How did the study come about?
Childhood asthma resembles a complex syndrome rather than a single disease. Clinical manifestations include cough, shortness of breath and wheeze.1 Because of its high prevalence, it is of major public health relevance.2,3 Presentation and disease course of childhood wheezing disorders vary,4 and its phenotypes are difficult to distinguish in early childhood despite the demand for diverse therapeutic strategies.5 Current treatments have no sustained effect on development of lung function and cannot prevent tracking of reduced lung function.6
Risk factors for childhood wheeze and subsequent asthma include genes, environmental factors and their interaction:1,7,8 all these affect lung development directly or indirectly (Figure 1). Data on the association between lung development and respiratory symptoms9 in early childhood are mostly derived from small hospital-based studies. Larger epidemiological studies have been based on questionnaires and most studies have been conducted in older children,2 in high-risk populations10 or retrospectively.11 There is a lack of prospectively assessed lung function studies in unselected healthy infants that take into account genetic and environmental factors. However, in order to disentangle the complex interaction of these predisposing factors on lung development and childhood wheeze, relatively large groups of infants need to be studied, with repeated lung function measurements, assessment of environmental and hereditary factors and clinical outcomes. The ‘Bern Infant Lung Development’ (BILD) cohort aims to contribute towards closing this gap. It was set up in 1999 to study physiological properties of the respiratory system and environmental and genetic risk factors affecting lung development in healthy individuals from infancy through childhood in relation to wheeze.9
Figure 1.
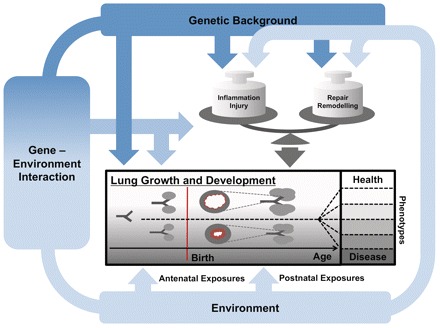
Proposed effect of environmental and genetic risk factors and their interaction on lung development and evolution of wheeze phenotypes. The Figure illustrates how genetic background, environmental exposures (ETS, air pollution, infections, allergens, active smoking) and their interaction might influence lung growth and development from branching of the airways to alveolarization. We propose a direct effect of these exposures on lung development, and an indirect effect via changes in the balance between inflammation, injury and repair mechanisms, leading to remodelling. Differences in these pathways might lead to different phenotypes of wheezing disorders
How is it funded?
Direct costs of the cohort are being paid by project grants from the Swiss National Science Foundation, the European Respiratory Society (ERS), the Austrian, German and Swiss Paediatric Respiratory Society and Swiss Governmental Anti-Tobacco Fund. The University Children’s Hospital in Bern, Switzerland, where the study centre is located, provides the infrastructure. All studies were approved by the local Ethics Research Committee.
What does it cover?
In a comprehensive approach, we search for early markers of common respiratory morbidity, including respiratory infections and asthma, by trying to disentangle predisposing and modifying factors of respiratory morbidity within the context of lung development. This includes the assessment of immune development and markers of airway inflammation in response to environmental triggers.
The strengths of the BILD cohort are its hallmarks: the prospective approach with standardized data collection starting before birth in unselected children; the accurate and standardized measurements of lung function and markers of airway inflammation; and the detailed assessment of incidence and determinants of respiratory symptoms especially during the first year of life.12
To explain developing properties of lung and airways during the vulnerable phases, lung function is measured at the age of 1 month and at the age of 6 years, with further lung function measurements planned for the age of 12 years.
We study the complex reaction of lung and airways to environmental triggers, which is possibly influenced both by inflammation and immune response. Here, we measure the association between exposure to environmental tobacco smoke (ETS), air pollution and lung function, markers of airway inflammation and components of the immune system. In addition, we prospectively assess viral pathogens of lower respiratory tract infections during the first year of life, as well as related respiratory symptoms and their severity.
Data covering genetic risk factors for childhood asthma are rapidly increasing.13,14 However, little is known about their effect on lung development and childhood wheeze.14–16 This is particularly true for genes known, from animal models, to be involved in lung development. Therefore, we measure the effect of single-nucleotide polymorphisms in these genes in comparison with genes associated with clinical manifestation of childhood allergy, asthma or wheeze.
Who is in the sample?
Recruitment for the BILD cohort is ongoing; about 35 unselected healthy neonates enter the cohort every year. Study participants are born after April 1999 in the agglomeration of Bern, the capital of Switzerland. One-third of the study population comes from the rural surroundings, and two-thirds from the urban area of Bern, which still offers relatively rural living conditions compared with other European cities.
Pregnant mothers are recruited at the four major maternity hospitals and practices of obstetricians in the agglomeration of Bern through advertisements and interviews. Interested families receive informative letters describing involved measurements and are contacted by the study centre. The following inclusion criteria apply: White ethnicity, term delivery (at least 37 weeks), ability of parents to speak one of the major Swiss languages (German, French), no severe maternal health problems, no maternal drug abuse other than nicotine, no known major birth defects or perinatal disease of the newborn, such as respiratory distress, airway malformation or other major respiratory diseases diagnosed after birth.
From 1999 until the end of 2009, a total of 42 110 mothers gave birth at the four major maternity hospitals in the agglomeration of Bern. By the end of 2009, we recruited 364 study participants (see Table 1 for anthropometric data of infants with lung function data), and 107 have been followed-up so far at the age of 6 years (Table 2). The others have not yet reached this age.
Table 1.
Anthropometric data of study participants in the BILD cohort with information on lung function (N = 344) at the age of 1 month
| Anthropometric data | Median | Interquartile range | Range |
|---|---|---|---|
| Gestational age at birth (weeks) | 39.9 | 38.9–40.6 | 37.0–42.3 |
| Birth weight (g) | 3410 | 3060–3660 | 2170–4915 |
| Age at study date (weeks) | 5.0 | 4.6–5.4 | 3.6–8.3 |
| Weight at study date (g) | 4325 | 4010–4750 | 2890–6400 |
| Length at study date (cm) | 54.6 | 53.1–56.5 | 48.0–61.5 |
Table 2.
Number of participants initially recruited, number completing study Phases 1 and 2 and number of drop-outs during study Phases 1 and 2 from 1999–2009 in the BILD cohort
| Year | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Total until 31/12/2009 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prenatally recruited | 26 | 40 | 20 | 21 | 46 | 44 | 37 | 29 | 30 | 45 | 25 | 364 |
| Completed study phase 1 | 23 | 37 | 19 | 20 | 45 | 44 | 36 | 28 | 29 | 44 | 25 | 350 |
| Completed study phase 2 | – | – | – | – | – | – | 28 | 14 | 11 | 18 | 36 | 107 |
| Drop-outs during study phase 1 | 3 | 3 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 14 |
| Drop-outs after study phase 1 | – | – | – | – | – | – | 8 | 8 | 7 | 1 | 8 | 32 |
How often have they been followed-up? What is attrition like?
The study design of the BILD cohort is shown in Figure 2. It includes three study phases with three questionnaires, weekly telephone interviews during the first year of life, two visits to the study centre and two visits to the families’ homes at the time of the first lower respiratory infection.
Figure 2.
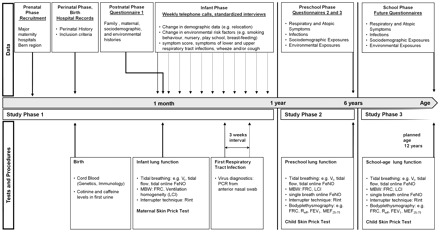
The BILD cohort: time-flow of recorded data, as well as of tests and procedures performed during the follow-up. Vt: tidal volume; FeNO: fraction of exhaled nitric oxide; MBW: multiple breath washout; FRC: functional residual capacity; LCI: lung clearance index; PCR: polymerase chain reaction; Rint: airway resistance by interrupter; Reff: effective airway resistance (measured by bodyplethysmography); FEV1: forced expiratory volume during the first second of expiration; MEF25-75: midexpiratory flow
Study Phase 1 starts with recruitment. After birth, mid wives extract perinatal history from medical records, and take cord blood and first urine samples if eligibility is confirmed. At the age of 1 month, the infants visit the study centre. On this occasion, lung function measurements and maternal skin-prick tests are performed, and Questionnaire 1 is used to assess pre- and perinatal history. During the first year of life, study nurses perform regular weekly telephone calls with standardized interviews.
After the first year of life, Study Phase 2 starts. The first follow-up is performed at the age of 6 years, when participants visit the study centre again to undergo lung function measurements and skin-prick tests. Questionnaires 2 and 3 assess the history during Study Phase 2. A further follow-up as part of Study Phase 3, also including lung function measurements and skin-prick tests, is planned for the age of 12 years.
Attrition was low during Phase 1 and relatively low during Phase 2. We lost only 13 of 364 recruited infants (3.7%) during Phase 1 and only 31 of 138 children (22.5%) during Phase 2 (Table 2). Drop-outs were almost exclusively due to personal reasons (8 during Phase 1, 25 during Phase 2), and to families moving away (1 during Phase 1, 5 during Phase 2). Five children were excluded because of heart disease diagnosed after the neonatal period (four in Phase 1 and one in Phase 2).
What has been measured?
The data are derived from four sources: (i) hospital records; (ii) questionnaires; (iii) telephone interviews; and (iv) objective measurements. A detailed description of data collected in the BILD cohort during Phases 1 and 2 is given in Table 3.
Table 3.
Detailed description of collected data and measurements in the BILD cohort during Phases 1 and 2
| Data collection from hospital records, questionnaires and telephone interviews | Study Phase 1 (pregnancy until age 1 year) | Study Phase 2 (age 1 year until age 6 years) |
|---|---|---|
| General health condition |
|
|
| Respiratory symptoms | Source: weekly telephone interviews during first year of life52,53 | Source: Questionnaires 2 and 3 |
|
|
|
| Environmental exposures | Source: Questionnaire 1, weekly telephone interviews during first year of life52,53 | Source: Questionnaires 2 and 3 |
|
|
| Objective measurements | Study Phase 1 (pregnancy until age 1 year) | Study Phase 2 (age 1 year until age 6 years) |
|---|---|---|
| Measurements of lung function and inflammation of airways |
|
|
| Skin-prick test | Maternal skin-prick test | Child skin-prick test |
|
|
|
| Immunology | Specimen: cord blood | |
|
||
| Microbiology | Specimen: anterior nasal swabs | |
|
||
| Genetics | deoxyribonucleic acid (DNA) extraction from white blood cells from cord blood | DNA extraction from mucosa cells retrieved per buccal swabs or from saliva collection |
|
|
|
| ETS and caffeine | Specimen: first urine after birth and urine during first lower respiratory-tract infection | |
|
||
| Air pollution at community level |
|
|
| Air pollution at individual level |
|
|
| Extraction of routine data | Study Phases 1–2 (until age 6 years) |
|---|---|
| Sources: Questionnaires 1–3, telephone interviews |
|
Hospital records (i): these are used to extract information about maternal warning signs during the delivery and perinatal data.
Questionnaires (ii): these assess information about health conditions, with an emphasis on respiratory and atopic symptoms, infections and socio-demographic and environmental exposures. Questionnaire 1 also collects information on pre- and perinatal risk factors including family and maternal past medical history. Questionnaires 2 and 3 assess the study participant’s history during Phase 2. While Questionnaire 1 contains validated questions from a preschool cohort study,17,18 Questionnaires 2 and 3 include questions for school-age children from the International Study of Asthma and Allergies in Childhood (ISAAC).19 All questionnaires have been validated and used, with little variation since 1999. Questionnaire 1 is programmed as an online database for data entry during interview, Questionnaires 2 and 3 are distributed to parents in paper form (see available as Supplementary Data at IJE online data A and B available as Supplementary Data at IJE online).
Weekly telephone interviews (iii): these interviews are carried out during the first year of life to collect information about respiratory symptoms. This includes a standardized score with a high sensitivity for lower respiratory-tract infections (Table 4).20 Changes in socio-demographic and environmental exposures are also assessed (listed in Table 5 for the study population with data from Phase 1). In the event of a first lower respiratory-tract infection, the study nurses visit the families twice within 3 weeks and perform two anterior nasal swabs for virus diagnostics by polymerase chain reaction (PCR).
Table 4.
Symptom score used in weekly telephone interviews during the first year of life20
| Symptom score | Day-time symptoms (cough, wheeze or breathing difficulties) | Night-time symptoms (cough, wheeze or breathing difficulties) |
|---|---|---|
| 0 | None | None |
| 1 | Slight; no treatment given | Slight; sleep not disturbed |
| 2 | Required treatment but no help from outside | Sleep disturbed once; no help required |
| 3 | Severe; required help from general practitioner (GP) | Sleep disturbed more than once or child needed help |
| 4 | Very severe; admitted to hospital | Sleep very disturbed or GP called |
Table 5.
Risk factors for respiratory disease of infants during Phase 1 of the BILD cohort
| Number of infants | Proportion of infants (%) | |
|---|---|---|
| History of maternal asthma | ||
| Negative | 320 | 88.6 |
| Positive | 39 | 10.8 |
| Unknown | 2 | 0.6 |
| History of paternal asthma | ||
| Negative | 323 | 89.0 |
| Positive | 36 | 9.9 |
| Unknown | 4 | 1.1 |
| History of maternal atopic disease a | ||
| Negative | 233 | 64.2 |
| Positive | 126 | 34.7 |
| Unknown | 4 | 1.1 |
| Skin-pick test results in mothers b | ||
| Negative | 197 | 65.0 |
| Positive | 106 | 35.0 |
| Maternal smoking during pregnancy | ||
| Negative | 316 | 87.5 |
| Positive | 42 | 11.6 |
| Unknown | 3 | 0.8 |
| Paternal smoking during pregnancy | ||
| Negative | 280 | 77.1 |
| Positive | 76 | 20.9 |
| Unknown | 7 | 1.9 |
| Older siblings | ||
| None | 181 | 50.1 |
| One | 114 | 31.6 |
| More than one | 58 | 16.1 |
| Unknown | 8 | 2.2 |
This Table shows the distribution of risk factors for respiratory disease of study participants during Phase 1 of the BILD cohort, i.e. during the first year of life (n = 364, information could not be retrieved for 3 out of 14 drop-outs during Phase 1).
aEither maternal history of allergic rhinitis, allergic asthma or atopic dermatitis.
bSkin-prick tests were performed in a subgroup of 303 mothers. Tests were positive in case of hives bigger than positive control (histamine) in any of the tested common allergens.
Objective measurements (iv): these measurements during infancy include, besides the mentioned virus diagnostics, a lung function test at the age of 1 month in unsedated infants during quiet natural sleep (tidal breathing measurements, multiple breath washouts, interrupter resistance measurements). At the age of 6 years, the same lung function tests are performed, supplemented by spirometry and bodyplethysmography. The fraction of exhaled nitric oxide (FeNO), as a marker of airway inflammation, is measured at both time-points. All measurements are performed according to current standards by the ERS and the American Thoracic Society (ATS).21–24 Skin-prick tests are performed in mothers during the first visit and in infants at the age of 6 years.
In addition to data from questionnaires, ETS exposure during pregnancy is objectively assessed by the analysis of the first urine after birth. Exposure to outdoor air pollution on the community level is measured by monitoring stations of the Swiss National Air Pollution Network (NABEL), including daily mean levels of particles with a 50% cut-off aerodynamic diameter of 10 µm (PM10) and nitrogen dioxide (NO2), as well as the daily maximum of mean hourly levels of ozone (O3). Air pollution at the individual level is measured in selected time periods by mobile passive samplers (daily mean levels of particles with a 50% cut-off aerodynamic diameter of 2.5 µm (PM2.5) and PM10) and stationary passive samplers at the measurement sites (daily mean levels of NO2).
What has been found? Key findings and publications
Development and standardization of non-invasive lung function tests
To assess lung development in a large infant cohort, one needs non-invasive tests that can be performed in uncooperative study participants. In addition to the standardized data collection, we have significantly added to current ERS/ATS standards of lung function measurements in infants23,24 and to the development and standardization of additional non-invasive lung function techniques.9,25–48
Early programmers of lung development
Since the beginning of our prospective birth cohort, we have investigated physical properties of the airways in infants prone to wheeze.9 We showed that not only bronchoconstriction, but also impaired airway growth, plays a role for the development of wheeze.27,29 Even without current wheeze, infants with a past history of wheeze display altered airway wall mechanics. This might be due to remodelling or independent developmental differences already present at birth. This emphasizes the importance of early programmers of lung development.30,49,50
Outdoor air pollution might be such an early programmer. By modelling outdoor air pollution exposure during pregnancy, we found that exposure to PM10 was associated with altered lung function in newborns and that NO2 levels were associated with elevated FeNO levels after birth, indicating the induction of inflammatory response in infants. This was enhanced if mothers smoked during pregnancy but independent of maternal atopy.12 Although atopy is a risk factor for allergic asthma, this adds to the hypothesis that lung development and evolution of allergic asthma might be partly independent processes, which are both influenced by early programmers.25,51
Prevalence, risk factors and prognosis of respiratory symptoms in early life
We were among the first to present prospective data on respiratory symptoms in a cohort of unselected infants. The incidence of symptoms was increased in infants of asthmatic mothers, whereas maternal hay fever was inversely related to the number of symptoms.52 High FeNO levels after birth were associated with severe symptoms during the first year of life in the offspring of atopic mothers, which was again pronounced if mothers smoked during pregnancy.53 Thus, FeNO after birth might be an important predictor of later respiratory symptoms and morbidity.
The role of viral triggers for early respiratory-tract infections
We analysed the pattern of viral pathogens causing respiratory infections during the first year of life and found that rhinoviruses were most common, followed by corona, parainfluenza and respiratory syncytial viruses. Patterns differed also with regard to the amount of viral shedding 3 weeks after incidence of symptoms.54–56 Additionally, we showed that Human Bocavirus (HBoV), a picornavirus isolated in children with lower respiratory-tract infections from several retrospective and hospital-based studies, is also associated with respiratory disease in healthy Swiss infants, and reported that HBoV circulates in an endemic fashion in the community, thus confirming the worldwide distribution.54
Early mechanisms and markers related to airway inflammation, the development of the immune system and childhood wheezing disorders
We were able to add towards the knowledge about the effects of environmental triggers on airway inflammation. We found that smoking during pregnancy has an effect on components of the innate immune system of the offspring. Total leucocyte counts in cord blood were significantly lower if mothers smoked during pregnancy. The decrease was most prominent in neutrophils, monocytes, dendritic cells and lymphocytes.57 Investigating the impact of outdoor air pollution on cytokine levels as of monocyte chemotactic protein-1 (MCP-1), interleukin-6 (IL-6), IL-10, for which large changes are seen after exposure to high pollution levels such as after bushfires, we found only small effects in cord blood of healthy term infants.58,59
Assessing the quantitative effect of the acute-phase plasma collection mannose-binding lectin (MBL) on respiratory morbidity, we found that low levels were only weakly associated, and high levels more strongly associated, with the incidence and severity of respiratory symptoms during the first year of life, particularly in infants with asthmatic parents.60
What are the main strengths and weaknesses?
The BILD cohort, with its unique data set of comprehensive lung function data, particularly during infancy, is an integrative and interdisciplinary framework to analyse how predisposing factors affect lung development from pregnancy through childhood and to predict later risk of respiratory disease. Especially with regard to the costly and time-consuming lung function measurements in infants, the BILD cohort has a number of methodological strengths, and normative data collected during these measurements in the BILD cohort have been published meanwhile.61 To ensure comparability, we measure lung function at any age in a standardized way, using the same technique and equipment on every subsequent occasion in the same order. Measurements are performed according to the latest recommendations by the ERS and ATS; often we were even stricter. For instance, for the analysis of tidal breathing at the age of 1 month, we used 100 instead of 30 breaths as currently recommended, for better accuracy.21 There is still enough flexibility to develop new lung function testing methods.32,36 To validate exposure to air pollutants, we have started to assess individual exposure to air pollutants using passive samplers, which is expensive and has yet been rarely used in studies assessing the effect of long-term exposure to air pollution on lung function.
The BILD cohort has a prospective design. It includes data assessments before and after birth, close follow-up with weekly standardized telephonic interviews until the age of 1 year and a detailed reassessment at the age of 6 years. Lung function tests are performed at several time points covering the vulnerable phases of lung development (growth of airways and alveoli) during gestation and childhood. This facilitates disentangling effects of pre- and perinatal risk factors from those of post-natal risk factors, such as exposure to airway pollutants.
With all included infants being healthy and of a narrow age range and all measurements in infants performed during quiet natural sleep, comparability within the cohort is given. Confounders such as physical activity, obesity and hypoxia important for older children are negligible in the studied infants. Nevertheless, these influences are prospectively assessed and will be included in the analysis of measurements at school age.
We have only minimal attrition during the study, especially during Phase 1, comparing favourably with other studies.62 This is probably due to the regular follow-up, particularly the weekly telephone interviews during the first year of life. The drawback of such a detailed time-consuming study is that it can only be done on a limited number of participants.
Selection bias is not a major issue, as all participants are recruited prenatally without knowledge of exposures and outcomes. These are assessed and analysed independently from each other. So far, we have been able to adjust for known biological, time-variant and further possible confounders.
All involved families were recruited in maternity hospitals and only a minority of children born during the study period in the region was recruited. Although the main reason for non-participation was lacking information about the study, participants are likely to be biased towards a well-educated middle-class population. This is the case with most studies involving detailed measurements. In our analyses, social class has so far neither been a risk factor nor a risk modifier for outcome measures. Therefore, although we believe that most results are fairly representative for the general population, it must be kept in mind that not all findings can be extrapolated to all Swiss or European children. Replication of the findings in independent cohorts is desirable.
Can I get hold of the data? Where can I find out more?
The Bern cohort studies are carried out at the Department of Paediatric Pulmonology at Children’s University Hospital Bern, Switzerland. The department’s homepage provides information regarding personal contact data, team members and publications (http://www.kinderkliniken.insel.ch/kiheil-pneumologie.html). The principal investigator is Prof. Dr Urs Frey, MD PhD, head of the University Children's Hospital (UKBB), Basel, Switzerland. Researchers interested in collaborative work or further information are invited to contact the principal investigator, Urs Frey, urs.frey@ukbb.ch.
Funding
Swiss National Science Foundation (32003B_124654/1, 3200-B0-12099, to O.F.; 3200-B0-12099, 3200-052197.97/1 to P.L.; 3200-B0-12099, 3200-052197.97/1, 3233-069348, 3200-069349 to C.K.; and 32003B_124654/1, 3200-B0-12099, 3200-052197.97/1, 3200-052197.97/2, 3200-068025 and 32-68025.02 to U.F.); European Respiratory Society (long-term research fellowship 675 to O.F.); Austrian, German and Swiss Paediatric Respiratory Society (training scholarship 2009 to O.F.).
Supplementary Material
Acknowledgements
We thank all study participants and their families in the canton of Bern for participating in the study and the staff of the four major maternity hospitals in the Bernese region (Klinik Engeried-Sonnenhof, Lindenhofspital, Salem-Spital, Universitätsklinik für Frauenheilkunde Bern) for support and recruitment. We also thank the study nurses Monika Graf, Barbara Hofer and Christine Becher and the lung function technicians Gisela Wirz and Sandra Luescher for their invaluable assistance and support.
Conflict of interest: None declared.
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–38. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Braun-Fahrlander C, Gassner M, Grize L, et al. No further increase in asthma, hay fever and atopic sensitisation in adolescents living in Switzerland. Eur Respir J. 2004;23:407–13. doi: 10.1183/09031936.04.00074004. [DOI] [PubMed] [Google Scholar]
- 3.Kuehni CE, Davis A, Brooke AM, Silverman M. Are all wheezing disorders in very young (preschool) children increasing in prevalence? Lancet. 2001;357:1821–25. doi: 10.1016/S0140-6736(00)04958-8. [DOI] [PubMed] [Google Scholar]
- 4.Bush A, Menzies-Gow A. Phenotypic differences between pediatric and adult asthma. Proc Am Thorac Soc. 2009;6:712–19. doi: 10.1513/pats.200906-046DP. [DOI] [PubMed] [Google Scholar]
- 5.Spycher BD, Silverman M, Brooke AM, Minder CE, Kuehni CE. Distinguishing phenotypes of childhood wheeze and cough using latent class analysis. Eur Respir J. 2008;31:974–81. doi: 10.1183/09031936.00153507. [DOI] [PubMed] [Google Scholar]
- 6.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–55. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 7.von Mutius E, Le Souef PN. Early gene-environment interactions: can they inform primary preventive strategies for asthma? Semin Respir Crit Care Med. 2007;28:255–63. doi: 10.1055/s-2007-981646. [DOI] [PubMed] [Google Scholar]
- 8.Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–58. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frey U. Why are infants prone to wheeze? Physiological aspects of wheezing disorders in infants. Swiss Med Wkly. 2001;131:400–6. doi: 10.4414/smw.2001.06137. [DOI] [PubMed] [Google Scholar]
- 10.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–86. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 11.Lopez Bravo IM, Sepulveda H, Valdes I. Acute respiratory illnesses in the first 18 months of life. Rev Panam Salud Publica. 1997;1:9–17. doi: 10.1590/s1020-49891997000100003. [DOI] [PubMed] [Google Scholar]
- 12.Latzin P, Roosli M, Huss A, Kuehni CE, Frey U. Air pollution during pregnancy and lung function in newborns: a birth cohort study. Eur Respir J. 2008;33:594–603. doi: 10.1183/09031936.00084008. [DOI] [PubMed] [Google Scholar]
- 13.Moffatt MF, Kabesch M, Liang L, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–73. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 14.Ober C, Hoffjan S. Asthma genetics (2006): the long and winding road to gene discovery. Genes Immun. 2006;7:95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 15.Guerra S, Martinez FD. Asthma genetics: from linear to multifactorial approaches. Annu Rev Med. 2008;59:327–41. doi: 10.1146/annurev.med.59.060406.213232. [DOI] [PubMed] [Google Scholar]
- 16.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–82. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 17.Kuehni CE, Brooke AM, Strippoli MP, Spycher BD, Davis A, Silverman M. Cohort profile: the Leicester respiratory cohorts. Int J Epidemiol. 2007;36:977–85. doi: 10.1093/ije/dym090. [DOI] [PubMed] [Google Scholar]
- 18.Strippoli MP, Silverman M, Michel G, Kuehni CE. A parent-completed respiratory questionnaire for 1-year-old children: repeatability. Arch Dis Child. 2007;92:861–65. doi: 10.1136/adc.2007.117978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 20.Silverman M, Wang M, Hunter G, Taub N. Episodic viral wheeze in preschool children: effect of topical nasal corticosteroid prophylaxis. Thorax. 2003;58:431–34. doi: 10.1136/thorax.58.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates JH, Schmalisch G, Filbrun D, Stocks J. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J. 2000;16:1180–92. doi: 10.1034/j.1399-3003.2000.16f26.x. [DOI] [PubMed] [Google Scholar]
- 22.Beydon N, Davis SD, Lombardi E, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–45. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 23.Frey U, Stocks J, Coates A, Sly P, Bates J. Specifications for equipment used for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J. 2000;16:731–40. doi: 10.1034/j.1399-3003.2000.16d28.x. [DOI] [PubMed] [Google Scholar]
- 24.Frey U, Stocks J, Sly P, Bates J. Specification for signal processing and data handling used for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J. 2000;16:1016–22. doi: 10.1183/09031936.00.16510160. [DOI] [PubMed] [Google Scholar]
- 25.Frey U. The high speed interrupter technique (HIT) and its role in wheezing disorders in infants. Pediatr Pulmonol Suppl. 1997;16:242–44. doi: 10.1002/ppul.19502308126. [DOI] [PubMed] [Google Scholar]
- 26.Frey U, Suki B, Kraemer R, Jackson AC. Human respiratory input impedance between 32 and 800 Hz, measured by interrupter technique and forced oscillations. J Appl Physiol. 1997;82:1018–23. doi: 10.1152/jappl.1997.82.3.1018. [DOI] [PubMed] [Google Scholar]
- 27.Frey U, Jackson AC, Silverman M. Differences in airway wall compliance as a possible mechanism for wheezing disorders in infants. Eur Respir J. 1998;12:136–42. doi: 10.1183/09031936.98.12010136. [DOI] [PubMed] [Google Scholar]
- 28.Frey U, Silverman M, Kraemer R, Jackson AC. High-frequency respiratory impedance measured by forced-oscillation technique in infants. Am J Respir Crit Care Med. 1998;158:363–70. doi: 10.1164/ajrccm.158.2.9703038. [DOI] [PubMed] [Google Scholar]
- 29.Frey U, Makkonen K, Wellman T, Beardsmore C, Silverman M. Alterations in airway wall properties in infants with a history of wheezing disorders. Am J Respir Crit Care Med. 2000;161:1825–29. doi: 10.1164/ajrccm.161.6.9812057. [DOI] [PubMed] [Google Scholar]
- 30.Henschen M, Stocks J, Brookes I, Frey U. New aspects of airway mechanics in pre-term infants. Eur Respir J. 2006;27:913–20. doi: 10.1183/09031936.06.00036305. [DOI] [PubMed] [Google Scholar]
- 31.Jackson AC, Tennhoff W, Kraemer R, Frey U. Airway and tissue resistance in wheezy infants: effects of albuterol. Am J Respir Crit Care Med. 1999;160:557–63. doi: 10.1164/ajrccm.160.2.9808137. [DOI] [PubMed] [Google Scholar]
- 32.Latzin P, Sauteur L, Thamrin C, et al. Optimized temperature and deadspace correction improve analysis of multiple breath washout measurements by ultrasonic flowmeter in infants. Pediatr Pulmonol. 2007;42:888–97. doi: 10.1002/ppul.20674. [DOI] [PubMed] [Google Scholar]
- 33.Schibler A, Hall GL, Businger F, et al. Measurement of lung volume and ventilation distribution with an ultrasonic flow meter in healthy infants. Eur Respir J. 2002;20:912–18. doi: 10.1183/09031936.02.00226002. [DOI] [PubMed] [Google Scholar]
- 34.Thamrin C, Latzin P, Sauteur L, Riedel T, Hall GL, Frey U. Deadspace estimation from CO2 versus molar mass measurements in infants. Pediatr Pulmonol. 2007;42:920–27. doi: 10.1002/ppul.20683. [DOI] [PubMed] [Google Scholar]
- 35.Thamrin C, Frey U. Effect of bacterial filter on measurement of interrupter resistance in preschool and school-aged children. Pediatr Pulmonol. 2008;43:781–87. doi: 10.1002/ppul.20865. [DOI] [PubMed] [Google Scholar]
- 36.Hutten GJ, van Eykern LA, Latzin P, Kyburz M, van Aalderen WM, Frey U. Relative impact of respiratory muscle activity on tidal flow and end expiratory volume in healthy neonates. Pediatr Pulmonol. 2008;43:882–91. doi: 10.1002/ppul.20874. [DOI] [PubMed] [Google Scholar]
- 37.Frey U, Silverman M, Barabasi AL, Suki B. Irregularities and power law distributions in the breathing pattern in preterm and term infants. J Appl Physiol. 1998;85:789–97. doi: 10.1152/jappl.1998.85.3.789. [DOI] [PubMed] [Google Scholar]
- 38.Schibler A, Schneider M, Frey U, Kraemer R. Moment ratio analysis of multiple breath nitrogen washout in infants with lung disease. Eur Respir J. 2000;15:1094–101. doi: 10.1034/j.1399-3003.2000.01518.x. [DOI] [PubMed] [Google Scholar]
- 39.Frey U, Silverman M, Suki B. Analysis of the harmonic content of the tidal flow waveforms in infants. J Appl Physiol. 2001;91:1687–93. doi: 10.1152/jappl.2001.91.4.1687. [DOI] [PubMed] [Google Scholar]
- 40.Cernelc M, Suki B, Reinmann B, Hall GL, Frey U. Correlation properties of tidal volume and end-tidal O2 and CO2 concentrations in healthy infants. J Appl Physiol. 2002;92:1817–27. doi: 10.1152/japplphysiol.00675.2001. [DOI] [PubMed] [Google Scholar]
- 41.Schibler A, Frey U. Role of lung function testing in the management of mechanically ventilated infants. Arch Dis Child Fetal Neonatal Ed. 2002;87:F7–F10. doi: 10.1136/fn.87.1.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frey U. Clinical applications of infant lung function testing: does it contribute to clinical decision making? Paediatr Respir Rev. 2001;2:126–30. doi: 10.1053/prrv.2000.0120. [DOI] [PubMed] [Google Scholar]
- 43.Frey U, Reinmann B, Stocks J. The infant lung function model: a mechanical analogue to test infant lung function equipment. Eur Respir J. 2001;17:755–64. doi: 10.1183/09031936.01.17407550. [DOI] [PubMed] [Google Scholar]
- 44.Frey U, Kuehni C, Roiha H, et al. Maternal atopic disease modifies effects of prenatal risk factors on exhaled nitric oxide in infants. Am J Respir Crit Care Med. 2004;170:260–65. doi: 10.1164/rccm.200307-1002OC. [DOI] [PubMed] [Google Scholar]
- 45.Baldwin DN, Suki B, Pillow JJ, Roiha HL, Minocchieri S, Frey U. Effect of sighs on breathing memory and dynamics in healthy infants. J Appl Physiol. 2004;97:1830–39. doi: 10.1152/japplphysiol.00298.2004. [DOI] [PubMed] [Google Scholar]
- 46.Baldwin DN, Pillow JJ, Stocks J, Frey U. Lung-function tests in neonates and infants with chronic lung disease: tidal breathing and respiratory control. Pediatr Pulmonol. 2006;41:391–419. doi: 10.1002/ppul.20400. [DOI] [PubMed] [Google Scholar]
- 47.Kondo T, Minocchieri S, Baldwin DN, Nelle M, Frey U. Noninvasive monitoring of chest wall movement in infants using laser. Pediatr Pulmonol. 2006;41:985–92. doi: 10.1002/ppul.20482. [DOI] [PubMed] [Google Scholar]
- 48.Hall GL, Reinmann B, Wildhaber JH, Frey U. Tidal exhaled nitric oxide in healthy, unsedated newborn infants with prenatal tobacco exposure. J Appl Physiol. 2002;92:59–66. doi: 10.1152/jappl.2002.92.1.59. [DOI] [PubMed] [Google Scholar]
- 49.Riedel T, Kyburz M, Latzin P, Thamrin C, Frey U. Regional and overall ventilation inhomogeneities in preterm and term-born infants. Intensive Care Med. 2009;35:144–51. doi: 10.1007/s00134-008-1299-x. [DOI] [PubMed] [Google Scholar]
- 50.Latzin P, Roth S, Thamrin C, et al. Lung volume, breathing pattern and ventilation inhomogeneity in preterm and term infants. PLoS One. 2009;4:e4635. doi: 10.1371/journal.pone.0004635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez FD. Asthma treatment and asthma prevention: a tale of 2 parallel pathways. J Allergy Clin Immunol. 2007;119:30–33. doi: 10.1016/j.jaci.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Latzin P, Frey U, Roiha HL, et al. Prospectively assessed incidence, severity, and determinants of respiratory symptoms in the first year of life. Pediatr Pulmonol. 2007;42:41–50. doi: 10.1002/ppul.20542. [DOI] [PubMed] [Google Scholar]
- 53.Latzin P, Kuehni CE, Baldwin DN, Roiha HL, Casaulta C, Frey U. Elevated exhaled nitric oxide in newborns of atopic mothers precedes respiratory symptoms. Am J Respir Crit Care Med. 2006;174:1292–98. doi: 10.1164/rccm.200606-782OC. [DOI] [PubMed] [Google Scholar]
- 54.Regamey N, Frey U, Deffernez C, Latzin P, Kaiser L. Isolation of human bocavirus from Swiss infants with respiratory infections. Pediatr Infect Dis J. 2007;26:177–79. doi: 10.1097/01.inf.0000250623.43107.bc. [DOI] [PubMed] [Google Scholar]
- 55.Regamey N, Kaiser L, Roiha HL, et al. Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J. 2008;27:100–5. doi: 10.1097/INF.0b013e31815922c8. [DOI] [PubMed] [Google Scholar]
- 56.Kaiser L, Regamey N, Roiha H, Deffernez C, Frey U. Human coronavirus NL63 associated with lower respiratory tract symptoms in early life. Pediatr Infect Dis J. 2005;24:1015–17. doi: 10.1097/01.inf.0000183773.80217.12. [DOI] [PubMed] [Google Scholar]
- 57.Pachlopnik Schmid JM, Kuehni CE, Strippoli MP, et al. Maternal tobacco smoking and decreased leukocytes, including dendritic cells, in neonates. Pediatr Res. 2007;61:462–66. doi: 10.1203/pdr.0b013e3180332d02. [DOI] [PubMed] [Google Scholar]
- 58.van Eeden SF, Tan WC, Suwa T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med. 2001;164:826–30. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 59.Schaub B, Frey U, Roosli M, Latzin P. Air pollution during pregnancy and coord blood cytokine secretion. European Respir J. 2008;32:246s. [Google Scholar]
- 60.Schlapbach LJ, Latzin P, Regamey N, et al. Mannose-binding lectin cord blood levels and respiratory symptoms during infancy: a prospective birth cohort study. Pediatr Allergy Immunol. 2009;20:219–26. doi: 10.1111/j.1399-3038.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuchs O, Latzin P, Thamrin C, et al. Normative data for lung function and exhaled nitric oxide in unsedated healthy infants. Eur Resp J. 2010 doi: 10.1183/09031936.00125510. published ahead of print, doi:10.1183/09031936.00125510. [DOI] [PubMed] [Google Scholar]
- 62.Douglas RM, Woodward A, Miles H, Buetow S, Morris D. A prospective study of proneness to acute respiratory illness in the first two years of life. Int J Epidemiol. 1994;23:818–26. doi: 10.1093/ije/23.4.818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


