Abstract
The epidemiology of Middle East respiratory syndrome coronavirus (MERS-CoV) since 2012 has been largely characterized by recurrent zoonotic spillover from dromedary camels followed by limited human-to-human transmission, predominantly in health-care settings. The full extent of infection of MERS-CoV is not clear, nor is the extent and/or role of asymptomatic infections in transmission. We conducted a review of molecular and serological investigations through PubMed and EMBASE from September 2012 to November 15, 2018, to measure subclinical or asymptomatic MERS-CoV infection within and outside of health-care settings. We performed retrospective analysis of laboratory-confirmed MERS-CoV infections reported to the World Health Organization to November 27, 2018, to summarize what is known about asymptomatic infections identified through national surveillance systems. We identified 23 studies reporting evidence of MERS-CoV infection outside of health-care settings, mainly of camel workers, with seroprevalence ranges of 0%–67% depending on the study location. We identified 20 studies in health-care settings of health-care worker (HCW) and family contacts, of which 11 documented molecular evidence of MERS-CoV infection among asymptomatic contacts. Since 2012, 298 laboratory-confirmed cases were reported as asymptomatic to the World Health Organization, 164 of whom were HCWs. The potential to transmit MERS-CoV to others has been demonstrated in viral-shedding studies of asymptomatic MERS infections. Our results highlight the possibility for onward transmission of MERS-CoV from asymptomatic individuals. Screening of HCW contacts of patients with confirmed MERS-CoV is currently recommended, but systematic screening of non-HCW contacts outside of health-care facilities should be encouraged.
Keywords: health-care workers, infection control, MERS-CoV, seroprevalence, subclinical infections
Abbreviations
- HCW
health-care worker
- MERS-CoV
Middle East respiratory syndrome coronavirus
- PCR
polymerase chain reaction
- WHO
World Health Organization
INTRODUCTION
Since 2012, the epidemiology of cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection reported to the World Health Organization (WHO) has been characterized largely by recurrent zoonotic spillover from the known animal reservoir—dromedary camels—and human-to-human transmission in health-care settings (1). Outbreaks in health-care settings on occasion have resulted in large outbreaks (2–9). Of the 2,260 cases (including 803 deaths) reported to WHO, 83% of cases have been reported in the Kingdom of Saudi Arabia (10).
The clinical presentation of MERS-CoV infection ranges from asymptomatic to severe respiratory illness, with approximately 35.5% of cases resulting in death (1). The role of asymptomatic or subclinical infections in human-to-human transmission of MERS-CoV is not well understood, but there is evidence that laboratory-confirmed MERS-CoV infection in patients who are reported as asymptomatic may be transmitted to other individuals (11).
For many novel infectious pathogens, surveillance initially focuses on individuals with disease who seek care at health-care facilities, which undoubtedly underestimates the true prevalence of infection, because it will not account for mild or asymptomatic infections not requiring medical care. Detailed outbreak investigations often include laboratory testing of close contacts and of health-care workers (HCWs), regardless of symptoms, and specialized serological investigations will include individuals thought to be at higher risk of infection, such as HCWs or those with occupational exposure to animal reservoirs. Estimates of the true prevalence of infection of high-risk pathogens are important to understand the populations required for vaccine candidates or specific therapeutic treatments as and when they become available. In addition, the role of subclinical or asymptomatic infection is critical in understanding chains of transmission missed by surveillance systems. For MERS-CoV, asymptomatic infection has been reported to WHO, but the possibility of transmission prior to symptom onset is critical for recommending effective infection prevention and control measures and for reducing secondary MERS-CoV transmission.
The role of asymptomatic infections in transmission of other respiratory viruses has been investigated previously. Highly pathogenic avian influenza H5N1 RNA, for example, has been detected by polymerase chain reaction (PCR) from asymptomatic family contacts of ill patients, suggesting the possibility for onward transmission, even in the absence of symptoms (12–15). For severe acute respiratory syndrome CoV, limited transmission to close contacts before symptom onset or hospitalization has been found in transmission-risk studies outside health-care settings, whereas human-to-human transmission within health-care settings was higher, likely due to higher viral load in hospitalized patients and more frequent exposure to the virus among HCWs (16–18).
Here, we have reviewed available evidence of the extent of subclinical and asymptomatic infection of MERS-CoV stratified by evaluating studies in which infection within and outside of health-care settings has been measured, and the potential role of onward human-to-human transmission from asymptomatic cases.
METHODS
We conducted a literature search in PubMed and EMBASE databases for observational epidemiologic studies of laboratory-confirmed MERS-CoV infection using the following search terms: “MERS-CoV” or “MERS” AND “seroprevalence” or “prevalence” or ”serological” or “infection” or “asymptomatic.” Additional studies were identified through consultation with the WHO MERS technical network and in the bibliography of a related recently published review (19). Publications in English dated before November 15, 2019, were considered, with no additional restrictions on year of publication. We assessed the titles and abstracts of identified studies to determine their eligibility for inclusion in the study. We stratified our analyses to evaluate subclinical and/or asymptomatic infection identified inside and outside health-care facilities. For descriptive analysis of WHO case-based data, we used the ggplot2 in R, version 3.4.2.0 (R Foundation for Statistical Computing, Vienna, Austria.).
For MERS-CoV infections studied outside health-care settings, we included studies in which evidence of MERS-CoV infection was reported, using molecular and/or serological methods in either individuals with occupational exposure to dromedary camels; familial, occupational, or social contacts of patients with confirmed MERS outside of health-care settings; the general population; or through national MERS surveillance records, when published. Eligible studies included reporting of the number of individuals tested and the number with molecular or serological evidence of MERS-CoV infection.
To evaluate MERS-CoV infections studied within health-care settings, we included studies in which the authors reported evidence of MERS-CoV infection, using molecular and/or serological methods among HCW and among non-HCW contacts (e.g., family contacts) of confirmed MERS cases treated in health care settings.
For each eligible study, we extracted information on the year of publication, the year biological samples were collected, the country in which the study was conducted, the number of individuals tested, characteristics of the individuals tested, and the total number of confirmed MERS-CoV infections by molecular or serological assay. Asymptomatic MERS-CoV infection was considered a laboratory-confirmed infection with no reported symptoms at the time of sampling.
In addition, we evaluated the symptomatic profile and place of reporting among laboratory-confirmed MERS-CoV infections reported to WHO from September 2012 to November 27, 2018. Within WHO databases, cases are classified as primary cases if they were reported as such by the reporting Member State; if direct or indirect contact with dromedary camels or dromedary products was reported in the case; and/or the exposures were under investigation without known contact with a patient with probable or confirmed MERS. Cases were classified as secondary cases due to human-to-human transmission if the patient reported recent direct contact with a patient known to have MERS-CoV infection and/or were identified as a household, occupational, or HCW contact of a patient known to have MERS-CoV infection.
RESULTS
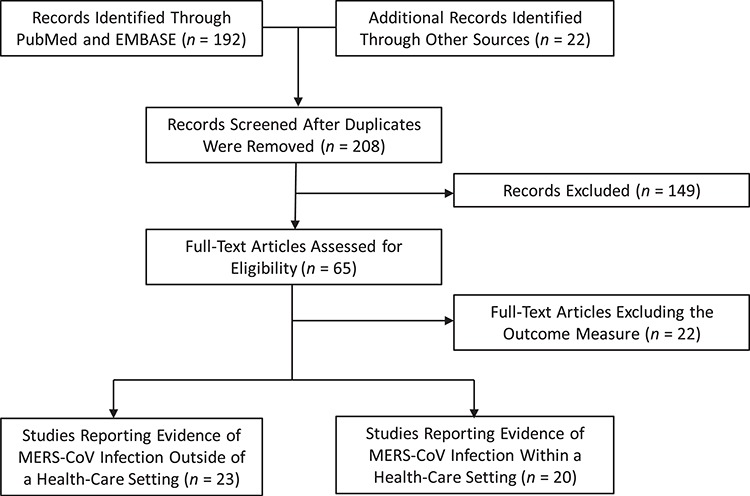
In total, we identified 43 studies in which MERS-CoV infections measured by serology and/or molecular testing were reported; 23 focused on individuals with exposures outside of health-care settings (4, 7, 11, 20–39), and 20 focused on individuals with exposures inside health-care facilities (5, 7, 29, 32, 40–54). The selection of identified and included studies is shown in Figure 1.
Figure 1. Flow diagram of selection of articles for the review of symptomatic and subclinical Middle East respiratory syndrome coronavirus (MERS-CoV) infections. Additional records identified through consultation with the World Health Organization MERS technical network and in the bibliography of a related review (19).
The 23 studies in which MERS-CoV infections were measured by serology and/or molecular testing outside of health-care settings are described in Table 1. The majority of studies focused on measuring seroprevalence of MERS-CoV in individuals with occupational exposure to dromedaries in the Middle East and Africa (20–28).
Table 1. Evidence of Middle East Respiratory Syndrome Coronavirus Infection Outside Health-Care Settings, 2012–2018.
| First Author, Year (Reference No.) | Years of Study | Location of Study | No. of Subjects | Description of Subjects | Laboratory Results | Evidence of Asymptomatic MERS-CoV Infection Among PCR/Serologically Positive Subjects |
|---|---|---|---|---|---|---|
| Occupational Exposure to Dromedaries | ||||||
| Aburizaiza, 2014 (20) | 2012 | KSA | 226 | Slaughterhouse workers | 0 (0%) had specific antibodies against MERS-CoV (immunofluorescence assay and neutralization) | |
| Chu, 2014 (21) | 2013 | Egypt | 179 | Camel abattoir workers | 0 (0%) had serological evidence of MERS-CoV infection | |
| Hemida, 2015 (22) | 2013–2014 | KSA | 191 | Occupational exposure to dromedary camels | 0 (0%) had specific antibodies against MERS-CoV (ppNT) | |
| Memish, 2015 (23) | 2012 | KSA | 75 | Direct contact with domestic animals, including camels | 0 (0%) had specific antibodies against MERS-CoV (ppNT) | |
| Reusken, 2015 (24) | 2013–2014 | Qatar | 294 | Daily occupational contact with dromedary camels | 10 (3.4%) had specific neutralizing antibodies against MERS-CoV (ELISA and PRNT90) | 10 (100%) reported no severe health problems |
| Liljander, 2016 (25) | 2013–2014 | Kenya | 1,222 | Livestock handlers in Kenya | 2 (0.2%) had confirmed serological evidence of MERS-CoV infection (recombinant ELISA, PRNT50 and PRNT90) | 2 (100%) reported no recent clinical symptoms, indication mild or subclinical infection |
| So, 2018 (26) | 2016 | Nigeria | 261 | Abattoir workers with exposure to dromedaries | 0 (0%) had specific neutralizing antibodies against MERS-CoV (ELISA and ppNT) | |
| Alshukairi, 2018 (27) | 2018 | KSA | 30 | Camel herders, truck drivers, and handlers | 20 (67%) seropositive for MERS-CoV infection (ELISA, PRNT50 and MERS-CoV–specific T-cell response) | 6 (20%) reported fever or cold in the previous 4 months |
| Zohaib, 2018 (28) | 2016–2017 | Pakistan | 840 | Camel herders | 0 (0%) had serological evidence of MERS-CoV infection (ELISA, PRNT50) | |
| Contacts of Patients Outside Health-Care Settings Who Had Confirmed MERS | ||||||
| Health Protection Agency, 2013 (29) | 2013 | United Kingdom | 33 | Close contacts of a confirmed case | 2 (6%) had molecular evidence of MERS-CoV infection (RT-PCR) | 0 (0%) were asymptomatic |
| Assiri, 2013 (4) | 2013 | KSA | 217 | Household contacts of confirmed cases | 5 (2%) had confirmed MERS-CoV infection (RT-PCR and viral load) | Not reported |
| Omrani, 2013 (30) | 2013 | KSA | 10 | Household contacts of confirmed cases | 0 (0%) had molecular evidence of MERS-CoV infection (RT-PCR) | |
| Mailles, 2013 (31) | 2013 | France | 162 | Contacts of a confirmed case | 1 (1%) had molecular evidence of MERS-CoV infection (RT-PCR) | 0 (0%) were asymptomatic |
| Memish, 2014 (32) | 2012–2014 | KSA | 462 | Family contacts of confirmed cases | 10 (2%) had molecular evidence of MERS-CoV infection (RT-PCR) | Not reported |
| Drosten, 2014 (11) | 2013 | KSA | 280 | Household contacts of confirmed cases | 12 (4%) had laboratory evidence of secondary MERS transmission (RT-PCR, ELISA, recombinant immunofluorescence assay, PRNT50, PRNT90) | 11 (92%) were asymptomatic |
| Arwady, 2016 (33) | 2014 | KSA | 79 | Relatives of patients infected with MERS-CoV | 11 (14%) had molecular evidence of MERS-CoV infection (RT-PCR); 8 (10%) additional contacts had serological evidence of MERS-CoV infection (ELISA) | 2 (11%) reported mild symptoms and 3 (16%) were asymptomatic |
| Plipat, 2017 (34) | 2015 | Thailand | 48 | High-risk contacts of a confirmed case | 0 (0%) had molecular evidence of MERS-CoV infection (RT-PCR) | |
| Al Hosani, 2019 (35) | 2013–2018 | United Arab Emirates | 124 | Case patients and household contacts of patients with MERS-CoV | 13 (54%) cases had MERS-CoV antibodies; 0 (0%) household contacts had serological evidence of MERS-CoV infection (ELISA and microneutralization) | 3 of 13 case patients (23%) were asymptomatic |
| General Population | ||||||
| Gierer, 2013 (36) | 2010–2012 | KSA | 268 | Children hospitalized for lower respiratory tract infection and male blood donors | 0 (0%) had specific neutralizing antibodies against MERS-CoV (lentiviral vector system) | |
| Müller, 2015 (37) | 2012–2013 | KSA | 10,009 | Healthy individuals across all 13 provinces of KSA | 15 (0.1%) had anti–MERS-CoV antibodies (recombinant ELISA, recombinant immunofluorescence assay, PRNT50 and PRNT90) | Not reported |
| Munyua, 2017 (38) | 2013 | Kenya | 760 | Households exposed to seropositive camels | 0 (0%) had specific neutralizing antibodies against MERS-CoV (ELISA and PRNT50) | |
| Table continues | ||||||
| Retrospective Review of National Surveillance Records | ||||||
| Al Hosani, 2016 (7) | 2013–2014 | United Arab Emirates | 1,586 | Case patients from surveillance records who were suspected to have MERS | 65 (4%) had molecular evidence of MERS-CoV infection (RT-PCR) | 23 (35%) were asymptomatic |
| Saeed, 2017 (39) | 2015–2016 | KSA | 57,363 | Case patients suspected to have MERS | 384 (1%) had molecular evidence of MERS-CoV infection (RT-PCR) | 19 (5%) were asymptomatic |
Abbreviations: ELISA, enzyme-linked immunoassay; KSA, Kingdom of Saudi Arabia; MERS-CoV, Middle East respiratory syndrome coronavirus; PCR, polymerase chain reaction; ppNT, pseudoparticle neutralization test; PRNT50, 50% plaque reduction neutralization test; PRNT90, 90% plaque reduction neutralization test; RT-PCR, reverse transcriptase polymerase chain reaction.
In the largest seroprevalence study conducted to date, 0.1% seroprevalence was calculated among general population samples collected in 2012–2013, 2% seroprevalence among shepherds of dromedaries, and 4% seroprevalence among slaughterhouse workers (37). Additional estimates of seroprevalence among occupational high-risk populations ranged from 0% to 67%, with seropositivity being detected in Kingdom of Saudi Arabia, Qatar, and Kenya, and from 0% to 54% among contacts of patients with confirmed MERS in household settings largely in countries of the Middle East. Within these studies, the majority of infections detected by serology appeared to be asymptomatic. Within these studies, a high proportion of seropositive camel workers reported no symptoms (80%–100% among seropositive individuals).
The 20 studies in which MERS-CoV infections were measured by serology and/or molecular testing within health-care settings are listed in Table 2 and include studies of HCWs and close contacts of patients with confirmed infection. The largest molecular and serological studies among HCWs were conducted among 1,695 and 1,169 HCWs in Kingdom of Saudi Arabia (32) and the Republic of Korea (40), respectively; the authors reported evidence of infection of 1% and 1.5%, respectively. Infection was more frequent among HCWs who did not use personal protective equipment when in contact with a patient with MERS (40).
Table 2. Evidence of Middle East Respiratory Syndrome Coronavirus Infection in Health-Care Settings, 2012–2018.
| First Author, Year (Reference No.) | Years of Study | Location of Study | No. of Individuals Tested | Description of Subjects | Laboratory Results | Evidence of Asymptomatic MERS-CoV Infection Among PCR/Serologically Positive Subjects |
|---|---|---|---|---|---|---|
| HCW Contacts of Patients With Confirmed MERS-CoV | ||||||
| Health Protection Agency, 2013 (29) | 2013 | United Kingdom | 59 | HCW | 0 transmission (RT-PCR) | |
| Memish, 2014 (32) | 2012–2013 | KSA | 1,695 | HCW | 19 (1%) had molecular evidence of MERS-CoV infection (RT-PCR) | 2 (11%) were asymptomatic; 5 (26%) had mild infection |
| Kim, 2016 (40) | 2015 | ROK | 1,169 | HCW | 17 (1%) had evidence of MERS-CoV infection, higher among HCWs who did not use PPE (ELISA and indirect immunofluorescence test) | |
| Cho, 2016 (41) | 2015 | ROK | 218 | HCW | 8 (4%) had molecular evidence of MERS-CoV infection (RT-PCR) | |
| Park, 2016 (42) | 2015 | ROK | 519 | HCW | 3 (1%) had molecular evidence of MERS-CoV infection (RT-PCR) | 3 (100%) were asymptomatic |
| Hastings, 2016 (43) | 2014 | KSA | 16 | HCW | 14 (88%) had molecular evidence of MERS-CoV infection (RT-PCR) | 13 (81%) were asymptomatic |
| Moon, 2017 (44) | 2015 | ROK | 82 | HCW | 0 transmission from asymptomatic HCWs (RT-PCR and ELISA) | |
| Alfaraj, 2019 (45) | 2015 | KSA | 153 | HCW | 7 (5%) had molecular evidence of MERS-CoV infection (RT-PCR) | 5 (71%) were asymptomatic |
| Amer, 2018 (46) | 2017 | KSA | 879 | HCW | 17 (2%) had molecular evidence of MERS-CoV infection (RT-PCR) | 17 (100%) were asymptomatic or had mild disease |
| Amer, 2018 (47) | 2017 | KSA | 107 | HCW | 9 (8%) positive for MERS-CoV (RT-PCR) | |
| Asymptomatic Infection Among Infected HCWs | ||||||
| Alshukairi, 2016 (48) | 2014–2016 | KSA | NR | HCW | 18 had molecular and serological evidence of MERS-CoV infection (RT-PCR, ELISA, IFA) | 6 (33%) were asymptomatic |
| Assiri, 2016 (49) | 2014–2015 | KSA | NR | HCW | 7 had molecular and serological evidence of MERS-CoV infection (RT-PCR, ELISA, IFA, MT) | 4 (57%) were asymptomatic |
| Balkhy, 2016 (50) | 2015 | KSA | NR | HCW | 43 had molecular evidence of MERS-CoV infection (RT-PCR) | 25 (58%) were asymptomatic |
| Table continues | ||||||
| Al Hosani, 2016 (7) | 2013–2014 | United Arab Emirates | NR | HCW | 31 had molecular evidence of MERS-CoV infection (RT-PCR) | 12 (39%) were asymptomatic |
| Alenazi, 2017 (51) | 2015 | KSA | NR | HCW | 43 had molecular evidence of MERS-CoV infection (RT-PCR) | 18 (42%) were asymptomatic |
| 2018a | 2012–2018 | Global | NR | HCW | 389 had laboratory-confirmed MERS-CoV infection | 94 (24%) were asymptomatic |
| MERS-CoV Infection in Health-Care Settings Among Non–HCWs | ||||||
| Al-Abdallat, 2014 (5) | 2012–2013 | Jordan | 124 | Contacts identified during MERS outbreak | 9 (7%) had serological evidence of MERS-CoV infection (ELISA, IFA, MT) | 0 (0%) were asymptomatic |
| Cho, 2016 (41) | 2015 | ROK | 675 | Patients in hospital, contacts of patients infected with MERS-CoV | 33 (5%) had molecular evidence of MERS-CoV infection (RT-PCR) | |
| Extent of Asymptomatic Infection Among Laboratory-Confirmed MERS Cases | ||||||
| Oboho, 2015 (52) | 2014 | KSA | NR | Confirmed MERS-CoV infection | 255 had molecular evidence of MERS-CoV infection (RT-PCR) | 64 (25%) patients were asymptomatic, although 26 patients interviewed reported at least 1 symptom consistent with respiratory illness |
| Assiri, 2016 (49) | 2014–2015 | KSA | NR | Confirmed MERS-CoV infection | 38 had molecular and serological evidence of MERS-CoV infection (RT-PCR, ELISA, IFA, MT) | 2 (5%) were asymptomatic |
| Alenazi, 2017 (51) | 2015 | KSA | NR | Patient contacts in hospital | 61 had molecular evidence of MERS-CoV infection (RT-PCR) | 3 (5%) were asymptomatic |
| Zhao, 2017 (53) | 2015 | KSA | NR | MERS survivors | 18 had molecular and serological evidence of MERS-CoV infection (RT-PCR, ELISA, IFA, MT, PRNT50, and MERS-CoV–specific T-cell response) | 3 (17%) were asymptomatic; patients with higher PRNT50 and T-cell responses had longer stays in the intensive care unit |
| Payne, 2018 (54) | 2015–2016 | Jordan | NR | Patient-contacts in hospital | 16 had laboratory-confirmed MERS-CoV infection (RT-PCR, ELISA, MT) | 3 (19%) were asymptomatic |
Abbreviations: ELISA, enzyme-linked immunoassay; HCW, health-care worker; IFA, immunofluorescence assay; KSA, Kingdom of Saudi Arabia; MERS-CoV, Middle East respiratory syndrome coronavirus; MT, microneutralization assay; NR, not reported; PPE, personal protective equipment; PRNT, plaque reduction neutralization test; ROK, Republic of Korea; RT-PCR, reverse transcriptase polymerase chain reaction.
a World Health Organization, unpublished data, 2018.
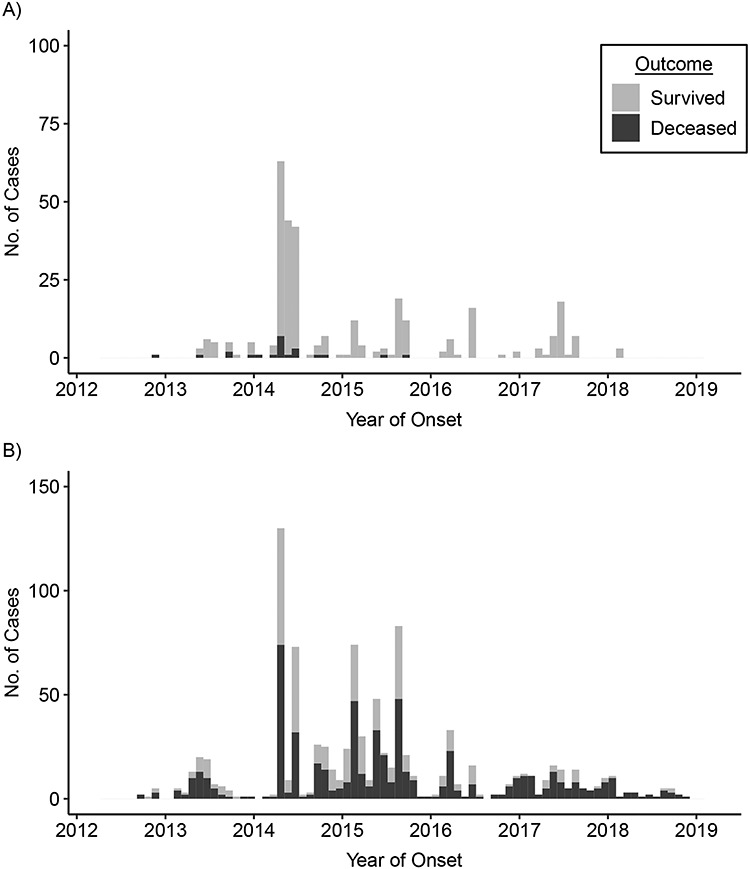
Since 2012, 298 of the 2,274 laboratory-confirmed cases (13.1%) reported to WHO have been reported as asymptomatic at the time of reporting, 164 of these patients were HCWs. The demographic characteristics and clinical presentation of primary and secondary cases of MERS-CoV infection are listed in Table 3. There were significantly more asymptomatic cases reported among secondary cases (n = 266 of 1,094; 24.3%) than among primary cases (n = 9 of 642 (1.4%); P < 0.001). Overall, no deaths were reported among patients with asymptomatic infections. Figure 2 shows the epidemic curve of MERS-CoV infections reported to WHO stratified by HCWs and non-HCWs. Of the 414 MERS-CoV infections among HCWs that were reported to WHO, 164 (39.6%) were reported to be asymptomatic.
Table 3. Description of Characteristics of Middle East Respiratory Syndrome Coronavirus Infection Reported to World Health Organization from September 2012 to November 27, 2018.
| Reported Source of Infection | ||||||
|---|---|---|---|---|---|---|
| MERS Case Characteristic | Outside Health-Care Setting | Within in Health-Care Setting | Not Known at the Time of Reporting to WHO | |||
| No. | % | No. | % | No. | % | |
| Case classification | 764 | 826 | 681 | |||
| Primary casea | 561 | 73.4 | 2 | 0.2 | 79 | 11.6 |
| Secondary caseb | 193 | 25.3 | 816 | 98.8 | 85 | 12.5 |
| Unknown at the time of reporting | 10 | 1.3 | 8 | 1.0 | 517 | 75.9 |
| Primary MERS-CoV infectiona | ||||||
| Age, yearsc | 55.9 (45.0–69.0) | 47.0 (39.0–55.0) | 57.8 (46.0–72.0) | |||
| Sex | ||||||
| Male | 459 | 81.8 | 2 | 100 | 72 | 91.1 |
| Female | 102 | 18.2 | 0 | 0 | 5 | 6.3 |
| Comorbidity | ||||||
| Any | 316 | 56.3 | 1 | 50 | 17 | 21.5 |
| None | 62 | 11.1 | 0 | 0 | 3 | 3.8 |
| Not reported | 183 | 32.6 | 1 | 50 | 59 | 74.7 |
| Clinical presentation | ||||||
| Asymptomatic | 7 | 1.2 | 0 | 0 | 2 | 2.5 |
| Symptomatic | 521 | 92.9 | 2 | 100 | 65 | 82.3 |
| Not reported | 33 | 5.9 | 0 | 0 | 12 | 15.2 |
| Outcome | ||||||
| Survived | 167 | 29.8 | 1 | 50 | 14 | 17.7 |
| Died | 277 | 49.4 | 0 | 0 | 32 | 40.5 |
| Not reported | 117 | 20.8 | 1 | 50 | 33 | 41.8 |
| Secondary MERS-CoV infectionb | ||||||
| Age, yearsc | 40.7 (27.0–54.0) | 49.3 (34.0–62.0) | 42.7 (28.0–54.0) | |||
| Sex | ||||||
| Male | 124 | 64.2 | 451 | 55.3 | 51 | 60 |
| Female | 69 | 35.8 | 365 | 44.7 | 34 | 40 |
| Comorbidity | ||||||
| Any | 47 | 24.4 | 281 | 34.4 | 13 | 15.3 |
| None | 43 | 22.3 | 104 | 12.7 | 10 | 11.8 |
| Not reported | 103 | 53.4 | 431 | 52.8 | 62 | 72.9 |
| Clinical presentation | ||||||
| Asymptomatic | 74 | 38.3 | 180 | 22.1 | 12 | 14.1 |
| Symptomatic | 103 | 53.4 | 482 | 59.1 | 51 | 60 |
| Not reported | 16 | 8.3 | 154 | 18.9 | 22 | 25.9 |
| Outcome | ||||||
| Survived | 127 | 65.8 | 337 | 41.3 | 28 | 32.9 |
| Died | 27 | 14.0 | 248 | 30.4 | 20 | 23.5 |
| Not reported | 39 | 20.2 | 231 | 28.3 | 37 | 43.5 |
Abbreviations: IQR, interquartile range; MERS-CoV, Middle East respiratory syndrome coronavirus; WHO, World Health Organization.
a Primary infection: reported direct or indirect contact with dromedary camels, no contact with a probable or confirmed MERS-CoV infected human case, no prior health care facility contact (n = 642).
b Secondary infection: direct epidemiologic link to a human MERS infection (n = 1,094).
c Values are expressed as mean (interquartile range).
Figure 2. Epidemic curve of laboratory-confirmed Middle East respiratory syndrome coronavirus infections among A) health-care workers and B) non–health-care workers and outcome reported to the World Health Organization from 2012 to November 27, 2018.
Evidence of human-to-human transmission from an asymptomatic infection
We found 4 studies that documented the duration of viral shedding from asymptomatic or mildly symptomatic individuals (55–58). Among asymptomatic, PCR-positive MERS-CoV infections, positive reverse transcriptase (RT)-PCR results were reported from the day of initial testing for as long as 28–42 days (55–58).
We found 1 study in which molecular and serological evidence of possible secondary transmission from asymptomatic individuals was reported (11). The study was conducted as part of an investigation of 12 household contacts, in whom upper respiratory tract samples from 7 were PCR positive and an additional 5 samples were seropositive using recombinant immunofluorescence or plaque reduction neutralization test (11). Eleven of these 12 individuals reported no symptoms at the time of sampling; this information, combined with epidemiologic data, indicated these people could have been involved in asymptomatic transmission within households.
We found 9 studies that described molecular evidence of MERS-CoV infection among asymptomatic individuals in health-care settings (7, 32, 42, 43, 45, 46, 50, 51, 53). Infectivity of an asymptomatic HCW infected with MERS-CoV was investigated in 1 study, but no evidence was found of secondary transmission to 82 HCWs with contact to the HCW with MERS-CoV infection (44).
DISCUSSION
In this review, we found 43 studies in which molecular and/or serological evidence of MERS-CoV infection was reported. Outside of health-care settings, the evidence of MERS-CoV infection has largely been focused on individuals with occupational exposure to dromedaries. The results to date are heterogenous, and although attempts have been made to evaluate MERS-CoV genetic diversity (59, 60), the differences in seroprevalence results to date likely reflect differences in the selection and characteristics of dromedary herds and humans tested. The available evidence of the MERS-CoV epidemiologic and genetic characteristics does not suggest there are differences in the virus’s ability to infect humans. Evidence supports that individuals with occupational exposure to dromedaries have higher rates of seroprevalence compared with household contacts of patients with confirmed MERS-CoV infection, likely reflecting more intense, unprotected exposures to MERS-CoV through dromedary secretions (61). That these individuals have subclinical infection and do not develop disease is likely because those with occupational exposure tend to be younger and healthier, without underlying high-risk conditions such as hypertension, diabetes, and renal failure. Variations in the seroprevalence rates by study are also likely due to variations in methodologies, including the timing of sample collection, serologic assays used, and interpretation of assay results.
Although the majority of human MERS-CoV infections have been reported to WHO from countries in the Arabian Peninsula, particularly Kingdom of Saudi Arabia, there is increasing evidence of infection in dromedary camels in herds throughout Africa and South Asia (62). Additional serological and molecular epidemiology studies at the dromedary-human interface using a standardized approach and consistent methodology, in the Arabian Peninsula and in Africa and South Asia, are needed to further understand this observed heterogeneity—that is, whether the observed differences in evidence of infection outside health-care settings may be attributable to genetic variation of the virus across different geographic regions and/or to factors and behaviors in human populations in these regions, which may change the susceptibility to infection. WHO is currently supporting studies underway in several countries in the Middle East, Africa, and South Asia in which the extent of infection in occupationally exposed persons is being evaluated . The results of such studies can contribute to better understanding the geographic reach of MERS-CoV in dromedaries and humans.
Within health-care settings, the detection of asymptomatic, PCR-positive infection has been reported to WHO from affected member states and also documented in 10 published studies. Although onward transmission was not investigated in those studies, the researchers did capture evidence of RNA shedding, which suggests human-to-human transmission is possible from individuals with no signs or symptoms of infection. This is supported by evidence documenting duration of viral shedding beyond 3 weeks in patients with subclinical MERS-CoV infection (55–58).
At the same time, the evidence for acute, asymptomatic MERS-CoV infection described in this review does not represent the full extent of subclinical infection. Asymptomatic contacts clear the virus more quickly than do symptomatic patients (58) and antibody titers in the former are likely to be lower, if they seroconvert at all, than in infected patients exhibiting symptoms (63). Timely and repeated biological specimen collection is needed to capture viral shedding and antibody kinetics of symptomatic and asymptomatic contacts (11). This can be achieved if all high-risk contacts of patients with confirmed MERS-CoV infection are identified during an outbreak and then tested using molecular and serologic laboratory assays, regardless of whether the individual exhibits symptoms. In outbreak settings, without the inclusion of testing of all contacts, the identification of chains of transmission may be incomplete.
Indeed, the latest WHO surveillance guidelines recommend that all contacts of patients with laboratory-confirmed MERS outside of health care facilities should be placed under active surveillance for 14 days after the last exposure to the confirmed case and that any contacts with symptoms of respiratory illness should be tested for MERS-CoV infection (64). If feasible, we recommend that follow-up should include molecular testing, regardless of the development of symptoms. In addition, studies conducted of high-risk workers, which have typically only included serologic testing, should also include molecular testing of upper respiratory samples in an attempt to capture viral carriage.
Despite these limitations in our current knowledge, the findings of our review reinforce the evidence that HCWs are more likely to be at risk of MERS-CoV infection due to close unprotected contact with patients with MERS patients prior to their diagnosis, particularly when aerosolizing procedures are performed. Because HCWs tend to be younger and healthier than patients in whom severe MERS develops, HCWs have fewer symptoms, if any, and present a silent risk of human-to-human transmission to others. Among HCW contacts, detailed studies of viral shedding and immune response of asymptomatic, PCR-positive MERS-CoV infections are urgently needed and should be conducted when outbreaks occur and enhanced surveillance is put in place by government and hospital officials.
Surveillance and testing for MERS-CoV have improved substantially since the virus was first discovered in 2012. In affected countries, visual respiratory triage systems before a patient enters the emergency department have been introduced; some emergency departments in affected countries have been restructured for enhanced triage of patients with respiratory symptoms; trainings specific to infection prevention and control of respiratory pathogens have been introduced and reintroduced in high-risk areas and hospitals with high turnover of HCWs; and audits of hospitals for compliance to specific infection prevention and control measures are regularly performed (6). In addition, the systematic testing of HCWs, extending beyond nurses and doctors to include reception staff, cleaners, technicians, and so forth, regardless of the development of symptoms, as required by the latest infection prevention and control guidelines for HCWs by WHO and Kingdom of Saudi Arabia, for example, has detected subclinical and asymptomatic infections that likely would have gone undetected in past outbreaks. Asymptomatic infections may have played a role in extensive secondary transmission in health-care settings before the latest guidelines were introduced, and the impact of such policies may be reflected in the lower peaks on the global MERS-CoV epidemic curve since 2016. Without this level of contact follow-up in community settings, the extent of asymptomatic infections in the community will remain unknown.
Screening of HCWs with exposure to patients infected with MERS-CoV may be feasible for preventing human-to-human transmission in health-care settings, and appears to be effective in Kingdom of Saudi Arabia and other affected countries in which this infection prevention and control measure has been introduced. Screening of non-HCW contacts in health-care settings should also be encouraged. Outside health-care settings, the feasibility of screening may be reduced, particularly given the difficulty in detecting asymptomatic infections. Therefore, transmission of MERS-CoV outside health-care settings should be expected to continue until zoonotic spillover from dromedaries can be interrupted.
ACKNOWLEDGMENTS
Author affiliations: Centre for Global Health, Institut Pasteur, Paris, France (Rebecca Grant); Department of Infectious Hazard Management, Health Emergencies Program, World Health Organization, Geneva, Switzerland (Rebecca Grant, Maria D. Van Kerkhove); and Infectious Hazard Management Unit, Department of Health Emergencies, World Health Organization Regional Office for the Eastern Mediterranean, Cairo, Egypt (Mamunur Rahman Malik, Amgad Elkholy).
We thank public health and animal health workers in affected and at-risk member states for their continuous work in identifying Middle East respiratory (MERS-CoV) syndrome coronavirus infections in humans and animals.
Conflict of interest: none declared.
REFERENCES
- 1. World Health Organization WHO MERS Global Summary and Assessment of Risk: August 2018. Geneva, Switzerland: World Health Organization; 2018http://www.who.int/csr/disease/coronavirus_infections/risk-assessment-august-2018.pdf. Accessed November 18, 2018. [Google Scholar]
- 2. Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. [DOI] [PubMed] [Google Scholar]
- 3. Hijawi B, Abdallat M, Sayaydeh A, et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19(suppl 1):S12–S18. [PubMed] [Google Scholar]
- 4. Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic and clinical description. Clin Infect Dis. 2014;59(9):1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drosten C, Muth D, Corman VM, et al. An observational, laboratory-based study of outbreaks of Middle East respiratory syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60(3):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al Hosani F, Pringle K, Al Mulla M, et al. Response to emergence of Middle East respiratory syndrome coronavirus, Abu Dhabi, United Arab Emirates, 2013–2014. Emerg Infect Dis. 2016;22(7):1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ki M. MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015;37:e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park HY, Lee EJ, Ryu YW, et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea. May to June 2015. Euro Surveill. 2015;20(25):1–6. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization Regional Office for the Eastern Mediterranean MERS situation update ,September 2018. http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-september-2018.html. Accessed September 29, 2018.
- 11. Drosten C, Meyer B, Müller MA, et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371(9):828–835. [DOI] [PubMed] [Google Scholar]
- 12. Lu C, Lu J, Chen W, et al. Potential infections of H5N1 and H9N2 avian influenza do exist in Guangdong populations of China. Chin Med J (Engl). 2008;121(20):2050–2053. [PubMed] [Google Scholar]
- 13. Vong S, Ly S, Van Kerkhove MD, et al. Risk factors associated with subclinical human infection with avian influenza a (H5N1) virus--Cambodia 2006. J Infect Dis. 2009;199(12):1744–1752. [DOI] [PubMed] [Google Scholar]
- 14. Cavailler P, Chu S, Ly S, et al. Seroprevalence of anti-H5 antibody in rural Cambodia 2007. J Clin Virol. 2010;48(2):123–126. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization WHO Consultation on Case Management and Research on Human Influenza A/H5. Hanoi, Vietnam; May 10–12, 2005. [Google Scholar]
- 16. Isakbaeva ET, Khetsuriani N, Beard RS, et al. SARS-associated coronavirus transmission, United States. Emerg Infect Dis. 2004;10(2):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau JT, Lau M, Kim JH, et al. Probable secondary infections in households of SARS patients in Hong Kong. Emerg Infect Dis. 2004;10(2):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu WC, Tsang TH, Tong WL, et al. Prevalence of subclinical infection by the SARS coronavirus among general practitioners in Hong Kong. Scand J Infect Dis. 2004;36(4):287–290. [DOI] [PubMed] [Google Scholar]
- 19. Hui DS, Azhar EI, Kim YJ, et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18(8):e217–e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aburizaiza AS, Mattes FM, Azhar EI, et al. Investigation of anti-Middle East respiratory syndrome antibodies in blood donors and slaughterhouse workers in Jeddah and Makkah, Saudi Arabia, Fall 2012. J Infect Dis. 2014;209(2):243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chu DK, Poon LL, Gomaa MM, et al. MERS coronaviruses in dromedary camels, Egypt. Emerg Infect Dis. 2014;20(6):1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hemida MG, Al-Naeem A, Perera RA, et al. Lack of Middle East respiratory syndrome coronavirus transmission from infected camels. Emerg Infect Dis. 2015;21(4):699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Memish ZA, Alsahly A, Masri MA, et al. Sparse evidence of MERS-CoV infection among animal workers living in southern Saudi Arabia during 2012. Influenza Other Respir Viruses. 2015;9(2):64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reusken CB, Farag EA, Haagmans BL, et al. Occupational exposure to dromedaries and risk for MERS-CoV infection, Qatar, 2013–2014. Emerg Infect Dis. 2015;21(8):1422–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liljander A, Meyer B, Jores J, et al. MERS-CoV antibodies in humans, Africa, 2013-2014. Emerg Infect Dis. 2016;22(6):1086–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. So RT, Perera RA, Oladipo JO, et al. Lack of serological evidence of Middle East respiratory syndrome coronavirus infection in virus exposed camel abattoir workers in Nigeria, 2016. Euro Surveill. 2018;23(32):1800175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alshukairi AN, Zheng J, Zhao J, et al. High prevalence of MERS-CoV infection in camel workers in Saudi Arabia. MBio. 2018;9(5):e011985-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zohaib A, Saqib M, Athar MA, et al. Countrywide survey for MERS-coronavirus antibodies in dromedaries and humans in Pakistan. Virol Sin. 2018;33(5):410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The Health Protection Agency UK Novel Coronavirus Investigation team Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. 2013;18(11):20427. [DOI] [PubMed] [Google Scholar]
- 30. Omrani AS, Matin MA, Haddad Q, et al. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17(9):e668–e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mailles A, Blanckaert K, Chaud P, et al. First cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill. 2013;18(24):20502. [PubMed] [Google Scholar]
- 32. Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: a prospective descriptive study. Clin Microbiol Infect. 2014;20(5):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arwady MA, Alraddadi B, Basler C, et al. Middle East respiratory syndrome coronavirus transmission in extended family, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22(8):1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plipat T, Buathong R, Wacharapluesadee S, et al. Imported case of Middle East respiratory syndrome coronavirus (MERS-CoV) infection from Oman to Thailand, June 2015. Euro Surveill. 2017;22(33):30598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Al Hosani FI, Kim L, Khudhair A, et al. Serologic follow-up of Middle East respiratory syndrome coronavirus cases and contacts - Abu Dhabi, United Arab Emirates. Clin Infect Dis. 2019;68(3):409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gierer S, Hofmann-Winkler H, Albuali WH, et al. Lack of MERS coronavirus neutralizing antibodies in humans, Eastern Province, Saudi Arabia. Emerg Infect Dis. 2013;19(12):2034–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Müller MA, Meyer B, Corman VM, et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional serological study. Lancet Infect Dis. 2015;15(6):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munyua P, Corman VM, Bitek A, et al. No serologic evidence of Middle East respiratory syndrome coronavirus infection among camel farmers exposed to highly seropositive camel herds: a household linked study, Kenya, 2013. Am J Trop Med Hyg. 2017;96(6):1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saeed AA, Abedi GR, Alzahrani AG, et al. Surveillance and testing for Middle East respiratory syndrome coronavirus, Saudi Arabia, April 2015-February 2016. Emerg Infect Dis. 2017;23(4):682–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim CJ, Choi WS, Jung Y, et al. Surveillance of the Middle East respiratory syndrome (MERS) coronavirus (CoV) infection in healthcare workers after contact with confirmed MERS patients: incidence and risk factors of MERS-CoV seropositivity. Clin Microbiol Infect. 2016;22(10):880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cho SY, Kang J-M, Ha YE, et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016;388(10048):994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park GE, Ko JH, Peck KR, et al. Control of an outbreak of Middle East respiratory syndrome in a tertiary hospital in Korea. Ann Intern Med. 2016;165(2):87–93. [DOI] [PubMed] [Google Scholar]
- 43. Hastings DL, Tokars JI, Abdel Aziz IZ, et al. Outbreak of Middle East respiratory syndrome at tertiary care hospital, Jeddah, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22(5):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moon SY, Son JS. Infectivity of an asymptomatic patient with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2017;64(10):1457–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alfaraj SH, Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus intermittent positive cases: implications for infection control. Am J Infect Control. 2019;47(3):290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amer H, Alqahtani AS, Alaklobi F, et al. Healthcare worker exposure to Middle East respiratory syndrome coronavirus (MERS-CoV): revision of screening strategies urgently needed. Int J Infect Dis. 2018;71:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Amer H, Alqahtani AS, Alzoman H, et al. Unusual presentation of Middle East respiratory syndrome coronavirus leading to a large outbreak in Riyadh during 2017. Am J Infect Control. 2018;46(9):1022–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alshukairi AN, Khalid I, Ahmed WA, et al. Antibody response and disease severity in healthcare worker MERS survivors. Emerg Infect Dis. 2016;22(6):1113–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Assiri A, Abedi GR, Bin Saeed AA, et al. Multifacility outbreak of Middle East respiratory syndrome in Taif, Saudi Arabia. Emerg Infect Dis. 2016;22(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Balkhy HH, Alenazi TH, Alshamrani MM, et al. Description of a hospital outbreak of Middle East respiratory syndrome in a large tertiary care hospital in Saudi Arabia. Infect Control Hosp Epidemiol. 2016;37(10):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alenazi TH, Al Arbash H, El-Saed A, et al. Identified transmission dynamics of Middle East respiratory syndrome coronavirus infection during an outbreak: implications of an overcrowded emergency department. Clin Infect Dis. 2017;65(4):675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oboho IK, Tomczyk SM, Al-Asmari AM, et al. 2014 MERS-CoV outbreak in Jeddah--a link to health care facilities. N Engl J Med. 2015;372(9):846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao J, Alshukairi AN, Baharoon SA, et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017;2(14): eaan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Payne DC, Biggs HM, Al-Abdallat MM, et al. Multihospital outbreak of a Middle East respiratory syndrome coronavirus deletion variant, Jordan: a molecular, serologic, and epidemiologic investigation. Open Forum Infect Dis. 2018;5(5):ofy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Al-Gethamy M, Corman VM, Hussain R, et al. A case of long-term excretion and subclinical infection with Middle East respiratory syndrome coronavirus in a healthcare worker. Clin Infect Dis. 2015;60(6):973–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Al-Abdely HM, Midgley CM, Alkhamis AM, et al. Infectious MERS-CoV isolated from a mildly ill patient, Saudi Arabia. Open Forum Infect Dis. 2018;5(6):ofy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62(4):477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014;29:307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chu DKW, Hui KPY, Perera RAPM, et al. MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc Natl Acad Sci USA. 2018;115(12):3144–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kiambi S, Corman VM, Sitawa R, et al. Detection of distinct MERS-coronavirus strains in dromedary camels from Kenya, 2017. Emerg Microbes Infect. 2018;7(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sikkema RS, Farag EABA, Himatt S, et al. Risk factors for primary Middle East respiratory syndrome coronavirus infection in camel workers in Qatar during 2013-2014: a case-control study. J Infect Dis. 2017;215(11):1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. FAO-OIE-WHO MERS Technical Working Group MERS: progress on the global response, remaining challenges and the way forward. Antiviral Res. 2018;159:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park WB, Perera RA, Choe PG, et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015;21(12):2186–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. World Health Organization Surveillance for Human Infection With Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Interim Guidance June 2018. Geneva, Switzerland: World Health Organization; 2018. http://www.who.int/csr/disease/coronavirus_infections/surveillance-human-infection-mers/en/. Accessed November 18, 2018. [Google Scholar]


