Why was the cohort set up?
The immune system of newborn children undergoes intensive development during the first years of life.1 However, both, adaptive and innate immunity do not reach full capacity until teenage years.2,3 The immune system is thus shaped by different external factors until adolescence.4 A malfunctioning immune system not only leads to higher susceptibility to infections but can also cause atopic diseases like asthma and allergies.5 According to the ‘hygiene hypothesis’ it was shown that children raised on farms or children in contact with dogs have a lower risk of developing asthma or allergies due to their environmental (e.g. microbial) exposures.4,6 Furthermore, infections with different respiratory viruses (e.g. rhinovirus, enterovirus and adenovirus) in infancy influence the development of chronic and immune-mediated diseases like asthma, type 1 diabetes and obesity in later life.7–9 Although many birth cohort studies have addressed asthma and allergies, only two studies, the INSPIRE study in the USA and the ORChID study in Australia, have focussed on infections and the development of atopic diseases.10–12 However, INSPIRE lacks comprehensive data on infections during childhood and ORChID has a small sample size and collected data on infections only for 2 years. In order to prospectively assess the complete infection history during the first 6 years of life, we have initiated the German multi-centre LoewenKIDS birth cohort study in 2015. The aim of this study is to combine the complete infection history with information on the development of the nasal and gut microbiome and the immune system, as well as the genetic background and information on the children’s environment (nutrition, pets, siblings, day-care attendance, medication etc.). These data will help us to understand the association between infections, vaccinations, the microbiome, and genetics on the one side and immune-mediated diseases like atopic dermatitis, allergic asthma, and allergies on the other side. Before initiating the cohort, we conducted a pilot study to assess willingness to participate and a feasibility study testing different study components.13,14 This study is funded by institutional resources of the Helmholtz Centre for Infection Research and the Martin-Luther-University Halle-Wittenberg.
Overall goals
The overall goal of our birth cohort is to record a complete history of respiratory and gastrointestinal infections in infancy in order to investigate the effect of pattern, timing and sequence of infections and other risk factors influencing the immune response on the development of asthma and atopic diseases from a life-course perspective. We further aim to study effects of timing and sequence of infections on the progression severity of subsequent infections, and how those affect the immune response to vaccinations and their waning.
Collaboration goals
We intend that several collaborations will allow us to address our study aims, but we also welcome further propositions. With a wide range of different biological samples and information on many aspects of children’s daily life, we are able to address a broad range of research questions.
Who is in the cohort?
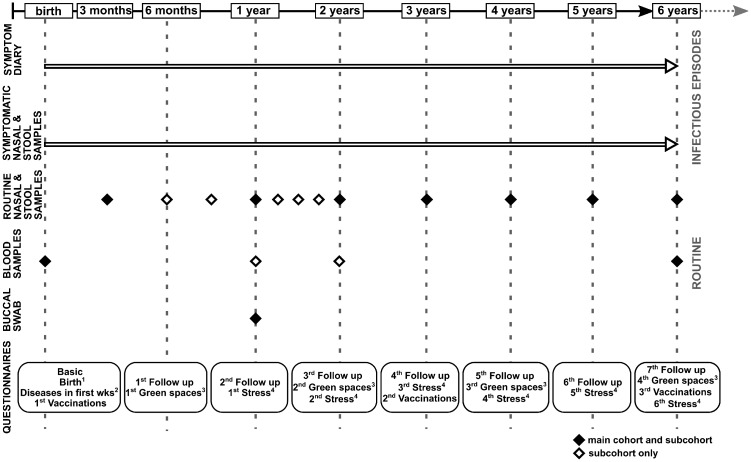
All newborn babies in five study regions in Germany [Braunschweig, Hannover, Bremen, Munich, Halle (Saale)] were eligible for enrollment in the study up to 3 months post-partum. Participants were recruited at antenatal preparation courses, information evenings in hospitals and in private practices, where approximately 35 000 families were reached. Overall, 782 newborns were enrolled, and of these 336 (43%) in Braunschweig, 174 (22%) in Hannover, 97 (12%) in Bremen, 91 (12%) in Munich and 76 (10%) in Halle (Saale) resulting in a response proportion of 2%. In total, 422 (54%) children were included before and 360 (46%) children after birth. The first enrolled child was born in November 2014 and the last in February 2018. The sex ratio of the participating children is 1:1. There are 27 sibling pairs within the cohort, including eleven pairs of twins. In total, 285 children (36%) were enrolled in a nested subcohort with more frequent collection of asymptomatic samples and additional blood sampling at the ages of 1 and 2 years (Figure 1).
Figure 1.
Study structure and materials over the whole study period of 6 years according to infectious episodes and tasks performed routinely in health. The timing of symptom diary, biological samples and questionnaires is shown separated for main cohort and subcohort and the subcohort only (depicted as full and empty diamonds, respectively). 1‘Birth’ questionnaires collect data on all birth aspects, 2‘Diseases in the first weeks’ questionnaires are completed by participants who have entered the study after their date of birth and could not fill in the symptom diary from the beginning, 3‘Green spaces’ questionnaires collect data on the living environment, 4‘Stress’ questionnaires address parental stress.
wks, weeks.
How often have they been followed up?
During the first 6 years, all parents are asked to fill in a symptom diary every day, take nasal or stool samples whenever their child shows predefined respiratory or gastrointestinal symptoms, and collect asymptomatic nasal and stool samples in asymptomatic children once a year (main cohort) or four times a year in the first 2 years and once a year in years 3–6 (subcohort). All participants are planned to provide blood samples at the age of 6 years and participants of the subcohort provide blood samples at ages 1 and 2 years in addition. All blood samples are taken by a physician. Parents fill in follow-up questionnaires at the age of 6 months, 1 year, and then annually until the age of 6 years. After the follow-up period of 6 years, participants will receive yearly questionnaires until the age of 15 with the option to further extend that period.
What has been measured?
In the LoewenKIDS study, data are collected in symptom diaries, questionnaires and through biological samples (Figure 1).
Symptom diary
Acute respiratory and gastrointestinal diseases are assessed in a daily symptom diary through ticking corresponding boxes. Symptoms include the precise temperature of fever, wheezing, runny/congested nose, chills, sore throat, loss of appetite, increased need to sleep, increased attachment, vomiting and diarrhoea. Here, parents also rate the symptom severity, and provide data on doctor visits, work absenteeism and medication on a daily basis. In case of shorter or longer symptomless time periods, crossing out the whole period simplifies the use of the symptom diary making it more feasible in everyday life. The symptom diary was developed based on the symptom diary used by the Australian birth cohort ORChID and adapted according to the results of our feasibility study.12,13 Participants can choose between a paper-based diary, an online version or an app. Changes between the different modes are allowed. With increasing immunocompetence of our participants, we expect a drop in days with symptoms, decreasing the parent’s workload over time.
So far, 754 (96%) of our children have turned 1 year old. Therefore, we have analysed the completeness of our symptom diary data in the first year of life based on the data we have to date. Of all 754 participants, at least 503 (67%) have filled out all quarter-yearly provided symptom diaries and another 62 (8%) have filled out at least three quarters of the symptom diaries in the first year of life. These proportions were similar in the subcohort and main cohort.
Questionnaires
Paper-based or online questionnaires collect data on the parents and child throughout the study (Table 1).
Table 1.
Content of the questionnaires in the LoewenKIDS study
| Topic | Pregnancy | Birth | 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | 6 years |
|---|---|---|---|---|---|---|---|---|---|
| Acute infections | Continuously in the daily symptom diary | ||||||||

|
|||||||||
| Demographics | x | x | |||||||
| Medication during pregnancy | x | ||||||||
| Selected diseasesa in mother’s family history | x | ||||||||
| Selected diseasesa in father’s family history | x | ||||||||
| Infection frequency mother | x | x | |||||||
| Infection frequency father | x | ||||||||
| Smoking of parents | x | x | x | ||||||
| Opinion on child’s vaccination | x | ||||||||
| Influenza vaccination mother | x | ||||||||
| Delivery | x | ||||||||
| Size and weight | x | x | x | x | x | x | |||
| Breastfeeding | x | x | x | x | |||||
| Vaccinations | x | x | x | ||||||
| Alcohol consumption during pregnancy | x | ||||||||
| Child’s development | x | x | x | x | x | x | x | ||
| General health | x | x | x | x | x | x | x | ||
| Diseases | x | x | x | x | x | x | x | ||
| Sleeping | x | x | x | x | x | x | x | ||
| Dietary habits | x | x | x | x | x | ||||
| Domestic pets | x | x | x | x | x | x | x | ||
| Siblings | x | x | x | x | x | x | x | ||
| Access to green areas | x | x | x | x | |||||
| Day-care attendance | x | x | x | x | x | x | |||
| Stress of parents | x | x | x | x | x | x | |||
| Atopic dermatitis | x | x | x | ||||||
| Allergy | x | x | x | x | |||||
| Asthma bronchiale | x | x | x | ||||||
| Social and emotional quality of life | x | x | x | ||||||
| Leisure and physical activities | x | x | |||||||
Selected diseases are neurodermatitis, asthma, diabetes mellitus type I, diabetes mellitus type II, rheumatoid arthritis, lupus erythematosus, as well as hay fever, and allegies against house dust mites and pet hair, and lactose, gluten and fructose intolerance.
Biological samples
We collect a vast amount of biological samples (Figure 1, Table 2). Our sample sizes will continuously increase as our participants get older and the number of biological samples is thus subject to change. Except for blood samples, all biological samples are self-collected by the parents. In the main cohort, routine nasal swabs and stool samples of the symptomless child are taken once a year for microbiome analyses. In the nested subcohort, routine samples are taken every 3 months in the first 2 years of life and yearly at ages 3–6 years. The collection of nasal swabs and stool samples during acute disease is triggered by signs of acute respiratory disease and signs of acute gastroenteritis, respectively. In detail, for respiratory symptoms, a swab is taken when the child shows one of the following symptoms: fever, wheezing, productive cough, or otitis media or pneumonia diagnosed by a physician, or two of the following symptoms: rhinitis, dry cough, sore throat, rigors, lack of appetite, increased need of sleep, or increased attachment according to a definition by Lambert et al.12 Swabs are taken using the Copan Liquid Amies Elution Swab (ESwab, Hain Lifescience) and transported via regular mail (typically 2–3 days) and stored at –80°C. For gastrointestinal symptoms, a sample is taken if diarrhoea occurs four or more times within 24 hours and/or vomiting occurs at least once within 24 hours. Stool samples are transported via mail and stored at –80°C in RNASepar Solution (Biosepar). For both, nose swabs and stool samples, we successfully tested the stability for up to 2 weeks at room temperature before starting collection. Four aliquots each containing at least 200 µl of the sample-storage solution mixture are available for nasal swabs and stool samples. Regarding completeness of biological samples, we have analysed the data of all 754 children (96%) who have turned 1 year old to date. In the main cohort, nasal swabs are 87% and stool samples 88% complete. In the subcohort, 67% and 75% have returned at least three of the four required nasal swabs and stool samples, respectively. Umbilical cord blood is taken whenever possible depending on the hospital chosen for birth. However, only 20 participants (3%) have provided samples, limiting the scope of research questions possible to address. Blood samples are taken by a physician within the subcohort at the age of 1 and 2 years and in all children at the age of 6 years (planned). We collect at least two aliquots each of 200 µl of serum and 500 µl of plasma and at least 3x106 peripheral blood mononuclear cells. Blood samples are stored at –80°C or in liquid nitrogen. Of 285 eligible participants in the subcohort, 150 (53%) have provided a blood sample after the first year of life. We expect blood samples in about 70% of these participants after the second year of life, resulting in around 106 complete blood sample sets from the first and second birthday (calculation based on the samples we have collected after the second year so far). Buccal swabs for genetic analyses are planned to be taken from all children (dry sterile swabs, Nuova Aptaca). We ask the parents to provide these samples when the child is 1 year old. Buccal samples are transported via regular mail and stored at –80°C. So far, 53% of the already contacted participants of the subcohort and 44% of the main cohort have provided buccal swabs. However, not all participants have been contacted yet, explaining the relatively low number in Table 2.
Table 2.
Overview of the available biological samples
| Sample typea | Main cohort (n = 497) |
Subcohort (n = 285) |
Total | ||
|---|---|---|---|---|---|
| Age (years) | n | Age (years) | n | n | |
| Nasal swab | |||||
| Symptomatic | 0–3 | 2698 | 0–3 | 2090 | 4788 |
| Asymptomatic, once per year | 0–3 | 892 | 2 –3 | 116 | 1008 |
| Asymptomatic, four times per year | – | – | 0–2 | 1533 | 1533 |
| Stool samples | |||||
| Symptomatic | 0–3 | 639 | 0–3 | 609 | 1248 |
| Asymptomatic, once per year | 0–3 | 949 | 2 –3 | 117 | 1066 |
| Asymptomatic, four times per year | – | – | 0–2 | 1653 | 1653 |
| Blood samples | |||||
| Umbilical cord blood samples | 0 | 17 | 0 | 3 | 20 |
| Whole blood | – | – | 1 | 150 | 150 |
| Whole blood | – | – | 2 | 60 | 60 |
| Genetic swabs | |||||
| 1 | 147 | 1 | 140 | 287 | |
Current sampling status as of 10/2018.
Planned use of collected data
Effects of certain infections on the immune system and the microbiome and vice versa are of special interest to us. Here, we aim to use symptomatic nasal swabs and stool samples to identify causal pathogens of respiratory and gastrointestinal symptoms. For pathogen identification, we plan to implement a wide assay including 39 respiratory and 36 gastrointestinal pathogens (Allplex™ Respiratory Panel Assays and Allplex™ Gastrointestinal Panel Assays, Seegene). Our focus is on viruses and we expect prevalence of human respiratory virus and respiratory syncytial virus as well as rotavirus, norovirus and adenovirus.13 Nevertheless, research on other causative agents of respiratory and gastrointestinal infections is planned or may evolve in future collaborations. Furthermore, all nasal swabs and stool samples will be analysed regarding changes of microbial community composition over time and with respect to influencing factors like infections, vaccinations, antibiotic intake and breastfeeding/nutrition over time. Analyses based on 16S rRNA gene region sequencing are part of the analysis plan. This can be further extended to metagenome or metatranscriptome analyses for specific questions in subsets of eligible samples. Identification of the virome and mycobiome will give insights into a holistic understanding of the overall microbial development within the first years of life. Blood samples will be used to determine cytokine and serological patterns as well as cellular immunoprofiles after infections and vaccination, but may also be analysed with regard to certain microbiota enterotypes or extrinsic factors like breastfeeding/nutrition. Buccal swabs will be used to determine the genetic background, i.e. single nucleotide polymorphisms, with regard to infection susceptibility, asthma predisposition or responsiveness to vaccinations. In the long-term, all of these data can be connected, and once asthma and atopic diseases become prevalent in the LoewenKIDS cohort, they will be linked to the development of atopic diseases and allergies. However, it is important to emphasize that we rely on laboratories of collaboration partners regarding analyses of all biological samples. Since our youngest participant is born in February 2018, we are still gathering data and samples and have not begun with the analysis of biological samples yet. We have several aliquots of each sample and are thus relatively flexible and open to providing our biological samples for different approaches and research questions.
What has it found? Key findings and publications
As the study is still ongoing, only limited findings are described here. Characteristics of parents and children of the main cohort and subcohort showed no differences, thus results are combined and reported in detail in Tables 3 and 4.
Table 3.
Data on participating parents from the basic questionnaire
| Parents | Total (n = 740) | Main cohort (n = 467) | Subcohort (n = 273) | P-value | Reference valuesa | Reference |
|---|---|---|---|---|---|---|
| Age mothers in years (mean ± SD) | 33 ± 4 | 33 ± 4 | 33 ± 4 | 0.496 | 30 | 15 |
| Age fathers in years (mean ± SD) | 35 ± 5 | 35 ± 5 | 35 ± 5 | 0.971 | n.a. | |
| Academic degree mothers | ||||||
| Others | 168 (23%) | 106 (22%) | 62 (23%) | 0.810 | 31% | 29 b |
| Apprenticeship | 110 (15%) | 67 (14%) | 43 (16%) | |||
| Master’s degree | 335 (45%) | 209 (45%) | 126 (46%) | 45% | ||
| PhD | 122 (16%) | 83 (18%) | 39 (14%) | |||
| 22% | ||||||
| Missing | 5 (1%) | 3 (1%) | 2 (1%) | |||
| 2% | ||||||
| Academic degree fathers | ||||||
| Others | 176 (24%) | 106 (23%) | 70 (25%) | 0.430 | 30% | 16 b |
| Apprenticeship | 119 (16%) | 84 (18%) | 35 (13%) | |||
| 48% | ||||||
| Master’s degree | 341 (46%) | 211 (45%) | 130 (48%) | |||
| PhD | 89 (12%) | 57 (12%) | 32 (12%) | 20% | ||
| Missing | 15 (2%) | 10 (2%) | 5 (2%) | |||
| 2% | ||||||
| Monthly household net income in Euro | ||||||
| Unknown/ n.a. | 104 (14%) | 77 (16%) | 27 (10%) | 0.055 | 4761c | 16 |
| <3000 | 127 (17%) | 88 (19%) | 39 (14%) | |||
| 3000–3999 | 166 (22%) | 102 (22%) | 64 (24%) | |||
| 4000–5000 | 154 (21%) | 91 (20%) | 36 (23%) | |||
| >5000 | 183 (25%) | 106 (22%) | 77 (28%) | |||
| Missing | 6 (1%) | 4 (1%) | 2 (1%) | |||
| First pregnancy | ||||||
| Yes | 467 (63%) | 298 (64%) | 169 (62%) | 0.675 | - | - |
| No | 273 (37%) | 170 (36%) | 103 (38%) | |||
| Siblings | ||||||
| Yes | 253 (34%) | 159 (34%) | 94 (35%) | 0.779 | - | - |
| No | 481 (65%) | 306 (65%) | 175 (64%) | |||
| Missing | 6 (1%) | 3 (1%) | 6 (1%) | |||
| Pets in the householdd | ||||||
| None | 465 (63%) | 297 (64%) | 168 (62%) | 0.645 | 43% | 18 |
| Cats | 116 (16%) | 68 (15%) | 48 (18%) | 0.261 | 12% | |
| Dogs | 75 (10%) | 52 (11%) | 23 (9%) | 0.249 | 12% | |
| Others | 98 (13%) | 61 (13%) | 37 (14%) | 0.826 | 10% | |
| Selected diseases in family historyd | ||||||
| Asthma | 239 (32%) | 139 (30%) | 100 (37%) | 0.048 | 9% | 19 , 21 , 22 |
| Diabetes type I | 42 (6%) | 30 (6%) | 12 (4%) | 0.257 | ||
| 7%e | ||||||
| Diabetes type II | 155 (21%) | 98 (21%) | 57 (21%) | 0.996 | ||
| Rheumatoid arthritis | 124 (17%) | 70 (15%) | 54 (20%) | 0.086 | <1% | |
| Lupus erythematosus | 11 (2%) | 5 (1%) | 6 (2%) | 0.218 | ||
| <1% | ||||||
| Neurodermatitis | 309 (42%) | 186 (40%) | 123 (45%) | 0.145 | ||
| 4% | ||||||
| Allergies in family historyd | ||||||
| Hay fever | 507 (69%) | 313 (67%) | 194 (71%) | 0.210 | 15% | 19 , 23 |
| House dust mite | 354 (48%) | 219 (47%) | 135 (50%) | 0.456 | 15%f | |
| Pet hair | 343 (46%) | 208 (44%) | 135 (50%) | 0.172 | 9%f | |
| Food intolerances in family historyd | ||||||
| Lactose | 169 (23%) | 105 (22%) | 64 (24%) | 0.733 | 15% | 24 , 25 |
| Fructose | 44 (6%) | 28 (6%) | 16 (6%) | 0.956 | n.a. | |
| Gluten (coeliac disease) | 38 (5%) | 20 (4%) | 18 (7%) | 0.164 | <1% | |
Reference values from the German general population.
Only age groups 30–40 were considered based on the age of our study population at childbirth.
Mean value.
Multiple entries were possible.
Diabetes mellitus type I and II combined.
Allergy sensitization only.
Differences between main cohort and subcohort were assessed using either the Chi2 test or the Student’s t-test. Of all 782 participants, a total of 740 basic questionnaires were available.
SD, standard deviation; n.a., not available.
Table 4.
Data on participating children from the birth questionnaire
| Children | Total (n = 715) | Main cohort (n = 452) | Subcohort (n = 263) | P-value | Reference valuesa | Reference |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 351 (49%) | 223 (49%) | 128 (49%) | 0.975 | 49% | 26 |
| Male | 356 (50%) | 224 (50%) | 132 (50%) | 51% | ||
| Missing | 8 (1%) | 5 (1%) | 3 (1%) | |||
| Birth weight (median; IQR) | ||||||
| Female | 3325 g; | 3330 g; | 3325 g; | 0.387 | 3390 g | 27 |
| 3027–3610 g | 3070–3600 g | 2995–3645 g | ||||
| Male | 3500 g; | 3490 g; | 3535 g; | 0.735 | 3530 g | |
| 3230–3800 g | 3210–3795 g | 3240–3800 g | ||||
| Birth length (median; IQR) | ||||||
| Female | 51 cm; | 51 cm; | 51 cm; | 0.653 | 51 cm | 27 |
| 50–52 cm | 50–52 cm | 50–52 cm | ||||
| Male | 52 cm; | 52 cm; | 52 cm; | 0.865 | 52 cm | |
| 50–53 cm | 50–53 cm | 51–53 cm | ||||
| Weight depending on gestational ageb | ||||||
| <10th percentile | 58 (8%) | 33 (7%) | 25 (9%) | 0.572 | n.a. | n.a. |
| 10th – 90th percentile | 602 (84%) | 383 (85%) | 219 (83%) | |||
| >90th percentile | 39 (6%) | 27 (6%) | 12 (5%) | |||
| Missing | 16 (2%) | 9 (2%) | 7 (3%) | |||
| Length depending on gestational ageb | ||||||
| <10th percentile | 26 (4%) | 16 (3%) | 10 (4%) | 0.663 | n.a. | n.a. |
| 10th – 90th percentile | 530 (74%) | 333 (74%) | 197 (75%) | |||
| >90th percentile | 140 (19%) | 93 (21%) | 47 (18%) | |||
| Missing | 18 (3%) | 10 (2%) | 9 (3%) | |||
| Mode of birth | ||||||
| Spontaneous | 473 (66%) | 305 (68%) | 168 (64%) | 0.749 | 61% | 28 |
| Forceps/suction | 50 (7%) | 29 (6%) | 21 (8%) | 7% | ||
| C-section (planned+unplanned) | 187 (27%) | 114 (26%) | 73 (28%) | 32% | ||
| Missing | 3 (0%) | 2 (0%) | 1 (0%) | |||
| Birth institution | ||||||
| Hospital, inpatient | 649 (91%) | 403 (89%) | 246 (94%) | 0.235 | n.a. | n.a. |
| Hospital, outpatient | 42 (6%) | 30 (7%) | 12 (4%) | |||
| Birthing centre | 13 (2%) | 10 (2%) | 3 (1%) | |||
| At home | 11 (1%) | 9 (2%) | 2 (1%) | |||
| Hospital admisssion in first 2 weeks | ||||||
| Yes | 77 (11%) | 46 (10%) | 31 (12%) | 0.736 | n.a. | n.a. |
| No | 636 (89%) | 405 (90%) | 231 (88%) | |||
| Missing | 2 (0%) | 1 (0%) | 1 (0%) | |||
Reference values from the German general population.
Percentile placement derived from the Fenton 2013 Preterm Growth Chart.20
Differences between main cohort and subcohort were assessed using the Chi2 test or the Wilcoxon test. Of all 782 participants, a total of 715 birth questionnaires were available.
IQR, interquartile range; n.a, not available.
The mean age of mothers and fathers at birth was 33 years [standard deviation (SD) ± 4 years] and 35 years (SD ± 5 years), respectively (Table 3). The average age of German mothers at birth is 30 years (not available for fathers).15 Our mothers are thus older than average. Concerning the parents’ education, 15% and 16% of the mothers and fathers completed an apprenticeship (German ‘Lehre’), 45% and 46% held a master’s degree or diploma and 16% and 12% a PhD (Table 3). In contrast, 45% of the German general population between 30 and 40 years of age completed an apprenticeship, 22% a master’s degree or diploma and only 2% hold a PhD.29 Our study is thus characterized by a highly educated study population, explaining the age of mothers at birth being above average. Furthermore, LoewenKIDS participants may have been intrigued to take part in our study because of their own background in academia. Of our study participants, 39% had a monthly household net income lower than 4000 Euro, 21% between 4000 and 5000 Euro and 25% above 5000 Euro (15% missing or unknown). With the average monthly net houshold income being 4761 Euro in Germany, the household income of our cohort is approximately average despite a high education level.16
Most prevalent diseases in the family histories of participating parents were allergies, with 69% affected by hay fever, 48% affected by house dust mite allergy and 46% affected by pet hair allergy. Lactose intolerance was the most common food intolerance with 23% of families affected. In the German general population, 15% are affected by hay fever and less by house dust mite and pet hair allergy, thus families of our cohort may be especially affected by allergies.17 Furthermore, neurodermatitis, asthma and diabetes type II were highly prevalent in the family histories of our study participants (42%, 32% and 21%, respectively). In the German general population over all age groups, the prevalences of neurodermatitis, asthma and diabetes (type I and II) are 4%, 9% and 7%, respectively.17,19 The high prevalence observed in our study may partly be due to biased sampling and an increased willingness to participate in parents with a history of these illnesses. However, it may also partly be due to the fact that we have asked for the complete family history, meaning diseases in parents, aunts and uncles, and grandparents of the participants.
In the cohort, 66% of the births were spontaneous, 27% had planned or unplanned C-sections (9% and 18%, respectively) and 7% were forceps- or vacuum-assisted deliveries, which is in line with data from the German general population (Table 4). Considering gestational age at birth and the <10th percentile and >90th percentile for weight according to Fenton and Kim,20 601 (84%) of the newborns had average weight, 39 (6%) were large for gestational age and 58 (8%) were small for gestational age, Table 4. Considering gestational age at birth and the same percentiles for length, 529 (74%) of the newborns were of average length, 140 (19%) above and 26 (4%) below averange length.
What are the main strengths and weaknesses?
LoewenKIDS is the first study to prospectively explore the sequence and cumulative load of infections in early childhood. Within this study, nasal and stool samples for microbiome and other omics analyses, blood samples for immune system analyses as well as buccal samples for genetic analyses are collected, providing an extensive view of biological processes during the first 6 years of life. Symptom diaries and multiple questionnaires covering a broad range of factors of the infantile development complete this holistic approach. The life-course perspective of this study enables a follow-up beyond the intensively monitored first 6 years of life that can provide valuable information on long-term effects. Similar to our study, the ORChiD study collected data from 154 children for 2 years in Brisbane, Australia. We designed the LoewenKIDS symptom diary to be similar to the one used in the ORChiD study and it will be interesting to compare or even combine our results with those obtained in Brisbane. In addition, we are looking forward to other suggestions for collaboration.
Due to the intensive study requirements we will be able to receive full datasets of only a fraction of participants for which we can combine all measured elements. Nevertheless, a higher number of complete data is available for several possible sub-analyses. As for biological samples, we were not able to collect samples more frequently. Therefore, highly dynamic events and variations in the microbiome will not be possible to assess. Basic characteristics of our study population are similar to the German general population except the mother’s age at birth, a higher education level and more allergic diseases in the family histories of mothers and fathers. However, our cohort holds enough participants with lower education levels and without diseases in the family histories for us to be able to assess possible effects derived from these selection biases.
Profile in a nutshell
LoewenKIDS is a birth cohort study prospectively assessing infectious diseases, the development of the microbiome and immune system as well as effects on the development of immune-mediated diseases like atopic dermatitis, allergic asthma and allergies during childhood and later life.
Children within the cohort were born from November 2014 to February 2018 and recruited in multiple centres throughout Germany [Braunschweig, Bremen, Halle (Saale), Hannover, Munich].
At baseline, the study included 782 new-born children, 285 thereof in an intensified nested subcohort.
Children are followed up until the age of 6 years with the option of further study participation.
Data are collected via symptom diaries, questionnaires and biological samples (nasal swabs, stool samples, buccal swabs, blood samples).
Collaborations are intended. Data are held by the LoewenKIDS research team and access can be applied for.
Can I get hold of the data? Where can I find out more?
The data are stored at the LoewenKIDS management centre. The reseach team is looking forward to potential collaborations, exchange of ideas, and suggestions. Access to the data can be obtained using the contact form on the website www.loewenkids-study.de and an application template. We are interested in collaborations based on specific expertise, particularly extending our own possibilities. The use of the data and samples must be approved and prioritized by our scientific steering committee.
Chief investigator: Prof. Dr med. Rafael Mikolajczyk.
Funding
This work was supported by the Helmholtz Centre for Infection Research (Braunschweig, Germany) and the Martin-Luther-University Halle-Wittenberg [Halle (Saale), Germany].
Conflict of interest: None declared.
References
- 1. Holt PG, Jones CA.. The development of the immune system during pregnancy and early life. Allergy 2000;55:688–97. [DOI] [PubMed] [Google Scholar]
- 2. Pirruccello SJ, Collins M, Wilson JE, McManus BM.. Age-related changes in naive and memory CD4+ T cells in healthy human children. Clin Immunol Immunopathol 1989;52:341–5. [DOI] [PubMed] [Google Scholar]
- 3. Ygberg S, Nilsson A.. The developing immune system - from foetus to toddler. Acta Paediatr 2012;101:120–7. [DOI] [PubMed] [Google Scholar]
- 4. McDade TW, Beck MA, Kuzawa C, Adair LS.. Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. Am J Clin Nutr 2001;74:543–8. [DOI] [PubMed] [Google Scholar]
- 5. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012;18:673–83. [DOI] [PubMed] [Google Scholar]
- 6. Jackson DJ, Gern JE, Lemanske RF Jr. Lessons learned from birth cohort studies conducted in diverse environments. J Allergy Clin Immunol 2017;139:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartlett NW, McLean GR, Chang YS, Johnston SL.. Genetics and epidemiology: asthma and infection. Curr Opin Allergy Clin Immunol 2009;9:395–400. [DOI] [PubMed] [Google Scholar]
- 8. Stene LC, Rewers M.. Immunology in the clinic review series; focus on type 1 diabetes and viruses: the enterovirus link to type 1 diabetes: critical review of human studies. Clin Exp Immunol 2012;168:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamada T, Hara K, Kadowaki T.. Association of adenovirus 36 infection with obesity and metabolic markers in humans: a meta-analysis of observational studies. PLoS One 2012;7:e42031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bousquet J, Gern JE, Martinez FD. et al. Birth cohorts in asthma and allergic diseases: report of a NIAID/NHLBI/MeDALL joint workshop. J Allergy Clin Immunol 2014;133:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larkin EK, Gebretsadik T, Moore ML. et al. Objectives, design and enrollment results from the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study (INSPIRE). BMC Pulm Med 2015;15:45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lambert SB, Allen KM, Druce JD. et al. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens. Pediatrics 2007;120:e929–37. [DOI] [PubMed] [Google Scholar]
- 13. Zoch B, Karch A, Dreesman J, Monazahian M, Baillot A, Mikolajczyk RT.. Feasibility of a birth cohort study dedicated to assessing acute infections using symptom diaries and parental collection of biomaterials. BMC Infect Dis 2015;15:436.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zoch B, Gunther A, Karch A, Mikolajczyk R.. Effect of disease definition on perceived burden of acute respiratory infections in children: a prospective cohort study based on symptom diaries. Pediatr Infect Dis J 2017;36:956–61. [DOI] [PubMed] [Google Scholar]
- 15.(Destatis) SB. Statistisches Jahrbuch 2018 - Kapitel 2 Bevölkerung, Familien, Lebensformen 2018. https://www.destatis.de/DE/Publikationen/StatistischesJahrbuch/StatistischesJahrbuch.html (12 November 2018, date last accessed).
- 16.(Destatis) SB. Einkommen, Einnahmen & Ausgaben 2016. https://www.destatis.de/DE/ZahlenFakten/GesellschaftStaat/EinkommenKonsumLebensbedingungen/EinkommenEinnahmenAusgaben/EinkommenEinnahmenAusgaben.html (12 November 2018, date last accessed).
- 17. Langen U, Schmitz R, Steppuhn H.. Prevalence of allergic diseases in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:698–706. [DOI] [PubMed] [Google Scholar]
- 18.Bevölkerung in Deutschland mit Tieren im Haushalt nach Tierart von 2014 bis 2018 (Personen in Millionen). 2018. Available from: https://de.statista.com/statistik/daten/studie/170901/umfrage/haustiere-im-haushalt/ (12 November 2018, date last accessed).
- 19. Heidemann C, Du Y, Schubert I, Rathmann W, Scheidt-Nave C.. Prevalence and temporal trend of known diabetes mellitus: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:668–77. [DOI] [PubMed] [Google Scholar]
- 20. Fenton TR, Kim JH.. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013;13:59.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuhn A, Bonsmann G, Anders H-J, Herzer P, Tenbrock K, Schneider M.. Diagnostik und Therapie des systemischen Lupus erythematodes. Dtsch Arztebl Int 2015;112:423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schneider M, Krüger K.. Rheumatoide arthritis – Frühdiagnose und Krankheitskontrolle. Dtsch Arztebl Int 2013;110:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haftenberger M, Laußmann D, Ellert U. et al. Prevalence of sensitisation to aeroallergens and food allergens. Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung 2013. [Google Scholar]
- 24. Flatz G, Howell JN, Doench J, Flatz SD.. Distribution of physiological adult lactase phenotypes, lactose absorber and malabsorber, in Germany. Hum Genet 1982;62:152–7. [DOI] [PubMed] [Google Scholar]
- 25. Schuppan D, Zimmer KP.. The diagnosis and treatment of celiac disease. Dtsch Arztebl Int 2013;110:835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geburten. 2017. Available from: https://www.destatis.de/DE/ZahlenFakten/GesellschaftStaat/Bevoelkerung/Geburten/Geburten.html (12 November 2018, date last accessed).
- 27.Robert-Koch-Institut. Beiträge zur Gesundheitsberichterstattung des Bundes: Referenzperzentile für anthropometrische Maßzahlen und Blutdruck aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS). 2013; 2.
- 28.Gesundheitswesen IfQuTi. Qualitätsreport. 2017.
- 29.(Destatis) SB, Bildungsstand der Bevölkerung 20162017. https://www.destatis.de/DE/Publikationen/Thematisch/BildungForschungKultur/AlteAusgaben/BildungsstandBevoelkerungAlt.html (26 November 2018, date last accessed).