ABSTRACT
Emerging viral infections represent a public health risk pointed out by the spreading of pathogens with potential zoonotic risk. Moreover, the risk of zoonosis has probably been underestimated in occupational settings. A literature review between 2007 and 2018 was performed to identify evidences concerning the epidemiological associations between some emerging viruses and occupational diseases. Observational studies and case-reports were selected and analyzed. West Nile Virus (WNV) disease, Crimean-Congo Hemorrhagic Fever (CCHF) disease and Hepatitis E virus (HEV) infection were included in the review for their potential zoonotic transmission. The most important risk factor for acquiring WNV infection and CCHF infection is the exposure to infected mosquitoes and ticks, respectively; therefore, outdoor workers are at risk of infection. HEV is responsible for epidemics and endemics of acute hepatitis in humans, that can become infected through waterborne, foodborne and zoonotic transmission routes. A total of 10, 34 and 45 eligible studies for WNV, CCHF virus (CCFHV) and HEV, respectively, were analyzed by year, country, study design, risk group and outcomes. The occupational risk groups mainly included farm and agricultural workers, veterinarians, slaughterers, animal handlers, healthcare workers and soldiers. These findings support the need to develop effective interventions to prevent transmission of emerging viruses.
Keywords: emerging viruses, infections, occupational exposure, workplace, workers, Hepatitis E, West Nile Virus disease, Crimean-Congo Hemorrhagic Fever
This systematic review summarizes epidemiological studies published from 2007 until October 2018 on West Nile Virus infection, Crimean-Congo Haemorrhagic fever and Hepatitis E in occupational settings
INTRODUCTION
It is well known that most infectious diseases in humans originate in animals, and the frequency of such diseases, named zoonoses, has been increasing over time (Belay et al. 2017).
A joint consultation of WHO/FAO/OIE held in 2004 (WHO 2004) defined emerging zoonosis as ‘a zoonosis that is newly recognized or newly evolved, or that has occurred previously but shows an increase in incidence or expansion in geographical, host or vector range’.
Drivers responsible for the emergence of zoonotic diseases include climate and environmental changes, human behavior, farming and trading practices, vector distribution and characteristics of the pathogens. Many zoonosis emerge from wildlife species, e.g. Severe Acute Respiratory Syndrome Coronavirus raised from bats and transmitted to civets before affecting humans. Examples of zoonotic virus of other origin include West Nile Virus (WNV), Chikungunya virus and Crimean-Congo Hemorrhagic Fever Virus (CCHFV), responsible for diseases with a high impact on public health (Wang and Crameri 2014; Belay et al. 2017). Hepatitis E virus (HEV) had been considered a sanitation problem in resource limited countries; however, the zoonotic form has emerged in industrialized countries with high seroprevalence, as detected in swine abattoir workers (Ukuli and Mugimba 2017).
Recent epidemiological data on zoonosis are also of concern regarding the occupational medicine. Mitigating the impact of viral emerging zoonotic diseases of occupational health importance is difficult because of several work and economic conditions worldwide, and requires multisectoral collaboration and interdisciplinary partnerships. In fact, control and prevention strategies for most zoonosis are effective according to a One Health approach at the human–animal–ecosystem interface.
In this study, a review was carried out to assess and summarize the scientific evidences concerning the epidemiological associations between some emerging viruses and occupational diseases. WNV and CCHFV were included as examples of pathogens responsible for vector-borne infections, transmitted by mosquitoes and ticks, respectively, and both viruses are spreading in Europe and neighboring countries at an increasing rate (Marcantonio et al. 2015). HEV infection was also included in the review since growing evidences show that zoonotic transmission through contact with infected animals or consumption of contaminated food is responsible for most of the autochthonous cases in industrialized countries (Clemente-Casares et al.2016). The purpose was to identify which occupational sector, job, population at risk are more vulnerable to three emerging zoonotic viruses and main clinical outcomes, according to the selected papers, in order to provide evidence for policy makers and stakeholders involved in occupational safety and health.
WNV
WNV is a neurotropic member of the family Flaviviridae, genus Flavivirus, maintained in enzootic cycles involving several species of birds, which act as amplifying reservoir host, and mosquitoes belonging principally to the Culex pipiens complex, although other species would also support the spread of the virus (Marcantonio et al.2015). A study conducted in Italy between 2008 and 2014 detected WNV in three mosquito species belonging to two genera: Culex pipiens s.l., Culex modestus and Ochlerotatus caspius (Mancini et al. 2017. Humans, horses and other mammals are incidental or dead-end host. First isolated in Uganda in 1937, starting from the 1990s WNV has spread rapidly across all the continents. Climate change (warmer temperature and higher cumulative rainfall) could be one of the drivers that contribute to the changing pattern of transmission of several vector-borne diseases (Riccardo et al. 2018). Following transmission via mosquito bites, WNV replicates in keratinocytes and in the skin dendritic cells (DCs), Langherans cells (LCs), which then migrates to the local lymphnodes from where the virus disseminate to the kidney, spleen and other visceral organs. In about 1% of all infected patients, the WNV infection evolves to severe neurologic disease, including encephalitis, meningitis, acute flaccid paralysis and death. The virus entry to the central nervous system can be either via blood stream as well as via trans-neural pathways. Infection can also happen by blood transfusion, organ, tissue and cell transplants. According to the above, although most human infections are subclinical, symptoms can vary from a self-limiting fever to severe neurological disease (Ulbert 2011).
It has been demonstrated, by in vitro and in mouse models in vivo, that WNV infection induces innate cell immune response through activation of the toll like receptors (TLR3) and retinoic acid-inducible gene I (RIG-I pathways) and induction of type I interferon (IFN-I) as well as of IFN λ. Type I IFN receptors signaling in astrocytes regulates the permeability of the blood–brain barrier and protects the cerebellum from neuroinvasive infection by WNV. Therefore, the negative regulation of IFN responses, by either host and viral factors, can contribute to the pathogenesis of WNV. In particular, NS1 protein of WNV, secreted upon infection, represses TLR3-induced IFN in mouse and human cells, thus favoring virus spreading in the CNS (Luo and Wang 2018).
CCHFV
CCHFV belongs to the genus Orthonairovirus, family Nairoviridae. Ticks of the genus Hyalomma are considered both main vector and natural reservoir; direct contact with fluids, tissue or blood of infected animals are also considered transmission routes of the infection to humans. CCHFV is maintained and transmitted in a vertical and horizontal transmission cycle involving a variety of wild and domestic animals that act as amplification hosts without showing signs of illness. Despite these animals have been considered reservoirs of the virus, they develop only a transient viremia, while the virus can persist in ticks for their entire lifespan, and can be vertically transmitted to the next generation. Therefore, ticks are considered both vector and reservoir for the virus (Gargili et al.2017). Nosocomial transmission may occur through direct contact with human infected blood or body fluids or contaminated medical equipment or supply. First recognized in 1944, human CCHFV infections have been reported in over 30 countries in Asia, Middle East, South-Eastern Europe and Africa. Clinical symptoms usually comprise mild and non-specific febrile illness; in some cases, severe hemorrhagic disease can develop (Wang and Crameri 2014).
The pathogenesis of CCHFV infection in humans is mainly based on immunopathogenetic mechanisms, mediated by either innate or adaptive immune responses. The RIG-I pathway is an immune sensor of CCHFV RNA. Studies in human patients have shown that TLRs, in particular TLR 7, 8, 9 and 10 polymorphisms correlate with the severity and outcome of the disease in some geographical areas (Turkey) (Hawman and Feldmann 2018). The virus itself is able to antagonize innate immune signaling through deubiquitinatin and cleavage of proteins involved in innate immunity, such as ISG15, mediated by a specific domain (OTU, ovarian tumor-like deubiquinase domain) in the L segment of CCHFV. With regard to the role of the adaptive immunity responses to CCHFV in human pathogenesis, it is not completely clear, due to the lack of suitable model in vivo. The evidence obtained from the recently developed cynomologus animal model suggested that neither the antibodies titer nor their neutralizing activity seem to correlate to the outcome of CCHFV infection. The role of T cell responses, such as cytolitic activity in hepatic injury and severity of the CCHFV associated haemorragic disease, seems not necessary but needs further studies.
HEV
Hepatitis E is an acute disease caused by HEV, classified in the family Hepeviridae, genus Orthohepevirus A. Genotypes HEV‐1 and HEV‐2 are restricted to humans and circulate in endemic area (Asia and Africa) causing outbreaks following the ingestion of contaminated water. In non-endemic areas (industrialized countries), HEV‐1 and HEV‐2 are linked to travel in endemic areas. In the last 10 years, an increasing number of autochthonous infections, linked to the zoonotic transmission of the genotypes HEV‐3 and HEV‐4, have been described (Kamar et al. 2017). There are evidences for the presence of autochthonous cases of HEV infections in Italy since 1980 (Stroffolini et al. 2015). The virus is transmitted via oral-fecal route, as well as by zoonotic transmission through direct contact with infected animals or food. Swine is the principal reservoir of HEV, mainly belonging to genotypes 3 and 4, with prevalence of anti HEV antibodies ranging from 8% to 93% (Huang et al. 2019). Other reservoirs are wild board, rabbits, deer, mongooses, yaks and camels, infected with different genotypes (Nan and Zhang 2016). Vertical transmission from mother to fetuses (Sharma et al. 2017), and bloodborne transmission of the virus has been reported (Al-Sadeq, Majdalawieh and Nasrallah 2017).
The virus probably replicates in extra-hepatic sites, such as intestinal tract, lymphnodes, colon, to reach the hepatocytes where it replicates in cytoplasm and then is released into the bloodstream and bile. The main liver damage by HEV is mediated by T cells and Natural Killer (NK) cells. The virus is shed in the stool (Lhomme et al. 2016).
Hepatitis E is usually a self-limiting illness, in most cases (95%) the infection is asymptomatic, as the HEV is non cytopathic, with mortality rate of 1–2% worldwide (WHO 2018). Sometimes symptoms of acute hepatitis can manifest.
HEV infection is associated with a number of extrahepatic manifestations, including kidney and a range of neurological injuries, in particular, Guillain–Barré syndrome, neuralgic amyotrophy and encephalitis/myelitis (Dalton et al. 2016). During pregnancy, HEV infection can take a fulminant course, resulting in fulminant hepatic failure, membrane rupture, spontaneous abortions and stillbirths. Studies from various developing countries have shown a high incidence of HEV infection in pregnancy, with a fatality rate of up to 30% (Pérez-Gracia, Suay-García and Mateos-Lindemann 2017).
Usually mild illness occurs in adult healthy individuals, whereas chronic severe disease occur in immunocompromised patients (transplant recipients, HIV immunocompromised patients) (Kamar et al.2014) and in pregnant women, in which HEV-1 and HEV-2 are likely to cause serious medical complications including liver failure, increased risk of miscarriage and premature delivery (Khuroo and Kamili 2003). Chronic HEV infection is defined as detection of HEV RNA in serum or stool for longer than 6 months and is typically associated to the genotype 3 of HEV.
It is thus evident that clinical features of HEV infection range from asymptomatic or acute liver failure to chronic infection without clinical symptoms but with increase in liver enzymes.
One of the critical point in HEV infection is clinical diagnosis of acute and chronic infection that is achieved by means of serologic and molecular tests, that are often non-specific.
Initially an anti-HEV IgM assay is used, whose positivity is confirmed by evidence of rising the IgG titers. Although the IgM appear in the early phase of clinical illness and last for 4 to 5 months in 90% of patients, serology may be negative in a considerable proportion of acutely infected patients. Anti-HEV IgG increase during the convalescent phase but it is not clear how long they persist. HEV RNA can be detected in stool about 1 week before the onset of illness and persists up to 2 weeks thereafter; serum viral RNA can persist up to 4 weeks in those who resolve the acute infection and for years in patients who develop chronic infection. The serological assays are easy to perform and relatively low expensive and several commercial and in-house ELISA assays are available; however, due to the cross-reactivity with other viruses and to the genotype variability of HEV, the sensitivity and specificity as well as performance of the serological tests are low and poor, providing inconclusive results. This is even more complicated in the case of immunosuppressed patients due to their delayed seroconversion upon HEV infection. The detection of HEV RNA by PCR and RT-PCR is therefore needed to confirm serological screening in persistent infection, especially in blood and organ donations (Al-Sadeq et al.2018).
METHODS
Eligibility criteria
The aim of this review is to identify observational studies that show evidence of association between human anti-HEV, anti-WNV and anti-CCHFV antibody seropositivity (IgG and/or IgM) and certain occupational groups at risk of exposure. For this review, we included studies meeting the following eligibility criteria.
Articles published in peer reviewed journals.
English language.
Epidemiological studies published from 2007 until October 2018.
Observational studies and case-reports (including cross-sectional, seroprevalence, retrospective, case-control and case-report).
Outdoor working population of all ages, sex and ethnic groups.
Well-defined and quantitative information source for the selected etiologic agents: Hepatitis E (HE), West Nile (WN), Crimean-Congo Hemorrhagic Fever (CCHF) viruses.
Outcome measures: seroprevalence of anti-HEV, anti-WNV and anti-CCHFV IgG and/or IgM among occupationally exposed populations.
Studies on humans only.
We excluded studies that did not report original results (reviews, letters and comments) or did not provide sufficient data (e.g. lack of information about the number of cases and controls or about the used method). Exploratory studies were not included.
Information sources
Search methods for identification of studies
Studies were identified by searching electronic database (PubMed, January 2007 to October 2018) and scanning reference lists of articles (from reviews not included). The following Medical Subject Headings (MeSH) terms were used: occupational groups; occupational medicine; industry; occupational diseases; disease; employment; occupational health; occupations; workplace; occupational exposure; workload and work. When building the search syntax, for prompt identification of studies conducted in the occupational setting, we referred to the strings developed precisely for this purpose by Mattioli et al. (Mattioli et al. 2010) and used the ‘more sensitive search strategy’:
(occupational diseases [MH] OR occupational exposure [MH] OR occupational exposure* [TW] OR ‘occupational health’ OR ‘occupational medicine’ OR work-related OR working environment [TW] OR at work [TW] OR work environment [TW] OR occupations [MH] OR work [MH] OR workplace* [TW] OR workload OR occupation* OR worke* OR work place* [TW] OR work site* [TW] OR job* [TW] OR occupational groups [MH] OR employment OR worksite* OR industry) AND name(s)-of-the-disease (namely: Hepatitis E, West Nile disease, CCHF).
The choice of this strategy allows either to assess diseases, which produce only a few articles or to explore scarcely studied disease in more depth, that is the case of the present review on emerging viruses among occupational populations.
Data extraction and assessment of bias
Two pairs of authors independently screened titles and abstracts of studies obtained by the search strategy. Each potentially relevant study located in the search was obtained in full text and assessed for inclusion independently by the two groups. Measure of inter-reviewer agreement was assessed via Cohen's κ statistics (Landis and Koch 1977). Disagreements between authors were resolved by consensus.
Data were collected from each relevant study. Extracted information included:
source (first author and year of publication);
general study details (citation, study design and year of publication);
setting (country/region considered, study population and job);
exposure measurement details (methodology including diagnostic tools used);
outcome data;
main findings.
RESULTS
We reviewed the scientific literature to give an overview of the evidence available from the last 12 years regarding the occupational risk of exposure to three emerging zoonotic viral infections: WNV disease, CCHF disease and Hepatitis E infection. Reports or studies were original papers suitable for inclusion; therefore, full-texts were analyzed and the following information was extracted (as applicable to study): type of study, geographical location, study population (number of cases, patients, control or risk groups), antibody positivity rates of exposed and control subjects, statistical significance comparing risk groups vs. non-risk groups, preventive measures.
WNV
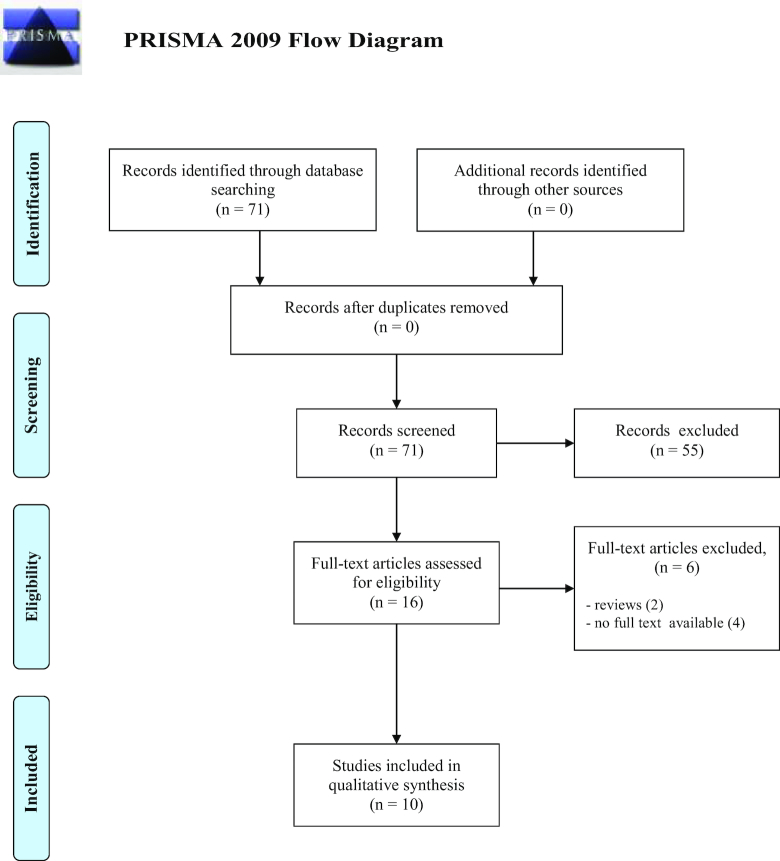
A total of 71 studies on WNV were collected and examined to determine if the inclusion criteria were met; 55 were discarded because did not meet the criteria. Two articles were reviews, therefore excluded; three studies whose abstracts were not found were also excluded. The full text of the remaining studies were searched and analyzed: one full text was not available, the remaining 10 fully met the inclusion criteria and were included in the systematic review. See flow diagram Fig. 1 (Moher et al. 2009). There was a significantly good measure for inter-reviewer agreement (Cohen's k = 0.860, P < 0.001).
Figure 1.
Flow diagram through the different phases of the review for WNV reports.
Summary data of selected studies are in Table 1.
Table 1.
Descriptive summary of epidemiological studies published from 2007 until October 2018 on WNV infection in occupational settings.
| References | Country year | Study design | Risk group | Outcomes (cases/deaths) | Main results |
|---|---|---|---|---|---|
| Smith (2016) | USA, na | Case report | 1 security guard | Diagnosis of WNV encephalopathy. | Employees with WNV complications may not be ready to return to work full duty and may need a flexible return to work or accommodations via a reduced schedule until fully recovered. |
| Vieira et al. (2015) | Brazil, 2014 | Case report | 52-year-old ranch worker | Encephalitis and flaccid paralysis at admission in hospital, high titres of antibodies against WNV. | This report exemplifies importance of acute viral encephalitis surveillance. |
| Venter and Swanepoel (2010) | South Africa, na | Case report | 1 veterinarian and 1 laboratorist | Both resulted in neurological disease. | Laboratorist acquired infection by needle stick injury. Veterinary student acquired infection while performing an autopsy, gloves were the only protective gear worn. Human cases of aseptic meningoencephalitis should be screened to determine if WNV cases can be detected. |
| Venter et al. (2009) | South Africa, na | Case report | 1 laboratorist | Symptoms included backache, neck stiffness and malaise; on day 8 rash, mild fever, meningoencephalitis and photophobia; and on day 9, arthralgia. | This case confirms the neurovirulent potential of lineage 2 WNV strain. |
| Remoli et al. (2018) | Italy, 2018 | Epidemiological study | 101 workers (farmers and agricultural workers) and 100 controls. | About 0% IgG positive. | No seropositivity for WNV was detected, although the study was carried out in a geographical area where outbreaks have been documented in the past; limited number of subjects may be responsible for the results. Surveys in outdoor workers could provide early warning on the emergence of arboviruses in specific regions and individuals. |
| van Eeden, Swanepoel and Venter (2014) | South Africa, 2011–2012 | Epidemiological study | 127 veterinarians | About 7.9% antibodies positive. | Indications that veterinarians might be at increased risk of WNV infection. |
| Karakoç et al. (2013) | Turkey, 2009 | Epidemiological study | 182 individuals at high risk (farmers, agricultural workers and traders); 125 at low risk (housewives, teachers, students and priest). | About 20.87% IgG positive (risk group workers). | In univariate analysis serologic positivity in the high-risk group was more statistically significant than in the low-risk group (73% versus 56%,P = 0.026). In multivariate analysis, being in an occupational risk group (OR = 2.2, CI 1.02–4.04, P = 0.044) was found to be a risk factor for WNV serologic positivity. |
| Barzon et al. (2009) | Italy, 2008–2009 | Epidemiological study | 321 farmers | About 0.9% IgG positive, n = 3, 0.6% IgM and IgG positive. | Workers employed in farms with WNV-positive horses; this infection appears to be widespread among horses in north-eastern Italy. Both 1998 and 2008 Italian outbreaks could be related to a continuous endemic circulation of WNV. |
| Spataro et al. (2008) | Italy, 2006 | Epidemiological study | 1280 (health care workers, hunters, stable workers as jockey and grooms, fowlers, veterinary surgeons and blood donors) | About 0% antibodies positive. | The study supplies an answer in considering the absence of risk infection in the category examined. However, programs of antibodies survey are useful to predict which effects could have the presence of WNV infection. |
| Bernabeu-Wittel et al. (2007) | Spain, na | Epidemiological study | 504 subjects from general population including farmers, stockbreeders, veterinarians, rangers, foresters, and extermination and pest control workers | About 2.8% IgG positive subjects at occupational risk. | P = 0.048 for activity in any risk profession (involving close contact with animals, nature and wetlands or mosquitoes). |
na = not available
Of the 10 paper included in our review, 4 were case report studies described in USA, Brazil and South Africa; the remaining were epidemiological studies conducted in Turkey, Italy, Spain and South Africa. We found that main transmission pathways were direct contact with animal's body fluids and indirect contact by infected mosquito bite. The first pathway was described in one veterinarian exposed to infected horse brain while performing an autopsy (Venter and Swanepoel 2010), and in one laboratorist who acquired the infection after a needlestick injury that exposed her to cell-culture fluid containing WNV strain SPU93/01 (Venter et al. 2009).
WNV infection acquired by mosquito bite occurred in one security guard (Smith 2016) and in one ranch worker (Vieira et al. 2015). Both zoonotic transmission pathways were also responsible for infections in farmers, agricultural workers and veterinarians enrolled in the epidemiological studies included in the review, with serologic IgG positivity ranging from 0.9% (Barzon et al. 2009) to 20.87% (Karakoç et al. 2013).
CCHFV
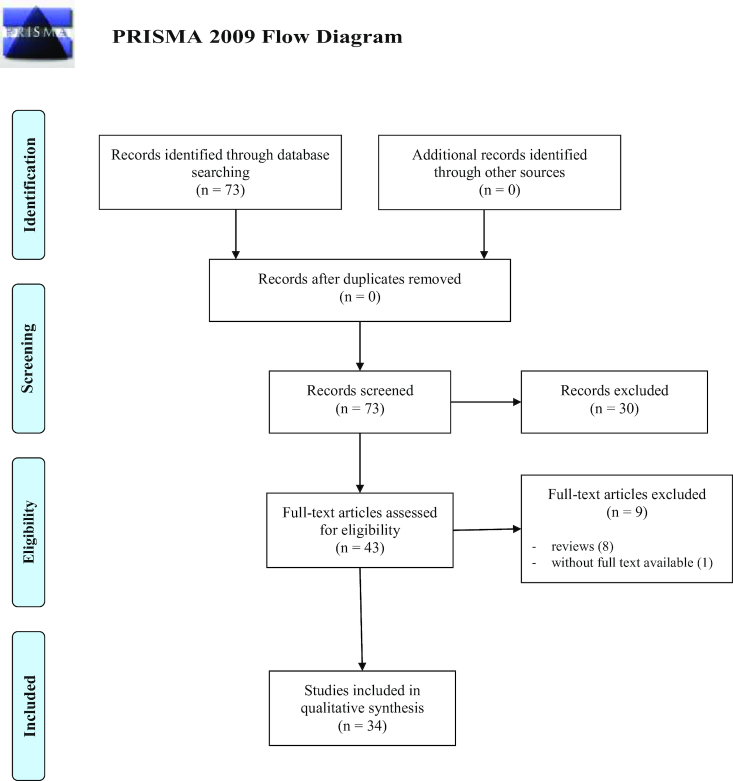
A total of 73 studies on CCHFV were identified for inclusion in the review; subsequently 30 were excluded because did not meet the criteria. Reviews and studies based on questionnaires were also excluded, abstract was not found for one study. Full text of the remaining papers were analyzed and 34 totally met the inclusion criteria; therefore, included in the review. See flow diagram Fig. 2 (Moher et al. 2009). There was 'moderate measure' for inter-reviewer agreement (Cohen's k = 0.565, P < 0.001).
Figure 2.
Flow diagram through the different phases of the review for CCHFV reports.
Summary data from selected studies are reported in Table 2.
Table 2.
Descriptive summary of epidemiological studies published from 2007 until October 2018 on CCHF infection in occupational settings.
| References | Country year | Study design | Risk group | Seroprevalence | Main results |
|---|---|---|---|---|---|
| Negredo et al. (2017) | Spain, 2016 | Case report | 1 nurse | IgM antibodies increased on the 6th day of illness and decreased after the 15th day. Titer for IgG antibodies remained constant. Index case and nurse both infected with the African lineage 3 of the virus. | The appearance of 2 autochthonous cases in a previously unaffected region of Europe reinforce the notion that CCHF is a re-emerging infectious disease. Importance of routine surveillance of vectors capable of spreading CCHF. |
| Yadav et al. (2017) | Saudi Arabia, 2016 | Case report | 1 supervisor on animal farm | The migrant worker returned home to India after becoming ill in Oman. | Travelers should be made aware of communicable diseases present in countries they visit, and patients should inform doctors if they have a recent travel history. Physician should consider CCHF in the differential diagnosis of patients with haemorrhagic signs and have recently returned from any area where CCHF is endemic or prevalent. |
| Yadav et al. (2016) | India, 2015 | Case report | 4 nurses | 1 nurse died. | HCWs are an important risk group. Infected patients should be isolated; HCWs should mandatorily wear minimum essential personal protective equipment (PPE). Need for syndrome-based surveillance of viral hemorrhagic fever (VHF) cases for CCHF and strict infection control measures in hospital environment. |
| Yildirmak, Tulek and Bulut (2016) | Turkey, na | Case report | 1 farmer (case index); 1 nurse; 1 doctor and 1 attendant to case index, and his uncle | Case index died. | HCWs visited index patient's room once without face-mask when the patient was on mechanical ventilation. All HCWs are required to follow barrier-nursing technique (gloves, masks, gowns, goggles and hand-washing). Besides contact and standard precautions, airborne precautions must be strictly followed, especially for patients with severe disease. Visitors must be restricted. |
| Fazlalipour et al. (2016) | Iran, 2015 | Case report | 1 butcher | The butcher recovered. | Traditional slaughtering and butchery may put workers at risk of CCHFV infection. As an important preventive measure, traditional slaughtering should be limited especially in places where there are not enough healthcare facilities to minimize the risk of infection. |
| Ozsoy et al. (2015) | Turkey, 2011–2012 | Case report | 1 forensic scientist | The forensic scientist recovered. | There is a need for education and training. The best protection for autopsy workers is good autopsy techniques and knowledge of safety procedures. |
| Pshenichnaya and Nenadskaya (2015) | Russia, 2011 | Case report | 8 HCWs: 3 nurses, 2 hospital attendants, 2 anesthetists and 1 obstetrician | All recovered. | Possibility of aerosol transmission of CCHF; when performing aerosol-generating medical procedures (AGMPs) for any CCHF patient airborne precautions should always be added to standard precautions for all HCWs who are in a patient's room. |
| Celikbas et al. (2014) | Turkey, 2004–2011 | Case report | 9 HCWs: 5 nurses and 4 physicians | 1 fatal, 2 asymptomatic. | In addition to standard precautions, airborne infection isolation precautions are essential during aerosol-generating procedures. Ribavirin is an effective treatment and beneficial for post-exposure prophylaxis. |
| Mardani, Namazee (2013) | Iran, 2011 | Case report | 6 patients: all consanguigne; 2/6 were livestock workers. | 1 livestock worker (index case) died. | This study only described manifestations, management and outcome of cases in an outbreak. Larger and controlled studies are necessary to evaluate the various aspects of the disease. |
| Naderi et al. (2011) | Iran, 2009 | Case report | 6 patients, of whom 4 were HCWs: 2 gynaecologists and 2 carers. | 2 patients dead, HCWs recovered. | Early diagnosis is not only important to prevent the spread of CCHF virus but also makes early administration of ribavirin possible, thus, reducing clinical manifestations and improving prognosis. |
| Mardani et al. (2009) | Iran, 1999–2009 | Case report | 1 shepherd, 1 farmer, 3 physicians | 3 dead, 2 recovered. | Risk of nosocomial transmission can be minimized by proper and timely infection-control measures, universal precautions including contact and droplet isolation for suspected patients, careful management of infected patients, and in some cases, administration of prophylactic therapy to healthcare workers after exposure. Based on data for our three cases, we do not recommend airborne isolation for CCHF patients. |
| Sargianou et al. (2013) | Greece, 2012 | Cross-sectional study | 207 individuals | About 3.4% (7/207) IgG positive; 4/7 occupied with either farming or animal husbandry, 2 housewives and 1 was a retiree. | Multivariate logistic regression showed that an agro-pastoral occupation was significantly associated with CCHFV seropositivity (OR 6.99, 95% CI 1.01–48.4, P = 0.049). |
| Andriamandimby et al. (2011) | Madagascar, 2008–2009 | Cross-sectional study | 1995 slaughterhouse workers | About 0.05% IgM positive, 0.75% IgG positive. | IgM 95% (confidence interval [CI]: 0–0.15%), IgG 95% (CI: 0.37–1.13%). Overall, the percentage of CCHFV infection in Madagascar among at-risk professionals is very low compared to those observed in endemic countries like Mauritania (7%) and United Arab Emirates (6%). This may be explained by the lack of ticks of the genera Hyalomma in Madagascar. The low percentage of detection of human antibodies against CCHFV and the scattered geographic distribution may be the consequence of repeated introductions of infected animals, large movements of domestic ruminants in the country and abortive circulations of CCHFV. |
| Leblebicioglu et al. (2016) | Turkey, 2002–2014 | Retrospective cross-sectional study | 9 hospitals, 51 healthcare-related exposure (22 physician trainees, 21 nurses, 2 physician specialists, 2 medical students, 2 ward-based staff and 2 laboratory technicians) extracted from chart of all personnel with a reported healthcare injury/accident related to CCHF | About 49% (25/51) of healthcare-related exposures resulted positive (laboratory confirmed) for CCHF. 16% (4/25) of mortality. | Advanced infection prevention and control training focused on sharp safety and personal protective equipment is vital for all clinical staff in endemic areas, accompanied by wider education of HCWs because CCHF was not initially considered in 25% of exposure cases. |
| Mourya et al. (2017) | India, 2014–2015 | Retrospective study | 69 patients suspected for CCHF | About 30.4% CCHF positive patients (21/69); and 9/21 (42%) workers (shepherds, farmers and staff nurses). | Hemorrhagic manifestations may provide a clue to early suspicion of CCHF in areas known to have this infection before the availability of confirmatory diagnosis. |
| Guner et al. (2014) | Turkey, 2007–2013 | Retrospective study | 7 HCWs (4 doctors and 3 nurses) | Ribavirin prophylaxis administered in 6 HCWs who had contact with blood or body fluid; 1 physician who did not receive ribavirin resulted in CCHF positive. | The virus has also been isolated from saliva; therefore, patient secretions, especially with reflexes that cause aerosolization (sudden cough or sneeze), may cause transmission by mucosal contact. HCWs should wear goggle or face shields to prevent mucosal contact in addition to mask, gloves and gown in intervention requiring close contact. |
| Duran et al. (2013) | Turkey, 2006–2012 | Retrospective study | 46 patients with CCHF and 38 patients without CCHF, but who had been bitten by ticks (control group). | Of 46 patients CCHF positive: 18 were housewives, 8 animal husbandry workers, 4 farmers, 2 HCWs and 14 other workers. Among HCWs 1 died and 1 survived. | The number of patients with a history of contact with animals or animal blood was significantly higher than that in the control group (P < 0.05). The number of patients with a history of tick bites was higher than that in the control group but the difference was not statistically significant (P > 0.05). |
| Vawda et al. (2018) | South Africa, 2012, 2016–2017 | Epidemiological study | 387 samples: 338 were workers (abattoir workers, slaughterers, veterinarians, horse handlers and farmers) and 49 recreational hunters | About 0.51% for IgG in workers (2 abattoir workers). | CCHFV remains uncommon in South Africa (seroprevalence results similar to those obtained 30 years ago). |
| Mostafavi et al. (2017) | Iran, 2011 | Epidemiological study | 190 butchers and slaughterhouse workers | About 16.49% for IgG (31 subjects). | Length of employment and age had a positive correlation with CCHF seropositivity. Average length of IgG seropostivity was 5 years, 11/31 had a previous record of infection. Total 39.7% of participants did not use any PPE: special training or information leaflets by butcher trade officials is required. |
| Akuffo et al. (2016) | Ghana, 2011 | Epidemiological study | 109 animal handlers | About 5.7% overall seropositivity | CCHFV was detected in ticks collected from cattle, one of the livestock known to play a role in the amplification of CCHF virus. Future studies targeted at understanding relationship between climatic conditions and occurrence of CCHF could be helpful in the establishment of early warning systems in the surveillance of the disease. |
| Todd et al. (2016) | Afghanistan, Pakistan and Iran, 2010–2011 | Epidemiological study | 809 Afghan National Army Recruits | About 4.1% (33/809) overall prevalence of antibodies to CCHF. | Need for geographical and temporal correlation with case reporting and screening. Continued surveillance of vector-borne aetiologies to monitor effectiveness of control measures. Future studies focused on both animals and humans to assess potential reservoirs of infection and to determine prevalence and risk factors for transmission of zoonotic diseases. |
| Wasfi et al. (2016) | Tunisia, 2014 | Epidemiological study | 38 slaughter workers | About 5.2% seroprevalence for IgG. | Seroprevalence suggests predominance of subclinical forms; similar seroprevalence in high-risk population reported from other endemic countries. Results provide strong evidence of circulation of CCHFV in Tunisia; further studies recommended on livestock, humans at high risk, birds and ticks to better understand dynamic transmission of CCHFV. |
| Cikman et al. (2016) | Turkey, 2002–2014 | Epidemiological study | 145 animal husbandry workes; 174 subjects exposed or bitten by ticks, 53 healthy subjects not exposed to livestock or ticks | About 12.4% workers IgG positive, 16.7% subjects exposed or bitten by ticks and 9.4% subjects not exposed. | Statistically significant difference between prevalence of CCHF in livestock workers or subjects exposed to CCHFV and those unexposed or residing in city (P < 0.05). High seroprevalence in Erzincan, where the disease is endemic. Educational and training programs targeted at high risk group should be developed and implemented. |
| Mohd Shukri et al. (2015) | Malaysia, 2012–2013 | Epidemiological study | 85 farm workers | About 0% farm workers IgG positive. | Results suggest that CCHFV is still not a threat to Malaysian farm workers; a possible explanation might relate to acaricides and rotational grazing systems used in 7 of the 8 farms studied. |
| Newman et al. (2014) | Afghanistan, 2008–2011 | Epidemiological study | 467 UK military personnel | About 0% for anti-CCHF antibodies in military personnel. | The need for continued surveillance of military personnel and for education of healthcare providers to help recognize and prevent illnesses and transmission of pathogens during and after overseas deployments. |
| Sisman (2013) | Turkey, 2007–2011 | Epidemiological study | 126 samples CCHF positive | About 3 (2.38%) HCWs and 118 (93.7%) workers in agriculture and animal husbandry. 1/3 HCWs died. | CCHF causes severe disease and has a mortality risk of about 10% in Turkey. High-risk groups are working in agriculture and animal husbandry in rural areas, especially those living at an altitude of 600 m or higher, in May, June and July. HCWs also have a higher risk. |
| Sidira et al. (2013) | Greece, 2010–2011 | Epidemiological study | 51 (19 slaughterhouse workers and 32 hunters); 277 subjects from general population | About 1.9% for IgG (0% in slaughterers, 3.1% in hunters (1/32); 2.2% for IgG in general population). | The fact that the disease emerged in Greece, endemic foci exist in the neighbouring countries and competent vector are present necessitates active surveillance of human CCHF infections. |
| Gozel et al. (2013) | Turkey, 2002–2012 | Epidemiological study | 190 HCWs: 57 nurses, 47 physicians, 45 laboratory technicians and 41 housekeeping staff | About 0.53% IgG positive (1 nurse). | Compliance of HCWs with the usage of PPE was high. Total rates of PPE usage were 93.7% for gowns, 77.4% for gloves and 38.9% for masks; the highest was found among the HCWs of infectious diseases ward: 100%, 88.6% and 82.9%, respectively. PPE usage in the hematology department was significantly lower than in the other departments (P < 0.05). |
| Mofleh, Ahmad (2012) | Afghanistan, 2008 | Epidemiological study | 30 cases CCHF positive: 1 nurse, 28 cases resulting from contact with animals ora animals products and 1 family contact | A total of 10 deads (2 butchers, 3 housewives, 1 farmer, 1 cook, 1 shopkeeper, 1 jobless and 1 daily wage worker). | More patients infected by contact with meat and body fluids died that those whose contact was through animal husbandry or ticks (P = 0.0048). Butchers are routinely exposed to the blood and other body fluids of animals, which suggest exposure to higher doses of the virus. |
| Memish et al. (2011) | Saudi Arabia, 2010 | Epidemiological study | 1026 soldiers | About 0.58% IgG positive. | Epidemiology and distribution of CCHFV in Saudi Arabia are unclear. However, this study provides systematic evidence that CCHFV is endemic to western provinces of Saudi Arabia. |
| Maltezou, Maltezos and Papa (2009) | Greece, 2008 | Epidemiological study | 21 HCWs: 2 physicians and 19 nurses | About 0% IgG positive. | Education of HCWs is needed about the epidemiology of CCHFV and appropriate implementation of PPE for containment of nosocomial transmission. |
| Gunes et al. (2009) | Turkey, 2006 | Epidemiological study | 782 with occupational risk (e.g. healthcare, slaughterhouse work and veterinary care); 100 controls. | About 12.8% IgG positive in workers; 2% in controls. | This study indicated that tick exposure is the most statistically significant transmission route for CCHFV in a high-risk population in Turkey. Effective tick prevention aids such as tick repellents may help reduce the risk. |
| Mardani et al. (2007) | Iran, 2003 | Epidemiological study | 129 HCWs, 94 unexposed subjects | About 3.87% (5/129) IgG positive in the exposed group, 0% in the unexposed group. | (95% CI: 0.55–7.20) (Fisher's exact test, P = 0.075). HCWs in contact with CCHF patients are more likely to test positive for anti-CCHF IgG. All HCWs should take all protective measures whenever they are likely to come in contact with CCHF patients or their blood and other body fluids. |
| Ergonul et al. (2007) | Turkey, 2002–2003 | Epidemiological study | 75 HCWs: 62 at risk of exposure to body fluids of patients, 13 controls | About 1.3% IgG positive in controls (HCW not at risk). | Simple barriers precautions are effective when rigorously applied. |
na = not available
Of the 34 paper included in our study, 11 were case reports (from Spain, Saudi Arabia, India, Turkey, Iran and Russia), 2 cross-sectional studies (from Greece and Madagascar), 4 retrospective studies (from India and Turkey), 17 epidemiological studies (from Turkey, Afghanistan, Ghana, Tunisia, Greece, South Africa, Iran, Malaysia and Saudi Arabia). CCHFV can follow different pathways while infecting humans: in the selected papers, worker's categories who acquired the virus by direct contact with infected animal's fluids or tissue included slaughterhouse workers with history of being splashed with fluids of animal viscera or of cutting their hands or other parts of the body (Mofleh and Ahmad 2012; Akuffo et al. 2016; Cikman et al. 2016; Wasfi et al. 2016; Mostafavi et al. 2017; Vawda et al. 2018), and butchers because of contact with infected meat (Mofleh and Ahmad 2012; Mostafavi et al. 2017). CCHFV seroprevalence among slaughterhouse workers and butchers ranged from 0.51% (Vawda et al. 2018) to 16.49% (Mostafavi et al. 2017).
Vector borne transmission of CCHFV following infected tick bite was found in agricultural and animal husbandry, farmers (Duran et al. 2013; Sargianou et al. 2013; Sisman 2013) and in military personnel deployed in areas where the virus is endemic (Memish et al.2011; Newman et al. 2014; Mostafavi et al. 2017). Human to human as well as nosocomial CCHFV transmission may occur through percutaneous or permucosal exposure to blood or body fluids from infected subjects. Health care Workers (HCWs) are at risk for contracting infection during patient care, as reported in papers included in the review (Ergonul et al. 2007; Mardani et al. 2007; Mardani et al. 2009; Naderi et al. 2011; Gozel et al. 2013; Guner et al. 2014; Ozsoy et al. 2015; Leblebicioglu et al. 2016; Yildirmak, Tulek and Bulut 2016; Yadav et al. 2016; Negredo et al. 2017). A probable CCHFV transmission occurred in HCWs after aerosol generating medical procedures in Russia (Pshenichnaya and Nenadskaya 2015).
HEV
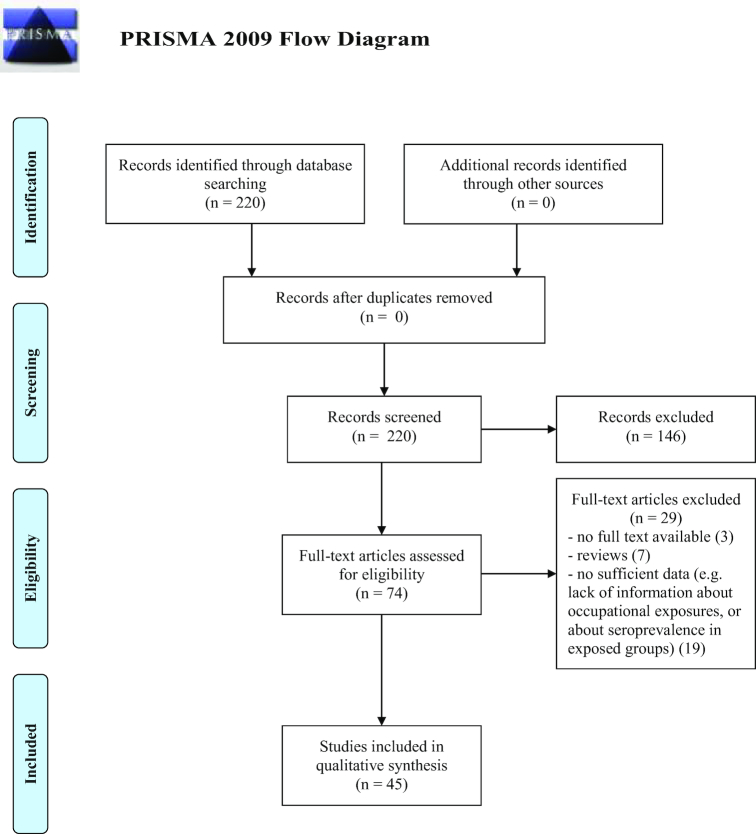
A total of 220 studies regarding HEV were identified for inclusion in the review. Subsequently, 146 studies were discarded because did not meet the criteria. Reviews and studies based on questionnaires were also excluded. For three studies the abstracts could not be retrieved and were not considered. The full text of the remaining studies were analyzed and 45 fully met the inclusion criteria and were included in the review. See flow diagram Fig. 3 (Moher et al. 2009). There was significantly good measure for inter-reviewer agreement (Cohen's k = 0.825, P < 0.001).
Figure 3.
Flow diagram through the different phases of the review for HEV reports.
Summary data from selected studies are reported in Table 3. The majority were epidemiological (24) and cross-sectional (15) studies from Africa (Uganda, Nigeria, Madagascar, Ghana and Burkina Faso), Asia (India, China, Korea, Indonesia, Taiwan and Thailand), Europe (Italy, Germany, Portugal, Norway, Finland, France, United Kingdom, Spain and Netherlands), Brazil and Cuba. Three were retrospective studies (from Switzerland, Italy and Spain), two case studies (Australia and Spain) and one case-control study (China). In occupational settings zoonotic transmission of HEV implies direct contact with swine, principal reservoir of HEV or other animals (wild boar, deer). Indirect contact in areas where animals live and roam or with objects or surfaces contaminated with HEV stools is also considered a transmission route, as well as contact with pig and slaughterhouse sewage. Articles selected in the review comprised mainly swine workers, including farmers and slaughterers, and veterinarians as occupational categories at risk of exposure to HEV; to a lesser extent food handlers (Appuhamy et al. 2014; Cui et al. 2016), workers exposed to wastewater (Tschopp et al. 2009; Albatanony and El-Shafie 2011; Martins et al. 2014) and forestry workers (Carpentier et al. 2012; Dremsek et al. 2012). Seroprevalence data for anti HEV IgG ranged between 2.3% for a group of abattoir workers in Sardinia (Masia et al. 2009) and 2.4% among farmers in UK (Meader et al. 2010) and 68.5% among pig farmers in Germany (Krumbholz et al. 2014) and 76% among butchers in Burkina Faso (Traoré et al. 2015). Significant risk factors for anti-HEV IgG positivity were age, amount of years of occupational exposure and direct swine contact. Hunting has been considered a possible risk factor for acquiring HEV infection, as reported in a study conducted in 144 hunters from Estonia that revealed the presence of HEV-specific IgG in 4.2% of the samples (Ivanova et al. 2015). Others found an anti-HEV prevalence significantly higher in Okinawa wild boar hunters (25.3%) than in the residents (male 7.7% and female 4.1%) (P < 0.0001) (Toyoda et al. 2008). However, the retrieved studies were not included in this review because we did not consider hunters as an occupational category.
Table 3.
Descriptive summary of epidemiological studies published from 2007 until October 2018 on HEV infection in occupational settings.
| References | Country year | Study design | Risk group | Seroprevalence | Main results |
|---|---|---|---|---|---|
| Appuhamy et al. (2014) | Australia, 2011 | Case study | 1 food handler. | IgM positive, IgG negative. | Clinical, epidemiological and laboratory features fitting the case definition for HEV infection. |
| Pérez-Gracia et al. (2007) | Spain, na | Case study | A 62-year-old male, type 2 diabetic, slaughterhouse worker. | Anti HEV IgG and IgM positive. | The patient recovered normal liver function uneventfully within 45 days after his admission to the hospital. |
| Kang et al. (2017) | China, 2013–2015 | Case-control study | 300 veterinarians, 600 farmers, 600 control subjects. | Farmers: 34.8% (32.8% anti-HEV IgG, 1.5% anti-HEV IgG); veterinarians: 26.7% (23.7% anti-HEV IgG, 2.3% anti-HEV IgM); and control subjects: 20.2%. | Significantly higher seroprevalence in farmers (P < 0.001) and veterinarians (P = 0.027) than controls. Highest seroprevalence detected in swine farmers (49.1%), lowest in cattle farmers (26.5%). Higher seroprevalence in farm animal than pet veterinarians. |
| Caruso et al. (2017) | Italy, na | Cross-sectional study | A total of 69 workers with swine contact (43 veterinarians and 23 farmers) and 73 without swine contact. | Total 3.52% anti-HEV IgG. | No difference in seropositivity between workers with (5.7%) and without swine contact (1.3%). Significant difference (OR: 10.1) between subjects exposed for short periods (veterinarians) and those for long periods (farmers), suggesting a correlation between time of exposure and the likelihood of HEV infection. |
| Ukuli, Mugimba (2017) | Uganda, 2015 | Cross-sectional study | A total of 45 swine abattoir workers. | Total 13.3% for anti HEV IgM. | Highest seroprevalence (50%) among slaughterers; lowest amongst sanitary cleaner, cloth cleaners and inspector. Seroprevalence increasing (>24 years). |
| Lassen B et al. (2017) | Estonia, 2012 | Cross-sectional study | A total of 115 veterinarians. | Total 2.6% for anti-HEV IgG. | The 3 anti-HEV IgG-positive veterinarians were small animal practitioners, worked in small animal clinics, and 1 also in an animal shelter. Professional experience up to 30 years. |
| Lange et al. (2017) | Norway, 2013 | Cross-sectional study | A total of 163 veterinarians, 79 swine farm workers and 1200 blood donors. | Total 30% anti-HEV IgG (farm workers), 13% veterinarians, 14% blood donors. Anti-HEV IgM in 4 farm workers and 3 blood donors. | Higher seroprevalence (2.5 times) in farm workers compared to blood donors (PR: 2.3, 95% CI: 1.6–3.2) and veterinarians (PR: 2.4, 95% CI: 1.4–4.0). Seroprevalence increasing with age in farm workers and blood donors and twice higher in veterinarians working with swine compared to those who did not work with swine. |
| Cui et al. (2016) | China, 2014–2015 | Cross-sectional study | A total of 1028 workers: 335 were in raw seafood processing; 287 in semi-finished products processing and 405 were less exposed workers (administrative staff, warehouse workers and packers). | Total 22.20% anti-HEV IgG (32.54%: direct contact with raw seafood; 24.74%: semi-finished products processing workers and 11.85%: less exposed group). | Age (40–49 years), working years (3–7 and >7), raw seafood processing workers and semi-finished products processing workers significantly associated with HEV infection. |
| Junaid, Agina and Abubakar (2014) | Nigeria, na | Cross-sectional study | A total of 110 farmers, 95 students, 24 housewife, 109 civil servant, 80 business and 8 others. | Farmers: 66.4% for anti-HEV IgG; business people: 48%; civil servant: 47.7%; house wives: 33.3%; students: 27.4%. Farmers: 7.3% for anti-HEV IgM. | Occupation significantly associated with IgG seropositivity (P < 0.001) but not with IgM (P > 0.05). Highest prevalence in animal handlers (66.7%). Age, location, farming as occupation, rural dwelling, attending to animals among risk factors significantly related to anti-HEV IgG seropositivity. |
| Yoon et al. (2014) | Republic of Korea, 2007–2009 | Cross-sectional study | 2450 individuals. | Total 5.9% for anti-HEV IgG (31.3%: skilled agricultural, forestry and fishery workers). | High frequency of agricultural, forestry and fishery workers associated with significantly high odd of HEV seropositivity (OR: 6.6; 95% CI: 3.1– 4.2). |
| Martins et al. (2014) | Brazil, 2010–2011 | Cross-sectional study | A total of 431 recyclable waste pickers. | Total 5.1% anti-HEV IgG and 0.7% anti-HEV IgM. | Significantly higher seroprevalence for age >40 years. |
| Tabibi et al. (2013) | Italy, 2010–2011 | Cross-sectional study | A total of 89 exposed workers (47 cow breeders, 31 pig breeders and 11 fish breeders), 14 controls. | Breeders: 1% for anti-HEV IgG (assay 1); 25.6% (assay 2). | Sensitivity and specificity of the assays used to test for IgG and IgM anti-HEV not well established in areas where hepatitis E is not endemic. |
| Lee et al. (2013) | Taiwan, 2012–2013 | Cross-sectional study | A total of 156 swine farmers, 314 health examination attendees, 100 pregnant women and 90 students. | Swine farmers: 29.5% for anti-HEV IgG; health examination attendees: 11.5%; pregnant women: 2%; student: 1.1%; 1 swine farmer and 1 health examination attendee for anti-HEV IgM. | Significantly higher seropositivity in swine farmers (P < 0.0001), with a higher risk (OR: 3.46; 95% CI: 1.91–6.27; P < 0.0001) than the general population. |
| Hinjoy et al. (2013) | Thailand, 2010–2011 | Cross-sectional study | A total of 171 pig farmers, 342 without occupational exposure to pigs in farms. | Exposed group: 22.8%; unexposed group: 23.1%. | No difference in seroprevalence between exposed and unexposed. Symptoms compatible with hepatitis reported in 10/171 pig exposed. |
| Temmam et al. (2013) | Madagascar, 2008–2009 | Cross-sectional study | A total of 427 slaughterhouse workers. | 14.1% | Significantly higher seropositivity for working years <5 (P = 0.03). |
| Albatanony and El-Shafie (2011) | Egypt, na | Cross-sectional study | A total of 43 workers at wastewater treatment plants (WWTPs) and 43 not exposed workers. | WWTPs workers: 51%; not exposed workers: 30%. | Significantly higher seropositivity (P < 0.05) in WWTPs than in not exposed. |
| Adjei et al. (2010) | Ghana, 2008 | Cross-sectional study | A total of 353 were swine exposed (feeding the pigs, cleaning barns, assisting the sows at birth and butchering on the farm). | Total 34.84% (19.26% anti-HEV IgG; 15.58% anti-HEV IgM, P < 0.05). | Significantly higher seroprevalence (P < 0.001) among swine exposed in the same farm setting for <6 months than >6 months (OR: 8.96; 95% CI: 5.43–14.80). |
| Adjei et al. (2009) | Ghana, 2008 | Cross-sectional study | A total of 105 swine workers. | Total 38.1% for anti-HEV IgM. | Higher seroprevalence associated with being employed on the farm for <6 months (OR: 9.1; 95% CI: 1.0–81.4) and having piped water (OR: 3.9; 95% CI: 0.4–90.8) and, among swine workers, with cleaning barns (OR: 2.67; 95% CI: 0.48–19.30), assisting sows at birth (OR: 2.10; 95% CI: 0.63–7.34) and butchering pigs at the farm (OR: 2.84; 95% CI: 1.05–7.92). |
| Tschopp et al. (2009) | Switzerland, 2004, 2006 | Prospective cohort study | A total of 332 workers exposed to waste water and 446 workers non exposed. | A total of 667 workers seronegative for HEV at the beginning of follow-up. During follow-up no diagnosis of clinical hepatitis E; seroconversion found in 26 subjects and 25 subjects with definite positive titres. | Seroconversion was not accompanied by clinical symptoms in 24 subjects. Liver disorders in two further patients, without diagnosis of hepatitis E. Workers never visiting endemic areas (13/26) suggesting HEV circulation in Switzerland. |
| De Sabato et al. (2017) | Italy, 2004 | Retrospective study | A total of 83 pig veterinarians and 170 blood donors. | Total 9.64% anti HEV IgG (veterinarians) and 8.82% (blood donors). | No statistically significant difference in seroprevalence comparing veterinarians (9.64%; 8/83) and blood donors (8.82%; 15/170). |
| Galiana et al. (2008) | Spain, 2004–2007 | Retrospective study | A total of 101 were exposed (swine farmers, pig handlers, swine veterinarians) and 97 unexposed. | Exposed: 18.8% anti-HEV IgG; unexposed: 4.1%. | People exposed to swine at risk of having anti-HEV IgG (OR: 5.4, P = 0.03). |
| Löve et al. (2018) | Iceland, na | Epidemiological study | A total of 291 (21 pig farm workers, 195 healthy volunteers and 75 patients with drug-induced liver injury). | Total 2.1% for anti HEV IgG. | Lower seroprevalence in Iceland than majority of other western countries (6 tested positive in 3 tests, 1 pig farm worker in 2 tests). |
| Mughini-Gras et al. (2017) | Italy, 2011–2014 | Epidemiological study | A total of 149 swine workers, 121 omnivores and 115 vegetarians/vegans. | Total 14.1% for anti-HEV IgG in swine workers, 0.8% in omnivores and 2.6% in vegetarians/vegans. | Seropositivity adjusted for age and gender in swine workers higher than omnivores (P = 0·007) and vegetarians/vegans (P = 0.041). Swine workers associated with HEV seropositivity (RR: 15.02; 95% CI: 2.17–104.15, P = 0.006). |
| Bansal et al. (2017) | India, na | Epidemiological study | A total of 32 slaughter house workers, 38 unorganized swine farmers, 20 organized swine farmers, 19 sewage workers/sweepers, 15 veterinary internes and 56 controls. | Total 60.48% were anti-HEV IgG (occupational risk groups), 10.71% (control/low risk) and 0.80% anti-HEV IgM (occupational risk groups). | Strong evidence (P < 0.05) of association between human anti-HEV IgG seropositivity and certain occupational exposure risk groups (sewage workers: 78.9%, unorganized swine farmers: 76.3%, swine slaughterhouse workers: 75%). |
| Sommerkorn et al. (2017) | Germany, 2013–2014 | Epidemiological study | A total of 139 were swine exposed (79 meat inspectors, classifiers or veterinarians, 29 slaughterers, 6 slaughterhouse workers and 25 who hunted regularly). | Total 18.7% anti-HEV IgG; 0–4.3% anti-HEV IgM. | Higher overall HEV IgG prevalence for slaughterer (20.7%, 95% CI: 8.0–39.7%) and veterinarians/meat inspectors/classifiers (20.3%, 95% CI: 12.0–30.8%) than for slaughterhouse workers (16.7%, 95% CI 0.42–64.1%). |
| Teixeira et al. (2017) | Portugal, 2015 | Epidemiological study | A total of 114 were swine exposed (96 slaughterhouse workers, 5 butchers, 11 pig farmers and 2 veterinarians working with pigs); 804 sera from anonymous. | Total 30.7% anti-HEV IgG (swine exposed); 19.9% (control group). | Significantly higher (P = 0.008) seroprevalence in swine workers compared to the general population. Professions with more than 16.5 years swine exposure as risk factor for being positive for anti-HEV IgG (OR: 5.4, 95% CI: 1.9–15.6, P = 0.002). |
| Kantala et al. (2017) | Finland, 2009 | Epidemiological study | A total of 333 veterinarians; 52 referred to as ‘non-veterinarians’. | Total 9.6% (10.2%: veterinarians; 5.8%: non-veterinarians). | No difference in seropositivity between veterinarians and non-veterinarians. Significantly higher total HEV seroprevalence (17.8%) in small animal practitioners than in any other veterinary practice specialty (3.6–8.7%). |
| Kim et al. (2015) | South Korea, 2012 | Epidemiological study | A total of 1434 slaughter workers and 414 residual products handlers. | Total 33.5% for anti-HEV IgG (slaughter workers: 32.8%; residual products handlers: 36.2%); 0.5% for anti-HEV IgM (0.5%: slaughter workers; 0.7%: residual products handlers). | Age, sex and working duration (for slaughter workers) and male sex and old age (for residual product handlers) significantly related to anti-HEV IgG seropositivity. |
| Traoré et al. (2015) | Burkina Faso, 2013 | Epidemiological study | A total of 100 volunteer butchers and 90 blood donors. | Butchers: 76% anti-HEV IgG, 1% anti-HEV IgM; blood donors: 47.8% anti-HEV IgG, 3.19% anti-HEV IgM. | Significantly higher seroprevalence in butchers than general population (OR = 3.46, 95% CI: 2.85–4.21, P < 0.001) and increasing with age. |
| Li et al. (2014) | China, na | Epidemiological study | A total of 1638 residents. | Farmers: 20.35% anti-HEV IgG positive; migrant: 16.5%; workers: 13.06%. | Highest HEV infection rate in farmers (20.35%) and migrants (16.50%). |
| Krumbholz et al. (2014) | Germany, 2009–2011 | Epidemiological study | A total of 537 individuals (302 with occupational swine contact and 235 controls). | Total 17.9% anti-HEV IgG (pig farmers: 68.5%; veterinarians: 10.6%; butcher/slaughterer: 1%); and controls: 8.5%. | Significantly, higher seroprevalence in subjects with swine contact (13.2–32.8%) compared with that in non-exposed humans (7.7–21.7%). |
| Mesquita et al. (2014) | Portugal, 2013 | Epidemiological study | A total of 493 individuals (373 veterinarians working in small animals clinic, 120 controls). | Total 9.7% anti-HEV IgG (veterinarians) and 13.3% (controls). | No difference between the two groups (P = 0.231). Highest seroprevalence for age >50 years. |
| Chaussade et al. (2013) | France, 2011–2012 | Epidemiological study | A total of 306 pig farm workers, 231 forestry workers and 322 controls. | Forestry workers: 36.4% for anti-HEV IgG; pig farm workers: 43.8%; controls: 26.1%. | Significantly higher seroprevalence for both occupations (OR: 1.58; P = 0.038 and OR: 2.51; P < 0.0001, respectively). Seroprevalence in pig farm workers increasing with the number of working years (26.9% for less than 14 years, 58.8% for more than 24 years, P = 0.008). |
| Widasari et al. (2013) | Indonesia, 2011 | Epidemiological study | A total of 137 swine workers, 100 blood donors (center of Java); 12 swine farm workers, 42 resident (east Java); 64 swine farm workers and 135 residents (Bali). | Total 6.7%: swine farm workers; 3.5%: local residents (central + east Java); 18.8%: workers; and 11.6%: local residents (Bali). | Significantly higher seroprevalence in swine farm workers from Bali than Java (P = 0.013). |
| de la Caridad Montalvo Villalba et al. (2013) | Cuba, 2007 | Epidemiological study | A total of 69 workers with and 37 without close swine contact. | Total 40.5% workers with close swine contact and 27.0% workers without swine contact. | Significantly higher seropositivity for age range of 60–70 years and 10–13 working years in pig farms (P = 0.033). Highest anti-HEV rate for swine contact and not keeping animal at home (60.0%). |
| Carpentier et al. (2012) | France, 2002–2003 | Epidemiological study | A total of 593 forestry workers (358 woodcutters, 105 sylviculturists, 130 game or fishing keepers or rangers), and 135 controls. | Forestry workers: 31% and controls: 19.2%. | Higher seroprevalence in game and fishing keepers and rangers (20.0%) and in silviculturists (24.8%) compared to controls (not statistically significant). Woodcutters at higher risk (37.2%; multivariate analysis: OR: 2.24 (P = 0.003)). |
| Silva et al. (2012) | Brazil, 2009–2010 | Epidemiological study | A total of 310 swine exposed and 101 blood donors. | Swine exposed: 8.4% for anti-HEV IgG and blood donors: 4%. | No difference in seroprevalence between the 2 groups. No association between type of property the participants worked in, performing swine slaughtering, or carcass handling and seroprevalence. |
| Dremsek et al. (2012) | Germany, 2008 | Epidemiological study | A total of 563 forestry workers and 301 blood donors. | Forestry workers: 17.8% for anti-HEV IgG; blood donors: 11.1% (commercial test); 21.4% and 12.3%, respectively (in-house test). | Slightly higher seroprevalence for male than female subjects (independently from the test used), not statistically significant. |
| Krumbholz et al. (2012) | Germany, 2007–2009 | Epidemiological study | A total of 106 workers (24 slaughterers, 14 meat inspectors, 46 pig farmers and 22 veterinarians with direct swine contact); 116 blood donors. | Workers: 28.3%; blood donors: 15.5%. | Difference in HEV-IgG seroreactivity between slaughterers and control group (41.7% vs. 15.5%, P = 0.009), and between the entire study group and the control group (28.3% vs. 15.5%, P = 0.023). |
| Geng et al. (2011) | China, 2006–2008 | Epidemiological study | A total of 247 workers in slaughterhouses and pig farms, and 2682 blood donors. | Pig farm workers: 58.73%; slaughterhouse workers: 35.87%; and blood donors: 20.06%. | Differences in positivity rates of anti-HEV between the 3 groups (P < 0.01). Significantly higher positivity in pig farm than in slaughterhouse workers (P < 0.01), and both significantly higher than general population (P < 0.01). |
| Meader et al. (2010) | United Kingdom, 1991, 1995, 1996 | Epidemiological study | A total of 413 farmers (PHLS Farm Cohort: sentinel group with close contact to domestic animals). | 2.4% | Highest seroprevalence for age 51–60 years (4.88%; RR: 3.3; 95% CI: 1.0–10.5). No association with exposure to pigs or water from a private supply. |
| Masia et al. (2009) | Italy, 2008 | Epidemiological study | A total of 35 abattoir workers, 95 laboratory workers and 402 blood donors. | Abattoir workers and/or laboratory workers: 2.3% and blood donors: 4.3%. | Neither differences (P > 0.05) between sex and age classes, nor according to occupation. |
| Chang et al. (2009) | China, na | Epidemiological study | A total of 247 workers (52 in livestock farms and 195 in slaughterhouses); 2572 blood donors. | Workers: 42.51%; blood donors: 20.29%. | Significantly higher seroprevalence (P < 0.0001) in pig farms (67.31%) and slaughterhouse (35.90%) than general population (20.29%). |
| Bouwknegt et al. (2008) | Netherlands, na | Epidemiological study | A total of 202 veterinarians and 648 from the general population. | Swine veterinarians: 11%; non-swine veterinarians: 6%; and general population: 2%. | Exposure to swine or their environment associated with elevated seroprevalence (Bayesian stochastical model, less conservative prior estimate: 13%, 95% CI: 1.6–40; more conservative: 7%, 95%: CI 0.1–20). |
| Vulcano et al. (2007) | Italy, 2005–2006 | Epidemiological study | A total of 92 workers were swine exposed (19 veterinarians, 39 farmers, 19 butchers, 6 workers processing animal carcasses and 9 abbattoir workers with no contacts with carcasses); 3511 healthy volunteers. | Total 3.3%: 1 pig farmer and 2 abbattoir workers. | Increasing prevalence (33%) in abattoir workers, while in pig farmers 3.6% below that of general population in the same city. |
na = not available
DISCUSSION
Infections continue to represent a global threat to human health. Some emerging viruses with potential zoonotic transmission seem to pose a risk not only for the general population but also for workers in specific settings and activities. WNV, CCHFV and HEV were key topics of our review, which aims to identify possible association between occupational exposure and increasing risk for acquiring these infections. The search of pertinent articles has excluded sources other than PubMed, considered exhaustive for the aim of summarizing current available evidences of occupational risks for the three selected viruses. This is a limit of our study; however, this intends to be a pilot study in the field, suitable for more detailed research in the future.
WNV
WNV transmission cycles through birds and mosquitoes, and mammals represent dead-end host of the infection, acquired through mosquito bites. The virus is able to amplify or replicate to high titre within birds, usually wetlands birds, which in turn transmit the infection to mosquitoes, primarily belonging to the Culex genus. Mosquitoes can reinfect birds, perpetuating enzootic infection, or can bridge the infection to mammals, humans and horses principally, representing a public health concern (Ahlers and Goodman 2018). Pointing out environmental conditions that favor WNV circulation and transmission to humans is quite difficult, mainly due to the complexity of its biological cycle. Factors contributing to the current epidemiological picture, characterized by an increasing rate of spread in Europe and neighboring countries, are several and include urbanization, variation in land use and climate changes (Marcantonio et al. 2015). Temporal extension of transmission season may increase the risk of exposure to the infection. In fact, in 2018, early WNV transmission has been observed in Italy and in other countries in South and South Eastern Europe, with a high number of cases (Riccardo et al. 2018). Moreover, changes in daily work activities caused by increased heat, as longer rest periods in the middle of the day and augmented work at dawn and dusk, could correspond to period when vectors are most active, therefore increasing the risk of disease transmission (Vonesch et al. 2016). Outdoor workers, such as farmers and agricultural workers, may be at increased risk of WNV infection.
Furthermore, workers in many other occupations could be at potential risk of exposure to WNV-infected humans and animals, their blood or other fluids and tissues. They comprise laboratory diagnosticians, researchers and technicians, veterinarians, wildlife rehabilitators, wildlife biologists, ornithologists, zoo and aviary curators, healthcare workers, emergency response and public safety personnel (NIOSH 2003. Few studies on the occupational risk caused by WNV have been performed; in fact, we identified only ten articles eligible for inclusion. The four case reports we included in the review concerned one US security guard who attended an off-site work event (Smith 2016), one Brazilian ranch worker (Vieira et al. 2015), one veterinarian and one laboratorist, both in South Africa, (Venter et al. 2009; Venter and Swanepoel 2010), all affected by the neuroinvasive illness that required hospitalization. In the first two cases, mosquito bites were responsible for the transmission of the virus; the veterinarian and the laboratorist acquired infection by needle stick injuries. The seroprevalence/seroepidemiological studies included in the study showed very low positivity for anti-WNV IgG in Italy and Spain, from 0% (Spataro et al. 2008; Remoli et al. 2018) to 2.8% (Bernabeu-Wittel et al. 2007), mainly regarding farmers, agricultural workers, veterinarians and foresters. Higher levels were reported in South Africa (7.9% for veterinarians) (van Eeden, Swanepoel and Venter 2014) and Turkey (20.87% for farmers and agricultural workers). In South Africa the virus is an endemic zoonotic agent and occurs where the principal vector (Culex univittatus) and avian host are present (van Eeden, Swanepoel and Venter 2014).
World Health Organization (WHO 2017) as well as experts in Europe are calling for greater awareness of WNV infection, since the number of cases rises because of demographic, environmental and social factors (Holt 2018). WNV outbreak in animals precede human cases; therefore, an active surveillance system to detect cases in birds and horses is essential to provide early warning for veterinary and human health authorities. In Italy entomological, veterinary and human surveillance systems for WNV infection have been implemented starting from 1998, when the disease was first detected in horses in Tuscany region. Starting from 2008, human cases have been reported in North-eastern Italy, an area now considered endemic for the virus: this is not unexpected since the geographical position of the country favors the distribution of arthropods as possible vectors of human pathogens. The few numbers of studies conducted in Italian workers could be explained by the difficulty in recruiting workers at risk of exposure to the infection, since they are mainly seasonal, often foreigners and cultural and language barriers could limit their participation to the studies (Remoli et al. 2018). Gloves and other protective clothing should be worn while handling sick animals or their tissues. Physicians should be alerted to detect clinical cases and educational programs raising awareness about the disease and the risk factors should be implemented. Healthcare workers caring for patients with suspected or confirmed WNV infection or handling their specimens, should implement standard infection control precautions; laboratorists should use effective personal protective equipment and apply biosafety measures (WHO 2017). Vaccines are not yet available for humans; treatment is supportive for patients with neuroinvasive disease (WHO 2017). It should be taken into account that although most WNV infections are subclinical, assessing the occupational risk is important not only for protecting workers’ health, but also for providing information on the potential spread of the virus.
CCHFV
CCHF is the most widely spread tick-borne viral infection of humans and is endemic in extensive geographical areas comprising many countries in Africa, southeastern Europe, Asia and the Middle East. Human infection can occur either by tick bites, mainly belonging to the genus Hyalomma, or by direct contact with blood or tissues of viremic humans or livestock (Sargianou, Papa 2013).
In recent years, the incidence of CCHFV infection has increased rapidly in countries of the World Health Organization Eastern Mediterranean Region (WHO EMR) and in Central Asia, probably due to a combination of biological, environmental and social factors, and enhanced awareness and diagnostic capability. Weather conditions may influence the timing of activation and densities of ticks, being ectothermic organisms. Migrating birds are sources of blood-meals for immature ticks, contributing to the dispersal of infected vectors and potential emergence of disease foci. Long-distance movement of livestock may also contribute to dispersal of CCHFV-infected ticks (Al-Abri SS et al. 2017; Gargili et al 2017). In endemic areas, farmers, veterinarians, livestock market workers, abattoir workers and other personnel engaged in activities in contact with animals and/or animal products are considered at risk for acquiring CCHFV infection, as well outdoor workers who could be exposed to infected ticks. Healthcare workers are at risk of exposure to the virus, when nursing infected patients with severe bleeding and hemorrhages without strict barrier procedures. Nosocomial transmission may therefore occur through direct contact with infected blood or body fluids, or through contaminated medical equipment or supply (ECDC 2015). Case reports attesting CCHFV transmission through direct contact with infected blood or tissue of animals regarded mainly farmers (Yadav et al. 2017) and livestock workers (Mardani et al. 2009; Mardani, Namazee 2013). Traditional slaughtering and butchery performed in some country (Iran) can be considered activities at risk (Fazlalipour et al. 2016).
Most articles sharing this transmission pathway are seroprevalence studies, carried out in Africa, Asia and Europe, and mainly regarding farmers, slaughterers, butchers and veterinarians (Gunes et al. 2009; Sidira et al. 2013; Akuffo et al. 2016; Cikman et al. 2016; Wasfi et al. 2016; Mostafavi et al. 2017; Vawda et al. 2018). The low seroprevalence for anti-CCHFV IgG antibodies (0.51%) found in South Africa among workers exposed to or in contact with animals seems to suggest that the virus is uncommon in this area (Vawda et al. 2018). The higher seroprevalence detected in Iran (16.49% among butchers and slaughterhouse workers) could be caused by the minimal use of personal PPE during daily work, as admitted by workers who completed a questionnaire (Mostafavi et al. 2017). A statistically significant difference between prevalence of CCHFV IgG antibodies in livestock workers and unexposed subjects was found in Turkey. CCHFV is endemic in central and north-eastern Anatolia and southern Black Sea regions of this country and several cases are emerging in other zones (Cikman et al. 2016). A cross sectional study conducted in Greece (Sargianou et al. 2013) showed that an agro-pastoral occupation, contact with sheep and goats, tick bites and increasing age were significantly associated with CCHFV seropositivity. Another cross sectional study performed in Madagascar (Andriamandimby et al. 2011) showed that here the percentage of CCHF infection is very low among at risk professionals because of the lack of ticks of the genera Hyalomma in this country. Four retrospective studies were conducted in India (Mourya et al. 2017) and Turkey (Duran et al. 2013; Guner et al. 2014; Leblebicioglu et al. 2016) on patients with a history of occupational exposure, suspected to have CCHFV infection, through a retrospective analysis of clinical and laboratory data. In endemic areas, hemorrhagic manifestations including melena, low platelet count and raised alanine aminotransferase may provide a suspicion of CCHFV infection. In Turkey, people living and actively working in rural areas (including housewives occupied in agriculture and animal husbandry) are particularly subjected to the infection. It was observed that public awareness about CCHFV has decreased the incidence of the disease (Duran et al. 2013). Nosocomial transmission of CCHFV to HCWs has been reported from different countries. The evidence that HCWs are at risk of exposure to CCHFV while caring infected patients is also supported by most case reports selected for the review (Mardani et al. 2009; Naderi et al. 2011; Celikbas et al. 2014; Ozsoy et al. 2015; Pshenichnaya and Nenadskaya 2015; Yadav et al. 2016; Yildirmak, Tulek and Bulut 2016; Negredo et al. 2017). In the differential diagnosis of subjects with hemorrhagic signs, physicians should consider CCHFV infection if these patients have recently returned from any area where the virus is endemic or prevalent. Of interest the concern regarding transmission of CCHFV via respiratory contact, as supposed by a case report from Russia (Pshenichnaya and Nenadskaya 2015) and one from Turkey (Yildirmak, Tulek and Bulut 2016), suggesting that airborne precautions could be essential during aerosol generating procedures. High mortality rate has been attested during nosocomial outbreaks; in some cases, ribavirin has been considered an effective treatment for the infection and could be used for postexposure prophylaxis (Celikbas et al. 2014). In our review, we included seroprevalence studies regarding seropositive HCWs from Turkey (Gozel et al. 2013), Greece (Maltezou, Maltezos and Papa 2009) and Iran (Mardani et al. 2007). Needle-stick injury, interventions for gastrointestinal bleeding, unprotected handling of infected materials, and emergency surgical interventions have been reported as high-risk activities for viral transmission. Military personnel that travel to and work in environments where they could be exposed to endemic or emerging infections, that are not present or prevalent in their native country, can be considered at high risk of contracting CCHFV. We selected 3 articles regarding Afghan National Army recruits (Todd et al. 2016) UK military personnel deployed to Afghanistan (Newman et al. 2014), and military units from Saudi Arabian Provinces. In these groups, seroprevalences were 4.1%, 0% and 0.58%, respectively. CCHFV infection has important public health implication due to the potential of human-to-human transmission; therefore, enhanced surveillance for tick vectors and CCHFV cases is essential. Control and prevention of the infection in ticks and animal is quite difficult since the tick-animal-tick cycle usually goes unnoticed and the infection in animals is usually not apparent. Educational and training programs addressed to workers with potential exposure to the virus aiming at increasing their knowledge, attitude and practice should be developed and implemented as preventive measures. Moreover, the use of approved acaricides on clothing and tick repellent on exposed skin and clothing, and wearing protective clothing are suggested for reducing the risk of tick to human transmission. Wearing gloves and other protective clothing while handling animals or their tissues in areas where CCHFV is endemic could minimize the risk of animal to human transmission (WHO 2013; ECDC 2015). In healthcare settings, implementation of standard infection control precautions by healthcare workers caring for patients with suspected or confirmed CCHFV infection or handling their specimens, should be recommended.
HEV
HEV is a major cause of epidemic viral hepatitis in developing countries and of sporadic and cluster cases in industrialized countries. According to the WHO, approximately one-third of the world population has been exposed to HEV, through the ingestion of contaminated water and food or the direct contact with infected animals, and in minor cases by blood-borne transmission (Sinakos et al. 2018). Serological evidence of prior exposure to the virus has been found in most areas, with higher seroprevalence rates (proportion of people who test positive for IgG antibodies to HEV) in regions with lower standards of sanitation and thus higher risk for transmission. However, presence of these antibodies does not imply presence or increased risk of disease.
Traditionally, industrialized countries were considered non-endemic, with most HEV infections in these regions being sporadic and considered to be imported. Nevertheless, in recent years, enhanced surveillance has detected an increasing number of non-travel-associated HEV infections.
Genotypes 3 and 4 of HEV are distributed worldwide, have a much wider host range and are considered to be zoonotic: HEV-3 is the principal genotype circulating in commercial swine herds, HEV-4, typical of the Asian continent, is believed to have recently introduced in Europe (Hakze-van der Honing et al. 2011; Monne et al.2015). Since the high pathogenicity of genotype 4, other studies should be performed to better understand to which extent this genotype has spread across Europe. The HEV-3 and HEV-4 genotypes circulate also in Europe and they have a high level of nucleotide identity between swine and human strains (Di Bartolo et al. 2012).
In the present review, available studies were carried out in Africa, Asia, Europe and Latin America.
Recently, piggish reservoirs and growing evidence of zoonotic transmission of HEV have been reported in these countries, suggesting the possibility of occupational transmission to humans. Exposed groups comprise swine farmers (organized—mixed feed feeders and unorganized—swill feeders), slaughterhouse workers, sewage workers and veterinarians (Bansal et al. 2017).
In this review, an increased risk was found also among food handlers (Appuhamy et al. 2014; Cui et al. 2016), workers exposed to wastewater (Tschopp et al. 2009; Albatanony and El-Shafie 2011; Martins et al. 2014) and forestry workers (Carpentier et al. 2012; Dremsek et al. 2012). Seroprevalence results were higher in individuals exposed to swine and/or wild animals, and increased with age and amount of years of occupational exposure. Humans with occupational exposure to wild animals and environmental sources of domestic animal wastes or with unexplained hepatitis showed an increased seroprevalence of anti-HEV antibodies. Poor environmental conditions in farms, occupation and low socioeconomic status might be risk factors in HEV infection. Wild boar stools may be responsible for a further source of HEV infection for people in close contact with the forestry environment. Forestry workers have already been identified to be at risk for HEV infection, as well as woodcutters. The finding of HEV and HEV-related RNA in a rising number of different animal species suggests a possible role for unidentified animal reservoirs, up to now as risk factors associated with HEV seropositivity in humans in areas where HEV is not endemic. Such reservoirs should be further explored by means of suitable diagnostic tools (Carpentier et al. 2012).
The fact that some European countries, such as Germany, have classified Hepatitis E as a notifiable infectious disease for several years and therefore have well defined records, whereas other countries, for example Italy, started in 2008, could explain difference in prevalence found in countries with a similar socio-economic and health status (Masia et al. 2009). Moreover, variations in seropositivity rates reported in studies from around the world, could be ascribed by the use of immunological assays with different sensitivity (Meader et al. 2010). The usefulness of such data for epidemiological purposes may also be limited due to variable and possible sub-optimal performance of available serological assays, and possible disappearance of the antibody with the passage of time among those exposed to the virus.
At population level, transmission of HEV and hepatitis E disease can be reduced by maintaining quality standards for public water reserves and proving appropriate removal systems for human feces. At individual level, infection risk can be diminished by maintaining hygienic habits such as hand washing with safe water, particularly before handling food; preventing consumption of water and/or ice of unknown pureness and adhering to WHO safe food procedures (WHO 2018). Standard biosecurity measures, including regular cleaning and disinfection, should be put in place to limit contamination of swine facilities. A vaccine against HEV, licensed in China in 2011, prevent symptomatic HEV-4 infections, but does not provide sterilising immunity. The vaccine seems to be safe in pregnant women, but the long-term efficacy in immunosuppressed and in subjects with chronic liver disease has to be determined. An important role of the vaccine could be the prevention of HEV outbreaks, e.g. in African refugee camps or other emergency settings (EASL 2018). In the absence of an effective vaccine against HEV, prevention for swine workers, farmers, butchers and veterinarians relies on the implementation of hygiene and individual protection. Raising awareness and improved education about the risk of acquiring HEV and the appropriate precautions may help to prevent the infection.
CONCLUSIONS
This review provides some evidences of risk for people exposed to emerging viruses in occupational setting. Although the raising number of publications regarding emerging infections, few are related to occupational health. Further studies should therefore be performed to gain more insight into the circulation of viruses in wider geographical area and in working scenarios. Moreover, such studies could contribute to evidence new risk factors for acquiring infections in exposed groups. This will be crucial in the development of effective interventions to prevent transmission of viruses potentially zoonotic.
Conflict of interest. None declared.
REFERENCES
- Adjei AA, Aviyase JT, Tett1ey Y et al.. Hepatitis E virus infection among pig handlers in Accra, Ghana. E Afr Med J. 2009;86:359–63. [DOI] [PubMed] [Google Scholar]
- Adjei AA, Tettey Y, Aviyase JT et al.. Unexpected elevated alanine aminotransferase, aspartate aminotransferase levels and hepatitis E virus infection among persons who work with pigs in Accra, Ghana. Virol J. 2010;7:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers LRH, Goodman AG. The Immune Responses of the Animal Hosts of West Nile Virus: A Comparison of Insects, Birds, and Mammals. Front Cell Infect Microbiol. 2018;8:96, DOI: 10.3389/fcimb.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuffo R, Brandful JA, Zayed A et al.. Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect Dis. 2016;16:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Abri SS, Abaidani IA, Fazlalipour M et al.. Current status of Crimean-Congo hemorrhagic fever in the World Health Organization Eastern Mediterranean region: issues, challenges, and future directions. Int J Infect Dis. 2017;58:82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadeq DW, Majdalawieh AF, Mesleh AG et al.. Laboratory challenges in the diagnosis of hepatitis E virus. J Med Microbiol. 2018;67:466–480. [DOI] [PubMed] [Google Scholar]
- Al-Sadeq DW, Majdalawieh AF, Nasrallah GK. Seroprevalence and incidence of hepatitis E virus among blood donors: A review. Rev Med Virol. 2017; 27:e1937. [DOI] [PubMed] [Google Scholar]
- Albatanony MA, El-Shafie MK. Work-related health effects among wastewater treatment plants workers. Int J Occup Environ Med. 2011;2:237–44. [PubMed] [Google Scholar]
- Andriamandimby SF, Marianneau P, Rafisandratantsoa JT et al.. Crimean-Congo hemorrhagic fever serosurvey in at-risk professionals, Madagascar, 2008 and 2009. J Clin Virol. 2011;52:370–2. [DOI] [PubMed] [Google Scholar]
- Appuhamy R, Moffatt C, Davis S et al.. Hepatitis E in a food handler – a rapid risk assessment to guide the public health response. Western Pac Surveill Response J. 2014;5:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal M, Kaur S, Deka D et al.. Seroepidemiology and molecular characterization of hepatitis E virus infection in swine and occupationally exposed workers in Punjab, India. Zoonoses Public Hlth. 2017;64:662–72. [DOI] [PubMed] [Google Scholar]
- Barzon L, Squarzon L, Cattai M et al.. West Nile virus infection in Veneto region, Italy, 2008–2009. Euro Surveill. 2009;14:19289. [DOI] [PubMed] [Google Scholar]
- Belay ED, Kile JC, Hall AJ, Barton-Behravesh C et al.. Zoonotic disease programs for enhancing global health security. Emerg Infect Dis. 2017;23:S65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu-Wittel M, Ruiz-Pérez M, del Toro MD et al.. West Nile virus past infections in the general population of Southern Spain. Enferm Infec Micr Cl. 2007;25:561–5. [DOI] [PubMed] [Google Scholar]
- Bouwknegt M, Engel B, Herremans MM et al.. Bayesian estimation of hepatitis E virus seroprevalence for populations with different exposure levels to swine in The Netherlands. Epidemiol Infect. 2008;136:567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier A, Chaussade H, Rigaud E et al.. High hepatitis E virus seroprevalence in forestry workers and in wild boars in France. J Clin Microbiol. 2012;50:2888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso C, Peletto S, Rosamilia A et al.. Hepatitis E virus: A cross-sectional serological and virological study in pigs and humans at zoonotic risk within a high-density pig farming area. Transbound Emerg Dis. 2017;64:1443–53. [DOI] [PubMed] [Google Scholar]
- Celikbas AK, Dokuzoğuz B, Baykam N et al.. Crimean-Congo hemorrhagic fever among health care workers, Turkey. Emerg Infect Dis. 2014;20:477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Wang L, Geng J et al.. Zoonotic risk of hepatitis E virus (HEV): A study of HEV infection in animals and humans in suburbs of Beijing. Hepatol Res. 2009;39:1153–8. [DOI] [PubMed] [Google Scholar]
- Chaussade H, Rigaud E, Allix A et al.. Hepatitis E virus seroprevalence and risk factors for individuals in working contact with animals. J Clin Virol. 2013;58:504–8. [DOI] [PubMed] [Google Scholar]
- Cikman A, Aydin M, Gulhan B et al.. Seroprevalence of Crimean-Congo hemorrhagic fever virus in Erzincan province, Turkey, relationship with geographic features and risk factors. Vector-Borne Zoonot. 2016;16:199–04. [DOI] [PubMed] [Google Scholar]
- Clemente-Casares P, Ramos-Romero C, Ramirez-Gonzalez E et al.. Hepatitis E Virus in Industrialized Countries: The Silent Threat. Biomed Res Int. 2016:9838041, DOI: 10.1155/2016/9838041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Sun Y, Xu A et al.. Hepatitis E seroprevalence and related risk factors among seafood processing workers: a cross-sectional survey in Shandong province, China. Int J Infect Dis. 2016;49:62–6. [DOI] [PubMed] [Google Scholar]
- Dalton HR, Kamar N, van Eijk JJ et al.. Hepatitis E virus and neurological injury. Nat Rev Neurol. 2016;12:77–85. [DOI] [PubMed] [Google Scholar]
- de la Caridad Montalvo Villalba M, Owot JC, Benedito EC et al.. Hepatitis E virus genotype 3 in humans and swine, Cuba. Infect Genet Evol. 2013;14:335–9. [DOI] [PubMed] [Google Scholar]
- De Sabato L, Di Bartolo I, Montomoli E et al.. Retrospective study evaluating seroprevalence of hepatitis E virus in blood donors and in swine veterinarians in Italy (2004). Zoonoses Public Hlth. 2017;64:308–12. [DOI] [PubMed] [Google Scholar]
- Di Bartolo I, Diez-Valcarce M, Vasickova P et al.. Hepatitis E virus in pork production chain in Czech Republic, Italy, and Spain, 2010. Emerg Infect Dis. 2012;18:1282–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dremsek P, Wenzel JJ, Johne R et al.. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med Microbiol Immunol. 2012;201:189–200. [DOI] [PubMed] [Google Scholar]
- Duran A, Küçükbayrak A, Ocak T et al.. Evaluation of patients with Crimean-Congo hemorrhagic fever in Bolu, Turkey. Afr Health Sci. 2013;13:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC. Factsheet About Crimean-Congo Hemorrhagic Fever. https://ecdc.europa.eu/en/crimean-congo-haemorrhagic-fever/facts/factsheet(16 January 2019, date last accessed), 2015. [Google Scholar]
- Ergonul O, Zeller H, Celikbas A et al.. The lack of Crimean-Congo hemorrhagic fever virus antibodies in healthcare workers in an endemic region. Int J Infect Dis. 2007;11:48–51. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver (EASL). Clinical practice guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256–71. [DOI] [PubMed] [Google Scholar]
- Fazlalipour M, Baniasadi V, Mirghiasi SM et al.. Crimean-Congo hemorrhagic fever due to consumption of raw meat: case reports from East-North of Iran. Jpn J Infect Dis. 2016;69:270–1. [DOI] [PubMed] [Google Scholar]
- Galiana C, Fernández-Barredo S, García A et al.. Occupational exposure to hepatitis E virus (HEV) in swine workers. Am J Trop Med Hyg. 2008;78:1012–5. [PubMed] [Google Scholar]
- Gargili A, Estrada-Peña A, Spengler JR et al.. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antiviral Res. 2017;144:93–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Wang L, Wang X et al.. Potential risk of zoonotic transmission from young swine to human: seroepidemiological and genetic characterization of hepatitis E virus in human and various animals in Beijing, China. J Viral Hepat. 2011;18:e583–90. [DOI] [PubMed] [Google Scholar]
- Gozel MG, Dokmetas I, Oztop AY et al.. Recommended precaution procedures protect healthcare workers from Crimean-Congo hemorrhagic fever virus. Int J Infect Dis. 2013;17:e1046–50. [DOI] [PubMed] [Google Scholar]
- Guner R, Hasanoglu I, Tasyaran MA et al.. Is ribavirin prophylaxis effective for nosocomial transmission of Crimean-Congo hemorrhagic fever? Vector-Borne Zoonot. 2014;14:601–5. [DOI] [PubMed] [Google Scholar]
- Gunes T, Engin A, Poyraz O et al.. Crimean-Congo hemorrhagic fever virus in high-risk population, Turkey. Emerg Infect Dis. 2009;15:461–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakze-van der Honing RW, van Coillie E, Antonis AF et al.. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS ONE. 2011;6:e22673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawman DW, Feldmann H.. Recent advances in understanding Crimean-Congo hemorrhagic fever virus. F1000Res. 2018;7, DOI: 10.12688/f1000research.16189.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinjoy S, Nelson KE, Gibbons RV et al.. A cross-sectional study of hepatitis E virus infection in healthy people directly exposed and unexposed to pigs in a rural community in northern Thailand. Zoonoses Public Hlth. 2013;60:555–62. [DOI] [PubMed] [Google Scholar]
- Holt E. West Nile virus spreads in Europe. Lancet Infect Dis. 2018;18:1184. [DOI] [PubMed] [Google Scholar]
- Huang X, Huang Y, Wagner AL et al.. Hepatitis E virus infection in swine workers: A meta-analysis. Zoonoses Public Health. 2019; 66::155–163. [DOI] [PubMed] [Google Scholar]
- Ivanova A, Tefanova V, Reshetnjak I et al.. Hepatitis E Virus in Domestic Pigs, Wild Boars, Pig Farm Workers, and Hunters in Estonia. Food Environ Virol. 2015;7:403–12. [DOI] [PubMed] [Google Scholar]
- Junaid SA, Agina SE, Abubakar KA. Epidemiology and associated risk factors of hepatitis E virus infection in plateau state, Nigeria. Virology (Auckl). 2014;5:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamar N, Dalton HR, Abravanel F et al.. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamar N, Izopet J, Pavio N et al.. Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3:17086. [DOI] [PubMed] [Google Scholar]
- Kang YH, Cong W, Zhang XY et al.. Hepatitis E virus seroprevalence among farmers, veterinarians and control subjects in Jilin province, Shandong province and Inner Mongolia Autonomous Region, China. J Med Virol. 2017;89:872–7. [DOI] [PubMed] [Google Scholar]
- Kantala T, Kinnunen PM, Oristo S et al.. Hepatitis E virus antibodies in Finnish veterinarians. Zoonoses Public Hlth. 2017;64:232–8. [DOI] [PubMed] [Google Scholar]
- Karakoç ZÇ, Tüzüner BM, Ergonul O et al.. West Nile virus infection in the Mesopotamia region, Syria border of Turkey. Vector-Borne Zoonot. 2013;13:739–43. [DOI] [PubMed] [Google Scholar]
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003; 10:61–9. [DOI] [PubMed] [Google Scholar]
- Kim BS, Lim HS, Lee K et al.. A survey on the status of hepatitis E virus infection among slaughterhouse workers in South Korea. J Prev Med Public Health. 2015;48:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz A, Joel S, Dremsek P et al.. Seroprevalence of hepatitis E virus (HEV) in humans living in high pig density areas of Germany. Med Microbiol Immunol. 2014;203:273–82. [DOI] [PubMed] [Google Scholar]
- Krumbholz A, Mohn U, Lange J et al.. Prevalence of hepatitis E virus-specific antibodies in humans with occupational exposure to pigs. Med Microbiol Immunol. 2012;201:239–44. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG.. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- Lange H, Øverbø J, Borgen K et al.. Hepatitis E in Norway: seroprevalence in humans and swine. Epidemiol Infect. 2017;145:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen B, Janson M, Neare K et al.. Prevalence of antibodies against hepatitis E virus in veterinarians in Estonia. Vector-Borne Zoonot. 2017;17:773–6. [DOI] [PubMed] [Google Scholar]
- Leblebicioglu H, Sunbul M, Guner R et al.. Healthcare-associated Crimean-Congo hemorrhagic fever in Turkey, 2002–2014: a multicentre retrospective cross-sectional study. Clin Microbiol Infect. 2016;22:387e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Shao PL, Chang LY et al.. Seroprevalence of hepatitis E virus infection among swine farmers and the general population in rural Taiwan. PLoS One. 2013;8:e67180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhomme S, Marion OR, Abravanel F et al.. Hepatitis E pathogenesis. Viruses. 2016;8:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LJ, Shen YY, Ai ZQ et al.. Seroepidemiology and genetic characterization of hepatitis E virus in western Yunnan province. Asian Pac J Trop Med. 2014;7:909–12. [DOI] [PubMed] [Google Scholar]
- Luo H, Wang T.. Recent advances in understanding West Nile virus host immunity and viral. F1000Res. 2018;7:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löve A, Björnsdottir TB, Olafsson S et al.. Low prevalence of hepatitis E in Iceland: a seroepidemiological study. Scand J Gastroenterol. 2018;53:293–6. [DOI] [PubMed] [Google Scholar]
- Maltezou HC, Maltezos E, Papa A. Contact tracing and serosurvey among healthcare workers exposed to Crimean-Congo hemorrhagic fever in Greece. Scand J Infect Dis. 2009;41:877–80. [DOI] [PubMed] [Google Scholar]
- Mancini G, Montarsi F, Calzolari M et al.. Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy. Vet Ital. 2017;53::97–110. [DOI] [PubMed] [Google Scholar]
- Marcantonio M, Rizzoli A, Metz M et al.. Identifying the environmental conditions favouring West Nile Virus outbreaks in Europe. PLoS ONE. 2015;10:e0121158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardani M, Keshtkar-Jahromi M, Ataie B et al.. Crimean-Congo hemorrhagic fever virus as a nosocomial pathogen in Iran. Am J Trop Med Hyg. 2009;81:675–8. [DOI] [PubMed] [Google Scholar]
- Mardani M, Namazee N. Close contact precautions could prevent an outbreak of Crimean-Congo hemorrhagic fever: a case series report from southern part of Tehran. Int J Prev Med. 2013;4:715–9. [PMC free article] [PubMed] [Google Scholar]
- Mardani M, Rahnavardi M, Rajaeinejad M et al.. Crimean-Congo hemorrhagic fever among health care workers in Iran: a seroprevalence study in two endemic regions. Am J Trop Med Hyg. 2007;76:443–5. [PubMed] [Google Scholar]
- Martins RM, Freitas NR, Kozlowski A et al.. Seroprevalence of hepatitis E antibodies in a population of recyclable waste pickers in Brazil. J Clin Virol. 2014;59:188–91. [DOI] [PubMed] [Google Scholar]
- Masia G, Orrù G, Liciardi M et al.. Evidence of hepatitis E virus (HEV) infection in human and pigs in Sardinia, Italy. J Prev Med Hyg. 2009;50:227–31. [PubMed] [Google Scholar]
- Mattioli S, Zanardi F, Baldasseroni A et al.. Search strings for the study of putative occupational determinants of disease. Occup Environ Med. 2010;67:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meader E, Thomas D, Salmon R et al.. Seroprevalence of hepatitis E virus in the UK farming population. Zoonoses Public Hlth. 2010;57:504–9. [DOI] [PubMed] [Google Scholar]
- Memish ZA, Albarrak A, Almazroa MA et al.. Seroprevalence of Alkhurma and other hemorrhagic fever viruses, Saudi Arabia. Emerg Infect Dis. 2011;17:2316–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita JR, Valente-Gomes G, Conceição-Neto N et al.. Pet veterinarians have no increased risk of hepatitis E compared to the general population. J Med Virol. 2014;86:954–6. [DOI] [PubMed] [Google Scholar]
- Mofleh J, Ahmad Z. Crimean-Congo hemorrhagic fever outbreak investigation in the western region of Afghanistan in 2008. East Mediterr Health J. 2012;18:522–6. [DOI] [PubMed] [Google Scholar]
- Mohd Shukri M, Ling Kho K, Ghane Kisomi M et al.. Seroprevalence report on tick-borne encephalitis virus and Crimean-Congo hemorrhagic fever virus among Malaysian's farm workers. BMC Public Health. 2015;15:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J et al.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monne I, Ceglie L, DI Martino G et al.. Hepatitis E virus genotype 4 in a pig farm, Italy, 2013. Epidemiol Infect. 2015;143:529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi E, Pourhossein B, Esmaeili S et al.. Seroepidemiology and risk factors of Crimean-Congo hemorrhagic fever among butchers and slaughterhouse workers in southeastern Iran. Int J Infect Dis. 2017;64:85–9. [DOI] [PubMed] [Google Scholar]
- Mourya DT, Viswanathan R, Jadhav SK et al.. Retrospective analysis of clinical information in Crimean-Congo hemorrhagic fever patients: 2014-2015, India. Indian J Med Res. 2017;145:673–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughini-Gras L, Angeloni G, Salata C et al.. Hepatitis E virus infection in north Italy: high seroprevalence in swine herds and increased risk for swine workers. Epidemiol Infect. 2017;145:3375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi HR, Sarvghad MR, Bojdy A et al.. Nosocomial outbreak of Crimean-Congo hemorrhagic fever. Epidemiol Infect. 2011;139:862–6. [DOI] [PubMed] [Google Scholar]
- Nan Y, Zhang YJ. Molecular Biology and Infection of Hepatitis E Virus. Front Microbiol. 2016;7:1419, DOI: 10.3389/fmicb.2016.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). Recommendations to Protect Laboratory and Field Workers from West Nile Virus Exposure, 2003. [Google Scholar]
- Negredo A, Calle-Prieto F, Palencia-Herrejón E et al.. Autochthonous Crimean-Congo hemorrhagic fever in Spain. N Engl J Med. 2017;377:154–61. [DOI] [PubMed] [Google Scholar]
- Newman EN, Johnstone P, Bridge H et al.. Seroconversion for infectious pathogens among UK military personnel deployed to Afghanistan, 2008–2011. Emerg Infect Dis. 2014;20: 2015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy S, Gokmen A, Ozdemir M et al.. Medical examiners and Crimean-Congo hemorrhagic fever contamination risk. J Forensic Leg Med. 2015;36:32–6. [DOI] [PubMed] [Google Scholar]
- Pshenichnaya NY, Nenadskaya SA. Probable Crimean-Congo hemorrhagic fever virus transmission occurred after aerosol-generating medical procedures in Russia: nosocomial cluster. Int J Infect Dis. 2015;33:120–2. [DOI] [PubMed] [Google Scholar]
- Pérez-Gracia MT, Mateos ML, Galiana C et al.. Autochthonous hepatitis E infection in a slaughterhouse worker. Am J Trop Med Hyg. 2007;77:893–6. [PubMed] [Google Scholar]
- Pérez-Gracia MT, Suay-García B, Mateos-Lindemann ML. Hepatitis E and pregnancy: current state. Rev Med Virol. 2017; 27:e1929. [DOI] [PubMed] [Google Scholar]
- Remoli ME, Fiorentini C, Marchi A et al.. Seroprevalence survey of arboviruses in workers from Tuscany, Italy. Med Lav. 2018;109:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardo F, Monaco F, Bella A et al.. An early start of West Nile virus seasonal transmission: the added value of one health surveillance in detecting early circulation and triggering timely response in Italy, June to July 2018. Euro Surveill. 2018;23, DOI: 10.2807/1560-7917ES.2018.23.32.1800427:pii = 1800427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargianou M, Panos G, Tsatsaris A et al.. Crimean-Congo hemorrhagic fever: seroprevalence and risk factors among humans in Achaia, western Greece. Int J Infect Dis. 2013;17:e1160–5. [DOI] [PubMed] [Google Scholar]
- Sargianou M, Papa A.. Epidemiological and behavioral factors associated with Crimea-Congo hemorrhagic fever virus infections in humans. Expert Rev Anti Infect Ther. 2013;11:897–08. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kumar A, Kar P et al.. Risk factors for vertical transmission of hepatitis E virus infection. J Viral Hepat. 2017;24:1067–75. 10.1111/jvh.1273028570034 [DOI] [PubMed] [Google Scholar]
- Sidira P, Nikza P, Danis K et al.. Prevalence of Crimean-Congo hemorrhagic fever virus antibodies in Greek residents in the area where the AP92 strain was isolated. Hippokratia. 2013;17:322–5. [PMC free article] [PubMed] [Google Scholar]
- Silva SM, Oliveira JM, Vitral CL et al.. Prevalence of hepatitis E virus antibodies in individuals exposed to swine in Mato Grosso, Brazil. Mem Inst Oswaldo Cruz. 2012;107:338–41. [DOI] [PubMed] [Google Scholar]
- Sinakos Ε, Gioula G, Liava C et al.. Prevalence of hepatitis E in liver transplant recipients in Greece. Epidemiol Infect. 2018;146:1619–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisman A. Epidemiologic features and risk factors of Crimean-Congo hemorrhagic fever in Samsun province, Turkey. J Epidemiol. 2013;23:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. A security guard with West Nile Virus Encephalitis. Workplace Health Saf. 2016;64:6–8. [DOI] [PubMed] [Google Scholar]
- Sommerkorn FM, Schauer B, Schreiner T et al.. Performance of hepatitis E virus (HEV)-antibody tests: a comparative analysis based on samples from individuals with direct contact to domestic pigs or wild boar in Germany. Med Microbiol Immunol. 2017;206:277–86. [DOI] [PubMed] [Google Scholar]
- Spataro P, Scoglio ME, Di Pietro Aet al. Seroprevalence study on the diffusion of the West Nile virus among blood donors, healthcare workers, jockeys, grooms and fowlers, veterinary surgeons and hunters in Messina (Italy). J Prev Med Hyg. 2008;49:22–5. [PubMed] [Google Scholar]
- Stroffolini T, Rapicetta M, Chionne P et al.. Evidence for the presence of autochthonous (locally acquired) cases of acute hepatitis E virus infections in Italy since the 80s. Eur J Intern Med. 2015;26:348–50. [DOI] [PubMed] [Google Scholar]
- Tabibi R, Baccalini R, Barassi A et al.. Occupational exposure to zoonotic agents among agricultural workers in Lombardy region, northern Italy. Ann Agric Environ Med. 2013;20:676–81. [PubMed] [Google Scholar]
- Teixeira J, Mesquita JR, Pereira SS et al.. Prevalence of hepatitis E virus antibodies in workers occupationally exposed to swine in Portugal. Med Microbiol Immunol. 2017;206:77–81. [DOI] [PubMed] [Google Scholar]
- Temmam S, Besnard L, Andriamandimby SF et al.. High prevalence of hepatitis E in humans and pigs and evidence of genotype-3 virus in swine, Madagascar. Am J Trop Med Hyg. 2013;88:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd CS, Mansoor GF, Buhler C et al.. Prevalence of zoonotic and vector-borne infections among Afghan National Army recruits in Afghanistan. Vector-Borne Zoonot. 2016;16:501–6. [DOI] [PubMed] [Google Scholar]
- Toyoda K, Furusyo N, Takeoka H et al.. Epidemiological study of hepatitis E virus infection in the general population of Okinawa, Kyushu, Japan. J Gastrenterol Hepatol. 2008;23:1885–90. [DOI] [PubMed] [Google Scholar]
- Traoré KA, Ouoba JB, Huot N et al.. Hepatitis E virus exposure is increased in pork butchers from Burkina Faso. Am J Trop Med Hyg. 2015;93:1356–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp A, Joller H, Jeggli S et al.. Hepatitis E, Helicobacter pylori and peptic ulcers in workers exposed to sewage: a prospective cohort study. Occup Environ Med. 2009;66:45–50. [DOI] [PubMed] [Google Scholar]
- Ukuli AQ, Mugimba KK.. Seroprevalence of hepatitis E in swine abattoir workers. Afr Health Sci. 2017;17:1022–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbert S, West Nile virus: the complex biology of an emerging pathogen. Intervirology. 2011;54:171–84. [DOI] [PubMed] [Google Scholar]
- van Eeden C, Swanepoel R, Venter M. Antibodies against West Nile and Shuni viruses in Veterinarians, South Africa. Emerg Infect Dis. 2014;20:1409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawda S, Goedhals D, Bester PA et al.. Seroepidemiologic survey of Crimean-Congo hemorrhagic fever virus in selected risk groups, South Africa. Emerg Infect Dis. 2018;24:1360–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter M, Burt FJ, Blumberg L et al.. Cytokine induction after laboratory-acquired West Nile virus infection. N Engl J Med. 2009;360:1260–2. [DOI] [PubMed] [Google Scholar]
- Venter M, Swanepoel R. West Nile virus lineage 2 as a cause of zoonotic neurological disease in humans and horses in southern Africa. Vector-Borne Zoonot. 2010;10:659–64. [DOI] [PubMed] [Google Scholar]
- Vieira MA, Romano AP, Borba AS et al.. West Nile virus Encephalitis: the first human case recorded in Brazil. Am J Trop Med Hyg. 2015;93:377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonesch N, D'Ovidio MC, Melis P et al.. Climate change, vector-borne diseases and working population. Ann Ist Super Sanita. 2016;52:397–405. [DOI] [PubMed] [Google Scholar]
- Vulcano A, Angelucci M, Candelori E et al.. HEV prevalence in the general population and among workers at zoonotic risk in Latium region. Ann Ig. 2007;19:181–6. [PubMed] [Google Scholar]
- Wang LF, Crameri G. Emerging zoonotic viral diseases. Rev Sci Tech. 2014,33:569–81. [DOI] [PubMed] [Google Scholar]
- Wasfi F, Dowall S, Ghabbari T et al.. Sero-epidemiological survey of Crimean-Congo hemorrhagic fever virus in Tunisia. Parasite. 2016;23:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Crimean-Congo Hemorrhagic Fever. https://www.who.int/news-room/fact-sheets/detail/crimean-congo-hemorrhagic-fever(16 January 2019, date last accessed), 2013. [Google Scholar]
- WHO. Report of the WHO/FAO/OIE Joint Consultation on Emerging Zoonotic Diseases. Geneva: Food and Agriculture Organization of the United Nations (FAO) and World Organisation for Animal Health (OIE), 2004. [Google Scholar]
- WHO. West Nile Virus. https://www.who.int/news-room/fact-sheets/detail/west-nile-virus(18 January 2019, date last accessed), 2017. [Google Scholar]
- WHO Hepatitis E. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e, (3 March 2019, date last accessed), 2018. [Google Scholar]
- Widasari DI, Yano Y, Utsumi T et al.. Hepatitis E virus infection in two different regions of Indonesia with identification of swine HEV genotype 3. Microbiol Immunol. 2013;57:692–03. [DOI] [PubMed] [Google Scholar]
- Yadav PD, Patil DY, Shete AM et al.. Nosocomial infection of CCHF among health care workers in Rajasthan, India. BMC Infect Dis. 2016;16:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav PD, Thacker S, Patil DY et al.. Crimean-Congo hemorrhagic fever in migrant worker returning from Oman to India, 2016. Emerg Infect Dis. 2017;23:1005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirmak T, Tulek N, Bulut C. Crimean-Congo hemorrhagic fever: transmission to visitors and healthcare workers. Infection. 2016;44:687–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Jeong HS, Yun H et al.. Hepatitis E virus (HEV) seroprevalence in the general population of the Republic of Korea in 2007–2009: a nationwide cross-sectional study. BMC Infect Dis. 2014;14:517. [DOI] [PMC free article] [PubMed] [Google Scholar]