Abstract
A distinct difference between veterinary and human medicine is the routine use of antimicrobial mass medications (prophylaxis, metaphylaxis) to healthy individuals. The need for antimicrobial mass medications is based on beliefs that group/s of animals will contract a bacterial disease (i.e. morbidity) and/or die (i.e. mortality). Bovine respiratory disease (BRD) represents the major indication for cattle antimicrobials worldwide. The objectives were to perform a systematic review and meta-analysis of randomised controlled clinical trials (RCTs) for naturally occurring BRD investigating antimicrobial prophylaxis/metaphylaxis to prevent morbidity/mortality. In total, 58 publications met the inclusion criteria summarizing 169 individual RCTs, spanning 50 years (1966–2016). Antimicrobial prophylaxis and metaphylaxis demonstrated moderate, yet highly variable relative risk reductions in BRD morbidity. These were dependent on the antimicrobial classes used, dependent on metaphylaxis definition, BRD attack rates and duration of the RCTs. Best relative risk reductions were from broad-spectrum critically important antimicrobials, or combinations. BRD prophylaxis/metaphylaxis represents major antimicrobial consumption for highly variable short-term gains in absolute risk reduction of morbidity/mortality. Despite widespread use of prevention products, the need for antimicrobial mass medications should be re-evaluated since the underlying problem is more likely the segmented infrastructure of the feedlot and veal calf industries compared to the disease itself.
Keywords: antimicrobial, prophylaxis, metaphylaxis, bovine
A systematic review and meta-analysis of randomised clinical trials investigating antimicrobial prophylaxis or metaphylaxis against naturally occurring bovine respiratory disease.
INTRODUCTION
Bovine respiratory disease (BRD) complex is the most common and extensively studied cattle disease of all ages, especially feedlot cattle and veal calves. BRD represents the major indication for cattle antimicrobials worldwide, especially for prophylaxis and metaphylaxis. Coupled with mortalities, treatment costs, decreased performance and reduced carcass value, BRD leads to major economic losses for cattle feeders (Ives and Richeson 2015).
The complex of viral, bacterial and/or mycoplasmal infections is described with blanket terms, ‘enzootic pneumonia’, ‘shipping fever’ or ‘bovine respiratory disease complex’, often used without precise definitions. Viruses and other stressors predispose cattle to opportunistic bacterial pneumonias, including bovine respiratory syncytial virus, parainfluenzavirus-3, infectious bovine rhinotracheitis virus, bovine herpes virus-1 and possibly bovine coronavirus (O’Neill et al.2014). These viruses trigger BRD by damaging upper respiratory tract mucosa and/or modifying host pro- and anti-inflammatory immune responses. Bacterial BRD pathogens include Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, Arcanobacterium pyogenes, Mycoplasma dispar and M. bovis that exist commonly as commensals, often as biofilms within the upper respiratory tract and tonsils.
Classical BRD field diagnosis are visual signs, including depression, anorexia, nasal and/or ocular discharge, coughing, laboured breathing (Buhman et al.2000) or a semi-objective four-point clinical illness score (Perino and Apley 1998). This is subjective, non-standardised but the typical basis for deciding on further antimicrobials treatments, or combined with rectal temperature, although bovine fever thresholds vary throughout the scientific literature (Ives and Richeson 2015). Comparing BRD field diagnostics to pulmonary lesions evident at slaughter reveals several cattle (e.g. >50%) with lung lesions, not previously identified and treated for BRD (Thompson, Stone and Schultheiss 2006; Tennant et al.2014). These limitations contribute largely to the justification for routine antimicrobial prophylaxis/metaphylaxis in place of identifying, pulling and treating sick calves/cattle. The culmination of stressors (e.g. recently weaned, auctioned, transported, environmental/nutritional changes, co-mingling, castrated) results in each cohort of feedlot cattle/veal calves expressing the majority of visual BRD cases (up to 90%) within the first 27 days after arrival (Edwards 1996; Buhman et al.2000).
Soon after the introduction of antimicrobial agents, their growth-promoting effects were discovered in animals (Moore et al.1946). Antimicrobials were approved for growth promotion in the USA since 1949 and since 1953 in the UK (Swann 1969). However, mounting evidence about antimicrobial resistance and transference of resistance genes from animal to human microbiota led to a full withdrawal of antimicrobial growth promoters in the European Union, since 2006 (Regulation 1831/2003). The European support to recommendations of the World Health Organization (WHO), the Food and Agriculture Organization (FAO) and the World Organization or Animal Health for a ban on antimicrobial growth promoters has encouraged other countries to follow (e.g. Canada, USA, South Korea). Since the ban, then antimicrobial ‘mass medications’ for the purposes of disease prevention has become the most common use in healthy food animals.
Different terms describe ‘mass medication’ concepts in food animals, such as prophylaxis, prevention and metaphylaxis. Antimicrobial prophylaxis for BRD first appeared in the scientific literature in the 1950s (King et al.1955), and metaphylaxis appeared later in the 1980s. Both concepts are believed to still be heavily used, but exact consumption figures for food animal antimicrobial mass medications are largely unknown.
Various definitions describe prophylaxis, prevention and metaphylaxis. Prophylaxis and prevention are defined similarly as the administration of a veterinary medicinal product (VMP) to healthy animals to prevent infection/s based on risk or possible consequences (ECDC/EFSA/EMA 2015; EMA 2016). Typically, the risk is neither clearly defined nor quantified, but common reasons include:
previous history of herd outbreaks
- traditional herd management practices or attitudes:
- introduction of new animals into groups (e.g. ‘welcome shots’)
- high stocking densities (i.e. increased ‘risk’ of disease)
- scheduled events in the production animal cycle (e.g. dry-off cow period, transport)
- stressful events (e.g. weaning, parturition, castration, dehorning)
- diet quality problems or diet changes
- intercurrent disease—e.g. viral or protozoal diseases
Originally, ‘metaphylaxis’ was defined the same as prophylaxis, the difference being that prophylaxis was applied to individuals and metaphylaxis was for whole groups/flocks/herds (Urban-Chmiel and Grooms 2012). Now, others re-define ‘metaphylaxis’ as mass medication of healthy animals when the disease of interest is present within the group/flock/herd (EMA 2016). Others define as, ‘Metaphylaxis is indicated for high-risk individuals, when the number of clinical cases within a group reaches a threshold, the remainder of the in-contact animals are treated simultaneously in order to restrict the spread and impact of the disease’ (Lees and Shojaee Aliabadi 2002). According to Edwards (2010) and Smith et al. (2001), the criterion for antimicrobial metaphylaxis occurs when morbidity (i.e. attack rate) exceeds 10% for two to three consecutive days. Thus, prophylaxis/prevention and metaphylaxis involves administering antimicrobials to ‘healthy’ individuals to ‘prevent’ infections, but for prophylaxis/prevention there is a perceived ‘risk’, whereas metaphylaxis could be a definable ‘hazard’.
Routine antimicrobial prophylaxis/metaphylaxis are uniquely veterinary concepts since equivalent modern examples in human medicine of routine antimicrobial mass medications to healthy individuals would be difficult to specify. Routine prophylaxis/metaphylaxis leads to substantial antimicrobial consumption since ‘healthy’ individuals will always outnumber sick individuals, in all but exceptional situations. Many food animal antimicrobial VMP formulations are designed for easy mass medication, for dissemination in either bulk animal feed or common drinking water. Since it is considered ‘inconvenient’ or impractical to separate diseased from healthy animals, under intensive livestock production conditions, then routine prophylaxis/metaphylaxis is employed commonly, even if unintended. For example, assuming antimicrobial VMPs for dissemination in either bulk animal feed or drinking water are preferred for prophylaxis/metaphylaxis (e.g. premixes, oral powders/granules/solutions for drinking water) then European data (29 countries for 2014: overall sales = 9,009.5 tonnes of active ingredient of antimicrobials) reveals just over 90% of antimicrobial sales reported, in mg/PCU (Population correction unit, in 1,000 tonnes : The estimated weight at treatment of livestock and of slaughter animals.), were these VMP formulations (ESVAC 2016). Furthermore, injectable antimicrobials can also be used for prophylaxis/metaphylaxis in food animals.
Evidence-based recommendations for food animal antimicrobial mass medications are sparse but mostly embody the antithesis of prudent use. This is at odds with the pleas of the United Nations, WHO, FAO and OIE calling for rational antimicrobial use in food animals. The need for antimicrobial mass medications is based on beliefs that a group/s of animals (especially newly received, stressed) will contract a major bacterial disease (i.e. morbidity) and/or die (i.e. mortality), resulting in economic and welfare consequences. Ancillary benefits are better average daily weight gains (i.e. growth promotion) and belief that less antimicrobial treatments are required later in the production cycle (i.e. reduced labour costs). Given the growing recognition of the one health agenda that animal and human systems are connected, it is relevant to better understand/question the original scientific evidence for antimicrobial prophylaxis/metaphylaxis in food animals to reduce morbidity and mortality from bacterial diseases. Beef production (feedlots or veal) is one of the world's largest food animal industries, and thus an appropriate choice for investigating antimicrobial prophylaxis/metaphylaxis.
The objectives were to perform a systematic review and meta-analysis of randomised controlled clinical trials (RCTs) investigating antimicrobial prophylaxis and/or metaphylaxis for preventing naturally occurring BRD. The primary objective was to assess RCTs investigating BRD antimicrobial prophylaxis/metaphylaxis for their impact on preventing morbidity. The secondary objective was to assess RCTs investigating BRD antimicrobial prophylaxis/metaphylaxis for their impact on preventing mortality. Other objectives were to assess the characteristics of antimicrobials (e.g. type, route of administration) on preventing BRD morbidity and mortality and compare prophylaxis to metaphylaxis.
MATERIALS AND METHODS
An open literature search involved online search engines, including Pub-Med, Google Scholar, Scopus, Web-of-Science, CAB abstracts and VetMed Resource, using the following search terms:
| At least one of: | cattle, calf, calve, veal, bovine, cow, steer, bull, |
| AND at least one of: | antibiotic, antimicrobial, antibacterial, cillin, mycin, cycline, sulpha, floxacin, ceftiofur, tilmicosin, tildipirosin, florfenicol, trimethoprim, tylosin |
| AND at least one of: | respiratory, shipping, pneumonia, |
| AND at least one of: | prophylaxis, prophylactic, prevent, prevention, preventive, preventative, control, metaphylaxis, metaphylactic |
Google searches were also performed to find other sources of potential publications. Prophylaxis and prevention were defined as the same type of mass medication.
Inclusion criteria for the publications were as follows:
Clinical trial involving visually healthy cattle comparing an antimicrobial prophylaxis/metaphylaxis group against a negative control group for naturally occurring BRD.
No antimicrobials administered to cattle immediately prior to transport or start of the trial.
Explanation of the establishment of the negative control group (e.g. group of cattle either not treated or given a placebo).
No antimicrobials given to healthy individuals in the negative control group during the course of the clinical trial.
-
Criteria (definition) for either prophylactic or metaphylactic medications. Prophylaxis was defined as group medication of asymptomatic cattle upon ‘arrival’ at the test facility. Too few publications were identified as defining prophylaxis as group medication ‘prior’ to transport of cattle, and thus excluded from this investigation. Definitions for metaphylaxis varied between publications, including:
- group medication of asymptomatic cattle upon arrival at the test facility.
- group medication of cattle with pyrexia and no other symptoms.
- group medication of cattle in contact with clinical BRD cattle.
- group medication of cattle when the BRD morbidity within the group ≥10%.
Publications defining metaphylaxis as group medication of asymptomatic cattle upon arrival were re-defined as prophylaxis to be consistent with the other prophylaxis RCTs. All other definitions were accepted as metaphylaxis.
Specification of randomisation in the study design. Randomisation was accepted if individuals were randomised to groups or pens of cattle were randomly chosen (i.e. random-block design). According to Perino and Apley (1998), every-other-calf or odd-and-even number schemes as cattle arrived and processed into pens were accepted as randomisation (i.e. systematic randomisation). The intent was not to bias cattle allocations but to serve as a practical method under field conditions. Random but unequal allocations were also acceptable if the allocations did not exceed a 2-to-1 ratio.
Disclosure of all treatments given during the clinical trial (i.e. type of antimicrobial, dosing regimen, route of administration, other medications).
Disclosure of relevant results for the primary objective of the study (i.e. total number of cattle in each group, morbidity data, animals excluded).
Other potential publications were identified by examining the reference lists of obtained articles. These in turn were further examined using the criteria described above. English translations were solicited for articles published in languages other than English.
Publications were excluded:
If clinical trials were focused on the treatment of clinical BRD cattle and not prophylaxis and/or metaphylaxis of visually healthy cattle.
If there was no negative control group as part of the study design.
If antimicrobials were given to healthy cattle in the negative control group (e.g. premixes) during the course of the clinical trial.
If the clinical trial involved artificial BRD infections instead of naturally occurring BRD.
If randomisation was not described as part of the study design.
If data could not be extracted for the primary objective of the study.
If other diseases occurred during the clinical trial and specific BRD morbidity could not be derived from the results.
Descriptive and quantitative data were extracted from each RCT described in publications meeting the inclusion criteria. Publications describing more than one RCT were specified with separate lines (rows) of data. Some publications described the RCTs performed at multiple geographically separate sites; if negative control and treatment groups were reported for each test facility, then separate lines of data were specified. If accepted RCTs were published in both a peer-reviewed and other types of articles, then only the peer-reviewed article was used.
Data extracted from each publication included:
- Total number of cattle in control and treatment groups.
- BRD morbidity after the observation period for control and treatment groups.
- BRD mortality after the observation period for control and treatment groups.
- Type of production system (e.g. feedlot, veal calf facility, etc.).
- Type and definition of mass medication (prophylaxis versus metaphylaxis).
- Description of randomisation method, if provided.
- Specification if blinding or double blinding was part of the study design. Blinding could have included personnel giving cattle treatments and/or personnel checking pens during the observation period.
- Disease definition of BRD used for morbidity data.
- Antimicrobial/s given to the treatment group (type, route of administration, dose).
- Observation period (days) of RCTs.
Data analysis
Analysis of the morbidity data was performed with a random effects model (R package ‘metafor’ 1.9–8) (Viechtbauer 2010) using restricted maximum likelihood as the method for estimation of τ2 (the true between-study variance). Heterogeneity between studies was identified by the I2 and τ2 statistics. Due to the heterogeneity between studies, the summary effect measure (in casu relative risk-RR) was calculated using random effect meta-analysis with inverse-variance weights. Potential factors affecting heterogeneity were analysed with a random effects meta-regression. Separate analysis was performed for morbidity and mortality data, respectively.
Data distribution from the RCTs was assessed with funnel plots (Viechtbauer 2010). Also, a trim-and-fill analysis (Duval and Tweedie 2000) was performed on the residuals using the trimfill function in metafor to test for funnel plot asymmetry and identify missing studies. The analysis assumes funnel plot symmetry and attempts to remove (trimming) smaller studies associated with asymmetry while filling the distribution with missing studies to balance the distribution.
RCTs morbidity data were further assessed using the number needed to treat (NNT = 1/ARR) and absolute risk reduction (ARR). To calculate the expected relation between the NNT and attack rate (CER), the following expression was used (Fig. 4):
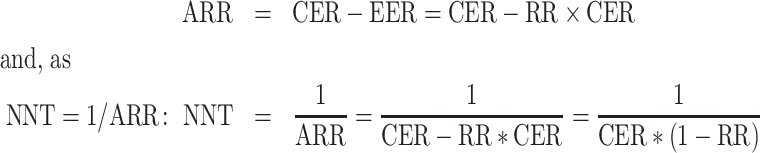 |
where CER is the control group event rate, EER is the experimental group event rate and RR is the relative risk estimate.
Figure 4.
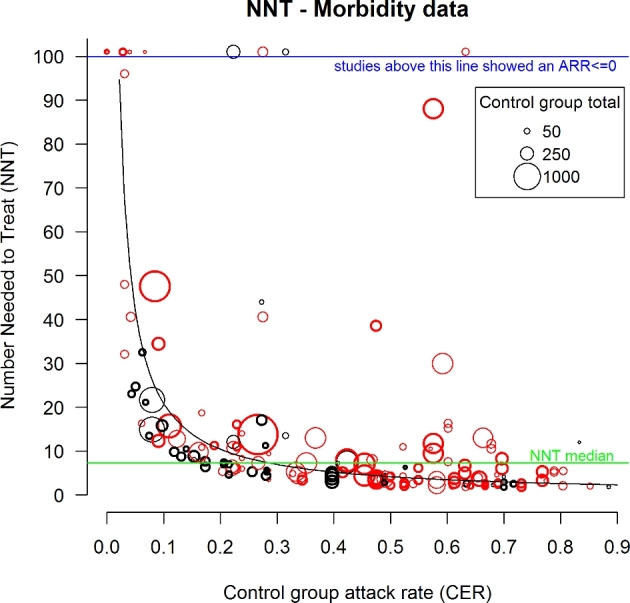
NNT plot of all morbidity data from RCTs involving either antimicrobial prophylaxis (red circles) or metaphylaxis (black circles). Size of the circles reflects the sample size of the RCT (see the legend). Bolded circles are blinded RCTs. Green line represents the overall NNT median value = 7.27. The curve represents the expected NNT as a function of CER assuming a uniform RR (=0.52) across all values of CER. NNT, number needed to treat; ARR, absolute risk reduction; RCT, randomised clinical trial.
Mortality data differed from morbidity data in that most studies showed very low mortality rates with several zero-event cells for mortality in the control and/or treatment groups. Therefore, mortality data were analysed with the Mantel-Haenszel method to calculate the summary RR and 95% confidence intervals (CI) (Higgins and Green 2011). For studies with zero-events, a value of 0.5 was added to all cells for zero-cell correction.
RESULTS
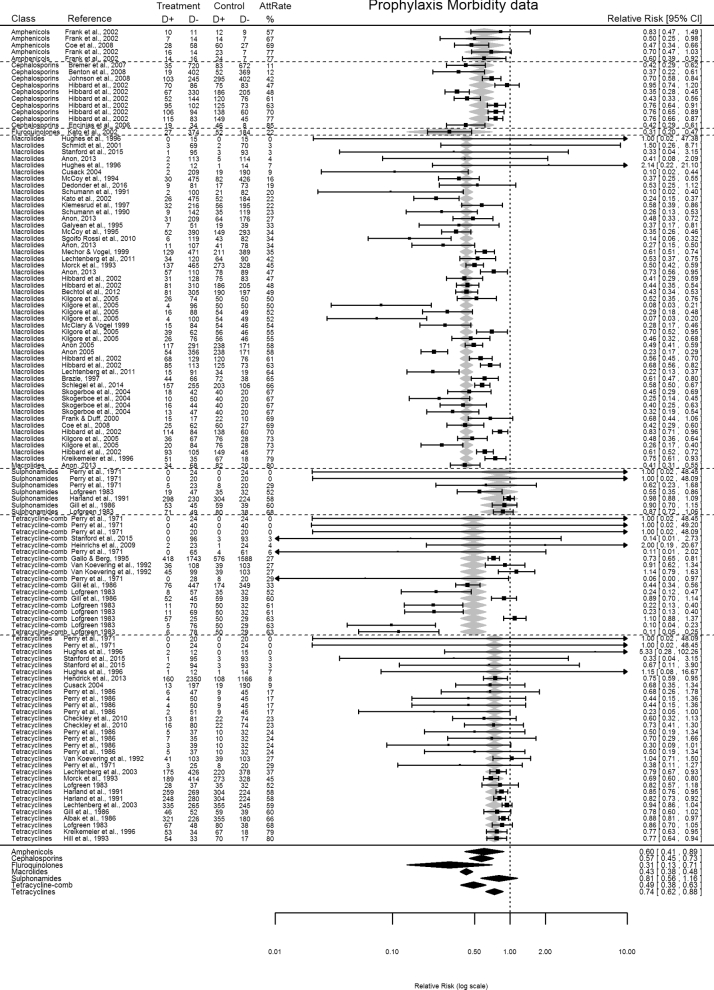
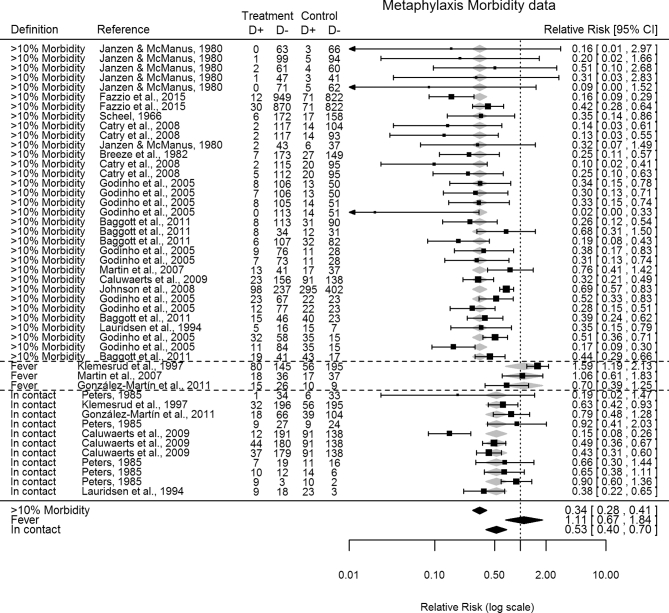
Each online search engines identified several publications, ranging from 62 to 706 titles/abstracts. A total of 58 publications (38 peer-reviewed + 5 international conferences + 15 other sources) met the criteria for the primary objective, summarizing 169 individual RCTs, and published between 1966 and 2016 (Scheel 1966; Perry et al.1971, 1986; Janzen and McManus 1980; Breeze et al.1982; Lofgreen 1983; Peters 1985; Albak, Bradstock and Cruise 1986; Gill et al.1986; Schumann, Janzen and McKinnon 1990; Harland et al.1991; Schumann, Janzen and McKinnon 1991; Van Koevering et al.1992; Hill, Gill and Ball 1993; Morck et al.1993; Lauridsen, Jorgensen and Olsen 1994; McCoy et al.1994, 1995; Gallo and Berg 1995; Galyean, Gunter and Malcolm-Callis 1995; Hughes, Tice and O’Connor 1996; Kreikemeier, Stokka and Marston 1996; Brazle 1997; Klemesrud et al.1997; McClary and Vogel 1999; Mechor and Vogel 1999; Frank and Duff 2000; Schmidt et al.2001; Frank et al.2002; Hibbard et al.2002; Kato et al.2003; Lechtenberg and Hanna 2003; Cusack 2004; Skogerboe et al.2004; Anonymous 2005, 2013; Godinho et al.2005; Kilgore et al.2005; Encinias et al.2006; Bremer et al.2007; Martín et al.2007; Benton et al.2008; Catry et al.2008; Coe et al.2008; Johnson et al.2008; Caluwaerts et al.2009; Heinrichs et al.2009; Checkley et al.2010; Sgoifo Rossi et al.2010; Baggott et al.2011; González-Martín et al.2011; Lechtenberg et al.2011; Bechtol et al.2012; Hendrick, Bateman and Rosengren 2013; Schlegel et al.2014; Fazzio et al.2015; Stanford et al.2015; Dedonder et al.2016). Other sources of publications included proprietary data described in two Freedom of Information Act summaries (3 RCTs), nine university research progress reports (18 RCTs) and four pharmaceutical companies technical bulletins (20 RCTs). Prophylaxis RCTs included 45 publications (27 peer-reviewed–77 RCTs + 3 international conferences–9 RCTs + 15 other sources–36 RCTs) (Fig. 1), and metaphylaxis RCTs included 14 publications (11 peer-reviewed-39 RCTs + 2 international conferences-6 RCTs + 1 other source-2 RCTs) (Fig. 2). Two publications assessed both prophylaxis and metaphylaxis.
Figure 1.
Forest plot of prophylaxis RCTs morbidity data sorted by antimicrobial class and control group attack rates. Random-effects meta-regression model RR predictions represented as shaded grey and black diamonds. AttRate—control group attack rate % (CER%); antimicrobials investigated included macrolides (tylosin, tilmicosin, tulathromycin, gamithromycin, tildipirosin), tetracyclines (oxytetracyline, chlortetracycline, doxycycline), amphenicols (florfenicol), cephalosporins (ceftiofur), sulfonamides (sulfadimethoxine, sulfamethazine, trimethoprim sulphonamide), and fluoroquinolones (enrofloxacin), tetracycline combinations (oxytetracyline and/or chlortetracycline + neomycin, or sulfadimethoxine and/or sulfamethazine).
Figure 2.
Forest plot of metaphylaxis RCTs morbidity data sorted by metaphylaxis definition. Random-effects meta-regression model RR predictions represented as shaded grey and black diamonds. ‘>10% Morbidity’—group medication of cattle when the BRD morbidity within the group ≥10%. ‘Fever’—group medication of cattle with pyrexia and no other symptoms. ‘In contact’—group medication of cattle in contact with clinical BRD cattle.
RCTs in feedlots were described in 51 publications (149 RCTs), four concerning veal/dairy calves (10 RCTs), two cow-calf operations (6 RCTs) and one bull-testing station (5 RCTs). Observation periods for RCTs ranged from 10 to 365 days (<20 days—26 RCTs; 20–39 days—91 RCTs; 40–80 days—40 RCTs; >80 days—11 RCTs).
The majority of publications described a uniform processing of cattle/calves upon arrival to the test facility, including injections with a variety of vaccines, vitamin products, dewormers and occasionally other products (e.g. growth implants). Afterwards, cattle were separated into treatment and control groups. These were considered typical field conditions for introduction of calves into feedlots and veal calf operations.
Most clinical trials described antimicrobial use according to the label dose (prophylaxis— 99 RCTs; metaphylaxis—44 RCTs), with a minority over the label dose (prophylaxis—11 RCTs; metaphylaxis—2 RCTs), and under the label dose (prophylaxis—12 RCTs; metaphylaxis—1 RCT). Thirteen BRD publications described the initial treatment of the negative control with saline using the same route of administration as the treatment group (45 RCTs), one with a placebo oral bolus (1 RCT), one with same formulation as the treatment group minus the antimicrobial substance (6 RCTs) and 43 publications with no placebo treatment of the control group (118 RCTs). The majority of RCTs (150) described two or more visual clinical signs in their BRD assessment (e.g. depression, poor body condition, anorexia, abnormal respiration, cough, oculo-nasal discharge). Of these, 118 RCTs also included the presence of fever in the assessment. No description was stated for 19 RCTs.
Methods of randomisation described included 20 publications (prophylaxis—60 RCTs; metaphylaxis—23 RCTs) with random-block design, 19 publications (prophylaxis—17 RCTs; metaphylaxis—20 RCTs) with systematic randomisation, 5 publications (prophylaxis—7 RCTs; metaphylaxis—2 RCTs) describing computer/statistical randomisation and 14 publications (40 RCTs) with no description. Also, 26 publications (prophylaxis—45 RCTs; metaphylaxis—34 RCTs) utilised blinding in the study design, with 4 publications (prophylaxis—2 RCTs; metaphylaxis—8 RCTs) describing the person/s giving the treatments as blinded and 22 publications (prophylaxis—43 RCTs; metaphylaxis—26 RCTs) describing the person/s checking cattle/pens for BRD as blinded.
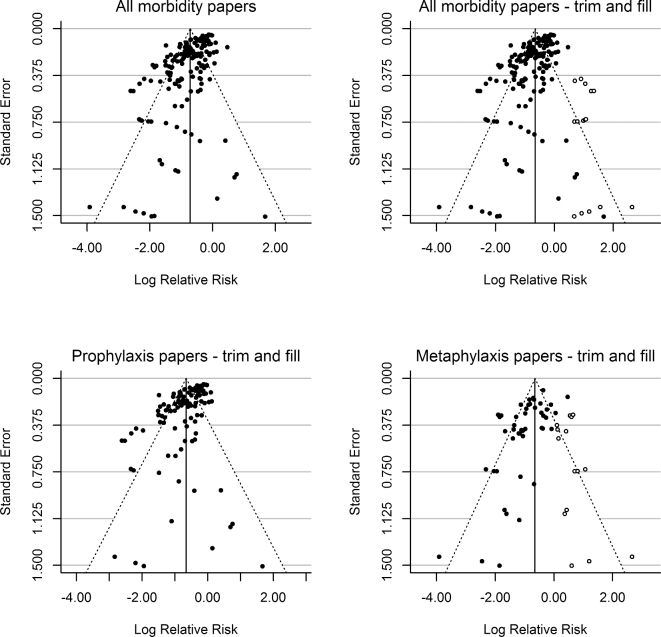
Many RCTs demonstrated a benefit in RR for reducing morbidity, with either antimicrobial prophylaxis or metaphylaxis (Figs 1 and 2), although results varied considerably between studies. The combined RR estimate (prophylaxis + metaphylaxis) was 0.49 (95% CI = 0.45–0.53). Data asymmetry was identified with funnel plots (Fig. 3). Using the Duval and Tweedie Trim and Fill method, the adjusted combined RR estimate was 0.52 (0.48–0.57). Funnel plot asymmetry was more evident for RCTs describing metaphylaxis, particularly a lack of small studies reporting poor efficacy (Fig. 3). Initially, metaphylaxis RCTs (RR, 95% CI = 0.42, 0.35–0.49) performed significantly better (P = 0.031) in reducing BRD morbidity than prophylaxis (RR, 95% CI = 0.52, 0.47–0.57). However, adjusted RR estimates revealed the two types of mass medications performed equally (metaphylaxis RR, 95% CI = 0.53, 0.43–0.64; prophylaxis RR, 95% CI = 0.52, 0.47–0.57). RCTs using parenteral antimicrobials (subcutaneous or intramuscular) alone or in combination with oral administrations performed significantly better at lowering morbidity compared to oral administration alone (i.e. RR, 95% CI = 0.47, 0.43–0.52 versus RR, 95% CI = 0.62, 0.49–0.79; P = 0.034). Blinded RCTs demonstrated significantly different results (P = 0.037) than non-blinded RCTs (blinded-RR, 95% CI = 0.45, 0.40–0.51; non-blinded-RR, 95% CI = 0.54, 0.48–0.61).
Figure 3.
Funnel plots of the morbidity data from RCTs. The upper panels show all morbidity data without (left) and with (right) Duval and Tweedie trim and fill to correct for potential publication bias (test for heterogeneity: Q (df = 168) = 1061.977, P-value < 0.0001). Published studies are represented with filled circles, where the added (fill) studies are shown with open circles. The lower panels show separate plots for prophylaxis studies (left) (test for heterogeneity: Q (df = 121) = 824.153, P-value < 0.0001) and metaphylaxis studies (right) (test for heterogeneity: Q (df = 46) = 195.687, P-value < 0.0001).
For prophylaxis RCTs, macrolides performed best in reducing BRD morbidity (RR, 95% CI = 0.43, 0.38–0.48; 28 pubs—50 RCTs), compared to tetracyclines (RR, 95% CI = 0.74, 0.62–0.88; 20 pubs—31 RCTs), tetracycline combinations (RR, 95% CI = 0.49, 0.38–0.63; 10 pubs—18 RCTs), amphenicols (RR, 95% CI = 0.60, 0.41–0.89; 2 pubs—5 RCTs), cephalosporins (RR, 95% CI = 0.57, 0.45–0.73; 7 pubs—10 RCTs), sulfonamides (RR, 95% CI = 0.81, 0.56–1.16; 7 pubs—7 RCTs) and fluoroquinolones (RR, 95% CI = 0.31, 0.13–0.71; 1 pub—1 RCT) (Fig. 1). For metaphylaxis RCTs, no significant differences were detected between antimicrobial classes (P = 0.86).
Metaphylaxis definitions included 11 publications (33 RCTs) defined as the BRD morbidity ≥10% within the group; 3 publications (3 RCTs) defined as pyrexia and no other symptoms; 5 publications (11 RCTs) as cattle in-contact within groups with visual BRD cattle. Metaphylaxis definitions demonstrated significantly different results. Best results on BRD morbidity were obtained with a ‘≥10%-morbidity’ definition (RR, 95% CI = 0.34, 0.28–0.41), compared to an ‘in-contact’ definition (RR, 95% CI = 0.53, 0.40–0.70; P = 0.011) or ‘fever’ definition (RR, 95% CI = 1.11, 0.67–1.84; P < 0.001) (Fig. 2).
The majority of RCTs (prophylaxis+metaphylaxis) fitted a correlation of decreasing NNT with increasing control group attack rates (Fig. 4). NNT for RCTs dropped below the NNT median at attack rates >25%. Observation periods (duration) of RCTs affected morbidity outcomes with short RCTs (<20 days-RR, 95% CI = 0.35, 0.28–0.42) demonstrating significantly better (P = 0.011) outcomes compared to longer RCTs (20–39 days-RR, 95% CI = 0.50, 0.45–0.56; 40–80 days-RR, 95% CI = 0.58, 0.49–0.67; > 80 days-RR, 95% CI = 0.68, 0.51–0.91). Best ARR results on BRD morbidity were clustered to only short RCT durations (e.g. ≤30 days) (Fig. 5a). Also, best ARR results on BRD morbidity were a feature of small rather than larger RCTs.
Figure 5.
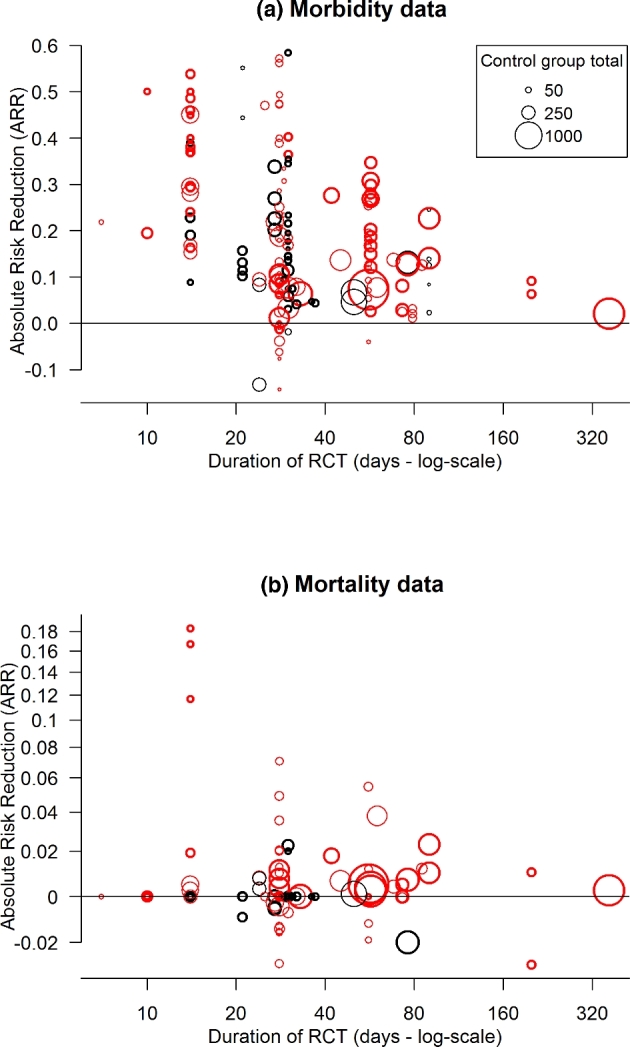
Plot of absolute risk reductions in (a) morbidity or (b) mortality for each RCT versus the duration of the RCT. For publications stating a range of duration days for RCTs, then the average value was calculated. RCTs involving either antimicrobial prophylaxis (red circles) or metaphylaxis (black circles). Size of the circles reflects the sample size of the RCT (see the legend). Bolded circles are blinded RCTs. For the purpose of visualisation of the wide range of ARR values for the mortality data, the y-values in (b) are shown on a modified log-scale (sign(y)*log10(10*y+ 1)). Labels at the tick marks are the original ARR values.
Publications described uniformly that identified BRD cattle were ‘pulled’ from the pen and treated with a pre-specified treatment protocol for that study, and returned. Thus, mortality data were confounded since all BRD cattle (i.e. treatment and control groups) were treated with antimicrobials, after recorded as morbidity data. Nevertheless, mortality data were analysed since it represented a treatment-only strategy, without prior mass medication (e.g. control group) compared to a treatment strategy, with prior mass medication (e.g. treatment group). From the original publications accepted for the morbidity data analysis, 47 publications (38 prophylaxis—89 RCTs, 11 metaphylaxis—39 RCTs) contained sufficient mortality data. BRD mortality rates were very low, with 37 of 89 (41.5%) prophylaxis RCTs and 32 of 39 (82%) metaphylaxis RCTs reported zero mortality in the control groups. The highest mortality rate was 18% (Fig. 5b).
Antimicrobial mass medications (prophylaxis + metaphylaxis) led to a relative reduction in mortality risk (RR, 95% CI = 0.62, 0.54–0.72). This was correlated with control group results, with RR declining with increasing mortality rates. A subgroup analysis revealed relative risk reductions with a control group mortality above 1.5% (RR, 95% CI = 0.55, 0.45–0.67) compared to below 1.5% (RR, 95% CI = 0.74, 0.57–0.96). However, the majority of RCTs demonstrated only minor effects in ARR (e.g. <5%) (Fig. 5b).
DISCUSSION
To the authors’ knowledge, this is the first critical appraisal of evidence for antimicrobial prophylaxis and metaphylaxis to prevent disease morbidity and mortality in a major food animal species. A systematic review and meta-analysis of RCTs concerning antimicrobial mass medications for preventing BRD identified advantages and disadvantages. Prophylaxis and metaphylaxis demonstrated moderate, yet highly variable relative risk reductions in BRD morbidity (adjusted combined RR, ±95% = 0.52, 0.48–0.57). BRD morbidity reductions were dependent on the antimicrobial classes used (Fig. 1), metaphylaxis definition criteria (Fig. 2), BRD attack rates (Fig. 4) and duration of the RCTs (Fig 5a). Disadvantages include that best positive RR results were achieved with antimicrobial classes (e.g. macrolides) considered as broad-spectrum critically important antimicrobials (CIAs) to human and veterinary medicine, or combination antimicrobials (Fig. 1). Also, the NNT to prevent one BRD case was very high under circumstances of low attack rates and variable when the infection pressure was too high for medication to prevent BRD transmission (Fig. 4).
Aetiologies for BRD outbreaks are founded in physiological, management and environmental factors in conjunction with viral and bacterial infectious agents. Genetic selection has resulted in domesticated cattle with small lungs relative to their metabolic demands, contributing to decreased respiratory compensation, particularly during periods of exertion or disease (Weekly and Veit 1995). Generally, high-risk calves/cattle are typically lightweight, recently weaned, highly co-mingled, or of auction market origin, extended transport times and unknown health/vaccination history. Despite widespread use of vaccines and antimicrobial mass medications, a sobering picture has suggested limited progress in North America to reduce the impact of BRD over the last 10–20 years (Hilton 2014). National Animal Health Monitoring System data show an increase in BRD feedlot mortality from 1.03% in 1994 to 1.6% in 2011, despite ∼16% were treated for BRD and up to 31% received prophylactic antimicrobials on arrival. Dairy heifer rearing data showed that 18.1% of pre-weaned dairy heifers were treated for BRD, including a BRD mortality of 2.3% (Dargatz 2014). Other data reveal that feedlot mortality increased by 0.05% per year over the last 13 years (Engler 2014). The reaction has been an increasing reliance on antimicrobial prophylaxis and/or metaphylaxis to control BRD, but the need for mass medications is more driven by the antiquated structure of the feedlot and veal calf industries compared to the disease itself. A major obstacle to control BRD remains the segmented infrastructure of these industries, as relatively unchanged for several decades (Ives and Richeson 2015). Calves/cattle progress through the production phase, changing ownership at any and all points, resulting in transporting, co-mingling from various sources, minimal biosecurity and other stressors, providing ample opportunity for immunosuppression and pathogen colonisation of the lower respiratory tract. It has been estimated the average number of middle men between the cattle rancher and the consumer is 15 (Ives and Richeson 2015). Cattle can be transported between two to five times throughout their lifetime, resulting in degrees of dehydration, physiologic stress and environmental/nutritional changes. This persistent infrastructure also impedes alternatives to antimicrobial mass medications, since the major BRD predisposition factors are present at the time of arrival to the facilities.
A BRD field diagnosis has major limitations for accurately identifying all BRD cattle. Common clinical signs (e.g. depression, anorexia, fever) are not pathognomonic. A recently published study using a hierarchical Bayesian latent class meta-analysis comparing BRD clinical signs to slaughter lung lesions revealed an estimated predicted diagnostic sensitivity and specificity of 0.27 (95% CI = 0.01–0.96) and 0.92 (0.14–1.00), respectively (Timsit et al.2016). There was much variability between studies due to different criteria for visual BRD diagnosis. This variability is further influenced by the number of cattle to be managed per employee, at large facilities. For example, the reported average number of cattle at high risk of developing BRD to be managed per employee at feedlots increased from 2739 to 3464 head between 2009 and 2014, in the USA and Canada (Lee et al.2015). The relevance of the RCTs from this systematic review and meta-analysis also reflects the decision-making criteria of producers, since every identified BRD case was further treated with antimicrobials. Based on NNT (Fig 4), antimicrobial mass medications had a minor effect on either reducing BRD morbidity risk or affecting the decision-making process for using further treatment antimicrobials. For example, the NNT median was 7.27, or an approximate 14% reduction in ARR. Pharmaco-economic benefits only occurred at high attack rates (e.g. ≥50%) where benefits were also variable between RCTs.
Concerns have been expressed previously about the quality of RCTs for BRD, primarily for treatment trials (O’Connor et al.2010b). Consensus statements exist for design specifications of clinical trials (e.g. CONSORT—Consolidated Standards of Reporting Trials), but the REFLECT statement for livestock clinical trials was not published until 2010 (O’Connor et al.2010a). Generally, veterinary RCTs lag behind human RCTs in quantity and quality study design features (e.g. blinding, description of randomisation, saline-treated placebo). Other limitations found in this investigation included a lack of RCTs for certain antimicrobial classes and metaphylaxis definitions. Certain types of beef production systems were under-represented (e.g. veal calves). Also, not all types of non-CIAs were represented by RCTs. More long-term studies could have better confirmed the declining benefits of initial antimicrobial mass medications. Significant explanatory variables were identified, but heterogeneity remained high in the models, due to the diversity of issues explored. Also, this heterogeneity further reflects the lack of foresight of old fashion practices of antimicrobial mass medication, based on beliefs of non-standardised risks without knowledge of the type/s of pathogens involved or antimicrobial susceptibilities. Like other food animal diseases, BRD is a disease complex encompassing multiple pathogens, and combined with a lack of foresight, will thus attract broad-spectrum antimicrobials for its successful prevention.
The results could be viewed further as general observations about antimicrobial mass medications under field conditions, describing cohorts of food animals with similar circumstances (e.g. transport, co-mingling, minimal biosecurity, broad disease definitions). Aspects of these results could fit concepts expressed in mathematical models of infectious disease transmission. BRD demonstrated disease patterns of low to high morbidity but consistently low mortality. Eventually, saturation (the resulting decline in the number of susceptible individuals to infection) occurs over time (up to 90 days for BRD) with more stable population dynamics (Grassly and Fraser 2008). The broad range of attack rates from RCTs reflects field variations in infectiousness (characteristics of infected individuals that determines the rate of spread to the susceptible population that can be broken down into biological, behavioural and environmental components) and susceptibility (biological, behavioural and environmental) of cohorts of cattle. For example, at low attack rates (<15%), the basic reproduction number (Ro) (the expected number of secondary infections resulting from infected individual/s in a population. Mathematical models typically incorporate variables that describe the probability of transmission per animal contact, the number of contacts with the infectious animal per unit time and the duration animal/s are infectious.) is sufficiently low (<1) based on a small offspring distribution (the number of secondary infections as a function of infectiousness over time) that does not economically justify prior antimicrobial mass medications (based on NNT). Under these circumstances, the disease could die out (Ro < 1) or become endemic (Ro = 1), but unlikely to progress to an epidemic (Ro > 1). A morbidity threshold does exist where antimicrobial mass medications provided more consistent reductions in morbidity risk (Figs 2 and 3), but negligible effect on mortality (Fig. 5b). This is broadly consistent with general observations that a disproportionate amount of disease transmission results from a small fraction of infected individuals (Grassly and Fraser 2008). The random effects among individuals tend to cancel each other out as the number of infected individuals increases, resulting in a more predictable progression to epidemic dynamics. Antimicrobial mass medications represent the main intervention for BRD (i.e. Ro – 1) to prevent/control an epidemic. The results of this investigation revealed that the impact of this intervention was influenced by the duration of the RCTs. For example, the highest impact clustered with only short and small RCTs (≤30 days) (Fig. 5), with progressively less impact over longer and larger RCTs. Longer RCTs better reflect feedlot/veal facilities, since cattle are typically kept for months. Also, high variability from antimicrobial interventions reflects the complex BRD infectious disease dynamics, where the likelihood of each of three possible outcome population scenarios (endemic, epidemic, disease die out) is dependent on factors, including:
Herd immunity—when a significant proportion of the population have immunity (e.g. vaccines, natural acquired or colostral immunity). Thus, more difficult for diseases to spread between individuals if a proportion are already immune, breaking the ‘chain of infection’.
Animal stress factors that promote immunosuppression (e.g. weaning, castration, dehorning)
Animal husbandry practices that promote contagious diseases (e.g. stocking density, transport of animals, co-mingling animals from different sources, poor biosecurity).
Characteristics of the bacterial clone involved in the disease (e.g. virulence factors, antigenicity, previous exposure to the population).
Although RCTs results for BRD mortality were confounded by previous treatment of BRD cattle, it does reveal an interesting aspect. The majority of RCTs reported zero mortality in control groups based on a ‘treatment-only’ strategy of visual BRD cases, with no prior mass medication, as just an effective method of preventing mortality.
CONCLUSIONS
Prudent use of antimicrobials is the judicious practice of medical principles, as ‘the cost-effective use of antimicrobials which maximises clinical therapeutic effect while minimizing both drug-related toxicity and the development of antimicrobial resistance’. (WHO 2001). This includes an accurate diagnosis, short-term effective first antimicrobial professional prescriptions based on microbial sensitivity or proven efficacy (RCTs, safety, PK/PD, spectrum of activity) and low impact on selecting antimicrobial-resistant bacteria. BRD antimicrobial mass medications fails in aspects of prudent use in that the disease to prevent is poorly distinguishable from primary viral or cases with lung lesions, and without knowledge of inciting pathogen/s or microbial sensitivity. This systematic review and meta-analysis of RCTs revealed that antimicrobial mass medications lead to a mean overall relative reduction in disease burden but did not economically lower the absolute risk for either displaying visual BRD symptoms or being selected for further antimicrobial treatments. Best relative risk reductions were primarily with broad-spectrum critically important antimicrobial classes. Metaphylaxis has a similar impact as prophylaxis, but the potential for lower antimicrobial consumption with an appropriate morbidity threshold definition that eliminates the least efficient (highest NNT) possibilities and prevents an epidemic. Prophylaxis/metaphylaxis for BRD represents major antimicrobial consumption for highly variable short-term gains in ARR, whereas RCTs of longer duration (e.g. >30 days) show progressive dampened variable ARR. Antimicrobial mass medications can also be associated with negligible improvements or worsened BRD morbidity/mortality.
Conflict of interest. None declared.
Supplementary Material
Supplementary data are available at FEMSPD online.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSPD online.
REFERENCES
- Albak C, Bradstock L, Cruise L. Prophylactic treatment of feedlot calves at processing with a long-acting oxytetracycline. Bovine Pr 1986;21:192–4. [Google Scholar]
- Anonymous. Comparative efficacy of DRAXXIN™ or Micotil® for the control of respiratory disease in cattle at high risk of developing undifferentiated bovine respiratory disease. Pfizer Animal Health Technical Bulletin 2005. [Google Scholar]
- Anonymous. Metaphylactic use of DRAXXIN® (tulathromycin) in weaned dairy calves at high risk for infectious respiratory disease. Technical Bulletin DRX13045 2013. [Google Scholar]
- Baggott D, Casartelli A, Fraisse F et al. . Demonstration of the metaphylactic use of gamithromycin against bacterial pathogens associated with bovine respiratory disease in a multicentre farm trial. Vet Rec 2011;168:241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtol DT, Johnson EG, Lechtenberg KF et al. . Multi-center field dose confirmation study of a single injection of 4 mg/kg body weight of 20, 23-di-piperdinyl-mycaminosyl-tylonolide (tildipirosin) in cattle at high risk for developing bovine respiratory disease. Study Number 2052-009-00. 2012;NADA 141–334:Freedom of Information Act Summary. [Google Scholar]
- Benton JR, Erickson GE, Klopfenstein TJ et al. . Effect of Excede® administered to calves at arrival in the feedlot on performance and respiratory disease. Nebraska Beef Cattle Reports 2008;37:99–101. [Google Scholar]
- Brazle FK. The effect of tilmicosin phosphate injection at arrival on newly purchased calves. The Professional Animal Scientist 1997;13:141–4. [Google Scholar]
- Breeze RG, Magonigle RA, McManus RF et al. . Control of shipping fever in feedlot cattle with a long-acting oxytetracycline injectable. Bovine Pr 1982;3:32–8. [Google Scholar]
- Bremer VR, Erickson GE, Klopfenstein TJ et al. . Evaluation of Excede® given at either initial processing or revaccination on bovine respiratory disease and pasture vs. feedlot receiving systems. Nebraska Beef Cattle Reports 2007;78:68–70. [Google Scholar]
- Buhman MJ, Perino LJ, Galyean ML et al. . Association between changes in eating and drinking behaviors and respiratory tract disease in newly arrived calves at a feedlot. Am J Vet Res 2000;61:1163–8. [DOI] [PubMed] [Google Scholar]
- Caluwaerts T, van de Ven J, Theeuwes P et al. . Different metaphylactic treatment schemes with Nuflor® compared to Draxxin® and control in a naturally occurring outbreak of Bovine Respiratory Disease in veal calves. European Buiatrics Forum (EBF). Marseille, France, 2009,126. [Google Scholar]
- Catry B, Duchateau L, Van de Ven J et al. . Efficacy of metaphylactic florfenicol therapy during natural outbreaks of bovine respiratory disease. J Vet Pharmacol Ther 2008;31:479–87. [DOI] [PubMed] [Google Scholar]
- Checkley SL, Campbell JR, Chirino-Trejo M et al. . Associations between antimicrobial use and the prevalence of antimicrobial resistance in fecal Escherichia coli from feedlot cattle in western Canada. Can Vet J 2010;51:853–61. [PMC free article] [PubMed] [Google Scholar]
- Coe PH, Grooms DL, Metz K et al. . Changes in antibiotic susceptibility of Escherichia coli isolated from steers exposed to antibiotics during the early feeding period. Vet Ther 2008;9:241–7. [PubMed] [Google Scholar]
- Cusack PMV. Effect of mass medication with antibiotics at feedlot entry on the health and growth rate of cattle destined for the Australian domestic market. Aust Vet J 2004;82:154–6. [DOI] [PubMed] [Google Scholar]
- Dargatz DA. Summary of BRD data from the 2011 NAHMS Feedlot and Dairy Heifer Studies. In: Proceedings of BRDS Symposium - New Approaches to BRD Prevention, Management and Diagnosis 2014; Denver, USA, 25–8. [DOI] [PubMed] [Google Scholar]
- Dedonder KD, Apley MD, Li M et al. . Pharmacokinetics and pharmacodynamics of gamithromycin in pulmonary epithelial lining fluid in naturally occurring bovine respiratory disease in multisource commingled feedlot cattle. J Vet Pharmacol Ther 2016;39:157–66. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), EFSA (European Food Safety Authority) and EMA (European Medicines Agency). ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. Stockholm/Parma/London: ECDC/EFSA/EMA. EFSA J 2015;13:4006, 114 pp. [Google Scholar]
- Edwards AJ. Respiratory diseases of feedlot cattle in the central USA. Bovine Practice 1996;30:5–7. [Google Scholar]
- Edwards TA. Control methods for bovine respiratory disease for feedlot cattle. Vet Clin N Am-Food A 2010;26:273–84. [DOI] [PubMed] [Google Scholar]
- Encinias AM, Walker DA, Murdock CW et al. . Effects of prophylactic administration of ceftiofur crystalline free acid on health and performance of newly received beef calves. Proc Western Section Am Soc Anim Sci 2006;57:160–3. [Google Scholar]
- Engler M. The impact of BRD: the current feedlot experience. Proceedings of BRDS Symposium. New Approaches to BRD Prevention, Management and Diagnosis 2014;Denver, USA, P12-16. [Google Scholar]
- European Medicines Agency (EMA). Revised guideline for the demonstration of efficacy for veterinary medicinal products containing antimicrobial substances (EMA/CVMP/627/01-Rev.1) 2016.
- European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) ‘Sales of veterinary antimicrobial agents in 29 European countries in 2014’. Trends from 2011 to 2014. 6th ESVAC report. (EMA/61769/2016) 2016.
- Fazzio LE, Giuliodori MJ, Galván WR et al. . A metaphylactic treatment with double dose oxytetracycline reduces the risk of bovine respiratory disease in feedlot calves. Rev Vet 2015;26:89–92. [Google Scholar]
- Frank GH, Briggs RE, Duff GC et al. . Effects of vaccination prior to transit and administration of florfenicol at time of arrival in a feedlot on the health of transported calves and detection of Mannheimia haemolytica in nasal secretions. Am J Vet Res 2002;63:251–6. [DOI] [PubMed] [Google Scholar]
- Frank GH, Duff GC. Effects of tilmicosin phosphate, administered prior to transport or at time of arrival, and feeding of chlortetracycline, after arrival in a feedlot, on Mannheimia haemolytica in nasal secretions of transported steers. Am J Vet Res 2000;61:1479–83. [DOI] [PubMed] [Google Scholar]
- Gallo GF, Berg JL. Efficacy of a feed-additive antibacterial combination for improving feedlot cattle performance and health. Can Vet J 1995;36:223–9. [PMC free article] [PubMed] [Google Scholar]
- Galyean ML, Gunter SA, Malcolm-Callis KJ. Effects of arrival medication with tilmicosin phosphate on health and performance of newly received beef cattle. J Anim Sci 1995;73:1219–26. [DOI] [PubMed] [Google Scholar]
- Gill DR, Smith RA, Hicks RB et al. . The effect of mass medication on health and performance of newly arrived stocker cattle. Okla AES Res Rep 1986;MP-118:260–8. [Google Scholar]
- Godinho KS, Wolf RM-LG, Sherington J et al. . Efficacy of tulathromycin in the treatment and prevention of natural outbreaks of bovine respiratory disease in european cattle. Vet Ther 2005;6:122–35. [PubMed] [Google Scholar]
- González-Martín JV, Elvira L, Cerviño López M et al. . Reducing antibiotic use: Selective metaphylaxis with florfenicol in commercial feedlots. Livest Sci 2011;141:173–81. [Google Scholar]
- Grassly NC, Fraser C. Mathematical models of infectious disease transmission. Nat Rev Microbiol 2008;6:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RJ, Jim GK, Guichon PT et al. . Efficacy of parenteral antibiotics for disease prophylaxis in feedlot calves. Can Vet J 1991;32:163–8. [PMC free article] [PubMed] [Google Scholar]
- Heinrichs AJ, Jones CM, Elizondo-Salazar JA et al. . Effects of a prebiotic supplement on health of neonatal dairy calves. Livest Sci 2009;125:149–54. [Google Scholar]
- Hendrick SH, Bateman KG, Rosengren LB. The effect of antimicrobial treatment and preventive strategies on bovine respiratory disease and genetic relatedness and antimicrobial resistance of Mycoplasma bovis isolates in a western Canadian feedlot. Can Vet J 2013;54:1146–56. [PMC free article] [PubMed] [Google Scholar]
- Hibbard B, Meeuwse DM, Bryson WL et al. . EXCEDE™ vs. Micotil® for control of bovine respiratory disease using a post-metaphylaxis interval. Pfizer Animal Health Technical Bulletin 2002. [Google Scholar]
- Higgins JP, Green S. Cochrane Handbook for Systematic review and meta-analysiss of Interventions version 5.1.0. The Cochrane Collaboration. 2011http://handbook.cochrane.org/ (20 July 2017, date last accessed). [Google Scholar]
- Hill WJ, Gill DR, Ball RL. Effects of aureomycin, delivered through the drinking water, on shipping stressed stocker cattle. Okla AES Res Rep 1993;304–7. [Google Scholar]
- Hilton WM. BRD in 2014- Where have we been, where are we now, and where do we want to go? In: Proceedings of BRDS Symposium - New Approaches to BRD Prevention, Management and Diagnosis 2014; Denver, USA, 7–11. [Google Scholar]
- Hughes TA, Tice GA, O’Connor D. An evaluation of the use of Micotil, metaphylactically, on arrival, for calf pneumonia. Irish Vet J 1996;49:622–4. [Google Scholar]
- Ives SE, Richeson JT. Use of antimicrobial metaphylaxis for the control of bovine respiratory disease in high-risk cattle. Vet Clin N Am Food A 2015;31:341–50. [DOI] [PubMed] [Google Scholar]
- Janzen ED, McManus RF. Observations on the use of a longacting oxytetracycline for in-contact prophylaxis of undifferentiated bovine respiratory disease in feedlot steers under Canadian conditions. Bovine Pr 1980;15:87–90. [Google Scholar]
- Johnson JC, Bryson WL, Barringer S et al. . Evaluation of on-arrival versus prompted metaphylaxis regimens using ceftiofur crystalline free acid for feedlot heifers at risk of developing bovine respiratory disease. Vet Ther 2008;9:53–62. [PubMed] [Google Scholar]
- Kato T, Saito M, Shoji K et al. . Prophylactic efficacy of enrofloxacin and tilmicosin on bovine respiratory disease in newly arrived beef cattle associated with Pasteurella multocida and Mycoplasma. J Jpn Vet Med Assoc 2003;56:7–11. [Google Scholar]
- Kilgore WR, Spensley MS, Sun F et al. . Clinical effectiveness of tulathromycin, a novel triamilide antimicrobial, for the control of respiratory disease in cattle at high risk for developing bovine respiratory disease. Vet Ther 2005;6:136–42. [PubMed] [Google Scholar]
- King NB, Edgington BH, Ferguson LC et al. . Preliminary results in the control and treatment of shipping fever complex in beef cattle. J Am Vet Med Assoc 1955;127:320–3. [PubMed] [Google Scholar]
- Klemesrud M, Apfel M, Klopfenstein TJ et al. . Synchronizing micotil treatment with time of sickness in newly received calves. Nebraska Beef Reports 1997;439:60–1. [Google Scholar]
- Kreikemeier K, Stokka G, Marston T. Influence of delayed processing and mass medication with either chlortetracycline (CTC) or tilmicosin phosphate (Micotil) on health and growth of highly stressed calves. In: Kansas State University Cattle Feeders Day Progress Report No. 773, 1996, 23–7. [Google Scholar]
- Lauridsen BH, Jorgensen J, Olsen L. Metaphylactic treatment against enzootic pneumonia of calves. In: Proceedings XVIII World Buiatrics Congress 1994; Bologna, Italy, 713–715. [Google Scholar]
- Lechtenberg KF, Daniels CS, Royer GC et al. . Field efficacy study of gamithromycin for the control of bovine respiratory disease in cattle at high risk of developing the disease. Int J Appl Res Vet Med 2011;9:184–92. [Google Scholar]
- Lechtenberg KF, Hanna MJ. A Study to evaluate the prophylactic efficacy of Norbrook's OXYTET 30% injectable formulation in reducing the incidence and severity of bovine respiratory disease (BRD). Study Number: MVS-4Q96-N-BRD-NB-01 2003;NADA 141–143: Freedom of Information Act Summary. [Google Scholar]
- Lee TL, Terrell SP, Bartle SJ et al. . Current feedlot cattle health and well-being program recommendations in the United States and Canada: the 2014 feedlot veterinary consultant survey. Bovine Pr 2015;49:124–31. [Google Scholar]
- Lees P, Shojaee Aliabadi F. Rational dosing of antimicrobial drugs: animals versus humans. Int J Antimicrob Ag 2002;19:269–84. [DOI] [PubMed] [Google Scholar]
- Lofgreen GP. Mass medication in reducing shipping fever-bovine respiratory disease complex in highly stressed calves. J Anim Sci 1983;56:529–36. [DOI] [PubMed] [Google Scholar]
- McClary D, Vogel G. Effect of timing of tilmicosin metaphylaxis on control of bovine respiratory disease and performance in feeder cattle. Bovine Pr 1999;33:155–61. [Google Scholar]
- McCoy RA, Stock RA, Shain DH et al. . Effect of Micotil® 300 on receiving and finishing performance and health of calves. Nebraska Beef Report 1994;33–5. [Google Scholar]
- McCoy RA, Stock RA, Shain DH et al. . Micotil® 300 on Health, Performance, and Feed Intake. Nebraska Beef Report 1995;38–41. [Google Scholar]
- Martín GJV, Partida EL, Villalobos PN et al. . Evaluation of mass and selective metaphylaxis medication with florfenicol at feedlot entry as a tool against bovine respiratory disease under commercial conditions in Spain. Cattle Practice 2007;15:309–11. [Google Scholar]
- Mechor GD, Vogel G. Variability in bovine respiratory disease (BRD) morbidity and mortality associated with arrival truckload. Tech Report Elanco Study T5CB39905 1999. [Google Scholar]
- Moore PR, Evenson A, Luckey TD et al. . Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J Biol Chem 1946;165:4376.al. [PubMed] [Google Scholar]
- Morck DW, Merrill JK, Thorlakson BE et al. . Prophylactic efficacy of tilmicosin for bovine respiratory tract disease. J Am Vet Med Assoc 1993;202:273–7. [PubMed] [Google Scholar]
- O’Connor AM, Sargeant JM, Gardner IA et al. . The REFLECT statement: Methods and processes of creating reporting guidelines for randomized controlled trials for livestock and food safety. Prev Vet Med 2010a;93:11–8. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Wellman NG, Ric M et al. . Characteristics of clinical trials assessing antimicrobial treatment of bovine respiratory disease, 1970–2005. J Am Vet Med Assoc 2010b;237:701–5. [DOI] [PubMed] [Google Scholar]
- O’Neill RO, Mooney J, Connaghan E et al. . Patterns of detection of respiratory viruses in nasal swabs from calves in Ireland: a retrospective study. Vet Rec 2014;175:351. [DOI] [PubMed] [Google Scholar]
- Perino LJ, Apley MD. Clinical trial design in feedlots. Vet Clin N Am Food A 1998;14:343–65. [DOI] [PubMed] [Google Scholar]
- Perry TW, Beeson WM, Mohler MT et al. . Value of chlortetracycline and sulfamethazine for conditioning feeder cattle after transit. J Anim Sci 1971;32:137–40. [DOI] [PubMed] [Google Scholar]
- Perry TW, Riley JG, Mohler MT et al. . Use of chlortetracycline for treatment of new feedlot cattle. J Anim Sci 1986;62:1215–9. [DOI] [PubMed] [Google Scholar]
- Peters AR. Use of a long-acting oxytetracycline preparation in respiratory disease in young beef bulls. Vet Rec 1985;116:321. [DOI] [PubMed] [Google Scholar]
- Scheel EH. Sulfamethazine prophylaxis in shipping fever. Mod Vet Pract 1966;47:75–6. [Google Scholar]
- Schlegel ER, Blasi DA, Hollenbeck WR et al. . Performance and health effects of Zuprevo 18% in newly received, highly stressed beef cattle. Kansas State University Agricultural Experiment Station and Cooperative Extension Service. Beef Cattle Research Report of Progress 2014;1101:16–9. [Google Scholar]
- Schmidt DG, Shirley JE, Titgemeyer EC et al. . The effects of metaphylactic treatment with tilmicosin on the incidence of bovine respiratory disease in growing dairy replacement heifers. J Dairy Sci 2001;84(suppl 1):187. [Google Scholar]
- Schumann FJ, Janzen ED, McKinnon JJ. Prophylactic tilmicosin medication of feedlot calves at arrival. Can Vet J 1990;31:285–8. [PMC free article] [PubMed] [Google Scholar]
- Schumann FJ, Janzen ED, McKinnon JJ. Prophylactic medication of feedlot calves with tilmicosin. Vet Rec 1991;128:278–80. [DOI] [PubMed] [Google Scholar]
- Sgoifo Rossi CA, Vandoni SL, Bonfanti M et al. . Effects of arrival medication with gamithromycin on bovine respiratory disease in feedlot cattle in Italy. Int J Appl Res Vet Med 2010;8:87–96. [Google Scholar]
- Skogerboe T, Evans N, Mann D et al. . Efficacy of tulathromycin in the treatment of cattle at high risk of bovine respiratory disease. Proceedings of the 23rd World Buiatrics Congress 2004;34:99. [Google Scholar]
- Smith RA, Stokka GL, Radostits OM et al. . Health and production management in beef feedlots. In: Radostits OM. (ed.), Herd Health: Food Animal Production Medicine. WB Saunders Company, Philadelphia, 2001, 581–633. [Google Scholar]
- Stanford K, Gibb DJ, Schwartzkopf-Genswein KS et al. . Feeding subtherapeutic antimicrobials to low-risk cattle does not confer consistent performance benefits. Can J Anim Sci 2015;95:589–97. [Google Scholar]
- Swann MM. Joint Committee on the Use of Antibiotics in Animal Husbandry and Veterinary Medicine. 1969, London, UK: HMSO. [Google Scholar]
- Tennant TC, Ives SE, Harper LB et al. . Comparison of tulathromycin and tilmicosin on the prevalence and severity of bovine respiratory disease in feedlot cattle in association with feedlot performance, carcass characteristics, and economic factors. J Anim Sci 2014;92:5203–13. [DOI] [PubMed] [Google Scholar]
- Thompson PN, Stone A, Schultheiss WA. Use of treatment records and lung lesion scoring to estimate the effect of respiratory disease on growth during early and late finishing periods in South African feedlot cattle. J Anim Sci 2006;84:488–98. [DOI] [PubMed] [Google Scholar]
- Timsit E, Dendukuri N, Schiller I et al. . Diagnostic accuracy of clinical illness for bovine respiratory disease (BRD) diagnosis in beef cattle placed in feedlots: A systematic literature review and hierarchical Bayesian latent-class meta-analysis. Prev Vet Med 2016;135:67–73. [DOI] [PubMed] [Google Scholar]
- Urban-Chmiel R, Grooms DL. Prevention and control of bovine respiratory disease. J Livest Sci 2012;3:27–36. [Google Scholar]
- Van Koevering MT, Gill DR, Smith RA et al. . Mass medication treatments for newly arrived stocker cattle. Okla AES Res Rep 1992;333–8. [Google Scholar]
- Viechtbauer W. Conducting meta-analysis in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- Weekly LB, Veit HP. Potential morphologic and physiologic factors that may predispose the bovine lung to respiratory disease. Comp Cont Educ 1995;17:974–82. [Google Scholar]
- World Health Organization. WHO Global Health Strategy for Containment of Antimicrobial Resistance. 2001http://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf (20 July 2017, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at FEMSPD online.