 2011;7:244–255. [DOI] [PubMed] [Google Scholar]
- 187. Christopherson KW, Cooper S, Hangoc G, Broxmeyer HE. CD26 is essential for normal G-CSF-induced progenitor cell mobilization as determined by CD26−/− mice. Exp Hematol. 2003;31:1126–1134. [DOI] [PubMed] [Google Scholar]
- 188. Fadini GP, Albiero M, Seeger F, et al. Stem cell compartmentalization in diabetes and high cardiovascular risk reveals the role of DPP-4 in diabetic stem cell mobilopathy. Basic Res Cardiol. 2013;108:313. [DOI] [PubMed] [Google Scholar]
- 189. Fadini GP, Boscaro E, Albiero M, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1α. Diabetes Care. 2010;33:1607–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Theiss HD, Gross L, Vallaster M, et al. Antidiabetic gliptins in combination with G-CSF enhances myocardial function and survival after acute myocardial infarction. Int J Cardiol. 2013;168:3359–3369. [DOI] [PubMed] [Google Scholar]
- 191. Theiss HD, Brenner C, Engelmann MG, et al. Safety and efficacy of SITAgliptin plus GRanulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction (SITAGRAMI-Trial)–rationale, design and first interim analysis. Int J Cardiol. 2010;145:282–284. [DOI] [PubMed] [Google Scholar]
- 192. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 193. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 194. Bhatt DL, Cavender MA. Do dipeptidyl peptidase-4 inhibitors increase the risk of heart failure [published online June 25, 2014]? JACC Heart Fail. 10.1016/j.jchf.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 195. McMurray J. The vildagliptin in ventricular dysfunction diabetes trial (VIVIDD). In: Proceedings from the European Society of Cardiology; August 31-September 4, 2013; Amsterdam, The Netherlands. [Google Scholar]
- 196. Kröller-Schön S, Knorr M, Hausding M, et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res. 2012;96:140–149. [DOI] [PubMed] [Google Scholar]
- 197. Nakamura K, Oe H, Kihara H, et al. DPP-4 inhibitor and α-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ayaori M, Iwakami N, Uto-Kondo H, et al. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J Am Heart Assoc. 2013;2:e003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Rieg T, Gerasimova M, Murray F, et al. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol. 2012;303:F963–F971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Hsieh J, Longuet C, Baker CL, et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia. 2010;53:552–561. [DOI] [PubMed] [Google Scholar]
- 201. Matikainen N, Mänttäri S, Schweizer A, et al. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 2006;49:2049–2057. [DOI] [PubMed] [Google Scholar]
- 202. Kojima Y, Kaga H, Hayashi S, et al. Comparison between sitagliptin and nateglinide on postprandial lipid levels: the STANDARD study. World J Diabetes. 2013;4:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Xiao C, Dash S, Morgantini C, Patterson BW, Lewis GF. Sitagliptin, a DPP-4 inhibitor, acutely inhibits intestinal lipoprotein particle secretion in healthy humans. Diabetes. 2014;63:2394–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Yan S, Marguet D, Dobers J, Reutter W, Fan H. Deficiency of CD26 results in a change of cytokine and immunoglobulin secretion after stimulation by pokeweed mitogen. Eur J Immunol. 2003;33:1519–1527. [DOI] [PubMed] [Google Scholar]
- 205. Vora KA, Porter G, Peng R, et al. Genetic ablation or pharmacological blockade of dipeptidyl peptidase IV does not impact T cell-dependent immune responses. BMC Immunol. 2009;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Yan S, Gessner R, Dietel C, Schmiedek U, Fan H. Enhanced ovalbumin-induced airway inflammation in CD26−/− mice. Eur J Immunol. 2012;42:533–540. [DOI] [PubMed] [Google Scholar]
- 207. Ansarullah, Lu Y, Holstein M, DeRuyter B, Rabinovitch A, Guo Z. Stimulating β-cell regeneration by combining a GPR119 agonist with a DPP-IV inhibitor. PLoS One. 2013;8:e53345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Kim SJ, Nian C, Doudet DJ, McIntosh CH. Dipeptidyl peptidase IV inhibition with MK0431 improves islet graft survival in diabetic NOD mice partially via T-cell modulation. Diabetes. 2009;58:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Kim SJ, Nian C, McIntosh CH. Sitagliptin (MK0431) inhibition of dipeptidyl peptidase IV decreases nonobese diabetic mouse CD4+ T-cell migration through incretin-dependent and -independent pathways. Diabetes. 2010;59:1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Makdissi A, Ghanim H, Vora M, et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. 2012;97:3333–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. van Poppel PC, Gresnigt MS, Smits P, Netea MG, Tack CJ. The dipeptidyl peptidase-4 inhibitor vildagliptin does not affect ex vivo cytokine response and lymphocyte function in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2014;103:395–401. [DOI] [PubMed] [Google Scholar]
- 212. Price JD, Linder G, Li WP, et al. Effects of short-term sitagliptin treatment on immune parameters in healthy individuals, a randomized placebo-controlled study. Clin Exp Immunol. 2013;174:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Goodwin SR, Reeds DN, Royal M, Struthers H, Laciny E, Yarasheski KE. Dipeptidyl peptidase IV inhibition does not adversely affect immune or virological status in HIV infected men and women: a pilot safety study. J Clin Endocrinol Metab. 2013;98:743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Shirakawa J, Fujii H, Ohnuma K, et al. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011;60:1246–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Ben-Shlomo S, Zvibel I, Rabinowich L, et al. Dipeptidyl peptidase 4-deficient rats have improved bile secretory function in high fat diet-induced steatosis. Dig Dis Sci. 2013;58:172–178. [DOI] [PubMed] [Google Scholar]
- 216. Panjwani N, Mulvihill EE, Longuet C, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(−/−) mice. Endocrinology. 2013;154:127–139. [DOI] [PubMed] [Google Scholar]
- 217. Stange T, Kettmann U, Holzhausen HJ. Immunoelectron microscopic single and double labelling of aminopeptidase N (CD 13) and dipeptidyl peptidase IV (CD 26). Acta Histochem. 1996;98:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Liu L, Liu J, Wong WT, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60:833–841. [DOI] [PubMed] [Google Scholar]
- 219. von Websky K, Reichetzeder C, Hocher B. Physiology and pathophysiology of incretins in the kidney. Curr Opin Nephrol Hypertens. 2014;23:54–60. [DOI] [PubMed] [Google Scholar]
- 220. Ceccarelli E, Guarino EG, Merlotti D, et al. Beyond glycemic control in diabetes mellitus: effects of incretin-based therapies on bone metabolism. Front Endocrinol (Lausanne). 2013;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kyle KA, Willett TL, Baggio LL, Drucker DJ, Grynpas MD. Differential effects of PPAR-γ activation versus chemical or genetic reduction of DPP-4 activity on bone quality in mice. Endocrinology. 2011;152:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bunck MC, Poelma M, Eekhoff EM, et al. Effects of vildagliptin on postprandial markers of bone resorption and calcium homeostasis in recently diagnosed, well-controlled type 2 diabetes patients. J Diabetes. 2012;4:181–185. [DOI] [PubMed] [Google Scholar]
- 223. Tinoco AD, Tagore DM, Saghatelian A. Expanding the dipeptidyl peptidase 4-regulated peptidome via an optimized peptidomics platform. J Am Chem Soc. 2010;132:3819–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Tagore DM, Nolte WM, Neveu JM, et al. Peptidase substrates via global peptide profiling. Nat Chem Biol. 2009;5:23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Tinoco AD, Kim YG, Tagore DM, et al. A peptidomics strategy to elucidate the proteolytic pathways that inactivate peptide hormones. Biochemistry. 2011;50:2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Tammen H, Hess R, Rose H, Wienen W, Jost M. Peptidomic analysis of blood plasma after in vivo treatment with protease inhibitors–a proof of concept study. Peptides. 2008;29:2188–2195. [DOI] [PubMed] [Google Scholar]